Abstract
The in vivo antibacterial activity of NO-releasing hyperbranched polymers was evaluated against Porphyromonas gingivalis, a key oral pathogen associated with periodontitis, using a murine subcutaneous chamber model. Escalating doses of NO-releasing polymers (1.5, 7.5, and 37.5 mg/kg) were administered into P. gingivalis-infected chamber once a day for three days. Chamber fluids were collected on Day 4, with microbiological evaluation indicating a dose-dependent bactericidal action. In particular, NO-releasing polymers at 37.5 mg/kg (1170 μg of NO/kg) achieved complete bacterial eradication (>6-log reduction in bacterial viability), demonstrating greater efficacy than amoxicillin (~4-log reduction in bacterial viability), a commonly used antibiotic. Time-kill assays further revealed that largest dose (37.5 mg/kg; 1170 μg of NO/kg) resulted in ~3-log killing of P. gingivalis after only a single dose. Based on these results, the potential clinical utility of NO-releasing hyperbranched polymers appears promising, particularly for oral health applications.
Introduction
Periodontitis is an oral disease associated with chronic inflammation and destruction of the tooth-supporting tissues (periodontium), affecting 11% of the population worldwide.1 If left untreated, periodontitis can cause permanent tooth loss and contribute to systemic health issues such as stroke, cardiovascular diseases, pulmonary diseases, and adverse pregnancy outcomes.2–5 Periodontitis is initiated by a synergistic and dysbiotic microbial community, which is orchestrated by keystone pathogens, such as Porphyromonas gingivalis, that remodel the microbiota from homeostasis to dysbiosis.6, 7 The systemic use of antibiotics (e.g., amoxicillin and metronidazole) is often combined with a non-surgical therapy to reduce the proportions of keystone pathogens, thereby facilitating an ecological shift in the oral microbial profile for preclusion of disease progression and further tooth loss.8, 9 Unfortunately, concomitant adverse side effects and the development of bacterial resistance limit extended antibiotic intervention.8, 10
Nitric oxide (NO), an endogenously produced free radical, possesses broad-spectrum antibacterial activity and plays a critical role in the immune response.11, 12 Both NO and its reactive byproducts (e.g., dinitrogen trioxide and peroxynitrite) exert nitrosative and oxidative stress on bacteria, resulting in DNA deamination, protein dysfunction, and lipid peroxidation.13, 14 The short biological half-life (few seconds) of NO permits localized action, avoiding adverse systemic effects common to conventional antibiotics.15 Equally important, NO’s multiple killing mechanisms avoids fostering bacterial resistance.16 Recent research has focused on the development of macromolecular scaffolds (e.g., nanoparticles and polymers) capable of storing and releasing NO in a controlled manner.17–22 Nitric oxide-releasing materials have proven effective against a wide range of pathogens, including antibiotic-resistant bacteria in vitro.20, 23 Despite promising in vitro results, in vivo antibacterial evaluation of NO-releasing materials is scarce in the literature. For example, NO-releasing coatings have been shown to reduce implant-associated bacterial infections in both rats and rabbits.24, 25 Friedman and co-workers investigated the utility of NO-releasing nanoparticles as a topical therapy for treating wound and burn infections in a number of rodent models.26–29 Although such materials were able to decrease the bacterial burden of both planktonic and biofilm-based pathogens at the infection sites, the bactericidal activity reported was less than desirable (i.e., not better than 1- or 2-log reduction). In addition, the in vivo antibacterial activity was not evaluated in a dose-dependent manner, leaving the role of NO dosing unknown.
Recently, we demonstrated the in vitro antimicrobial efficacy of NO-releasing silica nanoparticles, dendrimers, and hyperbranched polymers against periodontal pathogens.30–32 Of the materials studied to date, NO-releasing propylene oxide-modified hyperbranched polyamidoamines (HP-PO) exhibited superior bactericidal action and a favorable cytotoxicity profile relative to other systems. However, the in vivo evaluation of these materials has yet to be undertaken, and represents the next critical step for ascertaining therapeutic potential.
Herein, we report the in vivo antibacterial performance of NO-releasing HP-PO polymers using a murine subcutaneous chamber model, which has widely been used to study dental pathogen colonization and inflammatory response to oral infections.33–35 P. gingivalis was selected as the bacteria for study, given its importance in progressing chronic periodontitis.36 The bactericidal action of NO-releasing HP-PO polymers was studied for the first time in both dose- and time-dependent manners upon local administration into the chamber, and compared to amoxicillin administered in the same approach.
Materials and methods
Preparation of propylene oxide-modified hyperbranched polyamidoamine.
