Abstract
Amphibians, the most threatened group of vertebrates, are seen as indicators of the sixth mass extinction on earth. Thousands of species are threatened with extinction and many have been affected by an emerging infectious disease, chytridiomycosis, caused by the fungal pathogen, Batrachochytrium dendrobatidis (Bd). However, amphibians exhibit different responses to the pathogen, such as survival and population persistence with infection, or mortality of individuals and complete population collapse after pathogen invasion. Multiple factors can affect host pathogen dynamics, yet few studies have provided a temporal view that encompasses both the epizootic phase (i.e. pathogen invasion and host collapse), and the transition to a more stable co-existence (i.e. recovery of infected host populations). In the Sierra Nevada mountains of California, USA, conspecific populations of frogs currently exhibit dramatically different host/ Bd-pathogen dynamics. To provide a temporal context by which present day dynamics may be better understood, we use a Bd qPCR assay to test 1165 amphibian specimens collected between 1900 and 2005. Our historical analyses reveal a pattern of pathogen invasion and eventual spread across the Sierra Nevada over the last century. Although we found a small number of Bd-infections prior to 1970, these showed no sign of spread or increase in infection prevalence over multiple decades. After the late 1970s, when mass die offs were first noted, our data show Bd as much more prevalent and more spatially spread out, suggesting epizootic spread. However, across the ~400km2 area, we found no evidence of a wave-like pattern, but instead discovered multiple, nearly-simultaneous invasions within regions. We found that Bd invaded and spread in the central Sierra Nevada (Yosemite National Park area) about four decades before it invaded and spread in the southern Sierra Nevada (Sequoia and Kings Canyon National Parks area), and suggest that the temporal pattern of pathogen invasion may help explain divergent contemporary host pathogen dynamics.
Introduction
With thousands of amphibian species experiencing population declines around the world [1], amphibians are facing a global biodiversity crisis, and many suggest this is emblematic of a global mass extinction [2]. Though multiple factors play a role in these declines, the invasion and emergence of the fungal pathogen, Batrachochytrium dendrobatidis (Bd), and the ensuing epizootics (epidemics in wildlife) are implicated as major contributing factors [3]. Bd, and its recently described congener Batrachochytrium salamandrivorans (Bsal), are the only chytridiomycete known to be pathogenic to vertebrates. Since the description of Bd, [4], it has been detected on every continent except Antarctica and is frequently associated with amphibian die-offs [5–8], but not all species are susceptible. Bd infects the skin of amphibians and induces a thickening of the skin (hyperkeratosis) on the host, disrupting osmotic balance which, in highly infected individuals, often results in death [4, 9–11].
Two decades after its discovery, the dynamics and emergence of Bd are yet to be fully understood. Genomic studies have revealed that there are multiple lineages of Bd. For example, the Global Panzootic Lineage (Bd-GPL) is associated with Bd-epizootics and host population collapse [12], but other lineages are found in areas where epizootics have not been found (e.g. South Korea) [12–15] and many species survive infections. Asia is a geographic hotspot for Bd genetic diversity and is proposed as a possible source of the Bd-panzootic that began in the 20th century [16]. In the Americas (North, Central and South America), many of the reported declines of amphibians are attributed to Bd-GPL epizootics, yet most occurred decades before Bd was discovered [17, 18]. Thus, retrospective studies are needed to help create a timeline for Bd emergence and spread.
Studies of Bd epizootics in California were some of the first to describe host pathogen dynamics of chytridiomycosis in detail [7, 11, 19], yet, like many other areas that have suffered epizootics, the historical view of Bd in the region has not been fully described. The earliest evidence of Bd-infection in California is from an American bullfrog (Rana catesbeiana) specimen collected in 1928 [20]. Although this widely introduced species is identified as a reservoir species for Bd, and thus may have facilitated Bd invasion [21], there is no evidence that this infection case resulted in epizootics and may represent a failed invasion. Evidence suggests that the timing of Bd emergence (i.e. increase in infection prevalence and geographic spread) in California is from the late 1960s to the 1980s [22–24], a time period that coincides with declines of many species in the region [25–27].
In the Sierra Nevada mountain range of California, population declines and local extinctions have been documented in most of the amphibians that occur there [27]. Some population declines are attributed to introduced species like non-native fishes [28], though the causes of other declines are unknown [26]. Between 1976 and 1979, a mass die-off of Yosemite toads (Anaxyrus canorus) was documented near Yosemite National Park [29]. A later study suggested it could have been caused by a Bd epizootic, but the study was ultimately inconclusive [30]. Bradford (1991) documented a mass mortality event of the southern mountain yellow-legged frog (Rana muscosa) in Kings Canyon National Park in 1979; the cause of morality was not determined, but by 1989 the species was extirpated from that area of the park [25].
