Abstract
Uncontrolled interferon γ (IFNγ)-mediated T-cell responses to commensal microbiota are a driver of inflammatory bowel disease (IBD). Interleukin-10 (IL-10) is crucial for controlling these T-cell responses, but the precise mechanism of inhibition remains unclear. A better understanding of how IL-10 exerts its suppressive function may allow identification of individuals with suboptimal IL-10 function among the heterogeneous population of IBD patients. Using cells from patients with an IL10RA deficiency or STAT3 mutations, we demonstrate that IL-10 signaling in monocyte-derived dendritic cells (moDCs), but not T cells, is essential for controlling IFNγ-secreting CD4+ T cells. Deficiency in IL-10 signaling dramatically increased IL-1β release by moDCs. IL-1β boosted IFNγ secretion by CD4+ T cells either directly or indirectly by stimulating moDCs to secrete IL-12. As predicted a signature of IL-10 dysfunction was observed in a subgroup of pediatric IBD patients having higher IL-1β expression in activated immune cells and macroscopically affected intestinal tissue. In agreement, reduced IL10RA expression was detected in peripheral blood mononuclear cells and a subgroup of pediatric IBD patients exhibited diminished IL-10 responsiveness. Our data unveil an important mechanism by which IL-10 controls IFNγ-secreting CD4+ T cells in humans and identifies IL-1β as a potential classifier for a subgroup of IBD patients.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic relapsing-remitting disorder of the gastrointestinal tract caused by an abnormal immune response to commensal microbiota in genetically predisposed individuals. T cells of various subtypes (e.g. T helper 1 (Th1), Th2, Th17) are crucial for initiating and maintaining chronic inflammation in IBD. From a quantitative point of view, Th1 responses in IBD are highest and therefore an important driving force of inflammation. Conventional immunosuppressive therapies and anti-tumor necrosis factor (TNF) agents effectively maintain disease remission in IBD patients 1, 2. However, about 40–50% of pediatric IBD patients experience frequent relapses of inflammation, in some cases leading to treatment refractoriness thus necessitating surgical removal of intestinal segments. Defining the immune pathways that contribute to a complicated disease course is highly desirable for designing more effective and tailored therapeutic approaches.
The immunosuppressive cytokine interleukin-10 (IL-10) plays a crucial role in orchestrating intestinal immune homeostasis 3, 4. Animal studies have shown that IL-10 maintains intestinal tolerance to microbiota by controlling effector Th1 and Th17 responses 5, 6, and more recently, it was demonstrated that failure of innate immune cells to respond to IL-10 is critically involved in the development of such T cell-driven intestinal inflammation 7–9. In humans, the impact of the IL-10 pathway in intestinal inflammation has been fully appreciated by the discovery of monogenic defects in IL10 and IL10R genes 10–12. Given the emerging role for IL-10 in maintaining intestinal immune homeostasis, we hypothesize that a subgroup of pediatric IBD patients may exhibit milder forms of IL-10 dysregulation.
IL-10 is a dimer 13 and exerts its effects by binding to the IL-10 receptor, composed of two IL-10 binding chains (IL-10RA) and two accessory chains (IL-10RB) 14. Signal transducer and activator of transcription 3 (STAT3) is the major STAT protein activated by IL-10 and is essential for its immunosuppressive effects 15, 16. The mechanisms by which IL-10 prevents intestinal inflammation in humans are just beginning to be uncovered. Alterations in macrophage differentiation and function were initially described in IL10R-deficient patients 8, 17, and we have recently demonstrated that IL10RA deficiency can result in aberrant TNFα release by monocyte-derived dendritic cells (moDCs) and uncontrolled IFNγ and IL-17 release by peripheral blood cells 18. In mice, Th17 cells, but not Th1 cells, express the IL-10R and are controlled by IL-10 in a direct manner 6. Conversely, IL-10 suppresses murine Th1 cells indirectly via its actions on antigen-presenting cells 19. In humans, a direct inhibitory effect on T cells has been shown for anti-CD28-mediated T-cell proliferation and IL-2 production 20, 21, and moreover, IL-10 can directly interfere with T-cell receptor-induced IFNγ, but not IL-17 production in memory T cells 22. However, others demonstrate that the IL-10 inhibitory activity is mainly indirect by controlling antigen-presenting cell function 23–25. A precise understanding of how IL-10 controls T-cell responses in humans may allow identification of individuals with suboptimal IL-10 function among the heterogeneous IBD population.
Using cells from patients with an IL10RA deficiency or STAT3 mutations 26, we investigated the mechanisms by which IL-10 inhibits IFNγ secretion by human CD4+ T cells. We identified a signature characteristic for defective immune regulation by IL-10 and subsequently determined whether a similar immune signature and suboptimal IL-10 function could be detected in a cohort of pediatric patients with IBD. We identify antigen-presenting cells as essential targets of IL-10 action in controlling IFNγ-mediated T-cell responses in humans and to the best of our knowledge we are the first to identify altered IL-10 function in adolescent pediatric IBD patients with polygenic disease susceptibility.
