Abstract
Purpose
The highly vascular malignant brain tumor glioblastoma (GBM) appears to be an ideal target for anti-angiogenic therapy; however, clinical trials to date suggest the VEGF antibody bevacizumab affects only progression-free survival. Here we analyze a group of patients with GBM who received bevacizumab treatment at recurrence and are stratified according to tumor molecular and genomic profile (TCGA classification), with the goal of identifying molecular predictors of the response to bevacizumab.
Methods
We performed a retrospective review of patients with a diagnosis of glioblastoma who were treated with bevacizumab in the recurrent setting at our hospital, from 2006 to 2014. Treatment was discontinued by the treating neuro-oncologists, based on clinical and radiographic criteria. Pre- and post-treatment imaging and genomic subtype were available on 80 patients. We analyzed time on bevacizumab and time to progression. EGFR gene amplification was determined by FISH.
Results
Patients with classical tumors had a significantly shorter time on bevacizumab than mesenchymal, and proneural patients (2.7 vs. 5.1 vs. 6.4 and 6.0 months respectively, p = 0.011). Classical subtype and EGFR gene amplification were significantly associated with a shorter time to progression both in univariate (p < 0.001 and p = 0.007, respectively) and multivariate analysis (both p = 0.010).
Conclusion
EGFR gene amplification and classical subtype by TCGA analysis are associated with significantly shorter time to progression for patients with recurrent GBM when treated with bevacizumab. These findings can have a significant impact on decision-making and should be further validated prospectively.
Keywords: Bevacizumab, Classical, EGFR, Glioblastoma, Mesenchymal, Proneural
Introduction
Since its description in 1993 the monoclonal antibody against VEGF (bevacizumab) has been extensively studied [1, 2], and is in use for several cancer types. Due to its highly vascularized nature, glioblastoma was once considered an ideal target for anti-angiogenic therapies like bevacizumab (Avastin) [3]. However, data emanating from clinical trials as well as off trial experience have shown a modest and unsustained impact on disease progression. Two recent phase III trials showed that adding bevacizumab to standard chemo-radiotherapy in the upfront setting only improved progression-free survival with no overall survival benefit [4, 5]. A recent meta-analysis of randomized controlled trials of bevacizumab combined with chemotherapy in the recurrent setting also showed, an effect only on progression-free survival [6]. Nonetheless bevacizumab continues to be used in some settings, especially in recurrent tumors that have failed standard therapies and/or that do not qualify for targeted therapies. In view of increasing drug costs, the complications of the treatment and the lack of demonstrated survival benefit, identification of potential predictors of bevacizumab response, beyond common factors such as KPS could help guide treatment decisions [7].
Additional experience with bevacizumab has allowed for clarification of complications of treatment in GBM that include thrombocytopenia, cerebral hemorrhages, arterial thrombo-embolic events, proteinuria, hypertension, visceral perforation, and inhibition of wound healing [8–12]. Furthermore, as the experience with this therapy grew, a more invasive pattern of progression of GBM has been observed to develop while under anti-angiogenic therapy [13, 14]. A potential mechanism involving the inhibition of the HIF1α mediated hypoxic response in the tumor micro-environment has been proposed to underlie the more invasive or multifocal phenotype [15]. In light of these potential downsides, identifying which patients will benefit the most from bevacizumab treatment is crucial. We hypothesized that stratification by molecular profile, data that is increasingly available at many institutions, might provide a basis for prediction of treatment response, and eventually a rational parameter to be used for selecting patients to be treated with bevacizumab.
Recent large-scale genomic analyses have revealed patterns of molecular changes within tumor subclasses that harbor distinct underlying biology and clinical prognosis [16, 17]. Over the last decade or so, the TCGA (The Cancer Genome Atlas) classification was adopted by many centers, including ours. According to this schema, glioblastomas can be classified by transcriptomal features into classical, mesenchymal, neural or proneural subtypes that exhibit different gene mutation profiles, biological properties and treatment responses [17]. In addition, several well-known molecular markers such as IDH-1 mutation, EGFR amplification or mutation, and MGMT promoter methylation are often reported in GBM [18]. Molecular subtyping is increasingly incorporated into more traditional schemes, such as the latest WHO classification update [19]. The clinical challenge for the future will be to match treatment to the best molecular fit.