Nitric oxide-releasing and control (identical except not loaded with NO or capable of NO release) propylene oxide-modified hyperbranched polyamidoamine (HP-PO/NO and HP-PO/C, respectively) were prepared as previously reported.31 Briefly, hyperbranched polyamidoamine (h-PAMAM) was obtained through the polymerization of diethylenetriamine and methyl acrylate. The h-PAMAM polymer (300 mg) was subsequently modified with propylene oxide (97 μL) through a ring-opening reaction, yielding control HP-PO polymers. The conversion efficiency of PO modification was estimated to be 63% based on the integration of protons at 3.82 ppm to 2.2–3.60 ppm in the 1H NMR.31 The weight-average molecular weight of HP-PO polymers was measured to be 7.9 × 103 Da with polydispersity (PDI) of 1.62, using size exclusion chromatography coupled with a multi-angle light scattering detector.31 N-Diazeniumdiolate-modified NO-releasing HP-PO (HP-PO/NO) polymers were prepared by reacting HP-PO polymers with NO gas at high pressures (10 atm) under basic conditions (sodium methoxide).31 The resulting HP-PO/NO polymers were stored in anhydrous methanol (100 mg/mL) at −20 °C. Upon use, methanol was removed under vacuo to yield HP-PO/NO as a yellow solid. The successful formation of N-diazeniumdiolate was confirmed by the appearance of a characteristic NO donor absorbance peak at ~250 nm in the UV-vis spectra.31 The NO-release properties of HP-PO/NO were characterized in phosphate-buffered saline (PBS; 10 mM, pH 7.4) and Wilkins-Chalgren (W-C) anaerobic broth at 37 °C using a Sievers Chemiluminescence Nitric Oxide Analyzer (NOA; Boulder, CO). Of note, an aliquot (10 μL) of antifoam B emulsion (Sigma-Aldrich) was added into the 30 mL of W-C broth to prevent foaming during NO-release measurements.
In vitro planktonic bactericidal assays in broth.
Porphyromonas gingivalis (ATCC #33277) were cultured to 108 colony forming units per milliliter (CFU mL−1) in W-C broth as described previously.31 Control or NO-releasing HP-PO polymers were then introduced into those bacterial solutions at various concentrations. After 8 h, the bacterial solutions were serially diluted 100-, 10000-, and 100000-fold, and 100 μL of the diluted solutions was spiral plated onto Brucella Blood agars (Anaerobe Systems, Morgan Hill, CA). The agars were incubated under humidified anaerobic conditions (5% CO2, 10% H2, and 85% N2) for 96 h. The antibacterial activity of the materials was quantified using a plate counting method, with a detection limit of 1 × 103 CFU mL−1 (one colony in 100-fold dilution).33, 37
Subcutaneous chamber model:
Eight to ten-week old male C57B6/Ntac wild-type mice (body weight of 20 g) were purchased from Taconic Farms (Rensselaer, NY). Male mice were chosen to avoid undesired breeding and estrus-related changes of cytokines that might occur in female mice. The pathogen-free mice were single-housed with 12-h cycles of light and ad libitum access to regular mouse chow diet and water. The animal studies were approved and carried out in compliance with the Institutional Animal Care and Use Committee (IACUC) standards. Isoflurane inhalation was used to anesthetize the mice during the experiments. For chamber placement, an open-ended cylindrical coil spring (chamber) with a diameter of 0.4 cm and 1 cm in height (~100 μL of internal volume) was subcutaneously implanted in the flank region of each mouse using aseptic surgical techniques. After a 3-week healing period, a bacterial inoculum of P. gingivalis (ATCC #33277) at a dose of 108 CFU in 25 μl of PBS (10 mM, pH 7.4) was injected into the chamber of each mouse (Day 0). On the next day (Day 1), 30, 150, and 750 μg of NO-releasing or control (i.e., non-NO-releasing) HP-PO polymers dissolved in 30 μl of PBS (10 mM) with final pH of 7.4 was injected into the chamber. Analogous treatments were repeated on Day 2, and Day 3. Blank (only PBS) and amoxicillin in PBS injections were employed as negative and positive controls, respectively. On Day 4, chamber fluid was aseptically removed using a 27–gauge syringe (Becton Dickinson, Franklin Lakes, NJ). For time-course experiments, chamber fluids were removed on Day 2, Day 3, and Day 4, after the first, second, and third treatment, respectively. For bacterial viability quantification, an aliquot (15 μL) of chamber fluid was diluted into 35 μL of 35 vol% glycerol-PBS, and quantified CFUs of viable bacteria using the same plate counting method employed for the in vitro study. The remaining chamber fluid was used for cytokine level analysis.
Histological analysis.
Chambers and adjacent tissue were harvested after euthanasia, fixed in 10 vol% paraformaldehyde solution for 24 h, embedded in paraffin, and dissected into slices. The histological slices were subsequently stained with hematoxylin and eosin (H&E) and examined under light microscope.
Cytokine analysis in chamber fluids.
The levels of IL-1β and IL-6 in chamber fluids were quantified using an enzyme-linked immune-absorbent assay (ELISA) according to the manufacturer’s instruction (R&D Systems, Minneapolis, MN). Prior to the assay, aliquots (20 μL) of chamber fluids were diluted 40- and 10-fold using PBS for IL-1β and IL-6 analysis, respectively, such that the diluted concentrations fell within standard calibration ranges.
