Abstract
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis, and it is unclear whether its stromal infiltrate contributes to its aggressiveness. Here, we demonstrate that Dickkopf-3 (DKK3) is produced by pancreatic stellate cells and is present in most human PDAC. DKK3 stimulates PDAC growth, metastasis, and resistance to chemotherapy with both paracrine and autocrine mechanisms through NF-κB activation. Genetic ablation of DKK3 in an autochthonous model of PDAC inhibited tumor growth, induced a peritumoral infiltration of CD8+ T cells, and more than doubled survival. Treatment with a DKK3-blocking monoclonal antibody inhibited PDAC progression and chemoresistance and prolonged survival. The combination of DKK3 inhibition with immune checkpoint inhibition was more effective in reducing tumor growth than either treatment alone and resulted in a durable improvement in survival, suggesting that DKK3 neutralization may be effective as a single targeted agent or in combination with chemotherapy or immunotherapy for PDAC.
INTRODUCTION
The tumor microenvironment is an important mediator of progression for many cancers (1–6), and pancreatic ductal adenocarcinoma (PDAC) in particular is characterized by a dense fibrotic stroma in the tumor microenvironment. This fibrotic stroma consists primarily of pancreatic stellate cells (PSCs), which promote PDAC proliferation and metastasis (1, 4, 7) and reduce PDAC cell responses to therapeutics (1, 8). However, the precise mechanisms of how PSCs affect these processes are not well understood, and clinical trials targeting the stroma in PDAC have had largely disappointing results (9). Previous efforts to target PDAC stroma were directed at broadly eliminating stromal elements including fibroblasts, but a more effective strategy may be to inhibit specific tumor-promoting mechanisms elaborated by PSCs. To better understand the effects of the stroma on PDAC, we investigated the effects of Dickkopf-3 (DKK3), a factor secreted by PSCs, on PDAC.
DKK3 is a 38-kDa member of the dickkopf (DKK) family of glycoproteins (DKK1–4) that may be involved in regulating WNT pathways (10–12). The best-characterized member of the DKK family is DKK1, which is a natural soluble inhibitor of WNT signaling and is associated with tumor suppressor functions (13, 14). DKK3 shares a distinct N-terminal cysteine-rich domain and C-terminal colipase fold domain with other DKKs, but otherwise, DKK3 appears to be a divergent member of the DKK family with differences in DNA sequence, chromosome group location, and potentially receptor and signaling mechanisms as well (15, 16).
In contrast to DKK1, the functional role of DKK3 in cancer is not clear, with conflicting reports of its effect as either a tumor suppressor or promoter. In prostate cancer and osteosarcoma, DKK3 is described as a tumor suppressor, and its overexpression inhibits tumor growth and metastasis (17–23). However, data in head and neck cancer and other tumors suggest that DKK3 increases cancer aggressiveness (19, 24–26). Reports on the signaling mechanisms of DKK3 are similarly inconsistent, with studies showing no effect, potentiation, or inhibition of WNT (19, 25, 27).
Recent reports have demonstrated an immunomodulatory role for DKK3, including induction of CD8+ T cell tolerance. Exogenous DKK3 inhibited T cell activity, and when DKK3 function was blocked, CD8 T cell proliferation and interleukin-2 (IL-2) production were restored (28, 29). However, the precise role of DKK3 in the tumor immune response is far from clear, because conflicting reports also describe an immunostimulatory effect of DKK3 in lung and pancreatic cancer models (30–32).
In this study, we found that Dkk3 is highly expressed in human PDAC, specifically by PSCs rather than cancer cells. Given the conflicting literature on the role of DKK3 in cancer, we sought to characterize the contribution of DKK3 to PDAC using both genetic ablation in autochthonous models and pharmacologic inhibition with a monoclonal antibody (mAb) against DKK3. Last, we studied the effects of DKK3 on the tumor immune response in PDAC and investigated the efficacy of DKK3 blockade in improving response to immunotherapy.
RESULTS
DKK3 is overexpressed in PDAC
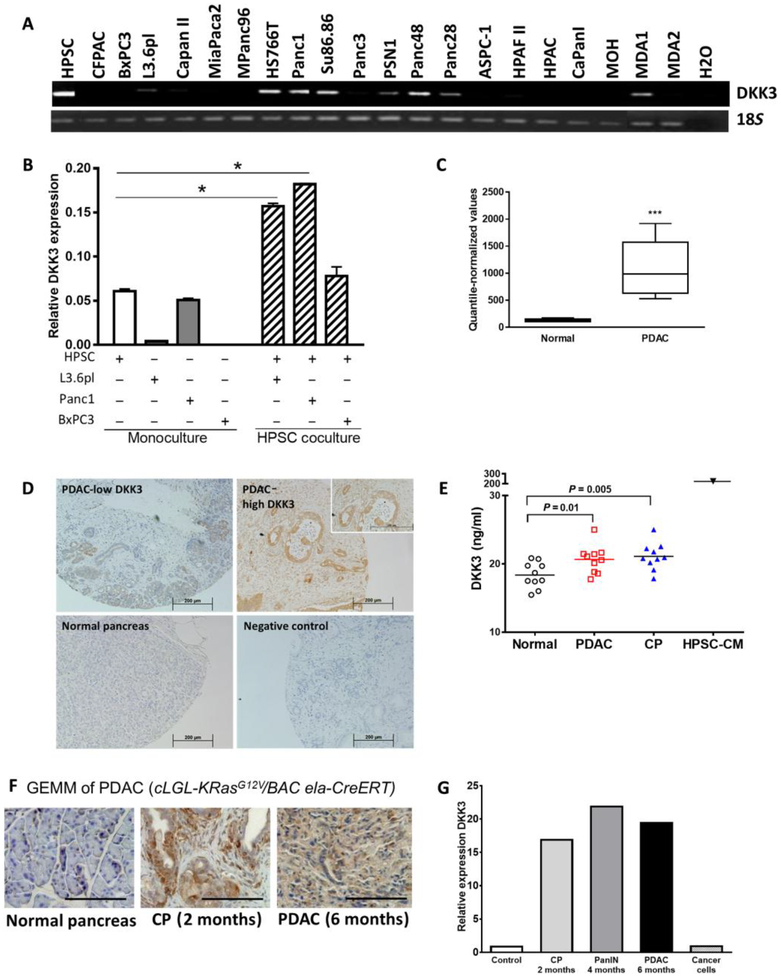
We examined the expression of Dkk3 in human PSCs (HPSCs) and 20 PDAC cell lines by reverse transcription polymerase chain reaction (RT-PCR; Fig. 1A). Expression was strongest in HPSCs, with lower expression in seven cell lines (HS766T, Panc1, SU86.86, Psn1, Panc48, Panc28, and MDA1) and no expression in the majority (14 of 21) of the cancer cell lines tested. DKK3 is secreted by HPSCs, as confirmed by Western blotting of HPSC-CM (fig. S1A). Dkk3 expression was similar in five HPSC preparations from different patients (fig. S1B).
Fig. 1. DKK3 is expressed by HPSCs in PDAC.
Dkk3 expression was measured in HPSCs and PDAC cell lines by RTPCR (A) and qPCR (B) in monoculture and coculture. Striped bars indicate expression in HPSCs after coculture with PDAC cells. (C) Dkk3 expression in human PDAC and normal pancreatic tissue was determined by Affymetrix array. (D) IHC of DKK3 in a tissue microarray of human PDAC. Shown are representative fields from PDAC expressing low and high amounts of DKK3 with normal pancreas and negative controls. (E) DKK3 concentrations were measured by enzyme-linked immunosorbent assay (ELISA) in plasma samples from patients with PDAC, CP, or no pancreatic disease and in conditioned medium (CM) from HPSCs (HPSC-CM). (F) In a GEMM of PDAC, DKK3 is expressed early in development with CP and PanIN lesions and increases in PDAC. Scale bars, 200 μm. (G) Relative expression of Dkk3 in the GEMM of PDAC and in cancer cells isolated from GEMM tumors was quantified by Affymetrix. *P < 0.05, ***P < 0.001. Data are means ± SEM.
Cross-talk between stromal fibroblasts and cancer cells has been described previously (2, 33). We investigated whether coculture of HPSCs and PDAC cells would affect DKK3 expression in either cell population. Quantitative RT-PCR (qRT-PCR) confirmed minimal to no expression of Dkk3 in L3.6pl and BxPC3 cells in monoculture, whereas expression in Panc1 cells was nearly equivalent to that of HPSCs (Fig. 1B). Coculture of HPSCs with either Panc1 or L3.6pl cells increased Dkk3 expression in HPSCs by threefold compared with culturing HPSCs alone (Fig. 1B, white striped bars). There was no increase in Dkk3 expression by HPSC after coculture with BxPC3. Conversely, Dkk3 expression in the cancer cells was not altered after coculture with HPSCs (fig. S1C). Thus, HPSCs have high basal expression of Dkk3, which is further augmented when they are cocultured with cancer cells, but PDAC cells express minimal amounts of Dkk3, which does not change with exposure to HPSCs.
Dkk3 expression was assessed in human PDAC and normal pancreas (n = 10 per group) using Affymetrix gene expression profiling (34), which showed 4.5 times higher expression in PDAC than in normal pancreas (Fig. 1C). In a tissue microarray of human PDAC, we confirmed the expression of DKK3 in 118 of 119 samples (99%), with moderate to high expression in 69 samples (58%) (Fig. 1D). Most samples showed that DKK3 expressed predominantly in the stroma, although several samples with more intense staining demonstrated staining in areas of carcinoma as well. Its expression was not restricted to cells positive for α-smooth muscle actin (α-SMA), which is one marker, but not a unique marker, of PSCs (fig. S1D). DKK3 is expressed in human umbilical cord endothelial cells (HUVECs) as well (fig. S1E), but its role there was not further explored in this study. When we examined DKK3 in plasma, patients with PDAC had significantly higher mean concentrations than did healthy volunteers (20.64 ng/ml versus 18.36 ng/ml, P < 0.01; Fig. 1E). DKK3 serum concentrations were similar in patients with chronic pancreatitis (CP) and with PDAC. The concentrations of DKK3 in HPSC-CM were 10 times higher than those in the plasma, suggesting that DKK3 may be highly concentrated in the local tumor microenvironment relative to the peripheral circulation.
