Abstract
Purpose
To determine disease-associated single-gene variants in conotruncal defects, particularly tetralogy of Fallot (TOF).
Methods
We analyzed for rare loss-of-function and deleterious variants in FLT4 (VEGFR3) and other genes in the vascular endothelial growth factor (VEGF) pathway, as part of a genome sequencing study involving 175 adults with TOF from a single site.
Results
We identified nine (5.1%) probands with novel FLT4 variants: seven loss-of-function, including an 8-kb deletion, and two predicted damaging. In ten other probands we found likely disruptive variants in VEGF-related genes: KDR (VEGFR2; two stopgain and two nonsynonymous variants), VEGFA, FGD5, BCAR1, IQGAP1, FOXO1, and PRDM1. Detection of VEGF-related variants (19/175, 10.9%) was associated with an increased prevalence of absent pulmonary valve (26.3% vs. 3.4%, p < 0.0001) and right aortic arch (52.6% vs. 29.1%, p = 0.029). Extracardiac anomalies were rare. In an attempt to replicate findings, we identified three loss-of-function or damaging variants in FLT4, KDR, and IQGAP1 in ten independent families with TOF.
Conclusion
Loss-of-function variants in FLT4 and KDR contribute substantially to the genetic basis of TOF. The findings support dysregulated VEGF signaling as a novel mechanism contributing to the pathogenesis of TOF.
Keywords: tetralogy of Fallot, genome sequencing, VEGF, FLT4, haploinsufficiency, congenital heart disease, conotruncal defects
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic heart malformation in humans. Approximately 20% of TOF patients are diagnosed with genetic syndromes.1 Recurrent 22q11.2 deletions, associated with 22q11.2 deletion syndrome, and other rare copy-number variants (CNVs) contribute substantially to the genetic burden, and have suggested disease-related mechanisms, such as disturbances of cell migration and vasculature development.2 The role of genetic factors is further supported by an increased risk of congenital heart defects (CHD) in first-degree relatives of TOF patients.3 However, for the majority of individuals with TOF, the etiology remains unknown. TOF-associated single-gene defects are rarely identified. A multisite collaborative study using exome sequencing recently identified FLT4 loss-of-function variants in 2.3% of children with TOF.4 Exome sequencing also revealed another FLT4 frameshift deletion in a TOF patient.5 As part of a genome sequencing study of the underlying genetic causes in adults with CHD, predominantly TOF, from a single site, we investigated rare and predicted damaging variants in FLT4 and other vascular endothelial growth factor (VEGF)-related genes.
Materials and methods
Study participants
The study was approved by the Research Ethics Boards at the University Health Network (REB 98-E156), Centre for Addiction and Mental Health (REB 154/2002), and The Hospital for Sick Children (REB 1000053844). Informed consent was obtained from all probands and/or their legal guardians.
Cohort 1: Study participants with microarray data available were selected from a well-characterized cohort of n = 552 unrelated adults with TOF or related congenital heart defects and no 22q11.2 microdeletion, recruited from the Toronto Congenital Cardiac Centre for Adults.2,6 We performed genome sequencing on n = 231 probands (175 TOF, 49 transposition of the great arteries, 7 other CHD). Of these, by design, n = 122 (92 TOF) had no rare (<0.1%) genic CNVs >10 kb, whereas n = 109 (83 TOF) had rare, autosomal CNVs >10 kb overlapping putative CHD candidate genes (Supplementary information, Tables S3 and S6, contain details on design and selection for sequencing).2,6
Cohort 2: We additionally performed genome sequencing of 11 individuals with TOF from ten families, eight of which were sequenced as parent–child trios. The families originated from a larger cohort of various CHD, recruited through the Ted Rogers Cardiac Genome Clinic.
Genome sequencing
DNA was sequenced on the Illumina HiSeq X system at The Centre for Applied Genomics (TCAG) in Toronto, Canada (Supplementary information, Table S1).7 Population allele frequencies were derived from 1000 Genomes, ExAC, and gnomAD (Supplementary information). Probability of loss-of-function intolerance (pLI) scores were derived from ExAC (http://exac.broadinstitute.org/); haploinsufficiency (HI) predictions were derived from DECIPHER (https://decipher.sanger.ac.uk/).
