Abstract
Background.
Hereditary angioedema (HAE) is a life-threatening, autosomal dominant disorder characterized by unpredictable, episodic swelling of the face, upper airway, oropharynx, extremities, genitalia and gastrointestinal tract. Almost all cases of HAE are caused by mutations in the SERPING1 gene resulting in a deficiency in functional plasma C1 esterase inhibitor (C1EI), a serine protease inhibitor that normally inhibits proteases in the contact, complement, and fibrinolytic systems. Current treatment of HAE includes long-term prophylaxis with attenuated androgens or human plasma-derived C1EI, and management of acute attacks with human plasma-derived or recombinant C1EI, bradykinin and kallikrein inhibitors, each of which require repeated administration. As an approach to effectively treat HAE with a single treatment, we hypothesized that a one-time intravenous administration of an adeno-associated virus (AAV) gene transfer vector expressing the genetic sequence of the normal human C1 esterase-inhibitor (AAVrh.10hC1EI) would provide sustained circulating C1EI levels sufficient to prevent angioedema episodes.
Methods.
To study the efficacy of AAVrh.10hC1EI, we used CRISPR/Cas9 technology to create a heterozygote C1EI deficient mouse model (S63+/−) that shares characteristics associated with HAE in humans including decreased plasma C1EI and C4 levels. Phenotypically, these mice have increased vascular permeability of skin and internal organs.
Results.
Systemic administration of AAVrh.10hC1EI to the S63+/− mice resulted in sustained human C1EI activity levels above the predicted therapeutic levels and correction of the vascular leak in skin and internal organs.
Conclusion.
A single treatment with AAVrh.10hC1EI has the potential to provide long term protection from angioedema attacks in affected individuals.
Keywords: C1 esterase inhibitor, complement, gene therapy, hereditary angioedema, vascular permeability
Introduction
Hereditary angioedema (HAE) is an autosomal dominant disorder characterized by episodic attacks of swelling of the face, extremities, genitalia, gastrointestinal tract and upper airways1–3. These attacks account for >20,000 emergency department visits annually in the US, often result in hospitalization, vary in frequency and severity, are unpredictable, and can last for up to 5 days2,4. Upper respiratory tract edema can result in laryngeal swelling and death by asphyxiation5,6. Over 50% of individuals with HAE have at least one laryngeal attack in their lifetime, and death by asphyxiation can occur in >25% of those affected1,3,6. Abdominal attacks secondary to edema of the walls of the gastrointestinal tract are accompanied by pain, nausea, vomiting, and diarrhea and are frequently misdiagnosed resulting in unnecessary surgery, delay in diagnosis, and narcotic dependence7–10. Disfiguring cutaneous attacks are associated with social stigma and depression requiring psychotropic or antidepressant medication and swelling may lead to impaired quality of life7,11.
HAE is caused by mutations in the SERPING1 gene, resulting in low levels of functional C1 esterase inhibitor (C1EI or C1INH), the largest member of the serine protease inhibitor (SERPIN) superfamily12–14. C1EI is produced primarily by the liver and secreted into blood where it functions as the major inhibitor of C1r, C1s, mannose binding lectin-associated serine protease MASP-1, MASP-2, factor XII and kallikrein in the contact system, factor XI and thrombin in the coagulation system, and tissue plasminogen-activator (tPA) and plasmin in the fibrinolytic system15–18. Low functional C1EI levels cause increased activation of C1, with consequent reduction in C2 and C416,18,19, and increased formation of kallikrein, leading to accumulation of bradykinin which triggers episodes of increased vascular permeability12,18,19. Over 200 different mutations in the SERPING1 gene have been reported worldwide6,20. HAE type I mutations (85% of cases) result in either a truncated or a misfolded protein that cannot be secreted. HAE type II (15% of cases) originates from mutations in the protein active site or nearby amino acids resulting in inactivity of the enzyme3,18,19.
Treatment strategies for HAE are targeted to treating acute attacks with on demand therapy or preventing attacks with prophylactic therapy21,22. Acute HAE attacks can be managed with early therapeutic intervention by administration of plasma derived C1INH (Berinert®), recombinant C1INH (Ruconest®), kallikrein inhibitor (Kalbitor®), or inhibiton of the bradykinin receptor (Firazyr®)23–26. However, long-term prophylaxis is required to sustain a normal quality of life in individuals with frequent and/or severe episodes of angioedema. Approved prophylactic therapy includes plasma derived C1INH (Cinryze®, Haegarda®), synthetic attenuated androgens (Danatrol®, Winstrol®) and antifibrinolytics23–29. Administration of plasma derived or recombinant C1INH is generally well-tolerated and is effective at reducing the incidence, severity and duration of HAE attacks, however these therapies are complicated by a high economic burden and the need for repeated administration carries a risk of limited compliance7,21,30–34. Although effective at reducing the frequency and severity of HAE attacks in many patients, chronic androgen use is associated with numerous side effects, which often leads to its discontinuation or patient noncompliance35,36.