To evaluate the expression of DKK3 at early stages of PDAC development, we examined a genetically engineered mouse model (GEMM) of PDAC with high expression of mutant Kras (cLGL-KrasG12VBAC Ela-CreERT) (35). In this model, mice develop CP and early pancreatic intraepithelial neoplasia (PanIN) lesions within 2 months, CP and late PanIN lesions within 4 months, and invasive PDAC with metastases within 6 months. Compared with control mice without pancreatic disease, Dkk3 expression was increased 18-fold in CP and early PanINs at 2 months and 21-fold in CP and late PanINs at 4 months (Fig. 1, F and G). When mice developed invasive PDAC at 6 months, Dkk3 expression by Affymetrix array was nearly 20 times higher than in controls (Fig. 1G). In contrast, Dkk3 expression was minimal in cancer cells that were isolated from invasive pancreatic tumors formed in this model (Fig. 1G), indicating that DKK3 is primarily derived from stromal cells.
DKK3 stimulates PDAC and stellate cell activity
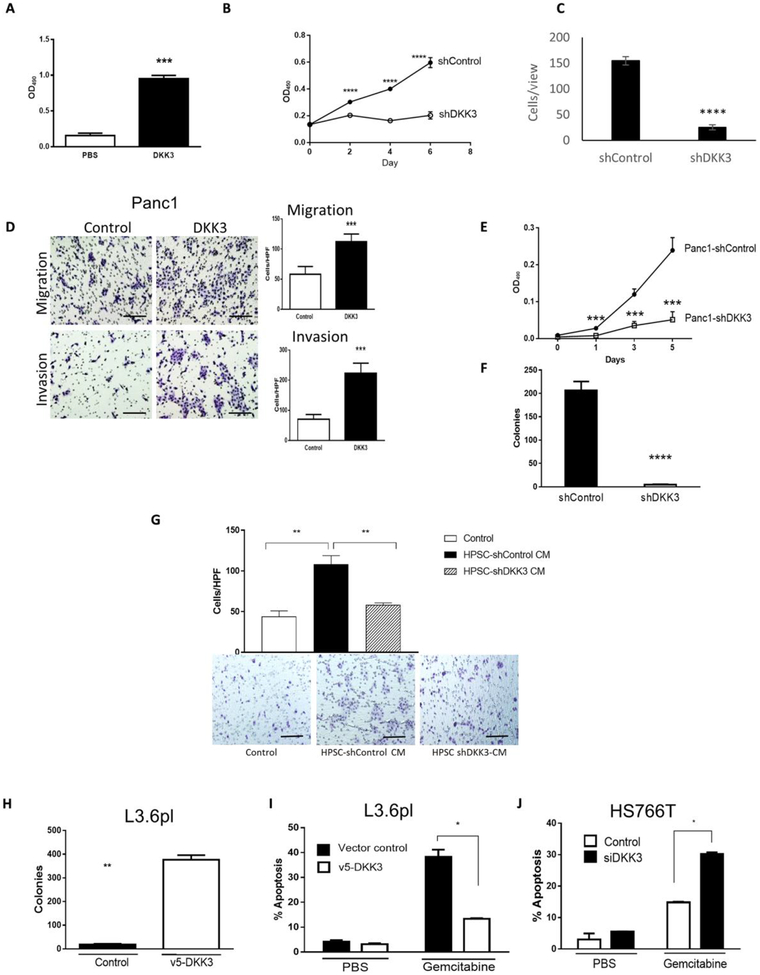
On the basis of our finding that DKK3 was produced primarily by HPSCs, we investigated whether DKK3 had a functional role in HPSC activity. Treatment with recombinant human DKK3 (rhDKK3; 10 μg/ml) for 48 or 72 hours significantly increased HPSC proliferation compared with phosphate-buffered saline (PBS) controls (P < 0.0001; Fig. 2A). Stable silencing of DKK3 in HPSCs (HPSC-shDKK3; fig. S1F) resulted in a 67% reduction in cell proliferation compared to control cells by day 6 (P < 0.00001; Fig. 2B) and a reduction in cell migration to 16.3% of controls (P < 0.00001; Fig. 2C). We confirmed DKK3 expression in primary HPSCs from a total of five separate patients (fig. S1B) and observed similar inhibitory effects on proliferation and migration when DKK3 was silenced as compared to our main “HPSC” cell line used throughout our experiments (fig. S2, A to D).
Fig. 2. DKK3 stimulates HPSC and PDAC activity and increases chemoresistance.
(A) HPSC proliferation was measured by MTT [3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide] after treatment with PBS or rhDKK3 (10 μg/ml). OD490, optical density at 490 nm. DKK3 was silenced in HPSCs by shDKK3 (fig. S1B), cell proliferation was measured by MTT assay (B), and migration was determined at 24 hours (C). Control cells were transfected with scrambled short hairpin RNA (shRNA). (D) Panc1 cells were treated with rhDKK3 (10 μg/ml) or serum-free medium control, and cell migration and invasion were measured after 24 hours. (E) Panc1 cells were stably silenced for DKK3, and (E) cell proliferation and (F) colony formation in soft agar were measured. (G) BxPC3 cell migration was measured after treatment with CM from HPSCs or HPSCs silenced for DKK3. HPF, high-power field. (H) Soft agar colony formation in gemcitabine and (I) apoptosis were determined in chemosensitive L3.6pl cells expressing DKK3 compared with transfection controls. (J) Gemcitabine-induced apoptosis was measured in chemoresistant HS766T cells silenced for DKK3. *P < 0.01, **P < 0.001, ***P < 0.0001, ****P < 0.00001. Scale bars, 200 μm.
Next, we investigated whether DKK3 had paracrine effects on PDAC cells. Treatment of Panc1 cells with rhDKK3 (10 μg/ml) resulted in a nearly 100% increase in migration and more than a threefold increase in invasion (P < 0.0001 versus PBS controls; Fig. 2D). Similar results were observed for BxPC3 cells, with induction of both migration (P < 0.001) and invasion (P < 0.0001) compared to controls (fig. S2E). Results of an initial dose-response experiment to test rhDKK3 in HPSC and BxPC3 functional assays are shown in fig. S2 (F and G). Unlike most other PDAC cell lines, Panc1 expresses a moderate amount of DKK3, and stable silencing of DKK3 resulted in inhibition of cell proliferation compared with cells transfected with control shRNA (Fig. 2E) and nearly completely eliminated their ability to grow in soft agar (Fig. 2F), suggesting that DKK3 is critical for anchorage-independent growth.
To confirm whether the effects of DKK3 secreted by HPSCs are similar to those of recombinant DKK3, we treated cells with CM from HPSCs transfected with shControl or shDKK3. BxPC3 cells treated with CM from HPSC-shControl showed an 87% increase in migration compared with serum-free medium controls, whereas migration with CM from HPSC-shDKK3 was similar to medium controls (Fig. 2G). A comparison of DKK3 in rhDKK3 and HPSCCM by Western blotting is shown in fig. S2H. In summary, these data suggest that DKK3 acts in a paracrine fashion to promote PDAC cell migration, invasion, anchorage-independent growth, and, to a lesser degree, proliferation.
DKK3 induces resistance to chemotherapy with gemcitabine
Our previous studies demonstrated that HPSCs produce secreted factors that enhance chemoresistance in PDAC (1), and therefore, we investigated whether DKK3 might contribute to this phenomenon. L3.6pl cells are relatively sensitive to gemcitabine and express minimal DKK3, whereas Panc1 and HS766T are relatively resistant to gemcitabine and express a moderate amount of DKK3 (36). When DKK3 was expressed in chemosensitive L3.6pl cells, colony formation in soft agar in the presence of gemcitabine increased by >90% compared with controls (P < 0.001; Fig. 2H), with concomitant reduction in apoptosis (P < 0.01; Fig. 2I). DKK3 was transiently silenced in relatively chemoresistant HS766T cells (HS766T-siDKK3; fig. S1F), and in the presence of gemcitabine, the rate of apoptosis in these cells doubled compared to control cells (P < 0.01; Fig. 2J). Together, these data suggest that DKK3 contributes to resistance to chemotherapy with gemcitabine.
Autocrine and paracrine effects of DKK3 are mediated by nuclear factor κB activation
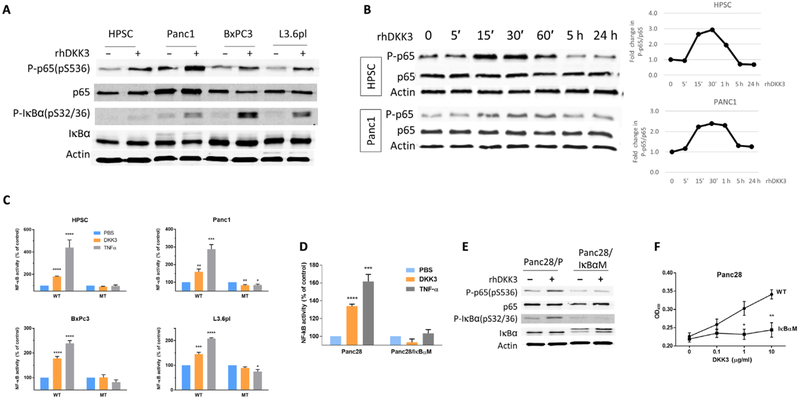
To investigate the mechanisms of DKK3 effects on HPSC and PDAC, we performed high-throughput Ab-based reverse phase protein assay (RPPA) analysis on primary HPSC cells (HPSC and HPSC20Aim) and PDAC cells (Panc1, BxPC3, and L3.6pl) that were treated with either PBS or rhDKK3 for 20 min. Unsupervised clustering revealed that one of the most activated pathways with DKK3 treatment was nuclear factor κB (NF-κB), which was validated with Western blot analysis (Fig. 3A). Stimulation with DKK3 in HPSC and PDAC cells also induced phosphorylation of nuclear factor of κ light polypeptide gene enhancer in B cells inhibitor, α (IκBα), which is regulated by NF-κB, providing additional evidence that DKK3 induced NF-κB activation. Peak phosphorylation of p65 in HPSC and Panc1 occurred at 15 to 30 min after stimulation with rhDKK3 (Fig. 3B). To confirm induction of NF-κB–dependent promoter activity, HPSC, Panc1, BxPC3, and L3.6pl cells were transfected with either wild-type (WT) or mutant (MT) κB-luciferase reporter gene constructs and stimulated with DKK3. Results of the dual-luciferase assay indicated that DKK3 induced NF-κB promoter activity in cells with the WT reporter but not in cells with the MT reporter (Fig. 3C). Tumor necrosis factor–α was used as a positive control. To further demonstrate the effect of DKK3 on NF-κB activation in PDAC, we used phosphorylation-defective Panc28 pancreatic cancer cells that stably express mutated IκBα and are incapable of NF-κB activation (Panc28/IκBαM) (37). Stimulation of parental Panc28 cells with DKK3 induced NF-κB promoter activity by 33% relative to PBS control, whereas no induction was seen in Panc28/IκBαM cells (Fig. 3D). These results were confirmed by Western blot analysis (Fig. 3E). Last, we investigated whether NF-κB–dependent effects of DKK3 affect PDAC cell function. Treatment with DKK3 stimulated Panc28 proliferation in a dose-dependent manner. However, proliferation of Panc28/IκBαM was not induced by DKK3, suggesting that NF-κB is required for DKK3-mediated effects on cell proliferation (Fig. 3F). Together, these results demonstrate that DKK3 activates NF-κB in both HPSC and PDAC cells and that inhibition of NF-κB blocks DKK3-mediated induction of PDAC cell activity.
