Supplemental Digital Content is Available in the Text.
Key Words: bioassay, isavuconazole, LC-FL, LC-MS/MS, LC-UV
Background:
Under certain circumstances, clinicians treating patients with isavuconazole for invasive aspergillosis or mucormycosis may use therapeutic drug monitoring. However, the accuracy and reproducibility of the various assays used by different laboratories for the quantification of isavuconazole plasma concentrations have yet to be determined.
Methods:
Human plasma samples spiked with known concentrations of isavuconazole were provided to 27 European laboratories that took part in a “round-robin” test (an interlaboratory test performed independently at least 2 times; 2 rounds performed in the current study). Assay methods included liquid chromatography–tandem mass spectrometry (LC-MS/MS), LC with ultraviolet detection (LC-UV), LC with fluorescence detection (LC-FL), and bioassay. The accuracy and reproducibility compared with the known concentrations for each sample in each round were compared overall, between assays, and between laboratories.
Results:
Twenty-seven laboratories participated in the study (LC-MS/MS, n = 15; LC-UV; n = 9; LC-FL, n = 1; bioassay, n = 2). In round 1, for nominal concentrations of 1000, 1700, 2500, and 4000 ng/mL, the mean (SD) determined concentrations were 1007 (183), 1710 (323), 2528 (540), and 3898 (842) ng/mL, respectively. In round 2, for nominal concentrations of 1200, 1800, 2400, and 4000 ng/mL, the mean (SD) determined concentrations were 1411 (303), 2111 (409), 2789 (511), and 4723 (798) ng/mL, respectively. Over both rounds, determined concentrations were consistently within 15% of the nominal concentrations for 10 laboratories (LC-MS/MS, n = 4; LC-UV, n = 5; bioassay, n = 1) and consistently exceeded the upper 15% margin for 7 laboratories (LC-MS/MS and LC-UV, n = 3 each; LC-FL, n = 1).
Conclusions:
Alignment of methodologies among laboratories may be warranted to improve the accuracy and reproducibility of therapeutic drug measurements.
INTRODUCTION
Triazole antifungal agents are an important class of drugs for the prophylaxis and treatment of invasive fungal infections, which are associated with high morbidity and mortality in immunocompromised patients.1 However, maintenance of safe and efficacious plasma or serum concentrations of these drugs can sometimes necessitate therapeutic drug monitoring (TDM). Factors inherent to a drug that dictate the need for TDM include a high level of intraindividual and interindividual variability of pharmacokinetics, a defined therapeutic range, and a narrow therapeutic window.2
Isavuconazole is the active moiety of the prodrug isavuconazonium sulfate, the newest available triazole antifungal agent with demonstrated efficacy and safety for the treatment of invasive aspergillosis or mucormycosis in adults.3–5 An early phase study demonstrated good predictability of the dose-exposure relationship6; in a population-pharmacokinetics analysis using data from the phase III SECURE trial in adult patients with invasive aspergillosis and other invasive mold diseases, no relationship of exposure with either efficacy or elevations/abnormalities in liver enzyme test results was found.7 This suggests that, in most adults, the clinical dose of isavuconazole results in exposures that are within the optimal therapeutic window, and so, routine TDM of isavuconazole may not be necessary. Nevertheless, the margins of the therapeutic window remain to be defined, and it has yet to be determined if and when TDM might be required during isavuconazole treatment.
Reliable interpretation of the results of TDM requires that the analytical assays are both accurate and reproducible. Several laboratories have published or presented methods for the analysis of isavuconazole concentrations using liquid chromatography with tandem mass spectrometry (LC-MS/MS) detection,8–12 LC with ultraviolet detection (LC-UV),13–15 or LC with fluorescence detection (LC-FL),16 or using bioassay.17 However, it has not been defined how the results of such analyses compare, both with respect to the different analytical methods used and to the interlaboratory differences using the same techniques. Therefore, we conducted a round-robin exercise (interlaboratory test performed independently at least 2 times) in 27 European laboratories to assess the accuracy and reproducibility of measurements of isavuconazole concentration measurements.
MATERIALS AND METHODS
This study commenced September 2016 and completed July 2017.
