Abstract
The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP), and North America (STP) to develop an internationally accepted nomenclature for proliferative and non-proliferative changes in rats and mice. The purpose of this publication is to provide a standardized nomenclature for classifying changes observed in the hematolymphoid organs, including the bone marrow, thymus, spleen, lymph nodes, mucosa-associated lymphoid tissues (MALT) and other lymphoid tissues (serosa-associated lymphoid clusters [SALCs] and tertiary lymphoid structures [TLSs]). The nomenclature for these organs is divided into three terminologies; descriptive, conventional and enhanced. Three terms are listed for each diagnosis. The rationale for this approach and guidance for its application to toxicologic pathology are described in detail below.
Keywords: INHAND, lymphoid, bone marrow, thymus, spleen, lymph node, MALT
Introduction (Figures 1 and 2)
Figure 1.
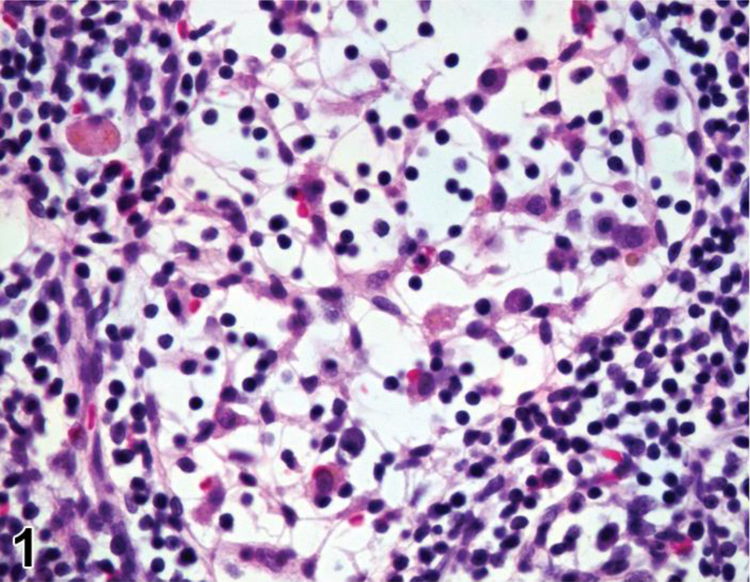
Rat, mesenteric lymph node, medullary sinus. Reticular meshwork.
Figure 2.

Rat, lymph node, paracortex. Lymphocyte crossing left wall of high endothelial venule (arrow).
The hematolymphoid organs produce and maintain the cells of acquired and innate immunity (lymphocytes, plasma cells, monocytes, macrophages, dendritic cells and granulocytes) and they also produce the cells that carry blood gases (erythrocytes) and maintain vascular integrity (megakaryocytes). The modifier ‘hematolymphoid’ acknowledges both the hematopoietic role of the bone marrow (and spleen in rodents) and the presence of lymphoid cells (lymphocytes, lymphoblasts, plasma cells) in the bone marrow, thymus, spleen, lymph nodes, MALT and other lymphoid tissues (SALC and TLS). The hematolymphoid organs are the organs of the immune system and they collectively produce the lymphocyte repertoire, conduct immune surveillance and mount immunologic reactions. These functions are distributed among the organs which are classified as primary/central and secondary/peripheral. The classic primary or central organs are the bone marrow and thymus where lymphocyte proliferation and maturation take place independent of stimulation by exogenous antigens. The spleen, lymph nodes, MALT and SALC are secondary lymphoid organs where exogenous antigen-dependent lymphocyte development and proliferation take place. TLS are tertiary lymphoid tissues that are induced in non-lymphoid organs.
The hematolymphoid organs share basic stromal and parenchymal features that enable them to function together as an integrated system. Each organ has a sponge-like stroma (reticular meshwork) that divides the organ into morphologically and functionally distinct compartments. 1–3 The interstices in the meshwork are populated by blood cells which serve as the organ’s parenchymal cells. The reticular meshwork is composed of fibroblastic reticular cells (FRCs) and their reticular fibers (except in thymus which has reticular cells of predominantly epithelial origin which do not produce reticular fibers). Reticular cells provide surfaces for blood cell adherence and produce trophic factors (chemokines) that direct lymphocyte movement (trafficking by means of haptotaxis) to B-cell and T-cell regions for further development and function.1 Cell trafficking occurs in primary, secondary and tertiary lymphoid organs. Reticular fibers act as conduits that conduct soluble mediators to specific locations in the secondary lymphoid organs (e.g. high endothelial venules in the lymph node) in order to enhance recruitment and migration of specific lymphocytes into the lymphoid organ for further development, function and interaction with other cell types. 4–6 The reticular meshwork is distensible and can expand to accommodate increased numbers of blood cells, as can be seen in an antigenically stimulated lymph node or a congested spleen. When depleted of blood cells, the meshwork can collapse and contract, as can be seen in lymphoid depletion in the thymus and contraction of the spleen. The reticular meshwork is difficult to appreciate in routinely stained tissues because its fine cytoplasmic processes are typically obscured by blood cells in its interstices, especially leukocytes, but it can be readily observed in lymph node sinuses that are not crowded with cellular traffic (Figure 1). The distinctive morphological appearances of the hematolymphoid organs are a function of the arrangement of their compartments and the number, types and distribution of the blood cells within the compartments.
A key feature of the hematolymphoid organs is that blood cells can move from one organ to another using the blood and lymph for transportation. Over the course of their long and complex life histories, lymphocytes move in and out of all the hematolymphoid organs via specialized adaptations in vascular and lymphatic endothelium (Figure 2). Mature naïve lymphocytes are particularly mobile and constantly cycle through secondary lymphoid organs in their continual search for cognate antigens. The life histories the blood cells include some time spent as constituents of organ parenchyma and some time spent as constituents of the blood. Even erythrocytes and platelets spend time in organ parenchyma, first during development in the bone marrow and then during periodic filtration through the spleen when they pass through the interstices of the red pulp. Erythrocytes, monocytes and platelets are also stored in the red pulp for ready release. Because of their mobility, blood cells can increase or decrease in a given location in response to demand. Lymph nodes can swell with newly recruited lymphocytes within hours of antigenic stimulation and the spleen can contract and expel erythrocytes into the blood within minutes in response to a drop in blood pressure or an increase in epinephrine. (Splenic contraction, a well known feature of muscular spleens, also can also occur to a lesser degree in the mouse and rat spleen by virtue of contractile proteins in the fibroblastic reticular cells.7,8) These types of rapid shifts in blood cell populations are due to migration and redistribution of existing blood cells and are not the result of a change in the absolute numbers of blood cells in the short term.
Blood cell migration creates special nomenclature challenges for the hematolymphoid system. Descriptive terminology (increased/decreased cellularity), now widely used in other organ systems, is particularly applicable to mobile blood cells of bone marrow origin (which include macrophages, mast cells and dendritic cells in addition to lymphocytes, erythrocytes and granulocytes) because it is often not possible to distinguish locally produced blood cells from those that arrived recently or to determine with certainty if missing blood cells died or emigrated elsewhere. Conventional terminology (hyperplasia/atrophy), historically the diagnostic standard, remains useful for diagnosing changes in carcinogenicity studies and is also used to diagnose changes in structures (e.g. HEVs). Enhanced terminology (cell type, increased/decreased, compartment) can be used in short term studies when a precise mechanistic approach to describing and evaluating the effect of exogenous substances on the hemolymphoid system is desired. This document defines and aligns these three lexicons to allow application of appropriate nomenclature based upon the needs of the individual study.
Diagnostic Challenges and Best Practices
In 2005, the STP Immunotoxicology Working Group published a “best practice” concept for examination of hematolymphoid organs using Enhanced Histopathology9 which has been described extensively elsewhere. The enhanced histopathology approach evaluates the compartments of each lymphoid organ individually in order to identify specific cellular and architectural changes.15–19 Descriptive diagnostic terms are used in a specifically proscribed way to quantify changes in individual cell types and localize these changes to the specific compartments of the organ(s) in which they occur (details about the compartments and their cellular constituents appear in tables in each organ section). Changes are reported using semi-quantitative descriptive terminology, rather than interpretive terminology. This methodology cannot directly measure immune function but it is a sensitive method for detecting subtle changes and has the potential to determine whether or not a specific treatment causes suppression or stimulation of the immune system.11 Moreover, evaluating the different cell types and changes within compartments may suggest the possible cause(s) or mechanisms for the findings. For example, a diagnosis of increased lymphocytes in the lymph node paracortex or increased follicles and germinal centers in the lymph node cortex provides more mechanistic information than a diagnosis of lymphoid hyperplasia.
This document regards descriptive terminology, conventional terminology and enhanced terminology as separate but complementary and evolving terminologies for the hematolymphoid organs. Table 1 indicates the recommended use of the descriptive, conventional and enhanced terminologies. For each diagnostic entity, the desdescriptive term is presented first, followed by the conconventional term and then by the enhenhanced term. This approach acknowledges the common practice of descriptive terminology along with the utility of standard interpretive diagnostic terms, such as hyperplasia and atrophy, and recognizes the scientific basis for enhanced descriptive diagnostic terms, such as increased or decreased cell type, which are more closely aligned with how the various cell types and compartments function. Descriptive terminology is recommended for short term (≤ 3 months) general toxicity studies. Conventional terminology is recommended for long term (chronic) studies such as 2-year bioassays (carcinogenicity studies). Enhanced terminology is recommended for characterizing immunomodulatory effects in short term studies, especially immunotoxicology studies. The level of detail generated by an Enhanced Histopathology evaluation is generally considered unnecessary or undesirable in chronic studies.
Table 1.
Recommended use of terminologies
| Terminology | Study Type | Examples |
|---|---|---|
| Descriptive | All general toxicity studies (≤ 3 months) except immunotoxicity | Increased cellularity, cortex, thymus |
| Conventional | Chronic toxicity or carcinogenicity studies | Hyperplasia, lymphoid, thymus |
| Enhanced | Immunotoxicity and short term (≤ 3 months) studies | Lymphocytes, increased, cortex, thymus |
The choice of which terminology to use is made at the discretion of the pathologist. Factors to consider include the length of the study, the expectation of an immunomodulatory effect, compliance with regulatory guidelines (e.g. OECD 407), additional studies to be performed, availability of ancillary data (e.g. immunohistochemistry, flow cytometry, anti-drug antibody assessment, concurrent disease processes, etc.) and the questions the study is addressing. Compartment locators and cell type are optional with descriptive and conventional terminology but should always be used with enhanced terminology.15–18,20 Regardless of which terminology is used, it is good practice to evaluate hematolymphoid organs in a compartment-aware manner. When a full Enhanced Histopathology evaluation is performed, the final interpretations and conclusions should be presented within the pathology narrative.
The selection of INHAND terms for the hematolymphoid organs was guided by the knowledge that these organs share similar structural and functional features and react in similar ways to physiological challenges. Terms were chosen that could generally be applied similarly across the organs. Synonyms and other closely related diagnostic terms in current use or of historical significance are listed under Other terms and are provided as a bridge from former diagnostic practices to the current recommended INHAND terminologies. Descriptive terminology and conventional terminology use common base terms that can be augmented with cell type and compartment modifiers when necessary. Enhanced terminology combines information about the process, the cell type(s) involved and the compartment(s) in which the process occurs.
All morphologically distinct areas are referred to as compartments, even when one compartment is nested within another compartment. In the spleen, for example, germinal centers are contained within follicles which are in turn contained within the white pulp. The spleen and lymph node are unique because they each have a non-lymphoid compartment that filters a body fluid; blood is filtered in the red pulp of the spleen and lymph is filtered in the sinuses of the lymph node. Changes in these filtration compartments are presented under the subheadings ‘Red Pulp’ in the spleen and ‘Sinuses and Lymphatics’ in the lymph node. Changes in lymphoid compartments are presented under the subheadings ‘White Pulp (PALS, follicles, germinal centers, marginal zones)’ in the spleen and ‘Cortex, Paracortex and Medullary Cords’ in the lymph node.
Macrophages present unique diagnostic challenges because they phagocytize, degrade and/or store cellular material. These physiological activities produce a wide array of cytoplasmic characteristics. Macrophage cytoplasm may contain apoptotic bodies (tingible body macrophages), erythrocytes (erythrophagocytosis), hemosiderin, lipofuscin, ceroid or other pigments (pigmented macrophages), or vacuoles (vacuolation) as well as granules, crystals, exogenous pigments or other manifestations of ingested xenobiotics. Macrophages can also become enlarged (hypertrophy) and can adhere together in clusters (macrophage aggregates). Macrophages are present in every hematolymphoid compartment but they may be difficult to identify when scattered among dense lymphocyte populations. Some populations are easily recognized, such as those in lymph node sinuses (traditionally referred to as sinus histiocytes). In this document, the term ‘macrophage’ is applied to macrophages in all locations to emphasize the similarity of the cell type across the organs. Because of the inherent variability of macrophages, their diagnoses are provided with a menu of modifiers and locators that can be selected to best describe a particular lesion. Macrophage diagnoses are listed in the General section and some are also listed under specific organs.
Lymphocytes present unique diagnostic challenges because the different lymphocyte subsets are functionally distinct but morphologically similar. They have differing sensitivities to toxicity and they can give rise to different subtypes of lymphomas, but the different lymphocyte subtypes generally cannot be identified in routine H&E slide preparations. Lymphocytes are best distinguished, when necessary, by using immunohistochemistry (IHC) to identify cellular markers (surface, cytoplasmic, nuclear).21 Information about using IHC is included under Diagnostic features for many diagnoses.
Immature lymphocytes (especially double-positive lymphocytes [CD4+/CD8+]) are sensitive to stress because endogenous cortisol triggers them to undergo apoptosis, especially in the thymus. Stress-related changes should be differentiated from immunomodulatory effects based on a combination of clinical signs (such as decreased body weight gain and activity), complete blood count results (increase in circulating neutrophils, decrease in circulating lymphocytes), increase in adrenal gland weight, decrease in thymus weight, decrease in thymic cortical cellularity with associated lymphocyte apoptosis, and changes in spleen and lymph node cellularity.22 Because the hematolymphoid organs and circulating blood cells are intimately intertwined, a complete evaluation of the hematolymphoid organs should always include clinical pathology (hematology) evaluation of the blood.
A background level of immune surveillance and response is always present in the hematolymphoid organs. Increases in cell numbers are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes in these organs do not, therefore, infer pre-neoplastic or pre-cancerous lesions. However, in unusual circumstances of severe or persistent hyperplasia, cell proliferation may increase the risk of neoplastic transformation, presumably due to accumulation of random mutations associated with DNA replication.23 Assessment of a hyperplastic change should include a thorough evaluation of body condition to rule out underlying conditions such as infection and inflammation and should consider whether or not the location, stage of maturation and/or morphology of the cells in question are unusual or unexpected. If there is a concern, clonality studies should be considered. 21
The level of background activity for each strain and group of animals is influenced by nutritional status, antigen load, age, genetics (rodent strain and genetically engineered mice), spontaneous lesions, steroid hormone status and infectious agents (opportunistic, incidental or concurrent). 24–26 As with all screening tests, comparison with concurrent control tissues is critically important in order to establish the range of normal tissue changes for a particular group of animals. It is therefore essential to compare treated animals to concurrent controls to accurately distinguish between background activity and changes attributable to xenobiotics. One recommended method of evaluation is to review all concurrent control tissues to determine the ‘‘range of normal’’ for overall tissue architecture and cellularity within that group of animals. The high-dose group is evaluated next, followed by the low- and mid-dose groups, constantly referring back to tissues from the control group to prevent diagnostic drift. Once all tissues from one lymphoid organ have been reviewed, the evaluation of each of the other organs is done in the same manner. 27 Another acceptable method, following examination of controls and high dose, is to combine slides from all dose groups and controls in a blinded fashion and re-examine all slides to determine if the hypothetical change cleanly segregates into treatment and control groups.
In summary, histopathologic evaluation of the hematolymphoid system requires a mechanistic understanding of normal histology and physiology and a holistic assessment of the entire distributed multi-organ hematolymphoid system. Differentiation and identification of background, individual, local or systemic effects requires accurate description and interpretation of histologic findings in conjunction with ancillary data such as clinical history, clinical pathology, organ weights and gross observations. If available, flow cytometry, immune function assays and anti-drug antibody assessment provide additional valuable ancillary data that may impact interpretation of morphologic assessments. Integration of all available data should result in an interpretive narrative in the written report. The goal of this document is to provide defined sets of terminology to enable clear communication of the histopathologic changes present in hematolymphoid organs.
General
There are a few changes, listed below, that may occur in one or more hematolymphoid organs as part of a localized or systemic condition. To avoid repetition in the individual organ sections, they are described in this section with the most commonly affected lymphoid tissues noted. In some cases, diagnoses along with diagnostic criteria and comments also appear in individual organs. Compartment location of the finding may be important and can be used to modify the process term at the discretion of the pathologist.
Vascular findings such as hemorrhage and inflammation of blood vessels occur in lymphoid tissues but are not included in this document because they are covered in detail in the cardiovascular INHAND document. Congestion, a common finding in the spleen, is included in the spleen section of this document.
des AMYLOID (N) (Figure 3) General hematolymphoid
Figure 3.

Mouse, spleen. Amyloid.
con AMYLOID
enh AMYLOID (indicate compartment)
Species
Mouse; Rat.
Other terms
Amyloidosis; Amyloid accumulation.
Pathogenesis
Deposition of twisted β-pleated sheet fibrils due to abnormal assembly of various proteins.
Diagnostic Features
Dense masses of eosinophilic hyaline material.
Deposits efface normal architecture and cause pressure atrophy.
- Distribution.
- Systemic deposition most common but localized deposits also occur.
- May occur in any tissue.
- Predilection for perivascular distribution.
- Mesenteric lymph node.
- Common site in mice.
- Subcapsular sinus area often affected first with extension to paracortex later.
- Medulla usually not involved.
- Spleen.
- Red pulp can be replaced by amyloid and white pulp may exhibit pressure atrophy.
- Staining properties.
- Congo red - stains pink or red with H&E, shows green birefringence under polarized light.
- Thioflavin T - fluoresces under UV light.
- Crystal violet or methyl violet - metachromasia.
- Electron microscopy.
- In humans, non-branching fibrils with indefinite length and a diameter of about 7.5 to 10 nm.
- In mice, 100A wide, rigid non-branching strands twisted into 2 filaments.
Differential Diagnoses
Deposition of collagen or fibrin:
Negative for Congo red.
Comment
Amyloid is not a chemically distinct entity. In experimental animals, amyloid protein is mostly of the AA-type. Amyloid was a common spontaneous finding in certain strains of mice (CD-1 and C57B6) in the past 28 but the incidence has decreased over time and it now occurs an incidental finding in occasional animals. It is uncommon in BALBC mice and is rarely observed as a spontaneous finding in rats. The kidney, ileum and adrenal gland are most often affected. 28 C57BL6 mice are susceptible to both senile (AApoAll) and secondary amyloid.
des APLASIA/HYPOPLASIA (N) (Figures 4 and 5) General hematolymphoid
Figure 4.

SCID mouse, thymus. Aplasia/hypoplasia.
Figure 5.

SCID mouse, lymph node. Aplasia/hypoplasia.
con APLASIA/HYPOPLASIA
enh APLASIA/HYPOPLASIA
Species
Mouse; Rat.
Synonym
Agenesis
Other terms
Congenital decreased lymphocytes.
Pathogenesis/cell of origin
Loss of specific gene function resulting in lack of normal development.
Diagnostic Features
Complete lack of development of a lymphoid organ.
Absence of tissue or organ.
Differential Diagnoses
Normal development:
Marginal zone is absent in mouse spleen until 4 weeks of age.
Normal aging:
Age-related involution in thymus.
Atrophy:
Loss of lymphocytes due to age, toxicity or disease.
At the gross and subgross level, the entire organ is small compared to concurrent controls.
Decreased lymphocyte cellularity.
Lymphocyte necrosis or apoptosis may be present.
Underlying stromal cells may be more prominent.
Comment
Aplasia of the thymus, spleen and Peyer’s patches has been reported in the mouse. These conditions in the mouse are generally congenital genetic disorders which can arise spontaneously or be found in strains of genetically engineered mice (GEM). Aplasia may be difficult to distinguish morphologically from severe lymphoid hypoplasia and decreased cellularity (atrophy), so age, species, history and changes observed in other tissues should be considered during diagnostic differentiation. If there is a congenital decrease in the development of an organ, then the term hypoplasia may be used.
Aplasia of the thymus occurs in nude mice that are homozygous null for the Foxnu1 gene. These mice either completely lack a thymus or have only severely small cystic thymic remnants. Affected mice are hairless from birth throughout life. Absence of the thymus is due to a failure of the thymus anlage to develop from the ectoderm of the third pharyngeal pouch and from premature degeneration of thymic epithelium in utero 29,30 (In humans with DiGeorge syndrome, the 3rd and 4th pharyngeal pouches fail to develop resulting in absence of the thymus and parathyroid glands). Lack of the thymus in homozygotes leads to many defects in the immune system, including depletion of lymphocytes from thymus-dependent areas of lymph nodes, spleen and Peyer’s patches, a much reduced lymphocyte population composed almost entirely of B cells, very poor response to thymic-dependent antigens, including failure to reject relatively allogeneic and xenogeneic skin and tumor grafts, and increased susceptibility to infection.
Aplasia of the spleen occurs in mice that are either homozygous or heterozygous for the Dh gene. Asplenic mice have enlarged Peyer’s patches and absolute lymphocytosis, granulocytosis and monocytosis and their serum protein concentrations and plasma high-density lipoprotein-cholesterol (HDL) levels are lower than normal. Homozygotes (Dh/Dh) are imperforate and die within 3 days of birth due to associated gastrointestinal anomalies. Heterozygotes (Dh/ +) can live for several months when housed in a specific pathogen free environment.
In the Sharpin null mouse, Peyer’s patch development occurs during embryogenesis but regresses spontaneously after birth resulting in a lack of distinct Peyer’s patches in the small intestine. The spleen, lymph nodes and nasal–associated lymphoid tissues are present but have architectural changes. Serum IgG, IgA and IgE concentrations are significantly decreased while serum IgM is normal. Inflammation involving multiple organs is a common feature in this genetic disorder.
In addition to these spontaneous mutant immune deficient mice, several genetically engineered mouse (GEM) strains are immune deficient such as NSG (NOD scid gamma; Nod. Cg-Prkdcscid IL2rgtm1Wjl/SzJ) 31 and NRG (NOD rag1 gamma; NOD.Cg-Rag1tm1MomIL2rgtm1Wjl/SzJ). 32
Immunodeficient mice (nude, SCID) and rats (nude) have hypocellular follicles and T lymphocyte-dependent areas in spleen and lymph nodes. The marginal zone is retained in nude mice and rats. Neonatal thymectomy of normal rodents does not affect marginal zone lymphocyte colonization.
SCID (Severe Combined Immune Deficient) mice (Prkdcscid/Prkdscid) are deficient for protein kinase enzyme activity involved in DNA repair, a deficiency that affects the function of lymphoid stem cells. Because they cannot generate T and B lymphocytes, SCID mice are lymphopenic and they cannot activate some components of the complement system. They have normal NK cells, macrophages and granulocytes, however. The thymus has a rudimentary medulla and no cortex, the spleen has no follicles and the lymph nodes and Peyer’s patches have undeveloped T zones and B zones. Some ‘leaky’ strains may produce small populations of functional B and/or T cells as they age29,30,33.
des APOPTOSIS, INCREASED, LYMPHOCYTE (N) (Figure 6) General hematolymphoid
Figure 6.
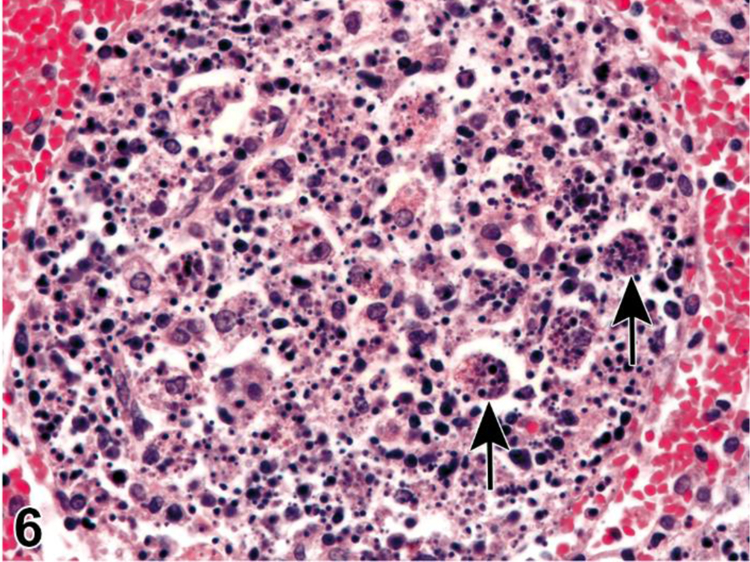
Rat, lymph node, medullary cord. Apoptosis, lymphocyte, increased and tingible body macrophages (arrows), increased.
con APOPTOSIS, INCREASED, LYMPHOCYTE
enh APOPTOSIS, INCREASED, LYMPHOCYTE
(indicate compartment and diagnose decreased lymphocytes, decreased area, tingible body macrophages, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Lymphocyte depletion; Atrophy.
Pathogenesis/cell of origin
Lymphocyte apoptosis may result from direct lymphocyte toxicity or from endogenous factors such as diet or stress (glucocorticoid release).
Diagnostic Features
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Differential Diagnoses
Necrosis, lymphocyte:
Necrotic cells are often contiguous, but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Involution, age-related (thymus):
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells.
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Apoptosis is a coordinated and often energy-dependent mode of cell death that is considered a vital component of various normal processes.34 Apoptosis eliminates activated or auto-aggressive immune cells during maturation, therefore a low level of lymphocyte apoptosis in the thymus is considered within normal physiological variation. Increased lymphocyte apoptosis may result from direct lymphocyte toxicity or from endogenous factors such as diet or stress (glucocorticoid release). Severe ongoing apoptosis results in severe decreased lymphocyte cellularity (lymphoid atrophy). Necrosis may occur together with apoptosis. While it is preferable to identify and record diagnoses of apoptosis and classic necrosis separately, this distinction may not be possible when one type of cell death histologically obscures the other. Also, necrotic cell debris can have some similarities to apoptotic debris, such as pyknosis and karyorrhexis. Apoptosis may predominate with conversion to a necrotic phenotype, or necrosis may predominate with scattered apoptosis. In these cases, it would be appropriate to use both terms together (apoptosis/necrosis) or only diagnose the predominate type of cell death and discuss the presence of the other type of cell death in the narrative.
des CELLULARITY, INCREASED, MAST CELL (N) General Hematolymphoid
conHYPERPLASIA, MAST CELL
enh MAST CELLS, INCREASED (indicate compartment)
Species
Mouse; Rat.
Pathogenesis/cell of origin
Develops from mast cells and their precursors present in the hematopoietic, mucosal and/or connective tissues.
Diagnostic Features
A loosely arranged collection of mature mast cells without nodule formation.
Mast cells are uniform, round or polygonal, medium-sized and well differentiated.
Nuclei are uniformly round but may be obscured by cytoplasmic granules.
Cytoplasm is abundant, granular and slightly to heavily basophilic.
Cytoplasmic granules may or may not be visible with hematoxylin and eosin depending on the type of fixation.
Cytoplasmic granules are metachromatic and generally stain with Giemsa, toluidine blue or other metachromatic stains.
No compression of adjacent tissues.
May involve one or more tissues or organs.
May be reactive to a tumor or associated with other inflammatory cells.
Mitotic figures are not present.
In lymph nodes, mast cells are located predominantly in the sinuses.
Differential Diagnoses
Mast cell tumor, benign:
A single, solitary, compact (dense) mast cell aggregate or nodule.
Compression of adjacent tissue.
Mast cell tumor, malignant:
Compact solitary nodule, local sarcomatous growth or sheet-like accumulation(s) of round, spindle shaped or immature mast cells.
Cytoplasm is often hypogranular, but may have typical basophilic granules.
May have atypical bilobed or polylobed nuclei.
Eosinophils may be associated with the mast cells.
Destructive growth pattern, may be locally infiltrative.
Multiple organs may be involved.
No bone marrow involvement.
No clear inflammatory stimulus.
Considered malignant.
Mast cell leukemia:
Atypical mast cells are present in the bone marrow and/or peripheral blood.
Mast cell accumulations with sheet-like or leukemic pattern present in one or more hematolymphoid organs.
Histiocytic sarcoma:
Nuclei are less regular.
Cytoplasm is eosinophilic.
Negative for metachromatic cytoplasmic granules.
Melanoma, malignant, amelanotic:
Differentiate from mast cells with IHC for expression of melanin (HMB45, PEP8).
Comment
Increased mast cell cellularity may occur in lymphoid, mucosal or connective tissues in response to cytokines associated with parasitic, allergic and other inflammatory lesions. The mast cells are usually mature with many metachromatic granules and do not form nodules. This finding may be seen in some mouse lines as an aging change without obvious cause.
des EXTRAMEDULLARY HEMATOPOIESIS (EMH) (N) (Figures 7 and 8) General hematolymphoid
Figure 7.
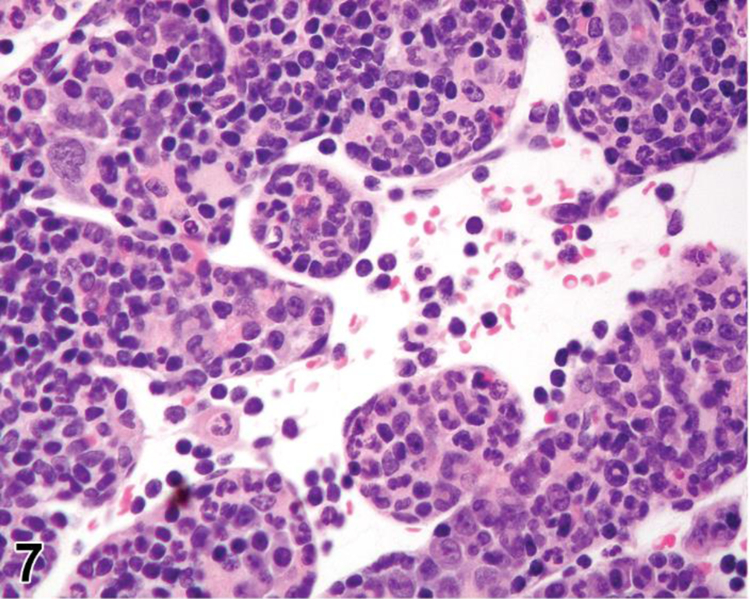
Mouse, lymph node, medullary cords. Extramedullary hematopoiesis.
Figure 8.
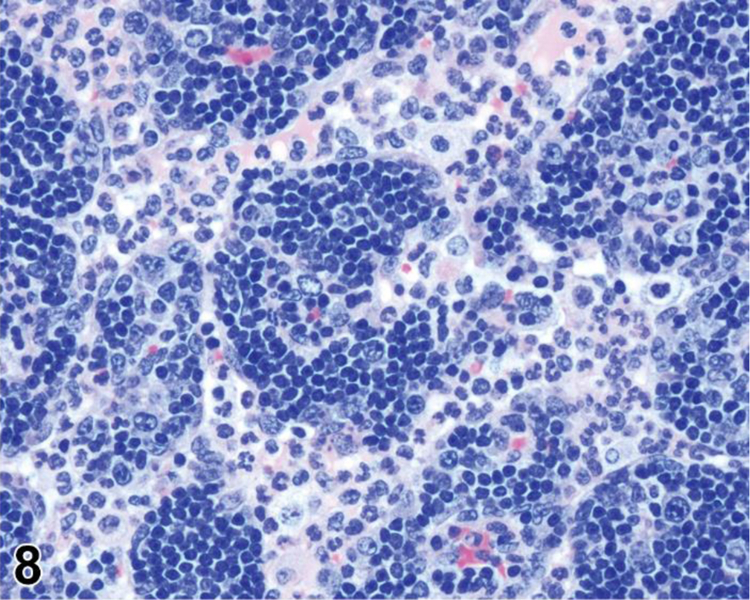
Rat mesenteric lymph node. Draining neutrophils from extranodal inflammation (contrast with extramedullary hematopoiesis).
conEXTRAMEDULLARY HEMATOPOIESIS
enh EXTRAMEDULLARY HEMATOPOIESIS
(indicate organ and compartment)
Modifier
Erythroid; myeloid
Species
Mouse; Rat.
Other terms
Increased hematopoiesis; Red pulp hyperplasia (spleen); Erythroid hyperplasia (lymph nodes, GALT, thymus); Erythropoiesis; Granulopoiesis; Myeloid metaplasia.
Pathogenesis/cell of origin
Circulating hematopoietic progenitor cells from the bone marrow and/or spleen.
Diagnostic Features
Varying proportions of mature and immature forms of myeloid, erythroid and megakaryocytic lineages, depending on etiology.
- EMH sites.
- Medullary cords in lymph nodes.
- Perivascular sites in thymus.
- Sinusoids in liver (refer to the INHAND monograph on the hepatobiliary system, see General introduction, objective and outline).
- Red pulp in spleen.
- EMH is normal in the rodent spleen
- EMH over background levels is diagnosed as EMH, Increased (see Spleen section)
Differential Diagnoses
Infiltrate, neutrophilic:
Infiltration of a relatively pure population of neutrophils into the tissue.
Presence of polymorphonuclear leucocytes but no other histological criteria of inflammation.
Leukemia; myeloid, erythroid or megakaryocytic:
Tumor cells are often all at one stage of differentiation (refer to neoplasia section).
Lymphoma
Distinguished by cell morphology and tissue distribution.
Comment
Extramedullary hematopoiesis is a response to increased hematopoietic demand that occurs in sites outside the bone marrow, such as in lymph nodes, thymus and some non-lymphoid organs, and at increased levels in the spleen. In lymph nodes, EMH shows a preference for the medullary cords. EMH in medullary cords should be distinguished from mature and degenerating neutrophils draining into the sinuses from inflamed tissues. Extramedullary hematopoiesis is commonly seen in the spleen in rodents where it may be recorded as ‘EMH, Increased’ when it is increased above background levels.
des INFILTRATE (indicate modifier) (N) (Figure 9) General hematolymphoid
Figure 9.

Rat, popliteal lymph node. Infiltrate, neutrophilic.
con INFILTRATE (indicate modifier)
enh Indicate cell type(s) present, increased (indicate organ and compartment)
Species
Mouse; Rat.
Modifier
Neutrophil; Eosinophil; Mast cell; Monocyte; Macrophage; Mixed cell.
Pathogenesis/cell of origin
Inflammatory cells from the circulating blood or local tissues.
Diagnostic Features
Infiltration of a relatively pure population of neutrophils, eosinophils, mast cells, macrophages or a mixture of these cell types into the tissue.
Presence of mononuclear or polymorphonuclear leucocytes but no other histological criteria of inflammation.
Differential Diagnoses
Cellularity, increased (cell type):
Increased normal cells with normal maturation in normal locations.
May be expansion of tissue architecture but without degeneration or distortion.
Reflects normal activity of the tissue or organ.
Inflammation:
Infiltrates are associated with degenerative and vascular changes such as necrosis, edema, hemorrhage, congestion and/or fibrosis.
Hematopoietic neoplasia:
Homogenous population of lymphocytes or granulocytes infiltrating the tissue.
Architecture effaced.
Other sites usually involved.
Extramedullary hematopoiesis (emh):
Population of mature/immature hematopoietic cells.
Response to a systemic condition.
Comment
Infiltrating inflammatory cells must be distinguished from hyperplasia of inflammatory cell types arising and maturing normally in hematolymphoid organs. Factors to consider when evaluating the presence of inflammatory cells include the stages of maturation present, the inflammatory cells normally present in the affected organ, inflammation in the local drainage field (for lymph nodes) or systemically (spleen), whether the cells are a pure or mixed population and whether there are degenerative changes present. In the bone marrow, orderly maturation of increased numbers of benign cells in situ is hyperplasia. Infiltrating cells are not associated with significant tissue damage. The term “infiltrate” is preferred over the term “inflammation” when the infiltrating cells are not accompanied by degenerative or vascular changes. The base term “infiltrate” is recommended, followed by the predominant cell type in the infiltrate, or by “mixed cell” if there is not a predominant cell type. Inflammatory changes in adjacent tissues should be considered when assessing infiltrates in lymph nodes. Neutrophils or other inflammatory cells draining through the sinuses and granulopoiesis in medullary cords do not constitute an infiltrate in lymph nodes. Increased lymphocytes in a lymphoid organ are not generally diagnosed as a lymphocyte infiltrate because they are normal constituents of lymphoid organs. Systemic inflammatory conditions should be considered when assessing infiltrates in bone marrow, thymus, lymph nodes and spleen. The choice of terminology should be left to the judgement of the pathologist.
des INFLAMMATION (indicate modifier) (N) (Figures 10 to 17) General hematolymphoid
Figure 10.

Rat, mesenteric lymph node. Inflammation, acute.
Figure 17.

Rat, mesenteric lymph node, medullary cord. Inflammation, granuloma.
con INFLAMMATION (indicate modifier)
enh Indicate cell type(s) present (Indicate organ and compartment
Species
Mouse; Rat.
Other terms
See below for the different types of inflammation.
Modifier
Neutrophil, mononuclear cell, lymphocyte, monocyte, mixed cell, lymphoplasmacytic, pyogranulomatous, granulomatous, acute, subacute, chronic, chronic active
Pathogenesis
See below for the different types of inflammation.
Diagnostic Features
des Inflammation, neutrophil
con Inflammation, acute
enh (indicate cell type(s) and compartment)
Other terms:
Acute lymphadenitis, splenitis, myelitis, etc.; purulent, fibrinopurulent or suppurative inflammation.
Pathogenesis:
Infectious agent or recent tissue damage.
Features:
Neutrophilic cellular infiltrates.
Edema.
Congestion.
Serous or fibrinous eosinophilic exudate.
- Necrosis.
- Increased single cell necrosis/apoptosis in germinal centers.
- Accumulation of karyorrhectic debris.
- Localized foci of necrosis associated with cellular infiltrates.
des Abscess
con Abscess
enh (indicate cell type(s) and compartment)
Other terms:
Purulent inflammation; suppurative inflammation.
Pathogenesis:
Localized neutrophilic inflammation that generally results from bacterial infection.
Features:
Localized focus of neutrophilic infiltrates.
Usually has a necrotic center with abundant karyorrhectic debris due to release of neutrophilic proteolytic enzymes.
Outer rim composed of macrophages, lymphoplasmacytic cells and/or connective tissue, depending on duration of lesion.
Considered acute or chronic, depending on the amount of surrounding connective tissue.
des Inflammation, mononuclear cell, lymphocyte, monocyte, eosinophil, mixed cell, lymphoplasmacytic or pyogranulomatous
con Inflammation, subacute, chronic or chronic active
enh (indicate cell type(s) and compartment)
Other terms:
Non-suppurative lymphadenitis, splenitis, myelitis, etc.
Pathogenesis:
Incomplete resolution of neutrophilic (acute) inflammation or infection with a low-grade infectious agent that is not easily cleared by the immune system.
Features (includes some combination of the following):
Mononuclear cell infiltrates of macrophages and lymphocytes with or without increased plasma cells.
Mixed cell inflammation may include neutrophils and/or eosinophils in addition to mononuclear cells.
Normal architecture is distorted/replaced.
Fibroplasia with or without neovascularization.
Congestion, edema and exudates minimal or absent.
des Inflammation, granulomatous
con Inflammation, granulomatous
enh (indicate cell type(s) and compartment)
Other terms:
Granulomatous lymphadenitis; splenitis; myelitis; etc.; histiocytic inflammation; Potter’s lesion35
Pathogenesis:
Infection or accumulation of a poorly digestible biologic agent or foreign material.
Features:
A chronic inflammatory response characterized by a significant component of activated macrophages, epithelioid cells and/or multinucleate giant cells (Langhans or foreign body types) along with other inflammatory cell types.
Epithelioid macrophages have abundant, pigmented, foamy or vacuolated cytoplasm.
Etiologic agent, e.g. fungi, mycobacteria, or phagocytized foreign material, may be evident in macrophage or giant cell cytoplasm.
May occur as a response to implanted biomaterials.
May exhibit lymphoid hyperplasia adjacent to the infiltrates.
May efface normal tissue architecture.
Can be characterized by additional modifiers as needed, e.g. pyogranulomatous, necrotizing, etc.
des Granuloma
con Granuloma
enh (indicate cell type(s) and compartment)
Other terms:
Granulomata, microgranuloma, pyogranuloma.
Pathogenesis:
A chronic unresolved inflammatory stimulus that isolates or walls off a poorly digestible biologic or infectious agent or foreign material.
Features:
Well demarcated, organized, focal lesion, often small and innocuous.
Often encapsulated by fibroblasts, lymphocytes and plasma cells.
Nodules or aggregates of enlarged macrophages (epithelioid cells) which may have a solid center or a necrotic center composed of cellular debris and/or neutrophils.
Epithelioid macrophages have abundant, pigmented, foamy or vacuolated cytoplasm.
Multinucleated giant cells (Langhans or foreign body types) often present.
May efface normal architecture of tissue.
Etiologic agent, e.g. fungi, mycobacteria, or phagocytized foreign material, may be evident in macrophage or giant cell cytoplasm.
May exhibit lymphoid hyperplasia adjacent to the nodules.
Differential Diagnoses
(for all types of Inflammation)
Infiltrate:
A relatively pure population of neutrophils, eosinophils, mast cells, macrophages or a mixture of these cell types infiltrates the tissue.
Absence of accompanying degenerative or vascular changes.
Necrosis:
Necrosis is the primary diagnosis; inflammation may or may not be present.
Localized foci of necrosis associated with cellular infiltrates.
Increased single cell necrosis/apoptosis in germinal centers with accumulation of karyorrhectic debris.
Absence of organizing macrophages.
Cellularity, increased, plasma cell
Relatively pure population of plasma cells.
Mott cells with Russell bodies may be present.
Typically, but not always, in medullary cords of lymph nodes
Germinal centers may be hypertrophic/hyperplastic.
May be associated with an acute or chronic disease process, including infectious etiology or neoplasm.
Cellularity, increased, macrophage
Increased abundance and/or size of macrophages
Most commonly in splenic red pulp and lymph node sinuses, but may occur in other compartments/organs.
Macrophages are generally individualized and have distinct cell borders.
Cytoplasm may or may not contain phagocytized material, pigment (commonly hemosiderin) or vacuoles.
May be associated with increased filtration and clearance.
Aggregates, macrophage
Adherent macrophages clustered together to form variably sized aggregates.
Macrophages may contain pigment.
Absence of accompanying degenerative or vascular changes.
Most commonly located in the PALS in spleen and medullary cords and paracortex in lymph nodes.
Lymphoplasmacytic or granulocytic neoplasia:
Homogenous population of lymphocytes or granulocytes infiltrating the tissue, effacing its architecture and usually involving other sites.
Atypical cells.
Mitosis may or may not be evident.
Comment
The term “inflammation” is generally accompanied by a modifier that characterizes the histologic features of the finding and is related to the duration of the pathologic process. The characteristics of the inflammation may also be described in the tissue comment, the data table and/or the report text. In the case of an infectious etiology, neutrophils may be intermixed with macrophages in a chronic response and the term “pyogranulomatous” inflammation may be appropriate. The term ‘chronic active’ can be used at the discretion of the pathologist when the histologic features of the inflammation demonstrate variable duration in different areas of the affected tissue. The significance of inflammation in a single site in lymph nodes should always be considered in the context of histologic findings in tissues that the lymph node drains. Transitory intrasinusoidal inflammatory cells originating from a draining site of inflammation must be differentiated from an intrinsic inflammatory process within the lymph node itself and can be indicated by using the modifier ‘reactive’. Abscesses occur occasionally in lymph nodes and GALT and are rarely observed in the spleen. Granulomas in lymph nodes usually occur in the paracortex and medullary cords. Granulomatous inflammation may be observed in response to sutures, catheters, nanoparticles, microspheres and biomedical devices 36 which may involve draining lymph nodes or the spleen (in the case of intravascular injection). The histologic presentation of inflammation can vary greatly and the use of modifiers to characterize it is strongly recommended. The choice of terminology and the decision on whether or not to diagnose should be left to the judgement of the pathologist.
desMETAPLASIA, OSSEOUS (N) (Figure 18) General hematolymphoid
Figure 18.

Mouse, spleen. Osseous metaplasia.
conMETAPLASIA, OSSEOUS
enhMETAPLASIA, OSSEOUS
(indicate organ and compartment)
Species
Mouse; Rat.
Other terms
Heterotopic ossification; Ectopic bone; Metaplastic bone.
Pathogenesis
Bone morphogenetic proteins are thought to stimulate the development of osseous metaplasia in association with focal tissue degeneration and/or neoplastic foci. Osseous metaplasia may also develop from foci of mineralization.
Diagnostic Features
Presence of osteoblasts.
Presence of bony trabeculae derived from a collagenous matrix.
Bone marrow may develop in larger foci of osseous metaplasia.
Differential Diagnoses
Mineralization:
Mineralized foci are amorphous and lack osteoblasts and typical bone structure.
Comment
Osseous metaplasia is an uncommon incidental finding.
desMINERALIZATION (N) (Figure 19) General hematolymphoid
Figure 19.

Rat, thymus, cortex. Mineralization.
conMINERALIZATION
enhMINERALIZATION
(indicate organ and compartment)
Species
Mouse; Rat.
Other terms
Calcification; Mineral deposits.
Pathogenesis
In lymphoid tissues, mineralization is generally dystrophic and occurs as a sequel to tissue degeneration or necrosis.
Diagnostic Features
Basophilic extracellular amorphous granular material and/or lamellated structures.
Rare sporadic finding within lymph nodes, spleen, thymus or Peyer’s Patches.
May be seen in infarcts, germinal center degeneration, paracortical lymphocyte necrosis, granulomas or tumors.
Positive with von Kossa silver method and alizarin red.
Differential Diagnoses
Metaplasia, osseous:
Contains osteoblasts and has typical bone structure.
Comment
Mineralization is rare in lymph nodes but can be seen in the germinal centers of Peyer’s patches, usually as an incidental finding.
des NECROSIS (N) (Figure 20) General hematolymphoid
Figure 20.
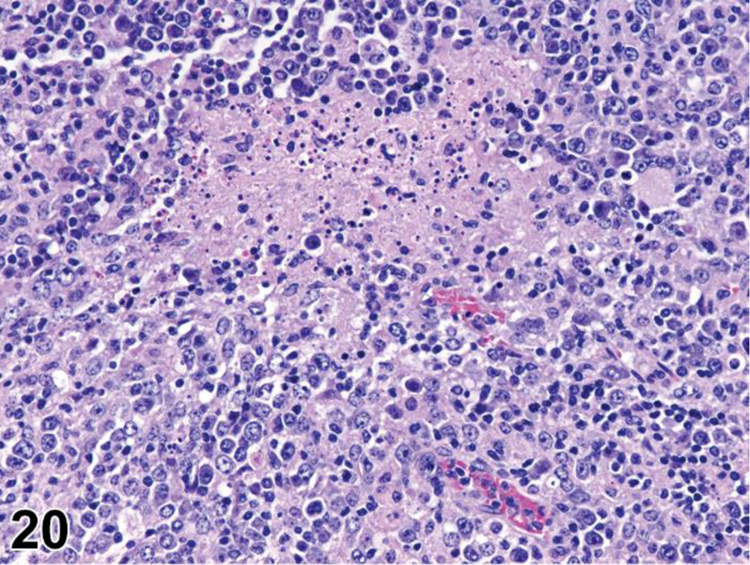
Mouse, mesenteric lymph node. Necrosis, lymphocyte.
con NECROSIS
enh NECROSIS
(indicate compartment and diagnose decreased lymphocytes, decreased area, pigment, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Necrotic cell death; Oncotic necrosis; Lymphocyte depletion.
Modifier
Lymphoid, Lymphocyte.
Pathogenesis/cell of origin
Necrosis can be seen in areas of infarction or as a direct treatment-related effect.
Diagnostic Features
Necrotic cells are often contiguous but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Differential Diagnoses
Apoptosis, lymphocyte, increased:
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Cellularity, decreased, lymphocyte; atrophy, lymphoid; involution, age-related (thymus):
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Lymphocyte necrosis is considered to be the result of a toxic process where the cell is a passive victim and follows an energy-independent mode of cell death. 34 Necrosis in the thymus is generally classic necrosis rather than single cell necrosis. Necrosis in the bone marrow can be seen as a direct treatment-related effect or as a result of ischemia. Ischemic necrosis of the distal femoral epiphysis has been associated with vascular necrosis in mice treated with corticosteroids. 37 Necrotic cell injury is mediated by three main, potentially overlapping, mechanisms: interference with the energy supply of the cell, direct damage to DNA and direct damage to cell membranes. If both necrosis and apoptosis are present, necrosis may predominate with scattered apoptosis or apoptosis may predominate with conversion to a necrotic phenotype. In such cases, necrosis and apoptosis may be diagnosed separately or may be diagnosed together as a single entity (apoptosis/necrosis). Alternatively, the predominant type of cell death can be diagnosed and the presence of the other type of cell death can be discussed in the narrative.
PHOSPHOLIPIDOSIS (N) General hematolymphoid
See Vacuolated Macrophages.
desPIGMENT, MACROPHAGE (N) (Figure 21) General hematolymphoid
Figure 21.