Fig. 3. NF-κB is activated by DKK3 in PSCs and PDAC cells and is necessary for DKK3-mediated stimulation of cell activity.
(A) Phosphorylation of p65 and IκBα induced by DKK3 treatment (10 μg/ml) was determined by Western blotting. Relative protein loading was shown by using anti–β-actin Ab. (B) Western blot showing the time course of p65 activation in HPSC and Panc1 cells. Cells were treated with recombinant DKK3 (10 μg/ml) for 0 to 24 hours, and changes in band density relative to baseline were quantified. (C) DKK3 stimulates NF-κB luciferase reporter in HPSC and PDAC cells, compared to the mutant luciferase reporter (MT). NF-κB activity induced by DKK3 was measured in Panc28 with phosphorylation-defective IκBαM by (D) luciferase reporter and (E) Western blotting. (F) Proliferation of Panc28 and Panc28/IκBαM was measured by MTT assay. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus PBS control.
Silencing of DKK3 in HPSCs inhibits tumor growth in vivo
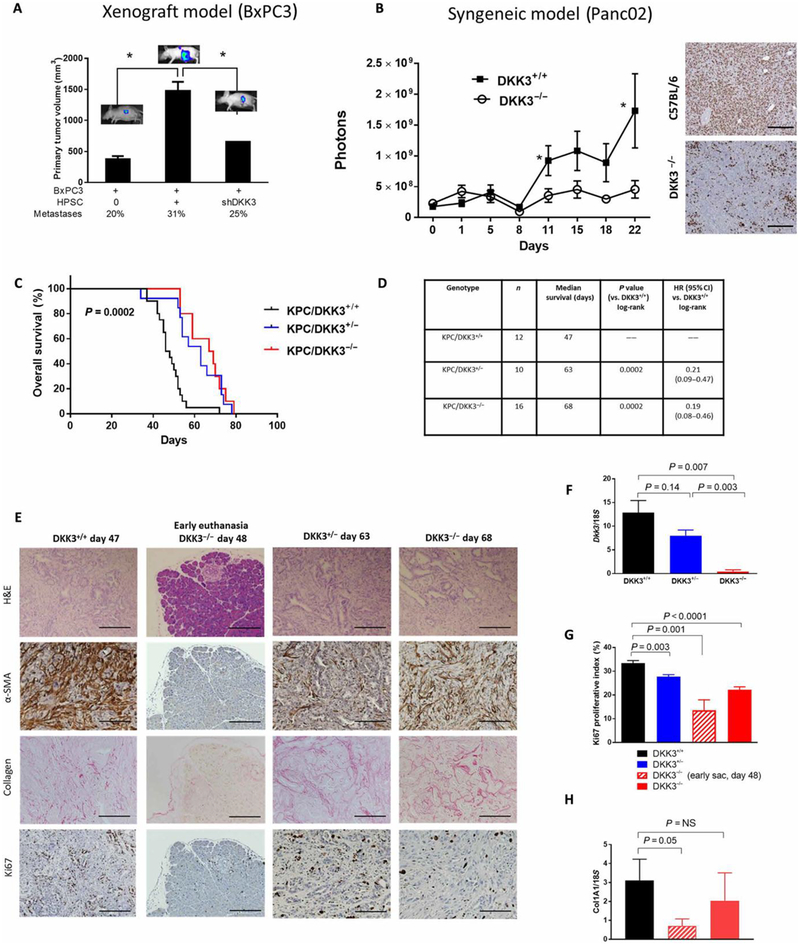
Using an orthotopic mouse model of PDAC in which HPSCs are coinjected with luciferase-labeled BxPC3 cells, we previously showed that the presence of HPSCs stimulates increased growth of the primary tumor and distant metastases in a dose-dependent fashion (1). To evaluate the role of DKK3 in tumor progression, nude mice were injected orthotopically with BxPC3 cells alone or in combination with HPSC-shDKK3 or control cells (HPSC-shControl) at a tumor-to-stroma ratio of 1:0 or 1:3. Consistent with our previous observations, mice injected with both HPSCs and BxPC3 developed larger primary tumors with a higher rate of peritoneal metastases than did those injected with BxPC3 alone (Fig. 4A). However, coimplantation with HPSC-shDKK3 resulted in significantly smaller primary pancreatic tumors compared to coimplantation with HPSC-shControl, with 67% reduction in tumor size (P < 0.05; Fig. 4A) and a lower incidence of peritoneal metastases (25% versus 31%). In light of recent reports suggesting a role for DKK3 in immunomodulation, we investigated the effects of DKK3 in immunocompetent models of PDAC. We first used a syngeneic implantation model with luciferase-labeled murine pancreatic cancer cells Panc02 (negative for Dkk3; fig. S3) injected into either DKK3−/− mice or control C57/BL6 mice. Tumor growth was exponential in control mice (Fig. 4B), but in DKK3−/− mice, growth was significantly inhibited, with a 3.8-fold decrease in luciferase signal at 22 days (P < 0.05) and far fewer Ki67-positive cells compared to controls (Fig. 4B). Together, these data support a stimulatory role of DKK3 in pancreatic tumor growth and metastasis.
Fig. 4. Neutralization of DKK3 inhibits tumor growth and prolongs survival.
BxPC3 tumor cells labeled with firefly luciferase were orthotopically implanted into nude mice, with or without either control HPSCs or HPSCs stably silenced for DKK3, in a 1:3 tumor/stroma ratio. (A) Average pancreas tumor volume and percentage of animals with metastases are shown at 35 days after injection. (B) IVIS imaging showing the growth of Panc02 tumor cells implanted subcutaneously in syngeneic C57/BL6 or DKK3-null mice, with Ki67 expression assessed by IHC. (C and D) Dkk3-deficient mice were crossed with KPC mice to produce P48-Cre; KrasLSL-G12D;Trp53fl/fl; Dkk3−/− progeny. Kaplan-Meier survival curve (C) and survival table (D) for mice with WT DKK3 (black), DKK3-null (red), or heterozygous DKK3 (blue). (E) Representative images of tumors from (C) from DKK3-WT mice (moribund, day 47), DKK3-heterozygous mice (moribund, day 63), or DKK3-null mice (early time point at day 48, or when moribund at day 68). (F) DKK3, (G) Ki67 proliferation index by IHC, and (H) collagen type I expression by qPCR. Data are means ± SEM [n = 8 to 15 mice per group in (A), 5 mice per group in (B), 10 to 16 mice per group in (C) to (D) and (F) to (H)]. *P < 0.05. Scale bars, 200 μm.
Depletion of DKK3 prolongs survival in an autochthonous model of PDAC
To further investigate the effects of DKK3 on PDAC in an immuno-competent model, we ablated Dkk3 in the P48-Cre;KrasLSL-G12D;Trp53fl/fl KPC model of PDAC. DKK3-deficient mice on a C57/BL6 background (DKK3−/− mice) have been extensively characterized and have only minor physiologic changes and no evidence of cancer development at 1 year (38). When they were bred with KPC mice, the resultant progeny, P48-Cre; KrasLSL-G12D;Trp53fl/fl; Dkk3−/− (termed “KPC/DKK3−/−”) and their DKK3-heterozygous and DKK3-WT littermates (KPC/DKK3+/− and KPC/DKK3+/+), had normal phenotypes at birth. Mice were monitored until they were moribund and then euthanized, and survival was calculated. When Dkk3 was depleted, either completely or partially (KPC/DKK3−/− or KPC/DKK3+/−), overall median survival was significantly prolonged compared to mice with WT DKK3 (68 days for KPC/DKK3−/− and 63 days for KPC/DKK3+/− versus 47 days for KPC/DKK3−/−; P = 0.0002; Fig. 4, C and D). The mice with at least partial DKK3 depletion had an 80% reduction in the risk of dying than mice with WT DKK3 {hazard ratio, HR, 0.21 [95% confidence interval (CI), 0.09 to 0.47] and HR, 0.19 (95% CI, 0.08 to 0.46); P = 0.0002; Fig. 4D}. A similar KPC model with heterozygous Trp53 has less aggressive disease and longer median survival (P48-Cre; KrasLSL-G12D; Trp53fl/+; Dkk3−/−). When Dkk3 was ablated in this slower-growing model, the difference in median survival between mice with intact or depleted Dkk3 was even more striking, with a greater than 2-fold increase in survival (83 days versus 177 days, P < 0.0001) and 25-fold difference in risk of dying (HR, 0.04, P < 0.0001; fig. S4).