Results
Rare FLT4 variants associated with tetralogy of Fallot
As an initial stage of this study on adults with congenital cardiac disease, we investigated genome sequencing data for disease-associated single-nucleotide variants (SNVs) and CNVs in the VEGF pathway. We identified nine previously unreported variants in FLT4, encoding vascular endothelial growth factor receptor 3 (VEGFR3). All were within the 175 individuals with TOF, thus the prevalence of FLT4 variants in this adult TOF cohort was 5.1% (9/175). Seven of the variants had loss-of-function effects (two stopgain, three frameshift insertion/deletions, one canonical splice site, one multiexon 8-kb deletion; Fig. 1a and Table 1). A missense variant p.(Leu1173Val) was predicted to be deleterious (CADD = 25, SIFT = 0, PolyPhen2 = 1), and was located in the terminal α-helix of the protein kinase domain, adjacent to a cluster of phosphorylated residues. An in-frame deletion p.(Glu741del) in immunoglobulin homology domain 7 (Ig7), close to the dimerization site Arg737 (ref. 8), was predicted to impact affinity for dimer formation. None of the nine individuals were considered syndromic (Table 1). One proband (TOF158) had a daughter with TOF, who had inherited the paternal FLT4 stopgain variant.
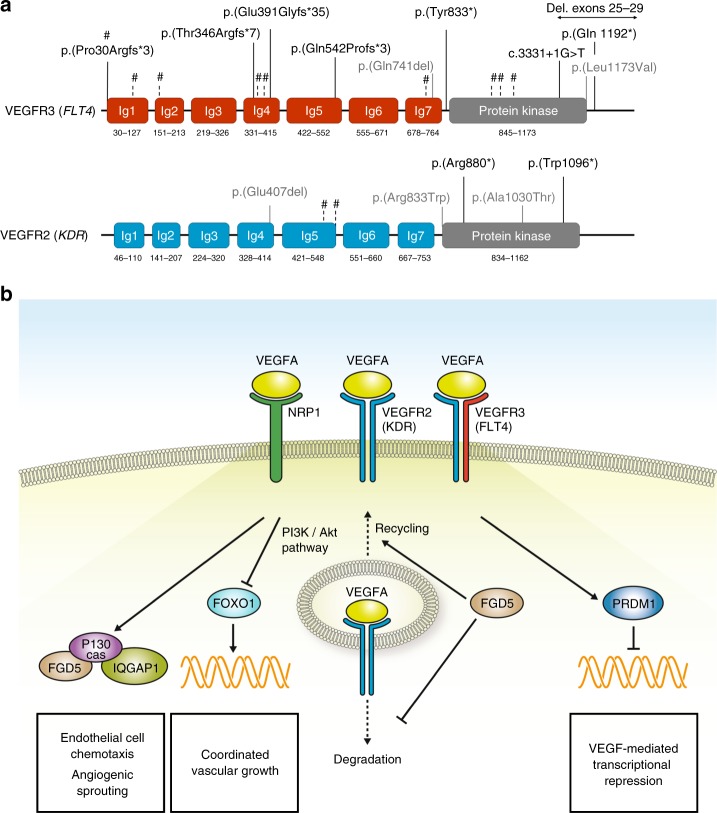
Fig. 1. VEGF pathway and genome sequencing in tetralogy of Fallot.