As a strategy to correct the consequences of the genetic defect with a one-time therapy, we hypothesized that a single intravenous administration of an adeno-associated virus coding for the normal, human C1 esterase inhibitor (hC1EI) would provide sustained, long-term therapeutic levels to protect from the unpredictable and debilitating attacks of angioedema. To evaluate this hypothesis, we created a C1EI deficient mouse model that mimics the clinical and molecular phenotype of HAE. After a single administration of a serotype rh.10 AAV vector coding for human C1EI (AAVrh.10hC1EI), there was persistent expression of hC1EI, resulting in long-term protection against the clinical phenotype.
Methods
Generation of a C1EI Deficient Murine Model
Clustered regularly interspaced short palindromic repeats (CRISPR) technology was used to generate a heterozygote C1EI mouse model utilizing the approach of Romanienko et. al37 (Mouse Genetics Core, Memorial Sloan-Kettering Cancer Center). The guide RNA GGCAGTACTGTAGGCTCTGG (gRNA51) in conjunction with CRISPR associated protein 9 (Cas9) mRNA were co-injected into the pronucleus of mouse zygotes (10 ng/µl) using conventional techniques38. Founder mice were first examined using a T7 endonuclease I digestion assay to detect nucleotide changes in the target region of the mouse genome. For this purpose, polymerase chain reaction (PCR) products of the target locus were synthesized using the following primers: SERPA – GCTTCTTGAACCACAGGATAGAGC, SERPB - CAGAAGGGTTCAGTAGTAGCCTG.
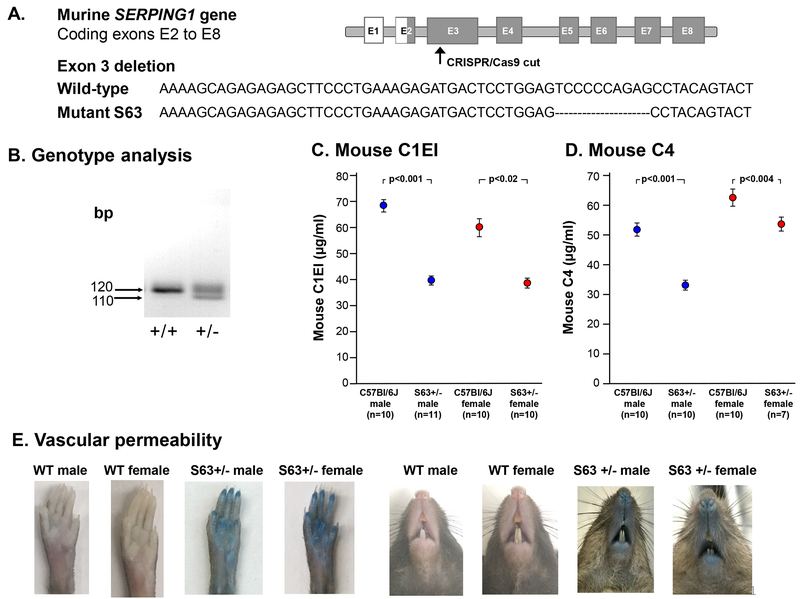
Of the 88 founder mice examined, 43 (49%) had indels based on cleavage by T7 endonuclease, suggesting a deletion and/or insertion. A subset of progeny mice was further examined for deletions by sequencing PCR amplicons of the mutated SERPING1 locus. Deletions ranging from 10 to 45 nucleotides were observed in 4 individual mouse lines. One SERPING1 knockout mouse chosen for further analysis (S63) had a deletion that created a frameshift, introducing an early stop codon in exon 3 (Figure 1A). S63 mice were bred with C57Bl/6J albino mice (Jackson Laboratory, Bar Harbor, ME). All mice were housed in microisolator cages and maintained according to standard guidelines.
Figure 1.
Characterization of the S63+/− mouse. A. CRISPR/Cas9 deleted sequences in exon 3 of the murine SERPING1 gene. B. PCR assessment of exon 3 of wild-type and S63 mice. C. Heterozygote S63+/− and wild-type serum C1EI levels as measured by ELISA. D. Heterozygote S63+/− and wild-type serum C4 levels as measured by ELISA. E. Increased vascular permeability in S63+/− mutant mice 30 min after Evans blue dye injection. Shown are photographs of wild-type (WT) and S63+/− males and female rear paws and snouts.