Rat, mesenteric lymph node. Pigment.
conPIGMENT, MACROPHAGE
enh PIGMENTED MACROPHAGES, INCREASED
(indicate organ and compartment)
Species
Mouse; Rat.
Other terms
Pigment deposits; Pigment deposition; Pigment accumulation; Hemosiderosis; Lipofuscinosis; Ceroidosis; Melanosis.
Pathogenesis
Pigments such as hemosiderin (iron derived from degradation of erythrocytes) and lipofuscin and ceroid (degradation products of phospholipid from cell membranes) are phagocytized and stored in macrophages. Melanin may also be an endogenous pigment of pigmented rodents.
Diagnostic Features
Hemosiderin:
Golden brown granular pigment within macrophages.
Positive with Perl’s iron stain or Prussian blue reaction.
Lipofuscin:
Tan to golden brown, may be granular or amorphous within macrophages.
Breakdown products of cell membrane lipids.
Associated with cell turnover, degeneration and/or necrosis.
Positive with Sudan black, Schmorl’s, Oil red O, carbol lipofuscin stain, Periodic Acid-Schiff, lysosomal acid phosphatase, esterase and Ziehl-Neelsen acid fast stains.
Orange auto-fluorescence under ultraviolet light.
Ceroid:
A wax-like, golden, or yellow-brown pigment similar in composition to lipofuscin which is often used in conjunction with the term lipofuscin.
A storage pigment that accumulates along with lipofuscin.
Alcohol-insoluble.
Positive with Sudan black and acid fast stains.
Auto-fluorescence under ultraviolet light.
Melanin:
Minute, rounded, light or dark brown granules.
May be intracytoplasmic or extracellular.
Non-refractile.
Black pigment may be found in the lymph nodes and spleen of pigmented rodents.
Positive with DOPA-oxidase, Fontana-Masson and Schmorl’s stains.
Melanin bleaching can be used for confirmation.
Differential Diagnoses
Test article-associated inert or insoluble pigments
HEMATOIDIN
Yellow, yellow-brown or orange-red refractile (not birefringent) granules 38
Derived from hemoglobin, chemically similar to bilirubin.
Does not contain iron.
Forms intracellularly but may be found extracellularly in areas of previous hemorrhage.
FORMALIN PIGMENT
(acid hematin pigment, acid formaldehyde hematin):
Dark brown extracellular birefringent granules or crystals.
Formed by the action of formaldehyde on hemoglobin.
Forms when aqueous formaldehyde fixitive solution is acidic (pH ≤ 6).
Commonly seen artifact in spleen, bone marrow, lung, liver, blood vessels and areas of hemorrhage.
Comment
Test article-associated pigment must be differentiated from naturally occurring pigments and artifactual pigments. Test article-associated pigments vary widely in color, granularity and refractility. None of the endogenous pigments are anisotropic (birefringent). Lymph nodes associated with the route of exposure should be given special attention. The association of pigmented macrophages in lymph nodes with intra-sinusoidal red blood cells is suggestive of hemosiderin. Hemosiderin may be increased in lymph nodes and thymus in association with hemorrhage. Hemosiderin tends to accumulate in the spleen and bone marrow of aged rodents as iron is recycled during erythropoiesis at these sites. Females tend to have more splenic hemosiderin than males. Hemosiderin in the thymus has been reported in rats fed an iron overload diet. Lymph node melanosis has been reported in transgenic mice with the tyrosine promoter fused to SV40. Pigment from identification tattoos applied to the skin can sometimes be observed in the local draining lymph nodes and is generally not diagnosed.
des TINGIBLE BODY MACROPHAGE, INCREASED (N) General hematolymphoid
con TINGIBLE BODY MACROPHAGE, INCREASED
enhTINGIBLE BODY MACROPHAGE, INCREASED
(indicate compartment, lymphocyte apoptosis, increased/decreased area, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Increased macrophages; Macrophage hyperplasia; Increased histiocytes; Histiocyte hyperplasia.
Pathogenesis/cell of origin
Macrophages engaged in phagocytic clearance of apoptotic cells.
Diagnostic Features
Large macrophages with abundant pale cytoplasm scattered among lymphocytes.
Pale cytoplasm contrasts with basophilic lymphocytes creating a ‘starry sky’ appearance.
- Contain intracytoplasmic apoptotic bodies.
- Darkly stained condensed nuclear material (tingible bodies) from apoptotic lymphocytes.
- Round to oval.
- Variable numbers and sizes.
- May be free or phagocytized depending on duration of process.
Tingible body macrophages increased compared to background levels in controls.
Positive for CD68 and lysozyme.
Differential Diagnoses
Increased cellularity, macrophages:
May be dispersed throughout compartment(s) or occur focally in aggregates.
Do not contain phagocytized apoptotic bodies.
Comment
Increased tingible body macrophages are typically seen anytime there is an increase in lymphocyte apoptosis. The pathogenesis may be treatment related (i.e. dexamethasone) or environmental (i.e. stress related, diet, etc.). The relative proportions of apoptotic lymphocytes, apoptotic bodies (free and phagocytized) and tingible body macrophages and the resulting decrease in lymphocyte cellularity will vary depending on severity and the timing of the insult. This is a temporary condition that generally returns to background levels once excess apoptotic lymphocytes have been cleared, although a severe and sustained insult can result in severe decreased lymphocyte cellularity (lymphoid atrophy).
desVACUOLATION, MACROPHAGE (N) (Figure 22) General hematolymphoid
Figure 22.
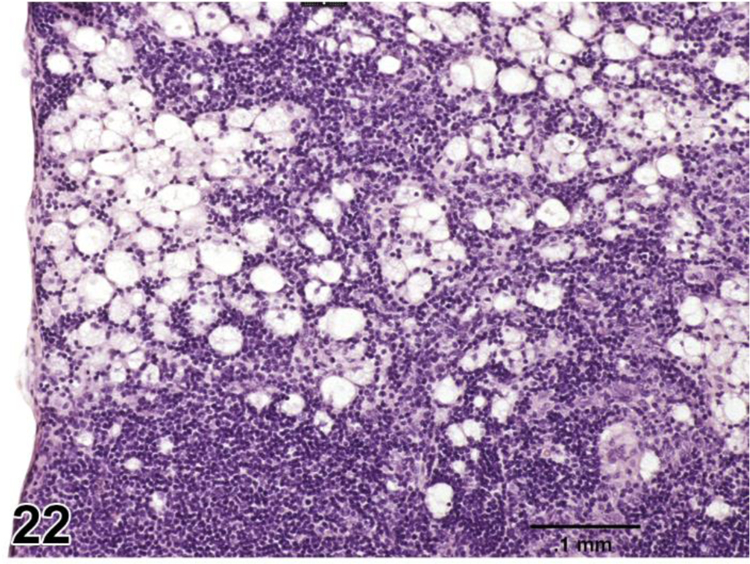
Rat, mesenteric lymph node. Vacuolation, macrophage.
conVACUOLATION, MACROPHAGE
enhVACUOLATION, MACROPHAGE
(indicate organ and compartment)
Species
Mouse; Rat.
Other terms
Foamy macrophages; Cytoplasmic vacuolation; Foam cells; Vacuolated histiocytosis; Vacuolated macrophage hyperplasia; Phospholipidosis.
Pathogenesis/cell of origin
Macrophages develop cytoplasmic vacuoles due to toxic or physiologic effect.
Diagnostic Features
Macrophages have vacuolated cytoplasm.
Vacuoles may be microvesicular, macrovesicular or both.
Can be focal, multifocal or diffuse.
Negative for LAMP-2 immunohistochemistry.
Differential Diagnoses
CELLULARITY, INCREASED, MACROPHAGE
Macrophage cytoplasm is not foamy.
Phospholipidosis:
Foamy macrophages with pale, finely vacuolated cytoplasm and eccentric nuclei.
Tissue architecture is preserved.
Mesenteric lymph nodes are a common site.
Non-lymphoid organs are involved, especially lungs, liver and kidneys.
- Definitive diagnosis can only be made by positive identification of secondary lysosomes.
- Lysosomal inclusion bodies (myeloid bodies) are visible by transmission electron microscopy.
- Lysosomal vacuoles are positive for lysosome-associated membrane protein (LAMP-2) by immunohistochemistry.
Fatty change:
Vacuoles are usually large.
Positive for fat stains.
Negative for LAMP-2 immunohistochemistry.
Erythrophagocytosis:
Phagocytized erythrocytes can have a ghost appearance imparting microvesicular appearance to the macrophages.
Diligent searching should identify pink erythrocytes or nuclei of nucleated erythrocytes within the macrophages.
Genetic storage disease:
Histochemical and IHC stains can be helpful in identifying the content within the vacuoles.
Comment
Cytoplasmic vacuolation occurs in mice with genetic lysosomal storage disorders and in animals exposed to certain xenobiotics. Phospholipidosis is a generalized lysosomal storage disorder induced by a variety of chemicals that interfere with lipid turnover and result in massive phospholipid accumulation in secondary lysosomes (myeloid bodies), particularly in macrophages. Cationic amphiphilic drugs (CAD) that often contain a hydrophilic ring and a hydrophobic side chain with a charged amine group may bind to phospholipids to form complexes that are resistant to degradation by phospholipases or they may inhibit phospholipases directly. Although phospholipidosis most commonly affects tissues with an abundance of macrophages, almost every tissue in the body can be affected. Lymphocytes in the peripheral blood, spleen and lymph nodes can also be affected by lysosomal inclusion bodies (myeloid bodies). Transmission electron microscopy or immunohistochemical staining is necessary to make a definitive diagnosis of phospholipidosis. The diagnosis of ‘vacuolation, macrophage’ can be used when phospholipidosis is suspected but not confirmed, and it can also be used as a descriptive diagnosis when phospholipidosis has been confirmed. In the latter case, positive results can be referenced in the report and the presence of phospholipidosis can be discussed in the text. Vacuolation is used as a base term followed by modifiers as appropriate. 39 Refer to the INHAND monograph on the hepatobiliary system for additional information (see General introduction, objective and outline).
BONE MARROW
Organization
Bone marrow is located within medullary cavities of bone and is considered to be a single compartment. It is variably distributed within the medullary cavity of long and flat bones and makes up approximately 3% of the body weight of adult rats.40 In rodents, it is most prominent and most easily evaluated in sternum, ribs, humerus and femur. The bone marrow is encapsulated by endosteum which lines the irregular scalloped inner surfaces and projecting cancellous bone spicules of the marrow cavities. The endosteum consists of osteoclasts, osteoblasts and flat “bone lining cells” which exert regulatory influence on adjacent hematopoietic cells.
Arteries and veins pierce cortical bone via nutrient canals specific to each bone. In general, the nutrient artery connects with the main central artery and together with the central vein they traverse the central core of the marrow parallel to the axis of the bone. Branching from the central artery are radial arteries that again branch into arterioles that either penetrate the inner surface of cortical bone and drain back into the marrow cavity vasculature or directly anastomose with the extensive venous sinus network. The venous sinus network drains into the central vein before exiting the marrow cavity. Nerves generally follow vascular structures. Bone marrow does not have recognized lymphatic drainage.
Function
Bone marrow is the major tissue for hematopoiesis and is responsible for production of erythrocytes, granulocytes, monocytes, platelets and dendritic cells. It is a primary lymphoid tissue and produces lymphocytes and lymphocyte precursors. The B-lymphocyte precursors migrate and mature in secondary lymphoid organs. The T-lymphocyte precursors migrate to the thymus (a primary lymphoid organ) where they mature and subsequently circulate to secondary lymphoid organs such as the lymph nodes and spleen.
Development
Hematopoietic stem cells (HSC) generate the cellular components of blood throughout the lifespan of the animal. This requires self-renewal and regulated differentiation of multiple cell lineages. Bone marrow serves as the primary microenvironment for this function in post-natal mammals. During embryogenesis in mice, hematopoietic progenitors arise within the extraembryonic yolk sac at ~E8.25 and within the placenta and other sites at ~E10. HSC are present in the fetal liver at day ~E11.0. Shortly before birth, HSC and hematopoietic cells are present in bone marrow. Within the post-natal bone marrow, HSC are closely associated with blood vessels, sinusoidal endothelial cells, perivascular cells and osteoclasts.41 Erythropoiesis, myelopoiesis and generation of platelets from megakaryocytes occurs within the post-natal marrow and blood cells cross the endothelium to get into the blood stream. Lymphocyte progenitors are produced in the marrow and migrate to the thymus and peripheral lymphoid organs. Relatively few mature lymphocytes and plasma cells return to reside in the marrow. In rodents, extramedullary hematopoiesis (EMH) occurs outside of the bone marrow in the spleen and is more pronounced in mice than in rats. In times of strong demand, EMH increases, primarily in the spleen.
Histology
Bone marrow consists of hematopoietic islands and cords enmeshed within a complex network of vascular sinuses supported by stromal cells, reticular fibers and extracellular matrix. Vascular sinuses are lined by endothelium. Adventitial reticular cells ensheath sinus endothelium and branch into the hematopoietic cords along reticular fibers that form the spongiform structural network of the hematopoietic space. 42 Hematopoiesis is a compartmentalized process 40 that is organized into microniches. Hematopoietic stem cells (HSC) are localized around vessels and along bone surfaces in association with osteoclasts. Erythropoiesis is clustered into islets, often in association with macrophages. Granulopoiesis is more diffusely distributed within hematopoietic cords. Lymphocytes and monocytes are aggregated near arterial vessels. Pre-T cells and immature B cells exit the marrow and home to the thymus and secondary lymphoid organs. Megakaryocytes are located adjacent to sinus endothelium. Macrophages, mature B cells and plasma cells are randomly and singly distributed. Adipocytes occur in association with adventitial cells surrounding vascular sinuses. Reticular fibers are composed of various types of collagen. Extracellular matrix is composed of water, salts, glycosaminoglycans and glycoproteins. Hematopoiesis is supported and regulated by soluble factors and cognate interactions. Cells differentiate in situ and then cross the venous sinus endothelium to enter the bloodstream. Platelets enter the blood stream as they are released from cytoplasmic processes of megakaryocytes that extend into the lumens of venous sinuses. Platelets are variable in size in mice which is evident on blood smears and flow cytometry.
Sampling and diagnostic considerations
Bone marrow for microscopic evaluation in rodents is typically collected from sternum, rib, humerus and/or proximal femur. Tissue is processed by standard techniques for H&E stained formalin fixed paraffin embedded decalcified bone. 43 Additionally, marrow casts may be collected from long bones and processed for histology. Bone marrow smears are routinely made for cytology. Histopathologic assessment of H&E stained bone marrow tissue sections is qualitative. Identifiable cellular components of the bone marrow compartment are given in Table 2. 17 General assessment of cellularity (cell density), hematopoietic activity, myeloid to erythroid ratio and orderly progression of maturation of erythrocytes and granulocytes can be made. Megakaryocytes and adipocytes are easily identified. Mature lymphocytes are not easily differentiated from other mononucleated bone marrow cells and therefore are not recommended to be part of the rodent bone marrow evaluation on H&E. Lymphocytes can be identified with immunohistochemistry. Plasma cells cannot be accurately quantified in bone marrow due to low numbers and non-uniform tissue distribution. Visualization of stromal cells and reticular fibers require special stains. Definitive identification of hematopoietic stem cells and specific immature stages of erythroid, myeloid, lymphoid, monocytoid and stromal cells is not routinely possible. 40,44 Pigments and abnormalities such as inflammation, necrosis and neoplasia are discernible. Cytology of Romanowsky stained bone marrow smears is quantitative and is required for definitive assessment of hematopoietic cell differentiation and maturation. Flow cytometry may be used to provide additional characterization of bone marrow subpopulations. Due to inherent variability in bone marrow morphology due to age, strain, sex, environmental and study conditions, i.e. blood collection, evaluation of bone marrow histology requires comparison of treatment groups to concurrent controls of the same anatomic site at the same time point within the same study.
Table 2:
Compartments and cellular components of the bone marrow
| Compartment | Components* |
|---|---|
| Bone Marrow | Myeloid |
| Erythroid | |
| Megakaryocytes | |
| Adipocytes | |
| Reticular adventitial cells | |
| Macrophages | |
| Granulocytes |
Lymphocytes are present but cannot be distinguished in H&E sections
Hematopoietic cellularity of the bone marrow is variable with a relatively wide range of reported values. One study reports approximately 70 – 80% of the marrow in rats and mice is composed of hematopoietic elements and 20 – 30% is composed of adipocytes. 45 In a separate study evaluating Fischer rats, hematopoietic cells varied from 33–88% depending upon the age of the rat and the anatomic site evaluated. 46 Active hematopoiesis in bone marrow continues throughout the lifespan of the rodent. However, hematopoietic cellularity of bone marrow is dependent upon anatomic site, age, sex and strain of the rodent. Cellularity is highest in young animals with modest declines with age. 40 Comparison to age and sex matched controls is therefore essential. The bone marrow is more cellular in the normal healthy mouse compared to the rat, making it difficult to differentiate specific structures, vasculature and cell types. Changes in bone marrow should always be interpreted in context of clinical pathology / hematology evaluation of peripheral blood. Bone marrow is the source of peripheral blood cells and therefore the marrow and the circulating blood are interconnected. Changes to bone marrow should also be interpreted holistically in context with findings in other organ systems, especially in circumstances of inflammation and neoplasia.
Non-proliferative Changes
des ANGIECTASIS (N) (Figure 23) Bone marrow
Figure 23.

Rat, bone marrow. Angiectasis. Abnormally dilated endothelial lined vascular spaces containing red and white blood cells.
con ANGIECTASIS
enh VESSEL DILATATION; SINUSOID DILATATION OR VESSEL/SINUSOID DILATATION
Species
Mouse; Rat.
Other terms
Vascular dilation; Vascular dilatation; Vascular ectasia.
Pathogenesis/cell of origin
Abnormally dilated endothelial lined vascular spaces may be seen with severe loss of hematopoietic tissue or associated with inflammation, neoplasia and vascular or cardiovascular disorders.
Diagnostic Features
Dilatation of bone marrow vessels or sinusoids with blood or serum.
May be diffuse or focal.
Follows vascular patterns throughout the marrow space.
Differential Diagnoses
Hemorrhage:
Abundant mature red blood cells outside of the endothelial-lined vessels.
Increased pigmented macrophages (hemosiderin) suggest chronicity.
Hemangioma:
Well circumscribed mass of dilated irregular endothelial-lined spaces.
Comment
Bone marrow angiectasis is characterized by dilated blood or serum filled vessels/sinusoids that are not increased in number and have normal structure and well-differentiated endothelial cells. If severe, the accumulation of blood within vascular compartments may be confused with hemorrhage where blood is present outside of endothelial lined spaces. Refer to the INHAND document for the circulatory system (see Introduction) for description of generalized changes to vascular structures applicable to bone marrow.
desCELLULARITY, DECREASED, ADIPOCYTE (N) Bone marrow
conATROPHY, ADIPOCYTE
enh ADIPOCYTES, DECREASED
Species
Mouse; Rat.
Other terms
Decreased adipocyte cellularity; Adipocyte hypocellularity; Hypoplasia; Depletion; Fat atrophy.
Pathogenesis/cell of origin
Decreased adipocytes in response to increased metabolic demand, decreased caloric intake or crowding/replacement by increased hematopoietic cells.
Diagnostic Features
Medullary adipocytes (adipocytes in bone marrow) decreased.
Medullary hematopoietic cells may appear increased due to relative decrease of adipocytes.
May be focal or diffuse.
Differential Diagnoses
Serous atrophy of fat:
Adipocytes and hematopoietic cells both decreased.
Bone marrow contains eosinophilic, seromucinous, gelatinous, hyaluronic acid-rich material.
Comment
Medullary adipose tissue can be decreased by crowding, obstruction and/or replacement by increased medullary hematopoietic tissue. A decrease in the relative proportion of adipocytes in the bone marrow is considered an adaptive response to an increase in the relative proportion of hematopoietic cells (increased medullary hematopoiesis). Decreased medullary fat stores may occur with decreased nutritional status. The overall health status of the animal and its systemic fat reserves should be taken into consideration when evaluating decreased adipose cellularity as bone marrow fat reserves are among the last to be mobilized. Decreased adipocyte cellularity should be diagnosed when the primary change is a decrease in adipocytes rather than when adipocytes decrease as a compensatory response to increased hematopoietic tissue. Comparison to concurrent control group animals is recommended.
desCELLULARITY, DECREASED, BONE MARROW (Figures 24 and 25) Bone marrow
Figure 24.
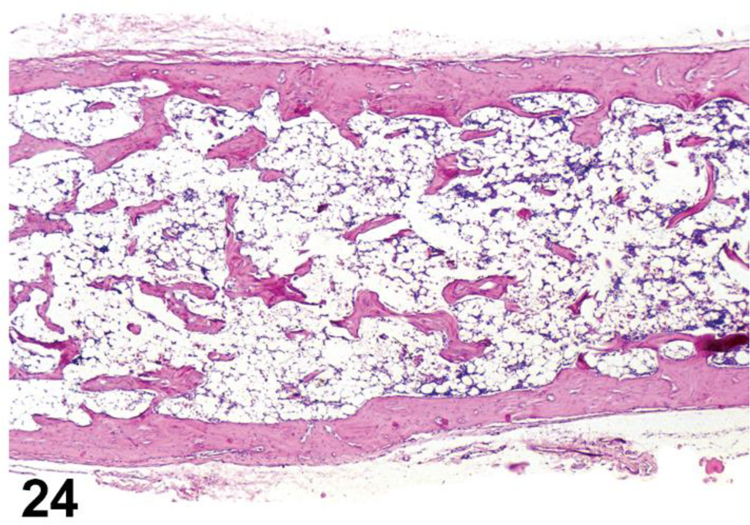
Rat, bone marrow. Cellularity, decreased (atrophy), diffuse. Hematopoietic cells decreased in number and density throughout the entire the medullary cavity.
Figure 25.

Rat, bone marrow. Cellularity, decreased, bone marrow sternum, focal. Hematopoietic cells absent or decreased within a focal area of the medullary cavity. Note prominent stroma and brown pigment.
conATROPHY (N)
enh HEMATOPOIETIC CELLS, DECREASED (Indicate cell type)
Species
Mouse; Rat.
Other terms
Hematopoietic hypocellularity; Hypoplasia; Aplasia; Depletion; Decreased cell numbers.
Modifier
Erythroid; Myeloid (granulocytic, monocytic); Megakaryocytic; NOS.
Pathogenesis/cell of origin
Decreased production or increased destruction of one or more hematopoietic cell lineages can be caused by toxicity, inanition, nutritional deficiencies, irradiation, autoimmune disease, inflammation, neoplasia, infectious agents, genetic defects and the normal aging process.
Diagnostic Features
Reduced hematopoietic cellularity.
Reduced area occupied by hematopoietic cells.
Real or apparent relative increase of adipose tissue, fluid or dilated bone marrow sinuses relative to hematopoietic cells.
Single or multiple cell lineages may be affected.
An entire cell lineage may be absent.
Maturation may be delayed or arrested at a specific stage of development.
Changes to myeloid/erythroid ratio (M:E ratio) may or may not be apparent dependent upon which cell lineages are affected.
Distribution may be focal, multifocal or diffuse.
Decreased cell count of affected cell type(s) may be present in peripheral blood.
Differential Diagnoses
Necrosis:
Decreased cellularity.
Necrotic cells and/or necrotic cellular debris present.
Comment
Cell type modifiers (erythroid, myeloid [granulocytic, monocytic], megakaryocytic) should be applied when decreases can be identified in specific cell populations. Decreased bone marrow cellularity (atrophy) usually occurs diffusely within the bone marrow cavity. Focal or multifocal can be used to characterize the change if it is localized. Atrophy (reduction in size, wasting), hypoplasia (decreased growth) and depletion (loss of cells) describe different dynamic processes that all manifest as fewer than normal numbers of cells in the bone marrow. These conditions may appear similar histologically but they have distinctly different pathogeneses. Use of enhanced terminology is suggested to avoid unintended or unsubstantiated interpretation of similar appearing clinical syndromes. Age-related decrease of hematopoietic cells and replacement by adipose tissue (see Adipocytes, increased) is part of the natural aging process. 47 Comparison of treatment groups to appropriate sex, strain and age matched control groups is essential for assessment of cellularity.
des DYSHEMATOPOIESIS (N) Bone marrow
con DYSHEMATOPOIESIS
enh DYSHEMATOPOIESIS
Species
Mouse; Rat.
Other terms
Altered hematopoiesis; Abnormal maturation; Myelodysplasia; Dysmyelopoiesis; Myeloid dysplasia; Dysgranulopoiesis; Granulocytic dysplasia; Myelomonocytic dysplasia; Dyserythropoiesis; Erythroid dysplasia; Erythrodysplasia; Red cell dysplasia; Thrombodysplasia; Dysthrombopoiesis; Dysmegakaryopoiesis.
Modifier
Erythroid; Myeloid (Granulocytic, Monocytic); Megakaryocytic; NOS.
Pathogenesis/cell of origin
Abnormal or defective differentiation of any of the hematopoietic cell lineages.
Diagnostic Features
Dyshematopoiesis (general):
Failure of normal maturation of erythroid, myeloid and/or megakaryocytic lineages.
Altered cell and/or nuclear size, morphology, nuclear/cytoplasmic ratio, and/or maturation; asynchronous nuclear and cytoplasmic development; lack of stages in maturation series, including early or later stages (maturation arrest).
Myeloid to erythroid (M:E) cell ratio may be altered.
Increased or decreased cellularity may be present.
Increased non-lymphoid immature forms/blasts may be present.
When present, immature forms/blasts are ≤ 20% of cell lineage.
Quantitative assessment best diagnosed by bone marrow smear evaluation or flow cytometry.
Maturation defect of non-lymphoid hematopoietic cells may manifest as cytopenia of one or more non-lymphoid hematopoietic cellular lineages in peripheral blood (e.g., decreased RBCs, neutrophils, and/or platelets).
Abnormal cells may be present in peripheral blood.
Non-hematopoietic tissues are not involved.
Diagnostic features generally best assessed by cytology (bone marrow smears) rather than H&E stained tissue.
Dyshematopoiesis, granulocytic:
Abnormal nuclear segmentation or asynchrony of chromatin maturation and nuclear segmentation.
Altered granule morphology and/or abnormal cytoplasmic features (e.g., size, shape, number, and/or tinctorial quality).
Altered cell and/or nuclear size, morphology, nuclear/cytoplasmic ratio, and/or maturation; asynchronous nuclear and cytoplasmic development; lack of stages in maturation series, including early or later stages (maturation arrest).
Dyshematopoiesis, erythroid:
Multiple or satellite nuclei, or nuclear fragmentation and/or abnormal nuclei shapes.
Abnormal cell size (ie: megaloblasts, abnormal sideroblasts).
Altered cell and/or nuclear size, morphology, nuclear/cytoplasmic ratio, and/or maturation; asynchronous nuclear and cytoplasmic development; lack of stages in maturation series, including early or later stages (maturation arrest).
Asynchronism between nuclear and cytoplasmic maturation.
Dyshematopoiesis, megakaryocytic:
Abnormal megakaryocyte size or nuclear morphology,
Asynchonism between nuclear and cytoplasmic maturation.
Differential Diagnoses
Leukemia; myeloid, erythroid, granulocytic, megakaryocytic, lymphoid:
Leukocytosis in the peripheral blood with immature forms/blasts.
Peripheral blood may contain abnormal megakaryocytes and/or atypical platelets.
Immature non-lymphoid hematopoietic forms/blasts are > 10 to 20% in hematopoietic tissues.
Neoplastic cells are often present in tissues in addition to blood, bone marrow, and spleen. May lead to diffuse leukemic involvement of tissue.
Comment
Dyshematopoiesis may be used as a general term encompassing abnormalities in one or more hematopoietic cell lineages. Dyshematopoiesis may be further characterized as abnormal maturation of specific cell lineages, e.g. erythroid, granulocytic and/or megakaryocytic lineages. Accurate diagnosis requires knowledge of normal maturation stages of all lineages. Dyshematopoiesis is characterized by the presence of precursors or abnormal cells in the absence of normal maturation. Histological evaluation of bone marrow in decalcified bone sections stained with H&E is used as a screening assay. Bone marrow smear evaluation (cytology) is used for quantitative assessment of relative cell numbers and to characterize cell morphology and confirm altered morphology when present. 48 Differentiation of dyshematopoiesis and leukemia can often be made. Dyshematopoiesis may occur de novo or develop secondary to xenobiotics, toxicities and/or irradiation.
Note: Dysplasia is strictly defined as abnormal growth or development. In the bone marrow, the terms dysplasia and myelodysplasia have historically been used to describe abnormal development of hematopoietic cells, specifically of myeloid cells. However, the term myelodysplasia is sometimes used to indicate a preneoplastic condition. While rare, myelodysplasia may be encountered in genetically modified mice. If this diagnosis needs to be used, it must be made in conjunction with clinical pathology data.
des FIBROSIS (N) (Figure 26) Bone marrow
Figure 26.

Rat, bone marrow. Fibrosis. Prominent extracellular matrix composed of collagen and fibroblasts focally displace hematopoietic cells within the medullary cavity.
con FIBROSIS
enh FIBROSIS (indicate reticulin, collagen or NOS if appropriate)
Species
Mouse; Rat.
Other terms
Fibroplasia; Reticular cell hyperplasia; Stromal hyperplasia; Myelopthisis; Myelofibrosis; Scar formation.
Pathogenesis/cell of origin
Fibroblasts, fibrocytes or adventitial reticular cells originating from pluripotential adventitial cells may proliferate in response to altered cytokine expression by resident cells, to inflammation, to injury or secondarily to neoplasia resulting in increased collagen or reticulin within the medullary cavity.
Diagnostic Features
Fibrosis (conventional) and enhFibrosis NOS (Not Otherwise Specified):
Increased extracellular matrix (collagen and/or reticulin) in the medullary cavity.
Increased extracellular matrix may or may not be accompanied by increased cellular elements (fibroblasts, fibrocytes, adventitial reticular cells) depending upon chronicity and activity of the process.
- Increased cellularity of fibroblasts/fibrocytes.
- Fibroblasts/fibrocytes may be morphologically heterogeneous depending on their activity.
- Fibroblasts are activated mesenchymal cells.
- Generally have an irregular or elongated cell body and an elliptical nucleus having two or more nucleoli.
- Synthesize/secrete collagen.
- Fibrocytes are mesenchymal cells not engaged in synthesis of extracellular fibers.
- Generally have less eosinophilic cytoplasm with a smaller, more fusiform cell body.
- Increased cellularity of adventitial reticular cells.
- Line the adventitial surface of vascular sinuses.
- Have pale cytoplasm, round vesicular nuclei and a single nucleolus.
- Synthesize/secrete reticulin.
May be diffuse, multifocal or focal.
Fibrosis, reticulinenh:
A specific term for increased reticular fibers in the medullary cavity.
Increased cellularity of adventitial reticular cells.
Reticular stroma may be more prominent without being increased.
Reticular fibers are difficult to identify in H&E stained tissue.
Reticular fibers stain black with silver stains.
Silver stain is required for definitive diagnosis of reticulin fibrosis.
Reticulin fibrosis may occur independently of, and often precedes, collagen fibrosis.
Fibrosis, collagenenh:
A specific term for increased collagen and reticulin fibers in the medullary cavity.
Increased reticulin fibers are observed concurrently with increased collagen.
Fibroblast and adventitial reticular cells may or may not be increased depending upon chronicity and activity of the process.
Diffuse, multifocal or focal.
Mature collagen stains positively with trichrome stains.
Reticulin fibers stain positively with silver stains.
Special stains are required for definitive diagnosis of collagen fibrosis.
Differential Diagnoses
Fibrous osteodystrophy (FOD):
Fibrosis of the medullary cavity, especially near the endosteal surface of cortical bone.
Associated with chronic renal disease.
Occurs in rats.
Fibro-osseous lesion (FOL):
Fibrovascular tissue replacement of bone marrow.
Often accompanied by lesions of the reproductive tract. 49
Occurs in mice.
Higher indicence in females than males
HYPERPLASIA, OSTEOBLAST
Localized increase in the production of osteoblasts.
Well differentiated localized proliferations of osteoblasts on bone surfaces.
May fill inter-trabecular spaces.
Sometimes admixed with focal fibroplasia.
Decreased Cellularity, bone marrow:
Decreased hematopoietic cellular components may result in increased prominence of stromal elements.
Should be differentiated from true increase in fibroblastic cells.
Comment
Bone marrow fibrosis is characterized as increased collagen and/or reticular fibers with or without proliferation of fibroblasts and adventitial reticular cells. Increased collagen and reticulin are extracellular matrix materials that often, but not always, occur concurrently with increased fibroblasts and/or increased adventitial reticular cells, depending upon the chronicity and activity of the fibrotic process. Differentiation of reticulin fibrosis and collagen fibrosis is encouraged if it would add value to the study. Fibrosis is a component of chronic inflammation but may also be due to perturbation of cytokine production by resident stromal cells including TGF-β, PDGF and other factors associated with megakaryocytes and platelets. 49 Focal fibrosis has been occasionally observed in young or aging rats and may be due to injury, inflammation, or necrosis. 50 Fibrosis has been reported in mice in response to administration of recombinant thrombopoietin. Fibroproliferative responses have been associated with a variety of conditions (e.g., pyruvate kinase deficiency, gamma radiation, drugs, infectious agents and malignancies). In humans, reticulin fibrosis is reported to be reversible with resolution of the inciting cause while collagen fibrosis is less likely to be so. 51 Abnormal bone metabolism may also affect the medullary cavity. For a more complete description of stromal changes associated with altered bone metabolism see the INHAND document on Bone (see I. Bone).
des HYPERSEGMENTATION, GRANULOCYTE (N) Bone marrow
con HYPERSEGMENTATION, GRANULOCYTE
enh HYPERSEGMENTATION, GRANULOCYTE
Species
Mouse; Rat.
Pathogenesis/cell of origin
Granulocytes.
Diagnostic Features
Increased numbers of mature granulocytes with nuclear hypersegmentation.
Hypersegmented granulocytes present in both bone marrow and peripheral blood.
Differential Diagnoses
Dyshematopoeisis:
Abnormal granulocyte precursor cellular morphology exclusive of nuclear hypersegmentation.
Comment
Granulocyte hypersegmentation in bone marrow is characterized by myeloid cells that have megaloblastic features with giant metamyelocytes and some hypersegmented mature cells. In peripheral blood, neutrophil nuclei are hypersegmented with at least six nuclear lobes. Similar changes have been reported in association with a variety of causes including infectious disease, dietary deficiencies, xenobiotics and corticosteroids. Corticosteroids cause retention of granulocytes allowing more time for maturation.
INFLAMMATION (N) (Figure 27) Bone marrow
Figure 27.

Mouse, bone marrow. Granuloma. Lesion comprised of a central zone of epithelioid macrophages, degenerate neutrophils and necrotic debris surrounded by concentric accumulations of mixed mononuclear cells.
See General Hematolymphoid
NECROSIS (N) (Figure 28) Bone marrow
Figure 28.

Rat, bone marrow. Necrosis/apoptosis. Dead and dying hematopoietic cells and amorphous eosinophilic cellular debris. Dying myeloid cells exhibit pyknosis, karyorrhexis and karolysis.
See General Hematolymphoid
desSEROUS ATROPHY OF FAT (N) (Figure 29) Bone marrow
Figure 29.

Rat, bone marrow. Serous atrophy of fat. Hematopoietic cells and adipocytes severely reduced in number with amorphous serous-like eosinophilic extracellular material.
conSEROUS ATROPHY OF FAT
enhSEROUS ATROPHY OF FAT
Species
Mouse; Rat.
Other terms
Gelatinous transformation.
Pathogenesis/cell of origin
Reduction of adipocytes associated with cachexia (e.g. secondary to neoplasia, endocrinopathies) and advanced severe malnutrition (e.g. maldigestion, malabsorption).
Diagnostic Features
Focal or diffuse depletion of adipocytes and hematopoietic cells.
Replacement of adipose tissue by eosinophilic gelatinous tissue.
Accumulation of extracellular gelatinous substances (hyaluronic acid, mucopolysaccharides) that usually stain positive with Alcian blue at pH 2.5.
Differential Diagnoses
Cellularity, decreased, adipocytes:
Decrease in adipocytes in the absence of eosinophilic gelatinous tissue.
Relative increase in hematopoietic cells.
Comment
Serous atrophy of fat is rarely encountered in standard toxicity studies because humane termination occurs at a predetermined degree of weight loss. The pathogenesis remains unclear, but is thought to be a basic bioregulatory process that is activated in states of advanced illness often associated with cachexia and weight loss.
Proliferative Changes (Non-neoplastic)
Hyperplastic changes in all the hematolymphoid organs, including the bone marrow, are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes do not infer preneoplastic or precancerous lesions in these organs (see Introduction). However, severe or persistent lymphoid hyperplasia may increase the risk of neoplastic transformation. If there is a concern, clonality studies should be considered.
desCELLULARITY, INCREASED, ADIPOCYTE (H) (Figure 30) Bone marrow
Figure 30.

Rat, bone marrow. Cellularity, increased, adipocyte. Increased numbers of contiguous adipocytes displace normally abundant hematopoietic cells within the bone marrow medullary cavity. Distinguish from increased prominence of adipocytes due to decreased hematopoietic cells.
conHYPERPLASIA, ADIPOCYTE
enh ADIPOCYTES, INCREASED
Species
Mouse; Rat.
Other terms
Increased adipocyte cellularity; Increased fat; Focal lipomatosis; Adipocyte accumulation.
Pathogenesis/cell of origin
Adipocytes.
Diagnostic Features
Increased number or cell density of adipocytes within medullary cavity.
Focal, multifocal or diffuse.
Differential Diagnoses
Cellularity, decreased, bone marrow
Reduced hematopoietic cellularity.
Focal/multifocal atrophy, hematopoietic cell:
Well delineated area containing reduced hematopoietic tissue.
Comment
Rodents generally have less fat and more hematopoietic elements in their marrow cavities compared to other mammals. Relative fat content of bone marrow varies with species, strain, sex, age, anatomic site and activity of hematopoietic elements. 52 It is generally more physiologically relevant to express changes in the relative proportions of adipocytes and hematopoietic cells in terms of increased or decreased hematopoietic cells. Increased adipocyte cellularity should be diagnosed when the primary change is an increase in adipocytes rather than when adipocytes increase as a compensatory response to decreased hematopoietic tissue. Comparison to concurrent control group animals is recommended.
desCELLULARITY, INCREASED, BONE MARROW (H) (Figures 31 to 33) Bone marrow
Figure 31.
Rat, bone marrow. Cellularity, increased (hyperplasia). Increased numbers and density of cells of undefined hematopoietic lineages fill the medullary cavity.
Figure 33.

Rat, bone marrow. Cellularity, increased, megakaryocyte. Increased number of megakaryocytes surrounded by erythroid cells and fewer immature myeloid cells. A megakaryocyte containing mitotic figures is present in the upper right quadrant of the photomicrograph.
conHYPERPLASIA, BONE MARROW
enh HEMATOPOIETIC CELLS, INCREASED (indicate cell type(s))
Species
Mouse; Rat.
Other terms
Increased hematopoiesis; Hematopoietic hypercellularity; Pan hyperplasia; Plasmacytosis; Reactive plasma cell hyperplasia; Regeneration.
Modifier
Erythroid; Myeloid (granulocytic, monocytic); Megakaryocytic; Lymphocytic; Plasma cell; NOS.
Pathogenesis/cell of origin
Proliferation of hematopoietic progenitor cells of one or more lineages.
Diagnostic Features
Increased hematopoietic cellularity involving single or multiple cell lineages.
Increased area occupied by hematopoietic cells.
Diffuse distribution within medullary cavity.
Morphology and maturation sequences are synchronous.
Altered myeloid/erythroid ratio (M:E ratio) may or may not be apparent dependent upon which cell lineages are affected.
Increased cell count of affected cell type(s) is usually, but not always, present in peripheral blood depending upon chronicity and/or peripheral consumption of the cell type.
Extramedullary hematopoiesis may or may not be present.
Lymphocytes and monocytes cannot be easily distinguished in routine H&E stained tissue sections.
- Plasma cells are rare and cannot reliably be quantified by histology due to limited sampling of tissue sections
- Identify plasma cells by IHC for IRF4 and CD138. 53
- Quantify plasma cells by flow cytometry.
Differential Diagnoses
Hematopoietic neoplasms:
Differentiation of granulocytic hematopoietic hypercellularity from well-differentiated granulocytic leukemia may be challenging.
Both lymphoid and non-lymphoid organs may be involved.
Abnormal forms of progenitor cells may be present.
Immature forms/blasts increased above 20% of a given lineage are suggestive of neoplasia. 54
Quantitative data from bone marrow smears and flow cytometry can help differentiate hypercellularity from hematopoietic neoplasia.
See Hematolymphoid Neoplasms for additional diagnostic criteria.
Dyshematopoiesis:
Lack of maturation.
Lack of normal production.
Need blood smear or bone marrow smear to confirm diagnosis.
Comment
Bone marrow cellularity may vary due to species, strain, sex, age and location (evaluation of both sternal and femoral marrow is recommended for rodents). Cellularity may be assessed by estimating the ratio of hematopoietic cells to medullary adipocytes. With increased bone marrow cellularity (hyperplasia), the proportion of hematopoietic cells is increased relative to adipocytes. Decreased adipocyte cellularity (atrophy) can also result in a similarly altered ratio and should be differentiated from increased hematopoietic cells. Mouse bone marrow generally has less fat and more hematopoietic cells than rat bone marrow, therefore the prominent venous sinusoids become compressed as the hematopoietic tissue expands. 55 Robust normal proliferation of hematopoietic cells may normally expand beyond the medullary cavity along perivascular spaces of nutrient blood vessels and must be differentiated from invasion by neoplastic cells. Multiple mechanisms may result in increased marrow cellularity. For example, increased erythroid cellularity may result from administration of exogenous erythropoietin (or potentially from an erythropoietin producing tumor), from increased endogenous expression of erythropoietin due to anemia from blood collection, hemorrhage or hemolysis or from hypoxia due to chronic heart or lung disease or congenital cyanotic heart defect. Increased myeloid cellularity may result from a variety of factors including excessive loss due to hemorrhage or from increased demand for neutrophils or otherwhite blood cells due to infection/inflammation in peripheral tissues. Increased megakaryocyte cellularity may be due to increased metabolic demand, pregnancy/lactation, endogenous overexpression or exogenous administration of thrombopoietin. 56 Plasma cells are a normal component of bone marrow and may be increased as part of an immune response to an inflammatory condition, infection or neoplasia.
des CELLULARITY, INCREASED, MACROPHAGE (H) (Figure 34) Bone marrow
Figure 34.