The southern mountain yellow-legged frog (Rana muscosa) and Sierra Nevada mountain yellow-legged frog (Rana sierrae) have undergone extensive population declines and local extinctions over the past 100 years [31]. Recently, population collapse and extinction in both species of frog have been shown to be primarily caused by Bd-epizootics [6, 7]; however, the extent of the effect across the entire Sierra Nevada range is unknown. For example, studies of host pathogen dynamics in both species of frog have shown that some host populations co-exist with the pathogen [19], while others go extinct < 1 year after pathogen invasion [7]. In the Yosemite area (central Sierra Nevada), mass die-offs associated with Bd-epizootics have not been observed in either R. sierrae or R. muscosa, yet populations are all infected with Bd. These frog populations exhibit moderately high Bd infection prevalence (60–75%) and very low Bd infection intensity (< 1 Zswab (zoospore equivalents of Bd DNA per swab) [19]). However, in the Sequoia-Kings Canyon area (southern Sierra Nevada), Bd- epizootics and die offs have been documented in those same species [7]. Here Bd infection prevalence rises rapidly to 100% soon after invasion and establishment of Bd. During this time, the Bd infection intensity rises 3–4 orders of magnitude higher than in Yosemite) (> 10,000 Zswab) [7], at which point, populations collapse. We hypothesize that Bd invaded and spread throughout the central Sierra Nevada (Yosemite area) long before it invaded and spread in the southern Sierra Nevada (Sequoia-Kings Canyon area) and propose that this may explain differences in present day host-pathogen dynamics. To test this, we conducted a retrospective survey using museum specimens to describe Bd-host dynamics in the Sierra Nevada mountain range over the past century. Because there are fewer available museum specimens from contemporary populations, we also present portions of previously published data on contemporary populations for comparison. Previous studies have hypothesized that Bd is an invasive pathogen in California, thus we also investigate the relationship between Bd infection and anthropogenic and abiotic factors (i.e., climate variables) that could help explain why Bd became established and spread in some areas. We also use statistical techniques to estimate when Bd invasion occurred in the Sierra Nevada, based on our available data dating back over a century.
Methods
Sampling of museum specimens
We collected skin swabs from 1165 formalin-fixed, ethanol-preserved, post-metamorphic anurans in museum archives from 1900 to 2005. Using the VertNet.org database, we identified and sampled specimens from permanent collections housed at the California Academy of Sciences, Museum of Vertebrate Zoology, Natural History Museum of Los Angeles County, the Slater Museum of Natural History, and the Carnegie Museum of Natural History. To maximize the probability of detecting Bd, we selected all available R. muscosa and R. sierrae museum specimens, because these species are known to have undergone dramatic Bd-related population declines [7]. In decades where there were <100 R. muscosa and R. sierrae specimens available, we randomly sampled other sympatric anuran species (Anaxyrus canorus, Anaxyrus boreas, and Hyliola regilla) within the Sierra Nevada range until we reached the 100-sample size for those decades. All of the data we collected and analyzed are freely accessible on the AmphibiaWeb amphibian disease portal online database (Butler, H. 2017 "Sierra Nevada Retrospective Analysis" AmphibiaWeb: Amphibian Disease Portal <https://n2t.net/ark:/21547/Ars2>).
We followed the museum swabbing technique described in Cheng et al. (2011) [32]. Each specimen was swabbed a total of 30 strokes with a sterile rayon-tipped swabs (MW113, Medical Wire and Equipment, Corsham, UK): 10 strokes on each side the ventral surface (running from the abdomen towards the pelvis and inner thighs), and 5 strokes on the toes and webbing of each hind foot. To reduce the possibility of cross contamination from multiple specimens kept in the same jar, each specimen was rinsed with 70% ethanol prior to swabbing, held in a unique plastic bag to prevent glove contamination, and gloves were changed between specimens. Swabs were stored in 1.5 mL microcentrifuge tubes and refrigerated at 4°C until extraction. Prior to extraction, swab vials were placed in a SpinVac (Savant Instruments, Farmingdale, NY, USA) for 15–20 min to evaporate any ethanol which could inhibit PCR. DNA extraction from the swabs was done using 40μL of Prepman Ultra (Applied Biosystems, Carlsbad, CA, USA) and diluted 1:10 with 0.25×TE Buffer. Presence of Bd was assayed by real-time qPCR, following the method described in Boyle et al. (2004) [33]. Samples were run in duplicate along with negative controls (H20, TE Buffer) and positive controls at dilutions of 100, 10, 1, and 0.1 Zoospore Equivalents. Raw qPCR output was multiplied by a factor of 80 to account for the dilution factor (1:80), giving a relative measure in terms of zoospore equivalents (Zswab) on the specimen. A sample was considered Bd positive if the amplification curve was sigmoidal with a Zswab value greater than zero.