Genotyping of the S63 SERPING1 mice was carried out using tail tissue. Samples were lysed in 65 µl of 25 mM NaOH, 0.2mM ethylenediaminetetraacetic acid (EDTA) at 95ºC for 45 min, then neutralized with 65 µl of 40 mM Tris-HCl. PCR was used to assess exon 3 of the SERPING1 gene (forward primer - GTTATTGTGATGGCTACACTGG, reverse primer - GATCCACTGGAGGCTCAAG). The PCR reaction mix (10 µl) contained 0.1 µg DNA polymerase (Clontech, Mount View, CA), 5 pmol forward and reverse primer pairs and 2 mM Nucleotide Mix (Thermo Fisher, Grand Island, NY). The standard PCR conditions included: denaturation (94 °C, 15 sec), annealing (64 °C, 30 sec), and extension (72 °C, 90 sec), repeated 35 times. PCR products were loaded onto a 4% agarose gel and run with 1X TAE. Amplification of DNA from wild-type mice result in a single band of 120 base pairs (bp). To detect the mutation introduced by CRISPR-Cas9, genomic DNA was extracted from tail biopsies, amplified by SERPING1 exon 3 flanking primers, and sequenced. Analysis revealed a deletion of ten nucleotides, creating a frameshift that introduced an early stop codon. The resultant protein was truncated at 43 amino acids in length. The genotype of the C1EI mice was verified by PCR amplification using the primers indicated above. DNA from the S63+/– mice genotyping appeared as two bands of 110 bp and 120 base pairs and wild-type S63+/+ mice appeared as a single band of 120 base pairs (Figure 1B).
Characterization of the S63+/− Murine Model
Murine C1EI (mC1EI) and murine C4 (mC4) were evaluated in mouse sera by ELISA according to manufacturer’s instructions (Biomatik, Wilmington, DE). To assess the vascular leak phenotype, Evans blue dye (Sigma-Aldrich., St. Louis, Missouri; 30 mg/kg in 100 µl PBS) was injected into the tail vein of 6 to 8 wk-old mice. Photographs of the rear paws and snout were taken 30 min after Evans blue dye injection.
AAV Vectors
The AAVrh.10hC1EI vector is comprised of the nonhuman primate-derived rh.10 capsid pseudotyped with AAV2 inverted terminal repeats, surrounding the hC1EI expression cassette. The expression cassette consists of the cytomegalovirus (CMV) enhancer chicken–β-actin promoter (CAG promoter), the human C1EI coding sequence, and rabbit β-globin polyadenylation signal (Supplemental Figure 1A). The hC1EI cDNA sequence was optimized for increased mRNA stability and to reduce the possibility of trans-inhibition by the mutant mRNA using human-biased codons and removal of mRNA instability elements, low (<30%) or high (>80%) GC regions, translation initiation sequences within the coding region and potential splicing signals. The optimized hC1EI cDNA was synthetized with an optimal Kozak consensus and cloned into the pAAV plasmid under control of the CAG promoter. The AAVrh.10hC1EI vector was produced by co-transfection of the pAAV plasmid together with a plasmid carrying the AAV Rep proteins derived from AAV2 (needed for vector replication), the AAVrh.10 viral structural (Cap) proteins VP1, 2 and 3 (which define the serotype of the produced rh.10 AAV vector) and the adenovirus helper functions of E2, E4 and VA RNA into human embryonic kidney 293T cells (HEK 293T; American Type Culture Collection). The AAVrh.10hC1EI vector was purified by iodixanol gradient and QHP anion exchange chromatography as previously described39. Vector genome titers were determined by quantitative TaqMan real-time PCR analysis. A vector coding for human α1-antitrypsin (AAVrh.10hα1AT), which is the same construct as described above with the hC1EI cDNA replaced by hα1AT cDNA, was used as a control for the in vivo expression studies40.
In Vivo Expression of AAVrh.10hC1EI in S63 Heterozygous (S63 +/−) Mice
All animal studies were conducted under protocols reviewed and approved by the Weill Cornell Institutional Animal Care and Use Committee. To assess AAVrh.10hC1EI-directed expression of hC1EI protein in vivo, S63+/− mice, age 6 to 8 wk, were injected (intravenously in 100 μl) with a one-time dose of AAVrh.10hC1EI (1011 gc), or AAVrh.10hα1AT at 1011 gc (control vector), or 100 μl PBS as a negative control. Activity of hC1EI was measured in serum at 0 wk, and at multiple time points over the course of 24 wk by a chromogenic activity assay that measured the activity of the protein based on its ability to inhibit its natural substrate, C1 esterase (TECHNOCHROM® C1-INH [CE], DiaPharma Group, West Chester, OH). The activity assay was conducted per the manufacturer’s protocol, and results expressed as a unit of function (U/ml). Native mouse C1EI was not detected by the activity assay.
Prevention of Vascular Leak
To demonstrate that AAVrh.10hC1EI therapy reversed increased vascular permeability observed in heterozygous S63+/– mice, Evans blue dye was injected intravenously and extravasation of dye from the vasculature was evaluated by photographing of rear paws and snouts with images taken 30 min after administration in both vector treated and non-treated mice at 2, 6, and 24 wk after therapy. At each time point, a subset of mice were euthanized, and rear paws, heart, lungs, spleen, small intestine, and kidneys were removed, blotted dry, and weighed. Evans blue dye was extracted from organs with 1 ml of formamide overnight at 55°C and measured spectrophotometrically at 600 nm41,42. The absorbance was measured three times for each organ and the average value was determined with formamide used as a blank. The amount of dye recovered was calculated by extrapolating from a standard curve prepared with different concentrations of Evans blue dye solution in 1 ml of formamide. Vascular permeability was quantified spectrophotometrically by measuring the amount of dye per mg of tissue.