In all mice regardless of their DKK3 status, hematoxylin and eosin (H&E) staining confirmed the presence of pancreatic carcinoma at the time of euthanization (Fig. 4E) despite the differences in survival with Dkk3 ablation. As expected, Dkk3 expression was virtually absent in homozygous KPC/DKK3−/− mice (red bar) and at intermediate levels in heterozygous KPC/DKK3+/− mice (blue bar) compared to control KPC/DKK3+/+ (black bar) mice as measured by qPCR (Fig. 4F). However, the Ki67 proliferative index was significantly reduced in the tumors in KPC/DKK3−/− mice compared with KPC/ DKK3+/+ mice (22% versus 33%, P < 0.0001; Fig. 4, E and G). The reduction in Ki67 correlated with the degree of Dkk3 ablation in a dose-dependent manner, from the highest expression in KPC/ DKK3+/+ mice to the lowest in the KPC/DKK3−/− mice. Together, these data indicate that pancreatic tumors eventually develop in all KPC mice regardless of DKK3 expression; however, when DKK3 expression is reduced, the tumors are less proliferative with less activated stroma.
When we examined the group of DKK3-heterozygous KPC mice, survival was surprisingly similar to DKK3−/− mice [63 days versus 68 days, P = NS (not significant); Fig. 4, C and D], although Dkk3 expression by qPCR in KPC/DKK3+/− tumors was significantly higher (P = 0.003; Fig. 4F). DKK3 expression in DKK3-heterozygous mice was 62% of that of KPC/DKK3+/+ by qPCR (Fig. 4F). In the less aggressive P48-Cre; KrasLSL-G12D;Trp53fl/+ model with heterozygous DKK3+/− expression, Dkk3 was similarly at 52.7% of DKK3+/+ mice (fig. S5A). These results suggest that even moderate depletion of DKK3 is effective in prolonging survival in this model of PDAC.
We then determined the extent to which DKK3 contributes to the earlier phases of PDAC development. We euthanized KPC/ DKK3−/− mice at about the same age (48 days) as when KPC/ DKK3−/− mice were dying (median survival, 47 days). At this time point, KPC/DKK3−/− mice appeared healthy, and H&E staining of their pancreas tissue was mostly normal, with a few focal areas of dysplasia, but no foci of PanINs or invasive carcinoma (Fig. 4E), whereas the KPC/DKK3+/+ mice were moribund with pancreatic carcinoma. Ki67 was significantly lower in KPC/DKK3−/− at early sacrifice compared to KPC/DKK3+/+ mice at the same age (13.5% versus 33.4%, P = 0.001; Fig. 4, E and G) but increased to 22% when the mice were moribund at 68 days (Fig. 4G).
At the early euthanasia time point for KPC/DKK3−/− mice, the stromal content also appeared to be lower compared with that for KPC/DKK3+/+ mice, as shown by IHC of α-SMA and collagen (Fig. 4E) and qPCR for collagen 1A (P = 0.05l; Fig. 4H). With time, however, activated stroma and cell proliferation increased, as shown in the tumors collected when the KPC/DKK3−/− mice were moribund [Fig. 4, E (day 68) and G and H (solid red bars)]. These findings suggest that DKK3 may be involved in the early stages of pancreatic tumorigenesis and that ablation of DKK3 delays the development of malignancy but does not completely prevent cancer formation. When tumors do form in DKK3-depleted mice, the tumors are slow growing with a low proliferative index and reduced activated stromal content, which may contribute to the improvement in overall survival. Histology and expression of α-SMA and Ki67 in DKK3-heterozygous tumors were similar to DKK3-null mice and are shown in Fig. 4 (E and G). Together, these data indicate that pancreatic tumors eventually develop in all KPC mice regardless of DKK3 expression; however, when DKK3 expression is reduced, the tumors are less proliferative with less activated stroma.
Inhibition of DKK3 with a neutralizing Ab suppresses PSCs and cancer cell function, enhances response to chemotherapy, and prolongs survival
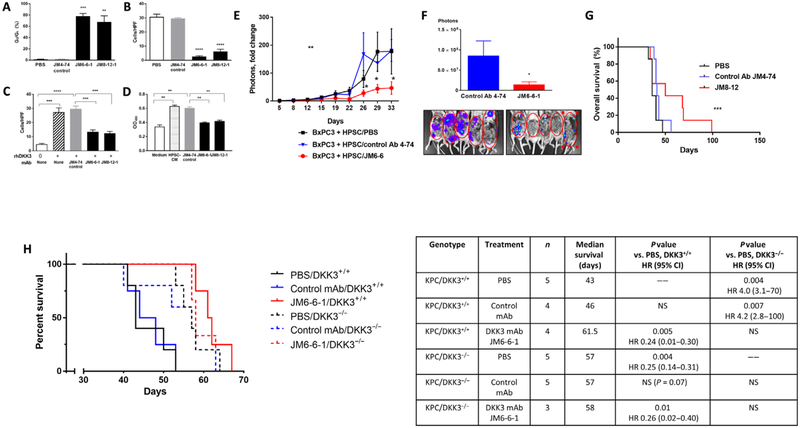
To further evaluate the therapeutic potential of DKK3 blockade, we generated mAbs (clones JM6-6-1 and JM8-12-1) against human DKK3 and tested their efficacy on HPSC and PDAC cell functions. Treatment of HPSCs with either JM6-6-1 or JM8-12-1 induced apoptosis by 70- to 80-fold (P < 0.01 and P < 0.001; Fig. 5A) and inhibited migration by 5- to 11-fold (P < 0.0001; Fig. 5B) compared with the irrelevant isotype control mAb. Treatment of BxPC3 cancer cells with JM6-6-1 or JM8-12-1 was able to reverse the DKK3-mediated induction of migration (P < 0.001; Fig. 5C) and also abrogated the DKK3-mediated induction of chemoresistance to gemcitabine in cancer cells (Fig. 5D). In this assay, survival of BxPC3 cells in gemcitabine with HPSC-CM treatment was nearly doubled over controls (P < 0.01), but the addition of either DKK3 mAb clone restored sensitivity to gemcitabine with a proliferation rate similar to that of media controls (Fig. 5D). Cell surface binding of DKK3 on PDAC cells was assessed by flow cytometry, which confirmed that addition of JM6-6-1 was effective in blocking binding of rhDKK3 to PDAC cells (fig. S5B).
Fig. 5. DKK3-blocking Abs inhibit PSC and cancer cell activity, chemoresistance, and tumor progression, with improved survival.
HPSCs and BxPC3 cells were treated with DKK3 mAb clones JM6-6-1 and JM8-12-1 or isotype control mAb or PBS. (A and B) HPSC apoptosis (A) and migration (B) as measured by fluorescence-activated cell sorting (FACS) and Transwell migration assay at 48 hours. (C) BxPC3 migration in response to rhDKK3 (10 μg/ml) as measured by Transwell migration assay at 48 hours. (D) BxPC3 resistance to gemcitabine (100 μM) as measured by MTT proliferation assay at 6 days. HPSC-CM (10 μg/ml). (E) The orthotopic coinjection BxPC3 + HPSC model of PDAC was used to test the efficacy of DKK3 mAb clones JM6-6-1 or JM8-12-1 (5 mg/kg ip, once every 5 days). Overall tumor progression was measured every 3 to 4 days by IVIS imaging. (F) Metastatic tumors in the peritoneal cavity after removal of the primary pancreatic tumor are shown by IVIS imaging. (G) Kaplan-Meier survival curve showing mice treated with DKK3 mAb clone JM8–12 (red), control Ab (blue), or PBS (black). KPC mice (P48-Cre; Kras LSL-G12D;Trp53fl/fl) either with WT DKK3 (solid lines) or deficient in DKK3 (dashed lines) were treated with DKK3 mAb JM6-6-1 (5 mg/kg, ip, once every 5 days), PBS, or control mAb. Kaplan-Meier survival curve (H) is shown with hazard ratios (log-rank test). Data are means ± SEM [n = 7 mice per group in (E) and (F), 6 to 7 mice per group in (G), 3 to 5 mice per group in (H)]. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus control Ab.
We tested the efficacy of DKK3 mAb in vivo using the previously described orthotopic coimplantation nude mouse model of PDAC with HPSCs mixed with luciferase-labeled BxPC3 cancer cells (1:3 tumor/PSC ratio). Mice were treated with PBS, isotype control mAb, or DKK3 mAb [clone JM6-6-1; 5 mg/kg intraperitoneally (ip), once every 5 days], and tumor growth was followed by IVIS imaging. Compared with either the PBS or control mAb groups, the JM6-6-1 mAb group showed a significant inhibition in tumor growth compared to baseline tumor signal at day 22 until day 33, when the mice were euthanized after completing 4 weeks of treatment (P < 0.01; Fig. 5E). Tumors in the PBS and control mAb groups grew rapidly from day 22 onward, with an eightfold increase in the luciferase signal. The tumors derived from BxPC3 alone without HPSCs showed no significant change in response to JM6-6-1 mAb treatment compared to either PBS or isotype control mAb (fig. S5C). Because HPSCs are the source of DKK3 in the coimplantation model, this suggests that JM6-6-1 is effective only when the target DKK3 is present. After pancreatic tumors were removed, examination of the peritoneal cavity also revealed a 6.5-fold lower volume of metastatic disease as measured by luciferase signal in the mice treated with JM6-6-1 compared with either PBS or the control mAb (P < 0.05; Fig. 5F).
In a separate survival experiment, treatment with JM8–12 (5 mg/kg ip, once every 5 days) resulted in significant improvement in median survival compared to either control group (50 days for JM8–12, 36 days for PBS, 41 days for control mAb; P < 0.0001; Fig. 5G). The hazard ratio for mice treated with JM8–12 mAb compared with that for the PBS control group was 0.26, indicating that, in this xenograft orthotopic model of PDAC, treatment with anti-DKK3 mAb was associated with decreased tumor growth and metastasis, as well as prolonged survival.