Preparation of Samples
All samples were prepared by Basilea Pharmaceutica (Basel, Switzerland). A 5-mg/mL stock solution was prepared by dissolving 7.102 mg of isavuconazole in 1.284 mL of dimethyl sulfoxide (DMSO). The stock solution was then diluted to prepare spiking solutions of 100,000, 170,000, 250,000, 400,000 ng/mL (round 1) or 120,000, 180,000, 240,000, 400,000 ng/mL (round 2) in a 1/1 solution of DMSO/acetonitrile + 0.05% trifluoroacetic acid (DMSO/ACN/TFA). Samples for the round robin were prepared by addition of 5 μL of the spiking solutions to 495-μL samples of human plasma containing K3-EDTA as anticoagulant. Final concentrations of samples used were based on the clinically relevant range of plasma concentrations for isavuconazole. Concentrations in round 1 were as follows: sample 1, 1000 ng/mL; sample 2, 1700 ng/mL; sample 3, 2500 ng/mL; and sample 4, 4000 ng/mL. Concentrations in round 2 were as follows: sample 1, 1200 ng/mL; sample 2, 1800 ng/mL; sample 3, 2400 ng/mL; and sample 4, 4000 ng/mL. For each round, all plasma samples of a given concentration were mixed together and re-aliquoted before distribution, such that each laboratory effectively received the same sample.
Laboratories and Testing
European laboratories with the facilities for and expertise in drug-concentration assays using LC-MS/MS, LC-UV, LC-FL, or bioassays based on diffusion of the drug into inoculated and incubated media were identified and invited to participate in the study. All laboratories included in the study were required to have developed and validated the assays used in this study before inclusion. Participating laboratories, listed in Supplemental Digital Content 1 (see Table S1, http://links.lww.com/TDM/A319), were provided with samples in the first and/or second round of the test. Details of some of the analytical methods used have been published previously8–10,13,15,16; others were adapted from methods established for the detection of other triazole antifungal agents18–20 or used/adapted a commercially available assay.21 All samples for each round were also tested by the Basilea Pharmaceutica bioanalytical laboratory using LC-MS/MS. Details of the methods used by Basilea Pharmaceutica and as provided by each laboratory are included in the Supplemental Digital Content 1 (see Methods, http://links.lww.com/TDM/A319). Results from Basilea Pharmaceutica were included in statistical analyses but were not considered when comparing different methods or laboratories.
Statistics
To determine the accuracy and precision of measurements made in each round, the mean, standard deviation (SD), and bias (percent deviation from nominal concentration) were calculated for all samples and for each assay method. The results were assessed with respect to the European Medicines Agency22 and the US Food and Drug Administration23 guideline requirements of accuracy (bias) and precision (relative SD) within a 15% limit.
RESULTS
In total, 34 laboratories in Germany, United Kingdom, Netherlands, Italy, France, Austria, Switzerland, and Denmark were invited to participate in the round-robin test. Of these, 27 laboratories provided results for the determination of isavuconazole concentrations in human plasma (see Table S1, Supplemental Digital Content 1, http://links.lww.com/TDM/A319) round 1, laboratories 1–10; and round 2, laboratories 11–27. The methods used for the determination were LC-MS/MS (round 1: laboratories 1–5; round 2: laboratories 11–20), LC-UV (round 1: laboratories 6–8; round 2: laboratories 21–26), bioassays (round 1: laboratories 9, 10; round 2, laboratory 10), and LC-FL (round 2: laboratory 27).
In round 1, the mean of determined values for the assessments from all laboratories for each of the samples with nominal concentrations of 1000, 1700, 2500, and 4000 ng/mL were within the 15% margins, although the SDs extended beyond those margins (Fig. 1). The overall biases at each of these concentrations were 0.7%, 0.6%, 1.1%, and −2.5%, respectively. Among all 40 samples assessed by the 10 laboratories, 29 determined concentrations were within 15% of the nominal concentrations (acceptance criterion), 7 were above the upper 15% margin, and 4 were below the lower 15% margin.
FIGURE 1.