Statistics
All data are presented as means ± standard error of the mean (SEM) unless otherwise stated; the “n” value for each group is stated in the figure or figure legend. Differences between groups were analyzed using an unpaired two-tailed Student’s t test. p values <0.05 were considered significant for all comparisons.
Results
C1EI Deficient Murine Model Phenotype
The heterozygous C1EI deficient S63+/− mice appeared normal at birth, and subsequently developed and bred normally with no differences in litter size. The serum level of murine C1EI in the S63+/− female and male mice was significantly less when compared with wild-type controls, (p<0.001; males and p<0.02; females, Figure 1C). Serum levels of C4 in the S63+/− mice were significantly lower than levels measured in wild-type controls (p<0.001, males; p<0.004, females, Figure 1D). The S63+/− mice did not develop spontaneous episodes of swelling. However, the S63+/− mice exhibited increased vascular permeability as evidenced by the extravasation of Evans blue dye in the paws and snout, far greater than that observed in wild-type controls (Figure 1E).
AAVrh.10hC1EI-mediated Therapy
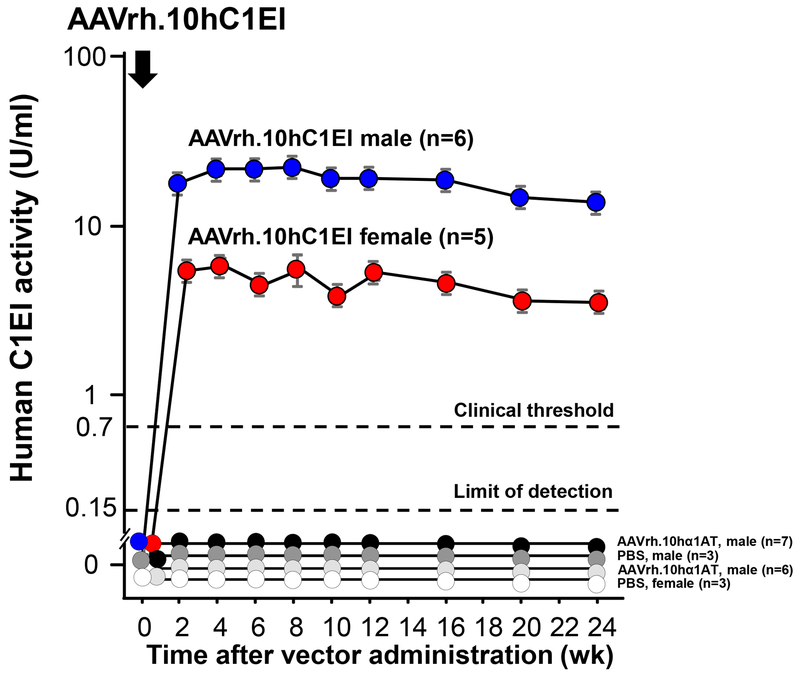
In vitro assessment demonstrated that the plasmids used to generate AAVrh.10hC1EI produced the AAVrh.10 capsid proteins and the human C1EI transgene (Supplemental Figure 1B, C). In vivo expression of hC1EI after AAVrh.10hC1EI gene transfer was demonstrated in S63+/− mice after a single intravenous administration of the vector (1011 gc; Figure 2). Human C1EI activity was observed in S63+/− mice for at least 24 wk, the last time point evaluated. As is typically observed in mice treated with experimental AAV vectors, the male mice had higher levels of the expressed protein compared to the female mice40,43–45. No hC1EI activity was detected in serum from control mice that received intravenous AAVrh.10hα1AT or PBS administration (Figure 2).
Figure 2.
AAVrh.10hC1EI-mediated persistent expression of human C1EI levels over time, following a single intravenous administration (1011 gc) to S63+/− mice. AAVrh.10hα1AT (1011 gc) and PBS were controls. Values are presented as means ± SEM. Numbers of mice in each treatment cohort are shown in the labels. The clinical threshold for C1EI is shown (0.7 U/ml) by the upper black dashed line. Limit of detection for the assay (0.15 U/ml) is shown by the lower black dashed line.