An earlier post-hoc analysis of the BELOB trial determined that tumors classified as “classical subtype” per TCGA criteria at first diagnosis, when treated with bevacizumab and CCNU at recurrence), led to an improvement in progression free survival [20]. Notably this cohort had almost exclusively classical tumors. In this report we sought to determine if molecular subclasses of glioblastoma (determined at the time of recurrence) respond in a distinct manner to bevacizumab treatment when given at recurrence.
Methods
Patient selection
Patients with pathologically confirmed glioblastoma treated with bevacizumab (Genentech South San Francisco, California, United States) in the recurrent setting and for whom genomic subtype analysis according to TCGA criteria (classical, proneural, mesenchymal, and neural) was available, were retrospectively identified. The classification was performed soon after tissue acquisition, independently of treatment decisions, and confirmed by CB and JH. Patients meeting these criteria treated at our hospital between 2006 and 2014 were retrieved by a search of a prospectively collected brain tumor registry database. The research protocol was approved by the IRB. KPS, gender, age, date of death and other treatment modalities were collected.
Molecular data
All molecular data was determined at recurrence. EGFR amplification was determined by FISH analysis, EGFR VIII by immunostaining, and MGMT promoter methylation status by real-time methylation specific PCR at our institution. IDH mutation analysis has been available on more recent cases but was not available on enough patients to do a meaningful analysis.
Nanostring analysis
Analysis of the Cancer Genome Atlas (TCGA) glioblastoma transcriptional subclasses was determined by using a semiquantitative gene expression profiling assay (Nanostring nCounter, Seattle, Washington, USA) based on 81 genes selected from the initial TCGA publication as described earlier [17, 21, 22]. Briefly, RNA from 192 GBM samples from TCGA was analyzed with codeset of 146 probes, including for 81 genes previously selected to distinguish TCGA expression subclasses. Raw code-set counts were normalized by a panel of GBM-invariant genes [22] and were used to generate centroids for four transcriptomal classes (proneural, classical, mesenchymal and neural) according to published classification by TCGA [17]. RNA was extracted from tumor tissue and assayed by the same Nanostring protocol and codeset. Patient sample RNA transcriptomal class assignments were assigned by the nearest TCGA class centroid by correlation.
Outcomes
Our main outcome measurement was time to progression on bevacizumab, defined as the time from the start of bevacizumab treatment until clinical progression as determined by the treating neuro-oncologist, and/or radiographic progression determined by RANO criteria [23]. Patients who stopped bevacizumab treatment for reasons other than progression were censored. In addition overall survival and time on bevacizumab were assessed. Bevacizumab was discontinued due to progression, toxicity or complications as determined by the individual treating neuro-oncologist. Since the determination of clinical progression is often based on MRI progression, we analyzed MRI changes during bevacizumab treatment. It is well known that bevacizumab can diminish gadolinium enhancement after contrast [24], so we included flair changes and multifocality changes in addition to contrast enhancement [23]. Radiographic progression while on bevacizumab was noted as either an increase in tumor volume on FLAIR or T1 weighed images with contrast or as the development of an additional lesion(s) (multifocal disease) on post treatment MRI, compared to pretreatment MRI. We included both clinical and radiographic progression in our outcomes evaluation, because clinical deterioration in this patient population is often attributed to disease progression in the absence of clear etiology such as seizures, even though it is not always extensively investigated. Other reasons for deterioration such as cognitive decline related to previous therapy (e.g. radiation), side effects of other medications or delirium, can therefore not be fully excluded.
Multifocal change
Since a more invasive behavior of GBM has been described in the setting of treatment with bevacizumab, we specifically looked at multifocal change [13]. We defined multifocal change as the appearance of a discrete new lesion (on FLAIR or T1 + contrast sequences), which have no visible connection to the existing lesion.