Mouse, bone marrow. Cellularity, increased, macrophage. Increased numbers of pigmented macrophages diffusely distributed within the medullary cavity.
conHYPERTROPHY/HYPERPLASIA, MACROPHAGE
enh MACROPHAGES, INCREASED
Species
Mouse; Rat.
Other terms
Macrophage accumulation; Macrophage infiltrate; Macrophage infiltration; Prominent macrophages; Histiocytosis; Histiocytic hyperplasia; Histiocytic infiltrate; Histiocytic aggregates.
Modifier
Tingible body; Pigmented; Vacuolated; Aggregates.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Increased abundance and/or size of macrophages within medullary cavity.
Cytoplasm may or may not contain phagocytized material, pigment or vacuoles.
Differential Diagnoses
GRANULOMA:
Organized structure with a compact collection of epithelioid macrophages or multinucleated giant cells and other inflammatory cells. May variably include necrosis, infectious agents or exogenous materials.
Associated with chronic inflammatory conditions and exposure to xenobiotics.
CELLULARITY, INCREASED, MAST CELL:
Cells have pale basophilic or eosinophilic cytoplasm containing abundant basophilic granules that stain metachromatically with Giemsa or toluidine blue stains.
Cytoplasm is not foamy or vacuolated.
Degranulated or immature mast cells may be difficult to differentiate from macrophages.
Histiocytic sarcoma:
Tumor cells are usually more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Macrophages may increase in response to demand for phagocytosis or in support of erythropoiesis. Increased macrophage cellularity (hypertrophy/hyperplasia) often occurs in combination with phagocytosis, pigment storage, vacuolation and aggregation as macrophages increase to meet the demand driving these processes. The diagnostic terminology for increased cellularity therefore includes these processes as modifiers to allow the pathologist to construct the most appropriate diagnosis for a particular constellation of features. These findings can also be diagnosed separately.
desCELLULARITY, INCREASED, MAST CELL (H) (Figure 35) Bone marrow
Figure 35.
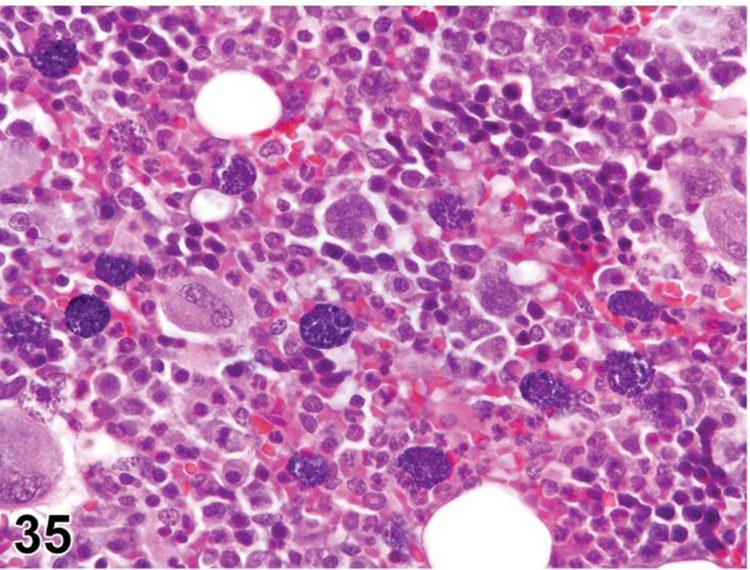
Rat, bone marrow. Cellularity, increased, mast cells. Increased numbers of mast cells diffusely distributed among normal appearing hematopoietic cells and adipocytes. Mast cells contain abundant basophilic cytoplasmic granules (granules are azurophilic with toluidine blue).
conHYPERPLASIA, MAST CELL
enh MAST CELLS, INCREASED
Species
Mouse; Rat.
Other terms
Increased mast cell cellularity; Mast cell infiltrate; Mast cell accumulation; Mastocytosis (see comment).
Pathogenesis/cell of origin
Increased mast cells in bone marrow may be associated with an inflammatory response, parasitism or hematologic diseases.
Diagnostic Features
Multifocal to diffuse increase of loosely arranged mast cells in the bone marrow.
Mast cell granules are metachromatic when stained with toluidine blue or Giemsa.
Mast cells are positive for chloroacetate esterase CAE and c-kit (CD117) by immunohistochemistry staining.
Differential Diagnoses
Mast cell tumor:
Focal nodular increase of mast cells.
Compression and loss of adjacent normal architecture.
Variable degrees of mast cell differentiation.
Often well differentiated in rodents.
See Hematopoietic neoplasia section for additional details.
Mast cell leukemia:
Mast cells present in bone marrow and peripheral blood.
Confirm mast cells and rule out basophils with special stains.
Mast cells are positive for both CAE and c-kit whereas basophils are negative for both stains.53
Comment
Mast cells are more prominent in the medullary cavity of the rat than the mouse. 48 Increased mast cells may represent an exaggerated inflammatory response and have been observed in infection models with or without parasites.
THYMUS
Organization
The thymus is a primary lymphoid organ and specialized gland that is composed of two identical lobes connected by an isthmus. In rats and mice, it is located in the cranial mediastinum in the chest, adjacent to the cranial part of the heart and dorsal to the sternum. A thin connective tissue capsule surrounds each lobe and gives rise to collagenous septa that, in rats, partially subdivides the lobes into lobules of variable size and orientation. Lobulation is absent in mice. Each lobule (or lobe in mice) is composed of a central medulla and peripheral cortex. The thymus is composed of cells of stromal and hematopoietic origin, and includes thymic epithelial cells, neural crest-derived mesenchymal cells, endothelial cells and dendritic cells (Table 3).
Table 3:
Compartments and cellular components of the thymus
| Compartment | Components |
|---|---|
| Subcapsular zone | CD4−/CD8− T cells |
| Epithelial cells | |
| Cortex | CD4+/CD8+ T cells |
| Epithelial cells | |
| Thymic nurse cells | |
| Dendritic cells | |
| Apoptotic cells | |
| Macrophages | |
| Plasma cells | |
| B cells | |
| Medulla | CD4+/CD8+ T cells |
| CD4+/CD8+ T cells | |
| Epithelial cells | |
| Dendritic cells | |
| Apoptotic cells | |
| Macrophages | |
| Hassall’s corpuscles | |
| B lymphocytes | |
| Myoid cells | |
| Neuroendocrine cells | |
| Corticomedullary Junction | Mature and immature T cells |
| B lymphocytes | |
| Plasma cells | |
| Epithelial Free Area (some rat strains only) | CD4+/CD8+ T cells |
Function
As a primary lymphoid organ, the thymus is a site where T lymphocytes (T cells) are formed and mature. T lymphocytes develop from a common lymphoid progenitor in the bone marrow. Those cells that are destined to give rise to T cells leave the bone marrow and migrate to the thymus where they divide and mature to produce T cells. During this process, they complete their antigen-independent maturation into functional naïve T cells. Once mature, the T cells emigrate from the thymus, join the recirculating pool of lymphocytes and begin searching for their cognate antigens in specific compartments of the secondary lymphoid organs, such as the paracortical regions of lymph nodes and the periarteriolar lymphoid sheaths (PALs) of the spleen. At this point, T cells have a critical role in the adaptive immune system, providing an immune response that is highly specific to a particular pathogen. Immunological memory occurs after an initial response to a specific pathogen, leading to an enhanced response to subsequent encounters of that same pathogen.
Development
The embryonic development of the thymus and the parathyroid glands is intimately linked. Both develop from the endodermal gut tube and are derived from outpockets of the most anterior region of the foregut, the pharynx, which is composed of a ventrally located thyroid diverticulum and a series of paired transient outpockets of the lateral foregut called the pharyngeal pouches. Each of the pharyngeal pouches of the third pair of outpockets forms a single epithelial organ primordium surrounded by a mesenchymal capsule. Neural crest cells migrate into the pharyngeal region and surround the third pouch during early development and become the mesenchymal cells that eventually form the thymic mesenchymal capsule and become associated with the thymic vasculature. As the paired primordia detach from the pharynx via apoptosis, they separate into individual bilateral primordial thymus and parathyroid organs while migrating to their final positions in the body. Soon after detachment, the parathyroids remain in the proximity of the thyroid gland. The primordial thymic lobes migrate caudally into the chest cavity and the two lobes meet at the midline, just above the heart. At this early stage, the thymus is composed only of thymic epithelial cells and is devoid of lymphocytes. Beginning at around embryonic day 11.5 in the mouse, bone-marrow derived lymphocyte progenitor cells are attracted to the thymus by factors (chemokines) secreted by the thymic epithelial cells. Lymphocyte progenitor cell immigration occurs at precise stages of organogenesis in successive discrete waves. The initial immigration occurs prior to vascularization of the thymus. In mice, the initial entry of the progenitor cells into the thymic anlage has been shown to be a two-step process. Progenitor cells accumulate in the mesenchymal layer at embryonic day 11 and then enter the epithelial cluster on embryonic day 12. Later, they enter via venules at the corticomedullary junction. Lymphocyte progenitor cells migrate centripetally through the cortex to the subcapsular region where they proliferate as lymphoblasts. Lymphoblasts mature into naïve T cells as they migrate back through the cortex and into the medulla. Lymphocyte progenitor cell entry into the thymus from the bone marrow continues in the postnatal period.
Histology
In the thymus, T cells develop their specific T cell markers, including the T cell receptor (TCR), CD3, CD4, CD8, and CD2. T cell maturation occurs via expression of the T cell receptor (TCR)-CD3 complex and its co-receptors CD4 and CD8 (differentiation antigens). Lymphocyte progenitor cells from the bone marrow enter the thymus at the corticomedullary junction as CD3−/TCR. At this stage they begin to express CD2, have not yet begun to rearrange their TCR genes, and have an immature double negative phenotype (CD4−/CD8−). They migrate to the subcapsular zone of the outer cortex and then mature as they migrate back through the cortex, becoming CD3/TCR+ and CD4/CD8 double positive (CD4+/CD8+), and then migrate to the medulla where they become single positive T cells (CD4+/CD8−, CD4−/CD8+).
As the T cells migrate through the cortex, T cell receptor gene rearrangement occurs and they undergo positive selection. Only the cells able to recognize antigen in the context of class I or class II MHC, expressed by the thymic epithelial cells (thymic nurse cells), will be “positively selected” to survive. Those that are unable to recognize antigen in the context of self-MHC (weakly binding cells) within 3–4 days undergo apoptosis, accounting in part for the apoptotic cells and tingible body macrophages that are scattered throughout the cortex in normal control animals. Those T cells that have a strong or medium binding capacity to either MHC class I/II or peptide molecules will survive. A small degree of negative selection also occurs in the cortex via exposure to self-antigens, allowing for the removal of auto-reactive T cells. To avoid autoimmunity, those T cells with high affinity are eliminated via apoptosis and those with intermediate affinity survive.
The T cells that survive then enter the medulla where there are fewer lymphocytes and relatively more thymic epithelial cells. In the medulla, T cells undergo further negative selection by interacting with thymic dendritic cells. These cells express transcriptional regulators AIRE and FEZ2 and this allows for the transcription of a variety of genes related to the expression of a more complex set of self-antigens than is present in the cortex. As with positive selection, those T cells that don’t survive during negative selection are eliminated via apoptosis. However, this process is not 100% effective so some autoreactive T cells will survive and enter the circulation.
Thymic (Hassall’s) corpuscles are present in the medulla. These structures consist of one or more central granular cells surrounded by concentric layers of epithelial cells. In the rat, they form whorls of flattened cells with central cell debris or concentrically arranged keratin; in mice they are less well defined and do not form central keratin. Thymic corpuscles are remnants of the epithelial tubes that grow out from the third pharyngeal pouches of the embryo to form the thymus. They seem to increase in number during times of increased lymphocyte apoptosis and may have a role in clearing cellular debris.
The thymic epithelial cells are present throughout the thymus and provide a supporting meshwork for lymphocyte migration. In addition to their role in the positive and negative selection process, thymic epithelial cells also secrete hormones such as thymopoietin, thymosin, thymulin and thymic humoral factor that support T cell maturation and enhance T cell function.
Sampling and diagnostic considerations
The thymus should be fixed in 10% formalin and then trimmed along the length of both lobes, to provide standardized longitudinal sections that show all anatomical structures. Cuts should be through the middle of the lobes to allow for evaluation of the largest surface area. The cortex and medulla should be evaluated separately for both conventional and enhanced histopathology. The cortex:medulla ratio can be estimated by determining the average of ratios across multiple lobules.
Because the thymus is sensitive to the effects of stress and aging, it is important to differentiate xenobiotic-induced thymic decreased cellularity (atrophy) from stress-related lymphocyte apoptosis and age-related thymic involution. Because of the effects of aging on the thymus, it is best to conduct enhanced histopathology on short-term studies.
Non-Proliferative Changes
desAPOPTOSIS, INCREASED, LYMPHOCYTE (N) (Figure 36) Thymus
Figure 36.

Sprague Dawley rat, thymus, cortex. Lymphocyte apoptosis with tingible body macrophages (arrows).
conAPOPTOSIS, INCREASED, LYMPHOCYTE
enhAPOPTOSIS, INCREASED, LYMPHOCYTE
(indicate compartment and diagnose decreased lymphocytes, decreased area, tingible body macrophages, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Lymphocyte depletion; Atrophy; Cell death; Single Cell Necrosis
Pathogenesis/cell of origin
Lymphocyte apoptosis may result from direct thymic lymphocyte toxicity or from endogenous factors such as diet or stress (glucocorticoid release).
Diagnostic Features
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Differential Diagnoses
Necrosis, lymphocyte:
Necrotic cells are often contiguous, but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Cellularity, decreased; atrophy:
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells.
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Apoptosis is a coordinated and often energy-dependent mode of cell death that is considered a vital component of various normal processes. Apoptosis eliminates activated or auto-aggressive immune cells during maturation, therefore a low level of lymphocyte apoptosis in the thymus is considered within normal physiological variation. Increased lymphocyte apoptosis may result from direct thymic lymphocyte toxicity or from endogenous factors such as diet or stress (glucocorticoid release). Severe ongoing apoptosis results in severe decreased lymphocyte cellularity (lymphoid atrophy). Necrosis may occur together with apoptosis. While it is preferable to identify and record diagnoses of apoptosis and classic necrosis separately, this distinction may not be possible when one type of cell death histologically obscures the other. Also, necrotic cell debris can have some similarities to apoptotic debris, such as pyknosis and karyorrhexis. Apoptosis may predominate with conversion to a necrotic phenotype, or necrosis may predominate with scattered apoptosis. In these cases, it would be appropriate to use both terms together (apoptosis/necrosis) or only diagnose the predominate type of cell death and discuss the presence of the other type of cell death in the narrative. 34
desCELLULARITY, DECREASED, LYMPHOCYTE (Figure 37) Thymus
Figure 37.

F344 rat, thymus, all compartments. Cellularity decreased (atrophy), lymphocyte. Treatment-related lesion in a subchronic study. Compare to Figure 49, concurrent control.
conATROPHY (N)
enh LYMPHOCYTES, DECREASED
(indicate compartments and diagnose apoptosis, tingible body macrophages, etc. separately if applicable)
Species
Mouse; Rat.
Locators
Cortex; Medulla.
enh Cortex; Medulla; Decreased area; etc.
Other terms
Lymphocyte depletion; Lymphoid depletion; Cortical depletion; Lymphoid atrophy.
Pathogenesis/cell of origin
Decreased lymphocyte cellularity (atrophy) may result from chronic direct thymic lymphocyte toxicity or may be related to endogenous cortisol released in response to stress.
Diagnostic Features
At the gross and subgross level, the entire organ is small compared to concurrent controls.
Decreased lymphocyte cellularity in the cortex and medulla.
Underlying stromal cells may be more prominent.
May have loss of corticomedullary distinction.
May have paler staining cortex due to decreased cellularity and darker staining medulla due to increased cellularity.
Increased lymphocyte apoptosis and/or tingible body macrophages may be present if decreased cellularity (atrophy) is ongoing.
Lymphoid decreased cellularity (atrophy) may be present in other lymphoid organs.
Fat (adipocyte) infiltration is not present.
Finding not present in concurrent controls.
Differential Diagnoses
Necrosis, lymphocyte:
Necrotic cells are often contiguous, but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Apoptosis, increased, lymphocyte:
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Involution, age-related:
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Treatment-related decreased cellularity is a common finding in toxicity studies when doses at or above the maximum tolerated dose (MTD) are used. Treatment-related decreased cellularity can be a direct effect of immunomodulation or a secondary effect of stress. It should not be attributed to stress unless there are other corroborating findings present, such as marked body weight loss, cortical hypertrophy in adrenal glands and effects in other lymphoid organs, such as reduced lymphoid cellularity and decreased germinal centers in lymph nodes and spleen. In chronic studies, decreased lymphocyte cellularity (atrophy) may overlap with age-related involution and distinguishing them can be challenging. Comparison with concurrent controls may help to make this distinction. A short term study evaluated with enhanced histopathology may be necessary to establish the etiology or mechanism with certainty. The term atrophy may be used in chronic studies, but atrophy may also be used in short-term studies if the pathogenesis/mechanism of cellular loss is not needed and enhanced histopathology is not performed.
desCORTICOMEDULLARY RATIO, DECREASED (N) (Figure 38) Thymus
Figure 38.

Rat, thymus cortex and medulla. Corticomedullary ratio, decreased.
conCORTICOMEDULLARY RATIO, DECREASED
enhCORTICOMEDULLARY RATIO, DECREASED
(diagnose lymphocyte apoptosis, necrosis, etc. and increased/decreased area separately if applicable)
Species
Mouse; Rat.
Other terms
Cortical depletion; Atrophy; Physiological involution; Age-related involution.
Pathogenesis/cell of origin
Most commonly results from apoptosis or necrosis in the cortex resulting in cortical lymphocyte depletion while the medulla often remains the same in size and cellularity.
Diagnostic Features
Thymus is grossly smaller (decreased size and weight) than normal or controls.
-
Loss of immature small lymphocytes from cortex.
Apoptosis (focal, multifocal or diffuse) of cortical lymphocytes.
Necrosis (focal, multifocal or diffuse) of cortical lymphocytes.
Medulla is normal in size and cellularity but may appear relatively larger.
Differential Diagnoses
Atrophy:
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells (see related diagnosis of focal B lymphocyte hyperplasia).
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
The corticomedullary ratio is generally determined subjectively by using the average of multiple ratios. The medulla normally occupies about ⅓ of the lobular volume in the adult rodent, so the normal ratio of cortex to medulla is approximately 2:1. However, the ratio may vary by species and strain so comparison should be made to concurrent controls. The ratio can also vary depending on orientation so a standardized trimming procedure should be followed. A decrease in the corticomedullary ratio may be due to either decreased cortical lymphocytes (most commonly) or increased medullary lymphocytes (or both). Dexamethasone and some organotin compounds such as dicotyl tin dichloride (DOTC) and 2-acetyl-4(5)-tetrahydroxybutylimidazole (THI) have been associated with decreased corticomedullary ratios. 57 If both decreased and increased corticomedullary ratios are present, then the term “altered corticomedullary ratio” may be used. 20,58,59
desCORTICOMEDULLARY RATIO, INCREASED (N) (Figure 39) Thymus
Figure 39.

Rat, thymus. Corticomedullary ratio, increased.
conCORTICOMEDULLARY RATIO, INCREASED
enhCORTICOMEDULLARY RATIO, INCREASED
(diagnose lymphocyte apoptosis, necrosis, etc. and increased/decreased area separately if applicable)
Species
Mouse; Rat.
Other terms
Cortical hyperplasia; Medullary depletion; Atrophy.
Pathogenesis/cell of origin
Regeneration of cortex after insult or treatment-related increase in cortical lymphocytes or decrease in medullary lymphocytes, or both.
Diagnostic Features
Cortex larger than normal and/or medulla smaller than normal.
Differential Diagnoses
Lymphoma:
Growth may extend beyond thymus, invade mediastinal fat or disperse throughout the lymphohematopoietic system and to other organs.
May present as a focal lesion in the cortex of one lobe or as diffuse infiltration in both thymic lobes with involvement of mediastinal lymph nodes.
Possesses a more homogeneous cell population, especially of lymphoblasts, compared to the mixture of large and small lymphocytes in lymphoid hyperplasia.
Comment
An increase in the corticomedullary ratio may be due to either increased cortical lymphocytes or decreased medullary lymphocytes (or both). Cyclosporin alters the corticomedullary ratio by increasing lymphocyte cellularity in the medulla so that it takes on the appearance of the cortex (cortification of the medulla). The original corticomedullary border is still located at its original position based on the vasculature. 12 If cortical lymphocytes are increased, lymphoma should be ruled out. If both decreased and increased corticomedullary ratios are present within a study, then the term “altered corticomedullary ratio” may be used. 20,58,59
desCYST, EPITHELIAL (N) (Figure 40 and 41) Thymus
Figure 40.

F344/N rat, thymus. Cyst, epithelial (ultimobranchial).
Figure 41.

F344/N rat, thymus. Cyst, epithelial (ultimobranchial). Higher magnification of Figure 40. Note ciliated epithelial lining (arrows).
conCYST, EPITHELIAL
enhEPITHELIAL CYSTS
(indicate compartment if applicable)
Species
Mouse; Rat.
Other terms
Thymopharyngeal duct remnants; Thymopharyngeal duct cyst; Epithelial cyst; Epidermal cyst.
Pathogenesis/cell of origin
Thymic tubular structures or remnants of thymopharyngeal duct.
Diagnostic Features
Cysts may contain homogeneous eosinophilic material in the lumen.
- Cysts may be congenital, age-related or associated with treatment.
- Congenital cysts arise from the thymopharyngeal duct.
- Generally singular with variable size.
- Lined by ciliated cuboidal epithelium.
- Generally found in the periphery of the thymus but may also be found in the medulla.
- Cysts that are not congenital.
- Generally arise from dilated tubules (see epithelial hyperplasia).
- Lined by non-ciliated epithelium.
- Generally are associated with increased epithelial structures (cords and tubules).
- Are found in the medulla.
- Have cuboidal to columnar epithelium.
- Vary in size.
In aging mice, cysts with or without ciliated epithelium may be found within the medulla.
Differential Diagnoses
Epidermal cyst, squamous:
Adjacent to thymus or within thymic capsule.
Squamous epithelial lining with hair shafts, sebaceous glands, and keratin.
Hyperplasia, epithelial:
Increased epithelial cell component with or without increased cords and tubules.
Cellularity, decreased, lymphocyte::
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells (see B cell lymphocyte hyperplasia).
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Epithelial cysts are a common background finding that can be congenital or may be associated with age-related physiological involution. Congenital epithelial cysts are derived from the thymopharyngeal duct which forms during the development of the thymus from the third pharyngeal pouch. Congenital cysts are common background findings in rodents of all ages. Cysts associated with aging are located in the medulla and are related to epithelial hyperplasia. Epithelial cysts can also be common in young mice from some mouse lineages. An increase in epithelial cysts may be related to treatment. At the pathologist’s discretion, all cysts may be lumped together under the term ‘epithelial cysts’. Thymopharyngeal cysts may also be diagnosed separately. Ultimobranchial cysts should not be diagnosed in the thymus; they are congenital cysts found in the thyroid gland. They are derived from remnants of the ultimobranchial body, an out pocketing of the fourth pharyngeal pouch that combines with the thyroid diverticulum, giving rise to calcitonin-producing C-cells. 50,60,61
desECTOPIC TISSUE, PARATHYROID (N) (Figure 42) Thymus
Figure 42.
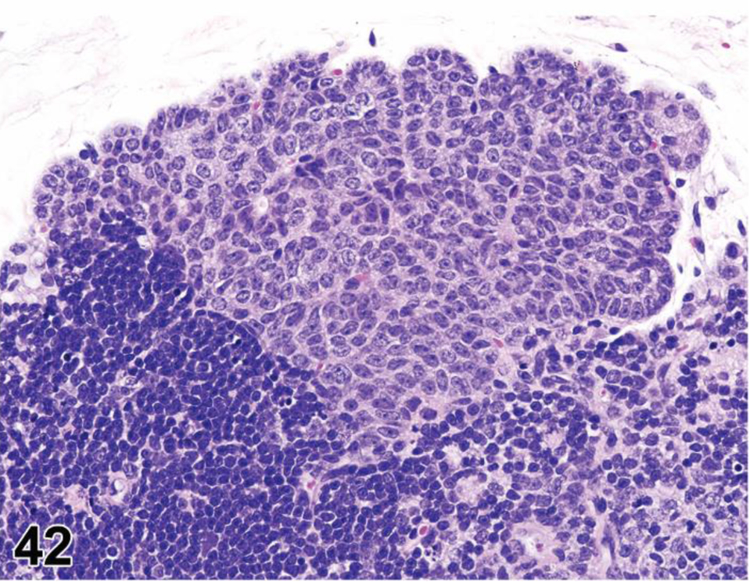
Mouse, thymus. Ectopic tissue, parathyroid gland.
conECTOPIC TISSUE, PARATHYROID
enhECTOPIC TISSUE, PARATHYROID
Species
Mouse; Rat.
Other terms
Accessory parathyroid tissue.
Pathogenesis/cell of origin
Genetic mutation of Hox genes results in aberrant migration of the parathyroid glands during development.
Diagnostic Features
Small focus of endocrine-like cells in the thymic capsule, connective tissue septa, or isolated foci within the thymic parenchyma.
Can occur in the thymus or dorsolateral to the esophagus near the larynx.
More common in mice, especially CD-1 mice.
Differential Diagnoses
None.
Comment
The parathyroid glands and the thymus are both derived from endoderm of the third pharyngeal pouch 62. It has been postulated that ectopic parathyroid tissue may be hormonally active. Small foci of parathyroid chief cells can be observed normal in the mouse thymus. 63,64
desECTOPIC TISSUE, (SPECIFY TISSUE) (N) Thymus
conECTOPIC TISSUE, (SPECIFY TISSUE)
enhECTOPIC TISSUE, (SPECIFY TISSUE)
Species
Mouse; Rat.
Pathogenesis/cell of origin
Pathogenesis and cell of origin varies depending on the type of tissue present.
Diagnostic Features
Other ectopic tissues such as skeletal muscle may be present within, or adjacent to, the thymus.
desECTOPIC TISSUE, THYMUS (N) (Figures 43 and 44) Thymus
Figure 43.

F344/N rat, thyroid. Ectopic tissue, thymus.
Figure 44.

Harlan Sprague Dawley rat, thymus. Ectopic tissue, thyroid.
conECTOPIC TISSUE, THYMUS
enhECTOPIC TISSUE, THYMUS
(indicate location e.g. parathyroid, ectopic tissue, thymus)
Species
Mouse; Rat.
Other terms
Aberrant thymic tissue; Thymic remnant.
Pathogenesis/cell of origin
Genetic mutation of Hox genes results in aberrant migration during development in some cases.
Diagnostic Features
Histologically normal ectopic thymus tissue located in the thyroid and/or parathyroid gland
The ectopic tissue is predominately cortical tissue.
Pale staining clusters of thymic epithelial cells and/or thymic (Hassell’s) corpuscles within the foci.
Differential Diagnoses
Simple lymphoid aggregates:
Aggregates of lymphocytes without structure (i.e. without cortex or medulla).
Lymphocytic thyroiditis:
Presence of interstitial inflammatory lymphocytes within the thyroid.
May include plasma cells.
May be extension of inflammation in adjacent soft tissues or part of a generalized disease.
Comment
Ectopic thymus (remnants of thymus tissue in the neck region near the thyroid gland) is common in rodents. Murine thymic tissue remnants can support T cell differentiation and can export T cells to the periphery. High incidence has been reported in BALB/c, CBA/J and C57BL/6 mice with no elevated incidence of autoimmunity. Prenatal exposure to retinoic acid has been associated with an increase in ‘ectopic’ thymus. 65 No toxicological significance has been attached to this finding. It can be a reason for failure of neonatal thymectomy in immunological studies. 50,62,66–70
desHYPOPLASIA (N) (Figures 45 and 46) Thymus
Figure 45.

SCID mouse, thymus, cortex and medulla. Hypoplasia.
Figure 46.
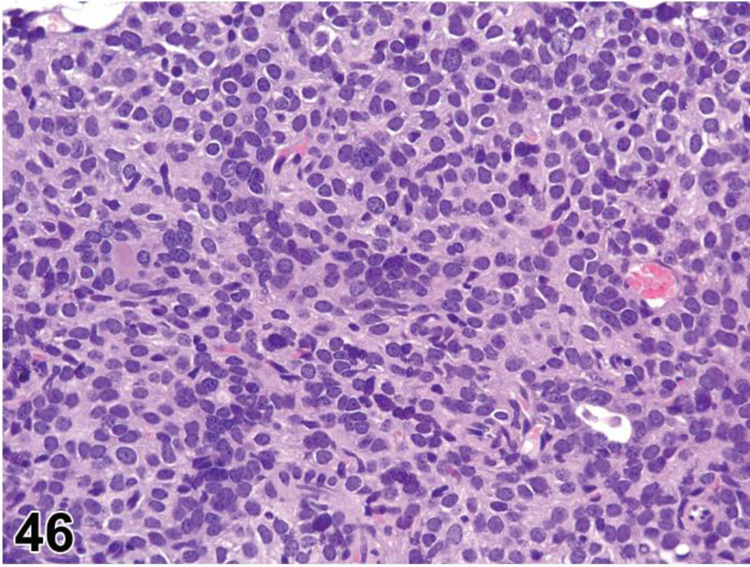
SCID mouse, thymus, cortex and medulla. Hypoplasia. Higher magnification of Figure 45.
conHYPOPLASIA
enh LYMPHOCYTES, DECREASED
(indicate compartments and diagnose decreased area separately if applicable)
Species
Mouse; Rat.
Other terms
Decreased cellularity; Aplasia.
Pathogenesis/cell of origin
Hypoplasia is a congenital condition characterized by underdevelopment or incomplete development of the organ.
Diagnostic Features
Decreased lymphocytes in the cortex and medulla.
Some thymic tissue present but not fully developed.
Differential Diagnoses
Atrophy/involution, age-related:
Thymus tissue may be absent when severe.
Not present in short term study with young animals.
Lymphocyte populations decline gradually with age beginning at puberty.
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Increased foci of B lymphocytes and plasma cells
Cellularity, decreased, lymphocyte:
Decreased lymphocyte cellularity in the cortex and/or medulla.
Lymphocyte necrosis or apoptosis may be present.
Underlying stromal cells may be more prominent.
May have loss of corticomedullary distinction.
Genetic thymic hypoplasia:
- Occurs in nude rat or mouse, SCID mouse, Rag1/2 nulls and other mutant strains.
- In nude rat, thymus is cystic.
- In SCID, Rag and NSG mice, thymus consists predominantly of cytokeratin positive epithelial cells.
aplasia/Hypoplasia:
Congenital absence of thymus tissue.
Comment
Hypoplasia must be distinguished from thymic decreased cellularity (atrophy) y or age-related thymic involution and phenotypes (chemical or genetically engineered models) that result in reduced/negligible thymic tissue.
INFLAMMATION (N) Thymus
See General Hematolymphoid
desINVOLUTION, AGE-RELATED (N) (Figures 47 to 49) Thymus
Figure 47.
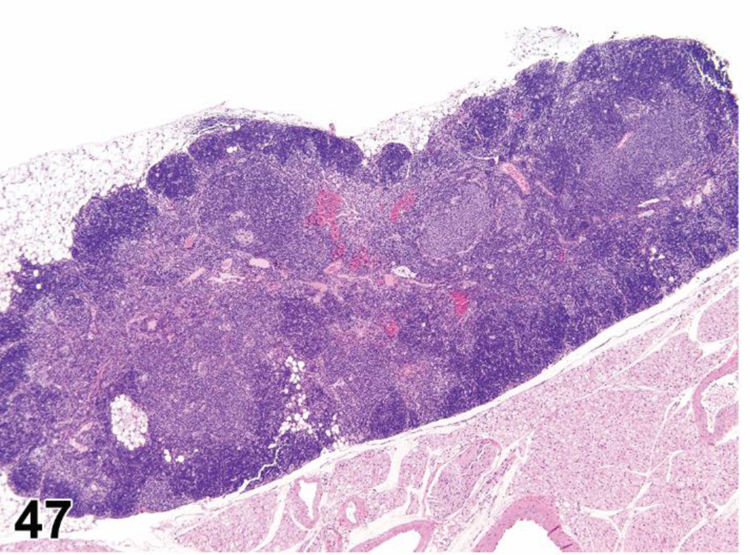
B6C3F1 mouse, thymus, all compartments. Involution, age related, moderate severity.
Figure 49.

F344 rat, thymus, all compartments. Normal control.
conINVOLUTION, AGE-RELATED
enhNot applicable
Species
Mouse; Rat.
Other terms
Physiological involution; Lymphocyte depletion; Lymphocyte atrophy; Aging atrophy; Atrophy.
Pathogenesis/cell of origin
Lymphocyte populations in the thymus gradually decline with age beginning at puberty.
Diagnostic Features
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium (see related diagnosis of epithelial hyperplasia).
Differential Diagnoses
Decreased cellularity (atrophy):
Loss of lymphocytes is often more prominent in cortex.
Treatment-related apoptosis or necrosis of lymphocytes.
Apoptosis, lymphocyte, increased:
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Necrosis, lymphocyte:
Necrotic cells are often contiguous, but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Comment
Age-related involution is characterized by a gradual decline in lymphocyte populations beginning at puberty and continuing with age in association with increased circulating levels of sex steroids. There are some species, strain and sex differences in the evolution of age-related thymus involution. Lymphocyte populations also decline with age in other lymphoid organs. Age-related thymic involution may share similar features with chronic experimentally-induced thymic lesions, such as a reduction in thymus weight and histological depletion of cortical lymphocytes. Since age-related involution can be a confounding factor in chronic studies, careful evaluation dose-relationship including concurrent controls and examination of other lymphoid organsis essential. Sometimes it may be necessary to conduct a short term study to distinguish the two diagnoses. Enhanced histopathology is not recommended for diagnosis of age-related involution in chronic studies since there is generally no need to characterize the finding or explain its mechanism of action. Age-related decline may be reversible; hormonal changes can reconstitute/restore the thymus morphologically and possibly functionally.
desLOSS OF CORTICOMEDULLARY DISTINCTION (N) (Figure 50) Thymus
Figure 50.
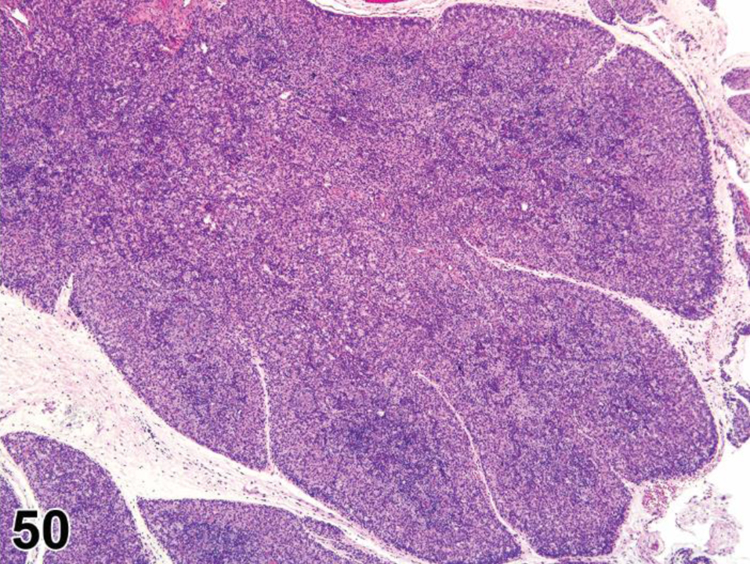
Rat thymus, cortex and medulla. Loss of corticomedullary distinction.
conLOSS OF CORTICOMEDULLARY DISTINCTION
enhLOSS OF CORTICOMEDULLARY DISTINCTION
(diagnose lymphocyte apoptosis, necrosis, etc. and increased/decreased area separately if applicable)
Species
Mouse; Rat.
Other terms
Atrophy; Hyperplasia.
Pathogenesis/cell of origin
Loss of lymphocytes may occur after toxins, irradiation and viral infection or as a congenital change in immunodeficient mice that results in loss of corticomedullary distinction in adult mice.
Diagnostic Features
Decreased cortical and medullary lymphocytes.
Little or no distinction between cortical and medullary cellularity/cell density.
Thymus size and weight grossly decreased.
Differential Diagnoses
Lymphoma:
Early lymphoma may present as diffuse infiltration in both thymic lobes with loss of corticomedullary distinction.
Growth may extend beyond compartments.
Cell population is more homogeneous, especially of lymphoblasts.
Comment
There is normally a clear demarcation between the cortex and medulla which can become obscured by changes in the lymphocyte populations of these compartments. Histologically, there may be a loss of cortical and medullary lymphocytes with a subsequent decrease in compartment areas. Depending on cause and severity, other lesions may be present (see HYPERPLASIA, EPITHELIAL and B cell HYPERPLASIA, LYMPHOID). The development of neoplasia may also result in loss of corticomedullary distinction. Rebound regeneration may be seen after the withdrawal of suppression and is not a pre-neoplastic lesion. Increased medullary lymphocytes can be induced by compounds that inhibit lymphocyte migration. 71
desNECROSIS, LYMPHOCYTE (N) (Figures 51 and 52) Thymus
Figure 51.

Sprague Dawley rat, thymus, cortex. Apoptosis, lymphocyte and necrosis, lymphocyte. Mouse was given cyclophosphamide. Because of the overwhelming apoptosis, there was caspase depletion and the apoptotic phenotype eventually became necrosis. Apoptosis and necrosis may be diagnosed separately or may be diagnosed together as a single entity (apoptosis/necrosis or apoptosis/single cell necrosis).
Figure 52.
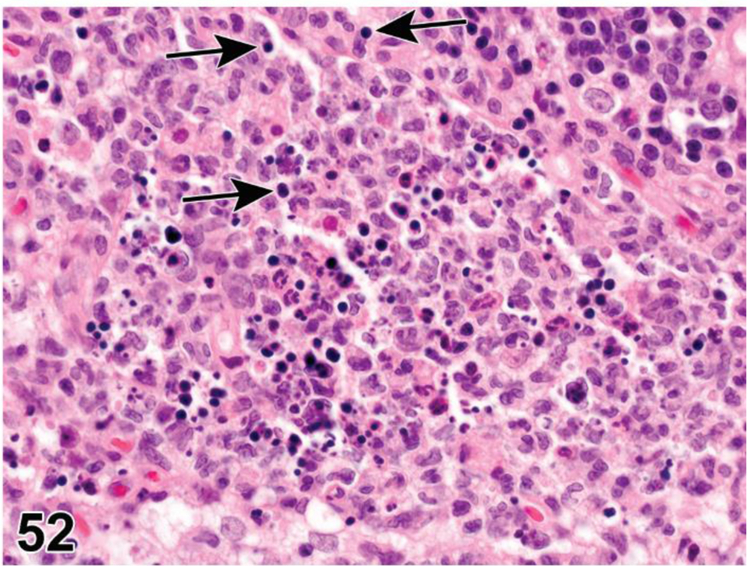
Sprague Dawley rat, thymus, cortex. Necrosis, lymphocyte. Note presence of cell debris, inflammation and minimal apoptosis (arrows).
conNECROSIS, LYMPHOCYTE
enhNECROSIS, LYMPHOCYTE
(indicate compartment and diagnose decreased lymphocytes, decreased area, pigment, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Necrotic cell death; Oncotic necrosis; Lymphocyte depletion.
Pathogenesis/cell of origin
Necrosis can be seen in areas of thymic infarction or as a direct treatment-related effect.
Diagnostic Features
Necrotic cells are often contiguous but pattern can be focal, multifocal or diffuse.
Cell swelling.
Cell rupture.
Karyolysis, pyknosis and karyorrhexis.
Inflammation usually present.
Differential Diagnoses
Apoptosis, increased, lymphocyte:
Single cells or small clusters of cells.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cytoplasm retained in apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis.
Nuclear fragmentation.
Intact cell membrane.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Age-related involution:
Decrease in overall size/weight of thymus.
Decrease in cortical lymphocytes.
Thinning and irregularity of the cortex.
Variable loss of corticomedullary demarcation.
Increase in perivascular spaces.
Increase in foci of B lymphocytes and plasma cells.
Infiltration of adipocytes in connective tissue capsule and septa.
Prominent epithelial cells in the medullary region that may form cords, ribbons, tubules or cysts lined by cuboidal to squamous epithelium.
Comment
Lymphocyte necrosis is considered to be the result of a toxic process where the cell is a passive victim and follows an energy-independent mode of cell death. Necrosis in the thymus is generally classic necrosis rather than single cell necrosis. Necrotic cell injury is mediated by three main, potentially overlapping, mechanisms: interference with the energy supply of the cell, direct damage to DNA and direct damage to cell membranes. If both necrosis and apoptosis are present, necrosis may predominate with scattered apoptosis or apoptosis may predominate with conversion to a necrotic phenotype. In such cases, necrosis and apoptosis may be diagnosed separately or may be diagnosed together as a single entity (apoptosis/necrosis or apoptosis/single cell necrosis). Alternatively, the predominant type of cell death can be diagnosed and the presence of the other type of cell death can be discussed in the narrative. 34
TINGIBLE BODY MACROPHAGE, INCREASED (N) (Figures 53 and 54) Thymus
Figure 53.

Sprague Dawley rat, thymus, cortex. Tingible body macrophages, increased (arrows).
Figure 54.

Sprague Dawley rat, thymus, corticomedullary junction. Tingible body macrophages. Note tingible body macrophages filled with apoptotic cell debris (arrows).
See General Hematolymphoid
Proliferative Changes (Non-Neoplastic)
Hyperplastic changes in all the hematolymphoid organs, including the thymus, are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes do not infer preneoplastic or precancerous lesions in these organs (see Introduction). However, severe or persistent lymphoid hyperplasia may increase the risk of neoplastic transformation. If there is a concern, clonality studies should be considered.
desCELLULARITY, INCREASED, EPITHELIAL CELL (H) (Figures 55 and 56) Thymus
Figure 55.
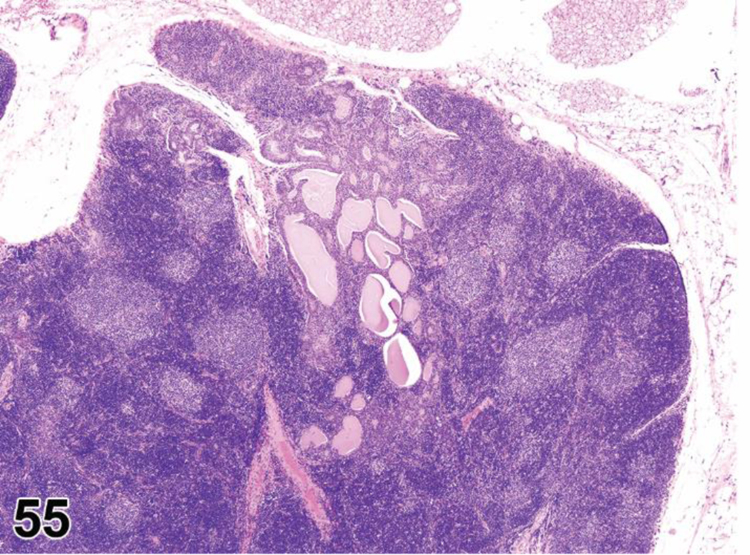
Rat, thymus, medulla. Cellularity increased, epithelial cell, focal. Note presence of cysts.
Figure 56.

Rat, thymus, medulla. Cellularity increased, epithelial cell, focal. Note presence of tubules. Higher magnification of Figure 55.
conHYPERPLASIA, EPITHELIAL
enh EPITHELIAL CELLS, INCREASED, MEDULLA
Species
Mouse; Rat.
Other terms
Hyperplasia, epithelial tubules and cords.
Pathogenesis/cell of origin
Epithelial component of the thymus; increased cords and tubules most likely derived from branchial remnants.
Diagnostic Features
One or more of these epithelial cell components are increased:
- Epithelial cords and tubules.
- Tubules.
- Gland-like structures composed of cuboidal to columnar cells.
- May be partially cystic and filled with a markedly eosinophilic colloid-like material (see Epithelial cysts).
- Lined by non-ciliated epithelial cells (subseptal thymic epithelial cells).
- May respond to hormonal stimulus in older females.
- Squamous metaplasia may be a feature.
- Localized in thymic medulla.
- Frequently located at the mid ventral portion of the thymus.
- Focal or diffuse.
- Pleomorphism may be considerable.
Epithelial pseudofollicles.
Invaginations of superficial epithelial cells into overlying septal connective tissue.
- Thymic (Hassall’s) corpuscles.
- Increased number and size.
- Increased calcification.
- May occur independently from other epithelial changes.
- See diagnosis of Thymic (Hassall’s) corpuscles, increased for additional information.
Differential Diagnoses
Thymoma, benign:
Epithelial component is neoplastic.
Lymphocytes may or may not be neoplastic.
Percentage of lymphocytes can vary.
May have a focal expansile mass of tubules with or without intertubular stroma, central areas of squamous epithelium and/or keratin formation.
May have nodules of keratinized squamous epithelium.
Thymoma, malignant:
May resemble a poorly differentiated carcinoma with sheets of pleomorphic epithelioid cells and scattered poorly formed tubules.
Cysts, epithelial:
Thymopharyngeal duct remnants forming cysts.
Lined by ciliated cuboidal epithelium containing variable amounts of homogenous eosinophilic material.
Comment
A variety of factors (neural, endocrine, growth factors, cytokines and chemokines) modulate the proliferative and secretory activity of medullary thymic epithelial cells (TEC). For example, TECs are stimulated by estrogen and inhibited by testosterone. Age-associated changes in the thymus can result in a disturbance of thymocyte-TEC interactions, resulting in increased proliferative and secretory activity of various epithelial subsets at different time points. There may be a relatively high incidence in some strains of rats, such as the Wistar and BN/Bi rat. In mice, the incidence is relatively high in some strains and rare in others. In mice, epithelial and lymphoid hyperplasia may be present simultaneously. In aged Wistar Hannover rats, a strain prone to developing thymomas, a focal or multifocal lobular hyperplasia has been described which displays a medullary zone and an enlarged and distorted cortical zone. This hyperplasia resembles a thymoma with lobular pattern but shows little or no compression or nodular growth pattern. The size is smaller or equivalent to the transverse axis of normal thymus. 9,72–79
desCELLULARITY, INCREASED, LYMPHOCYTE (H) (Figures 57 to 59) Thymus
Figure 57.

Rat, Thymus, cortex and medulla. Cellularity increased, T lymphocyte, cortex and medulla. Rat was treated with cyclosporine. The original corticomedullary border is still located at its original position based on the vasculature.
Figure 59.
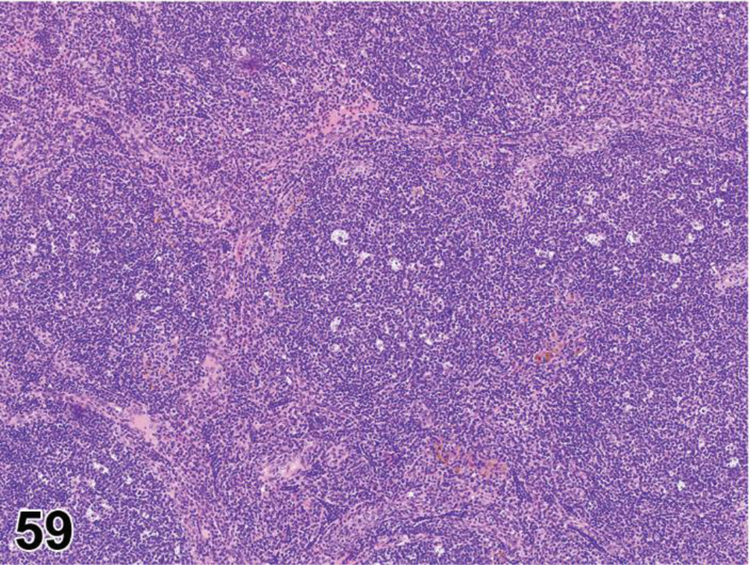
CD1 mouse, thymus, medulla. Cellularity increased, B cell. Note multiple germinal centers with tingible body macrophages.
conHYPERPLASIA, LYMPHOID
enh LYMPHOCYTES, INCREASED
(indicate compartment, increased area if applicable, etc.)
Species
Mouse; Rat.
Other terms
Lymphoid hyperplasia; Atypical hyperplasia; Focal hyperplasia; Nodular hyperplasia; Diffuse hyperplasia.
Modifier
T cell; B cell.
Pathogenesis/cell of origin
Lymphocytes; may be treatment related or associated with age-related involution (medullary B cell hyperplasia).
Diagnostic Features
Cellularity, increased, T cell:
Increased small, hyperchromatic, mostly uniform lymphocytes.
May be present unilaterally (one lobe) or bilaterally (both lobes).
May be present in cortex or medulla.
May be nodular, focal or diffuse.
May diffusely affect one or both compartments.
Normal delineation of compartments is retained.
No obvious proliferation of epithelial cells.
Distinct foci of proliferating subcapsular thymocytes may be present.
Positive for CD3, CD4 and/or CD8 by immunohistochemistry.
Cellularity, increased, B cell:
Increased small, mostly uniform lymphocytes.
Lymphocytes may diffusely infiltrate the medulla or form follicles or follicle-like nodules with or without germinal centers.
No extension beyond the thymic capsule.
Loss of normal corticomedullary demarcation.
Cortex generally shows patchy atrophic changes.
Epithelial and lymphoid hyperplasia may be present together, more so in rats than mice.
In mice, positive for CD45R/B220, CD79b, PAX5, CD79acy by immunohistochemistry (CD19 works in flow cytometry but does not work in formalin fixed paraffin embedded tissue. CD20 does not work in mice).
In rats, positive for CD45R/B220, CD79b CD45 RA and KiB1R by immunohistochemistry.
Differential Diagnoses
Lymphoma:
Growth may extend beyond compartments, invade mediastinal fat or disperse throughout the lymphohematopoietic system and to other organs.
May present as a focal lesion in the cortex of one lobe or as diffuse infiltration in one or both thymic lobes with or without involvement of mediastinal lymph nodes.
Usually lymphoblastic type when only the thymus is involved.
Possesses a more homogeneous cell population, especially of lymphoblasts, compared to the mixture of large and small lymphocytes in lymphoid hyperplasia.
Atypical hyperplasia, focal:
Preneoplastic precursor in treated B6C3F1 mice and p53 knockout mice.
May be unilateral or bilateral.
Diffuse change with loss of corticomedullary distinction.
Sheets of large atypical lymphocytes and fewer admixed small lymphocytes.
Heterogeneous cell population with a variable mitotic index that fails to extend beyond the thymic capsule.
Comment
Increased lymphocyte cellularity (hyperplasia) may be more definitively diagnosed as B or T cell lymphocyte hyperplasia if the identity of the lymphocyte population is known with certainty. Diffusely increased lymphocyte cellularity can be established only by comparison with age-matched controls. Increased cellularity may be induced by growth factors or cytokines. B cell hyperplasia in the medulla is a common component of age-related involution in both rats and mice (female mice may show a higher incidence than males). In some strains of mice (i.e. CD1), this change may be prolific with marked expansion of the medullary area. In old rats, the thymus occasionally is much larger (hyperplastic or persistent) than would be expected given the age of the animal. The architecture of both lobes is relatively normal. Atypical lymphocyte hyperplasia has been diagnosed in some studies where there is a confirmed chemical or genetically induced progression from thymic T cell lymphocyte hyperplasia to thymic lymphoma. The diagnostic term “atypical lymphocyte hyperplasia” has been used for a preneoplastic lesion in male and female haploinsufficient p16(Ink4a)/p19(Arf) mice and p53 (+/−) mice treated with phenolphthalein (NTP 2007, ntp.niehs.nih.gov/testing/types/altmodels/reports/gmm12/index.html, last accessed 8/23/16). This diagnosis should be used at the discretion of the pathologist, depending on the study requirements. 58,80–86
desEPITHELIUM-FREE AREAS, INCREASED (N) (Figures 60 and 61) Thymus
Figure 60.

Rat, thymus, medulla. Epithelium-free zones (arrows).
Figure 61.

Rat, thymus, medulla. Epithelium-free zones. Cytokeratin stain demonstrates absence of epithelium (arrows).
conEPITHELIUM- FREE AREAS, INCREASED
enh LYMPHOCYTES, T CELL, EPITHELIUM-FREE AREAS, INCREASED
Species
Rat.
Other terms
Epithelium-free zone
Pathogenesis/cell of origin
CD4+/CD8+ T lymphocytes.
Diagnostic Features
- Discrete cortical areas of densely packed lymphocytes.
- Higher than normal density of small T lymphocytes.
- No epithelial component.
- No obvious vascularization.
- Extend from capsular surface to deep cortex and often border the medulla (as seen with serial sections).
Populated by CD4+/CD8+ T lymphocytes and fewer TCR α/β and CD5 +/− cells.
High rate of proliferation assessed by BrDU.
Apoptotic cells and tingible body macrophages may be present.
Various macrophage types present (large and round to slightly dendritic or small and dendritic).
- May be difficult to discern with H&E.
- Negative for cytokeratin and laminin (no epithelial component).
- Negative for MHC Class II except for single cells.
Differential Diagnoses
Cellularity, increased, lymphocyte:
Increased amount of small, mostly uniform lymphocytes.
Epithelial cells intermixed, visualized with cytokeratin or laminin.
Comment
The function of epithelium-free areas (EFAs) is unknown. They may represent an alternate intrathymic pathway for T lymphocytes to move from the cortex to the corticomedullary region and medulla while avoiding cortical stromal elements and thus avoid positive/negative selection. They may also serve as reservoirs of lymphocytes. These areas are present in some strains of rats. The occurrence and extent varies between rat strains and by age, with more abundant EFAs in younger rats. There is no apparent difference between sexes. If an increase or decrease in the EFAs is suspected, IHC can be done to more fully characterize the changes. This finding is not a common change. 87
desTHYMIC CORPUSCLES, INCREASED (N) (Figure 62) Thymus
Figure 62.