Statistical analysis.
Statistical significance in bacterial viability and cytokine levels was assessed using a two-tailed student’s t-test for comparing two groups. For comparison of more than two groups, one-way ANOVA analysis using a Bonferroni post-test was employed. In all cases, p <0.05 was considered statistically significant.
Results and Discussion
Our goal was to evaluate the in vivo antibacterial efficacy of nitric oxide (NO)-releasing materials against P. gingivalis, a key pathogen associated with chronic periodontitis. Control (i.e., non-NO-releasing) and NO-releasing propylene oxide-modified hyperbranched polyamidoamines (HP-PO/C and HP-PO/NO, respectively) were prepared and characterized as reported previously.31 Prior to in vivo antibacterial evaluation, the NO-release properties of the HP-PO/NO polymers were investigated in a nutrient-rich environment (i.e., broth) to better mimic the in vivo environment (relative to PBS). As shown in Table 2, the HP-PO/NO polymers exhibited slightly reduced NO payloads and faster NO-release kinetics in nutrient-rich milieu compared to nutrient-free conditions (i.e., PBS). The discrepancies in the NO-release properties are attributed to the proteins present in the broth, that effectively scavenge NO and interact/alter the chemical properties of the functional groups (e.g., amines) responsible for stabilizing NO donors.38, 39 Nevertheless, the HP-PO/NO polymers released ~1 μmol/mg of NO with a half-life of 20 min in nutrient-rich W-C broth. The HP-PO/NO polymers also exhibited adequate NO storage stability when stored under low temperature and moisture-free conditions. Nitric oxide-release payloads after 6 months of storage in anhydrous methanol at −20 °C were nearly identical (i.e., <5% NO loss) to freshly prepared HP-PO/NO polymers, indicating adequate stability for potential future clinical applications.
Table 2.
The absolute amounts and their corresponding local concentrations, doses, and therapeutic NO amounts of the HP-PO polymers tested in the study.
| Absolute amount (μg)a | Concentration in PBS (mg/mL)b | Concentration in the chamber (mg/mL)c | Dose (mg/kg)d | Therapeutic NO amount (μg/kg)e |
|---|---|---|---|---|
| 30 | 1 | 0.3 | 1.5 | 47 |
| 150 | 5 | 1.5 | 7.5 | 234 |
| 750 | 25 | 7.5 | 37.5 | 1170 |
The absolute mass of the polymers injected into the chambers;
The concentration of polymers in the delivery vehicle (i.e., 30 μL of PBS);
The estimated concentration of polymers in the chamber (i.e., 100 μL) after injection;
The amount of polymers injected into the chambers normalized to the mass of the mice (20 g);
The amount of NO delivered into the chambers, which was estimated by multiplying the NO totals of HP-PO/NO polymers measured in the broth (Table 1) with the tested doses.
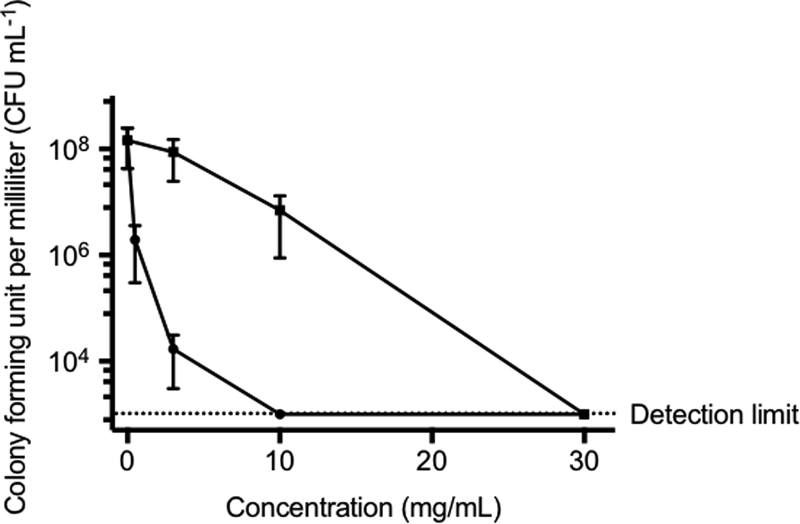
The in vitro antibacterial activity of HP-PO/NO and HP-PO/C polymers against P. gingivalis was first examined in broth (Figure 1). Analogous to the killing observed in PBS, HP-PO control polymers exhibited moderate activity due to abundant cationic amines capable of binding to negatively charged bacterial membranes and displacing essential metal cations.31 In comparison, the NO release significantly improved the antibacterial action of hyperbranched polymers. Indeed, HP-PO/NO at 3 and 10 mg mL−1 was able to achieve a ~4 log- (99.99%) and >5 log- (99.999%) reduction in bacterial viability, respectively. The antibacterial activity of HP-PO/C polymers was much less noticeable (<2 log reduction in bacterial viability) at the same concentrations. These promising in vitro results provided the motivation for investigating the bactericidal action of exogenous NO in vivo.