Volumetric analysis
Volumetric measurements were obtained using iPlan Net 3.0.0 software (BrainLAB AG, Germany 2009). Regions of interest (ROIs) were manually drawn by two neurosurgeons (KEH and YE) on each post-contrast axial T1-weighted and FLAIR image and used to compute the volume of the tumor in cubic centimeters. Tumor volume was measured on the MRI scan nearest to the start date and end point of bevacizumab treatment.
Statistical methods
Statistical testing was designed and performed by statisticians (JZ and KP). Associations between patient characteristics and MRI features with genomic subtype and molecular profile were examined using Fisher’s exact test and the Kruskal–Wallis test. Time on bevacizumab was compared between genomic subtypes using Kruskal–Wallis test since all patients stopped using bevacizumab at our last follow-up. The Kaplan–Meier method and Cox proportional hazards regression models were fit to evaluate associations of genomic subtype, age, gender, KPS, molecular profiles with time to progression on bevacizumab and overall survival (OS). Death was not a competing risk in the analysis of progression considering that all deaths occurred after stopping bevacizumab. Considering the number of patients who had undetermined EGFR amplification (19%) or EGFR VIII expression (48%), undeterminate status were treated as a separate group in above analyses.
A p value less than 0.05 was considered statistically significant. All analyses were performed in software packages SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and R version 3.1 (The R Foundation for Statistical Computing).
Results
Patient characteristics
This study identified 84 glioblastoma patients with transcriptomally-defined TCGA subtype who received bevacizumab for recurrent tumor. Four patients were excluded due to insufficient documentation of treatment and MRI dates. Table 1 describes characteristics of the 80 patients: 21% classical, 29% mesenchymal, 25% proneural and 5% neural. Clinical or radiographic progression was found in 65 patients. In 15 patients bevacizumab was stopped for reasons other than clinical or radiographic progression. Those included: wound dehiscence, nephrotoxicity, DVT, bowel perforation and intracranial hemorrhage. Among those patients, 8 had a mesenchymal subtype, 3 had a classical, 2 had a proneural and 2 had a neural subtype. Age and KPS were similarly distributed across the different genomic subtypes. The median time on bevacizumab was 4.6 months (range 0.2–32.2). The pre-treatment MRI was performed within 66 days (interquartile range (IQR6–16) prior to bevacizumab start, and post-treatment MRI was performed between 75 days prior to bevacizumab discontinuation and 209 days post bevacizumab end (IQR 2 prior—3 post). Although this is a wide range, most were done within a few days of treatment start and discontinuation; and time of MRI did not differ significantly among the different subtypes (p = 0.783). The majority of patients who progressed received other therapies during and after stopping treatment with bevacizumab, which were not statistically different amongst the different subtypes (Supplementary Table S1).
Table 1.