(a) Variant positions in vascular endothelial growth factor receptors 3 (VEGFR3; FLT4) and 2 (VEGFR2; KDR): loss-of-function variants (black; multiexon 8-kb deletion indicated by horizontal arrow), in-frame deletions or deleterious missense variants (gray). Loss-of-function variants in ref.4 indicated by vertical dashed lines and #; in FLT4 (NM_182925.4), from left to right: p.(Pro30Argfs*3) [1x inherited, 1x de novo], p.(Arg82*), p.(Thr168Serfs*76), p.(Tyr361*), p.(Pro364Alafs*63), p.(Gln736*), p.(Leu935Profs*72), p.(Cys949Argfs*53), p.(Gln999*); and in KDR (NM_002253.2): p.(Lys529*), c.1646-2A>T. Nomenclature as recommended by the Human Genome Variation Society (HGVS; http://varnomen.hgvs.org/). (b) Selected components of vascular endothelial growth factor (VEGF) signaling in endothelial cells, focusing on candidate genes for tetralogy of Fallot and their presumed roles in vascular development. VEGFA induces the formation of VEGFR2 homodimers (blue/blue), VEGFR2/ VEGFR3 heterodimers (blue/red), and binds to the coreceptor NRP1 (ref. 9). VEGFR1 (encoded by FLT1; not shown) may function as a negative regulator for VEGFA signaling, but also forms heterodimers with VEGFR2 (ref. 9). P130cas (encoded by BCAR1) mediates VEGFR2/NRP1 signaling and functions in the assembly of multiprotein complexes, among which are IQGAP1 and FGD5 (ref. 17). FGD5 also inhibits VEGFR2 degradation.18 VEGFR2 suppresses the activity of the transcription factor FOXO1, which is important for the regulation of coordinated vascular sprouting.19 The transcriptional repressor PRDM1 was linked to VEGF signaling in tumor vasculature and in wound healing;20 arterial pole defects in mutant mice indicate PRDM1 also functions in cardiovascular development (Table S5)
Table 1.
Individuals with tetralogy of Fallot and likely disruptive variants in genes in the vascular endothelial growth factor pathway
| Casea | Sex | Ageb (years) | Phenotype and family history of CHD | Gene (transcript) | Variant type | Variant | Chromosomal position (GRCh37/hg19) | Allele frequency (ExAC/gnomAD)c | Other variants of uncertain significanced | |
|---|---|---|---|---|---|---|---|---|---|---|
| SNVs | CNVs | |||||||||
| Cohort 1 (n = 19 individuals) | ||||||||||
| TOF293 | F | 23 | TOF, RAA, APV; stillborn offspring | FLT4 (NM_182925.4) | Deletion (multiexon) | Deletion of exons 25–29 | chr5:g.[180031767_180040470del] | 0 / 0 | ● | |
| TOF158 | M | 79 | TOF, RAA, paroxysmal atrial flutter requiring ablation, mild aortic dilatation; depression and/or anxiety, migraine, melanoma; daughter with TOFe | FLT4 (NM_182925.4) | Stopgain | c.3574C>T, p.(Gln1192*) | chr5:180038443G>A | 0 / 0 | ● | |
| TOF238 | M | 42 | TOF, RAA, MAPCA, PA; aortic dilatation | FLT4 (NM_182925.4) | Stopgain | c.2499C>G, p.(Tyr833*) | chr5:180047216G>C | 0 / 0 | ● | ● |
| TOF284 | M | 29 | TOF, MAPCA, inconclusive results about RAA; aortic valve replacement | FLT4 (NM_182925.4) | Duplication (frameshift) | c.1622dupG, p.(Gln542Profs*3) | chr5:180049766dupC | 0 / 0 | ||
| TOF254 | F | 32 | TOF, APV; bilateral femoral vein occlusions; depression and/or anxiety | FLT4 (NM_182925.4) | Deletion (frameshift) | c.1172_1173delAG, p.(Glu391 Glyfs*35) | chr5:180053196delCT | 0 / 0 | ● | |
| TOF68 | F | 20 | TOF, RAA, APV; depression and/or anxiety | FLT4 (NM_182925.4) | Deletion (frameshift) | c.1037delC, p.(Thr346Argfs*7) | chr5:180055948delG | 0 / 0 | ● | |
| TOF301 | F | 29 | TOF, RAA, paternal first cousin with suspected VSD | FLT4 (NM_182925.4) | Splice site | c.3331+1G>T, p.? | chr5:180041067C>A | 0 / 0 | ● | |
| TOF271 | M | 39 | TOF, obesity | FLT4 (NM_182925.4) | Missense | c.3517C>G, p.(Leu1173Val) | chr5:180039526G>C | 0 / 0 | ||