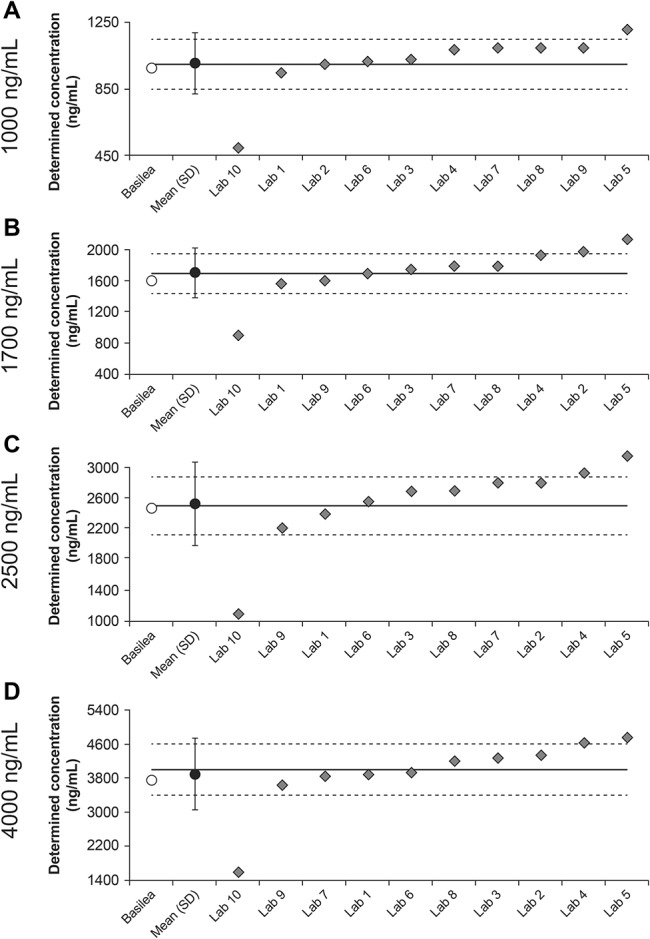
Isavuconazole plasma concentrations determined by Basilea and all laboratories in round 1 (labs 1–10) as a function of the nominal concentrations of 1000, 1700, 2500, and 4000 ng/mL (A–D, respectively). Solid lines represent nominal concentrations; dashed lines represent ±15% margins from nominal concentrations.
The results were also assessed as a function of the analytic method. For LC-MS/MS (laboratories 1–5), the mean of determined values for each nominal concentration was again within the 15% margins, although the SDs exceeded the upper margin at all tested concentrations (Fig. 2). Determined values from 2 laboratories were consistently within the 15% margins and those from 1 laboratory consistently exceeded the upper 15% margin. The overall biases at each of the tested concentrations (1000–4000 ng/mL) were 4.3%, 7.8%, 9.5%, and 6.9%, respectively. In total, 13/20 determined concentrations were within the 15% margins. The remaining 7/20 concentrations exceeded the upper 15% margin, including 1 sample for laboratory 2, 2 samples for laboratory 4, and all samples for laboratory 5. Determined values from the 3 laboratories that used LC-UV were consistently within 15% of the nominal concentrations (Fig. 2). The overall biases at each of the tested concentrations were 7.3%, 3.9%, 7.5%, and 0.2%, respectively. Of the 2 laboratories that used bioassays, all values determined from laboratory 9 were within 15% of the nominal concentrations, whereas those from laboratory 10 were all well below the lower 15% margin for all samples (overall bias at each concentration, −20%, −26.2%, −33.6%, and −34.5%, respectively) (Fig. 2).
FIGURE 2.
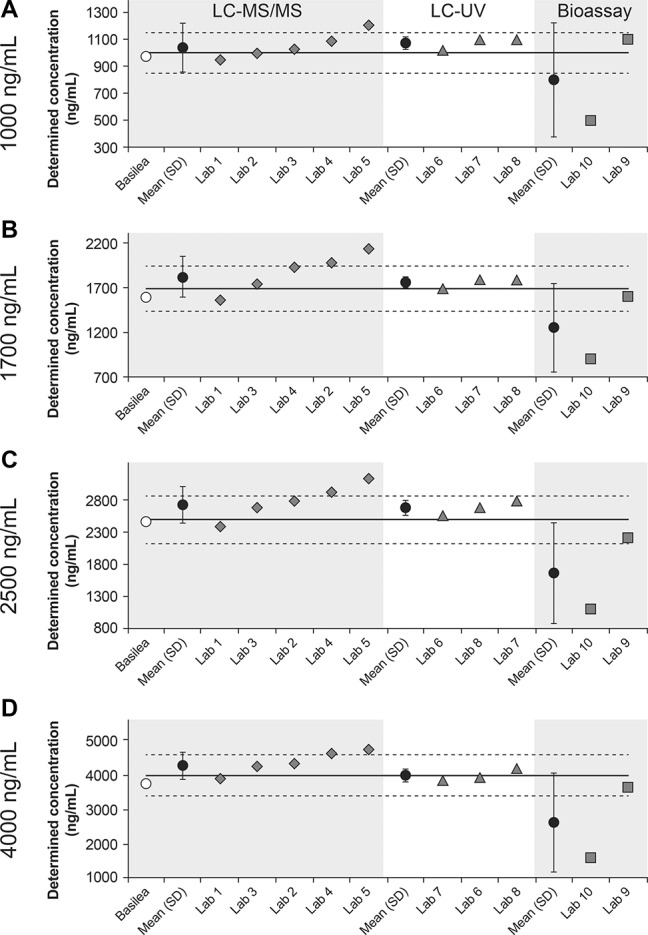
Isavuconazole plasma concentrations in round 1 determined using LC-MS/MS (labs 1–5), LC-UV (labs 6–8), or bioassay (labs 9, 10) as a function of the nominal concentration of 1000, 1700, 2500, and 4000 ng/mL (A–D, respectively). Solid lines represent nominal concentrations; dashed lines represent ±15% margins from nominal concentrations.