Patient, molecular and imaging characteristics by TCGA subtype (N = 80)
| All patients | Classical | Mesenchymal | Neural | Proneural | p value | |
|---|---|---|---|---|---|---|
| N | 80 | 21 | 29 | 5 | 25 | |
| Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | ||
| Age at treatment | 60.4 (25.1, 80.33) | 58.2 (40.1, 77.1) | 61.5 (34.4, 80.3) | 65.5 (45.6, 71.5) | 59.6 (25.1, 74.1) | 0.782 |
| Pre-treatment volume on MRI, cm3 | 20.03 (0, 96.36), n = 75 | 14.3 (0.5, 48.5) | 26.5 (0, 96.4) | 25.6 (6, 91.7) | 15.9 (0.7, 89) | 0.255 |
| Post treatment volume on MRI, cm3 | 17.59 (0, 155.56), n = 72 | 25.4 (0, 155.6) | 19.7 (0.2, 149.1) | 22.9 (3.1, 91.7) | 13.2 (0, 97) | 0.457 |
| % Volume change on MRI | 1% (−100%, 4484%), n = 72 | 120% (−100%, 4484%) | 12% (−98%, 2088%) | −66% (−90%, 1436%) | −26% (−100%, 1283%) | 0.272 |
| Pre-treatment volume on MRI flair, cm3 | 109.21 (2.66, 275.65), n = 73 | 101.1 (6.2, 235.3) | 119.9 (4.9, 275.6) | 52.2 (9.8, 275.3) | 88.7 (2.7, 229.2) | 0.513 |
| Post treatment volume on MRI flair, cm3 | 102.32 (22.73, 283.25), n = 70 | 68.1 (27.5, 278.3) | 114.9 (33.4, 283.2) | 104.8 (72.8, 173.5) | 96 (22.7, 210.9) | 0.165 |
| % Volume change on MRI flair | −2% (−74%, 1902%), n = 70 | −4% (−69%, 1902%) | 2% (−72%, 306%) | 133% (−51%, 639%) | −13% (−74%, 1396%) | 0.595 |
| Time to treatment failurea, months | 4.61 (0.23, 32.2) | 2.7 (0.9, 10.1) | 5.1 (0.2, 32.2) | 6.4 (3.7, 15.2) | 6 (0.5, 14.5) | 0.011 |
| N(%) | N(%) | N(%) | N(%) | N(%) | ||
| Gender | 0.677 | |||||
| F | 33 (41%) | 11 (33%) | 11 (33%) | 2 (6%) | 9 (27%) | |
| M | 47 (59%) | 10 (21%) | 18 (38%) | 3 (6%) | 16 (34%) | |
| Multifocal change | 0.690 | |||||
| N | 69 (92%) | 17 (25%) | 26 (38%) | 4 (6%) | 22 (32%) | |
| Y | 6 (8%) | 2 (33%) | 1 (17%) | 0 (0%) | 3 (50%) | |
| KPS | 0.480 | |||||
| 50–70 | 36 (45%) | 12 (33%) | 13 (36%) | 1 (3%) | 10 (28%) | |
| 80–100 | 44 (55%) | 9 (20%) | 16 (36%) | 4 (9%) | 15 (34%) | |
| MGMT methylated status | 0.011 | |||||
| No | 41 (51%) | 7 (17%) | 18 (44%) | 2 (5%) | 14 (34%) | |
| Yes | 14 (18%) | 6 (43%) | 0 (0%) | 2 (14%) | 6 (43%) | |
| Unknown | 25 (31%) | 8 (32%) | 11 (44%) | 1 (4%) | 5 (20%) | |
| EGFR gene amplified | < 0.001 | |||||
| No | 37 (46%) | 0 (0%) | 17 (46%) | 2 (5%) | 18 (49%) | |
| Yes | 28 (35%) | 16 (57%) | 5 (18%) | 3 (11%) | 4 (14%) | |
| Unknown | 15 (19%) | 5 (33%) | 7 (47%) | 0 (0%) | 3 (20%) | |
| EGRF VIII status | 0.207 | |||||
| No | 30 (38%) | 8 (27%) | 10 (33%) | 0 (0%) | 12 (40%) | |
| Yes | 12 (15%) | 4 (33%) | 2 (17%) | 2 (17%) | 4 (33%) | |
| Unknown | 38 (48%) | 9 (24%) | 17 (45%) | 3 (8%) | 9 (24%) |
KPS Karnofsky performance score, MGMT O6-methylguanin-DNA-methyltransferase, EGFR epidermal growth factor receptor
All patients were off Avastin at the last followup
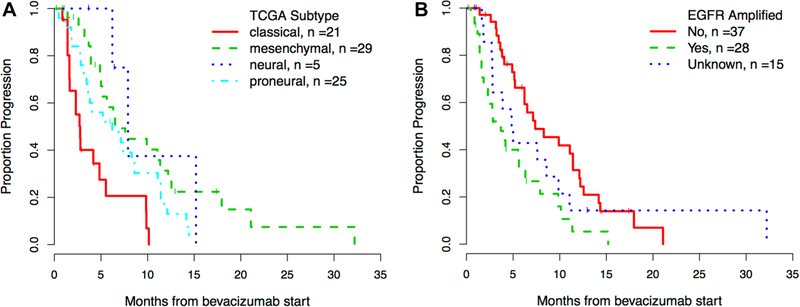
Classical glioblastomas have the shortest time on bevacizumab and a higher risk of progression
Time on bevacizumab was significantly shorter for the classical GBMs compared to the mesenchymal, neural and proneural subgroups (2.7 vs. 5.1, 6.4 and 6.0 months respectively, p = 0.011, Table 1). The classical subgroup also had a higher risk of progression than other subgroups in univariate analysis (p < 0.001, Fig. 1a; Table 2) and remained significant in multivariate analysis (p = 0.010). Of note, in the mesenchymal group 3 patients (10.3%) remained on bevacizumab 18 months or more versus none in the proneural and classical groups, suggesting some durability to this treatment in a select subgroup of patients.