Sprague Dawley rat, thymus, medulla. Thymic (Hassall’s) corpuscle (arrow).
conTHYMIC CORPUSCLES, HYPERPLASIA
enhTHYMIC CORPUSCLES, INCREASED (indicate medulla)
Species
Mouse; Rat.
Locators
Medulla.
Other terms
Increased Hassall’s bodies.
Pathogenesis/cell of origin
Type VI epithelial reticular cells.
Diagnostic Features
Corpuscle consists of concentric layers of flattened eosinophilic epithelial cells.
Cellular debris and/or keratin may be present in center of corpuscle.
Located in medulla.
Inapparent in some mouse strains.
Differential Diagnoses
None.
Comment
The function of thymic (Hassall’s) corpuscles is currently unclear, but they have been proposed to act in the removal of apoptotic thymocytes and the maturation of developing thymocytes. In rodent studies with a high degree of lymphocyte apoptosis, increased Hassall’s corpuscles with central cell debris may be present. In some mouse strains, Hassall’s corpuscles are inapparent unless there is increased apoptosis. Many studies suggest that these structures are active in antigen expression, cell signaling, transcription, and metabolism mediated by cytokines or growth factor receptors. In humans, they are a potent source of thymic stromal lymphopoietin (TSLP), a cytokine that directs the maturation of dendritic cells in vitro and increases the ability of dendritic cells to convert naive thymocytes to a Foxp3+ regulatory T cell lineage.
SPLEEN
Organization
The spleen is a highly vascular organ that has two grossly visible components, the red pulp and the white pulp. The splenic vasculature is complex and must be fully understood to appreciate normal structure and function as well as pathologic changes. Unlike other organs, medium-sized splenic arteries and veins do not routinely run side by side. Arterial vessels are located predominantly in the white pulp and venous vessels are located in the red pulp. The afferent splenic artery enters along the hilum and gives off central arteries which become ensheathed by white pulp. Each central artery and its accompanying white pulp sheath bifurcates repeatedly while descending from the hilus towards the parietal surface. The central arteries give off arterioles and capillaries which traverse the white pulp and either terminate at the periphery of the white pulp or pass out of the white pulp and terminate in the red pulp. Many of these blood vessels are open ended and release their blood into the reticular meshworks of the marginal zone and red pulp. In the red pulp, venous vessels coalesce into trabecular veins that empty into the splenic vein which exits the spleen along the hilum. Trabeculae are continuous with the splenic capsule; both are composed of fibroelastic tissue and smooth muscle. 88,89
The white pulp is subdivided into morphologically identifiable compartments with distinct cell populations. The central arteries are surrounded by periarterial lymphatic sheathes (PALS) populated primarily by T cells. Primary follicles populated by B cells are located along the PALS. A primary follicle becomes a secondary follicle when it forms a germinal center and a germinal center is considered a compartment in its own right. The marginal zone (MZ) is a distinct layer surrounding the PALS and follicles that is interposed between these compartments and the red pulp. The MZ is sometimes considered a third area along with the white pulp and red pulp. However, for the purposes of this document, the MZ is considered part of the white pulp. 90,91 The large white pulp compartment is therefore composed of four smaller compartments, the PALS, follicles, germinal centers and MZ, which may be evaluated together as a single large compartment (white pulp) or evaluated as separate smaller compartments using enhanced histopathology. 88 Table 4 indicates the various compartments and cellular components of the spleen.
Table 4:
Compartments and cellular components of the spleen
| Compartment | Components |
|---|---|
| Periarteriolar lymphoid sheath | Dominant T lymphocyte area |
| - T lymphocytes | |
| - B lymphocytes | |
| - Fibroblastic reticular cells (FRCs) | |
| - Interdigitating dendritic cells | |
| Follicle | Dominant B lymphocyte area |
| - B lymphocytes | |
| - Follicular dendritic cells (FDCs) | |
| Germinal center | Dominant B lymphocyte area |
| - B lymphocytes in several stages of development and maturation | |
| - T lymphocytes | |
| - Follicular dendritic cells (FDCs) | |
| - Tingible body macrophages | |
| Marginal Zones | - Marginal zone B cells |
| - Marginal zone macrophages (MZMs) | |
| - Marginal metallophilic macrophages (MMMs) | |
| - Fibroblastic reticular cells (FRCs) | |
| - T lymphocytes | |
| Red pulp | - Red pulp macrophages |
| - Red blood cells | |
| - Hematopoietic cells | |
| - Lymphocytes | |
| - Plasma cells | |
| - Fibroblastic reticular cells (FRCs) |
Function
The white pulp compartments serve as part of both the adaptive and the innate arms of the immune system. In mice and rats, the white pulp is composed of T-cell zones (the PALS) and two different B-cell zones (the follicles and the MZ). The B cells associated with the follicle and the marginal zone belong to the B-2 cell lineage. The T- and B-cell zones of the PALS and follicle respectively are involved with T cell-dependent adaptive immunity. In addition to B-2 cells, the marginal zone also has specific populations of macrophages and thus it is involved with both innate T cell-independent and adaptive T-cell-dependent immunity. The red pulp serves as a filter for the blood and is an important site of innate immunity by virtue of its population of red pulp macrophages. These macrophages clear aged and defective red blood cells and pathogens and store and recirculate iron. The splenic red pulp is also a normal site of low levels of hematopoiesis in the rat and mouse. 92–94
Development
In both the mouse and the rat, the spleen first develops as a composite of splenopancreatic mesenchyme around embryonic day (E) 10.5–11and separates from the pancreas around E12.5–13. Erythroblasts and F4/80 positive monocytes/macrophages are first detected in the fetal spleen around E12 and lymphoid progenitor cells are evident around E12.5–13.5. 95,96 Hematopoiesis occurs in the spleen around E14.5 before there is hematopoietic activity in the fetal bone marrow. Compartmentalization of the spleen into marginal zone, follicle and periarteriolar lymphoid sheaths (PALS) does not develop until after birth. Although T lymphocytes are localized around centroarterioles at birth, marginal zones (MZ) and (PALS) do not begin to develop until postnatal day (PND) 5 and PND 7 respectively. In the mouse, MZs and PALS are not fully developed until 3–4 weeks of age and mature follicles form around 6 weeks of age 97. Leukocytes are the most abundant cell population in the spleen during the first 4–5 days after birth. At PND 6, a massive accumulation of nucleated immature erythroid cells take place. This erythroid population remains the most dominant population in the neonatal spleen up to three weeks of age. 98
Histology
Blood released from capillary and arterial terminations deposit lymphocytes into the open circulation of the red pulp and marginal zone. Lymphocytes actively make their way back into the white pulp where they are directed to specific compartments by fibroblastic reticular cells (FRCs). 2 These stromal cells and their reticular fibers form a reticular meshwork that subdivides the white pulp into compartments. FRCs secrete specific chemokines that attract T and B cells to their respective compartments. Chemokines CXCL19 and CCL21 produced by stromal cells in the T-cell zone are crucial for attraction and retention of T cells and interdigitating dendritic cells (IDC). The T cell zone is composed of CD4+ and CD8+ T cells (CD4>CD8), IDCs and migrating B cells. In the T cell zone, T cells search for their cognate antigens by interacting with IDCs and migrating antigen presenting B cells. In follicular B-cell zones, follicles are composed primarily of naïve B cells, a few follicular dendritic cells (FDC) and a few CD4+ T cells. Chemokine CXCL13 is produced by the CD21+/CD35+ follicular dendritic cells and stromal cells of the follicular B-zone. This chemokine is required for B-cells to migrate to the B-cell follicles. Larger B cells are found in the center of the primary follicles and are surround by smaller lymphocytes. This difference in lymphocyte size is difficult to appreciate by H&E. B cells interact with follicular dendritic cells in their search for their cognate antigen. Follicular dendritic cells may collect soluble antigens from the FRC conduit system and present them to B cells. 99 Positive identification of a cognate antigen initiates primary follicular cell differentiation, clonal expansion and development of a germinal center (secondary follicle). Germinal centers are composed of centroblasts and centrocytes with subsequent differentiation into plasma cells and memory B-cells and the production of T-cell dependent humoral antibody. Upon plasma cell differentiation, the plasma cells migrate from the follicle through the marginal zone into the red pulp where they reside, often in close association with veins which carry their antibodies to the systemic circulation. Some long lived plasma cells migrate to the bone marrow where they reside for months. 89
The reticular meshwork of the marginal zone is populated predominantly by specific subsets of B cells and macrophages. Marginal zone B cells express IgM, CD45R/B220, CD1dhi and PAX5 but are negative for IgD. Marginal zone macrophages (MZM) express CD209b+ (SIGN-R1) and marginal metallophilic macrophages (MMM) line the marginal sinus and express CD169. These macrophages clear particulate pathogens and endogenous apoptotic debris from blood as it passes through the marginal zone and marginal sinuses. Loss of MZMs and MMMs will lead to marginal zone B cell loss and impaired trapping of particulate antigens. 91–93 Commonly, with marginal zone B cell hyperplasia, germinal center hyperplasia is concurrently present.
Marginal zones generally do not fully develop in mice until 3–4 weeks of age. The marginal zone is more prominent and larger in rats than in most mouse strains. Generally, the marginal zone in mice is 3 to 5 cells wide, but the width varies with strain and age. Marginal zone B cells and MZMs decrease in number as mice age. Mice 18–24 mo old have fewer marginal zone B cells and MZMs than mice <6 mo old.100 In aged mice MMMs diffuse into the white pulp follicle.100
The red pulp is composed of a large number of venous sinusoids (rat) or venules (mouse) interspersed with pulp cords (Cords of Billroth). The reticular meshwork in the pulp cords is populated by red pulp macrophages, hematopoietic cells and blood cells, predominantly red blood cells that are undergoing filtration. Blood released from capillary and arterial terminations enter the open circulation of the spleen. Blood cells move slowly through interstices of the marginal zone (MZ) and/or the splenic cords of the red pulp (Cords of Billroth) and then return to the closed circulation by passing through interendothelial slits in the walls of venous sinuses (rats) or pulp veins (mice). The rodent splenic red pulp is a normal site of extramedullary hematopoiesis and generally contains low numbers of myeloid, erythroid and megakaryocytic cells. In reactive and neoplastic conditions, one or more of these lineages may increase in volume substantially causing enlargement of the spleen. The mouse spleen is also reported to store half the body’s monocytes. 101 In addition to macrophages, the red pulp cords also contain scattered lymphoid cells consisting predominantly of CD8+ T-cells and occasional plasmablasts and plasma cells. The sinusoids predominantly contain red blood cells. Their endothelial cells express both phagocytic and vascular antigens, but unlike human sinus endothelial cells, they do not express CD8. Pigments (hemosiderin, ceroid, lipofuscin and melanin) may be present in both the cord macrophages and the phagocytic sinusoidal endothelial cells. The splenic capsule is composed of fibrous tissue, elastic fibers and a small amount of smooth muscle with a thin overlying layer of mesothelium.
Sampling and Diagnostic Considerations
A single cross-section of the spleen is generally used for routine histopathological assessment and is adequate for an evaluation of the red pulp and assessment of splenic enlargement. However, a splenic cross-section may not provide sufficient white pulp to render an adequate diagnosis. A longitudinal-section of the spleen will generally have an adequate amount of white pulp on which to base a diagnosis. Due to branching of the PALS as they course from hilar to parietal areas, the size of the PALS will vary with the depth of the longitudinal section. 102 Therefore, it is important to routinely sample cross sections and/or longitudinal sections consistently. 102,103
The white pulp compartments, red pulp and cellular components of the spleen may react individually or collectively in response to insults to cells or tissue. Optimal interpretation of a spleen is best done by reviewing the whole tissue section at low magnification in order to observe the anatomic compartments and to compare the compartments in treated tissue with those observed in normal or control tissue. 103 While clear diagnoses may be evident at low magnification for normal tissues, hyperplastic changes and neoplasia, some lesions will require evaluation of cytological detail at high magnification. Proliferative splenic lesions can involve the white and/or the red pulp and it can sometimes be difficult to differentiate reactive proliferations from lymphoma and/or leukemia. In making the differentiation between reactive and neoplastic proliferations, it is essential to interpret the changes in the spleen in conjunction with clinical history (age, sex, drug treatment, etc.), clinical pathological data and lesions in other tissues (systemic inflammatory lesions, tumor, etc.).
Immunohistochemistry can be helpful in the interpretation of histological changes in the spleen. Each compartment of the spleen has unique antigen expression patterns that can be used to compare expression patterns in control and treated tissues. 21
An interpretive diagnosis includes classical pathological terms such as atrophy, hyperplasia, etc., whereas a descriptive diagnosis uses enhanced histopathology to indicate an increase or decrease of cellularity or area within a specific anatomic compartment. 10 Use of enhanced terminology is recommended for immunotoxicity studies.
Non-Proliferative Changes
APLASIA/HYPOPLASIA (N) Spleen
See General Hemtolymphoid
White Pulp (PALS, follicles, germinal centers, marginal zones)
desAPOPTOSIS, INCREASED, LYMPHOCYTE (N) Spleen
conAPOPTOSIS, INCREASED, LYMPHOCYTE
enh INCREASED APOPTOSIS, LYMPHOCYTE
(indicate compartment and diagnose decreased lymphocytes, decreased area, tingible body macrophages, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Lymphocyte depletion; Atrophy; Single cell necrosis.
Pathogenesis/cell of origin
Lymphocyte death by apoptotic cell death pathway.
Diagnostic Features
Single cells or small clusters of cells affected.
Small, dark hyperchromatic cells.
Apoptotic bodies.
Cell shrinkage and convolution.
Pyknosis and karyorrhexis, nuclear fragmentation.
Intact cell membranes.
Cytoplasm retained in apoptotic bodies.
Increase in tingible body macrophages containing apoptotic bodies.
Inflammation usually not present.
Differential Diagnoses
Necrosis, lymphocyte:
Cell swelling.
Cell rupture.
Karyolysis, pyknosis, karyorrhexis.
Inflammation may be present.
Comment
Apoptosis is a coordinated and often energy-dependent mode of cell death that is considered a vital component of various normal processes. 34 Increased lymphocyte apoptosis may result from direct lymphocyte toxicity or from endogenous factors such as diet or stress (glucocorticoid release). Apoptosis may occur together with necrosis. While it is preferable to identify and record diagnoses of apoptosis and classic necrosis separately, this distinction may not be possible to make histologically when one type of cell death obscures the other. Also, necrotic cell debris can have some similarities to apoptotic debris, such as pyknosis and karyorrhexis. Apoptosis may predominate, with conversion to a necrotic phenotype, or necrosis may predominate with scattered apoptosis. In these cases, it would be appropriate to use both terms as a single entity (apoptosis/necrosis) or to only diagnose the predominate type of cell death and discuss the presence of the other type of cell death in the narrative.
desCELLULARITY, DECREASED, WHITE PULP (N) (Figures 63 to 67) Spleen
Figure 63.

B6129 mouse, spleen, white pulp and red pulp. Cellularity decreased (atrophy), diffuse with capsule and trabecula contraction.
Figure 67.

B6129 mouse, spleen, white pulp, marginal zone. Cellularity, decreased, lymphocyte. IBA1 stain illustrates marked decrease of marginal zone lymphocytes and prominence of the marginal zone macrophages (DAB positive cells) associated with the marginal zone, IBA1 stain.
conATROPHY, WHITE PULP
enh LYMPHOCYTES, DECREASED
(Indicate compartment and diagnose decrease in area if applicable)
Species
Mouse; Rat.
Other terms
Lymphoid depletion; Lymphoid degeneration; Decreased cellularity.
Modifier
Lymphoid, Lymphocyte.
Pathogenesis/cell of origin
Decreased lymphocytes as a result of apoptosis, necrosis, decreased lymphopoiesis (reduced, defective or deficient) or redistribution in response to stress, aging, toxicity or genetics.
Diagnostic Features
Decreased cellularity and/or area of one or more lymphoid compartment(s).
Lymphoid necrosis and/or apoptosis may be present.
Tingible body macrophages containing apoptotic bodies may be present.
- Hyaline material may be present in regressing germinal centers.
- Eosinophilic proteinaceous material.
- Negative for amyloid, may be positive for IgM.
- Other term - paramyloid.
Differential Diagnoses
Aplasia/Hypoplasia:
Congenital disorder.
Lymphoid tissue is completely absent.
History helpful in differentiating atrophy from hypoplasia.
Refer to General terms for additional information.
Comment
When using conventional terminology, the modifiers ‘lymphoid’ or ‘white pulp’ can be used to specify that decreased cellularity (atrophy) is limited to the white pulp. The absence of a modifier implies that the entire spleen is atrophic and that the white pulp and red pulp are similarly affected. Alternatively, the modifier ‘diffuse’ can be used to specify that white pulp and red pulp are both atrophic. Immunosuppressive drugs may cause decreased cellularity of specific compartment(s) of the spleen. Other causes of decreased cellularity include aging, cachexia, poor nutrition, toxins, chemotherapy, autoimmunity, irradiation or viral infections. Age-related atrophy may be species and strain related. Atrophic effects can be influenced by whether the spleen is in a resting or activated state at the time a test material is administered. Decreased lymphoid cellularity in the spleen can be a secondary effect of lymphocyte toxicity in the thymus due to decreased release of thymic lymphocytes into the circulation. Decreased lymphocyte cellularity via thymus depletion takes at least three weeks to manifest in the spleen.
NECROSIS, LYMPHOCYTE (N) Spleen
See General Hematolymphoid
TINGIBLE BODY MACROPHAGE, INCREASED (N) Spleen
See General Hematolymphoid
Red Pulp
desANGIECTASIS (N) (Figure 68) Spleen
Figure 68.

B6C3F1/N mouse, spleen, red pulp. Angiectasis.
conANGIECTASIS
enh VESSEL DILATATION; SINUSOID DILATATION OR VESSEL/SINUSOID DILATATION
(indicate compartment and diagnose increased area if applicable)
Species
Mouse; Rat.
Other terms
Dilatation, vascular.
Pathogenesis/cell of origin
Endothelium of splenic veins, sinusoids, arteries or arterioles.
Diagnostic Features
Dilatation of arteries, veins or sinusoids of the spleen.
Often only the red pulp sinusoids are involved.
Focal, multifocal or diffuse.
May be associated with thrombosis.
Endothelial cells are typically small, flat and spaced wide apart.
Endothelia cells of the spleen are CD34 negative.
Mitotic figures are not present.
Differential Diagnoses
Hemangioma:
Tumor cells are plump and form vascular structures which appear as new blood spaces.
May grow in a nodular pattern.
Tumor cells are CD34 positive.
Hemangiosarcoma:
No clear border.
Tumor cells are plump and form interconnecting vascular channels.
Tumor cells may or may not be invasive into adjacent tissues.
Tumor cell cytology is more pleomorphic and hyperchromatic and mitotic figures are present.
Tumor cells are CD34 positive.
Cyst:
Multiple blood filled or thrombi-filled cystic cavities of variable size in the red pulp.
Cystic cavities lacking an endothelial lining.
Peliosis – may be used as other term.
Comment
Hemangiomas and hemangiosarcomas (angiomas and angiosarcomas in humans) often arise in the red pulp from, or associated with, angiectasis. Unless there is a nodular or infiltrative pattern of growth or clear atypia of endothelial cells, it can be difficult to differentiate angiectasis from a vascular neoplasm. Irregular blood filled spaces can occur as a handling artifact during necropsy procedures or from trauma.
desCELLULARITY, DECREASED, RED PULP (N) (Figure 69) Spleen
Figure 69.
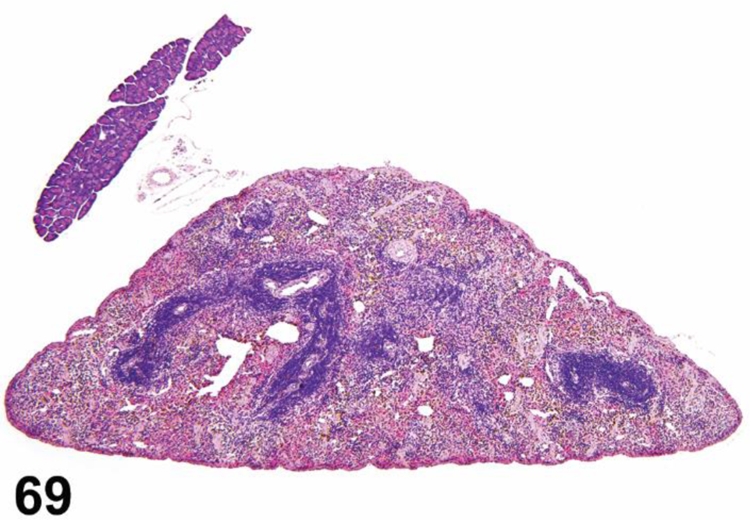
B6C3F1/N mouse, spleen, red pulp. Cellularity, decreased. Note wrinkling of capsule indicative of contraction due to atrophy of red pulp. There is a moderate amount of pigment within the red pulp.
conATROPHY, RED PULP
enh (INDICATE CELL TYPE), DECREASED, RED PULP
Diagnose decrease in area separately if applicable.
Species
Mouse; Rat.
Other terms
Decreased size.
Pathogenesis/cell of origin
Any or all of the red pulp cell types may be decreased due to physiological effects (i.e. aging, decreased body weight), changes in cell production/removal or toxicity.
Diagnostic Features
Decrease in one or more hematopoietic cell types (erythroid, myeloid, megakaryocytic, lymphoid and/or macrophages) compared to controls.
Fibroblastic reticular cell meshwork and venous sinuses may also be proportionally decreased.
Spleen capsule is not crenated or corrugated.
Differential Diagnoses
Contraction:
Erythrocytes are decreased in red pulp sinuses and cords.
Fibroblastic reticular cell meshwork is contracted and more prominent.
Splenic capsule may be crenated or corrugated.
History of cardiovascular insufficiency (hypotension, hemorrhage, moribundity) or exsanguination in the absence of barbiturate euthanasia.
Comment
Decreased cellularity (atrophy) of the red pulp may be observed in rats showing marked chemical-induced lesions, fasting or other factors affecting weight gain.
desCONGESTION (N) (Figure 70) Spleen
Figure 70.

F344/N rat, spleen, red pulp. Congestion, sinusoids.
conCONGESTION
enh RED BLOOD CELLS, INCREASED
(indicate red pulp and diagnose increased area if applicable)
Species
Mouse; Rat.
Other terms
Hyperemia; Chronic passive congestion.
Pathogenesis/cell of origin
Increased erythrocytes in red pulp cords and sinusoids due to active congestion (hyperemia, infection, inflammation, hypertension, increased clearance activity) or chronic passive congestion (venous obstruction, cardiac decompensation, chronic passive hyperemia).
Diagnostic Features
Enlargement of the red pulp; grossly, microscopically and by increased splenic weight.
Abundant erythrocytes within dilated red pulp sinusoids and pulp cords.
Usually diffuse but may be focal.
- Acute – dilated sinusoids.
- May involve arteries and veins in trabeculae.
- May involve marginal zone.
Chronic – red pulp fibrosis of the pulp cords may occur resulting in sinusoidal dilatation.
Differential Diagnoses
Leukemia, erythroid:
Tumor cells (primarily nucleated immature erythroid precursors) present in blood (intravascular) and/or red pulp sinusoids.
Tumor cells may be present in other tissues.
Idiopathic erythrocytosis:
Increased hematocrit and hemoglobin.
Focal hyperplasia, plasma cell, red pulp:
Focal lesion.
Lack of exsanguination:
Can be seen in animals found dead.
Comment
Congestion commonly occurs with barbiturate euthanasia due to smooth muscle relaxation. It can also be associated with both acute cardiac failure (acute congestion) and chronic cardiac failure (chronic passive congestion). Less commonly in rodents, chronic congestion can occur secondary to vascular abnormalities in the liver and portal vein obstruction. Vasoactive drugs such as histamine, bradykinin and prostaglandins increase storage of blood in the mouse spleen. 104
desCONTRACTION (N) (Figure 71) Spleen
Figure 71.
Mouse, spleen, capsule, mouse. Contraction. The capsule and trabecula are contracted due to atrophy of the red pulp.
conCONTRACTION
enhCONTRACTION
(indicate red pulp and diagnose decreased area if applicable)
Species
Mouse; Rat.
Pathogenesis/cell of origin
Smooth muscle contraction stimulated by epinephrine release, drop in blood pressure, hypoxia, etc.
Diagnostic Features
Erythrocytes in red pulp are decreased.
Venous sinuses may be open and prominent or collapsed and difficult to distinguish, depending on kinetics of contraction process.
Fibroblastic reticular cell meshwork contracted and more prominent.
Splenic capsule may be crenated or corrugated.
History of cardiovascular insufficiency (hypotension, hemorrhage, moribundity) or exsanguination without barbiturate euthanasia.
Spleen section has decreased area compared to controls.
Correlates with gross finding of small spleen.
Differential Diagnoses
Cellularity, decreased, red pulp:
Hematopoietic cell type(s) decreased.
Fibroblastic reticular cell meshwork and venous sinuses may also be decreased, but they lack prominence.
Capsule generally not crenated or corrugated.
Comment
The splenic capsule and trabeculae contain smooth muscle capable of rapid contraction to expel blood from the spleen. Fibroblastic reticular cells are considered to be myofibroblasts and play a role in splenic contraction in rats and mice. 7,105 Rodent spleens have less smooth muscle and less red pulp than some large animal species, so they tend to vary less in gross appearance when contracted. Contraction can be stimulated by drugs that lower blood pressure, acute blood loss, hypoxia, etc. This is a physiologic diagnosis that may be used to correlate with macroscopic observations of small spleen. Splenic contraction in an animal found dead or in the absence of barbiturate euthanasia is suggestive of cardiovascular insufficiency.
desECTOPIC TISSUE, SPLEEN (N) (Figures 72 and 73) Spleen
Figure 72.
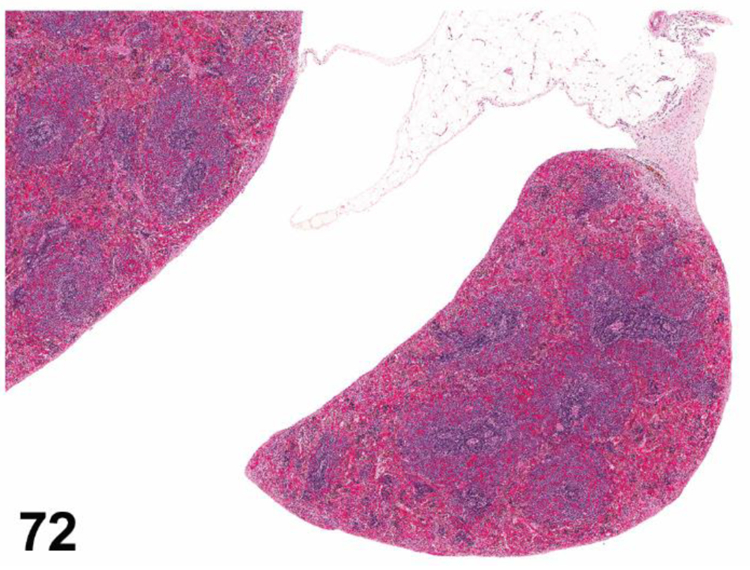
B6C3F1/N mouse, mesentery. Ectopic spleen.
Figure 73.

Rat, pancreas. Ectopic spleen.
co ECTOPIC TISSUE, SPLEEN
enhECTOPIC TISSUE, SPLEEN
Species
Mouse; Rat.
Other terms
Accesory spleen, Ectopic spleen; Spleen nodule; Supernumery spleen; Splenule; Splenunculus; Daughter spleen.
Pathogenesis/cell of origin
Normal splenic tissue in an abnormal location
Diagnostic Features
One or more dark red to black nodules ≥ 1 millimeter in diameter.
Embedded in the mesentery, mesenteric attachment of the spleen, pancreas or gonads.
Have all or some components of normal spleen (capsule, trabeculae, red pulp and/or white pulp).
Differential Diagnoses
Neoplasm of the pancreas, gonad
Comment
Ectopic spleen tissue is a rare finding in rodents and may be congenital or acquired. It may develop as a result of fusion failure of the splenic anlage during embryogenesis and may be aquired as a consequence of splenectomy or traumatic injury.
desERYTHROPHAGOCYTOSIS (N) (Figure 74) Spleen
Figure 74.
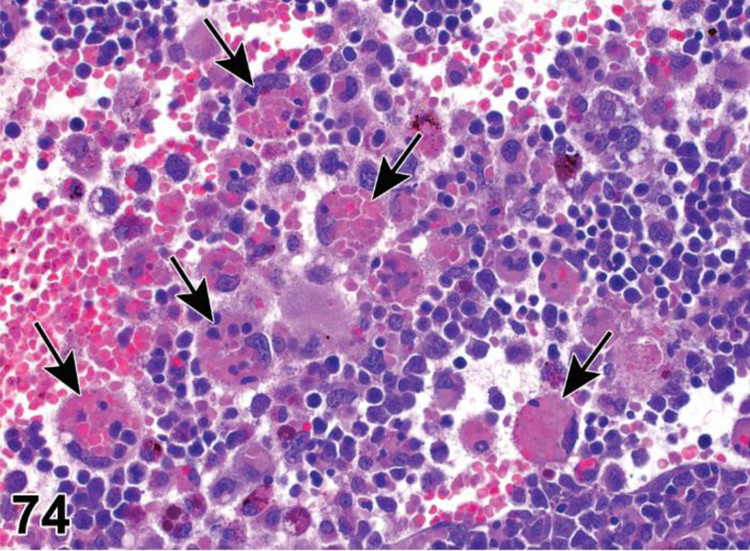
C57BL/6 mouse, spleen, red pulp. Macrophage erythrophagocytosis. Nucleated and mature erythrocytes are engulfed by macrophages in the red pulp (arrows).
conERYTHROPHAGOCYTOSIS
enhERYTHROPHAGOCYTOSIS
(indicate red pulp and diagnose increased macrophages if applicable)
Species
Mouse; Rat.
Other terms
Phagocytosis; Hemosiderosis; Increased pigment; Pigmented macrophages; Hemophagocytic syndrome.
Pathogenesis/cell of origin
Phagocytosis of erythrocytes by red pulp macrophages.
Diagnostic Features
Red pulp macrophages contain phagocytized intact or fragmented erythrocytes with or without nuclei or erythrocyte ghosts.
Often associated with increased pigmented macrophages containing hemosiderin.
May be associated with red pulp congestion if acute.
May be associated with increased red pulp macrophages if chronic.
Hemosiderin pigment positive for Prussian blue.
History of infection or autoimmune disorder.
Differential Diagnoses
VACUOLATION, MACROPHAGES:
Due to a variety of causes, including IL-4 exposure.
Histiocytic sarcoma:
Typical tumor cell morphology is seen.
Erythrophagocytosis and/or hematopoiesis may be observed (Magali 2003)
Presence of histiocytic sarcoma with similar cell morphology in other tissues.
Comment
Erythrophagocytosis is a normal phenomenon in the red pulp of the spleen. 106 The spleen’s open vascular systems provides a filter for removing senescent, parasitized, and otherwise altered erythrocytes in a physiological manner. Erythrophagocytosis occurs primarily by resident macrophages located in the pulp cords and venous sinuses. Fibroblastic reticular cells and cells lining the sinuses (littoral cells) may also be phagocytic. Physiological erythrophagocytosis is often difficult to appreciate on routine examination of the spleen. As a result of physiological erythrophagocytosis, iron is recycled by the macrophages resulting in accumulation of intracellular iron pigment (hemosiderin). In young adult rats, hemosiderin is more prominent in females than in males. Blood collection may also influence the amount of hemosiderin stored in macrophages. Splenic erythrophagocytosis can be stimulated by IL-4 exposure which also causes hepatic erythrophagocytosis. Extensive macrophage activation, which can occur with anemia, infection, immunodeficiency, neoplasia, etc, may lead to hemophagocytosis (engulfing of erythrocytes, leukocytes and platelets) by the splenic macrophages. Morphologically, there is splenic macrophage hyperplasia with the macrophages constipated with engulfed erythrocytes and/or other hematopoietic cells. Unlike physiologic erythrophagocytosis, hemophagocytosis is often fatal.
desFIBROSIS (N) (Figures 75 and 76) Spleen
Figure 75.

F344/N rat, spleen, red pulp. Fibrosis, red pulp. Splenic architecture is distorted due to contraction of the increased fibrous connective tissue in the red pulp.
Figure 76.

F344/N rat, spleen, red pulp. Fibrosis. Splenic white pulp in the upper left corner is atrophied and there is pigment associated with fibrous connective tissue replacing the normal red pulp elements.
conFIBROSIS
enhFIBROSIS
(indicate compartment and distribution i.e. focal, multifocal or diffuse)
Species
Mouse; Rat.
Other terms
Reticular cell hyperplasia; Stromal cell hyperplasia.
Pathogenesis/cell of origin
Stromal cells (of various cell types but usually fibroblasts), most often of the red pulp, also of capsule.
Diagnostic Features
Focal, multifocal or diffuse.
Generally localized in the red pulp but may occur in capsule and/or white pulp.
Expansive, larger lesions may enclose atrophic white pulp.
Not well circumscribed.
Consists of eosinophilic fibroblasts/fibrocytes with oval nuclei with small nucleoli and mature collagen fibers.
Collagen fibers may be markedly increased.
In denser fibrotic foci, sinuses are partly replaced by narrow capillaries.
Mitotic figures are rare.
May contain mature fat cells (lipomatosis or fatty change).
A silver stain and trichrome stains are helpful for assessment of reticular or collagen fiber content and structure of sinuses within the lesion.
Osseous metaplasia may occur in fibrotic areas.
Differential Diagnoses
Fibroma/Fibrosarcoma:
Fibroblasts and bands of dense mature collagen are the major components.
Fibrosarcoma often has a fascicular growth pattern which may or may not have a “herring bone” pattern.
Tumor cell nuclei are plump and have a distinct nucleolus.
Fibrosarcoma, pleomorphic:
Contains abundant collagen and storiform or cartwheel growth pattern.
Bizarre multinucleated neoplastic cells are present in the pleomorphic type.
Histiocytic sarcoma:
Cytoplasm is often more abundant than in stromal hyperplasia.
Multinucleated cells may be present, but they are not of a bizzare type.
Contains no prominent fibers and no mature fat cells.
Multiple foci present within the spleen and there may be simultaneous occurrence in other organs.
Comment
Chronic passive congestion and thrombosis of the venous sinusoids can lead to fibrosis of the red pulp with narrowing of the red pulp sinusoids. The presence of excessive nucleated cells in the sinusoids, such as those of LGL leukemia of the F344 rat, can result in sludging of blood leading to ischemia and subsequent fibrosis.
Capsular fibrosis is typically focal and usually occurs as a reparative process following inflammation or an injury such as an accidental needle puncture during intraperitoneal injection. It may also occur as a secondary process related to inflammatory, toxic or neoplastic lesions of the spleen. Pigment may be associated with the fibrosis and is usually secondary to hemorrhage related to the primary event. Fibrosis can be induced by a variety of chemicals (aniline compounds, azobenzene, o-toluidine hydrochloride, 4,4′-sulfonyldianiline, D & C Red No. 9). Spontaneous splenic fibrosis is very rare.
PIGMENT, MACROPHAGE (N) (Figure 77) Spleen
Figure 77.

CB-17 SCID mouse, spleen, red pulp. Pigment, hemosiderin, macrophages.
See General Hematolymphoid
VACUOLATION, MACROPHAGE (N) Spleen
See General Hematolymphoid
Non-Neoplastic Proliferative Changes
Hyperplastic changes in all the hematolymphoid organs, including the spleen, are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes do not infer preneoplastic or precancerous lesions in these organs (see Introduction). However, severe or persistent lymphoid hyperplasia may increase the risk of neoplastic transformation. If there is a concern, clonality studies should be considered.
White Pulp (PALS, follicles, germinal centers, marginal zones)
desAGGREGATES, MACROPHAGE, INCREASED (N) (Figure 78) Spleen
Figure 78.

Rat, spleen. Macrophage aggregates, increased. The animal had an infected catheter.
conAGGREGATES, MACROPHAGE, INCREASED
enh MACROPHAGE AGGREGATES, INCREASED
(indicate compartment and increased area if applicable)
Species
Mouse; Rat.
Locators
White pulp; Red pulp.
Other terms
Macrophage hyperplasia; Histiocytic aggregates; Histiocytic hyperplasia; Histiocytic granuloma; Granulomatous inflammation.
Modifier
Vacuolated; Pigmented.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Adherent macrophages clustered together to form variably sized aggregates.
Cell borders may be distinct or may appear syncytial.
Macrophages may or may not contain pigment.
Hemosiderin often increased if present.
Normal cellular elements are not displaced.
Most commonly located in the PALS.
Differential Diagnoses
GRANULOMA:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or foreign materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Increased Cellularity, mast cells:
Cells have pale basophilic or eosinophilic cytoplasm containing abundant basophilic granules that stain metachromatically with Giemsa or toluidine blue stains.
Cytoplasm is not foamy or vacuolated.
Degranulated or immature mast cells may be difficult to differentiate from macrophages.
Histiocytic sarcoma:
Tumor cells may be larger and are often more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Macrophages form aggregates when they cannot completely degrade microorganisms or ingested macromolecules, including some vehicles or test materials. Phagocytized test article may have a specific identifiable morphological character. Macrophages aggregates can be found anywhere in the spleen but occur most commonly in the PALS. Some red pulp macrophages form small aggregates, known as ellipsoids or PAMS (periarterial macrophage sheaths), which are centered around arterial terminations in the red pulp. They are small and inconspicuous in the rodent spleen but may enlarge in response to stimulus for increased clearance of cells or particulates from the blood.
desCELLULARITY, INCREASED, PLASMA CELL, WHITE PULP (H) (Figure 79) Spleen
Figure 79.

Mouse, spleen, periarteriolar lymphoid sheaths (PALS). Cellularity, increased, plasma cells.
con HYPERPLASIA, PLASMA CELL
enh PLASMA CELLS, INCREASED (indicate compartment)
Species
Mouse; Rat.
Other terms
Plasmacytosis.
Pathogenesis/cell of origin
In response to antigen, clonal expansion in germinal centers gives rise to antibody-secreting plasma cells.
Diagnostic Features
Increased plasma cells in the white pulp.
Cytoplasm is abundant and stains purple with routine hematoxylin and eosin stains.
Eccentric nuclei and prominent Golgi apparatus.
Mott cells with Russell bodies may be present.
May be associated with an acute or chronic disease process in the spleen or any other tissue, including infectious etiology or neoplasm.
Often occurs with blood born infections, systemic inflammatory conditions and autoimmune disorders.
Germinal centers may be hypertrophic/hyperplastic.
Mitotic figures are absent.
Differential Diagnoses
Plasmacytic Lymphoma:
Cells may have anaplastic plasmablastic features in less well differentiated plasmacytic lymphomas.
May be difficult to differentiate a well differentiated tumor from plasma cell hyperplasia based on morphologic features.
Ki67 or other proliferation markers can help differentiate because plasma cell hyperplasia does not have uniform Ki67 immunoreactivity.
Mitotic figures may be present but are rare in well differentiated tumors.
Tumor cells may be mature plasma cells, immature cells with an obvious plasmacytoid differentiation or a mixture of mature and immature plasma cells.
Formation of eosinophilic intracytoplasmic crystal-like structures considered diagnostic for plasmacytic lymphomas, although rarely seen.
Comment
Increased plasma cell cellularity (hyperplasia) is a common response of the spleen to acute and chronic inflammatory stimulation. Plasma cells are the mature end stage cell of a process involving antigen recognition and the production of mature and memory B cells in the follicle germinal centers or marginal zones. The resulting plasma cells are commonly located in the red pulp, especially around draining veins, but they may also be seen in the PALS and along the interface between the PALS and the marginal zone. Increased plasma cell cellularity is also a common aging change in the rodent spleen but is not well recognized unless immunohistochemical stains for plasma cell markers, heavy chain immunoglobulins or kappa light chains are performed.
desCELLULARITY, INCREASED, WHITE PULP (H) (Figures 80 to 82) Spleen
Figure 80.

CD1 mouse, spleen, white pulp, germinal centers. Cellularity, increased (hyperplasia) and abundant tingible macrophages.
Figure 82.

C56BL/6 mouse, spleen, white pulp, marginal zone and germinal center. Hyperplasia. Mouse was given influenza virus 7 day prior to tissue collection. The marginal zone hyperplasia typifies a T-cell independent innate immune reaction whereas the germinal center hyperplasia typifies a T-cell dependent adaptive humoral immune reaction. Based on the prominent centroblasts and high mitotic index the differential diagnosis would be marginal zone (MZ) lymphoma, but marginal zone hyperplasia in conjunction with germinal center hyperplasia is more consistent with MZ hyperplasia.
con HYPERPLASIA, WHITE PULP
enh LYMPHOCYTES, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Locators
White pulp.
Other terms
Nodular hyperplasia; Follicular hyperplasia; Marginal zone hyperplasia; Increased cellularity; Reactive lymph node.
Modifier
Lymphoid, Lymphocyte.
Pathogenesis/cell of origin
Increased lymphocytes in one or more lymphoid compartments due to antigenic stimulation, other immunomodulatory mechanisms, redistribution or aging changes; may include dendritic cell and macrophage hyperplasia to varying degrees.
Diagnostic Features
Increased cellularity and/or area of one or more lymphoid compartment(s).
Lymphoid compartments may be differentially affected.
Increased cellularity may involve lymphoid and/or antigen presenting cells.
Lymphoid cells can include mixtures of lymphoblasts, immunoblasts, medium sized lymphocytes, mature lymphocytes and/or plasma cells.
Antigen presenting cells can include dendritic cells, B cells and macrophages.
In rare cases, lymphoid hyperplasia can be due primarily to increased dendritic cells or macrophages.
May be associated with an acute or chronic disease process in the spleen or any other tissue, including infectious etiology or neoplasm.
May be a consequence of lymphoid regeneration after exposure to a toxin or from exposure to an immunomodulatory chemical.
Often occurs with blood borne infections, systemic inflammatory conditions and autoimmune disorders.
Differential Diagnoses
Normal aging process:
Can vary with strain or line of rat or mouse.
Compare with controls.
Lymphoma:
- Neoplastic cellular morphology of a uniform or pleomorphic population of lymphocytes.
- Early or late stages of differentiation.
- Focal, multifocal or diffuse.
Normal or atypical mitotic figures may be prominent.
Effacement of white pulp architecture with or without red pulp involvement.
Presence of lymphoma in other tissues.
Marginal zone Lymphoma:
May be difficult to distinguish from marginal zone hyperplasia.
Germinal center hyperplasia occurs more commonly with marginal zone hyperplasia than with marginal zone lymphoma.
Marked red pulp involvement with marginal zone lymphoma.
IHC for Pax5, kappa light chain, IgM or Ki 67 is often helpful in elucidating the degree of red pulp involvement and differentiating MZ lymphoma from MZ hyperplasia.
Pleomorphic/Follicular Lymphoma:
If present, germinal cells have no polarity and minimal to no mantle zone.
Germinal centers may be adjacent to each other and may fuse together.
Multinucleated cells may be present.
Blastic cells have prominent nucleoli.
IHC for IgG1, IgG2a and IgG2b may help establish clonality. 107
LYmphoproliferative disorder:103,108
Polyclonal lymphoproliferation.
Seen in genetic mutant mice.
Difficult to diagnose without knowing genetic background.
Comment
When using descriptive or conventional terminology, the modifiers ‘lymphocyte’, ‘lymphoid’ or ‘white pulp’ can be used to specify that the increased cellularity (hyperplasia) is limited to the white pulp. The absence of a modifier or the use of the term ‘diffuse’ implies that cellularity is increased in both the white pulp and the red pulp. An increase in size and/or cellularity of the entire white pulp or in any of its compartments may be diagnosed as lymphoid hyperplasia. When using enhanced terminology, the diagnostic term ‘cellularity, increased’ or ‘area increased’ should be modified by the appropriate compartment locator(s). Increased lymphoid cellularity (lymphoid hyperplasia) may be focal, multifocal or diffuse (i.e. involving all white pulp areas in a section), and may also include increases in white pulp dendritic cells and/or white pulp macrophages. Increased plasma cell cellularity is generally diagnosed separately (see Cellularity, increased, plasma cell).
Increased white pulp cellularity may be a response to immunomodulatory drugs or to antigens, infectious agents, toxins and infected, ulcerated and/or necrotic tissues or tumors. Lymphoid hyperplasia may be indistinguishable from early stages of lymphoma. Lymphoma should be diagnosed conservatively, preferably with overwhelming evidence of neoplasia, especially involvement of other tissues.
Increased cellularity is generally a reactive response that involves the production of additional cells and/or changes in the trafficking of existing cells, either at the site of the response or in a distant location. Lymphocyte proliferation and redistribution often occur together and may be morphologically indistinguishable, so the term ‘lymphoid hyperplasia’ is understood to encompass both processes in the spleen.
It is important to be familiar with the normal variation of lymphoid tissue within different sections of a spleen and between spleens of control animals, especially in very young and aging control mice and rats. Strain differences should also be considered. The marginal zone does not fully develop in the mouse until 3–4 weeks of age and its size (thickness i.e. the number of cell layers) varies among mouse strains and is often thin. In contrast, the marginal zone is very wide in most strains of rats. The spleen is larger and denser in aging rodents than in young animals. White pulp is oriented around arborizing central arteries and can vary in appearance depending on where the tissue section is taken relative to the arterial tree. The spleen is triangular in cross section, so careful attention should be given to standardizing the depth of longitudinal sections as the size and arrangement of white pulp structures vary with depth of sectioning, resulting in artifactual differences in size and cellularity.
Red Pulp
des CELLULARITY, INCREASED, ADIPOCYTE (N) (Figure 83) Spleen
Figure 83.

Rat, spleen, red pulp. Cellularity, increased, adipocytes. There are multiple foci of vacuolated adipocytes in the red pulp.
con HYPERPLASIA, ADIPOCYTE
enh ADIPOCYTES, INCREASED
(indicate compartment and distribution and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Lipomatosis; Lipidosis; Fatty infiltration; Fatty or lipid metaplasia; Fatty replacement; Fatty change.
Pathogenesis/cell of origin
Probably derived from local connective tissue, other stromal tissue, adipocytes or splenic pluripotent stem cells.
Diagnostic Features
Lipid-containing cells in the red pulp.
May be focal, multifocal or diffuse.
May be associated with collagen deposition.
Differential Diagnoses
Lipoma:
Nodular space occupying lesion composed of mature differentiated adipocytes.
Comment
Increased adipocyte cellularity is a rare lesion in the spleen of control mice and rats. It has been reported in rats exposed to aniline dyes in association with splenic fibrosis. 109,110
desCELLULARITY, INCREASED, MACROPHAGE (N) (Figure 84) Spleen
Figure 84.