| TOF236 | F | 33 | TOF, RAA; atrioventricular nodal reentry tachycardia requiring ablation; depression and/or anxiety; unilateral duplicated ureter; daughter with truncus arteriosus | FLT4 (NM_182925.4) | Deletion (in-frame) | c.2223_2225delGGA, p.(Glu741del) | chr5:180047950delTCC | 0 / 0 | ||
| TOF109 | M | 44 | TOF, PFO or ASD, atrial flutter; obesity; mild cognitive and memory problems attributed to cerebral ischemia; brother died in infancy of suspected cyanotic CHD | KDR (NM_002253.2) | Stopgain | c.3287G>A, p.(Trp1096*) | chr4:55955875C>T | 0 / 0 | ||
| TOF155 | M | 52 | TOF, PFO or ASD; depression and/or anxiety; gastroesophageal reflux | KDR (NM_002253.2) | Stopgain | c.2638C>T, p.(Arg880*) | chr4:55962486G>A | 0 / 0 | ● | ● |
| TOF326 | F | 46 | TOF, RAA, PFO or ASD; short stature; benign brain tumor | KDR (NM_002253.2) | Missense | c.2497C>T, p.(Arg833Trp) | chr4:55964316G>A | 0 / 0 | ||
| TOF359 | F | 30 | TOF, APV; learning difficulties; maternal uncle with unspecified cyanotic CHD | KDR (NM_002253.2) | Deletion (in-frame) | c.1219_1221delGAG, p.(Glu407del) | chr4:55976604delCTC | 0 / 0 | ||
| TOF241 | M | 29 | TOF, RAA, bicuspid pulmonic valve; short stature, obesity; learning difficulties; depression and/or anxiety; stillborn offspring | VEGFA (NM_001171623.1) | Stopgain | c.115G>T, p.(Glu39*) | chr6:43742126G>T | 0 / 0 | ||
| TOF89 | M | 53 (died 55) | TOF, PFO or ASD; inducible atrial flutter/fibrillation, systemic arterial hypertension, aortic dilatation, query BAV; ankylosing spondylitis | FGD5 (NM_152536.3) | Stopgain | c.3673C>T, p.(Arg1225*) | chr3:14963921C>T | 0 / 0 | ● | |
| TOF220 | F | 32 | TOF, RAA, APV; learning difficulties | BCAR1 (NM_001170715.1) | Deletion (multiexon) | Deletion of exons 2–7 | chr16:g.[75237177_75301117del] | 0 / 0 | ● | ● |
| TOF48 | F | 26 | TOF, PFO or ASD, bicuspid pulmonic valve | IQGAP1 (NM_003870.3) | Stopgain | c.309C>G, p.(Tyr103*) | chr15:90969495C>G | 0 / 0 | ● | |
| TOF62 | M | 54 | TOF, RAA; learning difficulties | FOXO1 (NM_002015.3) | Deletion (frameshift) | c.580_586delGTGCCCT, p.(Val194Thrfs*137) | chr13:41239764delAGGGCAC | 0 / 0 | ● | |
| TOF53 | F | 52 | TOF, PFO or ASD; coronary artery bypass grafts, systemic arterial hypertension; depression and/or anxiety | PRDM1 (NM_001198.3) | Stopgain | c.1824C>A, p.(Cys608*) | chr6:106554296C>A | 0 / 0 | ● | |
| Cohort 2 (n = 3 families) | ||||||||||
| CGC-034 | F | 1 | TOF, PA, MAPCA; lymphedema; maternal grandfather with bradycardia | FLT4 (NM_182925.4) | Deletion (frameshift) | c.89delC, p.(Pro30Argfs*3) | chr5:180058748delG | 0 / 4.12e-6 | ||
| CGC-001 | F, F | 1, 24 | TOF, PA, absent central pulmonary arteries, MAPCA; accessory bronchus; wide nasal bridge, broad nasal tip, downturned corners of the mouth, clinodactyly, short thumb; mother with TOF, PA, mild intellectual disability | KDR (NM_002253.2) | Missense | c.3088G>A, p.(Ala1030Thr) | chr4:55956227C>T | 0 / 0 | ● | |
| CGC-076 | M | 1 | TOF with severe PS, confluent pulmonary arteries, DORV, bilateral SVC (left SVC to the coronary sinus); esophageal atresia with tracheal fistula, bilateral inferior iris coloboma, clinodactyly of all fifth digits; short stature | IQGAP1 (NM_003870.3) | Stopgain | c.2296 C > T, p.(Arg766*) | chr15:91016189 C > T | 0 / 4.06e-6 | ● | |
All variants are heterozygous. Subjects in this table are of European descent, by design for cohort 1. No subject in cohort 1 had lymphedema or intellectual disability documented. Obesity was defined as body mass index (BMI) consistently >30 as an adult. Short stature was defined as height <3rd percentile using standard adult growth curves. Prevalence of liveborn offspring with major CHD in the adult cohort of 19 patients with TOF: 2/17 (11.8%), plus two stillborn offspring; prevalence of siblings with major CHD 1/40 (2.5%). The median age at TOF repair was 4 years (range 1–22) for this adult cohort of median age 33 (range 26–79) years.