In round 2, the mean of determined values for the assessments from all laboratories slightly exceeded the upper 15% margin for each of the samples with nominal concentrations of 1200, 1800, 2400, and 4000 ng/mL (Fig. 3). The overall biases at each of these concentrations were considerably larger than those observed in round 1 (17.6%, 17.3%, 16.2%, and 18.1%, respectively). Among all 72 determined concentrations from the 18 laboratories, 31 were within the 15% margins, 36 exceeded the upper 15% margin, and 5 were below the lower 15% margin.
FIGURE 3.
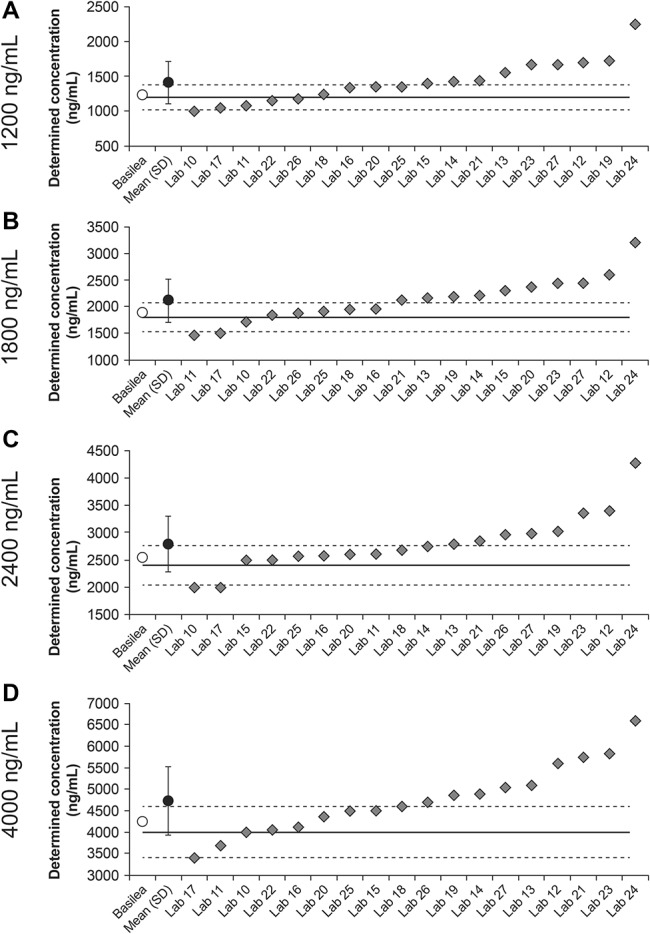
Isavuconazole plasma concentrations determined by Basilea and all laboratories in round 2 (labs 10–27) as a function of the nominal concentrations of 1200, 1800, 2400, and 4000 ng/mL (A–D, respectively). Solid lines represent nominal concentrations; dashed lines represent ±15% margins from nominal concentrations.