Mouse, spleen, red pulp. Cellularity, increased, macrophage.
conHYPERTROPHY/HYPERPLASIA, MACROPHAGE
enh MACROPHAGES, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Locators
Red pulp; White pulp.
Other terms
Increased macrophage cellularity; Macrophage accumulation; Macrophage infiltrate; Macrophage infiltration; Prominent macrophages; Histiocytosis; Histiocytic hyperplasia; Histiocytic infiltrate; Histiocytic aggregates.
Modifier
Tingible body; Pigmented; Vacuolated; Aggregates.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Increased abundance and/or size of macrophages in sinusoids and/or cords of red pulp.
Macrophages are generally individualized and have distinct cell borders.
Cytoplasm may or may not contain phagocytized material, pigment (commonly hemosiderin) or vacuoles.
Macrophages containing phagocytized apoptotic bodies are known as tingible body macrophages (see General section for additional information).
May be focal, multifocal or diffuse.
May also occur in white pulp.
Dendritic cells in the white pulp may be increased in size and/or number.
Differential Diagnoses
Aggregates, macrophage, increased:
Discrete clusters of adherent macrophages.
Lack organized structure or encapsulation.
Focal or multifocal.
Macrophages may transform into epithelioid cells.
GRANULOMA:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or injected materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Cellularity, increased, mast cell:
Cells have pale basophilic or eosinophilic cytoplasm containing abundant basophilic granules that stain metachromatically with Giemsa or toluidine blue stains.
Cytoplasm is not foamy or vacuolated.
Degranulated or immature mast cells may be difficult to differentiate from macrophages.
Histiocytic sarcoma:
Tumor cells are usually larger and more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Increased macrophage cellularity (hyperplasia/hypertrophy) occurs most commonly in the red pulp where the majority or the splenic macrophages are located, but it may also occur in the white pulp, including the marginal zone. In the red pulp, resident macrophages clear the blood of aged and damaged cells (especially erythrocytes) and particulates such as microorganisms. Increased macrophage cellularity may occur diffusely as part of a reactive response to a variety of conditions such as infectious diseases, immunological status, erythrocyte breakdown, metabolism of xenobiotics or distant neoplasia. Focal collections of macrophages, known as ellipsoids or PAMS (periarterial macrophage sheaths), are clusters of macrophages centered around arterial terminations in the red pulp that may enlarge in response to stimulus for increased clearance. In rodents, these macrophage collections are normally small and inconspicuous. Increased macrophage cellularity often occurs in combination with phagocytosis, pigment storage, vacuolation or aggregation as macrophages increase to meet the demand driving these processes. The diagnostic terminology therefore includes these processes as modifiers to allow the pathologist to construct the most appropriate diagnosis for a particular constellation of features. These findings can also be diagnosed separately.
desCELLULARITY, INCREASED, MAST CELL (H) (Figure 85) Spleen
Figure 85.

B6C3F1 mouse, spleen, red pulp. Cellularity, increased, mast cell.
conHYPERPLASIA, MAST CELL
enh MAST CELLS, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Mastocytosis (see comment).
Diagnostic Features
- Mast cell infiltrates in red pulp.
- No alteration or destruction of the splenic architecture.
- Focal, multifocal or diffuse.
Cells in the same stage of differentiation, usually well differentiated.
Large cells with abundant pale blue or vacuolated cytoplasm.
Abundant dark blue cytoplasmic granules.
Cytoplasmic granules stain metachromatically with Giemsa or Toluidine blue stain.
Differential Diagnoses
Cellularity, increased, macrophage:
Cells do not have pale blue cytoplasm or basophilic granules.
Tumor, mast cell, benign / Tumor, mast cell, malignant:
Neoplastic cytology and/or malignant growth pattern.
Comment
Increased mast cell cellularity (hyperplasia) is uncommon in the rodent spleen.
desCELLULARITY, INCREASED, MESOTHELIAL CELL (H) (Figure 86) Spleen
Figure 86.

Rat, spleen, capsule. Cellularity, increased, mesothelial cell.
conHYPERPLASIA, MESOTHELIAL CELL
enh MESOTHELIAL CELLS, INCREASED
(indicate compartment and distribution and diagnose increased area if applicable)
Species
Mouse; Rat.
Other terms
Hyperplasia; Capsule.
Diagnostic Features
Increased mesothelial cells on the surface of the spleen.
Often associated with inflammation including fibrosis.
May be focal, multifocal or diffuse.
Often associated with other peritoneal or splenic lesions including inflammation and fibrosis.
May be seen with tumors metastatic to the peritoneal cavity.
Differential Diagnoses
Mesothelioma, malignant:
Mesothelial tumor cells have characteristics of neoplastic cells and may be found on peritoneal surfaces of other organs in addition to the spleen.
Comment
Increased mesothelial cell cellularity (mesothelial hyperplasia) may be seen with chronic peritonitis, ascites, peritoneal tumor metastases and splenic inflammation.
desCELLULARITY, INCREASED, PLASMA CELL, RED PULP (H) (Figure 87) Spleen
Figure 87.

B6C3F1/N mouse, spleen, red pulp. Cellularity, increased, plasma cell.
conHYPERPLASIA, PLASMA CELL
enh PLASMA CELLS, INCREASED
(indicate compartment)
Species
Mouse; Rat.
Other terms
Plasmacytosis.
Pathogenesis/cell of origin
In response to antigen, clonal expansion in germinal centers gives rise to antibody-secreting plasma cells.
Diagnostic Features
Increased plasma cells in the red pulp.
Often arrayed around trabecular veins.
May see intermixed with other hematopoietic cell types as part of extramedullary hematopoiesis or as pure populations of plasma cells, depending on the nature of the inciting cause.
Cytoplasm is abundant and stains purple with routine hematoxylin and eosin stains.
Eccentric nuclei and prominent Golgi apparatus.
Mott cells with Russell bodies may be present.
May be associated with an acute or chronic disease process in the spleen or any other tissue, including infectious etiology or neoplasm.
Often occurs with blood borne infections, systemic inflammatory conditions and autoimmune disorders.
Germinal centers may be hypertrophic/hyperplastic.
Mitotic figures are absent.
Differential Diagnoses
Plasmacytic lymphoma:
Cells may have anaplastic plasmablastic features in less well differentiated plasmacytic lymphomas.
May be difficult to differentiate a well differentiated tumor from plasma cell hyperplasia based on morphologic features alone.
Ki67 or other proliferation markers can help differentiate because plasma cell hyperplasia does not have uniform Ki67 immunoreactivity.
Mitotic figures may be present but are rare in well differentiated tumors.
Tumor cells may be mature plasma cells, immature cells with an obvious plasmacytoid differentiation or a mixture of mature and immature plasma cells.
Formation of eosinophilic intracytoplasmic crystal-like structures considered diagnostic for plasmacytic lymphomas, although rarely seen.
Comment
Increased plasma cell cellularity (hyperplasia) is a common response of the spleen to acute and chronic inflammatory stimulation. Plasma cells are the mature end stage cell of a process involving antigen recognition and the production of mature and memory B cells in the follicle germinal centers or marginal zones. The resulting plasma cells are commonly located in the red pulp, especially around draining veins, but they may also be seen in the PALS and along the interface between the PALS and marginal zone. Plasmacytosis is also a common aging change in the rodent spleen but is not well recognized unless immunohistochemical stains for plasma cell markers, heavy chain immunoglobulins or kappa light chains are performed.
desCELLULARITY, INCREASED, STROMAL CELL (H) (Figure 88) Spleen
Figure 88.

Rat, spleen, red pulp. Cellularity, increased (hyperplasia), stromal cell. Extravasated red blood cells are interspersed among a proliferation of prominent red pulp stromal cells.
conHYPERPLASIA, STROMAL CELL
enh STROMAL CELLS, INCREASED
(indicate compartment and distribution, i.e. focal, multifocal or diffuse, and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Reticular cell hyperplasia; Fibroblastic reticular cell (FRC) hyperplasia.
Pathogenesis/cell of origin
Presumed to be increased fibroblastic reticular cells.
Diagnostic Features
Focal proliferation of stromal cells in the red pulp.
Cells have pale cytoplasm, round to oval vesicular nuclei and a single nucleolus.
- Reticulin (fiber) staining is present but collagen is not seen or is not prominent.
- FRCs are positive for smooth muscle actin, desmin and podoplanin. 2
- Reticular fibers are positive with silver stains.
Differential Diagnoses
Focal fibrosis:
Localized proliferation of fibroblasts with collagen deposition.
Focal lipomatosis:
Well-demarcated foci of adipocytes in the red pulp.
Cellularity, increased, macrophage:
IHC for F4/80 may be necessary to differentiate stromal cell hyperplasia from increased macrophage cellularity.
Comment
Induced stromal cell hyperplasia of the red pulp has been reported after long-term treatment of rats with aromatic amines. This lesion is milder than fibrosis and may be a precursor of fibrosis.
desEXTRAMEDULLARY HEMATOPOIESIS, INCREASED (N) (Figures 89 to 92) Spleen
Figure 89.

Mouse, spleen, red pulp. Extramedullary hematopoiesis, increased.
Figure 92.

NSG mouse, spleen, red pulp. Extramedullary hematopoiesis, increased, megakaryocyte. Pregnancy-lactation induced.
conEXTRAMEDULLARY HEMATOPOIESIS, INCREASED
enhEXTRAMEDULLARY HEMATOPOIESIS, INCREASED
(indicate red pulp and diagnose increase in area if applicable)
Species
Mouse; Rat.
Locators
Red pulp.
Other terms
Hematopoietic hyperplasia; Red pulp hyperplasia; Myeloid hyperplasia; Erythroid hyperplasia; Megakaryocytic hyperplasia; Hematopoietic cell proliferation; Myeloid metaplasia.
Pathogenesis/cell of origin
Hematopoietic cells normally present in the red pulp or that have migrated there from the bone marrow.
Diagnostic Features
Increased size/area of the red pulp.
Increased hematopoietic cells in the red pulp.
- Hematopoietic cells of 3 lineages (myeloid, erythroid and megakaryocytic) may be present.
- Proportions of cell types vary depending on the etiology of increased hematopoiesis.
- Any of the lineages may predominant.
- Each lineage is often represented by cells in various stages of differentiation.
Differential Diagnoses
Leukemia; myeloid, erythroid or megakaryocytic:
Tumor cells are often all at one stage of differentiation especially in less well differentiated tumors.
Other organs are often involved with the same neoplastic cells as in the spleen.
Some leukemias present with minimal tissue involvement but with a high white blood cell count and numerous immature forms/blasts in the blood.
Mitosis, apoptosis and necrosis may be present.
Lymphoma:
Cell morphology is different from that of myeloid, erythroid or megakaryocytic cells.
May be difficult to distinguish from undifferentiated myeloid leukemias, IHC would help.
Comment
Increased extramedullary hematopoiesis (EMH) is the commonly used term for diffuse hyperplasia of the red pulp. Increased EMH is usually diffuse but can also occur as a focal change. EMH consists of one or more of the three hematopoietic cell lineages i.e. erythroid precursors, myeloid precursors and megakaryocytes. In rodents, particularly in mice, the spleen shares hematopoietic function with the bone marrow to varying degrees throughout the animal’s life. Although some degree of EMH is normally present in the rodent spleen, it can increase in response to hematotoxic insult, anemia, bone marrow suppression, inflammation, tumors elsewhere in the body and pregnancy. Depending on the underlining initiator (anemia, inflammation, etc), one of the three cell lineages may predominate, and, depending on the dominant cellular lineage, the hyperplasia maybe referred as erythroid hyperplasia, myeloid (granulocyte) hyperplasia or megakaryocyte hyperplasia. Hyperplasia may be related to mouse or rat strain, sex and age.
desHYPERPLASIA, NODULAR (H) Spleen
conHYPERPLASIA, NODULAR
enhHYPERPLASIA, NODULAR (indicate red pulp)
Species
Mouse; Rat.
Other terms
Splenoma; Hamartoma; Malformation; Focal red pulp hyperplasia.
Pathogenesis/cell of origin
Red pulp of the spleen.
Diagnostic Features
Focal, solitary occurrence in the red pulp.
Nodule protrudes from the spleen.
Well demarcated, distinct border.
Consists of normal-appearing red pulp (sinusoids, hematopoietic foci, hemosiderin).
No white pulp elements/lymphoid tissue included in the nodule.
Expansile growth with focal expansion of the capsule.
Often grossly visible.
Differential Diagnoses
Hemangioma:
Tumor cells are plump and form vascular structures, which appear as blood spaces with varying amounts of connective tissue.
Consists predominately of endothelial-lined blood vessels with mature erythrocytes and few or no other hematopoietic cell lineages.
Hemangiosarcoma:
Lack sharp, distinct border.
Blood-filled vascular channels associated with varying amounts of connective tissue.
Cellular atypia of endothelia.
Solid areas with fibrosarcomatous appearance may be present.
Consists predominately of endothelial-lined blood vessels with mature erythrocytes and few or no other hematopoietic cell lineages.
Cellularity, increased, stromal cells:
Fibrostromal tissue.
No distinct border.
Reduced or absent sinuses replaced by capillaries.
Cellular pleomorphism may be present.
Comment
Nodular hyperplasia is a rare lesion in rats, possibly caused by splenic trauma and probably not a true hamartoma. Focal hyperplasia of the red pulp has been reported in F344 rats.
LYMPH NODE
Organization
The lymph nodes are part of an integrated network of lymph nodes and lymphatics which communicates with the body’s sites of contact with the outside world (the skin and the mucosae) and with the internal organs such as liver and kidneys. Some lymph nodes also drain other lymph nodes.
Function
Lymph nodes conduct antigen surveillance on tissue fluids (lymph), mount cellular and antibody responses against antigens and filter the lymph.105 Adaptive immune responses are initiated in the lymphoid compartments where recirculating lymphocytes are brought together with antigen presenting cells bearing antigens from the site of contact with the outside world or xenobiotic application (e.g. skin, mucosae). Lymphocytes that encounter their cognate antigens undergo clonal proliferation in germinal centers (B cells) and the paracortex (T cells). Activated B cells give rise to plasma cells that produce antigen specific antibodies and memory B cells that will augment the immune response to future encounters with the antigens. Activated T cells produce a variety of effector T lymphocytes. Innate immune responses occur in the sinuses where macrophages identify, phagocytize and eliminate pathogens, foreign material and cell debris.
Development
Nodes are present at birth and develop upon antigenic stimulation.
Histology
Lymph nodes have several lymphoid compartments and a filtration compartment (the sinuses). The cellular components of each compartment are outlined in Table 5.
Table 5:
Compartments and cellular components of lymph nodes
| Compartment | Components |
|---|---|
| Follicle | Dominant B lymphocyte area |
| - Primary follicles contain small resting naive B cells | |
| - Follicular dendritic cells (FDCs) | |
| Germinal center | - B lymphocytes in several stages of development and maturation |
| - T lymphocytes | |
| - Follicular dendritic cells (FDCs) | |
| - Tingible body macrophages | |
| Interfollicular cortex Paracortex | Dominant T lymphocyte areas |
| - T lymphocytes | |
| - B lymphocytes | |
| - Fibroblastic reticular cells (FRCs) | |
| - Interdigitating dendritic cells | |
| - High endothelial venules (HEV) | |
| Medullary cords | - Lymphocytes |
| - Plasma cells | |
| - Fibroplastic reticular cells (FRCs) | |
| - Macrophages | |
| Sinuses (subcapsular, transverse, paracortical, medullary) | - Macrophages |
| - Fibroblastic reticular cells (FRCs) |
The lymph node stroma consists of a reticular meshwork composed of fibroblastic reticular cells (FRCs) and reticular fibers. The reticular meshwork subdivides the lymph node into several functional compartments 4 and provides a scaffold for lymphocyte migration and segregation within the node. 1,2,5,6 FRCs provide surfaces for adherence and produce trophic factors (chemokines) that direct lymphocyte movement (trafficking by means of haptotaxis) to B-cell and T-cell regions. Lymphocytes enter the lymphoid tissue via high endothelial venules (HEVs) in the paracortex and interfollicular cortex and migrate to the follicles (B cells) and paracortex (T cells) where they survey antigens displayed on antigen presenting cells. If they do not find their cognate antigens, they exit into the sinuses and pass out of the node via the efferent lymphatics. Interdigitating dendritic cells collect antigen from the skin, mucosal surfaces etc., enter lymphatics and are transported in the lymph to the local draining lymph node. Lymph also transports soluble antigens, inflammatory mediators, cell debris, inflammatory cells, erythrocytes and microorganisms. Upon reaching the subcapsular sinus, interdigitating dendritic cells settle on the sinus floor and migrate into the lymphoid tissue. Inflammatory mediators and soluble antigens (<70 kd) are picked up by reticular fibers in the meshwork which form a system of conduits that carry these substances deep into the lymphoid tissue. 4,5 Inflammatory mediators stimulate HEVs to upregulate recruitment of recirculating lymphocytes which can cause increased lymphocyte cellularity, particularly around the HEVs in the peripheral paracortical and interfollicular areas. Soluble antigens are sampled from the conduit system by antigen presenting cells, including follicular dendritic cells in the follicles. When a lymphocyte encounters its cognate antigen on an antigen presenting cell, it is stimulated to remain in the node and undergo clonal proliferation.
Antigenic stimulation of B-cells leads to germinal center development within primary follicles. The trapping of antigen by follicular dendritic cells (FDC’s) and the migration of B lymphoblasts into the follicle are regarded as the first events in germinal center formation. Small, recirculating B cells in the primary follicles are pushed aside by the developing germinal center and form the follicular mantle that surrounds it. The germinal center with its surrounding mantle is referred to as a secondary follicle. In an H&E-stained section, the mantle of the secondary follicle stains densely basophilic because of the high number of small B cells. The germinal center is paler because of the presence of lymphoblasts and follicular dendritic cells. The antigen-stimulated B blasts proliferate exponentially and alter phenotypically, e.g. by losing their surface immunoglobulin (sIg). In this state they are called centroblasts. Centroblasts migrate apically and mature into non-dividing centrocytes that again express sIg. Centrocytes with high affinity for antigen express the cell survival gene bcl-2 and survive. Centrocytes with low or no affinity for antigen do not express bcl-2 and are lost by apoptosis. At this stage the dark zone (centroblasts) and light zone (surviving centrocytes) of the germinal center become apparent.111 Antibody isotype switching occurs in the apical light zone, most probably with help of CD4+ T lymphocytes. The presence of a dark and light zone depends on the phase of activation and also on the plane of section. The presence of tingible body macrophages reflect apoptosis of the centrocytes.112
Mature B cells leave the germinal center either as plasma cells or as B memory cells. Plasma cells can accumulate in the medullary cords and secrete antibodies into the lymph or they can leave the lymph node. B memory cells reside in the mantle zone. Germinal centers appear within a few days after antigen administration. In lymph nodes which have low background germinal center activity, germinal center reactions last about three weeks after antigen administration. The presence, size and number of germinal centers are valuable indicators of immune activation. The location of germinal centers in the lymph node can provide information on the amount of activation. A very active lymph node may have germinal centers in the paracortex and even in the medullary cords.105
Antigenic stimulation of T cells leads to enlargement of the central paracortical areas. T does not produce a distinctive structure like a germinal center and is less well characterized. Paracortical cellularity varies depending on the relative proportions of small basophilic recirculating lymphocytes, larger proliferating lymphoblasts, pale interdigitating dendritic cells and macrophages.
Lymph is cleared of particulates, cells and cell debris by resident macrophages (also known as sinus histiocytes) located in the reticular meshwork of the sinuses. Macrophages may contain pigment derived from erythrocytes (hemosiderin) or tattoo pigment. Sinuses vary in appearance depending on the numbers and types of blood cells passing through them. Lymphocytes on their way out of the lymph node are present in variable numbers in the sinuses. Draining erythrocytes, neutrophils, eosinophils and mast cells may be seen depending on activity in the drainage area. The sizes of medullary cords and medullary sinuses are inversely related. Sinuses narrow as medullary cords expand with plasma cells and sinuses become wider when cellularity in medullary cords decreases.
Sampling and diagnostic considerations
The lack of grossly visible features and the small size of lymph nodes often makes standardized mid-sagittal sections difficult to obtain. The complexity of normal nodal architecture, variation in lymphocyte numbers and variation in plane of section all contribute to the difficulty in evaluating lymph nodes. FRCs and lymphocyte subpopulations are not easily visualized in routine H&E-stained sections. The microscopic appearance of a lymph node can vary widely depending on the relative activity of surveillance/antigen presentation, lymphopoiesis and filtration functions, each of which expands a different compartment within the lymph node. The names to identify the different lymph nodes are not standardized and can present difficulty in the communication of effects. Harmonization of lymph node names has been proposed for the rat 113 and the mouse. 114
Mesenteric lymph nodes and/or respiratory tract-draining lymph nodes are generally sampled in toxicity studies, along with a quiescent peripheral lymph node that does not drain a mucosal surface, typically the axillary or popliteal lymph node. The use of peripheral lymph nodes in the evaluation of the immune system has been questioned 115 but their use has been suitable for evaluating immune stimulation. For evaluation of an immune-modulatory effect, it is of utmost importance to have concurrent controls for comparison. Descriptive or enhanced nomenclature is recommended for subacute (2 to 4 week) and subchronic (13 week) studies as a descriptive approach is the most useful in evaluating and characterizing immune modulatory effects. 13,15 For carcinogenicity studies and for special studies including those in transgenic animals, conventional interpretative nomenclature is suitable.
Knowledge about the area(s) drained by the lymph nodes is needed to interpret their morphology. For the evaluation of treatment-related effects, it is also important to realize that, although present at many sites, it is one integrated system together with the spleen, thymus, MALT and bone marrow. 116
Non-Proliferative Changes
Cortex, Paracortex and Medullary Cords
APLASIA/HYPOPLASIA (N) Lymph node
See General Hematolymphoid
desAPOPTOSIS, INCREASED, LYMPHOCYTE (N) (Figures 93 and 94) Lymph node
Figure 93.

Rat, mesenteric lymph node. Apoptosis, lymphocyte, increased, severe paracortex.
Figure 94.

Rat, mesenteric lymph node. Apoptosis, lymphocyte increased, follicle germinal center.
conAPOPTOSIS, INCREASED, LYMPHOCYTE
enh APOPTOSIS, INCREASED, LYMPHOCYTE
(indicate compartment and diagnose decreased lymphocytes, decreased area, tingible body macrophages, etc. separately if applicable)
Species
Mouse; Rat.
Other terms
Lymphocytolysis; Increased tingible body macrophages; Increased cell death.
Pathogenesis/cell of origin
Lymphocyte death by apoptotic cell death pathway.
Diagnostic Features
Apoptotic lymphocytes in various stages of shrinkage, nuclear condensation and fragmentation.
Apoptotic bodies free in tissue.
Increased tingible body macrophages (macrophages with phagocytized apoptotic bodies).
Pale tingible body macrophages scattered among basophilic lymphocytes create ‘starry sky’ appearance.
Often results in a reduction in cellularity of one or more lymphoid compartments (atrophy).
Differential Diagnoses
Necrosis, lymphoid:
Morphological appearance may include ghost cells or coagulation necrosis of multiple cells leading to areas without cellular detail.
Inflammatory response present.
Increased macrophages contain necrotic debris but not apoptotic bodies.
Macrophages often contain pigment or other phagocytosed material.
Normal:
Low level of apoptosis may be seen as part of normal lymphocyte development, e.g. in active germinal centers.
Comment
Apoptosis is a coordinated and often energy-dependent mode of cell death that is considered a vital component of various normal processes. Apoptosis eliminates activated or auto-aggressive immune cells during maturation, therefore a low level of lymphocyte apoptosis is considered normal. Lymphocyte apoptosis can be increased above background levels by viruses, obstruction (of blood, lymph, etc), radiation, cytotoxic drugs, corticosteroids and endogenous cortisol (stress). Increased apoptosis can also be seen in some tumors such as lymphoma. Increased lymphocyte apoptosis may result from direct nodal lymphocyte toxicity or may result from endogenous factors such as diet or stress (glucocorticoid release). In coordination with macrophages, apoptotic cells are cleared quickly and efficiently. Apoptotic lymphocytes, tingible body macrophages and/or decreased lymphoid cellularity are variably present, depending on the duration of the process. Ongoing and severe apoptosis results in lymph node decreased cellularity (atrophy). Differentiation of necrosis from apoptosis and other types of cell death can be difficult. There may be a mixture of cell death types (e.g. apoptotic cells may occur as a bystander effect with necrotic foci) and necrotic and apoptotic cell debris share some common features, such as pyknosis and karyorrhexis. While it is preferable to identify and record diagnoses of apoptosis and classic necrosis separately, this distinction may not be possible when these processes occur together. This may be because one type of cell death histologically obscures the other. Also, necrotic cell debris can have some similarities to apoptotic debris, such as pyknosis and karyorrhexis, or vice-versa. Apoptosis may predominate, with conversion to a necrotic phenotype, or necrosis may predominate with scattered apoptosis. In these cases, it would be appropriate to use both terms or only indicate the predominate type of cell death and discuss the presence of the other type of cell death in the narrative. 34
desCELLULARITY, DECREASED, LYMPHOCYTE (N) (Figures 95 and 96) Lymph node
Figure 95.

Rat, mesenteric lymph node. Cellularity, decreased, lymphocyte (atrophy), paracortex and interfollicular cortex. Note numerous inactive high endothelial venules (HEVs) crowded together.
Figure 96.

Rat, mesenteric lymph node. Cellularity, decreased, paracortex. Note absence of follicles with germinal centers.
conATROPHY, LYMPHOID
enh LYMPHOCYTES, DECREASED
(indicate compartment)
Species
Mouse; Rat.
Other terms
Depletion, lymphocyte; lymphoid depletion.
Modifier
Lymphoid; lymphocyte
Pathogenesis/cell of origin
Change in lymphocyte kinetics – increase in destruction or decrease in production (intranodal or systemic) and/or decrease in recruitment or retention (intranodal).
Diagnostic Features
- Decreased lymphocytes.
- Decreased size and/or cellularity of one or more lymphoid compartments.
- Decreased in size and/or number (involution) of germinal centers.
- Increased apoptosis and/or increased tingible body macrophages.
- Indicative of increased lymphocyte destruction.
- May affect one or more compartments.
- Distribution.
- Changes may be local involving one lymph node or systemic involving all lymph nodes.
- Other lymphoid organs may be affected, especially thymus.
- Decreased prominence of HEVs.
- Suggestive of decreased recruitment.
- Decreased lymphocytes in sinuses.
- Suggestive of decreased lymphocyte flux through sinuses.
Differential Diagnoses
Aplasia/Hypoplasia
Complete lack of development of a lymphoid organ.
Absence of tissue or organ.
Comment
The etiology of decreased lymphocyte cellularity (atrophy) is potentially complex. Decreased cellularity in the lymph node can be the direct result of atrophy (increased cell death or decreased cell production) in the lymph node. In some cases, however, it may be the result of decreased cellularity (atrophy) in the bone marrow or thymus or may reflect changes in lymphocyte trafficking and distribution patterns or may be due to effects on fibroblastic reticular cells. The conventional term ‘atrophy’ may be used in chronic studies or in cases where lymphocyte destruction is known to have occurred in the lymph node (evidence of apoptosis or necrosis is present). The descriptive term “cellularity, decreased” or the enhanced term ‘decreased lymphocytes’ are preferred for short term studies because they are purely descriptive and do not imply a mechanism for the reduction in lymphocytes or suggest where the reduction occurred. Decreased lymphoid cellularity can occur with advancing age, physiological stress, cachexia, toxins, chemotherapy, immunosuppressive drugs, irradiation, viral infections, and interference with lymphocyte trafficking. The extent of age-related atrophy is species and strain related. The extent of induced atrophy is dependent on dose, duration of administration/exposure, and whether the lymph node was in an activated or resting state during exposure. The diagnosis of atrophy depends heavily on the history and the anatomic lymph node examined, namely either continuously activated lymph nodes draining mucosal surfaces, such as the mesenteric or mandibular lymph nodes, or resting lymph nodes, such as the popliteal lymph nodes. Atrophy of the paracortex and medullary cords is easily recognized as decreased cellularity, whereas atrophy in follicles often presents as a decrease in size without prominent decreased cellularity. Lack of germinal center development (in activated lymph nodes) may accompany decreased cellularity. Necrosis and/or apoptosis may result in atrophy, so necrotic and/or apoptotic cells may be seen in atrophic lymph nodes depending on the stage of the process. If systemic immunomodulatory effects are present, generally all the lymphatic organs (lymph node, spleen, thymus) may show a similar effect reflecting the “one system but multiple sites” integrated interactions among these organs. 13,116
NECROSIS (N) Lymph node
See General Hematolymphoid
PIGMENT, MACROPHAGE (N) Lymph node
See General Hematolymphoid
TINGIBLE BODY MACROPHAGE, INCREASED (N) Lymph node
See General Hematolymphoid
INFLAMMATION (N) Lymph node
See General Hematolymphoid
Sinuses and Lymphatics
desDILATATION, SINUS (N) (Figure 97) Lymph node
Figure 97.

Rat, mesenteric lymph node. Dilatation, sinus(es).
conDILATATION, SINUS
enhDILATATION, SINUS
(indicate affected sinus, i,e. transverse, medullary, etc., if applicable)
Species
Mouse; Rat.
Other terms
Sinus ectasia; Sinus dilation; Widened sinuses; Cystic dilation; Cystic degeneration; Lymphatic cysts; Lymphangiectasia.
Pathogenesis/cell of origin
Diffuse or focal dilatation of subcapsular, paracortical or medullary sinuses.
Diagnostic Features
Focal or diffuse sinus enlargement.
Enlarged sinuses are often filled with an eosinophilic staining material, presumably lymph, containing lymphocytes and other cells.
Often associated with lymph node decreased cellularity/atrophy, especially of the medullary cords.
Medullary sinuses are most commonly affected.
Differential Diagnoses
Lymphangiectasis:
Dilation of afferent or efferent lymphatics.
Sinuses unaffected.
Edema:
Increase in interstitial fluid not restricted to sinuses.
Comment
Sinus dilatation is common in aged rodents. It may be of undetermined etiology or due to inflammatory lesions, edema or tumors in adjacent tissues. It is more common in the mesenteric and mediastinal lymph nodes. When medullary cords decrease in diameter due to lymphoid depletion, medullary sinuses may secondarily increase in actual or apparent size.
desERYTHROCYTES, INTRASINUSOIDAL (N) (Figures 98 and 99) Lymph node
Figure 98.

Rat, cervical lymph node. Erythrocytes, intrasinusoidal, medullary sinuses.
Figure 99.

Mouse, axillary lymph node. Hemorrhage, paracortex and follicles. Note relative absence of red blood cells in sinuses.
conERYTHROCYTES, INTRASINUSOIDAL
enhERYTHROCYTES, INTRASINUSOIDAL
(indicate affected sinus, i.e. transverse, medullary, etc., if applicable)
Species
Mouse; Rat.
Other terms
Sinus erythrocytosis/erythrophagocytosis; Draining erythrocytes, Sinusoidal hemorrhage.
Pathogenesis/cell of origin
Erythrocytes are transported to lymph node sinuses via lymphatic drainage from extranodal site of hemorrhage.
Diagnostic Features
Erythrocytes present in sinuses, either individualized or in aggregates.
Erythrocytes rosetted around and/or phagocytized by sinus macrophages.
Sinus macrophages contain hemosiderin pigment if exposure to draining erythrocytes is chronic or ongoing.
Differential Diagnoses
Hemorrhage:
True intranodal hemorrhage is rare.
Erythrocytes are present in lymphoid tissue instead of, or in addition to, the sinuses.
Localized lesion in the absence of other causes.
Vascular ectasia:
Dilation of congested blood vessels.
Absence of free blood cells in sinuses and lymphoid tissue.
Hemangioma:
Proliferation of endothelial cells and vascular structures forming a discrete, nodule-like lesion.
HEMAL LYMPH NODES (rats)
Comment
Intrasinusoidal erythrocytes generally originate from sites of extranodal hemorrhage and are carried to the lymph node in the draining lymph and are therefore not a true biological lesion per se. They are often focally located in the sinuses that were receiving lymph from hemorrhagic area(s) of the drainage field. Intrasinusoidal erythrocytes are commonly seen as a peri-mortem or post-mortem artifact secondary to terminal blood collection or necropsy manipulations, especially in mediastinal and bronchial lymph nodes. They may also be found in mesenteric, and to a lesser extent, mandibular lymph nodes. Acute hemorrhage in the lung is common with euthanasia and drainage is often observed in bronchial lymph nodes, especially in animals euthanized with CO2. 50,117 Hemosiderin-containing sinus macrophages (pigmented macrophages) are indicative of ante-mortem hemorrhage. If notable accumulations of erythrocytes are due to manipulations at necropsy or in-life handling (e.g. injection in the draining area), the incidence is expected to be distributed about equally among the groups, including controls. The association with handling can be discussed in the pathology narrative if deemed necessary to correlate with a gross lesion. Erythrocytes adherent to sinus macrophages (rosette formation) without erythrophagocytosis may be a sign of deficient phagocytosis, as found in studies with organotins. 118 Intranodal hemorrhage originating from nodal blood vessels is rare and can be distinguished by large numbers of free erythrocytes intermingled with lymphocytes in the lymphoid pulp tissue.
desLYMPHANGIECTASIS (N) Lymph node
conLYMPHANGIECTASIS
enh LYMPHATICS, DILATED
Species
Mouse; Rat.
Other terms
Lymphatic dilation; Lymphatic cysts; Lymphatic ectasia.
Pathogenesis/cell of origin
Lymphatics in soft tissue surrounding lymph nodes.
Diagnostic Features
Dilatation of any afferent or efferent lymphatic vessel(s) in connective tissue adjacent to lymph nodes.
Lymphatics may be cystic, especially in nude athymic rodents, but also seen in carcinogenicity studies.
Can be filled with acellular proteinaceous material.
Differential Diagnoses
Vascular dilation:
Dilation of blood vessels, characterized by the presence of erythrocytes in existing dilated vessels.
Edema:
Increased interstitial fluid not associated with a vessel.
Comment
Dilatation of lymphatic vessels is usually due to blockage of lymphatics by disease processes such as tumors or by inflammatory lesions.
PIGMENT, MACROPHAGE (N) Lymph node
See General Hematolymphoid
VACUOLATION, MACROPHAGE (N) Lymph node
See General Hematolymphoid
Proliferative Changes (Non-Neoplastic)
Hyperplastic changes in all the hematolymphoid organs, including the lymph nodes, are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes do not infer preneoplastic or precancerous lesions in these organs (see Introduction). However, severe or persistent lymphoid hyperplasia may increase the risk of neoplastic transformation. If there is a concern, clonality studies should be considered.
Cortex, Paracortex and Medullary Cords
desAGGREGATES, INCREASED, MACROPHAGE (N) (Figure 100) Lymph node
Figure 100.

Rat, mesenteric lymph node. Aggregates, macrophage, increased.
conAGGREGATES, INCREASED, MACROPHAGE
enhAGGREGATES, INCREASED, MACROPHAGE
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Macrophage hyperplasia; Histiocytic aggregates; Histiocytic hyperplasia; Histiocytic granuloma; Granulomatous inflammation; Potter’s lesion.
Modifier
Pigmented; Vacuolated.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Adherent macrophages clustered together to form variably sized aggregates.
Cell borders may be distinct or may appear syncytial.
Macrophages may or may not contain pigment.
Hemosiderin often increased if present.
Normal cellular elements are not displaced.
Most commonly located in medullary cords and paracortex.
Differential Diagnoses
Increased cellularity, macrophage, intrasinusoidal
Increased abundance and/or size of macrophages in one or more sinuses.
Macrophages are generally individualized and have distinct cell borders.
Inflammation, granuloma:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or injected materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Increased cellularity, mast cell:
Cells have pale basophilic or eosinophilic cytoplasm containing abundant basophilic granules that stain metachromatically with Giemsa or toluidine blue stains.
Cytoplasm is not foamy or vacuolated.
Degranulated or immature mast cells may be difficult to differentiate from macrophages.
Histiocytic sarcoma:
Tumor cells may be larger and are often more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Macrophages accumulate and form aggregates when they cannot completely degrade ingested macromolecules or microorganisms. Small scattered macrophage aggregates, often containing pigment, may be seen as a background finding.117 Aggregates can form in any of the lymphoid compartments, but they are most commonly located in the medullary cords and paracortex. Specific distribution patterns in the same node within a dose group may be consistent with a treatment-related effect. Phagocytized test article may have a specific identifiable morphological character. Some vehicles and dietary antigens can cause increased macrophage aggregates. Corn oil used in gavage studies will frequently result in small aggregates, commonly called “microgranulomas”, within the mesenteric lymph node. If macrophage aggregates are increased in the lymphoid compartments, sinusoidal macrophages may also be increased and should generally be diagnosed separately (see increased cellularity macrophage, intrasinusoidal). Enlarged lymph nodes containing sheets and bands of fusiform macrophages in the cortex and medulla (Potter’s Lesion) have been reported in NZB, CD1 mice and occasionally other mouse strains 35,117 This change has been associated with viral infection and is considered to be non-neoplastic.
desCELLULARITY, INCREASED, INTERDIGITATING DENDRITIC CELL (H)(Figure 101) Lymph
Figure 101.

Rat, lymph node. Cellularity, increased, interdigitating dendritic cells, paracortex, lymph node. Note prominent high endothelial venules (arrows).
conHYPERPLASIA/HYPERTROPHY, INTERDIGITATING DENDRITIC CELL
enh INTERDIGITATING DENDRITIC CELLS, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Cellularity, increased, non-lymphoid; Hyperplasia, non-lymphoid
Pathogenesis/cell of origin
Increased immigration of interdigitating dendritic cells to paracortex.
Diagnostic Features
Expansion of paracortical area.
Increased number of dendritic cells.
Dendritic cells initially accumulate around the periphery of the paracortex but may infiltrate the entire compartment.
Cells are larger than lymphocytes with irregularly contoured nuclei and pale cytoplasm.
Lymphocytes may be decreased in number or cell density.
Positive for S-100, variably and weakly positive for CD68 and CD45.
May be associated with inflammation or antigen in the drained tissues.
Differential Diagnoses
AGGREGATES, INCREASED, MACROPHAGE
Adherent macrophages clustered together to form variably sized aggregates.
Cell borders may be distinct or may appear syncytial.
Macrophages may or may not contain pigment.
Hemosiderin often increased if present.
Normal cellular elements are not displaced.
Most commonly located in medullary cords and paracortex.
OTHER TYPES OF INFLAMMATORY RESPONSE IN PARACORTEX:
Neutrophils and/or other inflammatory cells present.
Comment
The interdigitating dendritic cell (IDC) is a mature form of a tissue dendritic cell with increased antigen presenting capabilities. 119 IDCs originate from bone marrow and the immature cells are distributed in peripheral tissues including skin and mucosal epithelia. 120 They collect and process antigens in the peripheral tissues and then migrate via afferent lymphatics to the draining lymph nodes where they are distributed in the paracortical area as mature IDCs. 121 Increased IDC cellularity (hyperplasia) is thus a reactive change in response to the presence of antigens in the organs and tissues drained by the lymph node and is not considered a preneoplastic lesion. IDC hyperplasia has been observed in athymic nude mice and rats as a compensatory response to T cell deficiency. IDCs in superficial lymph nodes are derived from the Langerhans cells of the skin. Increased Langerhans cells, IDCs and paracortical hyperplasia have been observed in contact dermatopathic lymphadenopathy in humans. Similar paracortical hyperplasia due to increased IDCs has been observed in the superficial lymph nodes in a mutant strain of hairless rats. 122 In routine studies without special stains, the diagnosis of ‘cellularity, increased, non-lymphoid, paracortex’ or ‘hyperplasia, non-lymphoid’ can be used for this finding.
desCELLULARITY, INCREASED, LYMPHOCYTE (H) (Figure 102) Lymph node
Figure 102.

Rat, lymph node. Cellularity, increased, lymphocyte.
conHYPERPLASIA, LYMPHOID
enh LYMPHOCYTES, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Lymphoid hyperplasia; Lymphocyte proliferation; Lymph node activation; Reactive lymph node; Follicular hyperplasia; Increased follicular number.
Modifier
Lymphoid; lymphocyte
Pathogenesis/cell of origin
Change in lymphocyte kinetics –increase in production (intranodal or systemic) due to antigenic stimulation, increase in recruitment/retention (intranodal) and/or decrease in destruction.
Diagnostic Features
- Increased lymphocytes.
- Increased size and/or cellularity of one or more lymphoid compartments compared to concurrent controls exposed to the same environmental conditions.
- Increased in size and/or number of germinal centers.
Indicative of chronic antigenic stimulation.
- Distribution.
- Changes may be local involving one lymph node or systemic involving multiple lymph nodes.
- Other lymphoid organs may be affected.
- Increased prominence of HEVs.
- Suggestive of increased lymphocyte recruitment.
- Increased lymphocytes in sinuses.
- Suggestive of increased lymphocyte flux through sinuses.
Differential Diagnoses
Lymphoma:
Distortion of nodal architecture.
Starry sky appearance with tingible body macrophages.
Generally systemic with multiorgan involvement.
No obvious association with an inflammatory lesion.
Comment
Increased cellularity (lymphoid hyperplasia) can occur under a variety of circumstances which can be either specific, i.e. antigen driven by viruses or bacteria, or non-specific, e.g. driven by pollutants, particulates, tissue damage or drainage of inflammation from a distant site. The etiology of lymphoid hyperplasia is potentially complex, so the use of this interpretive diagnostic term should be considered carefully. Increased lymphocyte cellularity in the lymph node can be the direct result of hyperplasia (increased cell production and/or decreased cell death) in the lymph node. In some cases, however, it may reflect changes in lymphocyte trafficking and distribution patterns.
desCELLULARITY, INCREASED, PLASMA CELL (H) (Figures 103 and 104) Lymph node
Figure 103.
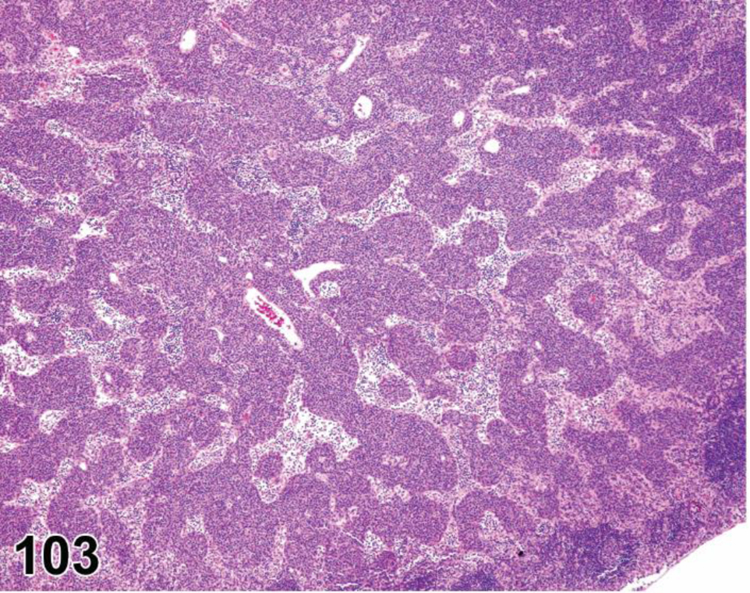
Rat, submandibular lymph node. Cellularity, increased, plasma cell (hyperplasia, plasma cell).
Figure 104.
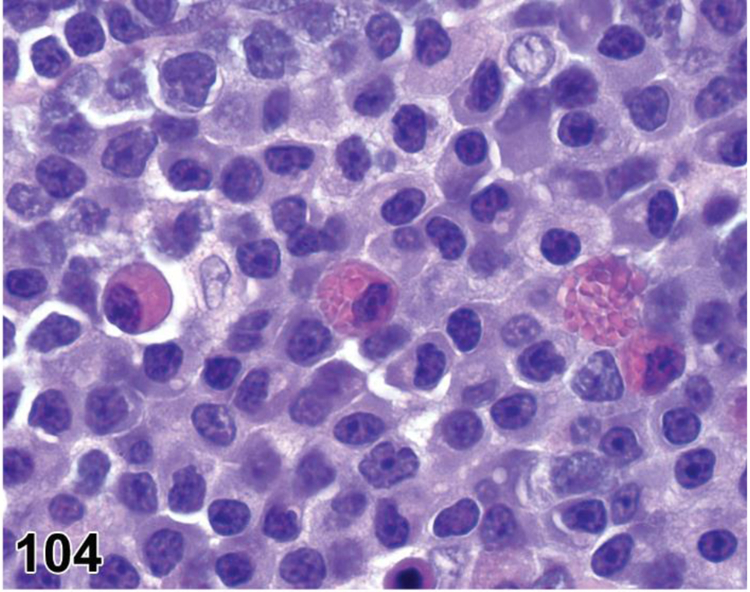
Rat, submandibular lymph node. Cellularity, increased, plasma cells. Note Mott cells containing eosinophilic Russell bodies scattered among plasma cells.
conHYPERPLASIA, PLASMA CELL
enh PLASMA CELLS, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Plasmacytosis.
Pathogenesis/cell of origin
Plasma cells, B lymphocytes.
Diagnostic Features
Medullary cords are expanded by well differentiated plasma cells.
Medullary sinuses may be collapsed by enlarged medullary cords and paracortex may be partially replaced by plasma cells.
Often observed as a reactive change in mandibular lymph nodes in chronic studies.
Not a systemic lesion.
Differential Diagnoses
Lymphoma:
Generally consists of lymphocyte populations without mature nuclear chromatin features.
Plasmacytic lymphoma:
Undifferentiated blasts rather than fully mature plasma cells.
Systemic lesion.
Affected tissues have distorted architecture.
Comment
Increased plasma cells are part of an inflammatory response to chronic antigenic stimulation that originates in a lymph node’s drainage field, such as an infected catheter or pododermatitis, etc. They are often seen in the mandibular lymph nodes in chronic studies. Affected lymph nodes may be macroscopically enlarged. Since mature plasma cells are fully differentiated, they are inherently not proliferative 123 and do not express Ki 67.
desCELLULARITY, INCREASED, STROMAL CELL (H) Lymph node
conHYPERPLASIA, STROMAL CELL
enh STROMAL CELLS, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Fibrous hyperplasia; Fibrosis, fibroplasia; Fibroblastic reticular cell hyperplasia.
Pathogenesis/cell of origin
Proliferation of fibroblastic reticular cells (FRCs).
Diagnostic Features
FRCs have large irregularly oval nuclei and pale cytoplasm and secrete reticular fibers.
Focal to multifocal change, most prominent in the paracortex.
FRCs are positive for cytokeratins 8 and 18 by immunohistochemistry. 124
Reticular fibers are positive for Gomori’s reticulin silver stain.
Definitive diagnosis may also be made by EM or IHC for desmin or smooth muscle actin.
Differential Diagnoses
Inflammation, granulomatous:
Presence of other inflammatory cells and possibly multinucleated giant cells.
Fibroplasia/Fibrosis:
Presence of fibrous tissue and mature collagen.
Collagen is positive for trichrome stains.
Histiocytic sarcoma:
Systemic neoplasm that often involves non-lymphoid organs.
Neoplastic proliferation of polymorphic cells with eosinophilic cytoplasm, often with multinucleated giant cells.
IHC for macrophage markers maybe required for a definitive diagnosis.
Hemangioma/Lymphangioma and Hyperplasia, angiomatous:
May look fibrotic with relatively few vascular spaces.
Focal lesion.
Vascular endothelia are often enlarged or slightly pleomorphic.
IHC for endothelial cell markers maybe required for a definitive diagnosis.
Comment
Definitive differentiation from fibrosis may require special stains, immunohistochemistry or electron microscopy. Fibrosis may be diagnosed in the absence of a definitive diagnosis. Reported cases of increased FRC cellularity (hyperplasia) in the lymph node of laboratory animals are rare. 124–127
desHYPERPLASIA, ANGIOMATOUS (H) (Figure 105) Lymph node
Figure 105.