Because we had observed an improvement in survival with genetic ablation of Dkk3 in the KPC model of PDAC (Fig. 4), we sought to determine whether pharmacologic neutralization of DKK3 with mAb would also be effective in this model. Human and murine DKK3 share 83% protein sequence homology by Basic Local Alignment Search Tool (BLAST) analysis, and therefore, we anticipated that JM6-6-1 would cross-react with host DKK3. We confirmed crossreactivity by a nondenaturing Western blot, which showed that JM6-6-1 recognizes murine DKK3, although more weakly than human DKK3 (fig. S6A). JM6-6-1 inhibited proliferation of PSCs that express DKK3 isolated from mouse pancreas (fig. S6, B and C). When KPC mice (P48-Cre; KrasLSL-G12D;Trp5fl/fl) were treated with JM6-6-1 (Fig. 5H, red solid line), median survival increased by 43% from 43 to 61.5 days compared with PBS or isotype control mAb (P = 0.005; HR, 0.24; 95% CI, 0.01 to 0.30; Fig. 5H). In contrast, in KPC/DKK3−/− mice that lack DKK3 (P48-Cre; KrasLSL-G12D;Trp53fl/fl; Dkk3−/−), JM6-6-1 had no effect on survival (red dashed line; 58 days) compared to PBS or control mAb treatment (black and blue dashed lines; 57 and 57 days, respectively; P = NS), indicating that the effects of JM6-6-1 are likely to be specific for DKK3. Consistent with our previous results, genetic depletion of DKK3 in KPC mice was associated with improved survival (black dashed line; 57 days) compared with KPC/DKK3+/+ mice treated with either PBS or isotype control mAb (black and blue solid lines; 43 and 46 days, respectively; P = 0.004 and P = 0.007, respectively). The median survival of KPC/DKK3+/+ mice treated with JM6-6-1 was not significantly longer than that of KPC/DKK3−/− mice (61.5 days versus 57 days), suggesting that pharmacologic neutralization of DKK3 using mAb was similar to genetic ablation in improving survival. JM6-6-1 was also effective in another experiment with larger sample sizes using another well-accepted GEMM model of PDAC that uses the Pdx1 promoter to drive oncogenic Kras (Pdx1-Cre; KrasLSL-G12D;Trp53fl/fl) (fig. S6D). However, this model was prone to development of benign papillomas in our laboratory, as others have reported (39, 40), and therefore, subsequent studies were performed using the KPC model with the more pancreas-specific P48-Cre allele, which did not develop papillomas.
DKK3 blockade is associated with increased tumor immune infiltrates and improves response to checkpoint inhibitor therapy
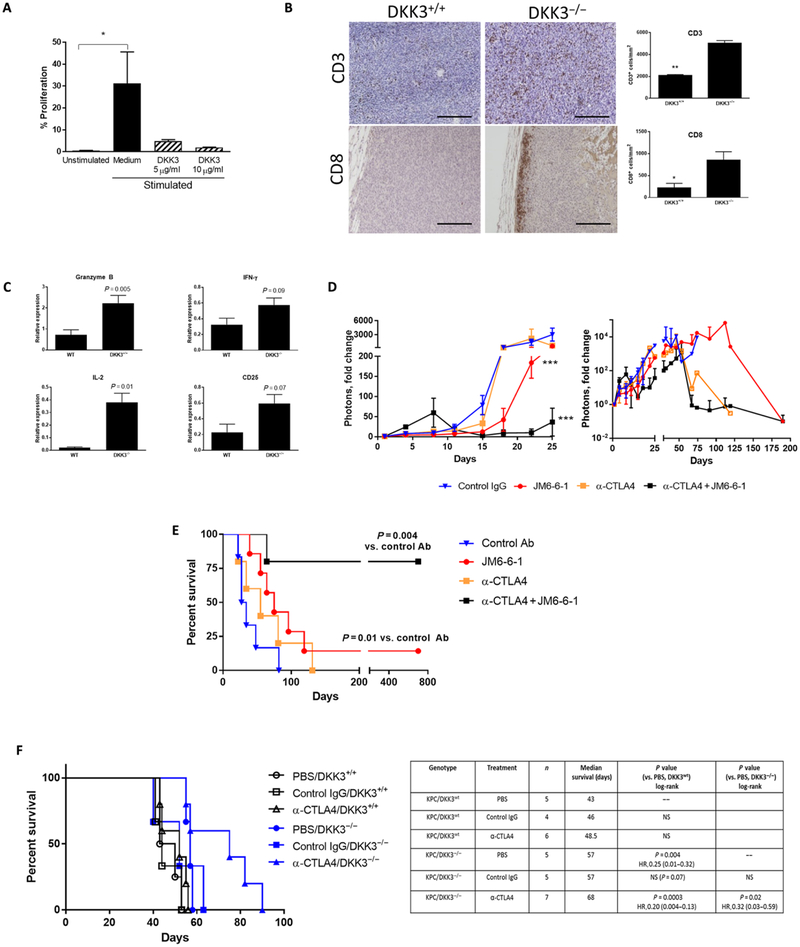
DKK3 has been shown to be an immune modulator and is associated with T cell tolerance (41, 42). When we stimulated CD3+ T cells with recombinant DKK3, cell proliferation was not significantly different compared to medium alone (P = 0.07; Fig. 6A). CD3- and CD8-expressing cells were analyzed in pancreatic tumors from a syngeneic orthotopic model with luciferase-labeled KPC cells implanted in either DKK3−/− or control C57/BL6 mice. IHC demonstrated a 2.4-fold increase in CD3+ cells in DKK3−/− mice compared with controls (Fig. 6B). CD8+ cells were rarely seen in tumors from C57/BL6 mice but were consistently identified in the periphery of tumors from DKK3−/− mice, with a nearly fourfold increase in expression (Fig. 6B). Additional markers of T cell activity were measured by qPCR, which showed significant increases in granzyme B and IL-2 in DKK3−/− tumors (P = 0.005 and P = 0.01, respectively; Fig. 6C). There were no significant differences in interferon-γ and CD25 (P = 0.09 and P = 0.07, respectively; Fig. 6C). These data suggest that DKK3 inhibited T cell proliferation and depletion of DKK3 was associated with increased CD3+ and CD8+ T cell numbers and activity in pancreatic tumors.
Fig. 6. DKK3 blockade is associated with increased tumor immune infiltrates and improves response to checkpoint inhibitor therapy.
(A) T cells were stimulated and treated with DKK3 (5 to 10 μg/ml), and proliferation was measured by carboxyfluorescein diacetate succinimidyl ester (CFSE) assay. In a syngeneic orthotopic model with luciferase-labeled KPC cells, (B) tumors were examined for CD3 and CD8 expression by IHC, and (C) additional markers of T cell activity were measured by qPCR. (D) Mice in the syngeneic orthotopic KPC-luciferase model were treated with control IgG, DKK3 mAb JM6-6-1, α-CTLA4, or the combination JM6-6-1 + α-CTLA4, and tumor growth was measured by IVIS imaging up to 190 days. Survival in this orthotopic implantation model is shown in (E). Using a GEMM (F), KPC/DKK3+/+ (black) or KPC/DKK3−/− (blue) mice were treated with α-CTLA4 or control IgG, and the Kaplan-Meier survival curve is shown. Data are means ± SEM [n = 5 mice per group in (B) and (C), 5 to 7 mice per group in (D) and (E), 4 to 7 mice per group in (F)]. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bars, 200 μm.
PDAC has been largely resistant to immune checkpoint therapies, and several studies suggested that this may be due to an immuno-suppressive microenvironment (43–48). To determine whether manipulation of DKK3 can affect the response to checkpoint inhibitors, mice bearing luciferase-labeled KPC tumors in the syngeneic orthotopic C57/BL6 model were treated with isotype control immunoglobulin G (IgG), DKK3 mAb JM6-6-1, anti-cytotoxic T lymphocyte–associated protein 4 (α-CTLA4), or the combination of JM6-6-1 with α-CTLA4. Treatment with α-CTLA4 alone was equivalent to control Ab treatment, with no effect on tumor growth by luciferase signal (Fig. 6D). Treatment with JM6-6-1 alone inhibited tumor growth at 18 days compared to control IgG or α-CTLA4 (mean photon fold change, 42.3 versus 277; P = 0.04), and after this time point, mice in the control IgG group began dying with large tumors. Mice in the combination group treated with JM6-6-1 + α-CTLA4 showed inhibition of tumor growth after 8 days, which was significant after day 18 (P < 0.0001). With additional follow-up to day 190 (Fig. 6D, right), tumors in the surviving mice in all groups continued to grow but at a slower rate in the JM6-6-1 and combination JM6-6-1 + α-CTLA4 groups (Fig. 6D). Survival for the four treatment groups is shown in Fig. 6E. Compared with control IgG, median survival was significantly longer with JM6-6-1 alone (75 days versus 30 days, P = 0.01) but was equivalent to α-CTLA4 treatment (P = 0.32). Combination treatment with JM6-6-1 + α-CTLA4 was associated with a significant improvement in survival (P = 0.004), and median survival was not reached in this group (Fig. 6E). Survival was also significantly better in the combination treatment group compared to JM6-6-1 alone (P = 0.04). At 726 days, 80% of mice were alive in the combination treatment group and 20% of mice in the JM6-6-1 group. At that time, all mice were electively sacrificed. IVIS imaging was not performed after 190 days, but at that point, there was minimal signal from the tumors in the surviving mice (Fig. 6D and fig. S6E), and no gross tumors were identified in the pancreata from the remaining mice in the JM6-6-1 and combination groups.
To further confirm our findings, we tested the effect of α-CTLA4 in the KPC/DKK3−/− model of PDAC in a survival study (Fig. 6F). Control IgG and α-CTLA4 had no effect on survival compared to PBS in WT KPC mice with intact DKK3 (black lines; median survival, 43 to 48.5 days). As we observed previously, median survival of KPC/DKK3−/− mice was significantly improved compared to KPC/ DKK3+/+ mice (57 days versus 43 days, P = 0.004). When KPC/ DKK3−/− mice were treated with α-CTLA4, survival increased to 68 days, representing a 58% improvement compared with WT DKK3 with PBS treatment (P = 0.0003; HR, 0.20; 95% CI, 0.004 to 0.13). The addition of α-CTLA4 to DKK3 ablation also improved survival relative to DKK3 ablation alone in KPC/DKK3−/− mice (68 days versus 57 days; P = 0.02; HR, 0.32; 95% CI, 0.03 to 0.59). In summary, depletion of DKK3 by either pharmacologic treatment with mAb or genetic ablation improved PDAC response to checkpoint inhibitor immunotherapy, with an improvement in survival.