APV, absent pulmonary valve; ASD, atrial septal defect; BAV, bicuspid aortic valve; CHD, congenital heart disease; CNV, copy-number variant; DORV, double outlet right ventricle; F, female; M, male; MAPCA, major aortopulmonary collateral arteries; PA, pulmonary atresia; PFO, patent foramen ovale; PS, pulmonary stenosis; RAA, right aortic arch; SNV, single-nucleotide variant; SVC, superior vena cava/cavae; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
aCase numbers for cohort 1 are those used for the same subjects in a previous report.2
bAge at last follow-up.
cAs of March 2018 for both ExAC and gnomAD databases (by design, allele frequencies in ExAC were null for cohort 1).
dSee Methods re study design with respect to CNVs, and Table S3 for details of putative CHD-related CNVs and SNVs identified.
eInherited paternal FLT4 variant (TOF158)
Variants in other vascular endothelial growth factor related genes
Assessing for rare variants in other genes encoding vascular endothelial growth factors (VEGFA, VEGFB, VEGFC, VEGFD, PGF) or their receptors (FLT1, KDR, NRP1, NRP2),9 we identified two stopgain and two nonsynonymous variants in KDR (encoding VEGR2; Fig. 1a and Table 1), and a stopgain variant p.(Glu39*) in VEGFA predicted to affect all isoforms. All variants were identified in individuals with TOF and absent in public databases. Like FLT4, both KDR and VEGFA were predicted to be intolerant to loss-of-function variants (KDR: pLI = 0.98, HI = 2.2%; VEGFA: pLI = NA, HI = 0.1%). The KDR missense variant p.(Arg833Trp) was predicted to be deleterious (CADD = 33, SIFT = 0, PolyPhen2 = 1), potentially through a disruption of the terminal protein kinase structure. The in-frame deletion p.(Glu407del) was in Ig4, a domain important for receptor activity and signaling.10
Under the hypothesis that haploinsufficiency of the VEGF signaling pathway is associated with TOF, and causative genes are likely intolerant to loss-of-function variation, we then systematically analyzed the data set for such variants. We screened unreported stopgain, frameshift, and canonical splice-site variants (n = 105) and coding deletions (n = 13), in 3230 genes with ExAC pLI >0.9 for known functions in the VEGF signaling pathway (Supplementary information). Thereby we identified five additional null variants (Fig. 1b and Table 1): a stopgain variant p.(Arg1225*) in FGD5 (pLI = 0.99), a deletion of exons 2–7 in BCAR1 (pLI = 0.99), a stopgain variant p.(Tyr103*) in IQGAP1 (pLI = 1), a frameshift deletion p.(Val194Thrfs*137) in FOXO1 (pLI = 0.97), and a stopgain variant p.(Cys608*) in PRDM1 (pLI = 0.98).
Clinical phenotype
Nineteen (nine males, 10 females) of 175 (10.9%) probands with TOF were identified with VEGF pathway-associated variants. Individuals with VEGF-related variants and TOF were enriched for absent pulmonary valve: 5/19 (26.3%) vs. 6/175 (3.4%) (Fisher’s exact test; FET: p < 0.0001, odds ratio 52.4, 95% confidence interval [5.4–2586.4]) and right aortic arch: 10/19 (52.6%) vs. 51/175 (29.1%) (FET: p = 0.029, odds ratio 3.1, 95% confidence interval [1.05–9.3]). None had lymphedema. We did not identify any other likely causal variants in these 19 probands. However, eight (42.1%) of the 19, including three with FLT4 variants, were amongst those with putative CHD-relevant CNVs. Phenotypic information and additional rare variants are summarized in Tables 1 and S3.