Fig. 1.
Time to progression on bevacizumab by a TCGA subtype b EGFR amplification status. Kaplan–Meier curve for time to progression on bevacizumab stratified by TCGA subtype (a) and EGFR amplification status (b)
Table 2.
Time to progression on bevacizumab (N = 80)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at treatment start | 1.00 (0.97, 1.02) | 0.829 | ||
| Baseline flair MRI volumea, cm3 | 1.01 (0.97, 1.04) | 0.742 | ||
| Gender | 0.854 | |||
| F | 1 | |||
| M | 1.05 (0.64, 1.73) | |||
| Nanostring | < 0.001 | 0.010 | ||
| Classical | 1 | 1 | ||
| Mesenchymal | 0.26 (0.13, 0.52) | 0.39 (0.17, 0.88) | ||
| Neural | 0.22 (0.06, 0.77) | 0.23 (0.06, 0.85) | ||
| Proneural | 0.43 (0.23, 0.82) | 0.76 (0.29, 1.95) | ||
| KPS | 0.805 | |||
| 50–70 | 1 | |||
| 80–100 | 1.07 (0.64, 1.76) | |||
| MGMT methylated status | 0.725 | |||
| No | 1 | |||
| Yes | 1.29 (0.66, 2.53) | |||
| Unknown | 1.16 (0.66, 2.04) | |||
| EGFR gene amplified | 0.007 | 0.010 | ||
| No | 1 | 1 | ||
| Yes | 2.39 (1.36, 4.18) | 4.00 (1.63, 9.77) | ||
| Unknown | 1.32 (0.67, 2.61) | 2.25 (1.03, 4.92) | ||
| EGRF VIII status | 0.014 | 0.001 | ||
| Absence | 1 | 1 | ||
| Presence | 0.67 (0.34, 1.32) | 0.35 (0.16, 0.79) | ||
| Unknown | 0.44 (0.25, 0.78) | 0.33 (0.17, 0.62) | ||
HR hazard ratio
Progression on bevacizumab was defined as either clinical or radiologic progression (N = 65)
The increment of hazard ratio estimates is every 10-unit increase in these size measurements
EGFR amplification is associated with a shorter time to progression
EGFR amplification was determined in 85% of the patients and in 43% (28/65) of them the gene was amplified. As expected, the amplified EGFR gene was significantly more often present in the classical subtype tumor (p < 0.001). Interestingly, amplified EGFR status was associated with a higher risk of progression on bevacizumab (p = 0.007, Fig. 1b; Table 2). This finding remained significant after controlling for genomic subtype and EGFR VIII expression in multivariate analysis (p = 0.010, Table 2). MGMT promoter methylation status was available in 69% of the patients and was more often unmethylated in patients with mesenchymal tumors (p = 0.011) but was not associated with a risk of progression (p = 0.725, Tables 1, 2).