Rat, mesenteric lymph node. Angiomatous hyperplasia.
conHYPERPLASIA, ANGIOMATOUS
enh INCREASED/DILATED, BLOOD VESSELS
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Angiomatosis; Vascular transformation.
Modifier
Lymphatic (if vessels contain only proteinaceous fluid and no erythrocytes)
Pathogenesis/cell of origin
Endothelial cell (both lymphatic and blood vessel origin).
Diagnostic Features
Increased numbers and/or size of blood vessels in the cortex, paracortex and/or medullary cords or adjacent perinodal connective tissue.
Cortex and medullary cords most commonly affected.
Focal or diffuse proliferation of (blood) vessels which are often dilated.
Dilated blood vessels filled with blood/erythrocytes which may be thrombosed.
In early stages, proliferating vessels may be filled with a proteinaceous fluid without the presence of erythrocytes suggesting lymphatic origin.
Differential Diagnoses
Erythrocytes, intrasinusoidal:
Erythrocytes located in the sinuses, not within vessels lined by endothelium.
Congestion:
Dilation of existing blood vessels of normal diameter, i.e. size is not increased.
Lymphangiectasis:
Dilation of existing lymphatics.
Devoid of erythrocytes.
Angioma and Angiosarcoma (Hemangioma and Hemangiosarcoma):
Increased number of vascular spaces.
Endothelial proliferation forming a nodular mass.
May extend out into the perinodal connective tissue.
Neoplastic vascular spaces contain RBCs.
Neoplastic cells with cytoplasmic microcapillary features.
Comment
Angiomatous hyperplasia is seen as a common aging lesion, especially in the mesenteric lymph nodes of B6C3F1 mice and in some strains of rats such as the Wistar strain. The lesion appears to start in vessels in the medullary cords and adjacent hilar tissue and is thought to be caused by the occlusion of efferent lymphatics. Larger lesions invariably contain erythrocytes. Angiomatous hyperplasia is generally not regarded as preneoplastic to hemangiomas and hemangiosarcomas, although there appears to be a progression. It has some morphological resemblance angiomatosis, a rare condition in humans. 128–131
desHYPERTROPHY/HYPERPLASIA, HIGH ENDOTHELIAL VENULES (HEVS) (N) (Figures 106 and 107) Lymph node
Figure 106.
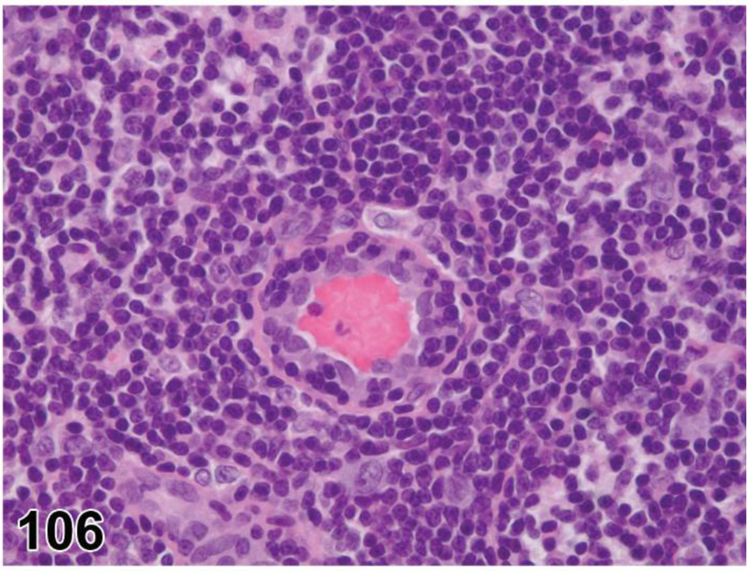
Rat, mesenteric lymph node. High endothelial venules (HEVs) hypertrophy/hyperplasia, cross section.
Figure 107.

Rat, mesenteric lymph node. High endothelial venules (HEVs) hypertrophy/hyperplasia, longitudinal section. Note the trafficking lymphocytes.
conHYPERTROPHY/HYPERPLASIA, HIGH ENDOTHELIAL VENULES (HEVS)
enh HIGH ENDOTHELIAL VENULES, INCREASED OR HIGH ENDOTHELIAL VENULES, HYPERTROPHY
(indicate compartment)
Species
Mouse; Rat.
Pathogenesis/cell of origin
HEV endothelial cells in lymph nodes.
Diagnostic Features
Hypertrophy of the normal ‘high’ vascular endothelium.
Increased lymphocyte migration across vessel walls.
HEVs are often dilated or increased in cross sectional area.
Hyperplasia, i.e. increased numbers of HEVs, may occur.
Differential Diagnoses
Atrophy, lymphoid, paracortex:
High endothelial venules may appear relatively more prominent when the surrounding paracortex is atrophic.
HEV endothelial cells are not hypertrophic.
Comment
HEV hypertrophy/hyperplasia occurs in response to immune stimulation, specifically to cytokines delivered to the HEVs via the FRC conduit system. Hypertrophy of the endothelial cells increases lymphocyte recruitment across the HEV wall and is usually associated with increased cellularity of one or more compartments of the lymph node. It can also be induced by xenobiotics such as hexachlorobenzene. 118,132
Sinuses and Lymphatics
desCELLULARITY, INCREASED, MACROPHAGE, INTRASINUSOIDAL (N) (Figure 108) Lymph node
Figure 108.

Rat, mesenteric lymph node. Cellularity, increased, macrophage, intrasinusoidal.
conHYPERTROPHY/HYPERPLASIA, MACROPHAGE, INTRASINUSOIDAL
enh INTRASINUSOIDAL MACROPHAGES, INCREASED OR INTRASINUSOIDAL MACROPHAGES, HYPERTROPHY
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Sinus histiocytosis; Histiocytic hyperplasia; Histiocytic infiltrate; Histiocytic aggregates; Increased macrophage cellularity; Macrophage accumulation; Macrophage infiltrate; Macrophage infiltration; Prominent macrophages.
Modifier
Pigmented; Vacuolated.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Increased abundance and/or size of macrophages in one or more sinuses.
Macrophages are generally individualized and have distinct cell borders.
Cytoplasm may or may not contain phagocytized material, pigment or vacuoles.
Macrophages tend to accumulate in subcapsular sinus first, then in transverse sinuses, then in medullary sinuses.
Differential Diagnoses
Aggregates, macrophage, increased:
Multiple discrete clusters of adherent macrophages.
Lack organized structure or encapsulation.
Focal or multifocal.
Generally not found in the sinuses.
GRANULOMA:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or injected materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Cellularity, increased, mast cell:
Cells have pale basophilic or eosinophilic cytoplasm containing abundant basophilic granules that stain metachromatically with Giemsa or toluidine blue stains.
Cytoplasm is not foamy or vacuolated.
Degranulated or immature mast cells may be difficult to differentiate from macrophages.
Histiocytic sarcoma:
Tumor cells may be larger and are often more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Macrophages residing in the sinuses are a distinct population of phagocytic cells that filter lymph and they are functionally different from macrophages located in the lymphoid compartments. 117 Note that the diagnosis is restricted to an increase in intrasinusoidal macrophages (hypertrophy/hyperplasia) without aggregate formation. An increase in intrasinusoidal macrophages (also known as sinus histiocytes) may be due to either an increase in resident macrophages or an influx of macrophages that traffic to the lymph node via afferent lymph or blood. 117,123 Sinus histiocytosis was a common term used for increased macrophages in the subcapsular, transverse, paracortical and/or medullary sinuses. Specific patterns in the same node within a dose group may be consistent with a treatment-related effect. Note that the number of macrophages within the sinuses can vary with the node and plane of section, variables which should be considered in the evaluation of this finding. Increased cellularity of intrasinusoidal macrophages (hypertrophy/hyperplasia) is often indicative of increased clearance of particulates from lymph. Sinus macrophages commonly phagocytize draining erythrocytes and tattoo pigment and then store the pigments as hemosiderin or carbon, respectively. These conditions may be diagnosed separately (erythrophagocytosis, pigmented macrophages), or may be included in the diagnosis of intrasinusoidal macrophage hypertrophy/hyperplasia as a modifier (pigmented) or simply noted as a comment in the data. The diagnostic terminology includes these processes as modifiers to allow the pathologist to construct the most appropriate diagnosis for a particular constellation of features.
desCELLULARITY, INCREASED, MAST CELL (H) (Figures 109 and 110) Lymph node
Figure 109.

Rat, mediastinal lymph node. Cellularity, increased, mast cells, subcapsular and medullary sinuses. Prominent basophilic granules.
Figure 110.

Rat, mediastinal lymph node. Cellularity, increased, mast cells, medullary sinuses. Faint basophilic granules.
conHYPERPLASIA, MAST CELL
enh MAST CELLS, INCREASED
(indicate compartment and diagnose increase in area if applicable)
Species
Mouse; Rat.
Other terms
Mast cell accumulation; Mastocytosis.
Pathogenesis/cell of origin
Mast cell proliferation, immigration or redistribution within the sinuses.
Diagnostic Features
Increased mature mast cells within sinuses without nodule formation.
No loss/distortion of normal architecture or compression of adjacent tissues.
Mitotic figures are not present.
Mast cells are uniform, round or polygonal, medium-sized well differentiated and non-cohesive.
Nuclei are uniformly round but may be obscured by cytoplasmic granules.
Cytoplasm is abundant, granular and slightly to heavily basophilic.
Cytoplasmic granules may or may not be visible with hematoxylin and eosin depending on the type of fixation.
Cytoplasmic granules are metachromatic and generally are positive for Giemsa, toluidine blue or other metachromatic stains.
Differential Diagnoses
Mast Cell Tumor, Benign:
A single, solitary, compact (dense) mast cell aggregate or nodule.
Compression of adjacent tissue.
Mast Cell Tumor, Malignant:
Compact solitary nodule, local sarcomatous growth or sheet-like accumulation(s) of round, spindle shaped or immature mast cells.
Cytoplasm is often hypogranular, but may have typical basophilic granules.
May have atypical bilobed or polylobed nuclei.
Located in the lymphoid tissue of the paracortex and medullary cords (not exclusively in the sinuses).
Eosinophils may be associated with the mast cells.
Destructive growth pattern, may be locally infiltrative.
Multiple organs may be involved.
No bone marrow involvement.
No clear inflammatory stimulus.
Considered malignant.
Mast cell leukemia:
Atypical mast cells are present in the bone marrow and/or peripheral blood.
Mast cell accumulations with sheet-like or leukemic pattern present in one or more hematolymphoid organs.
Comment
Mast cell numbers can vary according to location and species and strain of animal. The etiology and pathogenesis are often unknown. Mast cells in the peripheral tissues can migrate to the lymph node sinuses in response to certain hypersensitivity conditions. 123
desFIBROSIS (N) Lymph node
conFIBROSIS
enhFIBROSIS
Species
Mouse; Rat.
Other terms
Chronic inflammation.
Pathogenesis/cell of origin
Fibroblasts reactive to a local stimulus.
Diagnostic Features
Fibroblasts with oval to spindle shaped nuclei and eosinophilic extracellular matrix (collagen) when mature.
Increase in connective tissue and collagen with distortion of normal architecture/outline.
Often restricted to the capsule but may extend into subjacent node with loss of normal cells and architecture.
Differential Diagnoses
Plane of sectioning of normal tissue:
Tangential section of capsule.
Absence of fibroplasia, reactive cells.
Comment
Fibrosis restricted to the capsule/capsular surface of a lymph node is a sequel to inflammation or necrosis which may be localized to the node or secondary to local inflammation such as peritonitis.
MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT)
Organization
The mucosal immune system is organized into a) lymph nodes draining the mucosae, b) the more or less organized tissues associated with the mucosal epithelium, and c) mucosal single cells. Mucosal single cells include intraepithelial lymphocytes (IELs), macrophages and dendritic cells within the mucosal epithelium and lamina propria lymphocytes (LPLs), macrophages and dendritic cells in the mucosal lamina propria. Organized tissues associated with the intestinal mucosa are the Peyer’s patches (PP), colonic and rectal lymphoid aggregates, cryptopatches (CPs) and isolated lymphoid follicles (ILFs). Rodents do not have an appendix, but they have several variably developed lymphocytic aggregates associated with the cecum.133 The organized tissues associated with the respiratory mucosa are NALT (nose/nasopharynx-associated), LDALT (lacrimal duct-associated), LALT (larynx-associated) and BALT (bronchus-associated). BALT is normally present in (most strains of) rats, but needs to be triggered to become apparent in most strains of mice (inducible or iBALT). 134 In both rats and mice, BALT/iBALT are observed at a fixed location, namely at the bifurcations of bronchi and bronchioles. Although PP are already present at birth, their number increases after birth and can vary during life depending on antigenic stimulus. Thus, PP are also inducible as well as constitutive. Therefore the distinction between inducible and constitutive MALT is not strict.
Less well known in rodents is CALT/EALT (conjunctiva/eye-associated). Organized tissue associated with the urogenital mucosa has not been found in rodents, which suggests that the urogenital mucosae are served by MALT elsewhere, namely of the intestinal and/or respiratory tract.135 Non-lymphoid organs and tissues like mucosal glands are involved in the mucosal immune response as effector sites. 136 The mucosal immune system is connected to the systemic immune system via the mucosa-draining lymph nodes.
The above mentioned list of MALT is probably not complete as more MALT tissues will likely be identified in rodent mucosae in the future due to increased sampling or different planes of sectioning. Therefore it is important to keep in mind that aggregates of mononuclear/lymphocytic cells in the mucosae could be MALT rather than cell infiltrates responding to an inflammatory stimulus.
Definition and function of MALT
Several definitions are used for mucosa-associated lymphoid tissues, or MALT. Some definitions include the IELs and LPLs and the mucosa-draining lymph nodes. Here, the definition of MALT is restricted to the mucosa-associated, more or less organized tissues operating as immune-inductive or immune response-generating sites, 136 i.e. sites where naïve immune cells are triggered by antigens and where memory cells are generated. Therefore, MALT-like tissues in the salivary glands and elsewhere are not defined as MALT, because they are immune-effector, i.e. involved in the effectuation of the immune response, rather than immune-inductive sites. 137 It needs to be investigated if LDALT (lacrimal duct-associated) lymphoid tissue is really an immune-inductive site. However, the distinction between inductive and effector sites is not absolute, which really reflects the high plasticity of immune tissues and organs in general. Isolated lymphoid follicles and cryptopatches act as precursors of Peyer’s patches. IELs and LPLs are considered to be effector cells.
The mucosal immune system plays a decisive role in the relationship of the body itself with the enormous load of micro-organisms inhabiting the body (the microbiome). The immune system in the intestines needs to maintain a balance between tolerance of the microbiome and the uptake of nutrients on the one hand, and the exclusion of pathogens on the other hand.
In humans, certain B-cell lymphomas in gut, salivary glands and elsewhere, including kidneys, are being diagnosed ‘MALT lymphoma’ or Maltoma. There is often a background of chronic inflammation, either infective or of autoimmune character. 138 The term Maltoma or MALT lymphoma is linked to small B cells in the mantle zone of germinal centers. Since germinal centers can arise in mucosa-associated lymphoid tissues as well as in tertiary lymphoid structures (TLSs), the term MALT lymphoma is somewhat confusing.
Development
Most MALT is present at birth, and develops rapidly thereafter. 90 Age-related functional decline of the mucosal immune response has been described, but light-microscopic age-related changes in MALT of rodents have not been reported. Therefore, age-related involution is not described below as a separate item; comparison with concurrent controls is needed to decide whether or not age-related changes have occurred in a study. Abnormal MALT development has been reported in genetically engineered mice 139.
Histology
The compartments of MALT are outlined in Table 6.140 In general MALT is structured like lymph nodes, but MALT lacks afferent lymphatics. Instead, its epithelium (follicle-associated epithelium or FAE) and associated dendritic cells serve as porte d’entrée of antigens. 90,141–143. Species-dependent differences in the percentage of T and B cells in MALT have been described by Haley. 133
Table 6:
Compartments and cellular components of MALT*
| Compartment | Components |
|---|---|
| Follicle-associated epithelium (FAE) | - Microfold (M) epithelial cells within the epithelium (scarcity or absence of goblet cells) |
| - (dendrites of) Dendritic cells | |
| - Macrophages | |
| - Lymphocytes | |
| Subepithelial dome (SED) Syn. Dome |
- Dendritic cells |
| - Macrophages | |
| - T lymphocytes | |
| - Reticular cells | |
| Follicle | - Dominant B lymphocyte area |
| - Primary follicles contain small resting virgin B cells | |
| - Follicular dendritic cells (FDCs) | |
| Mantle | - Small resting B cells like in primary follicle |
| Germinal center | - B lymphocytes in several stages of development and maturation |
| - T lymphocytes | |
| - Follicular dendritic cells (FDCs) | |
| - Tingible body macrophages | |
| Interfollicular area Syn. Parafollicular area |
- Dominant T lymphocyte area |
| - T lymphocytes | |
| - B lymphocytes | |
| - Fibroblastic reticular cells (FRCs) | |
| - Dendritic cells | |
| - High endothelial venules (HEV) |
Adapted from Kuper et al. 2017. Compartmentalization is based on Peyer’s patches and NALT. Isolated lymphoid follicles (ILFs) and cryptopatches do not show such degree of organization.140
Sampling and diagnostic issues
Some guidance for sampling MALT may be helpful because sampling can influence the results. NALT sampled in situ within nasal cross sections can reveal prominent changes in NALT. However, the detection of more subtle effects requires dissected and longitudinally-embedded NALT, but this preparation damages nasal tissue (see 144 mice; 145 rat; 146 mice and rat). Peyer’s patches in the small intestine may not all react similarly to xenobiotics, thus standardized selection for microscopy is needed. 147 In rats, the number of grossly visible PPs in the 40 cm of ileum just proximal to the ileocecal junction can be counted as a general estimate, although it remains to be investigated if the distal PPs are sufficiently sensitive. An alternative method is the use of ‘Swiss rolls’ for microscopy, allowing examination of a considerable portion of the small intestines. 148 The use of ‘Swiss rolls’ also allows examination of solitary isolated lymphoid follicles and cryptopatches. Examination of cryptopatches can be facilitated by horizontal sectioning in a plane perpendicular to the villous axis. 149 When single lymphocytes in the epithelium or lamina propria are the focus of investigation, these cell populations can be isolated according to methods described by Sheridan and Lefrancois. 150 As mentioned under ‘Organization’, aggregates of mononuclear/lymphocytic cells at the mucosae could be MALT rather than being cell infiltrates due to an inflammatory stimulus, especially when they are observed consistently at a certain location.
Structures such as isolated lymphoid follicles (ILFs) and cryptopatches (CPs) in the intestines cannot be distinguished from tertiary lymphoid structures (TLSs) without the use of immunohistochemistry for specific lymphocyte subpopulations like innate lymphocytes. As a practical approach, TLSs in the gastrointestinal and respiratory tracts are only diagnosed when they are present outside the mucosa.
MALT is often not reported independently, but instead is recorded as part of the respiratory tract, nasal passages (NALT), lung (BALT) or intestines/gastrointestinal tract. Toxicity reports on Peyer’s patches and NALT are infrequent and reports on isolated lymphoid follicles and cryptopatches are essentially non-existent. 136,147,151 This could be due to sampling and diagnostic issues or because MALT is highly resistant to xenobiotic insults.
Non-Proliferative Changes
APLASIA/HYPOPLASIA (N) MALT
See General Hematolymphoid
APOPTOSIS, INCREASED, LYMPHOCYTE (N) MALT
See General Hematolymphoid
desCELLULARITY, DECREASED, LYMPHOCYTE (N) (Figures 111 and 112) MALT (mucosa-associated lymphoid tissue)
Figure 111.

Wistar rat, Peyer’s patch jejunum, follicle and interfollicular area. Cellularity decreased, lymphocytes.
Figure 112.

Wistar rat, Peyer’s patch jejunum, follicle and interfollicular area. Cellularity decreased, lymphocytes. Higher magnification of Figure 111.
conATROPHY, LYMPHOID
enh LYMPHOCYTES, DECREASED
(indicate compartment)
Species
Mouse; Rat.
Other terms
Lymphoid depletion.
Modifier
Lymphoid; lymphocyte
Pathogenesis/cell of origin
Decreased cellularity of lymphocytes.
Diagnostic Features
Decrease in size and/or cell density of individual Peyer’s Patches or other MALT (see comment), in a specific compartment.
Lymphoid necrosis or apoptosis.
Decreased germinal center development may accompany decreased cellularity or may be the most prominent feature.
Differential Diagnoses
Aplasia/Hypoplasia:
Incomplete or arrested development of one or more/all compartments.
Stroma may appear collapsed or prominent.
See General section for additional information.
Comment
Decreased lymphocyte cellularity (atrophy) can result from decreased lymphocyte and/or macrophage recruitment, direct immunotoxicity (may be accompanied by necrosis/apoptosis), decreased stimulation (activation) due to diminished antigenic presentation (e.g. by housing under SPF conditions), or stress and other endocrine disruption-related mechanisms. In the case of Peyer’s patches, the diagnosis of ‘decreased cellularity’ depends heavily on the plane of section and on the location in the small intestines.147,152 BALT is not normally seen histologically in most mouse strains unless there is antigenic stimulation (inducible BALT or iBALT) 139 so decreased cellularity is seldom an appropriate diagnosis in murine BALT.
desDEGENERATION, FOLLICLE-ASSOCIATED EPITHELIUM (N) (Figure 113) MALT (mucosa-associated lymphoid tissue)
Figure 113.

Wistar rat, nasal-associated lymphoid tissue (NALT). Degeneration, follicle-associated epithelium (FAE; arrows).
conDEGENERATION, FOLLICLE-ASSOCIATED EPITHELIUM
enh FOLLICLE-ASSOCIATED EPITHELIUM, DECREASED
Species
Mouse; Rat.
Other terms
Lympho-epithelial degeneration.
Pathogenesis/cell of origin
M cells and ciliated respiratory epithelium.
Diagnostic Features
Loss of cilia (ciliated respiratory epithelium).
Flattening and thinning of epithelial cells.
Differential Diagnoses
Necrosis:
Pyknosis or karyorhexis of nuclei.
Cytoplasmic eosinophilia.
Erosion/ulcer:
Loss of follicle-associated epithelium (erosion).
Loss of epithelium and underlying basement membrane (ulceration).
Metaplasia:
Change in epithelial cell types present, usually with a mixture of cell types in transition area.
Comment
Epithelial degeneration of ciliated respiratory epithelium and M (microfold) cells in NALT can be caused by exposure to xenobiotics or by an inflammatory condition such as rhinitis. 112,146 Degeneration of follicle-associated epithelium may alter uptake of antigens and thus may lead to changes in the underlying lymphoid tissue, such as atrophy or decreased or increased germinal center development. Follicle-associated epithelial alterations have not yet been reported for other MALT locations.
desHYALINE MATERIAL (N) (Figures 114 and 115) MALT (mucosa-associated lymphoid tissue)
Figure 114.

Rat, Peyer’s patch jejunum, subepithelial dome. Hyaline material, probably within macrophages (arrows).
Figure 115.

Rat, Peyer’s patch ileum, follicle and subepithelial dome. Hyaline material, capillary wall.
conHYALINE MATERIAL
enhHYALINE MATERIAL
(indicate compartment)
Species
Mouse; Rat.
Other terms
Eosinophilic material; Cysts; Hyaline change; Hyalinization; Paramyloid.
Pathogenesis/cell of origin
Deposition of eosinophilic material.
Diagnostic Features
Material may be associated with macrophages.
Predilection for the Peyer’s patch dome.
May be positive for immunoglobulin, amyloid, or other protein.
Positive for P component, protein AA, AL and FAP with immunohistochemistry.
Positive with Congo Red stain (green birefringence) to demonstrate amyloid.
Comment
Specific stains may help with identification of material (immunohistochemistry staining with antibodies against the different (sub)classes of immunoglobulins). The subepithelial dome appears to be a particularly sensitive area of the Peyer’s patch; fibrosis (scarring) and mineralization have also been observed in this area.
INFLAMMATION (N) MALT
See General Hematolymphoid
desLYMPHANGIECTASIS (N) (Figure 116)MALT (mucosa-associated lymphoid tissue)
Figure 116.

Wistar rat, mucosa-associated lymphoid tissue (MALT) lacrimal duct. Lymphangiectasis.
conLYMPHANGIECTASIS
enh LYMPHATICS, DILATED
(indicate compartment)
Species
Mouse; Rat.
Other terms
Dilatation of lymphatics, lymphatic ectasia.
Pathogenesis/cell of origin
Lymphatic vessels.
Diagnostic Features
Dilatation of the efferent lymphatics of MALT.
Differential Diagnoses
Angiectasis:
Vessels may contain red blood cells.
Neoplasia:
LYMPHANGIOMA, HEMANGIOMA or HEMANGIOSARCOMA
Extremely rare.
Plump endothelial cells, mitotic figures.
Comment
Dilatation of lymphatic vessels may be due to blockage of lymphatic outflow by diseases or to increased demand (increase in interstitial fluid), e.g. in (activated) lymphoid tissue associated with the lacrimal duct. 112
MINERALIZATION (N) MALT
See General Hematolymphoid
NECROSIS (N) MALT
See General Hematolymphoid
PIGMENT, MACROPHAGE (N) MALT
See General Hematolymphoid
TINGIBLE BODY MACROPHAGE, INCREASED (N) MALT
See General Hematolymphoid
Proliferative Changes (Non-Neoplastic)
Hyperplastic changes in all the hematolymphoid organs, including MALT, are generally reactive and are part of the normal physiological responses of these organs to acute and chronic insults or physiologic stimulation. Hyperplastic changes do not infer preneoplastic or precancerous lesions in these organs (see Introduction). However, severe or persistent lymphoid hyperplasia may increase the risk of neoplastic transformation. If there is a concern, clonality studies should be considered.
desAGGREGATES, MACROPHAGE (N) MALT (mucosa-associated lymphoid tissue)
conAGGREGATES, MACROPHAGE
enhAGGREGATES, MACROPHAGE
(indicate compartment)
Species
Mouse; Rat.
Other terms
Granulomatous inflammation; Histiocytic granuloma.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Adherent macrophages clustered together to form variably sized aggregates.
Cell borders may be distinct or may appear syncytial.
Macrophages may or may not contain pigment.
Hemosiderin often increased if present.
Normal cellular elements are not displaced.
Differential Diagnoses
Inflammation, granuloma:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or injected materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Histiocytic sarcoma:
Tumor cells are usually larger and more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Macrophages form aggregates when they cannot completely degrade microorganisms or ingested macromolecules, including some vehicles or test materials. Phagocytized test article may have a specific identifiable morphological character.
desCELLULARITY, INCREASED, LYMPHOCYTE (H) (Figures 117 and 118) MALT (mucosa-associated lymphoid tissue)
Figure 117.
Rat, Peyer’s patch ileum, follicle, interfollicular area, subepithelial dome. Cellularity increased, lymphocytes and macrophages. Note lymph vessels (lacteals) in villi filled with lymphocytes (arrows).
Figure 118.

Rat, gut-associated lymphoid tissue (GALT; also named lymphoglandular complex) colon, all compartments. Cellularity increased, lymphocyte.
conHYPERPLASIA, LYMPHOCYTE
enh LYMPHOCYTE, INCREASED
(indicate compartment)
Species
Mouse; Rat.
Other terms
Lymphocyte hyperplasia; Lymphocyte proliferation; Lymphocyte infiltration; Germinal center stimulation; Lymphoid accumulation.
Pathogenesis/cell of origin
Lymphocytes.
Diagnostic Features
Increased mucosal lymphoid tissue.
Increased cellularity.
Increased area.
- Increased size/number of germinal centers.
- Increased tingible body macrophages.
- Germinal centers may coalesce and form bizarre shapes surrounded by thin mantle zones.
- Increased macrophages in lymphoid tissue.
- Accumulation rather than aggregation.
- Diffuse or focal.
Efferent lymphatics are filled with lymphocytes.
HEV hypertrophy/hyperplasia.
HEVs prominent in interfollicular tissue.
HEVs may be increased in number.
Increased lymphocyte traffic across HEV walls.
Differential Diagnoses
Preneoplastic lymphocyte proliferation/lymphoma:
Normal MALT architecture is disturbed.
Lymphocytes invade the epithelial structures.
Comment
Local proliferation and influx of lymphocytes and/or increase in in macrophages occur in response to antigens or non-specific immune-stimulating compounds. Within a few days after stimulation, primary or resting follicles develop into secondary follicles with a germinal center. Germinal centers may last about three weeks after antigen administration. Increased germinal centers indicate increased activation of MALT. Generally, the Peyer’s patch closest to the cecum has the largest follicles with prominent germinal centers and distinct interfollicular areas, whereas those closer to the stomach are small and often without germinal centers. 147 Germinal centers in NALT are relatively uncommon. 153
desCELLULARITY, INCREASED, MACROPHAGE (N)MALT (mucosa-associated lymphoid tissue)
conHYPERTROPHY/HYPERPLASIA, MACROPHAGE
enh MACROPHAGES, INCREASED OR MACROPHAGES, HYPERTROPHY
(indicate compartment)
Species
Mouse; Rat.
Other terms
Macrophage accumulation; Macrophage infiltrate; Macrophage infiltration; Prominent macrophages; Histiocytosis; Histiocytic hyperplasia; Histiocytic infiltrate; Histiocytic aggregates.
Modifier
Tingible body; Pigmented; Vacuolated; Aggregates.
Pathogenesis/cell of origin
Monocyte/macrophages.
Diagnostic Features
Increased abundance and/or size of macrophages in lymphoid tissue.
Macrophages are generally individualized and have distinct cell borders.
Cytoplasm may or may not contain phagocytized material, pigment or vacuoles.
Can be diffuse or focal.
Differential Diagnoses
Aggregates, macrophage:
Multiple discrete clusters of adherent macrophages.
Lack organized structure or encapsulation.
Focal or multifocal.
Macrophages may transform into epithelioid cells.
Inflammation, granuloma:
Organized structure with a compact collection of epithelioid macrophages and other inflammatory cells which may include multinucleated giant cells.
Multifocal or diffuse lesions that may be variably associated with necrosis, other inflammatory cells, infectious agents or injected materials.
Associated with inflammatory conditions and exposure to xenobiotics.
Histiocytic sarcoma:
Tumor cells are usually larger and more atypical and pleomorphic than hyperplastic macrophages.
Multinucleated giant cells often present.
Nodular or coalescing sheets of neoplastic macrophages efface, displace or destroy normal architecture.
Other tissues may be involved.
Comment
Increased macrophage cellularity (hypertrophy/hyperplasia) in MALT may be a reactive response to a variety of conditions such as infectious diseases, immunological status, erythrocyte breakdown, metabolism of xenobiotics or distant neoplasia. Macrophages often increase in combination with phagocytosis, pigment storage or vacuolation so these processes are included as modifiers to allow the pathologist to construct the most appropriate diagnosis for a particular constellation of features. These findings can also be diagnosed separately.
desHYPERPLASIA, FOLLICLE-ASSOCIATED EPITHELIUM (H)MALT (mucosa-associated lymphoid tissue)
conHYPERPLASIA, FOLLICLE-ASSOCIATED EPITHELIUM
enh FOLLICLE-ASSOCIATED EPITHELIUM, INCREASED
Species
Mouse; Rat.
Other terms
Lympho-epithelial hyperplasia.
Pathogenesis/cell of origin
Follicle-associated epithelium (M cells intermingled with respiratory cells).
Diagnostic Features
Thickened epithelium as a result of an increase in number of respiratory and/or M cells.
Often in association with hyperplasia of the surrounding respiratory epithelium.
Epithelial hyperplasia may include proliferation of atypical, pleomorphic or undifferentiated cells.
Differential Diagnoses
Metaplasia, squamous, follicle-associated epithelium:
Hyperplasia of respiratory cells with a reduction in or even complete absence of M cells.
Comment
Hyperplasia of the follicle-associated epithelium may diminish or facilitate uptake of antigens, and thus may lead to inactivation or activation of underlying lymphoid tissue, seen as decreased cellularity (atrophy) with or without decreased germinal center development or increased cellularity (hyperplasia) with increased germinal center development. Follicle-associated epithelial hyperplasia has so far been reported for NALT, but not for Peyer’s patches. Exposure to xenobiotics or an inflammatory condition (such as rhinitis in the case of NALT) can cause these kind of epithelial alterations. 112,146 Distinction between M cells and respiratory cells is difficult in H&E-stained sections, unless M cells are numerous, have clustered together, and/or have enwrapped few or numerous lymphocytes (lymphocytes in cytoplasmic ‘pockets’ of the M cell) 154 In that case, M cells can be seen as a cluster of non-ciliated cells amidst ciliated epithelium in NALT. 155 In contrast, hyperplasia of respiratory cells can potentially lead to a decrease in number of M cells. In addition, granulocytes may occasionally be present in FAE.
desHYPERPLASIA, GOBLET CELL, FOLLICLE-ASSOCIATED EPITHELIUM (H)MALT (mucosa-associated lymphoid tissue)
conHYPERPLASIA, GOBLET CELL, FOLLICLE-ASSOCIATED EPITHELIUM
enh FOLLICLE-ASSOCIATED EPITHELIUM, GOBLET CELL, INCREASED
Species
Mouse; Rat.
Other terms
Lympho-epithelial goblet cell hyperplasia.
Pathogenesis/cell of origin
Goblet cells.
Diagnostic Features
Increased goblet cells.
Undulation of the epithelium by increased number of goblet cells.
Differential Diagnoses
None.
Comment
Normal follicle-associated epithelium contains a low number of goblet cells compared to the surrounding respiratory epithelium. Often, goblet cells are absent in the plane of section. Hyperplasia of goblet cells may present as an increase in the number of goblet cells or even as the presence of a few goblet cells in the plane of section. In more severe cases, the epithelium may undulate, due to clustering of goblets cells.
desHYPERTROPHY/HYPERPLASIA, HIGH ENDOTHELIAL VENULES (HEV) (N) (Figure 119) MALT (mucosa-associated lymphoid tissue)
Figure 119.
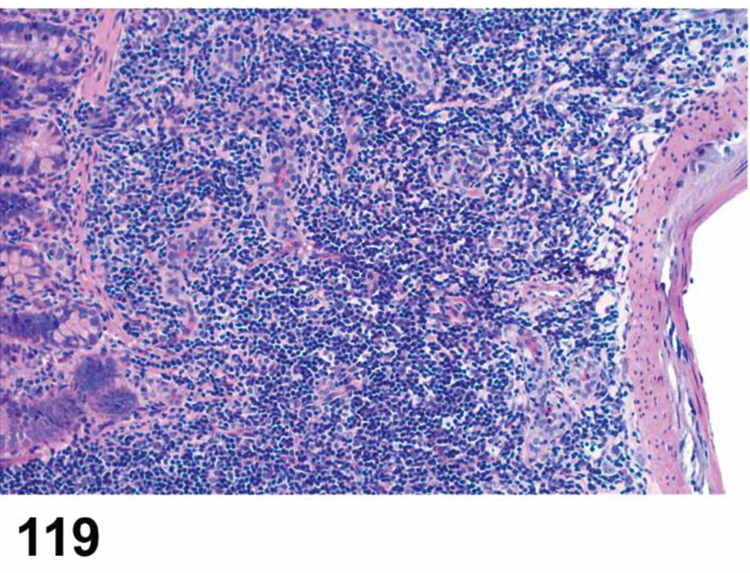
Rat, Peyer’s patch ileum, interfollicular area. Hypertrophy/hyperplasia high-endothelial venules (HEV).
conHYPERTROPHY/HYPERPLASIA, HIGH ENDOTHELIAL VENULES (HEV)
enh HIGH ENDOTHELIAL VENULES, INCREASED OR HIGH ENDOTHELIAL VENULES, HYPERTROPHY
(indicate compartment)
Species
Mouse; Rat.
Pathogenesis/cell of origin
Endothelium of HEVs.
Diagnostic Features
Prominent HEVs have an increased number of cross sections through the HEVs due to hyperplasia and (often associated) hypertrophy of the endothelium.
Increased height of endothelium and/or increased vascular elements.
Increased lymphocyte migration across vessel walls.
Associated MALT activation/lymphocyte hyperplasia occurs.
Comment
HEV hypertrophy/hyperplasia occurs in response to immune stimulation, specifically to cytokines delivered to the HEVs via the FRC conduit system. Hypertrophy of the endothelial cells increases lymphocyte recruitment across the HEV wall and is usually associated with increased cellularity of the lymphoid tissue. It is not known if HEV hypertrophy/hyperplasia is always part of activation of MALT, i.e. lymphocyte hyperplasia and germinal center development, but it is observed upon antigenic stimulation in lymph nodes and Peyer’s patches. 156–158
desMETAPLASIA, SQUAMOUS, FOLLICLE-ASSOCIATED EPITHELIUM (H)MALT (mucosa-associated lymphoid tissue)
conMETAPLASIA, SQUAMOUS, FOLLICLE-ASSOCIATED EPITHELIUM
enh FOLLICLE-ASSOCIATED EPITHELIUM, METAPLASIA, SQUAMOUS
Species
Mouse; Rat.
Other terms
Lympho-epithelial squamous metaplasia.
Pathogenesis/cell of origin
Follicle-associated epithelium.
Diagnostic Features
Replacement of the follicle-associated epithelium by squamous epithelium.
Several layers of stratified epithelial cells, with flattening of the more superficial cells.
Ciliated epithelial cells are absent.
Surface cells may contain keratohyaline granules or may be keratinized.
Desquamation of surface cells may occur.
Differential Diagnoses
Degeneration/regeneration:
Usually follows acute injury.
Cells are only one or two layers thick.
No horizontal layering of flattened superficial cells.
Comment
Squamous metaplasia of the follicle-associated epithelium is expected to alter uptake of antigens, and thus may lead to inactivation or activation of underlying lymphoid tissue, seen as atrophy with or without decreased germinal center development or increased cellularity and increased germinal center development. Follicle-associated epithelial metaplasia has so far been reported for NALT, but not for Peyer’s patches. Exposure to xenobiotics or an inflammatory condition (such rhinitis in the case of NALT) can cause these kind of epithelial alterations. 112,146
OTHER LYMPHOID TISSUES
Tertiary Lymphoid Structures (TLSs)
Organization
Under chronic inflammatory conditions, lymphocyte-specific microdomains can be formed in non-lymphoid organs like salivary glands, liver, pancreas, thyroids, joints and kidneys. 159 The liver has a hematopoietic function during embryonic development which is lost postnatally, but the liver can host TLS in adult life.160 The lymphocyte-specific microdomains are defined as tertiary lymphoid structures (TLSs) and can arise as a result of autoimmune responses (as for example in rheumatoid arthritis, Sjogren’s disease and Hashimoto’s thyroiditis), graft rejection, atherosclerosis (humans and ApoE knockout mouse), microbial infection, and neoplasia 161.
Function
TLSs function like lymph nodes or MALT. Their precise role in disease progression is still unknown. Their presence in specific areas suggest that they facilitate local antigen presentation and immune responses and likely play a role in epitope spreading by cross-reactivity of immune cells and antibodies with endogenous host cellular antigens. Formation of well-developed TLSs with germinal centers are often associated with increased severity of (autoimmune) disease and the local production of autoantibodies, but TLSs can have an immune-protective role as well, especially in resolving chronic inflammation. The latter has been observed in an atherosclerosis model in the APOE knock-out mouse. 162 TLSs are often indicators of good prognosis in cancer patients. 163
Development
TLSs are not present at birth and do not develop at a pre-destined location (anlage), although their formation is driven by many of the same cytokines which drive the development and maintenance of lymph nodes and MALT. 161 TLSs do not have specific developmental windows or anatomic locations, but instead arise in inflamed tissues. 161
Histology
TLSs contain B-and T- lymphocytes, follicular and non-follicular dendritic cells, fibroblastic reticular cells (FRCs) and high endothelial venules (HEVs) and may contain efferent lymphatic vessels. 164–169 Like MALT, they may not be supplied by afferent lymphatics and they are not encapsulated. The B- and T- lymphocytes are organized in similar fashion to lymph nodes and MALT, with T- and B-cell dominant areas including follicle-like compartments with germinal centers. Lymphoid cell movement into and through TLS appears similar to that seen in the lymph node and is regulated by the cytokines permitting cells to enter via HEVs, traffic along the FRC meshwork and exit via lymphatic sinuses. TLS can progress into a destructive inflammation and lose this lymphoid organ-like organization. 168
Sampling and Diagnostic Considerations
TLSs are not reported independently, but are instead regarded as chance findings in non-lymphoid organs. Lymphoid follicles with germinal centers that are located in non-lymphoid organs can be designated as TLS. Without follicle formation (and in the absence of distinct HEVs), it is impossible to distinguish TLSs from lymphocyte infiltrates in H&E-stained sections unless histochemistry/immunohistochemistry reveals a lymphoid tissue organization with dendritic cells, fibroblastic reticular cells, some (indistinct) HEV and lymphatics.
The distinction between TLS and MALT in the gastrointestinal tract and conducting airways can be complicated. Inducible BALT (iBALT) is sometimes considered to be TLS 134,170 because of its dependence on inflammatory signaling, but, unlike TLS, it has predestined locations (at the bifurcation of brochi/bronchioles). Likewise, isolated lymphoid follicles (ILFs) and cryptopatches (CPs) in the gastrointestinal tract resemble TLSs in their apparent dependence on inflammatory stimuli. This document considers all types of mucosal lymphoid accumulationsto belong to MALT. Increases in, or remarkable presence of, iBALT, ILFs and CPs or comparable structures are diagnosed according to ‘Proliferative changes, non-neoplastic’ in the MALT section. Lymphoid follicles outside typical MALT locations are diagnosed TLS.
desTERTIARY LYMPHOID STRUCTURES (TLSS) (N) (Figure 120) Other lymphoid tissues
Figure 120.

Mouse, tertiary lymphoid structure in Harderian gland. High endothelial venules (HEVs) are present (arrows).
conTERTIARY LYMPHOID STRUCTURES (TLSS)
enhTERTIARY LYMPHOID STRUCTURES (TLSS)
Species
Mouse; Rat.
Other terms
Tertiary lymphoid organs (TLOs); Tertiary lymphoid tissues; Lymphoid follicles; Germinal centers; Lymph node-like structures; Ectopic lymphoid structures; Lymphoid neogenesis.
Pathogenesis/cell of origin
Precise pathogenesisis unknown. Under chronic or unresolved inflammatory conditions, lymphocyte-specific microdomains can form in inflamed non-lymphoid organs or tissues.
Diagnostic Features
Ectopic i.e. in non-lymphoid organs such as salivary glands, thyroid, liver, pancreas, kidney or outside mucosa in the gastrointestinal and respiratory tracts.
Uncommon in brain and skin.
Follicle formation, preferably with some germinal center development.
Presence of distinct HEVs.
Presence of inflammatory condition involving the organ of concern.
Differential Diagnoses
Inducible BALT (iBALT) in the lungs and ILFs in the intestines:
iBALT: located between bronchus/bronchioles and artery and often in association with the airway epithelium.
ILF: located in the mucosa in association with the intestinal epithelium.
Increased SALCs in the body cavities:
Located in the serosa and adipose tissue depots of the abdominal and thoracic cavities.
Follicles (with germinal centers) absent or rare.
Lymphocyte inflammatory cell infiltrates:
Infiltrates lack organization, especially follicle formation and presence of HEVs.
May include granulocytes or other types of inflammatory cells.
Lymphoma:
Presence of lymphocyte accumulations elsewhere.
Involvement of primary and/or secondary lymphoid organs.
Absence of an inflammatory stimulus.
Comment
Immunohistochemical staining for follicular and non-follicular dendritic cells, fibroblastic reticular cells, HEVs and other structural elements can help in the diagnosis of TLS in case a distinct follicle formation is absent, because it can reveal the lymphoid organ-like organization of the lymphocyte accumulation(s).
Serosa-Associated Lymphoid Clusters (SALCS)
Organization
Serosa-associated lymphoid clusters (SALCs) include fat-associated lymphoid clusters (FALCs) and milky spots (MSs), also known as Kampmeier’s foci, which are considered to be identical structures that differ only in location. 171 Serosa-associated lymphoid clusters (SALCs) 172 are tiny white (‘milky’) lymphoid foci present in the serosa and the adipose tissue depots of the peritoneal, pleural and pericardial cavities.
Function
The presence of SALCs in the serosal surfaces in the peritoneal and thoracic cavities points to a role as first line defense in the cavities. During intraperitoneal inflammation, they increase greatly in number and size in the omentum. They may be observed in increased numbers following intraperitoneal injection and may function as ‘the MALT of the serosa’. Based on the abundant presence of innate lymphoid cells (ILC and B1 B cells) and innate B1 B cells, the clusters probablyform a special link between innate and adaptive immunity. The lymphoid clusters may play a role in tumor metastasis as well, since they are the major site of tumor dissemination in the peritoneal cavity. 173
Development
In the human omentum, SALCs are present before birth. 171 Although considered to be secondary lymphoid organs, they may develop via different molecular pathways than lymph nodes and spleen. 174 Part of the clusters may develop after birth and only upon an inflammatory stimulus, resembling tertiary lymphoid structures. 175
Histology
SALCs consist of clusters of lymphocytes (including innate lymphoid cells), macrophages, plasma cells and mast cells located immediately below, and covered by, the mesothelium. They can be highly vascularized and have HEVs and efferent lymphatics. SALCs are not well-organized; distinct T- and B-dominant areas are absent and follicles with or without germinal centers are rare or absent. 172,176
Sampling and Diagnostic Considerations
SALCs are not reported independently, but instead are often a chance finding in the serosa of the lungs, heart or abdominal organs. They may increase in response to intraperitoneal injections. They are generally not diagnosed unless they are abundant. It is often difficult to distinguish these lymphoid clusters from lymphocyte infiltrates in H&E-stained sections, let alone to establish whether the clusters are induced or pre-existent (hyperplastic). For practical reasons, in this document all lymphoid clusters in the adipose tissue depots of the abdominal and thoracic cavities are considered to be SALCs. The absence of inflammatory findings such as adipose tissue necrosis, abscesses, adhesions, increased granulocytes and hemorrhages distinguishes SALCs from peritonitis.
des SALCS, INCREASED (N) (Figure 121) Other lymphoid tissues
Figure 121.

Wistar rat, serosa-associated lymphoid clusters (SALCs, also named fat-associated lymphoid clusters or FALCs) outside the pleura. Well-vascularized and rich in lymphocytes.
con SALCS, INCREASED
enh LYMPHOCYTES, ADIPOSE TISSUE, INCREASED
Species
Mouse; Rat.
Other terms
Fat-associated lymphoid clusters (FALCs); Milky spots (MSs); Kampmeiers foci.
Pathogenesis/cell of origin
Precise histogenesis unknown.
Diagnostic Features
Lymphoid clusters composed of variable populations of B and T lymphocytes with macrophages and myeloid cells, depending on the stimulus.
Always in association with the serosa, most often in association with adipose tissue depots in the abdominal and thoracic cavities.
Clusters are covered by mesothelium.
May show some degree of organization.
Macrophages situated at the periphery.
HEVs are oftenpresent.
Follicles absent or rare.
Differential Diagnoses
Lymphocytic inflammatory cell infiltrates:
Infiltrates lack organization (but so may SALCs).
Necrotic adipocytes surrounded by macrophages and lymphocytes, in case of low-grade inflammatory cell infiltrates as seen in metabolic disease (called ‘crown-like structures’).
Absence of a distinct number of innate lymphoid cells (mainly nuocytes/natural killer cells and B1 B cells), as demonstrated by immunohistochemistry.
Lymphoma:
Presence of lymphocyte accumulations elsewhere.
Involvement of primary and/or secondary lymphoid organs.
Comment
Immunohistochemical staining for B1 B cells, dendritic cells, fibroblastic reticular cells, HEVs and other structural elements can help diagnose SALCs (FALCs/Milky spots) and distinguish them from infiltrates and lymphoma, especially when follicle formation is absent, because it can reveal the lymphoid organ-like organization of the lymphocyte accumulation(s).
HEMATOLYMPHOID NEOPLASMS
The WHO classification system of hematopoietic and lymphoid tumors is an advanced nomenclature for clinical purposes in humans. 177–179 In a multidisciplinary approach, this classification integrates immunophenotyping, morphology, degree of differentiation, genetic and molecular features for recognition of distinct disease entities and extends to early lesions identifiable by hematological investigations. The final goal of this classification is to connect pathology to clinical correlates, treatment, and prognosis. In an experimental setting with a scope of translational research, such as transgenic mouse models, the adoption of the most recent human classification could be applied. 21,54,108,180 In these cases, optimal and reproducible fixation conditions can be provided. The use of formalin-fixed paraffin embedded (FFPE) tissues is well established for mice, less so for rats. 103,181 An antibody panel for the investigation of murine hematopoietic tissues has been described by Kunder 181 and immunophenotyping using T- and B-cell markers can be applied. The pan-T-cell marker CD3 can be used after various fixatives such as Bouins, B-5, Zenkers, paraformaldehyde and formalin-fixed tissues.
In 18–24 month rodent carcinogenicity studies, the focus is on risk assessment rather than on translational research for clinical outcome and treatment. To meet the requirements of a classification system for rodent carcinogenicity studies, which are mainly based on H&E morphology, a more practical WHO-classification was developed with fewer subtypes for rat and mouse. 182,183 In risk assessment for a carcinogenic effect, fewer categories are advantageous as the overall incidence of lymphomas is often combined in the initial assessment of treatment-related effects. If an effect is observed, a further investigation of subtypes using IHC can be of interest to further characterize the type of tumor. Organs of decedents are often stored in formalin for prolonged and variable time periods over the course of the studies which may lead to inconsistent results of immunohistochemical stains. However, when fixation conditions are optimal, morphological classification can be further expanded, if desired, by immunotyping using T/B cell markers.
The progeny of hematopoietic stem cells such as myeloid and lymphoid lineages can give rise to autonomously growing cell clones forming systemic tumors including myeloproliferative neoplasms, histiocytic sarcoma, and lymphoma. Differentiation between these tumor entities is often difficult due to morphological similarities arising from relatedness of their cells of origin. It may be helpful to evaluate bone marrow cytology or blood smears, but they are not, however, routinely included in rodent carcinogenicity studies.
Hematopoietic tumors are inducible with various chemicals, viruses, and radiation, but they are also frequently observed spontaneously. Particularly high and variable incidences have been observed in aged mice. The etiology of spontaneous hematopoietic and lymphoid tumors in rodents is not well understood, and historical data from the same strain are helpful for an appraisal of tumor incidences. Notably, such tumors often arise as sporadic incidental findings earlier than most other tumors in rodents. In mice, T-cell lymphomas may arise spontaneously or by induction even younger than 3 months of age.
Besides tumors of hematopoietic and lymphoid cells, hematolymphoid organs can develop tumors of their stromal components. In the thymus, the epithelial reticular cells can give rise to proliferative lesions. These cells are not merely a mechanical scaffold, but support homing, proliferation, and maturation of mobile cells, 184 so proliferative stromal changes can be associated with increased or altered populations of lymphoid cells. Variable mixtures of epithelial and T-cells are reported in thymomas in humans 185, rats 183 and mice 182 according to the WHO-classification. Neoplastic stromal cells may lose their supportive abilities, as in malignant epithelial thymomas with squamous differentiation in which the lymphocytic component may be virtually absent. Reticulum cell sarcoma was reported in the mouse and rat in the early rodent literature and was divided into Types A and B by Dunn. 35 Further work on these tumors using modern techniques reclassified Dunn’s Type B tumor as B-cell origin lymphoma and Dunn’s Type A tumor as of histiocytic origin. 108,186 In recent years, the concept of reticular cell tumors has been modified and tumors of the fibroblastic reticular cell (FRC) have been described in humans 187–190 but they have not been demonstrated in the laboratory mouse or rat to date. Clones of C57BL/6 non-neoplastic FRCs have been immortalized and characterized. 190
Finally, vascular proliferative changes such as hemangioma or hemangiosarcoma often occur in lymphoid organs of aging rodents. These tumors are beyond the scope of the Hematolymphoid System and are presented in the Cardiovascular System. Vascular tumors should be considered in the differential diagnosis for tumors with a high amount of connective tissue and capillary vascular spaces, however.
Hematopoietic Neoplasms
LEUKEMIA, ERYTHROID (M) (Figures 122 to 124) Hematolymphoid neoplasms
Figure 122.