RESULTS
Interleukin-10 inhibits IFNγ-secreting CD4+ T cells indirectly via dendritic cells
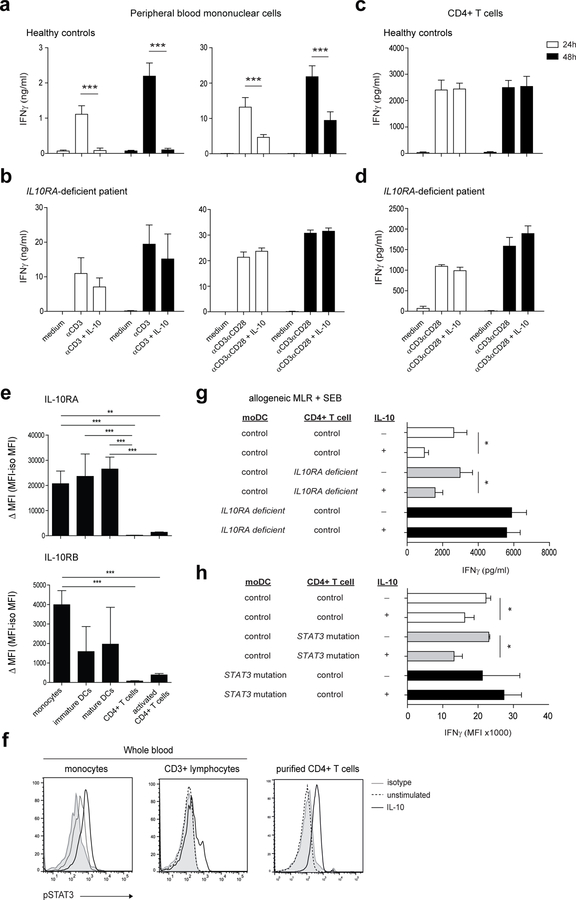
To investigate the basis for the IL-10-driven regulation of IFNγ production by CD4+ T cells, we used peripheral blood mononuclear cells (PBMCs) from an adolescent patient with a homozygous loss-of-function mutation in the IL10RA gene and normal controls. As expected 22, stimulation of PBMCs from healthy individuals with soluble anti-CD3 antibody or anti-CD3/CD28 beads in the presence of IL-10 resulted in an inhibition of IFNγ production (Figure 1a), and this inhibitory effect was absent in IL10RA-deficient cells (Figure 1b). Interestingly, IL-10 did not inhibit IFNγ production by purified CD4+ T cells from healthy individuals stimulated either with plate-bound anti-CD3 antibody or anti-CD3/CD28 beads at variable concentrations and bead-to-cell ratios (Figure 1c and d, Supplementary Figure S1a and b). Consistent with this, purified CD4+ T cells from healthy individuals showed very weak cell surface expression of both IL-10R chains compared to monocytes and mature moDCs (Figure 1e, Supplementary Figure S1d). However, the inability of IL-10 to inhibit IFNγ-secreting CD4+ T cells in a direct manner could not be explained by lack of IL-10 downstream signaling in CD4+ T cells. Although CD3+ T cells and monocytes in whole blood samples of healthy controls showed a different degree of IL-10-induced STAT3 phosphorylation, purified CD4+ T cells efficiently activated STAT3 upon IL-10 stimulation (Figure 1f). To conclusively demonstrate that the functional IL-10R on CD4+ T cells is not involved in the inhibition of IFNγ production, IL10RA-deficient moDCs were co-cultured, in the presence or absence of IL-10, with CD4+ T cells from healthy controls and vice versa. As shown in Figure 1g, IL-10 failed to inhibit IFNγ production by CD4+ T cells only when moDCs, and not CD4+ T cells themselves, were deficient for the IL-10 receptor. In agreement with this indirect inhibitory effect of IL-10, preincubation of moDCs from healthy controls with IL-10 before the addition of CD4+ T cells significantly inhibited IFNγ production, whereas this inhibitory effect was absent in the presence of IL10RA-deficient moDCs. (Supplementary Figure S1c). Moreover, compared to healthy control moDCs, IL10RA deficiency in moDCs caused a profound increase in IFNγ secretion (Figure 1g, Supplementary Figure S1c). Since STAT3 plays a critical role in IL-10 signaling we confirmed these results by performing an allogeneic mixed lymphocyte reaction (MLR) with moDCs and CD4+ T cells from healthy controls and autosomal dominant hyper-IgE syndrome (AD-HIES) patients carrying STAT3 mutations. Similar to IL10RA-deficient moDCs, IL-10 failed to inhibit IFNγ production by CD4+ T cells only when moDCs were carrying STAT3 mutations and defective in IL-10 signaling (Figure 1h). Altogether, these results demonstrate that IL-10 signaling in DCs, and not in CD4+ T cells, is crucial for controlling IFNγ secretion by human CD4+ T cells.
Figure 1. IL-10-mediated inhibition of IFNγ secretion by human CD4+ T cells requires dendritic cells.
(a, b) Peripheral blood mononuclear cells or (c, d) CD4+ T cells from an IL10RA-deficient patient and adult healthy controls (n=4) were stimulated with soluble anti-CD3 or anti-CD3/CD28 beads (bead-to-cell ratio 1:2) with or without IL-10. After 24 h or 48 h, supernatants were assayed for IFNγ using an ELISA. (e) Purified CD14+ monocytes, immature moDCs, LPS-matured moDCs, CD4+ T cells and anti-CD3/CD28-activated CD4+ T cells (48 h) were analyzed by flow cytometry for expression of IL-10RA and IL-10RB chains. Delta Mean fluorescence intensity (MFI) values (MFI-minus-MFI of the isotype control) are shown for n=4–8 adult healthy individuals per group. (f) Control whole blood or purified CD4+ T cells were stimulated with IL-10 for 60 min followed by quantification of STAT3 phosphorylation (Tyr705) by flow cytometry. (g) Allogeneic MLRs were performed using LPS-matured moDCs and CD4+ T cells from an IL10RA-deficient patient or healthy individuals. The bacterial superantigen Staphylococcal enterotoxin B (SEB) was added to all conditions in the presence or absence of IL-10. After 72h, supernatants were assayed for IFNγ using an ELISA. (h) Allogeneic MLRs were performed using moDCs and CD4+ T cells from healthy individuals or AD-HIES patients carrying STAT3 mutations. SEB was added to all conditions in the presence or absence of LPS and IL-10. After 72 h, supernatants were assayed for IFNγ using a Cytometric Bead Array. Results are mean ± SD (B, D, H) or mean ± SEM (A, C E, G) of a representative of at least two independent experiments. *P<0.05, ***P<0.001 using one-way ANOVA (E) or unpaired Student’s t test (A, G, H)
Loss of IL10R signaling increases IL-1β release by dendritic cells
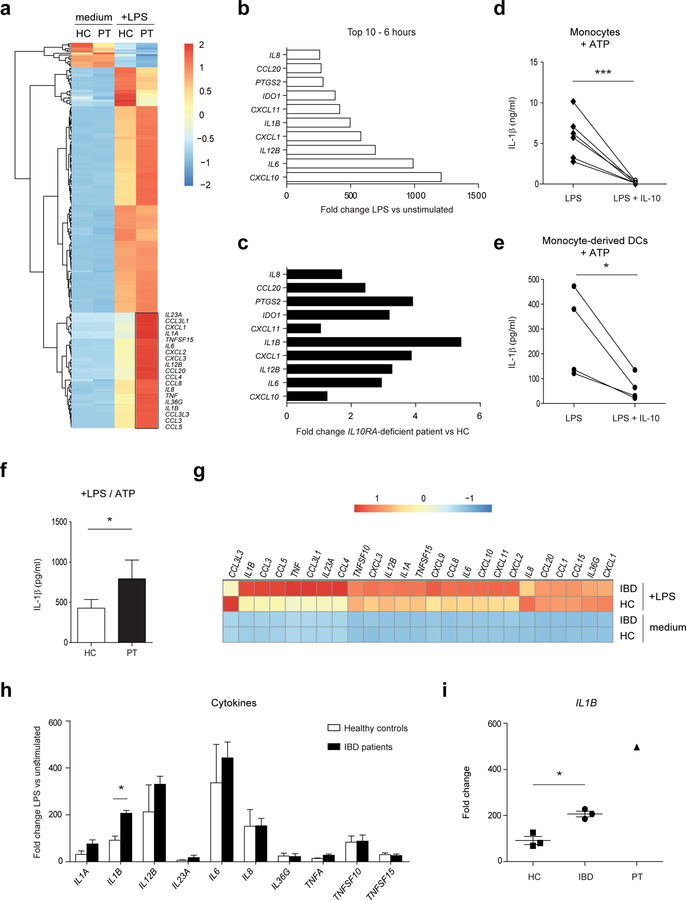