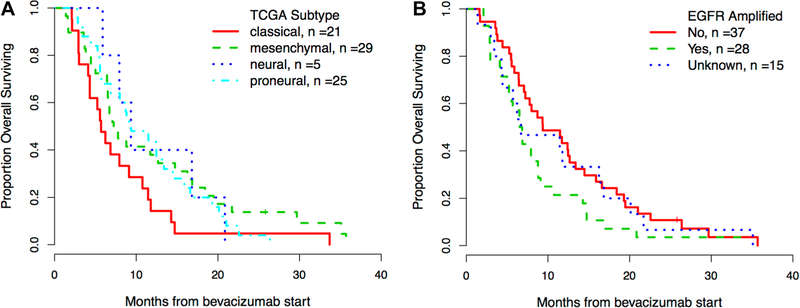
Overall survival by EGFR amplification status and subtype
All but 1 of the patients in this cohort died. The median overall survival from treatment initiation was 7.9 months (95% CI 6.5–11.4). Consistent with time to progression, patients with either the classical phenotype or EGFR-amplified tumors seemed to do worse although these differences were not statistically significant (Fig. 2a, b). These results should be interpreted with caution in light of the additional treatments that these patients received.
Fig. 2.
Overall survival (OS) by a TCGA subtype and b EGFR amplification status. Kaplan–Meier survival curve stratified by TCGA subtype (a) and EGFR amplification status (b)
No significant difference in tumor volume and multifocal change between subtypes
Large differences in tumor volume and in volume changes were noted in the different subtypes on T1 with contrast and FLAIR MRI images, but none reached statistical significance (Table 1). Multifocal change while under bevacizumab treatment was a relatively common finding (92%) but it was not different among the subtypes (Table 1).
Discussion
Our data suggest that glioblastomas that exhibit EGFR amplification or classical TCGA subtype are associated with a shorter time to progression on bevacizumab in the recurrent setting, in comparison to tumors with a proneural, neural and mesenchymal subtype or tumors without EGFR amplification. Patients with a classical subtype had a median time to progression of 2.8 months (95% CI 1.6–4.8) and in patients with EGFR amplified tumor, the median time to progression was 3.7 months (95% CI 1.6–5.6). A worse outcome in EGFR-amplified and classical tumors was statistically significant on both univariate and multivariate analysis. No difference in tumor volume change or multifocal change while on bevacizumab was observed among the different tumor subtypes. Our results therefore suggest that this treatment may be better reserved for patients without EGFR amplification or with a non-classical subtype.
This study has several limitations: the two most significant being the retrospective nature of the analysis and the different and intensive treatments that patients received in addition to bevacizumab. Even though receiving additional treatments was similarly distributed across the tumor sub-types, a treatment-related potential bias cannot be excluded. Another limitation is the fact that the decision to discontinue bevacizumab was often made on clinical grounds, as determined by the treating neuro-oncologist, and not necessarily always with clear evidence for radiographic progression. However the high volume of glioblastoma patients treated at MSKCC and the fact that treatment decisions are made in a multidisciplinary setting (e.g. tumor board) increases the uniformity of decision making across patients. Strengths of the study include the objective nature of the radiographic analysis and the fact that the treating physicians were blinded to the tumor subtype at the time of treatment. Another strength of the study is that the tissue analysis was done on the recurrent tumor. This is especially important since there are many genetic and epigenetic differences between the primary neoplasm at diagnosis and the recurrent tumor [25]. An important recent initiative to understand this process better is the Glioma Longitudinal Analysis (GLASS) Consortium, which has been initiated to better understand the recurrence process and, in doing so, discover vulnerabilities that can be used for therapeutic intervention [26].