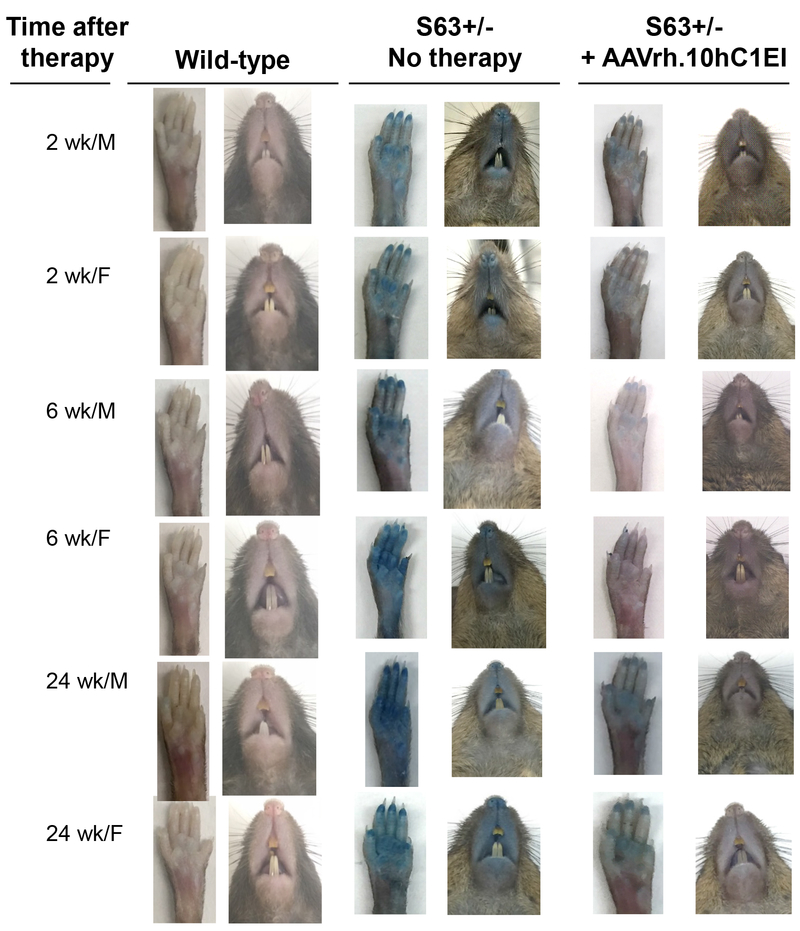
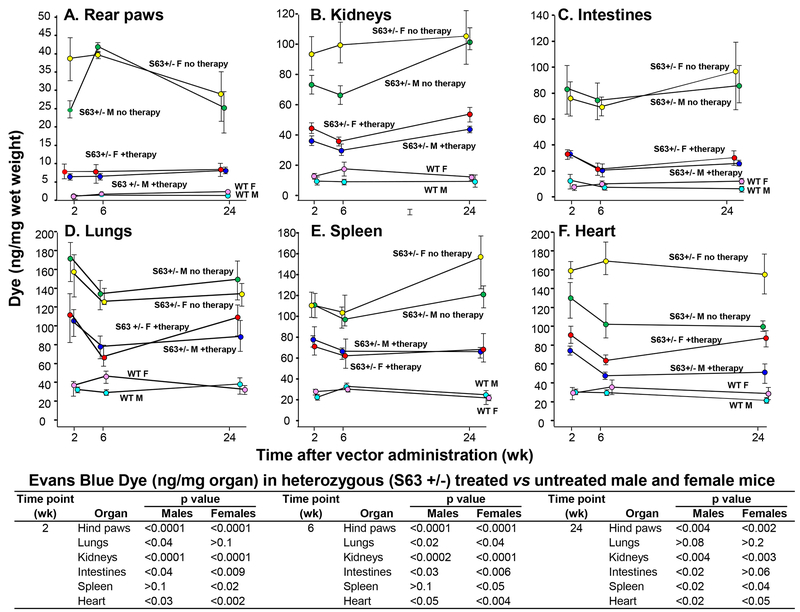
Vascular permeability of vector treated and untreated S63+/− and wild-type control mice was assessed using Evans blue dye at 2, 6 and 24 wk post-administration of AAVrh.10hC1EI (1011 gc). Untreated S63+/− mice visually exhibited greater extravasation of dye in their rear paws and snouts compared to wild-type controls (C57Bl/6J mice; Figure 3). In contrast, over 24 wk, the AAVrh.10hC1EI treated group displayed phenotypic results that were similar to the wild-type female and male controls (Figure 3). The observed phenotype was validated by quantitative spectrophotometric analysis of extracted dye from rear-paws (Figure 4A). The untreated S63+/− mice had significantly increased dye in the kidneys, intestines, lungs, spleen and heart compared to the wild-type controls (p<0.01, Figure 4B–F). In contrast, both male and female AAVrh.10hC1EI vector-treated mice had levels of dye extravasation comparable to the untreated wild-type controls. Comparisons of the S63+/− treated vs. untreated male and female mice rear paws and internal organs were significant (p<0.05) with the following exceptions; lungs (females, 2 and 24 wk), spleen (males, 2 and 6 wk) and intestines (female, 24 wk; Figure 4).
Figure 3.
AAVrh.10hC1EI correction of vascular permeability in S63+/− mice. Shown is assessment of S63+/− mice 2, 6 and 24 wk after treatment with AAVrh.10hC1EI (1011 gc) or PBS. Extravasation of dye in rear paws and snouts of S63+/− untreated and S63+/− treated mice 30 min after Evans blue dye administration. Age-matched C57BL/6J mice were used as wild-type controls. Shown are data for males and females.