Additional cohorts
Using genome sequencing data for another cohort (n = 11 individuals with TOF from ten families), we discovered three other variants in VEGF pathway genes. In a patient with TOF and congenital lymphedema, we identified a previously described frameshift variant p.(Pro30Argfs*3) in FLT4 (ref. 4), inherited from her mother with normal echocardiography results. We identified a predicted damaging KDR missense variant p.(Ala1030Thr) (CADD = 35, SIFT = 0, PolyPhen2 = 1), located in the protein kinase domain adjacent to the catalytic residues Asp1028 and Arg1032 (ref. 11) in a mother and daughter, both with TOF and pulmonary atresia. In a patient with complex congenital cardiac disease including TOF (Table 1), esophageal atresia with tracheal fistula, bilateral iris coloboma, and clinodactyly of all fifth digits, we identified another stopgain variant p.(Arg766*) in IQGAP1, inherited from his unaffected father.
Review of previously published microarray studies revealed several FLT4 and other VEGF-related genes impacted by rare CNVs in individuals with cardiac defects (Table S4). Apart from one frameshift insertion in BCAR1, there were no rare loss-of-function variants of FLT4, KDR, VEGFA, FGD5, IQGAP1, FOXO1, or PRDM1 identified in the genome sequencing data of 7231 individuals with autism from the MSSNG database (https://www.mss.ng/#).
Discussion
Our results support the hypothesis that dysregulated VEGF signaling contributes to the genetic etiology of TOF. We confirmed the importance of deleterious FLT4 variants,4 and identified null alleles in multiple haploinsufficiency-intolerant genes in the VEGF pathway.
For FLT4 variants, the results were overall consistent with a previous study4 that reported similar variants in 9 of 426 nonsyndromic TOF probands and one subject with an unspecified conotruncal defect, but no association with neurodevelopmental disorders or other congenital anomalies. FLT4 variants were more prevalent in our cohort than in the previous report (5.1% vs. 2.3%) (ref. 4). There could be several reasons for this beyond sampling variability. Genome sequencing results in more uniform and complete coverage of coding regions than exome sequencing, and enables the detection of structural variants (e.g., small CNVs, such as those identified in FLT4 and BCAR1; Fig. S1). Another difference in study design was that the adult cohort studied here had undergone extensive microarray studies, although we found no evidence to support enrichment for disease-associated single-gene defects in the n = 92 (52.6%) TOF patients with no cardiac disease–related rare CNVs (Table S3). Our analysis also considered missense variants and in-frame deletions/insertions, in addition to obvious loss-of-function alleles examined in the previous exome sequencing study.4
None of the loss-of-function FLT4 variants identified through genome sequencing in our adult TOF cohort overlapped with those previously reported.4 However, we identified one previously reported,4 recurrent frameshift deletion (Fig. 1a) in an infant with both TOF and lymphedema. Missense variants in the protein kinase domain reported to cause Milroy disease (hereditary lymphedema, OMIM-P 153100) provide evidence for allelic heterogeneity in FLT4. However, robust genotype–phenotype correlations are challenged by the abovementioned frameshift deletion and by a missense substitution in the protein kinase domain in an individual with isolated TOF (Fig. 1a). The absence of lymphedema history in our adult cohort, including those with FLT4 variants, would suggest at most a mild or fully remitted lymphedema phenotype.
The case-only adult cohort design did not allow for systematic segregation testing in family members; this will be the focus of future studies. However, as for most (6/9) families with incomplete penetrance of FLT4-associated TOF in a previous study,4 an FLT4 variant in our second TOF cohort was inherited from an unaffected mother. This evidence for reduced penetrance and variable expression may be related to other, as yet unidentified, genetic and perhaps nongenetic factors relevant to expression of the TOF phenotype, such as oligogenic inheritance models. Estimating recurrence risks and penetrance will require larger disease and population-based cohorts.