When assessed as a function of the analytic methods, the means of determined values for those using LC-MS/MS (laboratories 11–20) were slightly below the upper 15% margin (Fig. 4). Determined values from 2 laboratories were consistently within the 15% margins and those from 2 other laboratories consistently exceeded the upper 15% margin. The overall bias at each nominal concentration was again higher than observed for LC-MS/MS in round 1 (14.4%, 13.9%, 11.6%, and 12.1%, respectively). In total, 19/40 determined concentrations were within the 15% margins, 18/40 exceeded the upper 15% margin, and 3/40 were below the bottom 15% margin. Among laboratories that used LC-UV (laboratories 21–26), the mean values of determined values for all nominal concentrations exceeded the upper 15% margin (Fig. 4). Two laboratories consistently provided determined values that were within the 15% of the nominal concentrations, and 3 laboratories provided values that all exceeded the upper 15% margin. The overall bias at each nominal concentration was 25.6%, 24.3%, 28.6%, and 30.8%, respectively. Among the 24 total samples, 10 of the determined concentrations were within the 15% margins and 14 exceeded the upper 15% margin. For LC-FL (laboratory 27), determined values for all samples exceeded the upper 15% margin (Fig. 4). For bioassay, laboratory 10 was included again after having made technical adjustments. Although determined values for the 1200 and 2400 ng/mL nominal concentrations were each slightly below the lower 15% margin, determined values were within the 15% margins for the 1800 and 4000 ng/L nominal concentrations (Fig. 4).
FIGURE 4.
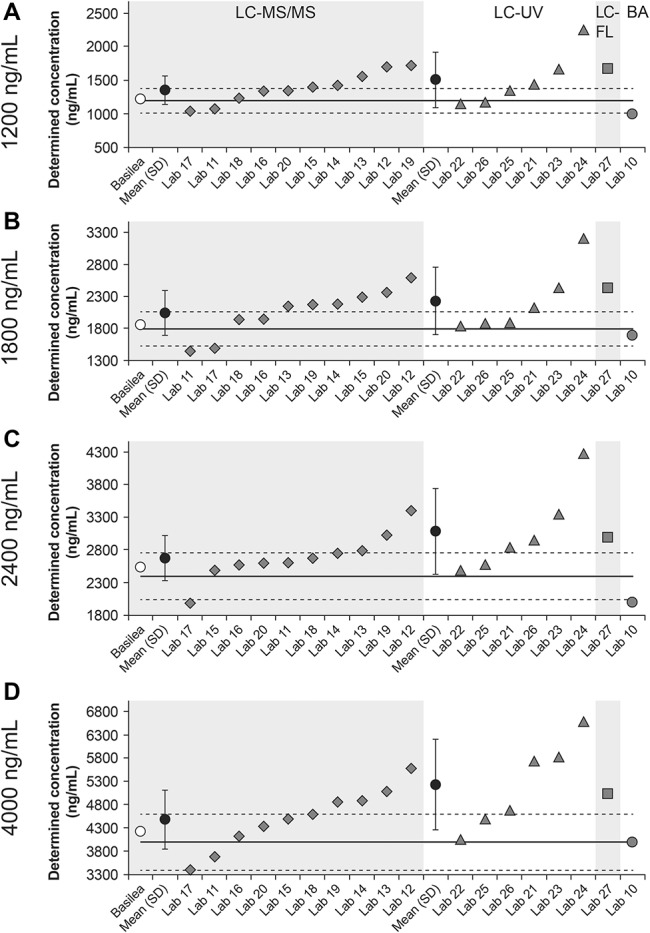
Isavuconazole plasma concentrations in round 2 determined using LC-MS/MS (labs 11–20), LC-UV (labs 21–26), LC-FL (lab 27), or bioassay (BA; lab 10) as a function of the nominal concentration of 1200, 1800, 2400, and 4000 ng/mL (A–D, respectively). Solid lines represent nominal concentrations; dashed lines represent ±15% margins from nominal concentrations.
DISCUSSION
In any instance for which isavuconazole TDM might be considered appropriate, its usefulness will require accurate measurement with regard to the analytic method used. In this round-robin exercise of European laboratories, 10 of the 27 laboratories included in both rounds provided determined plasma concentrations that were consistently within ±15% of the nominal concentrations. Of all total samples in both rounds, 54% (60/112) determined concentrations were within ±15% of the nominal concentrations. These data suggest that there is likely need for refinement and alignment for each of those analytical methods across the different laboratories.