Figure 1.
In vitro antibacterial activity of HP-PO (solid square) and HP-PO/NO (solid circle) polymers against P. gingivalis in W-C broth. The dashed line indicates the detection limit.
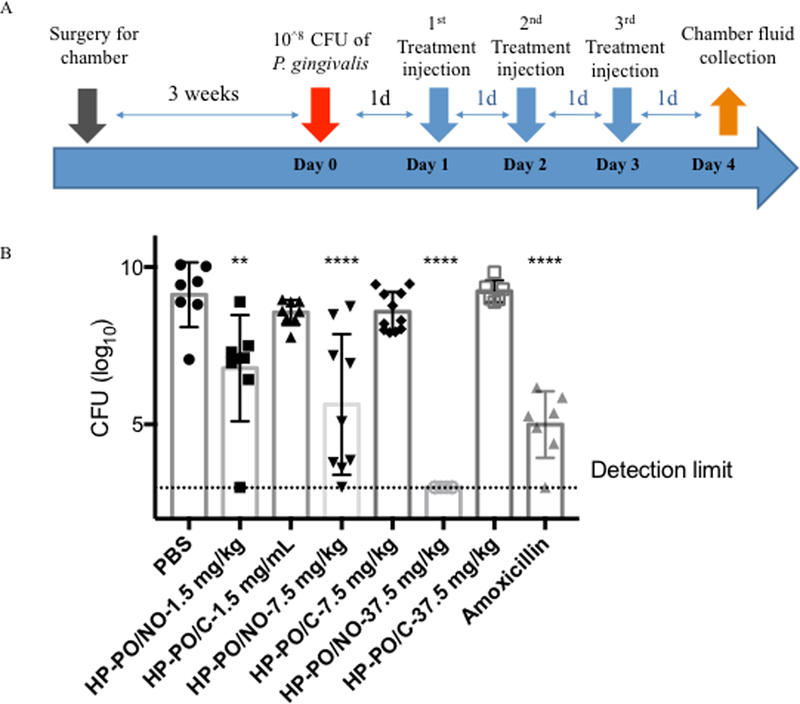
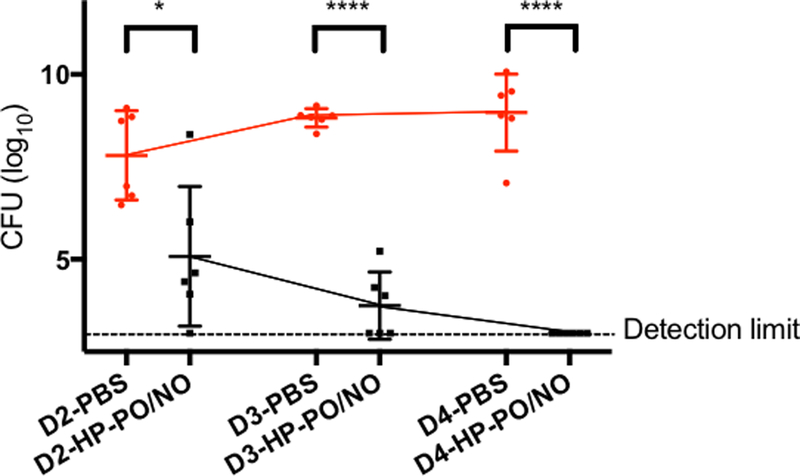
The in vivo antibacterial efficacy of HP-PO materials was evaluated against an P. gingivalis infection using a murine subcutaneous chamber model. The chambers were inoculated with 108 colony forming units (CFU) of P. gingivalis on Day 0 (Figure 2A). Subsequently, HP-PO/C and HP-PO/NO polymers at 1.5, 7.5, and 37.5 mg/kg were prepared in PBS (final pH = 7.4) and injected directly into the chambers once per day for three consecutive days. The absolute amounts and corresponding local concentrations and doses of the injected polymers are provided in Table 2. Of note, the therapeutic NO amounts were estimated by multiplying the NO totals measured in broth (Table 1) with the tested doses. Injection of blank PBS was performed as a negative control. Amoxicillin, a common antibiotic, was administered at its highest water-soluble concentration (5 mg/mL in PBS, equivalent to a therapeutic dose of 7.5 mg/kg) as a positive control. On Day 4, chamber fluids were harvested, followed by quantification of viable bacteria in the chamber fluids using a plate counting method.
Figure 2.
(A): The timeline for in vivo evaluation of HP-PO materials; (B) In vivo antibacterial efficacy of HP-PO/C and HP-PO/NO polymers against P. gingivalis (n ≥ 6 mice per group) on Day 4. Dashed line indicates the detection limit. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p <0.0001 compared to untreated negative control.
Table 1.
Nitric oxide-release properties of HP-PO/NO polymers at pH 7.4, 37 °C.a
| PBS | W-C Broth | |||||
|---|---|---|---|---|---|---|
| 1.11 ± 0.18 | 54 ± 5 | 14.0 ± 2.0 | 1.04 ± 0.23 | 19 ± 3 | 6.8 ± 1.0 | |
n ≥ 3 separate syntheses;
The total NO payloads (μmol NO per mg of polymer);
Time to release half of NO totals.