Mouse, liver. Erythroid leukemia.
Figure 124.

Mouse, spleen imprint. Erythroid leukemia.
Species
Mouse; Rat.
Other terms
Erythroleukemia; Erythroblastic leukemia.
Pathogenesis/cell of origin
Develops from the erythroid cell lineage in the spleen or bone marrow.
Diagnostic Features
Leukemia with excessive proliferation of erythroblasts, immature erythroid cells or normoblasts.
Undifferentiated blast cells are more common, but more well differentiated tumors may occur exhibiting all stages of erythroid differentiation.
Immature cells may have signs of atypia.
Often arises in splenic red pulp, particularly in mice; white pulp is compressed and atrophic.
Preferential spread to liver sinusoids.
Splenomegaly and hepatomegaly may be severe while lymph nodes are not involved.
Enlarged spleen may have numerous subcapsular hematomas.
Immature erythrocytic cells can be identified with specific antibodies and/or with benzidine (p-diaminobiphenyl). The expression intensity of antigens varies depending on the stage of differentiation of the erythroid cells. Some antigens increase in intensity with maturation (glycoprotein or glycophorin A [CD235a] and TER 119), while the intensity of other antigens decreases as the erythroid cells mature (GATA1, alpha hemoglobulin stabilizing protein, CD43, CD71, CD117).
Neoplastic cells may occur in the blood and diagnosis can be confirmed in blood or bone marrow smears, if available
Differential Diagnoses
Lymphoma:
No evidence of erythroid differentiation.
Differentiation from lymphoma may be difficult if erythroid development is arrested at a very primitive stage.
Definitive diagnosis of primitive erythroid cells generally requires the use of specific antibodies that recognize antigens expressed during normal erythropoiesis (CD117, CD43, GATA1, CD71, CD235a, TER119, alpha hemoglobin stabilizing protein) versus lymphoid antigen antibodies for lymphoid cells (PAX5, CD3, CD25, CD45, TDT).
Extramedullary hematopoiesis (EMH), Increased with predominant erythroid proliferation:
A hematopoietic stimulus is present such as anemia, inflammation, etc.
Mixture of cell lineages is present.
Erythroid leukemic cells are immature with signs of atypia.
Usually localized to the spleen, but foci of reactive hematopoiesis may be present in hepatic sinusoids and occasionally in other tissues.
Proliferating cells are not invasive and follow the normal distribution of EMH.
When severe, extramedullary erythropoiesis involving both the spleen and liver may be difficult to distinguish from erythroid leukemia.
Refer to General Terms for additional information.
Dyshematopoiesis, erythroid:
Erythroid hypercellularity.
Aberrant erythropoietic cell morphology such as multinuclearity, nuclear fragmentation, megaloblastosis or ringed sideroblasts.
Evidence of arrest in a stage of differentiation. 44
LEUKEMIA, MEGAKARYOCYTIC (M) (Figures 125 and 126) Hematolymphoid neoplasms
Figure 125.
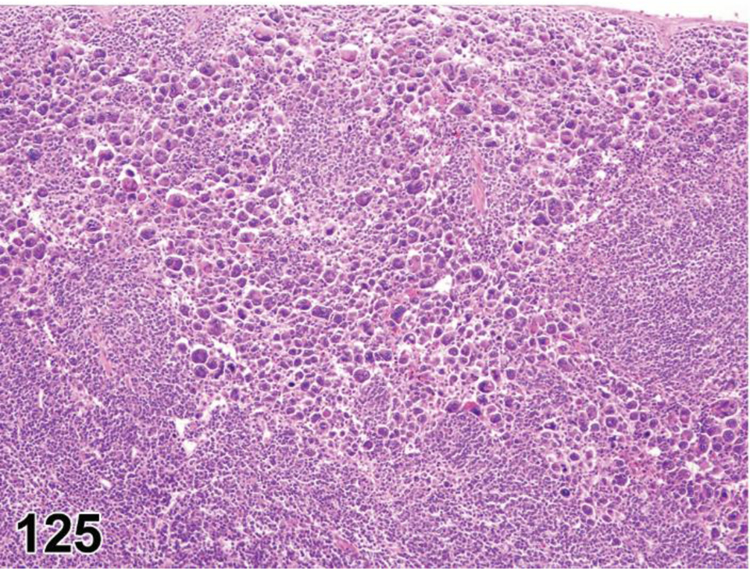
Mouse, spleen. Megakaryocytic leukemia.
Figure 126.

Mouse, spleen. Megakaryocytic leukemia.
Species
Mouse; Rat.
Other terms
Megakaryoblastic leukemia; Megakaryocytic myelosis.
Pathogenesis/cell of origin
Develops from the megakaryocytic cell lineage in the spleen and bone marrow.
Diagnostic Features
Increase in megakaryocytes in various stages of differentiation.
Marked increase of large mononuclear megakaryoblasts; smaller immature forms are often present.
Nuclei are often atypical and may or may not be multinucleated.
Tumor cells may be present in bone marrow, lymph nodes, spleen, liver and kidneys.
If lymph nodes are involved, megakaryocytes may be present in medullary sinuses.
Large dysplastic platelets are present in circulation.
Thrombocytopenia or pancytopenia may be evident.
Identification of a megakaryocyte lineage often cannot be made by morphologic features alone and immunophenotyping may be required. 191
Positive for platelet glycoprotein IIb/IIIa, CD41, GATA1, CD61 and von Willebrand factor (Factor VIII) by immunohistochemistry.
RUNX1 antibody can also be used to identify immature megakaryocytic stages of maturation.
Differential Diagnoses
Extramedullary hematopoiesis (EMH), megakaryocytic:
Megakaryocytic EMH in the splenic red pulp is usually associated with an increase in mature megakaryocytes with no evidence of blast cells.
Dysplasia, hematopoietic megakaryocytic:
Increased megakaryocytes with abnormal morphology and thrombocytopenia.
Differentiation arrest.
Leukemia, erythroid:
No multinucleated giant cells resembling normal or atypical megakaryocytes are present.
Differentiating megakaryoblastic leukemia from immature erythroid (erythroblastic) leukemia may require immunohistochemistry.
An IHC antibody panel consisting of CD41, CD61, von Willebrand factor and RUNX1 for megakaryocytic cells and glycophorin A (CD 235a) and alpha hemoglobin stabilizing protein (aHSP) for erythroid cells can be helpful in differentiating these two leukemias.
Comment
Megakaryocytic leukemia is rare in conventional mice. It has been described in genetically engineered mice and has been reported to be induced by a recombinant retrovirus (MuLV).
Comment
Erythroid leukemia is a rare spontaneous lesion in the mouse and is also inducible with MuLVs such as the Friend virus which induces Friend leukemia. Erythroid leukemia can also be induced by whole body radiation in C3H/He mice and RF mice. Erythroid leukemia is an extremely rare spontaneous lesion in the rat. 192 It is inducible after radiation and trimethylbenz[a]anthrene treatment.
LEUKEMIA, MYELOID (M) (Figures 127 to 129) Hematolymphoid neoplasms
Figure 127.

Mouse, liver. Myeloid leukemia.
Figure 129.
Mouse, spleen. Myeloid leukemia, moderately differentiated.
Species
Mouse; Rat.
Other terms
Granulocytic leukemia; Acute myeloid leukemia; Myeloblastic leukemia; Chloroleukemia; Granulocytic sarcoma (for localized growth).
Modifier
Neutrophilic; Eosinophilic; Basophilic; Myelomonocytic; Monocytic; NOS (not otherwise specified).
Pathogenesis/cell of origin
Develops from the granulocytic or monocytic cell lineage in the spleen (especially in the mouse) or bone marrow.
Diagnostic Features
Myeloid cell differentiation varies from immature (poorly differentiated) to mature with variable proportions of blastic to segmented forms.
Nuclei may have immature morphological features including indented nuclei and doughnut-shaped ring forms with a central opening.
Myeloblast nuclei have fine chromatin and prominent nucleoli while promyelocyte nuclei have dense coarse chromatin and less distinct nucleoli.
Myeloblasts frequently have azurophilic granules containing myeloperoxidase but they may not be visible in H&E sections.
Blood and/or bone marrow smear preparations may be helpful for the investigation of cytological details.
Some types of myeloid leukemia can develop very high white cell counts (up to 1 million cells/µl) consisting primarily of myelocytes and mature neutrophils, while other types (myeloblastic leukemia) consist primarily of cells resembling myeloblasts and promyelocytes.
Leukemia frequently occurs with diffuse organ enlargement, usually involving the spleen or bone marrow with secondary involvement of liver and other organs.
The spleen and liver may become very large.
Tissue masses may have a characteristic dull red color; infiltrated tissues may show a greenish discoloration macroscopically (hence the name chloroleukemia).
Late promyelocytes and later developmental stages are positive for lysosome markers, myeloperoxidase, chloroacetate esterase, lysozyme and other granulocytic antigens.
Kidneys may have increased eosinophilic granules (hyaline droplets) in the proximal tubules (as also occurs with histiocytic sarcoma).
Differential Diagnoses
Extramedullary hematopoiesis (EMH), Increased with predominant myeloid proliferation:
Differentiating extramedullary hematopoiesis from neutrophilic myeloid leukemia can be difficult.
Usually more mature stages of myeloid cells are present.
Often has an intermixture of relatively high numbers of erythroid precursors and megakaryocytes.
Morphology and maturation sequences are synchronous.
Peripheral white blood cell counts usually do not reach the extremely high levels more commonly seen in myeloid leukemia.
Generally occurs as a reactive response to conditions such as chronic dermatitis, ulcerated skin tumors, repeated blood loss, necrotic tumors, abscesses and increased red cell destruction.
Lymph nodes are frequently involved, especially mesenteric lymph nodes and regional draining nodes of a lesion.
In rare cases, other organs may be involved such as adrenal, ovary, pituitary and perirenal fat.
Distribution tends to follow normal anatomical boundaries and does not appear invasive except for proliferation in the epidural space and in the extramedullary sinus along bones.55
Dyshematopoiesis, Granulocytic:
Abnormal or defective differentiation of myeloid lineage.
- Increased numbers of granulocytic precursor cells.
- Abnormal nuclear segmentation or asynchrony of chromatin maturation and nuclear segmentation.
- Altered granule morphology and/or abnormal cytoplasmic features (e.g., size, shape, number, and/or tinctorial quality). 33
Comment
Modifiers may be used to indicate the lineage of the tumor cells if desired. The modifier NOS (not otherwise specified) can be used when cell lineage is desired but cannot be determined. Lineage is based on the tinctorial characteristics of the cytoplasmic granules, when present, but definitive subtyping may require immunohistochemistry. Smear preparations may be helpful for the investigation of cytological details.
Myeloid leukemias that consist of predominantly blasts and immature forms, i.e. myeloid leukemia without maturation (<10% of mature granulocytes), myeloid leukemia with maturation (>10% of mature forms), myelomonocytic leukemia and monocytic leukemia, are extremely difficult to differentiate from one another without cellular lineage characterization by either flow cytometry, immunohistochemistry or histochemistry for myeloperoxidase. Therefore, the most appropriate diagnosis for these types of leukemia is simply myeloid leukemia. Chronic myeloid leukemias have been described in genetically engineered mice with a Philadelphia chromosome. 193
The term ‘myeloid’ is commonly used to refer to the white blood cell component of bone marrow. Histologically, the granulocytic series is the predominant lineage of the white blood cell component. The most common form of myeloid leukemia arises from the neutrophilic lineage. Thus, myeloid leukemia, which originates from the myeloblast, is often called granulocytic leukemia. Myeloid leukemia can also be used in a broader sense for any leukemic hematopoietic neoplasm derived from a common myeloid progenitor cell, including granulocytic, granulocytic/monocytic, erythroid and megakaryocytic leukemia, mast cell tumors and histiocytic sarcoma.
LEUKEMIA, NOS (M) Hematolymphoid neoplasms
Species
Mouse; Rat.
Synonym(s)
Leukemia.
Other terms
Unclassifiable leukemia; Undifferentiated leukemia; Anaplastic leukemia.
Pathogenesis/cell of origin
Develops from cells of the hematopoietic tissue.
Diagnostic Features
Leukemia with leukemic infiltration in various organ systems.
Liver, spleen and/or lymph nodes may be enlarged at necropsy.
Differential Diagnoses
Leukemoid reaction (leukocytosis):
Mature granulocytes in capillary beds.
Most often seen in lungs and liver.
Usually associated with a gross lesion in another tissue, i.e. abscess, ulcerated skin, etc.
Comment
Leukemia NOS (not otherwise specified) can be used when classification is desired but a definitive lineage cannot be determined by IHC or other lab techniquessuch as FACS and gene expression studies. Leukemia NOS can also be used when the leukemia is unclassifiable due to the condition of the specimen (autolysis, poor fixation or technique). Leukemia (with no modification) may be used when classification is not desired.
Lymphoid Neoplasms
LYMPHOMA (M) (Figures 130 to 151) Hematolymphoid neoplasms
Figure 130.

Mouse, spleen. Lymphoblastic lymphoma, with the starry sky effect.
Figure 151.

Rat, liver. LGL leukemia, OX-8 (CD8α).
Species
Mouse; Rat.
Other terms
Malignant lymphoma; Lymphosarcoma
Modifier
Subtype modifiers:
Lymphoblastic; Pleomorphic; Follicular; Immunoblastic; Lymphocytic; Plasmacytic; Epitheliotrophic cutaneous T-cell; Marginal zone; LGL, NOS.
Cell type modifiers:
T cell; B cell.
Qualifier (optional):
Leukemic (can be used when there is an obvious leukemia present judging from the peripheral blood smears or tissue sections)
Pathogenesis/cell of origin
Develops from T or B lymphocytes or their precursors in the spleen, lymph nodes, thymus or bone marrow.
Diagnostic Features
Lymphoblastic lymphoma:
Medium-sized to large lymphoblasts.
Cytoplasm is scant to moderate, basophilic and may be vacuolated.
Nuclei are round, oval, irregular or convoluted with finely stippled chromatin.
A central nucleolus ranges from inconspicuous to 1–3 distinct small nucleoli.
Nuclear to cytoplasmic ratio is high.
Mitotic figures are variable in number, but are often numerous.
Cells are non-cohesive but form homogeneous sheets.
Starry-sky appearance is often present with tingible body macrophages and apoptosis.
May become leukemic in later stages.
Aggressive behavior with diffuse infiltration of liver, kidneys and ovaries and infiltration along the vascular tree in the lung (similar to immunoblastic lymphoma).
- May invade the central nervous system when in a leukemic phase.
T-cell lymphoblastic lymphoma
- Originates in the thymus in young rats and mice.
- Occurs at a high frequency in some strains and genetic targeted mice.
B-cell lymphoblastic lymphoma
- Arises in the spleen or lymph nodes in old mice.
- Positive for PAX5 and generally positive for CD45R but may be negative for CD45R by IHC when cells are of the pro-B stage.
Pleomorphic lymphoma:
Neoplastic cell population can consist of several cell types including small lymphocytes, small and large follicular center cells (centrocytes and centroblasts), immunoblasts and small and large cells of non-follicular origin.
The proportion of each cell type varies in each lymphoma and sometimes even in each anatomical site.
Multinucleated cells may be present.
Blastic cells have prominent nucleoli.
Cells with cleaved nuclei (centrocytes) are seen in variable proportions to cells with round nuclei (centroblasts).
Cells may be admixed with variable proportions of T helper CD4+ cells, macrophages and/or eosinophils.
Single or multicentric origin.
Most common lymphoma subtype in aged mice (>12 months old) in most strains.
Early single-site pleomorphic lymphomas common in mesenteric lymph nodes and spleen.
Occur in Peyer’s patches in aged rodents.
Growth pattern is typically diffuse and generally follicles and germinal centers are not apparent (in contrast to humans where they are apparent).
When present, follicles may result in a nodular appearance, especially in spleen.
Rarely leukemic in distribution.
- Tumor cells are most often of follicular B-cell origin, but may rarely be of T cell origin.
- IHC or flow cytometry is necessary to determine B or T cell origin.
- Pleomorphic lymphoma may be diagnosed when the tumor cell type is mixed or unknown.
- Follicular lymphoma is correct and preferred terminology if B-cell origin is determined.
- Pleomorphic lymphomas of B cell origin may produce heavy chain immunoglobulins and express kappa light chains far more frequently than lambda light chains.
Follicular lymphoma
Follicular lymphoma is of B cell origin and is the preferred terminology if B-cell origin is determined, especially by immunohistochemistry and/or with clonal assays.
Neoplastic cell population can consist of several cell types including small lymphocytes, small and large follicular center cells (centrocytes and centroblasts),
The proportion of each cell type varies in each lymphoma and sometimes even in each anatomical site
Multinucleated cells may be present
Blastic cells have prominent nucleoli
Cells with cleaved nuclei (centrocytes) are seen in variable proportions to cells with round nuclei (centroblasts).
Cells may be admixed with variable proportions of T helper CD4+ cells, macrophages and/or eosinophils
Single or multicentric origin
Positive for CD45R/B220, PAX5 CD79a, Bcl6 and/or PNA
Immunoblastic lymphoma:
Cells are large, non-cohesive and monotypic.
Cytoplasm is conspicuously amphophilic.
Nuclei are large and vesicular with one large, sometimes bar shaped, central or peripheral nucleolus.
Mitotic figures may be numerous.
May be of B cell (more commonly) or T cell origin.
Plasmacytoid cells and plasma cells may be present.
Rare in most non-genetically engineered strains.
Pattern of organ involvement shows diffuse infiltration of lymph nodes, spleen, liver, kidneys and ovaries and along the vascular tree in the lung (similar to lymphoblastic lymphoma).
Not primarily leukemic in distribution.
When of B cell origin, cells stain positive for heavy chain immunoglobulins or kappa light chains and, rarely, for lambda light chains.
Has been reported in the BB/E rat in association with translocations in the c-myc oncogene. 194
Lymphocytic lymphoma:
Tumor cells are small to medium-sized and well-differentiated with a narrow rim of cytoplasm and densely clumped chromatin.
Cells differ little, if at all, from normal circulating small lymphocytes.
Cells are uniform and non-cohesive.
Mitotic figures are rare.
No tingible body macrophages.
Can be of T or B cell origin.
Normal architecture of involved lymphoid organs may or may not be maintained.
Plasmacytic lymphoma:
Synonyms: Plasma cell tumor; Plasmacytoma.
Tumor cells may be mature plasma cells, immature cells with an obvious plasmacytoid differentiation or a mixture of mature and immature plasma cells.
Cytoplasm is basophilic and pyroninophilic and a small perinuclear halo (Golgi apparatus) may be present.
Nuclei are round and have a cartwheel appearance.
Mitotic figures are rare in well differentiated tumors.
Formation of eosinophilic intracytoplasmic crystal-like structures considered diagnostic for plasmacytic lymphomas, although rarely seen.
- May be difficult to differentiate a well differentiated tumor from plasma cell hyperplasia based on morphologic features.
- Ki67 can help differentiate because plasma cell hyperplasia does not have uniform Ki67 immunoreactivity.
May arise in spleen or lymph nodes, rarely in bone marrow.
Rare in most mouse strains (more common in NZB mice and inducible in BALB/c mice).
Positive for heavy chain immunoglobulins by immunocytochemistry.
More than 95% of tumors are positive for kappa light chains.
Since the majority of normal mouse cells express kappa, light chain determination is unsuitable for clonality determination in mice (in contrast to man, where the kappa to lambda ratio is 2:1). Demonstration of a restricted IgH isotype (IgG1, IgG2a, IgG2b, or IgA) would be required for a relatively strong indication of clonality. 195
Epitheliotropic cutaneous lymphoma:
Presents as a dermal gross lesion with hair loss and encrusted skin.
Tumor cells are small to medium sized with little cytoplasm and indented nuclei.
Mitotic figures are rare.
Infiltration of dermis and squamous epithelium.
Loose lichenoid infiltrates in the epidermis and adnexa.
Pautrier’s microabsesses in the epidermis and hair follicles.
Inflammatory histiocytes and plasma cells may be present in the dermis.
Resembles “mycosis fungoides”
Later stages may involve multiple extracutaneous sites.
Rare lesion reported in rats.
Tumor cells expressing Pan T cell markers (CD2,CD3 and CD8) are consistent with tumors of T-cell origin.
Marginal zone lymphoma:
- Progressive lesion.
- Marginal zone hyperplasia.
- Widened marginal zone in the absence of germinal center hyperplasia.
- Uniform population of lymphocytes with clear cytoplasm (monocytoid cells).
- The width of the marginal zone in mice varies with the strain so comparison to concurrent controls is important.
- Germinal center hyperplasia accompanies marginal zone hyperplasia, but not common with MZL.
- May be focal, multifocal or diffuse.
- Marginal zone lymphoma.
- Further widening of the marginal zone with cellular atypia (medium to large cells with prominent nucleoli and occasional mitotic figures.
- Very homogenous to pleomorphic population of medium sized cells.
- Diffuse infiltration of the red pulp with complete or incomplete bridging of follicles.
- Further progression.
- Marginal zones increase in width with coalescence and bridging of the follicles.
- Larger cells with vesicular nuclei with and without prominent nucleoli.
- Invasion of the white pulp, compression of PALS, obliteration of the white pulp (in rare cases).
- Generally restricted to the spleen but may metastasize to liver and lymph nodes.
Large granular lymphocyte (LGL) leukemia:
Synonyms: LGL lymphoma; LGL-NK lymphoma.
Uniform populations of medium sized lymphocytes with morphology similar to large granular lymphocytes (NK cells).
Nuclei are round, oval, slightly irregular or reniform with varying degrees of differentiation, clumped nuclear chromatin and small nucleoli.
- Cytoplasm is basophilic and has variable numbers/size of granules.
- Granules appear reddish in Giemsa-stained peripheral blood smears and tumor imprints.
- Not visible in routine H&E-stained sections.
- Granules differentiate LGL-leukemia from other types of lymphoma.
High incidence (10–50%) in aging Fischer 344 rats.
Reported in other rat strains such as the Wistar or Sprague Dawley.
Not reported as a spontaneous lesion in mice but has been induced in genetically engineered mice.
The tumor is almost by definition leukemic but WBC counts vary.
Liver and spleen most frequently involved, but other organs may also be infiltrated.
Early, and possibly primary, lesions are frequently found in the spleen, especially in the marginal zone. 196
Positive for OX-8 (CD8) by immunohistochemistry.
- Three stages are recognized based upon extent and severity. 197
- Stage 1:
- Clinically and morphologically prelymphoma/leukemia.
- Spleen is normal in size or only slightly enlarged.
- Mild number of LGL cells present in red pulp and marginal zone.
- Few or no neoplastic LGL cells in liver sinusoids.
- No identifiable neoplastic LGL cells in any other organ.
- Stage 2:
- Moderately enlarged spleen.
- Numerous LGL cells in red pulp.
- Splenic architecture preserved.
- Mild to moderate involvement of liver with aggregates of LGL cells in sinusoids.
- Minimal to no involvement of other organs.
- Stage 3:
- Advanced stage.
- Spleen and liver markedly enlarged.
- Normal splenic architecture effaced by neoplastic cells.
- Liver markedly infiltrated by LGL cells.
- Liver frequently has degenerative changes which may be accompanied by nodular regenerative hyperplastic lesions.
- Neoplastic cell infiltrates in other organs such as lungs, lymph nodes, kidneys, brain, and adrenals.
Differential Diagnoses
Hyperplasia, lymphoid:
A reactive response to tumor, ulceration, infection.
Involves the draining lymph node.
Polyclonal proliferation.
Hyperplasia, marginal zone:
A reactive response to tumor, ulceration and infection
Widened marginal zone with germinal center hyperplasia
No cellular atypia or increased mitotic figures
Generally occurs diffusely.
Fibrosarcoma, pleomorphic:
Differential for pleomorphic lymphoma.
Soft tissue tumor (see INHAND classification soft tissue/skeletal muscle).
Also known as pleomorphic/undifferentiated sarcoma.
Tends to have a more marked fibrous structure.
Often has an obvious soft tissue primary site (e.g. subcutaneously on the rear leg).
Does not have a primarily lymphoid distribution pattern in most cases.
Does not stain with any IHC markers
Histiocytic sarcoma:
Cytoplasm is eosinophilic and abundant.
May sometimes resemble a follicular lymphoma.
May have a more fibrous structure (in rats) and it often has areas with large multinucleated cells which may show foreign body type patterns.
Usually has a different anatomical distribution and distribution pattern compared to lymphomas.
Is sometimes admixed with a lymphoma such that the animal has two systemic tumors simultaneously.
Often has a primary site, e.g. either retroperitoneally or in the liver, spleen, skin, bone marrow, brain, mesenteric lymph node, uterus, vagina etc.
Stains with one or more IHC markers for macrophages/histiocytes.
Thymoma, malignant:
Has an epithelial component.
Location of lesion is not systemic.
Comment
The early stages of lymphoma development can be diagnostically challenging. Systemic tumors may start at a single site, e.g. in a solitary lymph node, before spreading to other organs. The diagnosis of a single-site tumor can be difficult and is based on abnormal cytological features, disturbance of tissue architecture and the absence inflammation or infection. At the other extreme, widely disseminated lymphoma may involve multiple hematolymphoid organs and extra nodal tissues. When numerous lymph nodes are affected, it is sufficient to evaluate routine lymph nodes (i.e. mesenteric and axillary) and a representative sample of up to five other enlarged lymph nodes.
Some types of lymphomas cannot be distinguished morphologically and can only be identified by IHC. For example, pleomorphic lymphoma consists of several subtypes of B and T cell lineages, the majority of which are the follicular lymphoma type. Some of the spontaneous B cell lymphomas in mice resemble the diffuse large B-cell lymphoma seen commonly in humans. These include histiocyte-associated B cell lymphoma and B-cell lymphoblastic lymphoma, especially in CD-1 mice. 180 Lymphoma NOS (not otherwise specified) can be used when classification is desired but a definitive lineage cannot be determined by IHC or other lab techniques (FACS, gene expression studies). Lymphoma NOS can also be used when the lymphoma is unclassifiable due to the condition of the specimen (autolysis, poor fixation or technique). Lymphoma (with no modification) may be used when classification is not desired.
Differentiating between lymphoma and lymphoid hyperplasia can be diagnostically challenging. Hyperplasia is usually a reactive change but, on rare occasions, may be a pre-neoplastic change if it is severe or prolonged in duration, atypical, distorts normal architecture and exhibits cellular atypia. In cases of concern, additional diagnostic tests for immunoglobulin and T cell receptor clonality should be considered to differentiate hyperplasia from lymphoma.
Histiocytic Neoplasm
HISTIOCYTIC SARCOMA (M) (Figures 152 to 154) Hematolymphoid neoplasms
Figure 152.

Mouse, liver. Histiocytic sarcoma.
Figure 154.

Mouse, liver. Histiocytic sarcoma with multinucleated tumor cells.
Species
Mouse; Rat.
Other terms
Reticular cell sarcoma-Type A; Malignant histiocytosis; Kupffer cell sarcoma.
Pathogenesis/cell of origin
Cells of the mononuclear phagocyte system.
Diagnostic Features
Uniform population of round or oval cells with abundant eosinophilic cytoplasm.
Cells may also be spindle-shaped.
Nuclei are round, irregular, elongated, folded or indented; ring-shaped nuclei with a central opening may occasionally be present.
Multinucleate giant cells are often scattered throughout the tumor.
Phagocytosis may be present.
Mitotic figures may vary from few to numerous.
Atypical cells are sparse and pleomorphism is usually absent.
Areas of necrosis surrounded by palisading tumor cells are common and characteristic.
Minimal fibrosis may be present.
Perivascular tumor cell infiltrates are commonly found in the lungs.
Tumor cells may be present in blood smears.
Vascular tumor emboli are common.
Infiltrates can occur in lymphoid organs, in non-lymphoid organs (such as uterus, vagina, liver, skin, brain), retroperitoneally and on serosal surfaces.
- Kidneys often have an increase in intracytoplasmic eosinophilic hyaline granules (hyaline droplets) in the proximal tubules.
- Severity is dependent upon the degree of tumor load in the animal.
- Hyaline droplets are positive for lysozyme and negative for alpha1-antitrypsin, alpha 2µ-globulin, rat or mouse immunoglobulin and albumin.
F4/80, lysozyme and MAC-2 are reliable markers for histiocytic sarcoma in mice.
ED1 (CD68), ED2 (CD163), ED3 (CD169) and lysozyme are reliable markers for histiocytic sarcoma in rats.
In a rare histiocytic sarcoma, sporadic neoplastic histiocytes expressed S-100. 21
Differential Diagnoses
Fibrosarcoma, pleomorphic/ undifferentiated pleomorphic sarcoma:
A mixed cell population of histiocyte-like cells, bizarre tumor giant cells, fibroblasts and undifferentiated cells in various proportions.
The fibrous component is always prominent.
More pleomorphic and more fusiform cell type with less tendency for multinucleated cells than histiocytic sarcoma.
Fibrosarcoma:
More uniform fibroblast pattern with long bundles (fascicles) of cells with or without a herring bone pattern.
Fibrous component often prominent.
Lymphoma (pleomorphic, follicular, lymphoblastic):
Lymphoma cells have less cytoplasm.
Multinucleated giant cells are not usually present.
Spleen and lymph nodes are frequently involved.
H&E-stained sections of lymphoma often appear bluish macroscopically due to the relatively high nuclear:cytoplasmic ratio of the neoplastic lymphocytes.
H&E-stained sections of histiocytic sarcoma appear reddish macroscopically due to the relatively low nuclear:cytoplasmic ratio of the neoplastic histiocytes.
Schwannoma, malignant:
Schwannomas have typical growth patterns consisting of solid areas (Antoni A) and areas with large cystic spaces (Antoni B).
Multinucleate giant cells are not present.
Eosinophilic cytoplasm is not abundant.
Often occurs in the uterus.
S-100 immunoreactivity is diffuse in schwannomas but is more sporadic in histiocytic sarcoma when it occurs.
Tumor, mast cell, malignant:
Nuclei are generally bizarre, bilobed and/or polylobed, often with prominent nucleoli.
Cytoplasm is granular and basophilic or may be hypogranular.
Metachromatic cytoplasmic granules are positive for Giemsa or Toluidine blue.
Solitary type: a single local sarcomatous growth of atypical mast cells.
Systemic type: compact nodular or sheet-like accumulations of round, spindle shaped or immature mast cells.
Multiple organs are often involved.
Eosinophils may be associated with the mast cells.
May be difficult to differentiate from other sarcoma types without the use of IHC.
Adenocarcinoma, spindle cell:
Can be confused with an immature histiocytic sarcoma that has spindle cell morphology.
Can be differentiated with IHC for cytokeratin and macrophage biomarkers.
Growth pattern tends to be more expansive than invasive.
Melanoma, malignant:
Non-pigmented melanomas can be confused with a mature histiocytic sarcoma with round cells.
Can be differentiated with IHC for melanin (HMB45, PEP8) and macrophage markers (F4/80).
Mac 2 and CD68 are not helpful since they are expressed in both tumor types.
S100 is also expressed in both tumors, but the pattern of expression is sporadic in histiocytic sarcoma and diffuse in melanoma.
Comment
Histiocytic sarcomas arise most frequently in subcutaneous tissues in the Wistar rat and in the liver and lungs in F344 and Sprague-Dawley rats. Lymph nodes have been reported as primary sites in some rat strains. The frequency of tumor infiltrates in mice is liver > spleen > lung > bone marrow > uterus > lymph node > kidney, but primary organ involvement is very much strain related. In some mouse strains, histiocytic sarcoma and lymphoma may both occur in a single animal, either in the same tissue or in different tissues. Histiocytic sarcomas most often spread hematogenously but may also undergo lymphogenous spread.
Mast Cell Neoplasms (Figures 155 to 157)
Figure 155.

Mouse, spleen. Mast cell tumor, malignant.
Figure 157.
Mouse, spleen. Mast cell tumor, malignant. Giemsa.
LEUKEMIA, MAST CELL (M) Hematolymphoid neoplasms
Species
Mouse; Rat.
Pathogenesis/cell of origin
Develops from mast cells and their precursors present in the hematopoietic, mucosal and/or connective tissues.
Diagnostic Features
Atypical mast cells are present in the liver, spleen, bone marrow and/or peripheral blood.
Mast cell accumulations with sheet-like or leukemic pattern present in one or more hematolymphoid organs.
Histochemical stains and immunohistochemistry can be used to diagnosis the atypical cells as mast cells.
Morphological differentiation between mast cells and basophils is difficult due to the heavy cytoplasm granularity often seen in both cell types.
Unlike mast cells, basophils are generally tryptase negative and are always CD117 negative.
Considered malignant.
Differential Diagnoses
Histiocytic sarcoma:
Nuclei are less regular.
Cytoplasm is eosinophilic.
Negative for metachromatic cytoplasmic granules.
Melanoma, malignant, amelanotic:
Differentiate from mast cells with IHC for expression of melanin (HMB45, PEP8).
Comment
Little is known about mast cell proliferations in rodents and the terminology for proliferative conditions is confusing. The classification presented here is proposed as a standardized terminology for use by the toxicologic pathology community. This classification is considered a work in progress and changes are anticipated as knowledge and understanding of rodent mast cell proliferations evolve. Nodular mast cell accumulations are not present in normal tissue, hence their neoplastic classification. Such lesions are relatively rare, so there is little information on progression. Presently, there are no clear criteria for differentiating benign from malignant tumors in rodents. In humans, a threshold of 15 or more mast cells is set for aggregates in biopsies indicating systemic mastocytosis. 198 As mast cells are normal tissue constituents, it may be helpful to define a similar threshold for mast cell lesions in rodents. Because well differentiated mast cell tumors often show malignant growth characteristics in other species, a tumor with multiple nodules in several organs with or without leukemia is considered malignant, while a solitary nodular tumor is considered benign in the absence of pleomorphism. Mastocytosis is the diagnosis used in human terminology for a malignant mast cell tumor. Mast cell tumors are rare in rats and mice. 199 Most reported cases are generally localized and well differentiated. In rats, two cases have been reported in the mesentery 200,201 and a primary subcutaneous eyelid nodule generalized to other organs including lymph nodes, liver and kidneys. 202 “Retikulose mit reicher Mastzellbeteiligung” (reticulocytosis with prominent mast cells) was reported by Hunstein after whole-body radiation in a Wistar rat. 203 In mice, reported cases showed distribution to multiple organs. 204
There are at least three types of mast cells in the mouse. The most common is the connective tissue type with basophilic metachromatic granules, which is generally seen in the hematolymphoid tissues, interstitial tissues, the serosa and the intestinal submucosa. There also are two mucosal mast cell types, one of which has eosinophilic granules that are not metachromatic and this type is generally located within the mucosal epithelium. The other mucosal mast cell type is located in the lamina propria and is not generally apparent histologically in H&E sections of formalin fixed paraffin embedded tissue. The two mucosal mast cell types can be differentiated from one another and the connective tissue type with immunohistochemical stains. 205
Histochemical stains and immunohistochemical antibodies can be used to differentiate mast cells from histiocytes, lymphocytes and melanocytes. A panel of histochemical assays that includes toluidine blue, alcian blue, chloroacetate esterase and safarin and/or a panel of antibodies for the mast cell markers CD117, CD34 and mast cell proteases (Mcp)-1, 4 and 6 may be used when diagnosing mast cell disorders in rodents. CD117 and CD34 are expressed in mouse mast cells throughout their development and maturation, but they are not specific for mast cells. Anti-tryptase (anti-Mcp-6) has been shown to be the most specific marker for diagnosing mast cell disorders in tissue or decalcified specimens that are of the connective tissue mast cell subtype. Both the connective tissue and lamina propria mucosal mast cells express Mcp-4 and tryptase where as Mcp-1 is the most specific marker for the intraepithelial mucosal mast cell disorders.. Anti-CD117 and anti-tryptase (anti-Mcp-6) will also aid in determining whether cell infiltrates are mast cells or basophils. 21 Note that some decalcification solutions may inhibit some mast cell histochemical reagents and immunohistochemistry antibodies.
TUMOR, MAST CELL, BENIGN (B) Hematolymphoid neoplasms
Species
Mouse; Rat.
Other terms
Mastocytoma; Systemic mastocytosis.
Pathogenesis/cell of origin
Develops from mast cells and their precursors present in the hematopoietic, mucosal and/or connective tissues.
Diagnostic Features
A single, solitary, compact (dense) aggregate or nodule.
Compression of adjacent tissue.
No systemic involvement.
No clear inflammatory stimulus.
Considered benign in the absence of pleomorphism.
Comment
See comment under Mast Cell Leukemia.
TUMOR, MAST CELL, MALIGNANT (M) Hematolymphoid neoplasms
Species
Mouse; Rat.
Other terms
Mastocytoma; Mast cell sarcoma; Malignant mastocytoma; Malignant mastocytosis; Systemic mastocytosis.
Pathogenesis/cell of origin
Develops from mast cells and their precursors present in the hematopoietic, mucosal and/or connective tissues.
Diagnostic Features
Solitary type:
A single, local, sarcomatous growth of atypical mast cells.
Cytoplasm may be hypogranular.
Nuclei are generally bizarre, bilobed and/or polylobed, often with prominent nucleoli.
May be difficult to differentiate from other sarcoma types without the use of IHC.
Systemic type:
Compact nodular or sheet-like accumulation(s) of round, spindle shaped or immature mast cells.
Multiple compact/sheet-like accumulations are present in at least one organ.
Multiple organs are often involved.
In lymph nodes, located in the lymphoid tissue of the paracortex and medullary cords (not exclusively in the sinuses).
Cytoplasm is often hypogranular, but may have typical basophilic granules.
May have atypical bilobed or polylobed nuclei.
Destructive growth pattern, may be locally infiltrative.
Eosinophils may be associated with the mast cells.
No bone marrow involvement.
No clear inflammatory stimulus.
Considered malignant.
Comment
See comment under Mast Cell Leukemia.
Thymus Neoplasms
THYMOMA, BENIGN (B) (Figures 158 to 164) Thymus
Figure 158.
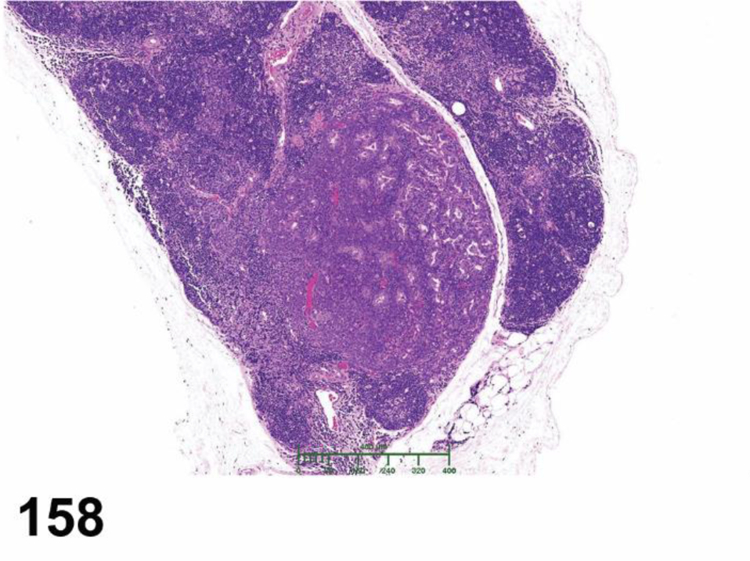
Rat, thymus. Thymoma, benign.
Figure 164.

Rat, thymus. Thymoma, malignant, squamous.
Species
Mouse; Rat.
Modifier
Epithelial; Spindeloid.
Pathogenesis/cell of origin
Thymic epithelial cells.
Diagnostic Features
Solitary lesion.
Primarily a tumor of thymic epithelial cells.
In rats, tumor differentiation ranges from a predominantly normal thymic structure with medullary differentiation to a mixture of epithelial cells and lymphocytes without medullary differentiation.
- Thymoma with medullary differentiation.
- Tumor subdivided into small lobules.
- Each lobule has a central medullary area of large pale epithelial cells surrounded by large numbers of small uniform lymphocytes.
- Thymic corpuscles may be present in medullary areas.
- Medullary areas are associated with fibrous trabeculae.
- Well circumscribed, non-invasive, may be fully or partially encapsulated.
- Common in Wistar rats, females more than males.
- Thymoma without medullary differentiation.
- Neoplastic epithelial cells are diffusely admixed with variable numbers of lymphocytes.
- Relative proportions of epithelial cells and lymphocytes vary between tumors and within a given tumor
- At least partially encapsulated.
- IHC for pancytokeratin helps visualize the epithelial component in lymphocyte-rich thymomas.
- Relatively rare.
Mice have a solid growth of tubules and epithelial cords located centrally within the lobules of the thymus.
Slight local invasion beyond the confines of the tumor capsule or thymus can occur in benign thymomas.
- Types of thymoma in rodents.
- Epithelial type: Neoplastic epithelial cells constitute more than 80% of the tumor
- Spindeloid type: Neoplastic epithelial cells are fusiform and can mimic mesenchymal cells with a sarcomatous growth pattern.
Differential Diagnoses
Hyperplasia, epithelial:
Growth is present between and within the lobular structure of the thymus.
Cysts containing eosinophilic colloid are present.
Thymoma, malignant:
Marked invasion of adjacent tissues.
Lymphoma:
No neoplastic epithelial component.
Other lymphoid organs often involved.
More common in mice than thymomas.
Hyperplasia, lymphoid:
No neoplastic epithelial component.
May have follicle formation.
No gross enlargement of the thymus.
Very common in some mouse strains.
Mesothelioma, malignant:
No neoplastic epithelial component.
Rare tumor with epitheloid or mesenchymal features.
Comment
The epithelial component of a thymoma is an inherent component of the tumor that is present even when the majority of the tumor mass is composed of lymphocytes. Special stains for pancytokeratin can be used to distinguish an early thymoma from lymphoid hyperplasia and lymphoma, especially when the epithelial component is not clearly apparent. The epithelial component is often more clearly recognizable in rats than in mice. In both species, areas within the lesion that are densely populated by small lymphocytes can mimic epithelial free areas (EFAs). As the proportion of epithelial cells and lymphocytes can vary considerably and lymphocytes are often abundant, the use of a modifier “lymphoid” is not encouraged. The incidence of benign thymomas varies considerably depending on strain, sex and breeding source. Benign thymomas with medullary differentiation are common in certain Wistar strains and rare in Sprague Dawley and Fischer rats and in many mouse strains. In humans, thymomas have been associated with myasthenia gravis and can include myoid types with striated muscle fibers and neuroendocrine types with small epithelial nests separated by thin bands of connective tissue. Large benign thymomas may cause dyspnea and other clinical signs due to compression of thoracic organs but this is not indicative of histologically-based malignancy.
THYMOMA, MALIGNANT (M) (Figures 174 and 175) Thymus
Species
Mouse; Rat.
Modifier
Epithelial; Spindeloid.
Pathogenesis/cell of origin
Thymic epithelial cells.
Diagnostic Features
Thymic epithelial cells are the primary neoplastic cell type.
Malignant thymomas often have more epithelial cells than benign thymomas.
Tumor differentiation ranges from tumors composed of a mixture of epithelial cells and lymphocytes with or without medullary differentiation to tumors composed exclusively of epithelial cells.
Epithelial cells with squamous differentiation are more common in malignant thymomas than in benign thymomas.
Marked local invasion and/or metastases.
Use of a pan-cytokeratin marker for epithelial cells can be helpful in determining malignancy in infiltrative or invasive lymphocyte-rich thymomas.
- Additional types of thymoma.
- Epithelial type: Neoplastic epithelial cells constitute more than 80% of the tumor
- Spindeloid type: Neoplastic epithelial cells are fusiform and can mimic mesenchymal cells with a sarcomatous growth pattern.
Differential Diagnoses
Hyperplasia, epithelial:
No evidence of marked invasion of adjacent tissues.
Thymoma, benign:
Differentiation between a malignant thymoma and a benign thymoma is based upon the degree of differentiation and invasive growth pattern.
A malignant thymoma shows a markedly invasive growth pattern and/or distant metastasis.
Carcinoma, squamous cell (e.g. Skin Carcinoma, squamous cell)
Differentiation between a malignant epithelial thymoma with squamous differentiation and a squamous cell carcinoma is difficult in the absence of a lymphocytic component and evidence of an extrathymic primary squamous cell carcinoma.
The diagnosis is made on the anatomical localization of the tumor; thymomas must originate in the thoracic cavity.
Lymphoma:
Epithelial component is pre-existing and not malignant.
Other lymphoid organs are typically affected.
Lymphoma cells are large with severe atypia, whereas lymphocytes in thymoma are small with no atypia. 206,207
Mesothelioma, malignant:
Mesotheliomas are rare tumors with epitheloid or mesenchymal features.
No epithelial component.
Comment
Malignant thymomas are relatively rare tumors in mice and rats.
Figure 11.

Rat, mesenteric lymph node. Inflammation, acute (higher magnification of Fig. 10).
Figure 12.

Mouse, mesenteric lymph node. Inflammation, abscess.
Figure 13.