It may be a concern that determined concentrations were consistently within the 15% margins stipulated in guidelines from the European Medicines Agency22 and Food and Drug Administration23 for fewer than half of the laboratories. Moreover, determined concentrations outside of those margins were far more likely to be overestimated (43/112) than underestimated (9/112). Overestimation in clinical practice might result in a decision to reduce the dose and thereby pose a risk for less efficacious treatment. From a methodological perspective, this suggests that a common systematic error across a subset of the laboratories is possible. Data regarding the assay calibrations performed were not available for all laboratories. As isavuconazole is a relatively new drug, it may be that some have not yet implemented the adjustments necessary to obtain the desired accuracy. For example, it is unclear whether all laboratories using LC controlled for possible matrix effects during elution.24 Controls for matrix effects were reported by 4 of the laboratories in the current study, and among those laboratories, determined concentrations were consistently within the 15% margin for 3 (LC-MS/MS: laboratories 1,8 3,10 and 189) but consistently exceeded the upper 15% margin for 1 (LC-FL: laboratory 2716). Another potentially important source of systemic error is failure to account for the content of isavuconazole in locally prepared, concentrated stock solutions for preparation of calibration/quality control samples. Therefore, it seems likely that there is a need for standardization of methods, including extraction and dilution of samples, use of appropriate internal standards, working/stock solutions, and use of solvents. The use of an internal standard would help to enhance the accuracy of the different assays used in this study to varying degrees. The use of a stable labeled internal standard is best practice for LC-MS/MS. For an LC-UV approach, an internal standard would need to be chromatographically separated from all UV peaks of interest. However, for a bioassay method, an internal standard would not be necessary for concentration determination.
It is noteworthy that the largest overestimations were observed with LC-UV in round 2 and seemed to be pronounced at higher nominal concentrations. This may involve a systematic error in purification steps (ie, steps also used for LC-MS/MS), but that alone may not account for the greater magnitude of bias. One potential explanation is that uncontrolled ionization effects, which might have affected LC-MS/MS measurements by some laboratories, might have an even greater effect on UV absorption. That might also explain the overestimations observed using LC-FL, although no firm conclusions could be drawn regarding the overall accuracy and reproducibility of this analytic approach based on results from 1 laboratory.
As only 2 laboratories in the round-robin test used bioassay, it was also not possible to draw any firm conclusions from those results. In round 1, the determined concentrations of all samples from one of the laboratories were all within the 15% margin; however, the second laboratory provided values that were underestimated for all samples. In round 2, after consultation with the first laboratory and alignment of methodology, the second laboratory provided results that were all much closer to the nominal concentrations. Nevertheless, 2 of the 4 values were still below the lower 15% margin, and so, it may be that this approach is prone to interuser variability.
Although the second laboratory that used bioassay was provided an opportunity to review and adjust their methodology, other laboratories in this study were not, and so, that might be considered a limitation of this study. In fact, multiple assessments by each laboratory for each concentration might possibly have resulted in greater consistency. However, this study was designed to more closely follow normal practice in a clinical setting, during which replicates are not usually performed. It is also important to note that, although at least one laboratory in this study had possible effects of commonly co-administered drugs,8 it is not known whether all the other laboratories had the same issue.
CONCLUSIONS
Taken together, the results of this study suggest that improving the accuracy and reproducibility of each method is very likely to require the standardization of methodologies across analytical laboratories. This process would require more open discussion regarding protocols and might be facilitated by implementing international standards. Ultimately, it would increase the confidence of clinicians in the event that isavuconazole TDM becomes necessary.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all personnel at the participating analytical laboratories (see Materials, Supplemental Digital Content 1, http://links.lww.com/TDM/A319). Isavuconazole has been co-developed by Astellas Pharma Global Development Inc and Basilea Pharmaceutica International Ltd.
Footnotes
The study was funded by Basilea Pharmaceutica International Ltd, Basel, Switzerland. Medical writing support was provided by Ed Parr, PhD, CMPP, of Envision Scientific Solutions and funded by Basilea Pharmaceutica International Ltd.
F. Pea has received personal fees from Basilea Pharmaceutica, Gilead, MSD, and Pfizer unrelated to the current study. R. Krause has received research grants from Merck and served on the speakers' bureau of Pfizer, Gilead, Astellas, Basilea, and Merck. C. Müller has received personal fees from Astellas unrelated to the current study. M. Richardson has received grants and personal fees from Basilea related to the current study and personal fees from Gilead Sciences, Astellas, MSD, and Pfizer unrelated to the current study. T. Wiktorowicz and J. Spickermann are full-time employees of Basilea Pharmaceutica International Ltd. A. S. Henriksen was a full-time employee of Basilea Pharmaceutica International Ltd at the time of the study. The remaining authors declare no conflict of interest.