We next aimed to identify moDC-derived factors that may drive IFNγ secretion by human CD4+ T cells in the absence of a functional IL-10 pathway and conducted a full transcriptome analysis of IL10RA-deficient and healthy control moDCs using RNA sequencing. A total of 237 genes were differentially expressed between IL10RA-deficient and healthy control moDCs upon LPS stimulation, with 15 genes downregulated and 222 upregulated genes in IL10RA-deficient cells. Many of the LPS-induced genes were highly upregulated in IL10RA-deficient moDCs (Figure 2a, Supplementary Table 1), especially genes encoding proinflammatory cytokines and chemokines, with IL12B, IL6, IL1B, IL8 and CXCL1 being among the top 10 ranked genes (Figure 2b). Compared to moDCs from healthy controls, IL1B showed the largest fold change difference upon LPS stimulation in IL10RA-deficient moDCs (Figure 2c). In agreement, LPS-induced IL-1β secretion in monocytes (Figure 2d) and moDCs (Figure 2e) from healthy controls could be downregulated by IL-10 (Figure 2d and e), and IL10RA-deficient moDCs secreted significantly higher levels of IL-1β protein (Figure 2f). These data are in line with recent evidence showing that IL-1 neutralization may be beneficial for controlling inflammation in patients with IL10R deficiency 17. As IL10R-deficient patients do not respond to conventional immunosuppressive IBD treatments 10, 27, it can be envisaged that polygenic IBD patients showing similar unresponsiveness to these immunosuppressive regimens may exhibit a similar proinflammatory expression signature. We therefore selected a small number of pediatric IBD patients having a severe disease course with eventually all having undergone a resection (Table 1) and used RNA-Seq in moDCs to uncover the proinflammatory expression signature. Comparison of the gene-expression profiles of moDCs from IBD patients, IL10RA-deficient patient and healthy controls revealed that a distinct set of genes are upregulated in both the pediatric IBD patients and the IL10RA-deficient patient compared to healthy controls (Supplementary Figure S2a, Supplementary Table 1). Differentially expressed genes were identified with the R package “edgeR” using the following criteria: FPKM ≥ 5 in at least one sample, fold change was ≥1.5, and FDR ≤ 0.1. Notably, moDCs from IBD patients displayed enhanced expression of several cytokines and chemokines upon LPS stimulation (Figure 2g, Supplementary Table 1). In accordance with the expression profile of IL10RA-deficient moDCs, of all proinflammatory cytokines, IL1B was most differentially expressed between moDCs from healthy controls and the selected pediatric IBD patients (Figure 2h) and present in intermediate transcript levels compared to IL10RA-deficient moDCs (Figure 2i). Importantly, the in vitro generation of moDCs from patients with IBD and the IL10RA-deficient patient occurred independently from any secondary effects of ongoing inflammation in these patients, suggesting that a cell-intrinsic effect causes the increased IL-1β production by these cells. Collectively, our data demonstrate that lack of IL-10R expression on moDCs results in enhanced IL-1β secretion, and that IL1B is highly expressed in moDCs from pediatric IBD patients with a severe disease manifestation, suggesting that IL-1β may drive IFNγ secretion by CD4+ T cells and contribute to the disease process.
Figure 2. Dendritic cells with an IL10RA deficiency express high levels of IL-1β upon bacterial stimulation.
(a-c, g-i) Monocyte-derived dendritic cells from healthy controls (n=3), IBD patients (n=3) and an IL10RA-deficient patient (PT) were stimulated with LPS and high throughput RNA sequencing was performed after 6 hours of stimulation. (a) Heat map representing color-coded expression levels (FPKM values) of differentially expressed genes in IL10RA-deficient moDCs upon LPS stimulation. (b) Top 10 genes overexpressed in IL10RA-deficient moDCs upon LPS stimulation. Fold change values are calculated by dividing LPS-stimulated samples by unstimulated samples. (c) Comparison of the LPS-induced genes in (b) with expression levels in moDCs from healthy controls. (d, e) Monocytes and moDCs from adult healthy controls (n=4–6) were stimulated with LPS in the presence or absence of IL-10 for 20 h. Supernatants were assayed for IL-1β using an ELISA. To enhance IL-1β secretion, ATP was added during the last 15 minutes of stimulation. (f) Adult healthy controls (n=4) and IL10RA-deficient patient moDCs were stimulated with LPS for 20 h and ATP during the last 15 minutes of stimulation. Amount of IL-1β secreted by moDCs was determined by ELISA. (g) Heat map representing color-coded expression levels (FPKM values) of cytokine and chemokine genes in moDCs. (h, i) Cytokine mRNA expression in moDCs from (h) pediatric IBD patients and adult healthy controls and (i) adult healthy controls, pediatric IBD patients and IL10RA-deficient patient. Fold change values are calculated by dividing LPS-stimulated samples by unstimulated samples. Results are mean ± SD. *P<0.05, ***P<0.001 using one-way ANOVA (H, I) or unpaired Student’s t test (D, E, F). HC=healthy control, IBD=inflammatory bowel disease, PT=IL10RA-deficient patient.
Table 1.