DISCUSSION
In this study, we identified DKK3 as a protein expressed in nearly all human PDACs. In an autochthonous model of PDAC, DKK3 was also present in CP and premalignant PanIN lesions. DKK3 is a secreted factor produced by PSCs in the tumor-associated stroma of PDAC and acts in both an autocrine and paracrine manner, not only to stimulate PSC activity but also to increase PDAC cell proliferation, migration, and invasion. In addition, DKK3 protects cancer cells from undergoing apoptosis induced by chemotherapy. These effects are mediated, at least in part, by NF-κB activation in both PSCs and PDAC cells. Inhibition of DKK3 in xenograft and syngeneic models of PDAC by both genetic ablation and pharmacologic depletion using mAb resulted in inhibition of tumor growth and metastases, improvement in response to chemotherapy, and prolongation of survival.
However, ablation of DKK3 did not result in a complete cure, and all DKK3-null mice had pancreatic tumors when they died, although the tumors were smaller, less proliferative, and with less active stroma, which likely contributed to the animals’ prolonged survival. As a therapeutic approach, the combination of DKK3-targeted therapy with other treatments, including chemotherapy, targeted agents, or immunotherapy, would likely result in a more durable response than DKK3 neutralization alone. Another potential application of DKK3 targeting is to intervene at early stages of PDAC development. KPC/DKK3−/− mice had essentially normal pancreata compared with control littermates, who had their maximal tumor burden at the same age, suggesting that the absence of DKK3 may have affected tumor initiation or progression. DKK3 was also expressed during the PanIN stage of development in cLGL-KrasG12V/BAC Ela-CreERT mice, which suggests that DKK3 may be involved at an early time point. On the basis of these observations, it is possible that targeting DKK3 at an early stage of PDAC development could be an effective preventive strategy, and additional studies are needed to test this hypothesis.
Our results contradict other reports that describe DKK3 as a tumor suppressor, and indeed, adenoviral vector delivery of DKK3 has been proposed as a treatment approach in xenograft models of prostate, testicular, breast, and gastric cancers, as well as PDAC (17–23, 49–52). However, the models used in those studies lacked stromal elements, whereas we found that stromal fibroblasts were the primary source of DKK3 in PDAC. When PSCs were absent in the coinjection orthotopic model of PDAC, DKK3 blocking Abs had no effect on tumor progression. DKK3 also has a stimulatory effect on prostate stromal cells and retinal ganglion and Muller glia cells (25, 53, 54). In the retina, DKK3 potentiates WNT signaling, which parallels our observations in PSCs in PDAC (25). It is conceivable that DKK3 has diverse and even conflicting roles in tumor progression that are cell context dependent, similar to what is known about transforming growth factor–β (55).
Others have demonstrated that the tumor-associated stroma in various malignancies, including PDAC, can contribute to an immuno-suppressive microenvironment. Kaneda et al. (47) showed that macrophage lipid kinase PI3Kγ (phosphatidylinositol 3-kinase γ) promotes an immunosuppressive tumor microenvironment in PDAC, resulting in tumor progression, metastasis, and fibrosis. Inhibition of PI3Kγ restored an antitumor immune response and decreased tumor growth with improved survival. Focal adhesion kinase (FAK) has also been shown to be correlated with low amounts of CD8+ T cell infiltration and fibrosis in human PDAC samples (48), and treatment with a FAK inhibitor resulted in decreased tumor growth with improved survival in the KPC model of PDAC. Moreover, FAK inhibition improved responsiveness to T cell immunotherapy and PD-1 (programmed cell death protein 1) inhibitors in the previously unresponsive KPC model. Similarly, we found that DKK3 produced by the PSCs inhibits CD8+ cytotoxic T cells, and ablation of DKK3 in the KPC model resulted in a robust infiltration of cytotoxic T cells into the tumors. Tumor inhibition with anti-DKK3 mAb was more effective in immunocompetent syngeneic models and GEMMs of PDAC compared with immunodeficient xenograft models, which also suggests that the effects of DKK3 blockade may be amplified in the presence of an intact immune system. The observation that survival was equal in KPC mice with either homozygous or heterozygous depletion of DKK3 was unexpected. Whether partial ablation of DKK3 results in a similar degree of CD8+ T cell recruitment as in DKK3-null mice is unknown, and more studies are needed to address this question. What is clear is that checkpoint inhibitor therapy was not effective in the KPC model of PDAC, mirroring the results seen in clinical trials. However, DKK3 neutralization by either genetic ablation or pharmacologic blockade with mAb was able to overcome resistance to immunotherapy and prolong survival. The combination of DKK3 blockade with immunotherapy was superior to DKK3 blockade alone in improving survival.
Our study is limited by the sample size of several in vivo studies, which is a recognized challenge of using GEMMs. Our studies also did not use a humanized anti-DKK3 Ab, which potentially could show different results than our current Ab clones. Last, it remains unclear how DKK3 can have such widely pleiotropic effects in various malignancies, as either a tumor suppressor or a tumor promoter as shown in our studies. Although we demonstrate that DKK3 activity in pancreatic cancer is at least partly dependent on NF-κB activation, the signaling mechanisms have not been fully elucidated, and the receptor for DKK3 has not been firmly established. Additional insight into these questions would be important not only to understand the diverse functions of DKK3 but also to improve the specificity of DKK3-targeted therapies in clinical trials to increase their efficacy and minimize toxicities.
In conclusion, our study demonstrates that DKK3 is frequently expressed in PDAC and promotes tumor progression, metastasis, and chemoresistance that depends at least in part on NF-κB activation. We found that inhibition of DKK3 by either genetic ablation or pharmacologic mAb blockade was effective in slowing down pancreatic tumor growth with an improvement in survival. Furthermore, inhibition of DKK3 was able to overcome resistance to immunotherapy with anti-CTLA4 inhibitor, resulting in long-term durable improvement in survival. Together, our results indicate DKK3 may be a therapeutic target as monotherapy or in combination with immunotherapy.
MATERIALS AND METHODS
Study design
This study was designed to evaluate the role of DKK3 in PDAC progression and metastasis and to evaluate the preclinical efficacy of DKK3 inhibition using in vitro and in vivo models of PDAC. These objectives were addressed by (i) determining the expression of DKK3 in human PDAC cells and tissue samples and mouse models of PDAC, (ii) assessing the effect of DKK3 on HPSC and PDAC activity and chemoresistance, (iii) evaluating the role of NF-κB in DKK3 activity, and (iv) determining whether DKK3 neutralization by shRNA, genetic ablation, or a blocking Ab is effective in inhibiting PDAC growth and metastasis, improving survival, and improving response to chemotherapy or immunotherapy.
In animal studies, mice were randomly assigned to treatment and control groups. Numbers of tested mice are specified in each figure. Outliers were removed only if tumor implantation by orthotopic injection was not successful as assessed by IVIS luciferase imaging. The primary end points were tumor size and survival. Mice were euthanized when moribund or at the end of the prespecified treatment period. All procedures were performed in accordance with institutional protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Pathology analysis was performed in a blinded fashion.
Cell culture
Cells were confirmed Mycoplasma free before experiments. HPSCs were developed in our laboratory and have been described previously (1) from residual PDAC surgical tissue in accordance with the policies and practices of the Institutional Review Board (University of Texas MD Anderson Cancer Center). Cell purity was determined by immunohistochemistry for α-SMA, vimentin, and desmin, as well as morphology and positive staining with Oil Red O. PDAC cell lines were obtained from the American Type Culture Collection (CFPAC, BxPC3, Capan2, MiaPaca2, MPanc96, HS766t, Panc1, SU86.86, ASPC-1, HPAFII, HPAC, and CapanI), I. J. Fidler (L3.6pl; Department of Cancer Biology, University of Texas MD Anderson Cancer Center), and C. Logsdon (Panc3, PSN1, Panc48, and Panc28) (56–58). MDA1 and MDA2 primary human PDAC cell lines were developed by passage in a murine xenograft model. KPC murine pancreatic cancer cells were isolated from tumors formed in a genetically engineered KPC mouse model of PDAC (59), provided by S. Ullrich (Department of Immunology, University of Texas MD Anderson Cancer Center). MOH cells were obtained from R. Mohamed (Wayne State University, Detroit, MI). All cells were maintained in 10% fetal bovine serum (FBS)/Dulbecco’s modified Eagle’s medium at 37°C in a humidified atmosphere of 5% CO2. HUVECs were obtained from American Type Culture Collection and cultured on plates coated with 0.5% Gelatin A in minimal essential medium (Thermo Fisher Scientific) containing 15% FBS, 1 mM sodium pyruvate (Sigma), 1× vitamin solution (Thermo Fisher Scientific), 1× nonessential amino acids, and bFGF (10 ng/ml; Thermo Fisher Scientific). For coculture studies, HPSCs and PDAC cells were cultured in a 10-cm Transwell coculture system (Corning Incorporated), and after 96 hours, cells were harvested for RNA isolation.
Reverse transcriptase polymerase chain reaction
Total RNA was isolated from cells with the RNeasy mini kit (Qiagen), and complementary DNA was synthesized from total RNA with the QuantiTect reverse transcription kit (Qiagen). Dkk3 transcripts were amplified by using specific primer pairs: DKK3, 5′-CGGCTTCTG-GACCTCATC-3′ and 5′-CGGCTTGCACACATACAC-3′; Collagen1 (COL1A1), 5′-CATGAGCCGAAGCTAACCCC-3′ and 5′-GGGAC-CCTTAGGCCATTGTG-3′; and 18S (primer pair), 5′-GAGCGGTC-GGCGTCCCCCAACTTC-3′ and 5′-GCGCGTGCAGCCCCGGA-CATCTAA-3′ as the internal control. qPCR was performed in an iCycler IQ multicolor real-time PCR detection system (Bio-Rad Laboratories).
Western blotting
To confirm that DKK3 is secreted by HPSCs, CM was collected as described previously (2), protein concentration was measured by Bradford assay (Bio-Rad Laboratories), and 50 μg of protein was loaded onto a SDS–polyacrylamide gel electrophoresis (PAGE) gel and blotted against goat anti-human DKK3 Ab (Abcam). To evaluate pathways activated by DKK3, HPSCs were serum-starved overnight and treated with rhDKK3 (10 μg/ml). Protein lysates were separated by SDS-PAGE and blotted against total and phosphorylated p65 (Cell Signaling Technology) and total and phosphorylated IκBα (Cell Signaling Technology).