A. S. Henriksen affiliation at the time of the study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
REFERENCES
- 1.Lass-Florl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405–2419. [DOI] [PubMed] [Google Scholar]
- 2.Lewis R, Brüggemann R, Padoin C, et al. Triazole Antifungal Therapeutic Drug Monitoring. Presented at the Sixth European Conference on Infections in Leukaemia; September 11, 2015; Sophia Antipolis, France.
- 3.Natesan SK, Chandrasekar PH. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist. 2016;9:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 5.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt-Hoffmann A, Roos B, Maares J, et al. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother. 2006;50:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai AV, Kovanda LL, Hope WW, et al. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother. 2017;61:e01037–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatiguso G, Favata F, Zedda I, et al. A simple high performance liquid chromatography-mass spectrometry method for therapeutic drug monitoring of isavuconazole and four other antifungal drugs in human plasma samples. J Pharm Biomed Anal. 2017;145:718–724. [DOI] [PubMed] [Google Scholar]
- 9.Müller C, Gehlen D, Blaich C, et al. Reliable and easy-to-use liquid chromatography-tandem mass spectrometry method for simultaneous snalysis of fluconazole, isavuconazole, itraconazole, hydroxy-itraconazole, posaconazole, and voriconazole in human plasma and serum. Ther Drug Monit. 2017;39:505–513. [DOI] [PubMed] [Google Scholar]
- 10.Toussaint B, Lanternier F, Woloch C, et al. An ultra performance liquid chromatography-tandem mass spectrometry method for the therapeutic drug monitoring of isavuconazole and seven other antifungal compounds in plasma samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1046:26–33. [DOI] [PubMed] [Google Scholar]
- 11.Kovanda LL, Petraitiene R, Petraitis V, et al. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: implications for clinical breakpoints. J Antimicrob Chemother. 2016;71:1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farowski F, Cornely OA, Vehreschild JJ, et al. Quantitation of azoles and echinocandins in compartments of peripheral blood by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2010;54:1815–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wissen CP, Burger DM, Verweij PE, et al. Simultaneous determination of the azoles voriconazole, posaconazole, isavuconazole, itraconazole and its metabolite hydroxy-itraconazole in human plasma by reversed phase ultra-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;887-888:79–84. [DOI] [PubMed] [Google Scholar]
- 14.Cozzi V, Baldelli S, Castoldi S, et al. Development and validation of a chromatographic ultraviolet method for the quantification of isavuconazole in human plasma samples. Ther Drug Monit. 2018;40:512–514. [DOI] [PubMed] [Google Scholar]
- 15.Nannetti G, Pagni S, Palù G, et al. A sensitive and validated HPLC-UV method for the quantitative determination of the new antifungal drug isavuconazole in human plasma. Biomed Chromatogr. 2018;32:e4333. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen R, Andersen SR, Astvad KMT, et al. Implementation of isavuconazole in a fluorescence-based high-performance liquid chromatography kit allowing simultaneous detection of all four currently licensed mold-active triazoles. mSphere 2017;2:e00098–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rautemaa-Richardson R, Moore C, Richardson M. Isavuconazole levels in non-immunocompromised patients treated for chronic pulmonary aspergillosis. Presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases; April 24, 2017; Vienna, Austria.
- 18.Gordien JB, Pigneux A, Vigouroux S, et al. Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal. 2009;50:932–938. [DOI] [PubMed] [Google Scholar]
- 19.Jourdil JF, Tonini J, Stanke-Labesque F. Simultaneous quantitation of azole antifungals, antibiotics, imatinib, and raltegravir in human plasma by two-dimensional high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;919-920:1–9. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi L, D'Aronco S, Furlanut M. Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of voriconazole and posaconazole in serum samples from patients with invasive mycoses. J Bioanal Biomed. 2011;3:92–97. [Google Scholar]
- 21.Chromsystems Instruments and Chemicals GmbH. TDM of isavuconazole using MassTox TDM Series A kit. Available at: https://www.chromsystems.com/tdm-of-isavuconazole. Accessed August 10, 2018.
- 22.European Medicines Agency. Guideline on bioanalytical method validation. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed November 2, 2017. [DOI] [PubMed]
- 23.US Food and Drug Administration. Guidance for industry: bioanalytical method validation. Available at: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Accessed November 2, 2017.
- 24.Gosetti F, Mazzucco E, Zampieri D, et al. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2010;1217:3929–3937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