Rat, mesenteric lymph node. Inflammation, chronic.
Figure 14.
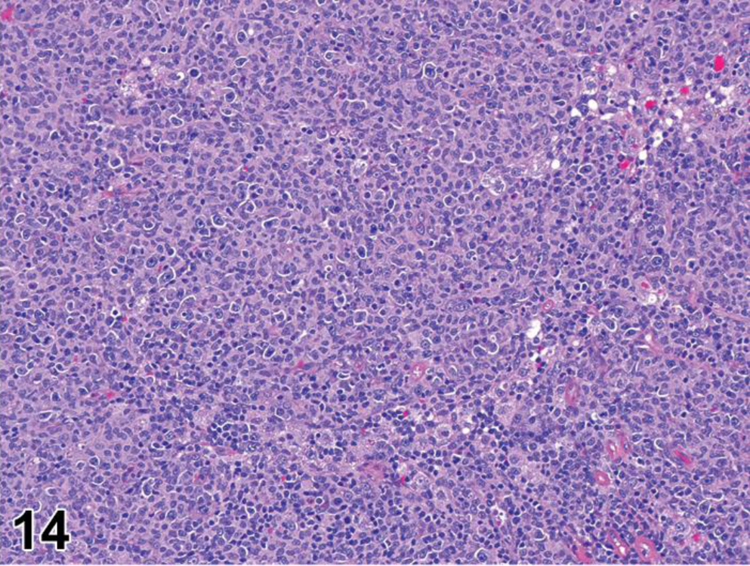
Rat, mesenteric lymph node. Inflammation, chronic (higher magnification of Fig. 12).
Figure 15.
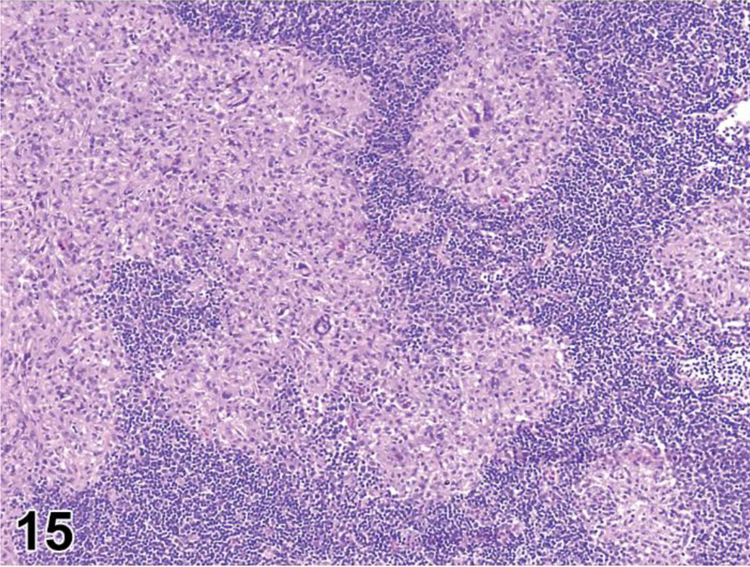
Rat, mesenteric lymph node. Inflammation, granulomatous.
Figure 16.

Rat, mesenteric lymph node. Inflammation, granulomatous (higher magnification of Fig. 15).
Figure 32.

Mouse, bone marrow. Cellularity, increased, granulocytic. Increased numbers of densely packed myeloid cells fill the medullary space, increasing the myeloid to erythroid (M:E) ratio. Megakaryocytes are present (arrows). Abundant band cells identify granulocytic precursor cells. Response to a peripheral abscess and demand for neutrophils.
Figure 48.
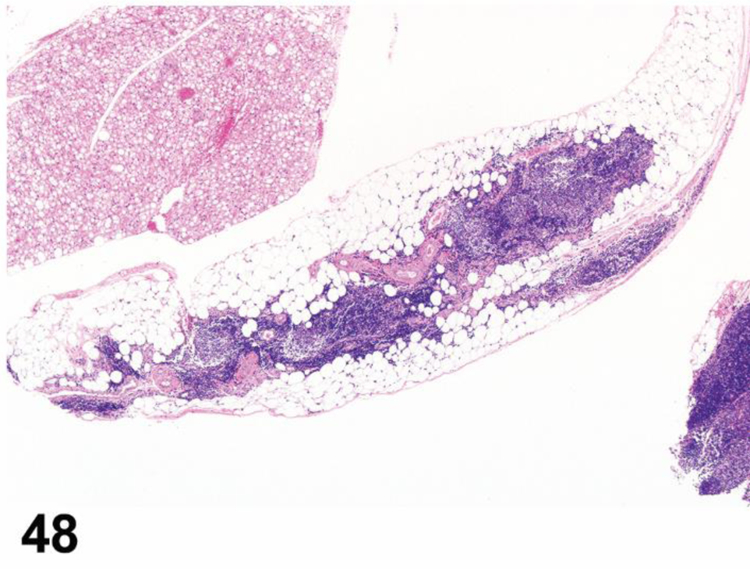
B6C3F1 mouse, thymus, all compartments. Involution, age related, marked severity.
Figure 58.

CD1 mouse, thymus, medulla. Cellularity increased, B cell. Medulla expanded by multiple germinal centers and is often associated with age related involution.
Figure 64.

B6129 mouse, spleen, white pulp, marginal zone. Cellularity, decreased, lymphocyte. Marked decrease in marginal zone lymphocytes exposes the marginal zone macrophages (arrows).
Figure 65.

B6129 mouse, spleen, white pulp, marginal zone. Cellularity, decreased, lymphocyte. Decrease of marginal zone lymphocytes reveals the marginal zone macrophage population, the reticulated network of pale pink cells, and prominent marginal metallophilic macrophages that surround the follicle (arrows).
Figure 66.

B6129 mouse, spleen, white pulp, marginal zone. Cellularity, decreased, lymphocyte. CD45R/B220 stain illustrates marked decrease of marginal zone lymphocytes and presence of the marginal zone macrophages associated with the marginal zone, CD45R/B220 stain.
Figure 81.

Mouse, spleen, white pulp, germinal centers. Cellularity, increased, lymphocyte.
Figure 90.

Rat, spleen, red pulp. Extramedullary hematopoiesis, increased, consists of all three lineages with erythropoiesis being dominant.
Figure 91.

Rat, spleen, red pulp. Extramedullary hematopoiesis, increased, consists of all three lineages with granulopoiesis being dominant.
Figure 123.

Mouse, liver. Erythroid leukemia.
Figure 128.

Mouse, spleen. Myeloid leukemia, undifferentiated.
Figure 131.
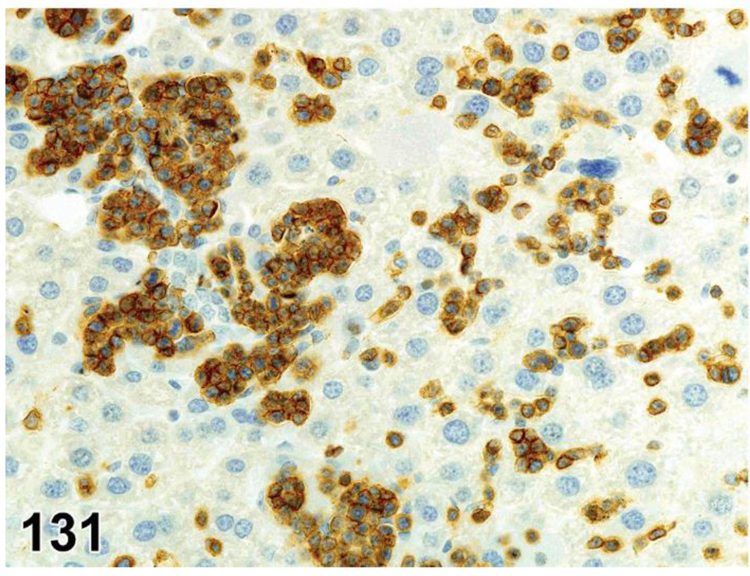
Mouse, liver. Lymphoblastic lymphoma, CD3.
Figure 132.
Mouse, spleen. Follicular (pleomorphic) lymphoma.
Figure 133.

Mouse, spleen. Follicular (pleomorphic) lymphoma.
Figure 134.

Mouse, small intestine. Follicular (pleomorphic) lymphoma.
Figure 135.

Mouse, spleen. Follicular (pleomorphic) lymphoma.
Figure 136.

Mouse, spleen. Follicular (pleomorphic) lymphoma, CD45R (B220).
Figure 137.

Mouse, spleen. Immunoblastic lymphoma.
Figure 138.

Mouse, spleen. Lymphocytic lymphoma, diffuse.
Figure 139.

Mouse, spleen. Lymphocytic lymphoma, well differentiated.
Figure 140.

Mouse, spleen. Plasmacytic lymphoma.
Figure 141.
Mouse, skin. Epitheliotrophic cutaneous lymphoma.
Figure 142.

Mouse, spleen. Marginal zone hyperplasia.
Figure 143.

Mouse, spleen. Marginal zone lymphoma.
Figure 144.

Mouse, spleen. Marginal zone lymphoma.
Figure 145.

Rat, spleen. LGL leukemia, stage 1.
Figure 146.

Rat, spleen. LGL leukemia, stage 2.
Figure 147.
Rat, spleen. LGL leukemia, stage 3.
Figure 148.

Rat, liver. LGL leukemia.
Figure 149.

Rat, liver. LGL leukemia, crenated (darker) tumor cells.
Figure 150.

Rat, blood. LGL leukemia.
Figure 153.

Mouse, liver. Histiocytic sarcoma.
Figure 156.
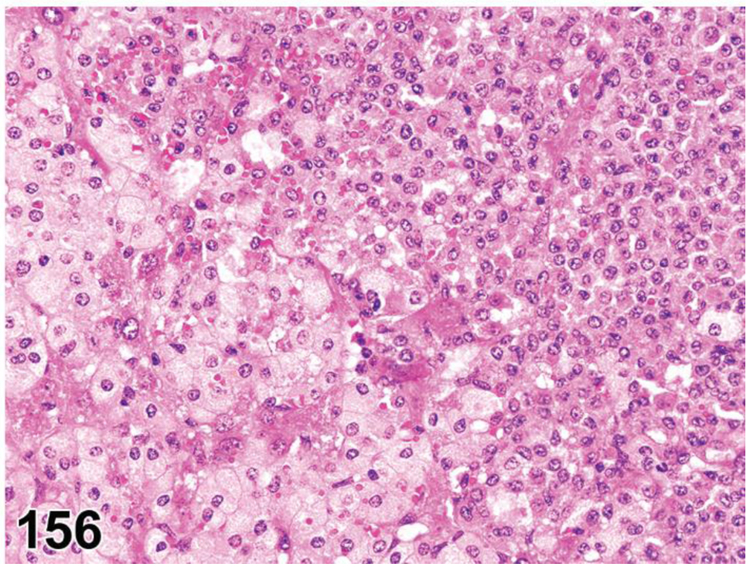
Mouse, liver. Mast cell tumor, malignant.
Figure 159.

Rat, thymus. Thymoma, benign with medullary differentiation.
Figure 160.

Rat, thymus. Thymoma, benign, epithelial.
Figure 161.

Rat, thymus. Thymoma, benign, epithelial.
Figure 162.

Rat, thymus. Thymoma, benign, spindeloid.
Figure 163.

Rat, thymus. Thymoma, malignant, squamous.
Acknowledgement:
The authors wish to express their thanks to the STP membership for comprehensive reviews, excellent comments and helpful edits. Photographs used in this document were either provided from coauthors, the National Toxicology Program Archives, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina, USA, Drs. Armando Irizarry (Eli Lilly and Company), Virginie Piccicuto (Covance, Harrogate, UK), Christopher Gray (Covance, Harrogate, UK), Catherine Ross (Covance, Harrogate, UK), and Jonathan Carter (Covance, Harrogate, UK).
Footnotes
Declaration of Conflicting Interests Statement
The author(s) declared no potential, real, or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bajénoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006;25(6):989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Haan JM, Mebius RE, Kraal G. Stromal cells of the mouse spleen. Frontiers in immunology 2012;3:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruehl-Fehlert C, Hartmann E, Rinke M. Reactive and proliferative changes of splenic reticulum cells of rats investigated with special staining methods and immunohistochemistry. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2008;59(5):281–290. [DOI] [PubMed] [Google Scholar]
- 4.Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. Journal of immunology (Baltimore, Md : 1950) 1996;157(2):495–499. [PubMed] [Google Scholar]
- 5.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. The Journal of experimental medicine 2000;192(10):1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. International immunology 2008;20(12):1483–1487. [DOI] [PubMed] [Google Scholar]
- 7.Saito H, Yokoi Y, Watanabe S, Tajima J, Kuroda H, Namihisa T. Reticular meshwork of the spleen in rats studied by electron microscopy. The American journal of anatomy 1988;181(3):235–252. [DOI] [PubMed] [Google Scholar]
- 8.McCuskey RS, McCuskey PA. In vivo and electron microscopic studies of the splenic microvasculature in mice. Experientia 1985;41(2):179–187. [DOI] [PubMed] [Google Scholar]
- 9.Schuurman HJ, Kuper CF, Kendall MD. Thymic microenvironment at the light microscopic level. Microscopy research and technique 1997;38(3):216–226. [DOI] [PubMed] [Google Scholar]
- 10.Elmore SA. Enhanced histopathology evaluation of lymphoid organs. Methods in molecular biology (Clifton, NJ) 2010;598:323–339. [DOI] [PubMed] [Google Scholar]
- 11.Germolec DR, Kashon M, Nyska A, et al. The accuracy of extended histopathology to detect immunotoxic chemicals. Toxicological sciences : an official journal of the Society of Toxicology 2004;82(2):504–514. [DOI] [PubMed] [Google Scholar]
- 12.ICICIS. Report of validation study of assessment of direct immunotoxicity in the rat. The ICICIS Group Investigators. International Collaborative Immunotoxicity Study. Toxicology 1998;125(2–3):183–201. [DOI] [PubMed] [Google Scholar]
- 13.Kuper CF, Harleman JH, Richter-Reichelm HB, Vos JG. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol Pathol 2000;28(3):454–466. [DOI] [PubMed] [Google Scholar]
- 14.Ruehl-Fehlert C, Bradley A, George C, Germann PG, Bolliger AP, Schultee A. Harmonization of immunotoxicity guidelines in the ICH process--pathology considerations from the guideline Committee of the European Society of Toxicological Pathology (ESTP). Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2005;57(1):1–5. [DOI] [PubMed] [Google Scholar]
- 15.Elmore S Enhanced histopathology of the lymph nodes. Toxicol Pathol 2006;34(5):634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmore S Enhanced histopathology of the spleen. Toxicol Pathol 2006;34(5):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmore S Enhanced histopathology of the bone marrow. Toxicol Pathol 2006;34(5):666–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmore S Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol Pathol 2006;34(5):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmore SA. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol Pathol 2006;34(5):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmore S Enhanced histopathology of the thymus. Toxicol Pathol 2006;34(5):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehg JE, Bush D, Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol 2012;40(2):345–374. [DOI] [PubMed] [Google Scholar]
- 22.Everds NE, Snyder PW, Bailey KL, et al. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 2013;41(4):560–614. [DOI] [PubMed] [Google Scholar]
- 23.Newkirk KM, Brannick EM, Kusewitt DF. Chapter 6- Neoplasia and Tumor Biology IN: Pathologic Basis of Veterinary Disease (Sixth Edition), Zachary JF ed. 6th ed: Elsevier, pp 286–321.; 2017. [Google Scholar]
- 24.Gopinath C Pathology of toxic effects on the immune system. Inflammation research : official journal of the European Histamine Research Society [et al] 1996;45 Suppl 2:S74–78. [PubMed] [Google Scholar]
- 25.Levin S, Semler D, Ruben Z. Effects of two weeks of feed restriction on some common toxicologic parameters in Sprague-Dawley rats. Toxicol Pathol 1993;21(1):1–14. [DOI] [PubMed] [Google Scholar]
- 26.Odio M, Brodish A, Ricardo MJ Jr. Effects on immune responses by chronic stress are modulated by aging. Brain, behavior, and immunity 1987;1(3):204–215. [DOI] [PubMed] [Google Scholar]
- 27.Elmore SA. Enhanced histopathology of the immune system: a review and update. Toxicol Pathol 2012;40(2):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frith CH, Chandra M. Incidence, distribution, and morphology of amyloidosis in Charles Rivers CD-1 mice. Toxicol Pathol 1991;19(2):123–127. [DOI] [PubMed] [Google Scholar]
- 29.Groscurth P, Muntener M, Tondury G. [Histogenesis of the immune system of the “nude” mouse. II. Postnatal development of the thymus: a light microscopical study (author’s transl)]. Beitrage zur Pathologie 1975;154(2):125–139. [PubMed] [Google Scholar]
- 30.Groscurth P, Müntener M, Töndury G. The postnatal development of the thymus in the nude mouse. Paper presented at: Proceedings of the first international workshop on nude mice1974; Stuttgart. [Google Scholar]
- 31.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. Journal of immunology (Baltimore, Md : 1950) 2005;174(10):6477–6489. [DOI] [PubMed] [Google Scholar]
- 32.Pearson T, Shultz LD, Miller D, et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clinical and experimental immunology 2008;154(2):270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annual review of immunology 1991;9:323–350. [DOI] [PubMed] [Google Scholar]
- 34.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn TB. Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. Journal of the National Cancer Institute 1954;14(6):1281–1433. [PubMed] [Google Scholar]
- 36.Wallig MA, Janovitz EB. Chapter 4. Morphologic manifestations of toxic cell injury. In: Haschek WM, Rousseaux CG, Wallig MA, Bolon B, Ochoa R, eds. Haschek and Rousseaux’s Handbook of Toxicologic Pathology Vol Volume I 3rd ed.: Academic Press; 2013:77–105. [Google Scholar]
- 37.Janke LJ, Liu C, Vogel P, et al. Primary epiphyseal arteriopathy in a mouse model of steroid-induced osteonecrosis. The American Journal of Pathology 2013;183(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S Artefacts in histopathology. Journal of oral and maxillofacial pathology : JOMFP 2014;18(Suppl 1):S111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mann PC, Vahle J, Keenan CM, et al. International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol 2012;40(4 Suppl):7S–13S. [DOI] [PubMed] [Google Scholar]
- 40.Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol 2006;34(5):548–565. [DOI] [PubMed] [Google Scholar]
- 41.Coskun S, Chao H, Vasavada H, et al. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell reports 2014;9(2):581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss L The hematopoietic microenvironment of the bone marrow: an ultrastructural study of the stroma in rats. The Anatomical record 1976;186(2):161–184. [DOI] [PubMed] [Google Scholar]
- 43.Morawietz G, Ruehl-Fehlert C, Kittel B, et al. Revised guides for organ sampling and trimming in rats and mice--Part 3. A joint publication of the RITA and NACAD groups. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2004;55(6):433–449. [DOI] [PubMed] [Google Scholar]
- 44.Travlos GS. Histopathology of bone marrow. Toxicol Pathol 2006;34(5):566–598. [DOI] [PubMed] [Google Scholar]
- 45.Valli VE, McGrath JP, Chu I. Hematopoietic System. In: W.M. H, Rousseaux CG, Wallig MA, eds. Handbook of Toxicologic Pathology Vol 2 2nd ed. San Diego: Academic Press; 2002:647–679. [Google Scholar]
- 46.Cline JM, Maronpot RR. Variations in the histologic distribution of rat bone marrow cells with respect to age and anatomic site. Toxicol Pathol 1985;13(4):349–355. [DOI] [PubMed] [Google Scholar]
- 47.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging cell 2004;3(6):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramaiah L, Bounous DI, Elmore SA. Chapter 50 - Hematopoietic System. In: Haschek WM, Rousseaux CG, Wallig MA, Bolon B, Ochoa R, eds. Haschek and Rousseaux’s Handbook of Toxicologic Pathology Vol III 3rd ed.: Academic Press; 2013:1863–1933. [Google Scholar]
- 49.Wancket LM, Devor-Henneman D, Ward JM. Fibro-osseous (FOL) and degenerative joint lesions in female outbred NIH Black Swiss mice. Toxicol Pathol 2008;36(2):362–365. [DOI] [PubMed] [Google Scholar]
- 50.Frith CH, Ward JM, Chandra M, Losco P. Non-proliferative Lesions of the Hematopoietic System in Rats. In: Guides for Toxicologic Pathology Washington, DC: STP/ARP/AFIP; 2000:1–22. [Google Scholar]
- 51.Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. British journal of haematology 2007;139(3):351–362. [DOI] [PubMed] [Google Scholar]
- 52.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging cell 2010;9(5):667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehg J, Ward JM. Application of immunohistochemistry in toxicologic pathology of the hematolymphoid system. In: Parker GA, ed. Immunopathology in Toxicology and Drug Development: Volume 1, Immunobiology, Investigative Techniques, and Special Studies New York: Springer; 2017. [Google Scholar]
- 54.Kogan SC, Ward JM, Anver MR, et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood 2002;100(1):238–245. [DOI] [PubMed] [Google Scholar]
- 55.Fredrickson TN, Harris AW. Chapter 3. Normal Histology. In: Fredrickson TN, Harris AW, eds. Atlas of Mouse Hematopathology Amsterdam, Netherlands: Harwood Academic Publishers; 2000. [Google Scholar]
- 56.Everds NE. Chapter 5: Hematology of the Laboratory Mouse. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, eds. The Mouse in Biomedical Research Vol III 2 ed. Amsterdam: Elsevier; 2007:133–170. [Google Scholar]
- 57.Houben GF, Penninks AH, Seinen W, Vos JG, Van Loveren H. Immunotoxic effects of the color additive caramel color III: immune function studies in rats. Fundamental and applied toxicology : official journal of the Society of Toxicology 1993;20(1):30–37. [DOI] [PubMed] [Google Scholar]
- 58.Pearse G Histopathology of the thymus. Toxicol Pathol 2006;34(5):515–547. [DOI] [PubMed] [Google Scholar]
- 59.Pearse G Normal structure, function and histology of the thymus. Toxicol Pathol 2006;34(5):504–514. [DOI] [PubMed] [Google Scholar]
- 60.Stefanski SA, Elwell MR, Stromberg PC. Spleen, Lymph Nodes, and Thymus. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds. Pathology of the Fischer Rat San Diego: Academic Press; 1990:369–393. [Google Scholar]
- 61.Ward J, Mann P, Morishima H, Frith C. Thymus, spleen and lymph nodes. In: Maronpot R, ed. Pathology of the Mouse Vienna, IL, USA: Cache River Press; 1999:333–360. [Google Scholar]
- 62.Cordier AC, Haumont SM. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. The American journal of anatomy 1980;157(3):227–263. [DOI] [PubMed] [Google Scholar]
- 63.Hardisty JF, Boorman GA. Thyroid and parathyroid glands. In: Maronpot RR, Boorman GA, Gaul BW, eds. Pathology of the Mouse Vienna, IL: Cache River Press; 1999:537–554. [Google Scholar]
- 64.Seely JC, Hildebrandt PC. Parathyroid gland. In: Boorman GA, Eustis SL, Elwell MR, C.A. M, MacKenzie WF, eds. Pathology of the Fischer Rat 1990.
- 65.Yu J, Gonzalez S, Martinez L, Diez-Pardo JA, Tovar JA. Effects of retinoic acid on the neural crest-controlled organs of fetal rats. Pediatric surgery international 2003;19(5):355–358. [DOI] [PubMed] [Google Scholar]
- 66.Caturegli P, Rose NR, Kimura M, Kimura H, Tzou SC. Studies on murine thyroiditis: new insights from organ flow cytometry. Thyroid : official journal of the American Thyroid Association 2003;13(5):419–426. [DOI] [PubMed] [Google Scholar]
- 67.Dooley J, Erickson M, Gillard GO, Farr AG. Cervical thymus in the mouse. Journal of immunology (Baltimore, Md : 1950) 2006;176(11):6484–6490. [DOI] [PubMed] [Google Scholar]
- 68.Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Developmental biology 1998;195(1):1–15. [DOI] [PubMed] [Google Scholar]
- 69.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science (New York, NY) 1969;166(3906):753–755. [DOI] [PubMed] [Google Scholar]
- 70.Vladutiu AO, Rose NR. Aberrant thymus tissue in rat and mouse thyroid. Experientia 1972;28(1):79–81. [DOI] [PubMed] [Google Scholar]
- 71.Takai K, Takahara S, Isoyama N, et al. Effects of FTY720 on rat lymphoid organs. Transplantation proceedings 2004;36(8):2453–2456. [DOI] [PubMed] [Google Scholar]
- 72.Brelinska R Thymic epithelial cells in age-dependent involution. Microscopy research and technique 2003;62(6):488–500. [DOI] [PubMed] [Google Scholar]
- 73.Cherry CP, Eisenstein R, Glucksmann A. Epithelial cords and tubules of the rat thymus: effects of age, sex, castration, of sex, thyroid and other hormones on their incidence and secretory activity. British journal of experimental pathology 1967;48(1):90–106. [PMC free article] [PubMed] [Google Scholar]
- 74.Marković L [Interaction involving the thymus and the hypothalamus-pituitary axis, immunomodulation by hormones]. Srpski arhiv za celokupno lekarstvo 2004;132(5–6):187–193. [DOI] [PubMed] [Google Scholar]
- 75.Murakami M, Hosoi Y, Araki O, et al. Expression of thyrotropin receptors in rat thymus. Life sciences 2001;68(25):2781–2787. [DOI] [PubMed] [Google Scholar]
- 76.Nancy P, Berrih-Aknin S. Differential estrogen receptor expression in autoimmune myasthenia gravis. Endocrinology 2005;146(5):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez-Puebla ML, LaCava M, Miliani De Marval PL, Jorcano JL, Richie ER, Conti CJ. Cyclin D2 overexpression in transgenic mice induces thymic and epidermal hyperplasia whereas cyclin D3 expression results only in epidermal hyperplasia. The American Journal of Pathology 2000;157(3):1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savino W, Postel-Vinay MC, Smaniotto S, Dardenne M. The thymus gland: a target organ for growth hormone. Scandinavian journal of immunology 2002;55(5):442–452. [DOI] [PubMed] [Google Scholar]
- 79.Tomonari Y, Sato J, Kurotaki T, Wako Y, Kanno T, Tsuchitani M. Thymomas and Associated Hyperplastic Lesions in Wistar Hannover Rats. Toxicol Pathol 2019;47(2):129–137. [DOI] [PubMed] [Google Scholar]
- 80.Hematopoietic System. In: Mohr U, ed. International Classification of Rodent Tumors. The Mouse Berlin Heidelberg New York: Springer; 2001:432. [Google Scholar]
- 81.Dunnick JK, Hardisty JF, Herbert RA, et al. Phenolphthalein induces thymic lymphomas accompanied by loss of the p53 wild type allele in heterozygous p53-deficient (+/−) mice. Toxicol Pathol 1997;25(6):533–540. [DOI] [PubMed] [Google Scholar]
- 82.Faccini J, Abbott D, Paulus G. In: Mouse histopathology. A glossary for use in toxicity and carcinogenicity studies Amsterdam: Elsevier; 1990:18–47. [Google Scholar]
- 83.Frith C, Pattengale P, Ward J. Color atlas of neoplastic and non-neoplastic lesions in aging mice In: Toxicology Pathology Associates; Little Rock, AR: 1985:12. [Google Scholar]
- 84.Frith CH, Ward JM. Color atlas of neoplastic and non-neoplastic lesions in aging mice Amsterdam, Oxford, New York, Tokyo: Elsevier; 1988. [Google Scholar]
- 85.Frith CH, Wiley LD. Morphologic classification and correlation of incidence of hyperplastic and neoplastic hematopoietic lesions in mice with age. Journal of gerontology 1981;36(5):534–545. [DOI] [PubMed] [Google Scholar]
- 86.Greaves P Hematopoietic and lymphatic systems. In: Histopathology of preclinical toxicity studies Amsterdam: Elsevier Academic Press; 2007:99–159. [Google Scholar]
- 87.Bruijntjes JP, Kuper CF, Robinson JE, Schuurman HJ. Epithelium-free area in the thymic cortex of rats. Dev Immunol 1993;3:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol 2006;34(5):455–465. [DOI] [PubMed] [Google Scholar]
- 89.Mebius RE, Kraal G. Structure and function of the spleen. Nature reviews Immunology 2005;5(8):606–616. [DOI] [PubMed] [Google Scholar]
- 90.Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol 2006;34(5):599–608. [DOI] [PubMed] [Google Scholar]
- 91.Steiniger B, Timphus EM, Barth PJ. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochemistry and cell biology 2006;126(6):641–648. [DOI] [PubMed] [Google Scholar]
- 92.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature reviews Immunology 2013;13(2):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garraud O, Borhis G, Badr G, et al. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond. BMC immunology 2012;13:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steiniger BS. Human spleen microanatomy: why mice do not suffice. Immunology 2015;145(3):334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN. Development and function of the mammalian spleen. BioEssays : news and reviews in molecular, cellular and developmental biology 2007;29(2):166–177. [DOI] [PubMed] [Google Scholar]
- 96.Golub R, Cumano A. Embryonic hematopoiesis. Blood cells, molecules & diseases 2013;51(4):226–231. [DOI] [PubMed] [Google Scholar]
- 97.Parker GA, Picut CA, Swanson C, Toot JD. Histologic Features of Postnatal Development of Immune System Organs in the Sprague-Dawley Rat. Toxicol Pathol 2015;43(6):794–815. [DOI] [PubMed] [Google Scholar]
- 98.Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen. International journal of biological sciences 2012;8(5):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nolte MA, Belien JA, Schadee-Eestermans I, et al. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. The Journal of experimental medicine 2003;198(3):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in Marginal Zone Macrophages and Marginal Zone B Cells in Old Mice. Journal of immunology (Baltimore, Md : 1950) 2011;186(6):3441–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science (New York, NY) 2009;325(5940):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suttie AW. Histopathology of the spleen. Toxicol Pathol 2006;34(5):466–503. [DOI] [PubMed] [Google Scholar]
- 103.Ward JM, Rehg JE, Morse HC 3rd. Differentiation of rodent immune and hematopoietic system reactive lesions from neoplasias. Toxicol Pathol 2012;40(3):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reilly FD, McCuskey RS. Studies of the hemopoietic microenvironment. VI. Regulatory mechanisms in the splenic microvascular system of mice. Microvascular research 1977;13(1):79–90. [DOI] [PubMed] [Google Scholar]
- 105.Willard-Mack C Normal structure, function, and histology of lymph nodes. Toxicol Pathol 2006;34(5):409–424. [DOI] [PubMed] [Google Scholar]
- 106.Edwards VD, Simon GT. Ultrastructure aspects of red cell destruction in the normal rat spleen. Journal of ultrastructure research 1970;33(1):187–201. [DOI] [PubMed] [Google Scholar]
- 107.Fredrickson TN, Tang Y, Chattopadhyay SK, Morse HC 3rd, Hartley JW. Retrovirus-induced lymphoproliferation as a model for developing diagnostic criteria for malignant lymphoma in mice. Toxicol Pathol 1993;21(2):219–228. [DOI] [PubMed] [Google Scholar]
- 108.Morse HC 3rd, Anver MR, Fredrickson TN, et al. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 2002;100(1):246–258. [DOI] [PubMed] [Google Scholar]
- 109.Goodman DG, Ward JM, Reichardt WD. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4’-sulfonyldianiline, or D & C red No. 9. Journal of the National Cancer Institute 1984;73(1):265–273. [PubMed] [Google Scholar]
- 110.Ward JM, Reznik G, Garner FM. Proliferative lesions of the spleen in male F344 rats fed diets containing P-chloroaniline. Veterinary pathology 1980;17(2):200–205. [DOI] [PubMed] [Google Scholar]
- 111.Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunology today 1992;13(1):17–21. [DOI] [PubMed] [Google Scholar]
- 112.Kuper CF. General aspects of immunotoxicology including validation issues. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2006;57:363–366. [DOI] [PubMed] [Google Scholar]
- 113.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. Journal of anatomy 1971;109(Pt 3):369–383. [PMC free article] [PubMed] [Google Scholar]
- 114.Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. Journal of immunological methods 2006;312(1–2):12–19. [DOI] [PubMed] [Google Scholar]
- 115.Lapointe JM, Valdez RA, Ryan AM, Haley PJ. Evaluation of the utility of popliteal lymph node examination in a cyclophosphamide model of immunotoxicity in the rat. Journal of immunotoxicology 2016;13(4):449–452. [DOI] [PubMed] [Google Scholar]
- 116.Harleman JH. Approaches to the identification and recording of findings in the lymphoreticular organs indicative for immunotoxicity in regulatory type toxicity studies. Toxicology 2000;142(3):213–219. [DOI] [PubMed] [Google Scholar]
- 117.Elmore S Histopathology of the lymph nodes. Toxicol Pathol 2006;34(5):425–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vos J, Karjnc-Franken M. Toxic effects on the immune system, rat. In: Jones T, Ward J, Mohr U, Hunt R, eds. Hematopoietic System Berlin: Springer Verlag; 1990:168–183. [Google Scholar]
- 119.Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Seminars in immunology 2005;17(4):262–272. [DOI] [PubMed] [Google Scholar]
- 120.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997;90(9):3245–3287. [PubMed] [Google Scholar]
- 121.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature reviews Immunology 2005;5(8):617–628. [DOI] [PubMed] [Google Scholar]
- 122.Shamoto M, Shinzato M, Qian B, Hosokawa S, Ishibashi M. Paracortical hyperplasia of superficial lymph nodes in a new mutant strain of hairless rats (ISh): a lesion similar to human dermatopathic lymphadenopathy. Pathology international 1999;49(4):305–309. [DOI] [PubMed] [Google Scholar]
- 123.Frith C, Ward J, Harleman J, et al. Proliferative and Nonproliferative Lesions of the Haematopoietic System in Mice. In: Guides for Toxicologic Pathology Washington, DC: STP/ARP/AFIP; 1997. [Google Scholar]
- 124.Franke WW, Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation; research in biological diversity 1987;36(2):145–163. [DOI] [PubMed] [Google Scholar]
- 125.Thomazy VA, Vega F, Medeiros LJ, Davies PJ, Jones D. Phenotypic modulation of the stromal reticular network in normal and neoplastic lymph nodes: tissue transglutaminase reveals coordinate regulation of multiple cell types. The American Journal of Pathology 2003;163(1):165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ushiki T, Ohtani O, Abe K. Scanning electron microscopic studies of reticular framework in the rat mesenteric lymph node. The Anatomical record 1995;241(1):113–122. [DOI] [PubMed] [Google Scholar]
- 127.Weidman F Hyperplasia of reticulum tissue in lymph sinuses of monkeys (cebus fatuellus). The Anatomical record 1924;27(5):269–272. [Google Scholar]
- 128.Losco P, Harleman H. Normal development, growth and aging of the lymph node. In: U M, DL D, CC C, eds. Pathobiology of the Aging Rat Washington, DC: ILSI Press; 1992:49–73. [Google Scholar]
- 129.Mitsumori K Blood and lymphatic vessels In: Boorman G, Eustis S, Elwell M, Montgomery C, MacKenzie W, eds. Pathology of the Fischer Rat San Diego: Academic Press; 1990:473–484. [Google Scholar]
- 130.Moonim MT, Al-Riyami M, Tungekar MF. Nodular spindle cell vascular transformation in a retroperitoneal lymph node: morphological approach and differential diagnosis. Histopathology 2008;53(4):476–479. [DOI] [PubMed] [Google Scholar]
- 131.Plendl J, Kolle S, Sinowatz F, Schmahl W. Nonneoplastic Lesions of blood vessels. In: U M, DL D, CC C, WW C, JP S, JM W, eds. Pathobiology of the Aging Mouse Washington, DC: ILSI Press; 1996:385–391. [Google Scholar]
- 132.Vos JG. Immunotoxicity of hexachlorobenzene. IARC scientific publications 1986(77):347–356. [PubMed] [Google Scholar]
- 133.Haley PJ. Species differences in the structure and function of the immune system. Toxicology 2003;188(1):49–71. [DOI] [PubMed] [Google Scholar]
- 134.Plasticity Pabst R. and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunology letters 2007;112(1):1–8. [DOI] [PubMed] [Google Scholar]
- 135.Mestecky J, Fultz PN. Mucosal immune system of the human genital tract. The Journal of infectious diseases 1999;179 Suppl 3:S470–474. [DOI] [PubMed] [Google Scholar]
- 136.Brandtzaeg P, Pabst R. Let’s go mucosal: communication on slippery ground. Trends in immunology 2004;25(11):570–577. [DOI] [PubMed] [Google Scholar]
- 137.Brandtzaeg P Secretory immunity with special reference to the oral cavity. Journal of Oral Microbiology 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bacon CM. Extranodal lymphomas. Diagnostic Histopathology 2010;16:82–98. [Google Scholar]
- 139.Seymour R, Sundberg JP, Hogenesch H. Abnormal lymphoid organ development in immunodeficient mutant mice. Veterinary pathology 2006;43(4):401–423. [DOI] [PubMed] [Google Scholar]
- 140.Kuper C, Wijnands V, Zander S. Mucosa-associated Lymphoid Tissues. In: Parker G, ed. Immunopathology in Toxicology and Drug Development Vol 2 Springer International Publishing AG: Humana Press; 2017:83–123. [Google Scholar]
- 141.Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V. The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PloS one 2014;9(1):e86656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuper CF, Koornstra PJ, Hameleers DM, et al. The role of nasopharyngeal lymphoid tissue. Immunology today 1992;13(6):219–224. [DOI] [PubMed] [Google Scholar]
- 143.van der Brugge-Gamelkoorn GJ, van de Ende MB, Sminia T. Non-lymphoid cells of bronchus-associated lymphoid tissue of the rat in situ and in suspension. With special reference to interdigitating and follicular dendritic cells. Cell and tissue research 1985;239(1):177–182. [DOI] [PubMed] [Google Scholar]
- 144.Asanuma H, Thompson AH, Iwasaki T, et al. Isolation and characterization of mouse nasal-associated lymphoid tissue. Journal of immunological methods 1997;202(2):123–131. [DOI] [PubMed] [Google Scholar]
- 145.Koornstra PJ, de Jong FI, Vlek LF, Marres EH, van Breda Vriesman PJ. The Waldeyer ring equivalent in the rat. A model for analysis of oronasopharyngeal immune responses. Acta oto-laryngologica 1991;111(3):591–599. [DOI] [PubMed] [Google Scholar]
- 146.Kuper CF, van Oostrum L, Ma-Hock L, Durrer S, Woutersen RA. Hyperplasia of the lymphoepithelium of NALT in rats but not in mice upon 28-day exposure to 15 ppm formaldehyde vapor. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2011;63(1–2):25–32. [DOI] [PubMed] [Google Scholar]
- 147.Bruder MC, Spanhaak S, Bruijntjes JP, Michielsen CP, Vos JG, Kuper CF. Intestinal T lymphocytes of different rat strains in immunotoxicity. Toxicol Pathol 1999;27(2):171–179. [DOI] [PubMed] [Google Scholar]
- 148.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Laboratory animals 1981;15(1):57–59. [DOI] [PubMed] [Google Scholar]
- 149.Taylor RT, Williams IR. Lymphoid organogenesis in the intestine. Immunologic research 2005;33(2):167–181. [DOI] [PubMed] [Google Scholar]
- 150.Sheridan BS, Lefrancois L. Isolation of mouse lymphocytes from small intestine tissues. Current protocols in immunology 2012;Chapter 3: Unit 3 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends in immunology 2012;33(6):289–296. [DOI] [PubMed] [Google Scholar]
- 152.Kuper CF, Van Zijverden M, Klaassen C, Tegelenbosch-Schouten M, Wolterbeek AP. Effects of cyclosporin A and cyclophosphamide on Peyer’s patches in rat, exposed in utero and neonatally or during adult age. Toxicol Pathol 2007;35(2):226–232. [DOI] [PubMed] [Google Scholar]
- 153.Kunisawa J, Nochi T, Kiyono H. Immunological commonalities and distinctions between airway and digestive immunity. Trends in immunology 2008;29(11):505–513. [DOI] [PubMed] [Google Scholar]
- 154.Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer’s patches. Journal of immunology (Baltimore, Md : 1950) 2003;170(2):816–822. [DOI] [PubMed] [Google Scholar]
- 155.Spit BJ, Hendriksen EG, Bruijntjes JP, Kuper CF. Nasal lymphoid tissue in the rat. Cell and tissue research 1989;255(1):193–198. [DOI] [PubMed] [Google Scholar]
- 156.Dabak DO, Ozturk G. Antigen-induced changes on high endothelial venules in rat cervical lymph nodes. Lymphology 2003;36(2):62–68. [PubMed] [Google Scholar]
- 157.Ichikawa S, Gu S, Yamashita A. Correlation of rectum-associated lymph nodules with the development of experimentally induced acute colonic inflammation in rats. Journal of gastroenterology and hepatology 2001;16(12):1360–1367. [DOI] [PubMed] [Google Scholar]
- 158.Mebius RE, Breve J, Duijvestijn AM, Kraal G. The function of high endothelial venules in mouse lymph nodes stimulated by oxazolone. Immunology 1990;71(3):423–427. [PMC free article] [PubMed] [Google Scholar]
- 159.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nature immunology 2006;7(4):344–353. [DOI] [PubMed] [Google Scholar]
- 160.Pipi E, Nayar S, Gardner DH, Colafrancesco S, Smith C, Barone F. Tertiary Lymphoid Structures: Autoimmunity Goes Local. Frontiers in immunology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aloisi F, Columba-Cabezas S, Franciotta D, et al. Lymphoid chemokines in chronic neuroinflammation. Journal of neuroimmunology 2008;198(1–2):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hu D, Mohanta SK, Yin C, et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin beta Receptors. Immunity 2015;42(6):1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weinstein AM, Storkus WJ. Therapeutic Lymphoid Organogenesis in the Tumor Microenvironment. Advances in cancer research 2015;128:197–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ager A High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Frontiers in immunology 2017;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity 2004;21(5):655–667. [DOI] [PubMed] [Google Scholar]
- 166.Eberl G. Development and function of secondary and tertiary lymphoid tissues. European journal of immunology 2007;37(2):300–301. [DOI] [PubMed] [Google Scholar]
- 167.Link A, Hardie DL, Favre S, et al. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. The American Journal of Pathology 2011;178(4):1662–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. The Journal of clinical investigation 2014;124(3):953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: Parallels with lymph node stroma. Frontiers in immunology 2012;3:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Seminars in immunology 2008;20(1):26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cruz-Migoni S, Caamano J. Fat-Associated Lymphoid Clusters in Inflammation and Immunity. Frontiers in immunology 2016;7:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuper CF, van Bilsen J, Wijnands MVW. The Serosal Immune System of the Thorax in Toxicology. Toxicological sciences : an official journal of the Society of Toxicology 2018;164(1):31–38. [DOI] [PubMed] [Google Scholar]
- 173.Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2016;37(5):5715–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature reviews Immunology 2010;10(9):664–674. [DOI] [PubMed] [Google Scholar]
- 175.Benezech C, Luu NT, Walker JA, et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nature immunology 2015;16(8):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jackson-Jones LH, Duncan SM, Magalhaes MS, et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nature communications 2016;7:12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117(19):5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. Lyon, France: IARC Press; 2008. [Google Scholar]
- 179.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rehg JE, Rahija R, Bush D, Bradley A, Ward JM. Immunophenotype of Spontaneous Hematolymphoid Tumors Occurring in Young and Aging Female CD-1 Mice. [Corrected]. Toxicol Pathol 2015;43(7):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kunder S, Calzada-Wack J, Holzlwimmer G, et al. A comprehensive antibody panel for immunohistochemical analysis of formalin-fixed, paraffin-embedded hematopoietic neoplasms of mice: analysis of mouse specific and human antibodies cross-reactive with murine tissue. Toxicol Pathol 2007;35(3):366–375. [DOI] [PubMed] [Google Scholar]
- 182.Jaffe ES, Harris NL, Stein H, Vardiman JW. Classification of Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues Lyon: IARC Press; 2001. [Google Scholar]
- 183.Stromberg P, Harlemann JH, Ward JM, Hailey JR. Chapter 4. Hematopoietic System In: Mohr U, Capen CC, Dungworth DL, Griesemer RA, Ito N, Turusov VS, eds. International classification of rodent tumours. Part I: The Rat Lyon: IARC Scientific Publications No. 122; 1993:1–27. [Google Scholar]
- 184.Buettner M, Pabst R, Bode U. Stromal cell heterogeneity in lymphoid organs. Trends in immunology 2010;31(2):80–86. [DOI] [PubMed] [Google Scholar]
- 185.Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2015;10(10):1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hao X, Fredrickson TN, Chattopadhyay SK, et al. The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyte-associated lymphoma of mice. Veterinary pathology 2010;47(3):434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Andriko JW, Kaldjian EP, Tsokos M, Abbondanzo SL, Jaffe ES. Reticulum cell neoplasms of lymph nodes: a clinicopathologic study of 11 cases with recognition of a new subtype derived from fibroblastic reticular cells. The American journal of surgical pathology 1998;22(9):1048–1058. [DOI] [PubMed] [Google Scholar]
- 188.Jones D, Amin M, Ordonez NG, Glassman AB, Hayes KJ, Medeiros LJ. Reticulum cell sarcoma of lymph node with mixed dendritic and fibroblastic features. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2001;14(10):1059–1067. [DOI] [PubMed] [Google Scholar]
- 189.Martel M, Sarli D, Colecchia M, et al. Fibroblastic reticular cell tumor of the spleen: report of a case and review of the entity. Human pathology 2003;34(9):954–957. [DOI] [PubMed] [Google Scholar]
- 190.Ng CT, Nayak BP, Schmedt C, Oldstone MB. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proceedings of the National Academy of Sciences of the United States of America 2012;109(20):7823–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hao X, Shin MS, Zhou JX, et al. Histologic and molecular characterizations of megakaryocytic leukemia in mice. Leukemia research 2006;30(4):397–406. [DOI] [PubMed] [Google Scholar]
- 192.Nonoyama T, Hayashi S, Urano T, Yagami K, Miyajima H. Spontaneous erythroleukemia in a 16-wk-old female Slc:SD rat. Toxicol Pathol 1993;21(3):335–339. [DOI] [PubMed] [Google Scholar]
- 193.Sontakke P, Jaques J, Vellenga E, Schuringa JJ. Modeling of Chronic Myeloid Leukemia: An Overview of In Vivo Murine and Human Xenograft Models. Stem cells international 2016;2016:1625015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meehan CJ, Krajewski AS, Butcher GW, Smith W, Baird JD. Lymphoma in the BB/E rat: c-myc translocation identified. The Journal of pathology 1993;170(1):87–93. [DOI] [PubMed] [Google Scholar]
- 195.Fredrickson TN, Hartley JW, Morse HC 3rd, Chattopadhyay SK, Lennert K. Classification of mouse lymphomas. Current topics in microbiology and immunology 1995;194:109–116. [DOI] [PubMed] [Google Scholar]
- 196.Losco PE, Ward JM. The early stage of large granular lymphocyte leukemia in the F344 rat. Veterinary pathology 1984;21(3):286–291. [DOI] [PubMed] [Google Scholar]
- 197.Dunnick JK, Eustis SL, Huff JE, Haseman JK. Two-year toxicity and carcinogenicity studies of ampicillin trihydrate and penicillin VK in rodents. Fundamental and applied toxicology : official journal of the Society of Toxicology 1989;12(2):252–257. [DOI] [PubMed] [Google Scholar]
- 198.Akin C Mast cell activation syndromes. The Journal of allergy and clinical immunology 2017;140(2):349–355. [DOI] [PubMed] [Google Scholar]
- 199.Maronpot RR. Pathology of the mouse. In: Maronpot RR, Boorman GA, Gaul BW, eds. Pathology of the mouse Vienna, IL: Cache River Press; 1999:699. [Google Scholar]
- 200.Tuch K, Pueschner H. Mastocytom bei der Ratte. Berl Muench Tieraerztl Wochenschr 1992;105:27. [Google Scholar]
- 201.Yamagishi Y, Katsuta O, Tsuchitani M. Mastocytoma in a Fischer 344 rat. The Journal of veterinary medical science / the Japanese Society of Veterinary Science 1992;54(4):783–785. [DOI] [PubMed] [Google Scholar]
- 202.Baselmans AH, Kuijpers MH, van Dijk JE. Brief communication, Histopathology of a spontaneously developing mast cell sarcoma in a Wistar rat. Toxicol Pathol 1996;24(3):365–369. [DOI] [PubMed] [Google Scholar]
- 203.Hunstein W, Stutz E, Reincke U. [RADIATION-INDUCED LEUKEMIA IN WISTAR RATS AFTER FRACTIONATED TOTAL-BODY IRRADIATION]. Blut 1963;9:389–404. [PubMed] [Google Scholar]
- 204.Lewis DJ, Offer JM. Malignant mastocytoma in mice. Journal of comparative pathology 1984;94(4):615–620. [DOI] [PubMed] [Google Scholar]
- 205.Vogel P, Janke L, Gravano DM, et al. Globule Leukocytes and Other Mast Cells in the Mouse Intestine. Veterinary pathology 2018;55(1):76–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elmore SA, Cora MC, Gruebbel MM, et al. Proceedings of the 2014 National Toxicology Program Satellite Symposium. Toxicol Pathol 2015;43(1):10–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Matsuyama M Thymoma lymphocytic, rat. In: Jones TC, Ward JM, Mohr U, Hunt RD, eds. Monographs on pathology of laboratory animals. Hemopoietic system Berlin, Heidelberg, New York, Tokyo: Springer; 1990:275–280. [Google Scholar]