Time to reach the measured NO level ≤ 10 PPB mg−1 s−1.
As shown in Figure 2B, P. gingivalis successfully colonized the chambers and maintained high viability (i.e., ~109 CFU mL−1) in the negative control group (i.e., PBS) for the duration of the in vivo experiments. In contrast to the behavior observed in vitro, HP-PO/C polymers did not influence bacterial viability in vivo up to the highest tested dose of 37.5 mg/kg. The lack of antibacterial activity is likely due to the complex in vivo environment (e.g., high protein content) impeding any appreciable association of HP-PO/C polymers with bacteria, the primary antibacterial mechanism for cationic polymers. In contrast, HP-PO/NO polymers resulted in a significant reduction in bacterial viability, identifying NO as the key antibacterial agent in vivo. The bactericidal action of HP-PO/NO polymers was dose-dependent, with higher doses resulting in greater antibacterial efficacy. Specifically, the use of 1.5 mg/kg (47 μg of NO/kg), 7.5 mg/kg (234 μg of NO/kg), and 37.5 mg/kg (1170 μg of NO/kg) HP-PO/NO polymers resulted in a ~2.3-, ~3.5-, and ~6.1-log reductions in bacterial viability relative to untreated negative controls, respectively. Relative to control (non-NO-releasing) HP-PO polymers, the HP-PO/NO also exhibited significant improvement in bacterial killing at each tested concentration as determined by two-tailed student t-test, further demonstrating the bactericidal efficacy and drug-like activity of NO-releasing polymers. Numerous studies have indicated that NO plays an important role in the natural immune response to bacterial infection.12, 40 For example, mice lacking adequate endogenous NO production exhibit impaired host defense against P. gingivalis in the murine subcutaneous chamber.41 The antimicrobial activity of NO stems from its ability to introduce both nitrosative stress and oxidative stress on bacteria, leading to bacterial death.12, 20 As such, the local administration of exogenous NO via HP-PO/NO polymers (injected into the chamber) was expected to facilitate killing of P. gingivalis. The largest dose, 37.5 mg/kg (1170 μg of NO/kg) HP-PO/NO polymers, achieved complete eradication of P. gingivalis. In contrast, amoxicillin did not achieve the same bactericidal efficacy (~4.1-log killing of P. gingivalis for amoxicillin) as the HP-PO/NO, suggesting the superiority of the NO-release drug as a novel antibacterial agent for infection clearance.
To elucidate the bactericidal kinetics of the HP-PO/NO polymers at the most effective dose (37.5 mg/kg), the antibacterial action was quantified as a function of time by collecting chamber fluids on Day 2, Day 3, and Day 4. The HP-PO/NO polymers elicited a significant reduction (~2.7-log reduction) in bacterial viability after the first treatment (on Day 2) compared to the negative control. The rapid bactericidal action of HP-PO/NO polymers against P. gingivalis indicates another merit of their potential pharmacological use as an antibacterial therapy. Subsequent treatments continued to lower bacterial viability until complete eradication after the third dose on Day 4.
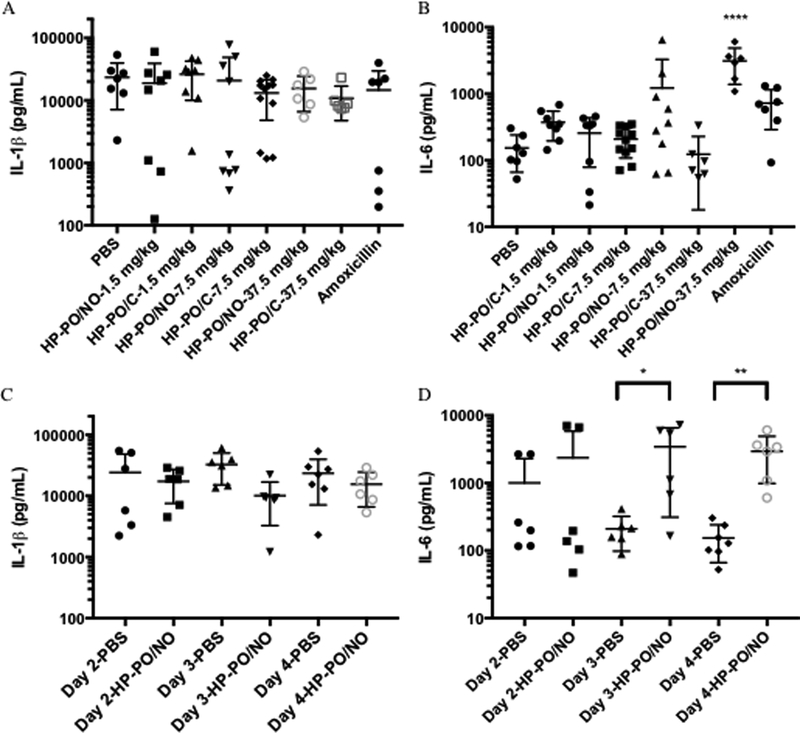
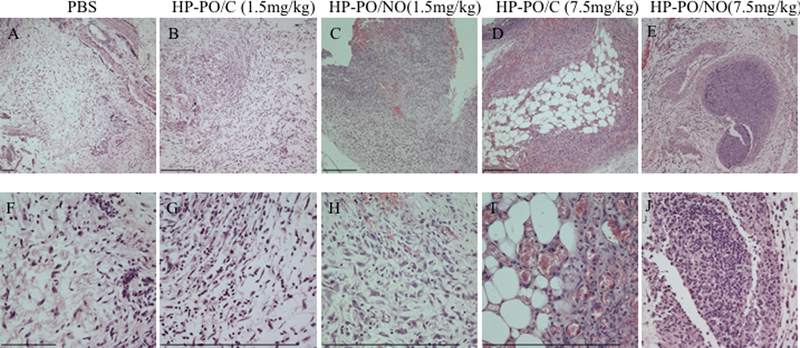