Immunohistochemical analysis
Primary rabbit anti-mouse DKK3 Ab was obtained from Proteintech Inc., goat anti-human DKK3 was obtained from Abcam, and rabbit anti-goat secondary Ab was obtained from Jackson ImmunoResearch Laboratories. mAbs against mouse α-SMA, Ki67, CD3, and CD8 were purchased from Abcam, Thermo Fisher Scientific, Santa Cruz Biotechnology, and BioLegend, respectively. Slides were blindly evaluated and scored by a dedicated gastrointestinal pathologist (H.W.).
DKK3 plasma concentrations by ELISA
Plasma samples from patients with either PDAC or CP (or normal controls) were obtained under an Institutional Review Board–approved protocol. DKK3 concentrations were detected with the RayBio Human DKK-3 ELISA Kit (RayBiotech Inc.) according to manufacturer-provided instructions.
Expression and silencing of DKK3
Human pcDNA3.1/V5-His A-DKK3 construct was provided by L.Zhang (Department of Pharmacology and Chemical Biology, University of Pittsburgh Cancer Institute, Pittsburgh, PA) (60). DKK3 was silenced by lentiviral transfection with shDKK3 (Open Biosystems) and by transfection with siDKK3 (Qiagen). Stable silencing of DKK3 in HPSCs was achieved by cotransfection of lentiviral plasmid control vector or shDKK3 (HPSC-shControl or HPSC-shDKK3) with packaging vectors into 293T cells with Lipofectamine 2000 (Invitrogen). Viral supernatant (200 μl) was added to HPSCs with polybrene (8 μg/ml; hexadimethrine bromide) in a six-well plate for 2 days, and stably transduced cells were selected in puromycin (1 μg/ml). Transient silencing of DKK3 in HPSCs and PDAC cells was achieved by transfection with a mixture of 5 nM siDKK3 and 3 μl of HiPerFect agent according to the manufacturer’s recommendations (Qiagen).
Cell-based assays
Cell proliferation was measured by the MTS assay (Promega). Cell migration and invasion studies with stimulation by rhDKK3 (R&D Systems) were performed using a 6.5-mm Transwell with an 8.0-μm pore membrane insert (Corning Incorporated) and BioCoat Matrigel-coated invasion chambers (BD Biosciences).
To assess growth in soft agar, cells were seeded into low–melting point agarose culture dishes and allowed to grow for 14 days. After staining with p-iodonitrotetrazolium violet (Sigma), the total number of colonies was counted in 10 high-power fields.
DKK3 was silenced in relatively chemoresistant HS766T using small interfering RNA (Qiagen) and overexpressed in chemosensitive L3.6pl by transfection with Lipofectamine LTX (Invitrogen) and pcDNA3.1/V5-His A-DKK3 construct, as described (60). The expression construct was provided by L. Zhang (Department of Pharmacology and Chemical Biology, University of Pittsburgh Cancer Institute, Pittsburgh, PA). In the presence of gemcitabine, cell viability was determined by soft agar colony formation. Apoptosis was determined by flow cytometry. Briefly, cells were fixed in 70% ethanol, washed, and resuspended in 200 μl of staining buffer [propidium iodide (50 μg/ml) and ribonuclease (50 units/ml) in PBS]. Propidium iodide–stained cells were detected with FACS, and sub-G1% was calculated as percentage of apoptotic cells.
Reverse-phase protein assay
Primary HPSC cell lines from two different patients (HPSC and HPSC-20Aim) were plated in triplicate in six-well dishes at 5 ×105 cells per well and treated with either rhDKK3 (10 μg/ml) or PBS for 20 min. Cells were lysed, prepared, and analyzed at the University of Texas MD Anderson Cancer Center Functional Proteomic core facility (Houston, TX) as described at www.mdanderson.org/education-and-research/resources-for-professionals/scientific-resources/core-facilities-and-services/functional-proteomics-rppa-core/index.html. Serial dilutions of samples were arrayed on nitrocellulose-coated slides and run against 220 validated Abs.
NF-κB activity
NF-κB activity was determined by using a luciferase reporter assay. Briefly, cells stably expressing a lenti–NF-κB luciferase reporter construct (61) in a 96-well plate were treated with rhDKK3 (10 μg/ml) under serum-free conditions for 5 hours. Luciferase signal was detected by using d-luciferin firefly potassium salt (Caliper Life Sciences) and the IVIS imaging system (Xenogen Corp.).
Binding assay
Cell surface binding of DKK3 was performed using flow cytometry. In brief, BxPC3 or L3.6pl cells (0.5 × 106) were incubated for 30 min with His-labeled rhDKK3 (10 μg/ml) with or without JM6-6-1 (70 μg/ml). After washing with binding buffer (3% bovine serum albumin in PBS), cells were stained with 1:100 anti-His Ab (Abcam) for 30 min, followed by staining with secondary Ab conjugated with DyLight 488 for 20 min. The cells were fixed with 2% paraformaldehyde and subjected to fluorescent signal detection by BD FACSCalibur.
PDAC mouse models
Nude mice and C57BL/6 mice were obtained from the Jackson laboratory and maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with current regulations and standards of the Department of Agriculture, Department of Health and Human Services, and National Institutes of Health (NIH). All animal procedures were reviewed and approved by the University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
An orthotopic nude mouse model of PDAC using BxPC3 cancer cells labeled with firefly luciferase (BxPC3-FL) coinjected with HPSCs has been previously described (1). Mice received intrapancreatic injections of BxPC3-FL (1 × 106 per mouse) with HPSC-shControl or HPSC-shDKK3 in a tumor-to-stroma ratio of 1:0 or 1:3 in 50 μl of Hanks’ balanced salt solution, and tumor growth was monitored using IVIS imaging (1). In addition, we used a syngeneic immunocompetent DKK3-null model provided by C. Niehrs (DKK3−/−) (38), which was injected with murine PDAC cells (termed “KPC cells”) isolated from tumors arising in a GEMM of PDAC (Pdx1-Cre; KrasLSL-G12D;Trp53R172H/+) (59, 62) and labelled with luciferase.
Two GEMMs of PDAC that differ in the pancreas-specific promoter to target oncogenic Kras expression (59, 62) were used in the experiments to evaluate the effect of DKK3 neutralizing mAb (Pdx1-Cre; KrasLSL-G12D;Trp53R172H/+ and P48-Cre; KrasLSL-G12D;Trp53fl/fl). To evaluate the effects of genetic ablation of DKK3 on pancreatic cancer development, DKK3-null mice were crossed with P48-Cre; KrasLSL-G12D; Trp53R172H or P48-Cre; KrasLSL-G12D;Trp53fl/fl mice (59, 62), and the progenies were monitored until they were moribund. Tissue and blood samples were collected after the mice were euthanized. A GEMM of PDAC with high expression of MT Kras (cLGL-KrasG12V/ BAC Ela-CreERT) (35) was used to evaluate the expression of DKK3 at various stages of PDAC development.
DKK3-mAb
We generated neutralizing mAbs to DKK3 by immunizing A/J mice with purified recombinant human DKK-3/His (R&D). Initial screening was performed by using ELISA high-throughput screening, by which more than 30 hybridoma clones showed strong and specific DKK-3 binding. After subcloning of anti-DKK3 mAb hybridomas, 12 purified mAbs were further tested with functional assays. Data are shown using two of the most effective clones (JM6-6-1 and JM8-12-1), and a nonspecific IgG1 isotype control clone was used as a negative control.
T cell division assay
Peripheral blood mononuclear cells were obtained from buffy coat by density centrifugation using Histopaque 1077 (Sigma), and CD3+ T cells were isolated using a pan T cell isolation kit (Miltenyi Biotec). Cells were labeled with CFSE (Invitrogen) and combined 1:1 with CD3/CD28 human T cell activator beads (Life Technologies) in a 96-well plate. DKK3 or HPSC-CM was added, and after 96 hours, cells were stained for CD3, CD4, and CD8 to define lineage and analyzed by flow cytometry. Data are reported as percentages of proliferating CD3+ T cells.
Statistical analysis
Results are shown from at least three independent experiments and presented as means ± SEM. Data were analyzed by two-tailed Student’s t test, and a significant difference was defined as P < 0.05. Survival analysis was performed by using the Kaplan-Meier method (log rank). All statistical analyses were performed in GraphPad Prism 6.
Supplementary Material
Acknowledgments:
We thank Y. Wang for assistance in the production of DKK3 mAbs; I. J. Fidler, S. Ullrich, R. Mohamed, and L. Zhang for providing valuable reagents; R. Bassett for statistical support; and L. Bradford for assistance in preparing the manuscript. We are grateful to E. Dmitrovsky, S. Swisher, C. Toniatti, D. Zha, and P. Hwu for critical discussions.
Funding: This study was funded by the NIH (5K08CA138912-05), the American Cancer Society Institutional Research Grant, the Lustgarten Foundation, the MD Anderson Knowledge Gap Seed Funding, and the MD Anderson Provost Funds (to R.F.H.). This work was supported in part by the NIH/NCI Cancer Center Support Grant (P30CA016672) for the Flow Cytometry and Cellular Imaging, Genetically Engineered Mouse Facility, Monoclonal Antibody Core, Research Animal Support, Research Histopathology, and Tissue Biospecimen and Pathology Core facilities.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/464/eaat3487/DC1
Fig. S1. DKK3 expression and silencing.
Fig. S2. DKK3 expression and function in HPSCs and PDAC cells.
Fig. S3. DKK3 expression in syngeneic models of PDAC.
Fig. S4. DKK3 depletion in the P48-Cre; KrasLSL-G12D;Trp3fl/+ model of PDAC.
Fig. S5. DKK3 expression in an autochthonous model of PDAC and effects of DKK3 mAb on cell surface binding of DKK3 and an orthotopic model.
Fig. S6. DKK3 expression in mouse PSCs and effects of treatment with DKK3 mAb on survival.