Patient characteristics
| I | II | III | |
|---|---|---|---|
| Number | 3 | 38 | 20 |
| Age in years, range | 13–15 | 8–18 | 8–17 |
| Male (n) | 1 | 19 | 10 |
| Female (n) | 2 | 19 | 10 |
| Diagnosis | |||
| ulcerative colitis | 16 | 9 | |
| Crohn’s disease | 3 | 20 | 11 |
| IBD unclassified | 2 | ||
| Age at diagnosis (years) | 7–11 | 8–18 | 5–16 |
| Treatment at time of analysis | |||
| no | 38 | 3 | |
| azathioprine | 2 | 7 | |
| mesalazine | 2 | ||
| infliximab | 6 | ||
| methotrexate | 1 | 1 | |
| prednisolone | 4 | ||
| adalimumab | 1 | 3 | |
| 6-mercaptopurine | 1 | ||
| Treatment history | |||
| azathioprine | 3 | ||
| mesalazine | 2 | ||
| infliximab | 3 | ||
| methotrexate | 1 | ||
| prednisolone | 3 | ||
| adalimumab | 3 | ||
| 6-mercaptopurine | |||
| Surgery | 3 | 10 |
IL-1β stimulates IFNγ release by human CD4+ T cells in both a direct manner and indirectly through induction of IL-12 production
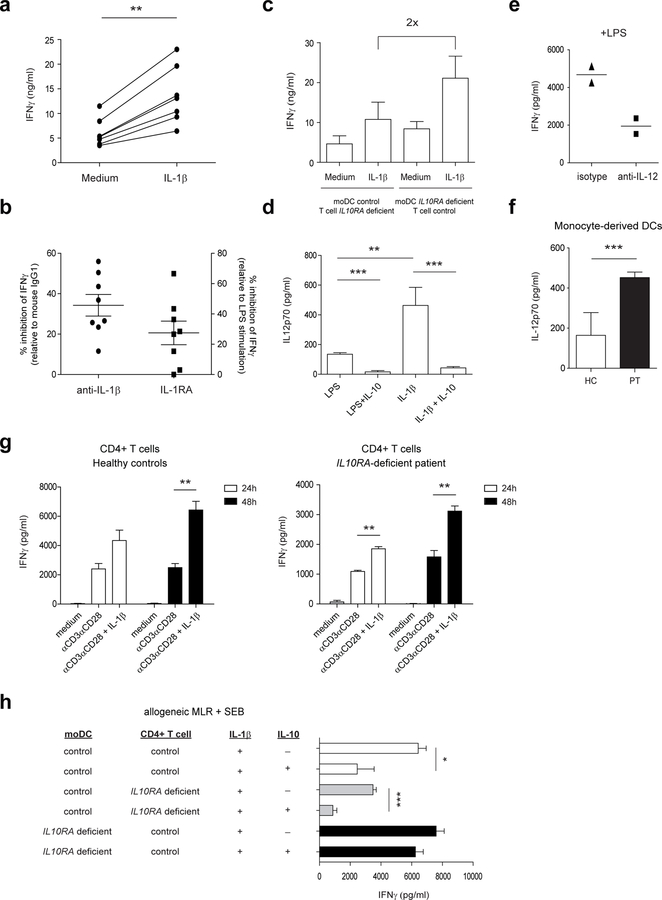
IL-1β is well known for its involvement in the differentiation of Th17 cells 28, 29, but has also been shown to stimulate antigen-induced expansion of murine Th1 cells 30. More recently, it was reported that T cell-intrinsic MyD88 activation by IL-1β is required for the functionality of murine Th1 memory cells 31. However, since the relationship between IL-1β and Th17 cells in various animal models of autoimmune disorders is most evident, the activity of IL-1β on human Th1 cells has not been thoroughly investigated. Therefore, we examined the effect of IL-1β on IFNγ-secreting CD4+ T cells by performing an allogeneic MLR using healthy control cells in the presence of recombinant IL-1β, neutralizing IL-1β antibodies, or IL-1 receptor antagonist (IL-1Ra). IL-1β exposure significantly increased IFNγ production (Figure 3a), and endogenous IL-1β neutralization and IL-1 receptor blockade inhibited IFNγ secretion by ~30–40% (Figure 3b). Interestingly, in the presence of IL10RA-deficient moDCs, the levels of IFNγ were two times higher upon IL-1β stimulation compared to healthy control moDCs (Figure 3c). IL-1β stimulates IL-12 secretion by human moDCs in conjunction with IFNγ and CD40 ligand 32, 33, and thereby may augment IFNγ production from T cells. In agreement with this, IL-1β significantly increased IL-12 secretion by moDCs in an allogeneic MLR using healthy controls (Figure 3d). Blocking of IL-12 reduced the LPS-induced IFNγ release (Figure 3e) and inhibited the IL-1β induced IFNγ release by CD4+ T cells in the MLR (Supplementary Figure S2b). Additionally, IL-10 significantly inhibited the IL-1β -induced IL-12 production (Figure 3d) and IL10RA-deficient moDCs released increased amounts of IL-12p70 (Figure 3f). In parallel, however, CD4+ T cells from both healthy controls (Figure 3g, left panel) and the IL10RA-deficient patient (Figure 3g, right panel) released significantly enhanced amounts of IFNγ upon IL-11β stimulation, suggesting that both direct and indirect effects account for the increased IL-1β -driven IFNγ release by CD4+ T cells. To investigate the mechanism by which IL-10 controls the IL-1β -driven IFNγ production, IL10RA-deficient moDCs were co-cultured, in the presence of IL-1β and IL-10, with healthy control CD4+ T cells and vice versa. In line with our data identifying the IL-10 control of moDCs crucial in suppressing IFNγ-secreting CD4+ T cells, IL-10 was unable to inhibit the IL-1β -driven IFNγ production by CD4+ T cells when moDCs were deficient for the IL-10 receptor (Figure 3h). Together, these data demonstrate that IL-1β stimulates IFNγ release by human CD4+ T cells in both a direct manner and indirectly through induction of IL-12 production. The IL-1β indirect effects only are tightly controlled by IL-10.
Figure 3. IL-1β-driven IFNγ secretion by CD4+ T cells is controlled by IL-10 via dendritic cells.
Allogeneic MLRs were performed using moDCs and CD4+ T cells from healthy individuals. SEB was added in the presence or absence of (a) IL-1β and (b) neutralizing IL-1β antibodies, IL-1 receptor antagonist or appropriate isotype control. After 72 h, supernatants were assayed for IFNγ using an ELISA. Percentage inhibition values are calculated by considering the percent of cytokine secretion upon LPS stimulation alone (for IL-1RA) or LPS in combination with mouse IgG1 isotype (for anti- IL-1β) as 100%. (c) Allogeneic MLRs were performed using moDCs and CD4+ T cells from an IL10RA-deficient patient or healthy individuals in the presence of SEB with or without IL-1β. (d, e) Allogeneic MLRs were performed using moDCs and CD4+ T cells from healthy individuals. SEB was added in the presence or absence of (d) LPS, IL-1β and IL-10 or (e) LPS with or without neutralizing IL-12 antibodies or appropriate isotype control. After 72 h, supernatants were assayed for (d) IL-12p70 and (e) IFNγ using an ELISA. (f) Adult healthy controls (n=4) and IL10RA-deficient patient moDCs were stimulated with LPS for 20 h and IL-12p70 was determined by ELISA. (g) CD4+ T cells from healthy controls (n=4) and an IL10RA-deficient patient (PT) were stimulated with anti-CD3/CD28 beads in the presence or absence of IL-1β. (h) Allogeneic MLRs were performed using moDCs and CD4+ T cells from an IL10RA-deficient patient or healthy individuals in the presence of SEB with or without IL-1β and IL-10. Results are mean ± SD (D-H) or mean ± SEM (B, C, G left) of a representative of at least two independent experiments. *P<0.05, **P<0.01, ***P<0.001 using unpaired Student’s t test.