Lastly, the impact of polymer treatments on host immune response was evaluated by measuring the cytokine levels in the chamber fluids (Figure 4). Consistent with a previous study, P. gingivalis infection resulted in high levels of IL-1β and IL-6 in the chamber fluids.41 The administration of HP-PO/C polymers and amoxicillin had minimal impact on the cytokine production. Upon HP-PO/NO treatments, the levels of IL-1β were not influenced regardless of NO dose. However, the largest dose of NO (1170 μg of NO/kg) significantly up-regulated the production of IL-6. The elevation of IL-6 levels is attributed to the host response to exogenous NO. Previous studies suggest that NO plays a complex, often paradoxical role in modulating cytokine levels, exerting both inductive and suppressive cytokine expression depending on concentration.40, 42–44 Demirel et al. investigated the impact of exogenous NO on the production of IL-6 in vitro as a function of NO dose, using N-diazeniumdiolate-modified diethylenetriamine (DETA/NO) as a NO donor.42 Although 0.1 mM of DETA/NO did not influence IL-6 levels, 1 mM of DETA/NO was able to up-regulate the production of IL-6 significantly.42 These results are consistent with our observations that the largest NO dose (1170 μg of NO/kg) stimulates IL-6 production. Histological analysis (H&E staining) was used to visualize the infiltration of inflammatory cells in tissue surrounding the infected chambers (Figure 5). Regardless of the treatment, the inflammatory cells exhibited a polymorphonuclear structure indicative of neutrophil infiltration, suggesting that the high levels of cytokine production in the chambers result in acute inflammation.45 The immune response to HP-PO/NO polymers at 37.5 mg/kg (1170 μg of NO/kg) was also evaluated as a function of time. IL-1β levels remained stable over the duration of treatments (Figure 4C), while a significant increase in IL-6 production was observed on Day 3 after the second treatment (Figure 4D). The kinetic data suggests that the high levels of IL-6 are likely a result of repeated challenges with large NO doses. Although high levels of pro-inflammatory cytokines (IL-1β and IL-6) are important for bacterial infection clearance, the long-term impact of these cytokine concentrations and NO exposure on gingival tissues should be evaluated carefully in the oral cavity. Previous studies have demonstrated the utility of a ligature model for introducing periodontitis and concomitant tooth loss via binding a ligature comprised of bacterial plaque in the gingival sulcus around the molar teeth of a rodent.46–49 In this manner, the ligature model should be employed to further elucidate the therapeutic potential of NO-releasing HP-PO polymers in the mouth.
Figure 4.
Cytokine levels in chamber fluids on Day 4: (A) IL-1β; (B) IL-6. Cytokine levels in chamber fluids for negative control and treatments with 37.5 mg/kg HP-PO/NO polymers as a function of time: (C) IL-1β; (D) IL-6. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p <0.0001 compared to untreated negative control.
Figure 5.
Representative histological images of the tissues around the infected chambers: (A-E) under low magnification; (F-J) under high magnification.
Conclusion
The local administration of NO-releasing HP-PO polymers in a murine subcutaneous chamber model was capable of achieving complete eradication of a P. gingivalis infection (>6-log reduction in bacterial viability), with greater efficacy than was possible using amoxicillin. The superior in vivo bactericidal efficacy of NO-releasing HP-PO polymers suggests their utility as a potent antibacterial agent for periodontitis management. Additional in vivo studies (e.g., ligature model) are still required to further understand the true potential clinical benefits of these NO-releasing materials for oral health applications.
Figure 3.
In vivo bactericidal kinetics of HP-PO/NO polymers at 37.5 mg/kg (n ≥ 6 mice per group). Dashed line indicates the detection limit. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p <0.0001 compared to untreated negative control.