REFERENCES AND NOTES
- 1.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD, Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 68, 918–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M, Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, Keogh G, Merrett N, Pirola R, Wilson JS, Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 29, 179–187 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Apte MV, Wilson JS, Dangerous liaisons: Pancreatic stellate cells and pancreatic cancer cells. J. Gastroenterol. Hepatol 27 Suppl 2, 69–74 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Moses HL, Tumor-stroma interactions. Curr. Opin. Genet. Dev 15, 97–101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorak HF, Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med 315, 1650–1659 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS, Apte MV, Role of pancreatic stellate cells in pancreatic cancer metastasis. Am. J. Pathol 177, 2585–2596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA, Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijlsma MF, van Laarhoven HW, The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: A systematic review and critical appraisal. Cancer Metastasis Rev. 34, 97–114 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Macheda ML, Stacker SA, Importance of Wnt signaling in the tumor stroma microenvironment. Curr. Cancer Drug Targets 8, 454–465 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Moon RT, Kohn AD, De Ferrari GV, Kaykas A, WNT and β-catenin signalling: Diseases and therapies. Nat. Rev. Genet 5, 691–701 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Taipale J, Beachy PA, The Hedgehog and Wnt signalling pathways in cancer. Nature 411, 349–354 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Cowling VH, D’Cruz CM, Chodosh LA, Cole MD, c-Myc transforms human mammary epithelial cells through repression of the Wnt inhibitors DKK1 and SFRP1. Mol. Cell. Biol 27, 5135–5146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS, Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 21, 878–889 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Guder C, Pinho S, Nacak TG, Schmidt HA, Hobmayer B, Niehrs C, Holstein TW, An ancient Wnt-Dickkopf antagonism in Hydra. Development 133, 901–911 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Niehrs C, Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH, Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 65, 9617–9622 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Edamura K, Nasu Y, Takaishi M, Kobayashi T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T, Watanabe M, Huh NH, Kumon H, Adenovirus-mediated REIC/Dkk-3 gene transfer inhibits tumor growth and metastasis in an orthotopic prostate cancer model. Cancer Gene Ther. 14, 765–772 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R, Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-β-catenin pathway. Cancer Res. 64, 2734–2739 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK, Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 25, 5027–5036 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Nozaki, Tsuji T, Iijima O, Ohmura Y, Andou A, Miyazaki M, Shimizu N, Namba M, Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int. J. Oncol 19, 117–121 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi M, Kataoka K, Abarzua F, Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y, Ochiai K, Nasu Y, Kumon H, Huh NH, Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J. Biol. Chem 284, 14236–14244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh S-Y, Hsieh P-S, Chiu C-T, Chen W-Y, Dickkopf-3//REIC functions as a suppressor gene of tumor growth. Oncogene 23, 9183–9189 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Katase N, Lefeuvre M, Gunduz M, Gunduz E, Beder LB, Grenman R, Fujii M, Tamamura R, Tsujigiwa H, Nagatsuka H, Absence of Dickkopf (Dkk)-3 protein expression is correlated with longer disease-free survival and lower incidence of metastasis in head and neck squamous cell carcinoma. Oncol. Lett 3, 273–280 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura RE, Hunter DD, Yi H, Brunken WJ, Hackam AS, Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol. 8, 52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Glinka A, Delius H, Niehrs C, Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/β-catenin signalling. Curr. Biol 10, 1611–1614 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Caricasole A, Ferraro T, Iacovelli L, Barletta E, Caruso A, Melchiorri D, Terstappen GC, Nicoletti F, Functional characterization of WNT7A signaling in PC12 cells: Interaction with A FZD5 × LRP6 receptor complex and modulation by Dickkopf proteins. J. Biol. Chem 278, 37024–37031 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Meister M, Papatriantafyllou M, Nordström V, Kumar V, Ludwig J, Lui KO, Boyd AS, Popovic ZV, Fleming TH, Moldenhauer G, Nawroth PP, Gröne HJ, Waldmann H, Oelert T, Arnold B, Dickkopf-3, a tissue-derived modulator of local T-cell responses. Front. Immunol 6, 78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu KH, Tounsi A, Shridhar N, Kublbeck G, Klevenz A, Prokosch S, Bald T, Tuting T, Arnold B, Dickkopf-3 contributes to the regulation of anti-tumor immune responses by mesenchymal stem cells. Front. Immunol 6, 645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzawa K, Shien K, Peng H, Sakaguchi M, Watanabe M, Hashida S, Maki Y, Yamamoto H, Tomida S, Soh J, Asano H, Tsukuda K, Nasu Y, Kumon H, Miyoshi S, Toyooka S, Distant bystander effect of REIC/DKK3 gene therapy through immune system stimulation in thoracic malignancies. Anticancer Res. 37, 301–307 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Uchida D, Shiraha H, Kato H, Sawahara H, Nagahara T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K, Nasu Y, Kumon H, Yamamoto K, Synergistic anti-pancreatic cancer immunological effects by treatment with REIC/DKK3 protein and peripheral blood mononuclear cells. J. Gastroenterol. Hepatol 31, 1154–1159 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Uchida D, Shiraha H, Kato H, Sawahara H, Nagahara T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K, Nasu Y, Kumon H, Yamamoto K, Synergistic anti-pancreatic cancer immunological effects by treatment with reduced expression in immortalized cells/dickkopf-3 protein and peripheral blood mononuclear cells. J. Gastroenterol. Hepatol 31, 1154–1159 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Mueller MM, Fusenig NE, Friends or foes-bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S, Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 63, 2649–2657 (2003). [PubMed] [Google Scholar]
- 35.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD, Ras activity levels control the development of pancreatic diseases. Gastroenterology 137, 1072–1082 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W, Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 69, 5820–5828 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, Tsao MS, Chiao PJ, Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-κB transcription factors. J. Biol. Chem 282, 6001–6011 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Barrantes Idel B, Montero-Pedrazuela A, Guadaño-Ferraz A, Obregon MJ, Martinez de Mena R, Gailus-Durner V, Fuchs H, Franz TJ, Kalaydjiev S, Klempt M, Holter S, Rathkolb B, Reinhard C, Morreale de Escobar G, Bernal J, Busch DH, Wurst W, Wolf E, Schulz H, Shtrom S, Greiner E, Hrabé de Angelis M, Westphal H, Niehrs C, Generation and characterization of dickkopf3 mutant mice. Mol. Cell. Biol 26, 2317–2326 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampson BL, Kendall SD, Ancrile BB, Morrison MM, Shealy MJ, Barrientos KS, Crowe MS, Kashatus DF, White RR, Gurley SB, Cardona DM, Counter CM, Targeting eNOS in pancreatic cancer. Cancer Res. 72, 4472–4482 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westphalen CB, Olive KP, Genetically engineered mouse models of pancreatic cancer. Cancer J. 18, 502–510 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig J, Federico G, Prokosch S, Küblbeck G, Schmitt S, Klevenz A, Gröne HJ, Nitschke L, Arnold B, Dickkopf-3 acts as a modulator of B cell fate and function. J. Immunol 194, 2624–2634 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Papatriantafyllou M, Moldenhauer G, Ludwig J, Tafuri A, Garbi N, Hollmann G, Küblbeck G, Klevenz A, Schmitt S, Pougialis G, Niehrs C, Grone HJ, Hämmerling GJ, Arnold B, Oelert T, Dickkopf-3, an immune modulator in peripheral CD8 T-cell tolerance. Proc. Natl. Acad. Sci. U.S.A. 109, 1631–1636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson III BA, Yarchoan M, Lee V, Laheru DA, Jaffee EM, Strategies for increasing pancreatic tumor immunogenicity. Clin. Cancer Res. 23, 1656–1669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laheru D, Jaffee EM, Immunotherapy for pancreatic cancer – science driving clinical progress. Nat. Rev. Cancer 5, 459–467 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Zheng L, Xue J, Jaffee EM, Habtezion A, Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 144, 1230–1240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Hegde S, DeNardo DG, Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol. Immunother 66, 1037–1048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, Schmid MC, Sun P, Mose E, Bouvet M, Lowy AM, Valasek MA, Sasik R, Novelli F, Hirsch E, Varner JA, Macrophage PI3Kγ drives pancreatic ductal adenocarcinoma progression. Cancer Discov. 6, 870–885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG, Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22, 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki K, Watanabe M, Sakaguchi M, Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH, Kumon H, Date H, REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 16, 65–72 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Tanimoto R, Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kumon H, Huh NH, REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int. J. Mol. Med 19, 363–368 (2007). [PubMed] [Google Scholar]
- 51.Than SS, Kataoka K, Sakaguchi M, Murata H, Abarzua F, Taketa C, Du G, Yashiro M, Yanagihara K, Nasu Y, Kumon H, Huh NH, Intraperitoneal administration of an adenovirus vector carrying REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric carcinoma. Oncol. Rep 25, 989–995 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Uchida D, Shiraha H, Kato H, Nagahara T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K, Nasu Y, Yagi T, Kumon H, Yamamoto K, Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer. J. Gastroenterol. Hepatol 29, 973–983 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Nakamura RE, Hackam AS, Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 28, 232–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zenzmaier C, Sampson N, Plas E, Berger P, Dickkopf-related protein 3 promotes pathogenic stromal remodeling in benign prostatic hyperplasia and prostate cancer. Prostate 73, 1441–1452 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padua D, Massague J, Roles of TGFβ in metastasis. Cell Res. 19, 89–102 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S, Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 9, 991–997 (2003). [PubMed] [Google Scholar]
- 57.Frazier ML, Pathak S, Wang ZW, Cleary K, Singletary SE, Olive M, Mackay B, Steck PA, Levin B, Establishment of a new human pancreatic adenocarcinoma cell line, MDAPanc-3. Pancreas 5, 8–16 (1990). [DOI] [PubMed] [Google Scholar]
- 58.Yamada H, Yoshida T, Sakamoto H, Terada M, Sugimura T, Establishment of a human pancreatic adenocarcinoma cell line (PSN-1) with amplifications of both c-myc and activated c-Ki-ras by a point mutation. Biochem. Biophys. Res. Commun 140, 167–173 (1986). [DOI] [PubMed] [Google Scholar]
- 59.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA, Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J, Zhang L, Downregulation of Dkk3 activates β-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 29, 84–92 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Arumugam T, Ramachandran V, Logsdon CD, Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J. Natl. Cancer Inst. 98, 1806–1818 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA, Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.