The signatures of high IL-1β expression and suboptimal IL-10 function classify a subgroup of pediatric IBD patients
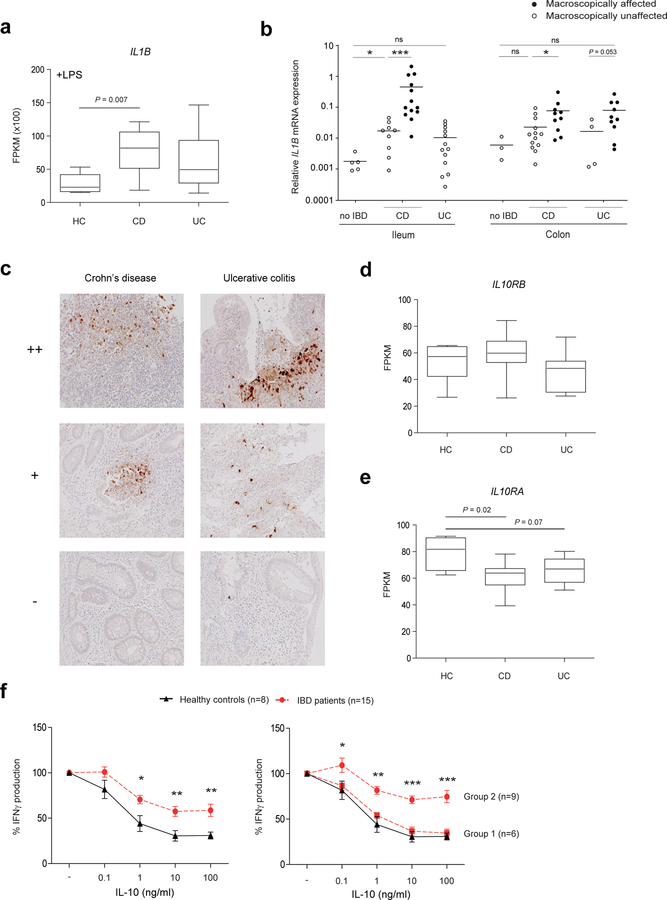
High levels of IL-1β in the colon has been detected in murine models of colitis, and treatment with IL-1 blocking agents or using Il1r1−/− mice successfully ameliorated or prevented disease 34–36. Given that IL-1β is highly expressed in the absence of a functional IL-10 pathway and a strong stimulator of CD4+ T-cell responses, we examined the expression of IL-1β in a large cohort of pediatric IBD patients and selected pediatric CD and UC patients who received either conventional immunosuppressive therapy, with or without anti-TNF treatment, and patients that had undergone resection (Table 1). We generated RNA-seq data of PBMCs from 6 healthy individuals, 11 patients with CD, and 9 patients with UC. Full transcriptome analysis showed significantly enhanced IL1B transcript levels in LPS-stimulated PBMCs from CD patients compared to healthy controls. A similar trend was found for LPS-stimulated PBMCs from UC patients, but the UC patients showed greater variation in the degree of IL1B mRNA expression (Figure 4a). Importantly, within the intestine, IL1B mRNA expression was increased in biopsies from macroscopically affected tissue compared to unaffected regions in treatment-naïve CD and UC patients associating IL1B mRNA expression with the site of inflammation (Figure 4b and Supplementary Figure S3a). Immunohistochemical analysis of paraffin-embedded biopsies revealed heterogeneity in IL-1β expression in inflamed regions. While lesional biopsies of some IBD patients were negative, IL-1β protein expression was clearly detectable in a subgroup of CD and UC patients (Figure 4c). Given that IL-10R signaling is crucial for controlling IL-1β action and production, we next questioned whether IL-10 responses are dysregulated in pediatric IBD patients. We therefore investigated expression of both chains of the IL-10 receptor in PBMCs from CD and UC patients. While the expression of IL10RB was not affected (Figure 4d), IL10RA expression was lower in PBMCs from both CD and UC patients (Figure 4e). To provide a proof of concept that variation in IL-10 responsiveness can be detected amongst therapy naïve IBD patients, we performed a dose-response assay in which PBMCs from treatment-naive CD and UC patients (n=15) were stimulated with anti-CD3/CD28 beads in the presence of different IL-10 concentrations for 48 hours. Compared to PBMCs from healthy individuals, cells derived from IBD patients were less sensitive to IL-10-induced inhibition of IFNγ production (Figure 4f left panel). However, there was a clear dichotomy in the IBD patient group with a subgroup of patients (group 1) responding equally well to IL-10 as healthy controls, whereas PBMCs from a second group of patients (group 2) were clearly less sensitive to IL-10 inhibition of IFNγ release (Figure 4f right panel). In the group of pediatric IBD patients that were less sensitive to the IL-10 inhibitory effects, IL1B mRNA levels in diagnostic intestinal biopsies and plasma IL-6 concentrations were higher compared to the group showing normal IL-10 responsiveness (Supplementary Figure S3b, S3c). Clinically, more patients had severe disease behavior and more patients received biological therapy compared to the group showing normal IL-10 responsiveness (Supplementary Figure S3d). These data support the concept that IL-10 responsiveness is detectably variable amongst IBD patients. As the number of patients in our study is very small this concept should be further investigated in a larger prospective cohort study. Collectively, we demonstrate that high expression of IL-1β is associated with lesional inflammation in a subgroup of pediatric IBD patients. Moreover, we provide evidence for reduced IL-10 responsiveness in some pediatric IBD patients, which among other mechanisms may be caused by altered IL-10RA expression and may contribute to the increased IL-1β expression.
Figure 4. Enhanced IL-1β expression and suboptimal IL-10 function in pediatric IBD patients.
(a) Peripheral blood mononuclear cells from adult healthy controls (n=6), CD patients (n=11) and UC patients (n=9) were stimulated with bacterial LPS and high throughput RNA sequencing was performed after 6 hours of stimulation. FPKM values of IL1B mRNA expression are shown. (b) IL1B mRNA expression was measured in biopsies from macroscopically affected intestine (closed symbol) and biopsies from unaffected regions (open symbol) derived from treatment-naive CD and UC patients and no IBD controls. (c) Representative immunohistochemical staining for IL-1β in inflamed sections of paraffin-embedded biopsies of pediatric IBD patients (n=33). (d, e) IL10RA and IL10RB mRNA expression was determined in peripheral blood mononuclear cells from adult healthy controls (n=6), CD patients (n=11) and UC patients (n=9) using high throughput RNA sequencing. FPKM values of IL10RA and IL10RB mRNA expression are shown. (f) Peripheral blood mononuclear cells from healthy controls (n=8) and treatment-naive IBD patients (n=15) were stimulated with anti-CD3/CD28 beads in the presence of different concentrations of IL-10 (0.1, 1, 10, 100 ng/ml). After 48 h, supernatants were assayed for IFNγ using an ELISA. The relative difference was calculated considering the percent of cytokine secretion upon anti-CD3/CD28 beads stimulation without IL-10 as 100%. Results are mean ± SEM (F). *P<0.05, **P<0.01, ***P<0.001 using one-way ANOVA (A, B, E) or unpaired Student’s t test (F).
DISCUSSION
This study reveals that IL-10 signaling in DCs, but not T cells, is essential for controlling IFNγ secretion by human CD4+ T cells. Our data identify moDC-derived IL-1β as a potent T-cell stimulatory factor. IL-1β stimulates the production of IFNγ by CD4+ T cells either directly or acts indirectly by stimulating IL-12 release in moDCs which in turn increases CD4+ T cell-derived IFNγ release. Notably, the IL-1β indirect effects on IL-12 production are tightly controlled by IL-10. Translation of these findings to pediatric IBD revealed high IL-1β expression in a subgroup of patients. Additionally, we provide evidence for reduced IL10RA expression and diminished IL-10 responsiveness in pediatric IBD patients, likely explaining the selectively enhanced IL-1β expression.