Figure 4.
Spectrophotometric analysis of vascular permeability of various organs of S63+/− mice following AAVrh.10hC1EI therapy. Shown is data over time for S63+/− mice and wild-type controls. Evans blue dye was administered intravenously and tissues sampled following necropsy at 30 min. Whole tissue was incubated with 1 ml formamide to extract the extravasated Evans blue dye. Optical density was measured at 600 nm and the measurements converted into ng dye extravasated per mg tissue. A. Rear paws; B. kidneys; C. small intestines; D. lung; E. spleen; and F. heart. p values between treated and untreated S63+/− mice are presented in the table for all time-points. n=3–5 mice/group at 2, 6, and 24 wk time-points.
Discussion
Hereditary angioedema (HAE), is a rare autosomal dominant disorder resulting from mutations in the C1-inhibitor gene (SERPING1)12–14,18. The disorder afflicts 1 in 10,000 to 1 in 50,000 persons and has been reported in all races without gender predominance3,46. Affected individuals face a number of challenges including living with the uncertainty of when the next attack will present, the variability of the disease and a lack of identifiable triggers2,8,47. The goals of treatment for HAE are focused on life-saving efforts, slowing the progression and severity of attacks, reducing the number of future attacks, and their impact on quality of life3,19,21,48,49. Current therapies with plasma derived and recombinant C1INH therapies are well tolerated, however, the main challenge is that the protection provided by a single administration of these products is short, estimated at 3–4 days, requiring repeated drug administration to maintain persistent efficacy21,48. Other limitations of current angioedema therapies, in addition to the inconvenience of repeated administration, include product side-effects, safety profiles, and the high cost of most of the available therapies7,8,21,32–34,50.
To circumvent the requirement for frequent C1EI administration, we have developed a novel AAV-mediated gene therapy strategy to mediate persistent therapeutic serum levels of C1EI with a single administration of a gene transfer vector coding for human C1EI in a novel murine model of HAE that mimics the human disease. The data demonstrates that AAV-mediated C1EI therapy markedly reduces the vascular permeability phenotype in these mice.
In the present study, we established and characterized a mouse model that mimics hereditary angioedema at the molecular level. In particular, mice had markedly decreased circulating levels of C1EI and C4. Intra-strain gender-dependent differences were detected in C4 and C1EI levels, but overall, as in humans, C1EI levels in most mice were approximately 50% of normal and C4 levels were overall decreased. On average, C4 levels in the female S63+/− mice, did not drop below 50% of wild type controls, but there were no differences in the vascular permeability data between male and female S63+/− mice. Although there are no intergender differences in C4 levels in humans with HAE, the mouse data suggests dysregulated activation of the complement system, akin to the human disease. S63+/− mice did not have spontaneous or triggered episodes of swelling involving the skin or organs as in the human disease, but mice displayed increased vascular permeability, as demonstrated by analysis of the extravasation of intravenously injected Evans blue dye in multiple organs. Although there is a previously described C1INH-deficient mouse41,51, we developed the S63+/− mouse because the previously described mouse is owned by Lexicon Pharmaceuticals (The Woodlands, TX) and is not freely available to the academic community41,51.
Our study demonstrates that a one-time therapy with AAVrh.10hC1EI provided sufficient human C1EI systemically, in both male and female mice, to abrogate the increased vascular permeability phenotype associated with HAE. With the goal of translating this therapy to human clinical trials, human C1 esterase inhibitor was used. Given the high homology between murine and human SERPING1 at the protein level (78% identity), the murine studies used human C1 esterase inhibitor, instead of mouse C1 esterase inhibitor, as we hypothesized that the human therapy would successfully reverse the mouse phenotype52. Therapy with AAVrh.10hC1EI was effective at protecting against increased vascular permeability in the paws, snout, and internal organs of these mice. Although the C1EI deficient mice did not display any obvious spontaneous clinical phenotypic abnormalities, after intravenous injection of Evans blue dye, which binds to serum albumin, the mice displayed increased vascular permeability compared with wild-type controls. Shortly after Evans blue dye injection, the rear paws, snout, and internal organs in C1EI deficient mice turned blue; whereas, wild-type control mice turned only slightly blue, as observed both clinically and by spectrophotometric measurement of the extracted dye. A one-time dose of AAVrh.10hC1EI reversed the increased vascular permeability after Evans blue dye injection up to 24 wk after therapy, the last time point evaluated. While the current study is limited to 24 weeks, AAV-based gene therapies in our lab and others have demonstrated long term expression measured in years and limited only by the longest time point evaluated53,54,55. This study establishes that a gene therapy expressing C1EI provides protection from increased vascular permeability in the mouse model, but translation to drug development requires formal safety and toxicology studies that will inform the viability of this approach with respect to dose and associated drug safety.