Specimen collection localities were plotted in Quantum GIS 3.0 Girona (www.qgis.org). Because not all museum databases or collection labels have been updated to reflect the 2007 taxonomic split of R. muscosa (sensu lato) into allotypic northern R. sierrae and a southern R. muscosa (sensu stricto) [31], all specimens of “Rana muscosa” were checked against range information and were assigned to a species given morphology (i.e. R. muscosa having a longer leg length to body size ration compared to R. sierrae) and locality data [31].
Statistical analyses
All statistical analyses were performed using the statistical software R (version 3.5.0). For each decade we calculated Bd prevalence with 95% binomial confidence intervals (CI). We also calculated the probability of not detecting Bd in each decade based on our sample size, assuming a binomial distribution.
We performed a stepwise binomial logistic regression using Bd infection status as the response (dependent) variable on the historical survey data, as individuals are either infected (Zswab > 0) or not infected (Zswab = 0). The data were analyzed in two categories: 1) all decades and 2) only the decades before emergence, which we defined as the decades prior to the decade with the greatest change in Bd prevalence. We did not perform a regression for post-emergence decades since this study was focused on understanding factors associated with the Bd invasion. We used the following explanatory variables in our model: annual mean temperature, annual minimum temperature, annual maximum temperature, elevation, annual precipitation, human footprint, distance to the closest water body, category of the closest water body, croplands, built environment, population density, roads, railways, and pastures. Using the scale function in R’s base package, we scaled the covariates using their mean and standard deviation to enable a better comparison of coefficients [34]. Elevation and topographic information for the distance to the closest water body were downloaded from the US Geological Survey National Hydrography Dataset (nationalmap.gov, https://nhd.usgs.gov/data.html) using the package FedData (version 2.4.6) and distance to the closest water body was calculated using the gdistance package (version 1.2–1). Annual temperature and precipitation information were downloaded from PRISM (PRISM Climate Group, Oregon State University, http://prism.oregonstate.edu, created 10 Feb 2018), and human footprint and land use information were accessed through the Dryad digital repository listed in Venter et al. (2016) [35], and the data extracted using the R package raster (2.5–8). We performed a Pearson correlation test to determine if any of the explanatory variables were highly correlated with each other (r > 0.9 or r < -0.9) and eliminated the variables that were highly correlated to reduce multicollinearity. The eliminated highly correlated variables were minimum temperature, maximum temperature, and elevation–all of which are highly correlated with mean temperature. We validated the models using k-fold cross validation.
We conducted Bayesian hierarchical modeling using Markov Chain Monte Carlo (MCMC) to estimate the arrival year of Bd in the Sierra Nevada with the R package rjags (version 4–6). In this model, Bd arrival is described using a threshold model where Bd switches from absent to present in the population with some mean prevalence. The number of infected individuals in each year was treated as a draw from a binomial distribution with a sample size equal to the number of individuals sampled that year [36, 37].
As a baseline for Bd infection prevalence comparison, we used a conservative probability of 0.05. This probability is based on a previous study that used the same qPCR technique on museum specimens and showed a mean of 11% infection prevalence in a population where Bd was determined to be endemic (Illinois, USA) for specimens collected over a 100-year timespan [38].