Our finding of a strong association between EGFR amplification and a shorter time to progression on bevacizumab is novel. The fact that EGFR amplification alone also resulted in a statistically powerful difference in terms of progression on bevacizumab is important, as it is more readily determined by a simple widely available test (FISH analysis). TCGA class analysis might not be routinely available in every institution. Also, EGFR amplification is usually retained from primary to recurrent tumors [27]. It is interesting to note that some patients with mesenchymal tumors, that were all MGMT unmethylated, seemed to do better on bevacizumab in this study. In a few cases some patients managed to stay on this treatment for more than 18 months, suggesting that the mesenchymal subtype may be more suitable to this treatment. This is especially promising since this subtype was shown to have the worst prognosis and recurrent tumors have often shown a switch to this sub-type [28]. Although the mesenchymal subtype has the most “angiogenic” signature, thus was expected to respond most to bevacizumab, a recent report suggested that the proneural subclass benefited more from bevacizumab treatment in the upfront setting. In a retrospective analysis of the AvAglio trial, patients with wild type IDH proneural glioblastomas had a significant overall survival advantage of 17.1 versus 12.8 months versus placebo. The underlying mechanism of this preferential response is still unclear [29]. Interestingly, another phase II trial, that looked at hypofractionated stereotactic radiotherapy schedule with temozolomide and bevacizumab for newly diagnosed glioblastomas, found that proneural tumors did worse than the other subtypes, although this did not reach statistical significance for overall survival [21]. As mentioned earlier, molecular analysis of patients in the BELOB trial led the authors to conclude that in the recurrent setting classical tumors had the longest progression free survival when treated with bevacizumab and CCNU [20]. Even though this patient cohort was much more uniform, the tissue analysis was done on the original tumor and not in the recurrent setting as in our study which is known to change [25]. By far the most tumors (68%) in the BELOB trial were classical and other types were grouped as “non-classical” because of low numbers, which may have impacted the results. Also their findings were only in the combination treatment group and not bevacizumab alone. The finding of mesenchymal tumors responding for an extended period of time to bevacizumab in the recurrent setting has not been reported previously, to our knowledge.
It has become evident in recent years that bevacizumab treatment is not without risk, in particular from wound breakdown and the possibility of inducing a more infiltrative/aggressive tumor after treatment [14]. Our analysis suggests that bevacizumab should be considered more carefully in classical and EGFR-amplified tumors and may be more beneficial for mesenchymal, neural and proneural tumors. Nevertheless bevacizumab remains one of the few options available to patients with recurrent tumors that have failed standard of care and available trials. Neuro-oncologists have thus maintained this drug in their armamentarium, especially since it also offers a significant anti-edema activity. It is therefore highly relevant to investigate potential predictors of high failure risk. Further work will be needed to identify a potential mechanistic antagonism in the context of EGFR amplification that might explain these results. Alternatively, confounding factors such as the stage at which bevacizumab is used in these tumors, specific prior or concomitant treatment regimens may contribute to the worse results seen in this group.
Conclusion
Classical subtype and EGFR-amplified glioblastomas show a worse response to bevacizumab in the recurrent setting. Additional investigations are required to further validate this finding as well as to investigate the mechanistic basis for bevacizumab propensity to fail in the setting of amplified EGFR.
Supplementary Material
Footnotes
Conflict of interest All the authors declare that there is no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent The research protocol was submitted to the institutional research board and deemed exempt. No patient consent was required.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-019-03102-5) contains supplementary material, which is available to authorized users.