There is accumulating evidence that innate immune responses are crucial for initiation and maintenance of intestinal T-cell inflammation in mice 7–9. We identify antigen-presenting cells as essential targets of IL-10 action in controlling IFNγ-mediated T-cell responses in humans. We demonstrate that upon a bacterial trigger IL10RA-deficient moDCs express enhanced levels of proinflammatory cytokine transcripts, including IL1B. As a similar phenomenon has been described for monocyte-derived macrophages we anticipate that our findings may be relevant for multiple subpopulations of intestinal antigen presenting cells.17 Interestingly, combined genome-wide analysis has shown that among the 163 IBD susceptibility loci, the most significantly enriched Gene Ontology term is ‘regulation of cytokine production’. Cell-type expression specificity analysis revealed that especially dendritic cells show the strongest enrichment of genes in IBD loci 37. We demonstrate that even high dose of IL-10 did not overcome the lower sensitivity of CD4+ T cells. Multiple mechanisms can contribute to the increased IFNγ production by CD4+ T cells in the absence of IL-10 regulation. As the effects of defective IL-10 regulation on IFNγ production were detected as early as 24h of MLR culture without substantial changes in CD4+ T cell proliferation (data not shown) we anticipate that reactivation of memory cells is largely responsible for the observed effects in this experimental setup. In the absence of IL-10-mediated moDC inhibition IL-1β can directly impact IFNγ secretion in memory T cells or first induce moDC derived IL12p70 that in turn enhances IFNγ secretion by memory T cells. Even though IL-1β is known to enhance IL-17 production, low concentrations of IL-17 were measured in the MLR cultures and no consistent IL-17 upregulation occurred in the absence of IL-10 regulation of moDC.
Besides establishing the importance of IL-10 mediated antigen presenting cell regulation to control CD4+ T cell activation in IBD, our data argue that downregulation of IL-10R expression may underlie resistance to IL-10 regulation in a subgroup of IBD patients. Differences in IL10RA expression were not dependent on age (data not shown). Suboptimal IL-10 receptor mediated inhibition could in turn lead to the enhanced IL-1β expression levels found in IBD patients creating a vicious circle as proinflammatory cytokines like IL-1β and TNFα can alter IL-10 responsiveness of DCs 38. Hence, the inflammatory milieu in IBD patients may cause IL-10 receptor downregulation on circulating mononuclear cells in turn amplifying the production of proinflammatory cytokines like IL-1β. In this light it is interesting that mRNA expression of other inflammatory mediators IL-6, cyclooxygenase-2 and oncostatin-M (OSM) had a tendency to be increased in colonic biopsies and plasma of patients with lower IL-10 responsiveness when compared to the group showing normal responsiveness (Supplementary Figure S3b, S3c). Suboptimal IL-10 responsiveness also occurs in other chronic inflammatory diseases and arises through diverse mechanisms. In rheumatoid arthritis patients, synovial DCs down-regulate the IL-10 receptor thus evading the immunosuppressive effects of IL-10 38. In type 2 diabetes, in vitro IL-10 hyporesponsiveness of macrophages in the presence of high glucose was linked to reduced intracellular signal transduction through STAT3 without changes in IL-10 receptor expression 39. Whether such mechanisms are also operative in IBD patients awaits further studies.
Although the precise etiology has not yet been fully elucidated, there have been significant advances in uncovering the cause of IBD, including the discovery of over 160 IBD susceptibility loci and more than 50 monogenic defects 37, 40. However, IBD is characterized by a remarkable heterogeneity in disease manifestation and response to therapy. Defining immune pathways that should be targeted is highly desirable for the design of more effective clinical approaches. The abnormalities caused by monogenic defects in IBD-like diseases are valuable for identifying relevant pathways involved in the pathogenesis and classification of polygenic IBD. In agreement with IL10RA deficiency, in pediatric IBD patients, we observed higher IL1B expression in activated PBMCs and macroscopically affected intestinal tissue compared to unaffected regions with a tendency of higher expression in CD than UC patients. The expression and balance between IL-1β and IL-1RA in IBD patients was first examined over 20 years ago with limited follow-up studies 41–46. The expression profile we found shows heterogeneity for IL-1β expression in pediatric IBD patients, suggesting possible relevance for a subgroup of patients. Recently, it was shown that in inflamed tissue of CD and UC patients, oncostatin M (OSM) is highly expressed and predicts response to anti-TNFα therapy. Interestingly, in addition to OSM, both IL1A and IL1B mRNA transcripts were significantly enriched in the inflamed tissue when compared to non-IBD controls 47. These data together with our evidence suggest that IL-1β can be an additional biomarker and potential therapeutic target in a subgroup of IBD patients. Identifying this subgroup of patients is a crucial step in determining clinical efficiency of anti-IL-1 therapy.
Although the primary limitation of this study is that it involves a small cohort of pediatric IBD patients, we provide a proof of concept that IL-10 responsiveness is heterogeneous amongst IBD patients and anticipate to find similar subgroups of suboptimal IL-10 responder patients in larger pediatric and adult IBD patient cohorts. The group of pediatric IBD patients that were less sensitive to the IL-10 inhibitory effects exhibited clinical and immunological differences at time of diagnosis. Whether these differences reflect a difference in immune pathology, disease severity or time to diagnosis remain to be elucidated in these future cohort analyses Given the crosstalk between IL-1β and other proinflammatory cytokines active in IBD (e.g. TNFα), it is crucial to predict in which patients IL-1β acts as the primary upstream regulator/enhancer. While IL-1 blocking agents are currently not used in clinic for IBD patients, neutralizing IL-1 therapy has already been shown to be effective in some IL10R-deficient patients 17. In patients with moderate to severe CD that are refractory to either anti-TNF agents or immunosuppressive conventional therapy, neutralizing IL-12-IL-23 therapy has been shown to have clinical effect 48. Given the role of IL-1β in stimulating IL-12 production by moDCs, we consider that the IL-1/IL-12/IFNγ axis may play a major pathogenic role in a subgroup of IBD patients. Further studies and larger patient cohorts are needed to identify and further characterize the pediatric IBD patients who would benefit from targeting the IL-1/IL-12/IFNγ pathway.
Unraveling the underlying pathogenic immune pathways in the diverse subgroups of IBD patients will contribute to future therapeutic approaches tailoring therapy to the patients’ individual disease. Here, we demonstrate that abnormalities in monogenic disease are useful for the functional understanding of polygenic IBD pathogenesis and can advance our ability to classify varying clinical pathologies.
METHODS
Patients.