Acknowledgement
This work was supported by the National Institutes of Health (DE025207 and DE027087). Mark H. Schoenfisch declares a competing financial interest: he is a co-founder and maintains a financial interest in Novan, Inc. and Vast Therapeutics, Inc. Both companies are commercializing macromolecular nitric oxide storage and release vehicles as therapeutics for dermatological and respiratory indications, respectively.
References
- (1).Marcenes W; Kassebaum NJ; Bernabé E; Flaxman A; Naghavi M; Lopez A; Murray CJL Global Burden of Oral Conditions in 1990–2010:A Systematic Analysis. J. Dent. Res 2013, 92, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Beck JD; Offenbacher S Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J. Periodontol 2005, 76, 2089–2100. [DOI] [PubMed] [Google Scholar]
- (3).Sen S; Sumner R; Hardin J; Barros S; Moss K; Beck J; Offenbacher S Periodontal disease and recurrent vascular events in stroke/transient ischemic attack patients. J. Stroke Cerebrovasc. Dis 2013, 22, 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Southerland JH; Moss K; Taylor GW; Beck JD; Pankow J; Gangula PR; Offenbacher S Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis 2012, 222, 196–201. [DOI] [PubMed] [Google Scholar]
- (5).Kassebaum NJ; Bernabé E; Dahiya M; Bhandari B; Murray CJL; Marcenes W Global burden of severe periodontitis in 1990–2010 a systematic review and meta-regression. J. Dent. Res 2014, 93, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Paster BJ; Olsen I; Aas JA; Dewhirst FE The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2006, 42, 80–87. [DOI] [PubMed] [Google Scholar]
- (7).Khan SA; Kong EF; Meiller TF; Jabra-Rizk MA Periodontal diseases: bug induced, host promoted. PLOS Pathogens 2015, 11, e1004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Feres M; Figueiredo LC; Soares GMS; Faveri M Systemic antibiotics in the treatment of periodontitis. Periodontology 2015, 67, 131–186. [DOI] [PubMed] [Google Scholar]
- (9).Herrera D; Alonso B; León R; Roldán S; Sanz M Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol 2008, 35, 45–66. [DOI] [PubMed] [Google Scholar]
- (10).Slots J; Rams TE Antibiotics in periodontal therapy: advantages and disadvantages. J. Clin. Periodontol 1990, 17, 479–493. [DOI] [PubMed] [Google Scholar]
- (11).Carpenter AW; Schoenfisch MH, Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev 2012, 41, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jones ML; Ganopolsky JG; Labbé A; Wahl C; Prakash S Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol 2010, 88, 401–407. [DOI] [PubMed] [Google Scholar]
- (13).Wink D; Kasprzak K; Maragos C; Elespuru R; Misra M; Dunams T; Cebula T; Koch W; Andrews A; Allen J; et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991, 254, 1001–1003. [DOI] [PubMed] [Google Scholar]
- (14).Burney S; Caulfield JL; Niles JC; Wishnok JS; Tannenbaum SR The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res 1999, 424, 37–49. [DOI] [PubMed] [Google Scholar]
- (15).Holt J; Hertzberg B; Weinhold P; Storm W; Schoenfisch M; Dahners L Decreasing bacterial colonization of external fixation pins via nitric oxide release coatings. J. Orthop. Trauma 2011, 25, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Seabra AB; Justo GZ; Haddad PS State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol. Adv 2015, 33, 1370–1379. [DOI] [PubMed] [Google Scholar]
- (18).Quinn JF; Whittaker MR; Davis TP Delivering nitric oxide with nanoparticles. J. Control. Release 2015, 205, 190–205. [DOI] [PubMed] [Google Scholar]
- (19).Kim J; Saravanakumar G; Choi HW; Park D; Kim WJ A platform for nitric oxide delivery. J. Mater. Chem. B 2014, 2, 341–356. [DOI] [PubMed] [Google Scholar]
- (20).Yang L; Feura ES; Ahonen MJR; Schoenfisch MH Nitric oxide–releasing macromolecular scaffolds for antibacterial applications. Adv. Healthcare Mater. 2018, 7, 1800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Yang L; Lu Y; Soto RJ; Shah A; Ahonen MJR; Schoenfisch MH S-Nitrosothiol-modified hyperbranched polyesters. Polym. Chem 2016, 7, 7161–7169. [PMC free article] [PubMed] [Google Scholar]
- (22).Soto RJ; Yang L; Schoenfisch MH Functionalized mesoporous silica via an aminosilane surfactant ion exchange reaction: controlled scaffold design and nitric oxide release. ACS Appl. Mater. Interfaces 2016, 8, 2220–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sadrearhami Z; Nguyen T-K; Namivandi-Zangeneh R; Jung K; Wong EHH; Boyer C Recent advances in nitric oxide delivery for antimicrobial applications using polymer-based systems. J Mater Chem B 2018, 6, 2945–2959. [DOI] [PubMed] [Google Scholar]
- (24).Nablo BJ; Prichard HL; Butler RD; Klitzman B; Schoenfisch MH Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005, 26, 6984–6990. [DOI] [PubMed] [Google Scholar]