The protection against increased vascular permeability coupled to the long term gene expression studies suggests that AAVrh.10hC1EI has a long-lasting protective effect and indicates that protection can be achieved against HAE attacks by a single dose, an advantage of the delivery system. Thus, in a clinical setting, AAVrh.10hC1EI therapy could offer long-term protection to individuals affected by HAE following a single administration of the vector.
Supplementary Material
Acknowledgments
We thank N Mohamed for help preparing this manuscript. These studies were supported, in part, by NIH R03 AI22040 and Adverum Biotechnologies, Menlo Park, CA. The MSKCC Mouse Genetics Core is supported in part by an NCI Cancer Center Support Grant 5 P30 CA008748.
Footnotes
Conflicts statement. Cornell University has licensed the patent disclosure relating to gene therapy for C1EI deficiency to Adverum Biotechnologies. RGC is a shareholder and a consultant to Adverum. OP, MC and RGC are inventors on the patent disclosure. TQ, ASW, ARR, DS and SMK have no conflicts
References
- 1.Agostoni A, ygoren-Pursun E, Binkley KE et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol 2004;114:S51–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med 2008;359:1027–1036. [DOI] [PubMed] [Google Scholar]
- 3.Zuraw BL, Bernstein JA, Lang DM et al. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol 2013;131:1491–1493. [DOI] [PubMed] [Google Scholar]
- 4.Gower RG, Busse PJ, ygoren-Pursun E et al. Hereditary angioedema caused by c1-esterase inhibitor deficiency: a literature-based analysis and clinical commentary on prophylaxis treatment strategies. World Allergy Organ J 2011;4:S9–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bork K, Hardt J, Schicketanz KH et al. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med 2003;163:1229–1235. [DOI] [PubMed] [Google Scholar]
- 6.Bork K, Korger G, Kreuz W. Review of the long-term safety of a human pasteurized C1 inhibitor concentrate. J Allergy Clin Immunol 2012;129;2S:AB222. [Google Scholar]
- 7.Banerji A The burden of illness in patients with hereditary angioedema. Ann Allergy Asthma Immunol 2013;111:329–336. [DOI] [PubMed] [Google Scholar]
- 8.Banerji A, Busse P, Christiansen SC et al. Current state of hereditary angioedema management: a patient survey. Allergy Asthma Proc 2015;36:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bork K, Staubach P, Eckardt AJ et al. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol 2006;101:619–627. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein E, Stolz LE, Sheffer AL et al. Abdominal attacks and treatment in hereditary angioedema with C1-inhibitor deficiency. BMC Gastroenterol 2014;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol 2014;112:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cugno M, Zanichelli A, Foieni F et al. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med 2009;15:69–78. [DOI] [PubMed] [Google Scholar]
- 13.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 2009;78:147–176. [DOI] [PubMed] [Google Scholar]
- 14.Lomas DA. Molecular mousetraps, alpha1-antitrypsin deficiency and the serpinopathies. Clin Med 2005;5:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicardi M, Zingale L, Zanichelli A et al. C1 inhibitor: molecular and clinical aspects. Springer Semin Immunopathol 2005;27:286–298. [DOI] [PubMed] [Google Scholar]
- 16.Pappalardo E, Zingale LC, Terlizzi A et al. Mechanisms of C1-inhibitor deficiency. Immunobiology 2002;205:542–551. [DOI] [PubMed] [Google Scholar]
- 17.Walford HH, Zuraw BL. Current update on cellular and molecular mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol 2014;112:413–418. [DOI] [PubMed] [Google Scholar]
- 18.Zuraw BL, Christiansen SC. HAE Pathophysiology and Underlying Mechanisms. Clin Rev Allergy Immunol 2016;51:216–229. [DOI] [PubMed] [Google Scholar]
- 19.Zuraw BL. The pathophysiology of hereditary angioedema. World Allergy Organ J 2010;3:S25–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.C1 inhibiTor gene muTATion dATAbAse. 2018. http://hae.enzim.hu/. [last accessed 4/19/18]
- 21.Zuraw BL, Banerji A, Bernstein JA et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 2013;1:458–467. [DOI] [PubMed] [Google Scholar]
- 22.Zuraw BL, Christiansen SC. How we manage persons with hereditary angioedema. Br J Haematol 2016;173:831–843. [DOI] [PubMed] [Google Scholar]
- 23.Berinert. [package insert]. Kankakee, IL: CSL Behring LLC; . 2009. http://labeling.cslbehring.com/PI/US/Berinert/EN/Berinert-Prescribing-Information.pdf [last accessed 12/1/2017] [Google Scholar]
- 24.European Medicines Agency. Firazyr: Annex I: Summary of Product Characteristics. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000899/WC500022966.pdf [last accessed 12/1/2017]
- 25.Kalbitor. [package insert]. Cambridge, MA: Dyax Corp; . 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125277s071lbl.pdf [last accessed 12/1/2017] [Google Scholar]
- 26.Ruconest. [package insert]. https://81b77e9a9bc9711e90b1-f416dd7f832b6e1ad8c969a90667ca99.ssl.cf1.rackcdn.com/shared/pi/ruconest-pi.pdf . 2015. [last accessed 12/1/2017]