Blood was collected in EDTA tubes from an IL10RA-deficient patient 18, AD-HIES patients carrying STAT3 mutations, cohorts of treatment-naïve or treatment-experienced pediatric IBD patients (Table 1), pediatric control (no IBD) patients suspected of IBD but having a normal intestinal histology, and adult healthy volunteers. At time of the analysis, the intestinal inflammation of the IL10RA-deficient patient was in clinical remission while receiving thalidomide, intravenous immunoglobulin and colchicine. All IBD patients were diagnosed by endoscopy and histology of biopsies according to the Porto criteria 49. All participants and/or their parents gave written informed consent. For the participants with IBD and IL10RA deficiency, the study was approved by the Medical Ethical Committee of the Erasmus University Medical Center. The participants with AD-HIES were enrolled in a National Institutes of Health Institutional Review Board approved protocol ().
Cell isolation.
Peripheral blood mononuclear cells, CD14+ monocytes and moDCs were isolated and cultured as described 18. CD4+ T cells were isolated using the negative selection Dynal CD4+ T cell isolation kit (Invitrogen). Purified CD4+ T cells and moDCs were used either directly in stimulation assays or co-cultured as indicated in an allogeneic mixed lymphocyte reaction (MLR) for 72 h. The various cell populations were stimulated for the indicated time-points with: purified lipopolysaccharide from Escherichia coli Serotype 0111:B4 (100 ng/ml, Sigma-Aldrich, St. Louis, MO), adenosine 5’-triphosphate disodium salt (ATP, 5 mM, InvivoGen, San Diego, CA), soluble anti-CD3 antibody (500 ng/ml; Sanquin, the Netherlands), plate-bound anti-CD3 antibody (clone OKT3 – 0.1,1, 10 µg/ml, Biolegend, San Diego, CA), anti-CD3/CD28 beads (bead-to-cell ratio 1:2 or 1:4, Invitrogen), Staphylococcal enterotoxin B (SEB, 0.05 µg/ml, Sigma-Aldrich or List Biological laboratories Inc, Campbell, CA), IL-10 (25 ng/ml, R&D Systems), IL-1β (10 ng/ml, R&D Systems), neutralizing IL-1β antibodies (canakunimab, 10 µg/ml), IL-1 receptor antagonist (anakinra, 5 µg/ml), neutralizing human IL-12p70 antibody (10 µg/ml, R&D systems, Minneapolis, MN) or appropriate isotype controls.
Cytokine Measurements.
Cytokine production was measured in supernatants using enzyme-linked immunosorbent assays for IL-1β, R&D systems), IFNγ (eBiosciences, San Diego, CA), IL-12p70 (BD biosciences, San Diego, CA), BD Cytometric Bead Array (BD biosciences), or the LEGENDplex Human Th Cytokine Panel (Biolegend).
Flow Cytometry.
For intracellular phosphorylated-STAT3 staining, whole blood samples or CD4+ T cells were stimulated for 60 min with IL-10 (25 ng/ml, R&D systems). Samples were stained for phosphorylated-STAT3 (Tyr705; BD biosciences) or the appropriate isotype control according to the BD Phosflow protocol. For IL-10 receptor staining, Fcγ receptors were first blocked by preincubation with saturating amounts of normal human serum. Subsequently, cells were stained with anti-IL-10RA (Mouse IgG2B Clone # 714212) and anti-IL-10RB (Mouse IgG1 Clone # 90220) antibodies (R&D systems) or matched isotype controls (R&D systems).
Stained cells were analyzed using the FACSCanto II (BD Biosciences) and FlowJo software. Delta MFI is obtained by gating on the population of interest (for example CD4+ cells and calculating the IL10RA or IL10RB MFI-minus-the isotype control MFI (also see Supplementary Figure S1d.)
RNA isolation and Quantitative PCR.
Intestinal tissues were pre-prepared by homogenization using mechanical pressure. Total RNA isolation and quantitative real-time PCR was performed as described 50. Quantification of the signal was achieved by correcting the cycle threshold value (Ct) of the gene of interest with the Ct value of the reference gene GAPDH (∆Ct). The relative expression to GAPDH for the gene of interest was measured as 2(-∆Ct). Primer sets used were GAPDH; Fw: 5’-GTCGGAGTCAACGGATT-3’, Rv: 5’-AAGCTTCCCGTTCTCAG-3’, IL1B; Fw: 5’-CCGCGTCAGTTGTTGT-3’, Rv: 5’-GGAGCGTGCAGTTCAG-3’.
RNA Sequencing Library Preparations.
For moDC analysis, RNA quality was ensured using the RNA6000 PicoAssay for the Bioanalyzer 2100 (Agilent), followed by RNA amplification using the Ovation RNA-Seq System V2 (NuGen Technologies, San Carlos, CA) and library preparation using the Ovation SP Ultralow Library System (NuGen) according the manufacturers’ protocols. For PBMC analysis, mRNA was enriched using KAPA mRNA capture beads followed by preparation of libraries using the KAPA Stranded mRNA Seq kit (Kapa Biosystems, Wilmington, MA) according the manufacturers’ protocols.
RNA Sequencing Data Analysis.
Bar-coded PCR products (indexed) were sequenced on an Illumina HiSeq 2500 platforms at the NHLBI DNA Sequencing and Genomics Core. Sequenced reads (50 bp, paired-end) were obtained with the Illumina CASAVA pipeline and mapped to the human genome hg19 (GRCh37, Feb. 2009) using Tophat 2.0.11 supplemental ref 51. Raw counts on exons of each gene were calculated using Cufflinks 2.1.1 supplemental ref 52 and normalized by using FPKM (Fragments Per Kilobase per Million mapped reads). Differentially expressed genes were identified with the R package “edgeR” using the following criteria: FPKM ≥ 5 in at least one sample, fold change was ≥1.5, and FDR ≤ 0.1. The gene expression heat maps were generated with the R package “pheatmap”.
Immunohistochemistry.
Paraffin embedded biopsies were processed and stained as described.18 Antibodies used were anti-IL-1β (clone 3A6, Cell Signalling Technology, Danvers, MA) and mIgG1 isotype control antibody. Images were acquired using a Leica DM5500B upright microscope and LAS image acquisition software (Leica Microsystems, Rijswijk, the Netherlands).
Statistics.
Significance was determined using unpaired Student’s t-test (two-tailed) or Mann–Whitney U-test performed on GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Each figure legend describes the statistical test used for each experiment. P-values of <0.05 were regarded as significant.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge B. Prakken (UMC Utrecht, the Netherlands) for providing neutralizing IL-1β antibodies and IL-1 receptor antagonist, and R. Spolski (National Institutes of Health, USA) and R. Debets (Erasmus University Medical Center, the Netherlands) for critical reading of the manuscript. Supported by the Dutch Sophia Foundation grant S14–17, EUR Fellowship from the Erasmus University Rotterdam (SV), Ter Meulen reFund of the Royal Netherlands Academy of Arts and Sciences (SV), ECCO-IOIBD Fellowship from the European Crohn’s and Colitis Organisation and the International Organization for the Study of Inflammatory Bowel Disease (SV), Netherlands Organisation for Scientific Research grant 2013/09420/BOO (MEJ), Dutch Sophia Foundation grants 557 (MAvL) and 671 (LdR) and by the Division of Intramural Research, NHLBI (PL, AF, and WJL). We thank the IBD patients and their families for participating and supporting our research.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
The sequencing data have been deposited at the European Genome-Phenome Archive (https://ega-archive.org/). Dataset: EGAD00001005117 Study: EGAS00001003741.