FLT4 encodes VEGFR3, one of three main cell surface receptors for vascular endothelial growth factors. We conjectured that variants in other genes involved in VEGF signaling could disrupt this network and may also be involved in the etiopathogenesis of TOF. We identified KDR (encoding VEGFR2) as a novel TOF-associated gene, with five novel damaging variants in our data set (Fig. 1a). This was supported by loss-of-function variants in two individuals with conotruncal defects reported in supplementary data of a previous study.4 We also detected a stopgain variant in VEGFA as a further candidate for TOF pathogenesis. VEGFA perturbation has previously been linked to cardiac development and TOF.12,13 VEGFA and VEGFR2 are the best studied regulators of vascular development under physiological and pathological conditions. VEGFA induces the formation of VEGFR2 homodimers and VEGFR2/VEGFR3 heterodimers, both of which are involved in the regulation of angiogenic sprouting.9,14
Our analyses identified null alleles in additional candidate genes that link the VEGF signaling pathway to TOF: FGD5, BCAR1, IQGAP1 (2x), FOXO1, and PRDM1 (Fig. 1b and Supplementary information). Mouse constitutive knockout models support a role for these VEGF-related genes in cardiovascular development (Table S5). Mutant Prdm1 mice show arterial pole defects and pharyngeal arch anomalies that are more severe on a Tbx1 heterozygous background, reflecting interaction between these two genes. Complete deletion of any of Flt4, Kdr, Vegfa, Fgd5, Bcar1, or Foxo1 is embryonically lethal with impaired cardiac and/or vessel development.
We found that TOF probands with VEGF-related variants were enriched for the presence of absent pulmonary valve and right aortic arch. Impairment of asymmetric VEGF signaling and blood flow were previously linked to right aortic arch.15 Further studies are required to confirm that haploinsufficiency of VEGFA, FGD5, BCAR1, IQGAP1, FOXO1, and PRDM1 are associated with TOF, and to delineate the associated phenotypes. The functional impacts of the missense and in-frame variants in FLT4 and KDR require elucidation. We did not identify deleterious variants in other promising candidate genes such as NRP1 (encoding Neuropilin-1, a VEGFR2 coreceptor) or FLT1 (encoding VEGFR1) in this data set, and statistical evaluation of the VEGF pathway awaits final analyses of all rare variants and gene pathways for the entire cohort sequenced. However, previous studies reported loss-of-function variants in FLT1 (n = 2) or BCAR1 (n = 1) in subjects with conotruncal defects (supplemental data4), and a heterozygous deletion encompassing NRP1 cosegregating with TOF in a single family.16
Our findings, in the context of previously published data, support the hypothesis of deficient VEGF signaling as a novel and plausible pathomechanism of TOF and related cardiovascular defects. Loss-of-function variants in FLT4 and KDR contribute substantially to the disease prevalence and warrant consideration for clinical diagnostic testing, particularly in patients with TOF and normal extracardiac development.
Electronic supplementary material
Acknowledgements
We thank the patients and their families for participating in this study. We thank Celine Aziz for assistance with Sanger sequencing. This study made use of data generated by the DECIPHER community (http://decipher.sanger.ac.uk), a project funded by the Wellcome Trust.
Funding
This work was funded by a donation from the W. Garfield Weston Foundation (A.S.B., C.S.), a Canadian Institutes of Health Research grant (MOP-89066), University of Toronto McLaughlin Centre (A.S.B., C.S.), and the Ted Rogers Centre for Heart Research. E.O. holds the Bitove Family Professorship of Adult Congenital Heart Disease. S.W.S. is funded by the GlaxoSmithKline-CIHR Chair in Genome Sciences at the University of Toronto and The Hospital for Sick Children. A.S.B. holds the Dalglish Chair in 22q11.2 Deletion Syndrome at the University Health Network and University of Toronto.
Disclosure
The authors declare no conflicts of interest.
Electronic supplementary material
The online version of this article (10.1038/s41436-018-0260-9) contains supplementary material, which is available to authorized users.