Results
Museum sampling
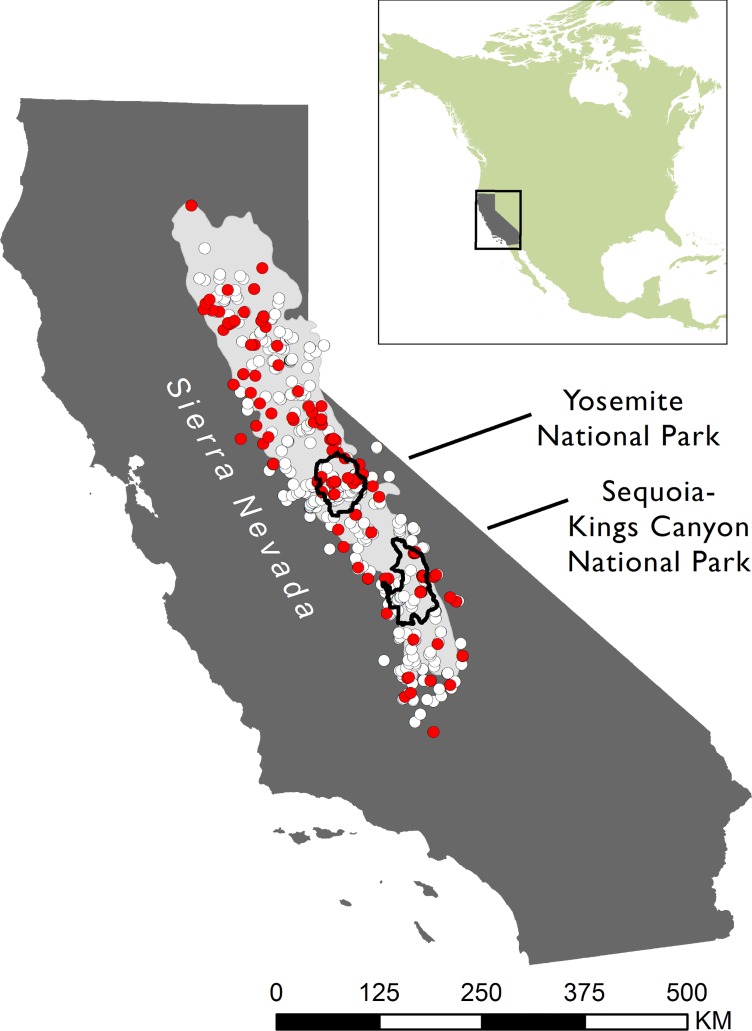
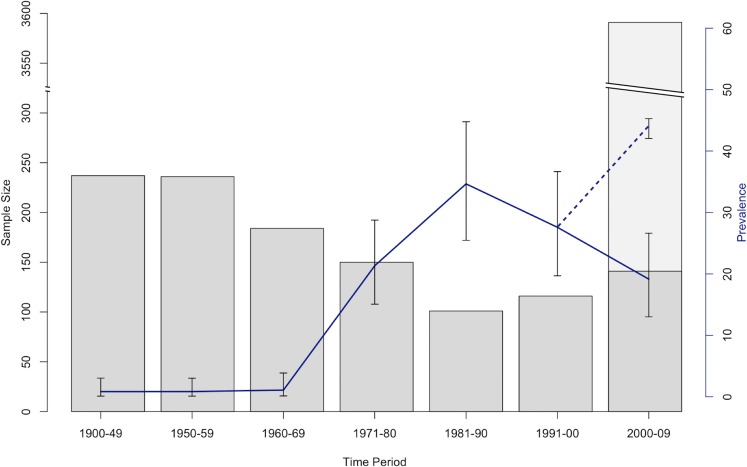
A total of 132 out of 1164 archived specimens sampled across the Sierra Nevada were Bd-positive (Fig 1); six were collected before 1970 and the remaining 126 positives were collected after 1970 (Table 1; Fig 2). The probability of not detecting Bd, given our sample size, was low (p < 0.01) for each time period (Table 1). The pre-1970 Bd-positive specimens were collected in 1939, 1942, 1955, 1959, 1962, and 1965, at isolated and widely distributed sites across the mountain range (Fig 3). The overall Bd infection prevalence by decade ranged from 0.8% in the 1950s to 34.7% in the 1980s, with the greatest change occurring in the 1970s (Fig 2). When we included previously published data collected from live animals in the field (n = 3492) [7], the prevalence in the 2000s was over 40% (Fig 2). Based on the museum data, the Bayesian hierarchical modeling iterations predicted that Bd would have likely arrived between 1932–1939 (95% of iterations; = 1936) in the Sierra Nevada, though this arrival time may also signal a failed invasion attempt.
Fig 1. Spatial distribution of 1165 amphibian museum specimens tested for Bd-infection collected between 1900–2005 in the Sierra Nevada.
Red and gray circles denote individuals tested positive and negative for Bd; respectively.
Table 1. Sample size per decade and the probability of detecting no Bd based on a 5% conservative probability of Bd detection.