- (25).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta Biomater. 2016, 44, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Martinez LR; Han G; Chacko M; Mihu MR; Jacobson M; Gialanella P; Friedman AJ; Nosanchuk JD; Friedman JM Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J. Invest. Dermatol 2009, 129, 2463–2469. [DOI] [PubMed] [Google Scholar]
- (27).Schairer DO; Chouake JS; Nosanchuk JD; Friedman AJ The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mihu MR; Cabral V; Pattabhi R; Tar MT; Davies KP; Friedman AJ; Martinez LR; Nosanchuk JD Sustained nitric oxide-releasing nanoparticles interfere with methicillin-resistant Staphylococcus aureus adhesion and biofilm formation in a rat central venous catheter model. Antimicrob. Agents Chemother 2017, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mordorski B; Costa-Orlandi CB; Baltazar LM; Carreño LJ; Landriscina A; Rosen J; Navati M; Mendes-Giannini MJS; Friedman JM; Nosanchuk JD; Friedman AJ Topical nitric oxide releasing nanoparticles are effective in a murine model of dermal Trichophyton rubrum dermatophytosis. Nanomedicine 2017, 13, 2267–2270. [DOI] [PubMed] [Google Scholar]
- (30).Backlund CJ; Sergesketter AR; Offenbacher S; Schoenfisch MH Antibacterial Efficacy of Exogenous Nitric Oxide on Periodontal Pathogens. J. Dent. Res 2014, 93, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Yang L; Wang X; Suchyta DJ; Schoenfisch MH Antibacterial activity of nitric oxide-releasing hyperbranched polyamidoamines. Bioconjugate Chem. 2018, 29, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yang L; Schoenfisch MH Nitric oxide-releasing hyperbranched polyaminoglycosides for antibacterial therapy. ACS Appl. Bio Mater 2018, 1, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Metzger Z; Lin Y-Y; Dimeo F; Ambrose WW; Trope M; Arnold RR Synergistic Pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. J. Endod 2009, 35, 86–94. [DOI] [PubMed] [Google Scholar]
- (34).Wang Q; Jotwani R; Le J; Krauss JL; Potempa J; Coventry SC; Uriarte SM; Lamont RJ Filifactor alocis infection and inflammatory responses in the mouse subcutaneous chamber model. Infect. Immun 2014, 82, 1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Gyurko R; Boustany G; Huang PL; Kantarci A; Van Dyke TE; Genco CA; Gibson FC Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect. Immun 2003, 71, 4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).How KY; Song KP; Chan KG Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front. Microbiol 2016, 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sutton S Accuracy of plate counts. J. Validation Technol 2011, 17, 42–46. [Google Scholar]
- (38).Shin JH; Schoenfisch MH Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold. Chem Mater 2007, 20, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhou Z; Annich GM; Wu Y; Meyerhoff ME Water-soluble poly (ethylenimine)-based nitric oxide donors: preparation, characterization, and potential application in hemodialysis. Biomacromolecules 2006, 7, 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bogdan C Nitric oxide and the immune response. Nature Immunol. 2001, 2, 907. [DOI] [PubMed] [Google Scholar]
- (41).Gyurko R; Boustany G; Huang PL; Kantarci A; Van Dyke TE; Genco CA; Gibson FC, Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect Immun. 2003, 71, 4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Demirel I; Vumma R; Mohlin C; Svensson L; Säve S; Persson K, Nitric oxide activates IL-6 production and expression in human renal epithelial cells. Am. J. Nephrol 2012, 36, 524–530. [DOI] [PubMed] [Google Scholar]
- (43).Obregon C; Graf L; Chung KF; Cesson V; Nicod LP Nitric oxide sustains IL-1β expression in human edndritic cells enhancing their capacity to induce IL-17–producing T-Cells. PloS one 2015, 10, e0120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kobayashi Y The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol 2010, 88, 1157–1162. [DOI] [PubMed] [Google Scholar]
- (45).Zeng Q; Zhu Y; Yu B; Sun Y; Ding X; Xu C; Wu Y-W; Tang Z; Xu F-J Antimicrobial and antifouling polymeric agents for surface functionalization of medical implants. Biomacromolecules 2018, 19, 2805–2811. [DOI] [PubMed] [Google Scholar]
- (46).Hasturk H; Kantarci A; Goguet-Surmenian E; Blackwood A; Andry C; Serhan CN; Van Dyke TE, Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. Methods 2007, 179, 7021–7029. [DOI] [PubMed] [Google Scholar]
- (47).Hajishengallis G; Liang S; Payne MA; Hashim A; Jotwani R; Eskan MA; McIntosh ML; Alsam A; Kirkwood KL; Lambris JD Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Abe T; Hajishengallis G Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Marchesan J; Girnary MS; Jing L; Miao MZ; Zhang S; Sun L; Morelli T; Schoenfisch MH; Inohara N; Offenbacher S; Jiao Y An experimental murine model to study periodontitis. Nat Protoc 2018, 13, 2247–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]