- 27.CINRYZE. [package insert]. Exton, PA: ViroPharma Biologics, Inc; . 2010. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM129918.pdf [last accessed 12/1/2017] [Google Scholar]
- 28.Longhurst H, Cicardi M, Craig T et al. Prevention of Hereditary Angioedema Attacks with a Subcutaneous C1 Inhibitor. N Engl J Med 2017;376:1131–1140. [DOI] [PubMed] [Google Scholar]
- 29.CSL Behring. HAEGARDA, C1 esterase inhibitor subcutaneous (human). 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM564335.pdf [last accessed 12/1/2017]
- 30.Bernstein JA. HAE update: epidemiology and burden of disease. Allergy Asthma Proc 2013;34:3–6. [DOI] [PubMed] [Google Scholar]
- 31.Craig T, Riedl M, Dykewicz MS et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol 2009;102:366–372. [DOI] [PubMed] [Google Scholar]
- 32.Craig T, Shapiro R, Vegh A et al. Efficacy and safety of an intravenous C1-inhibitor concentrate for long-term prophylaxis in hereditary angioedema. Allergy Rhinol (Providence) 2017;8:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riedl MA, Banerji A, Busse PJ et al. Patient satisfaction and experience with intravenously administered C1-inhibitor concentrates in the United States. Ann Allergy Asthma Immunol 2017;119:59–64. [DOI] [PubMed] [Google Scholar]
- 34.Wilson DA, Bork K, Shea EP et al. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol 2010;104:314–320. [DOI] [PubMed] [Google Scholar]
- 35.Riedl MA. Critical appraisal of androgen use in hereditary angioedema: a systematic review. Ann Allergy Asthma Immunol 2015;114:281–288. [DOI] [PubMed] [Google Scholar]
- 36.Zuraw BL, Davis DK, Castaldo AJ et al. Tolerability and Effectiveness of 17-alpha-Alkylated Androgen Therapy for Hereditary Angioedema: A Re-examination. J Allergy Clin Immunol Pract 2016;4:948–955. [DOI] [PubMed] [Google Scholar]
- 37.Romanienko PJ, Giacalone J, Ingenito J et al. A Vector with a Single Promoter for In Vitro Transcription and Mammalian Cell Expression of CRISPR gRNAs. PLoS One 2016;11:e0148362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Second ed Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 1994. [Google Scholar]
- 39.Sondhi D, Johnson L, Purpura K et al. Long-term expression and safety of administration of AAVrh.10hCLN2 to the brain of rats and nonhuman primates for the treatment of late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther Methods 2012;23:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De BP, Heguy A, Hackett NR et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006;13:67–76. [DOI] [PubMed] [Google Scholar]
- 41.Han ED, MacFarlane RC, Mulligan AN et al. Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest 2002;109:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thurston G, Suri C, Smith K et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514. [DOI] [PubMed] [Google Scholar]
- 43.Davidoff AM, Ng CY, Zhou J et al. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 2003;102:480–488. [DOI] [PubMed] [Google Scholar]
- 44.Inagaki K, Fuess S, Storm TA et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006;14:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nietupski JB, Hurlbut GD, Ziegler RJ et al. Systemic administration of AAV8-alpha-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol Ther 2011;19:1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med 2001;161:2417–2429. [DOI] [PubMed] [Google Scholar]
- 47.Zotter Z, Csuka D, Szabo E et al. The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency. Orphanet J Rare Dis 2014;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craig T, Aygoren-Pursun E, Bork K et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J 2012;5:182–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu MA, Zanichelli A, Mansi M et al. Current treatment options for hereditary angioedema due to C1 inhibitor deficiency. Expert Opin Pharmacother 2016;17:27–40. [DOI] [PubMed] [Google Scholar]
- 50.Craig TJ, Levy RJ, Wasserman RL et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009;124:801–808. [DOI] [PubMed] [Google Scholar]
- 51.Han Lee ED, Pappalardo E, Scafidi J et al. Approaches toward reversal of increased vascular permeability in C1 inhibitor deficient mice. Immunol Lett 2003;89:155–160. [DOI] [PubMed] [Google Scholar]
- 52.NCBI. HomoloGene:44. Gene conserved in Euteleostomi. National Library of Medicine. https://www.ncbi.nlm.nih.gov/homologene/44 [last accessed 6/5/18]
- 53.Nathwani AC, Rosales C, McIntosh J et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011;19:876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sondhi D, Peterson DA, Giannaris EL et al. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther 2005;12:1618–1632. [DOI] [PubMed] [Google Scholar]
- 55.Chiuchiolo MJ, Kaminsky SM, Sondhi D et al. Intrapleural administration of an AAVrh.10 vector coding for human alpha1-antitrypsin for the treatment of alpha1-antitrypsin deficiency. Hum Gene Ther Clin Dev 2013;24:161–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.