References
- 1.Morgenthau A, Frishman WH. Genetic origins of tetralogy of Fallot. Cardiol Rev. 2018;26:86–92. doi: 10.1097/CRD.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 2.Silversides CK, Lionel AC, Costain G, Merico D, Migita O, Liu B, et al. Rare copy number variations in adults with tetralogy of Fallot implicate novel risk gene pathways. PLoS Genet. 2012;8:e1002843. doi: 10.1371/journal.pgen.1002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Digilio MC, Marino B, Giannotti A, Toscano A, Dallapiccola B. Recurrence risk figures for isolated tetralogy of Fallot after screening for 22q11 microdeletion. J Med Genet. 1997;34:188–190. doi: 10.1136/jmg.34.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. 2017;49:1593–1601. doi: 10.1038/ng.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szot JO, Cuny H, Blue GM, Humphreys DT, Ip E, Harrison K, et al. A screening approach to identify clinically actionable variants causing congenital heart disease in exome data. Circ Genom Precis Med. 2018;11:e001978. doi: 10.1161/CIRCGEN.117.001978. [DOI] [PubMed] [Google Scholar]
- 6.Costain G, Lionel AC, Ogura L, Marshall CR, Scherer SW, Silversides CK, et al. Genome-wide rare copy number variations contribute to genetic risk for transposition of the great arteries. Int J Cardiol. 2016;204:115–121. doi: 10.1016/j.ijcard.2015.11.127. [DOI] [PubMed] [Google Scholar]
- 7.Reuter MS, Walker S, Thiruvahindrapuram B, Whitney J, Cohn I, Sondheimer N, et al. The Personal Genome Project Canada: findings from whole genome sequences of the inaugural 56 participants. CMAJ. 2018;190:E126–E36. doi: 10.1503/cmaj.171151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppanen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, et al. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc Natl Acad Sci U S A. 2013;110:12960–12965. doi: 10.1073/pnas.1301415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 10.Hyde CA, Giese A, Stuttfeld E, Abram Saliba J, Villemagne D, Schleier T, et al. Targeting extracellular domains D4 and D7 of vascular endothelial growth factor receptor 2 reveals allosteric receptor regulatory sites. Mol Cell Biol. 2012;32:3802–3813. doi: 10.1128/MCB.06787-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McTigue MA, Wickersham JA, Pinko C, Showalter RE, Parast CV, Tempczyk-Russell A, et al. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure. 1999;7:319–330. doi: 10.1016/S0969-2126(99)80042-2. [DOI] [PubMed] [Google Scholar]
- 12.Lambrechts D, Devriendt K, Driscoll DA, Goldmuntz E, Gewillig M, Vlietinck R, et al. Low expression VEGF haplotype increases the risk for tetralogy of Fallot: a family based association study. J Med Genet. 2005;42:519–522. doi: 10.1136/jmg.2004.026443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters TH, Sharma V, Yilmaz E, Mooi WJ, Bogers AJ, Sharma HS. DNA microarray and quantitative analysis reveal enhanced myocardial VEGF expression with stunted angiogenesis in human tetralogy of Fallot. Cell Biochem Biophys. 2013;67:305–316. doi: 10.1007/s12013-013-9710-9. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson I, Bahram F, Li X, Gualandi L, Koch S, Jarvius M, et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 2010;29:1377–1388. doi: 10.1038/emboj.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 16.Duran I, Tenney J, Warren CM, Sarukhanov A, Csukasi F, Skalansky M, et al. NRP1 haploinsufficiency predisposes to the development of Tetralogy of Fallot. Am J Med Genet A. 2018;176:649–656. doi: 10.1002/ajmg.a.38600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans IM, Kennedy SA, Paliashvili K, Santra T, Yamaji M, Lovering RC, et al. Vascular endothelial growth factor (VEGF) promotes assembly of the p130Cas interactome to drive endothelial chemotactic signaling and angiogenesis. Mol Cell Proteomics. 2017;16:168–180. doi: 10.1074/mcp.M116.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhan MA, Azad AK, Touret N, Murray AG. FGD5 regulates VEGF receptor-2 coupling to PI3 kinase and receptor recycling. Arterioscler Thromb Vasc Biol. 2017;37:2301–2310. doi: 10.1161/ATVBAHA.117.309978. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arulanandam R, Batenchuk C, Angarita FA, Ottolino-Perry K, Cousineau S, Mottashed A, et al. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell. 2015;28:210–224. doi: 10.1016/j.ccell.2015.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.