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- 1.Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. Journal of Crohn’s & colitis 2014; 8(10): 1179–1207. [DOI] [PubMed] [Google Scholar]
- 2.Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. Journal of pediatric gastroenterology and nutrition 2012; 55(3): 340–361. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993; 75(2): 263–274. [DOI] [PubMed] [Google Scholar]
- 4.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM et al. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. The Journal of experimental medicine 1998; 187(4): 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011; 34(5): 794–806. [DOI] [PubMed] [Google Scholar]
- 6.Huber S, Gagliani N, Esplugues E, O’Connor W Jr., Huber FJ, Chaudhry A et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011; 34(4): 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014; 40(5): 720–733. [DOI] [PubMed] [Google Scholar]
- 8.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 2014; 40(5): 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard-Madoux MJ, Ober-Blobaum JL, Costes LM, Kel JM, Lindenbergh-Kortleve DJ, Brouwers-Haspels I et al. IL-10 control of CD11c+ myeloid cells is essential to maintain immune homeostasis in the small and large intestine. Oncotarget 2016; 7(22): 32015–32030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine 2009; 361(21): 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigneur B, Escher J, Elawad M, Lima R, Buderus S, Kierkus J et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: a survey of the Genius Working Group. Inflammatory bowel diseases 2013; 19(13): 2820–2828. [DOI] [PubMed] [Google Scholar]
- 12.Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. The American journal of gastroenterology 2011; 106(8): 1544–1555. [DOI] [PubMed] [Google Scholar]
- 13.Zdanov A, Schalk-Hihi C, Gustchina A, Tsang M, Weatherbee J, Wlodawer A. Crystal structure of interleukin-10 reveals the functional dimer with an unexpected topological similarity to interferon gamma. Structure (London, England : 1993) 1995; 3(6): 591–601. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 15.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. Journal of immunology (Baltimore, Md : 1950) 2004; 172(1): 567–576. [DOI] [PubMed] [Google Scholar]
- 16.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. The Journal of biological chemistry 1999; 274(23): 16513–16521. [DOI] [PubMed] [Google Scholar]
- 17.Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID et al. Interleukin 1beta Mediates Intestinal Inflammation in Mice and Patients With Interleukin 10 Receptor Deficiency. Gastroenterology 2016; 151(6): 1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veenbergen S, van Leeuwen MA, Driessen GJ, Kersseboom R, de Ruiter LF, Raatgeep RHC et al. Development and Function of Immune Cells in an Adolescent Patient With a Deficiency in the Interleukin-10 Receptor. Journal of pediatric gastroenterology and nutrition 2017; 65(1): e5–e15. [DOI] [PubMed] [Google Scholar]
- 19.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. European journal of immunology 1997; 27(5): 1229–1235. [DOI] [PubMed] [Google Scholar]
- 20.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. Journal of immunology (Baltimore, Md : 1950) 1993; 150(11): 4754–4765. [PubMed] [Google Scholar]
- 21.Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. European journal of immunology 2000; 30(6): 1683–1690. [DOI] [PubMed] [Google Scholar]
- 22.Naundorf S, Schroder M, Hoflich C, Suman N, Volk HD, Grutz G. IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. European journal of immunology 2009; 39(4): 1066–1077. [DOI] [PubMed] [Google Scholar]
- 23.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of experimental medicine 1991; 174(5): 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. European journal of immunology 1994; 24(4): 1007–1009. [DOI] [PubMed] [Google Scholar]
- 25.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. Journal of immunology (Baltimore, Md : 1950) 1993; 151(3): 1224–1234. [PubMed] [Google Scholar]
- 26.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N et al. STAT3 mutations in the hyper-IgE syndrome. The New England journal of medicine 2007; 357(16): 1608–1619. [DOI] [PubMed] [Google Scholar]
- 27.Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 2012; 143(2): 347–355. [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nature immunology 2007; 8(9): 942–949. [DOI] [PubMed] [Google Scholar]
- 29.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology 2007; 8(9): 950–957. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(17): 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenten D, Nish SA, Yu S, Yan X, Lee HK, Brodsky I et al. Signaling through the adaptor molecule MyD88 in CD4+ T cells is required to overcome suppression by regulatory T cells. Immunity 2014; 40(1): 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara T, Urabe K, Fukagawa S, Uchi H, Inaba K, Furue M et al. Engagement of human monocyte-derived dendritic cells into interleukin (IL)-12 producers by IL-1beta + interferon (IFN)-gamma. Clinical and experimental immunology 2005; 139(3): 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesa AK, Galy A. IL-1 beta induces dendritic cells to produce IL-12. International immunology 2001; 13(8): 1053–1061. [DOI] [PubMed] [Google Scholar]
- 34.Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. The Journal of experimental medicine 2012; 209(9): 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science (New York, NY) 2005; 307(5710): 734–738. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008; 456(7219): 264–268. [DOI] [PubMed] [Google Scholar]
- 37.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491(7422): 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald KP, Pettit AR, Quinn C, Thomas GJ, Thomas R. Resistance of rheumatoid synovial dendritic cells to the immunosuppressive effects of IL-10. Journal of immunology (Baltimore, Md : 1950) 1999; 163(10): 5599–5607. [PubMed] [Google Scholar]
- 39.Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, Cheung ST et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Scientific reports 2016; 6: 21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlig HH, Schwerd T. From Genes to Mechanisms: The Expanding Spectrum of Monogenic Disorders Associated with Inflammatory Bowel Disease. Inflammatory bowel diseases 2016; 22(1): 202–212. [DOI] [PubMed] [Google Scholar]
- 41.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut 1990; 31(6): 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. Journal of immunology (Baltimore, Md : 1950) 1995; 154(5): 2434–2440. [PubMed] [Google Scholar]
- 43.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut 1989; 30(6): 835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut 1998; 42(2): 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satsangi J, Wolstencroft RA, Cason J, Ainley CC, Dumonde DC, Thompson RP. Interleukin 1 in Crohn’s disease. Clinical and experimental immunology 1987; 67(3): 594–605. [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA et al. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clinical and experimental immunology 2004; 138(2): 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nature medicine 2017; 23(5): 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. The New England journal of medicine 2016; 375(20): 1946–1960. [DOI] [PubMed] [Google Scholar]
- 49.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. Journal of pediatric gastroenterology and nutrition 2014; 58(6): 795–806. [DOI] [PubMed] [Google Scholar]
- 50.Veenbergen S, van Berkel LA, du Pre MF, He J, Karrich JJ, Costes LM et al. Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103(+) dendritic cells. Mucosal immunology 2016; 9(4): 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.