References
- 1.Kim KJ, Li B, Winer J et al. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423):841–844. 10.1038/362841a0 [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Adamis AP (2016) Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov 15(6):385 10.1038/nrd.2015.17 [DOI] [PubMed] [Google Scholar]
- 3.Thomas AA, Brennan CW, DeAngelis LM, Omuro AM (2014) Emerging therapies for glioblastoma. JAMA Neurol 71(11):1437–1444. 10.1001/jamaneurol.2014.1701 [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Dignam JJ, Armstrong TS et al. (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708. 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W et al. (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. 10.1056/NEJMoa1308345 [DOI] [PubMed] [Google Scholar]
- 6.Yang S-B, Gao K-D, Jiang T, Cheng S-J, Li W-B (2017) Bevacizumab combined with chemotherapy for glioblastoma: a meta-analysis of randomized controlled trials. Oncotarget 8(34):57337 10.18632/oncotarget.16924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaub C, Tichy J, Schäfer N et al. (2016) Prognostic factors in recurrent glioblastoma patients treated with bevacizumab. J Neurooncol 129(1):93–100. 10.1007/s11060-016-2144-7 [DOI] [PubMed] [Google Scholar]
- 8.Saran F, Chinot OL, Henriksson R et al. (2016) Bevacizumab, temozolomide, and radiotherapy for newly diagnosed glioblastoma: comprehensive safety results during and after first-line therapy. Neuro-Oncol 18(7):991–1001 10.1093/neuonc/nov300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laviv Y, Rappaport ZH (2014) Extremely late wound dehiscence following bevazicumab treatment in a long term survival glioblastoma patient. Clin Neurol Neurosurg 127:125–127. 10.1016/j.clineuro.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 10.Lai A, Tran A, Nghiemphu PL et al. (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29(2):142–148. 10.1200/JCO.2010.30.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladha H, Pawar T, Gilbert MR et al. (2015) Wound healing complications in brain tumor patients on Bevacizumab. J Neurooncol 124(3):501–506. 10.1007/s11060-015-1868-0 [DOI] [PubMed] [Google Scholar]
- 12.Friedman HS, Prados MD, Wen PY et al. (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. 10.1200/JCO.2008.19.8721 [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain MC (2011) Radiographic patterns of relapse in glioblastoma. J Neurooncol 101(2):319–323. 10.1007/s11060-010-0251-4 [DOI] [PubMed] [Google Scholar]
- 14.Mamo A, Baig A, Azam M et al. (2016) Progression pattern and adverse events with bevacizumab in glioblastoma. Curr Oncol 23(5):468 10.3747/co.23.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blouw B, Song H, Tihan T et al. (2003) The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4(2):133–146. 10.1016/S1535-6108(03)00194-6 [DOI] [PubMed] [Google Scholar]
- 16.Brennan CW, Verhaak RGW, McKenna A et al. (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhaak RGW, Hoadley KA, Purdom E et al. (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omuro A, DeAngelis LM, KR P et al. (2013) Glioblastoma and other malignant gliomas. JAMA 310(17):1842 10.1001/jama.2013.280319 [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Perry A, Reifenberger G et al. (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 20.Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y et al. (2016) Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res 76(3):525–534. 10.1158/0008-5472.CAN-15-0776 [DOI] [PubMed] [Google Scholar]
- 21.Omuro A, Beal K, Gutin P et al. (2014) Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 20(19):5023–5031. 10.1158/1078-0432.CCR-14-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastenhuber ER, Huse JT, Berman SH et al. (2014) Quantitative assessment of intragenic receptor tyrosine kinase deletions in primary glioblastomas: their prevalence and molecular correlates. Acta Neuropathol 127(5):747–759. 10.1007/s00401-013-1217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang RY, Rahman R, Ballman KV et al. (2016) The impact of T2/FLAIR evaluation per RANO criteria on response assessment of recurrent glioblastoma patients treated with Bevacizumab. Clin Cancer Res 22(3):575–581. 10.1158/1078-0432.CCR-14-3040 [DOI] [PubMed] [Google Scholar]
- 24.Verhoeff JJC, van Tellingen O, Claes A et al. (2009) Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer 9:444 10.1186/1471-2407-9-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eskilsson E, Verhaak RGW (2016) Longitudinal genomic characterization of brain tumors for identification of therapeutic vulnerabilities. Neuro Oncol 18(8):1037–1039. 10.1093/neuonc/now064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldape K, Amin SB, Ashley DM et al. (2018) Glioma through the looking GLASS: molecular evolution of diffuse gliomas and the Glioma Longitudinal Analysis Consortium. Neuro Oncol 20(7):873–884. 10.1093/neuonc/noy020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Gao Y, Kerkhof M et al. (2015) Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol 17(7):935–941. 10.1093/neuonc/nov013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips HS, Kharbanda S, Chen R et al. (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neuro-genesis. Cancer Cell 9(3):157–173. 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 29.Sandmann T, Bourgon R, Garcia J et al. (2015) Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol 33(25):2735–2744. 10.1200/JCO.2015.61.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.