Credible intervals (CI) were calculated using binomial confidence intervals.
| Time Period | Negatives | Positives | n | Lower CI | Upper CI | Pr |
|---|---|---|---|---|---|---|
| 1900–1949 | 235 | 2 | 237 | 0.1 | 3.01 | < 0.01 |
| 1950–1959 | 234 | 2 | 236 | 0.1 | 3.03 | < 0.01 |
| 1960–1969 | 182 | 2 | 184 | 0.13 | 3.87 | < 0.01 |
| 1970–1979 | 118 | 32 | 150 | 15.07 | 28.76 | < 0.01 |
| 1980–1989 | 66 | 35 | 101 | 25.46 | 44.77 | < 0.01 |
| 1990–1999 | 84 | 32 | 116 | 19.69 | 36.65 | < 0.01 |
| 2000–2009 | 114 | 27 | 141 | 13.01 | 26.62 | < 0.01 |
Fig 2. Bd infection prevalence in anurans of the Sierra Nevada from 1900–2009.
Bar graphs denote sample size from each time period. Dark gray bars denote samples from museum specimens, and light gray bars denote samples collected from live animals in the field (live animal data from [7]). Blue line denotes Bd infection prevalence calculated from museum specimens only, and dotted blue line denotes Bd infection prevalence including both museum specimens and live animals in the field (i.e. museum specimens and data from [7]).
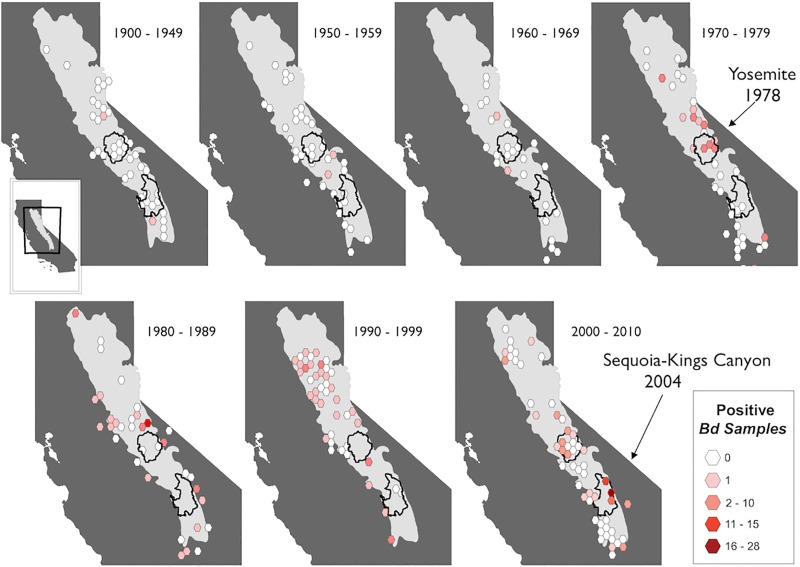
Fig 3. New incidences of Bd-positive amphibians in the Sierra Nevada mountain range per time period in California from 1900–2009.
The earliest Bd positives (12/26) were detected in Yosemite National Park were in the 1970s (mass die offs documented there in 1978 [Sherman and Morton 1993]). The first Bd positives (1586/3492) detected in Sequoia- Kings Canyon National Parks were in the 2000s (mass die offs documented there beginning in 2004; [7]). The Sierra Nevada mountains are denoted by light gray shading.
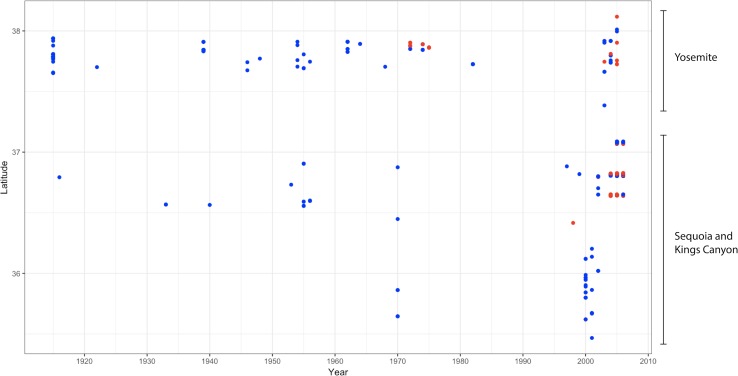
We found that Bd-positive specimens appeared earlier and in higher numbers in the central and northern part of the Sierra Nevada range (e.g. the vicinity of Yosemite National Park and northwards), compared to the southern areas (e.g. the vicinity of Sequoia-Kings Canyon National Parks; Fig 3), but few samples were collected in the southern areas between the late 1970s and 1990s. In the Yosemite National Park area, the first positive was detected in 1972, and the first mass declines noted in 1978. In the Sequoia-Kings National Parks area the first detection of Bd was in 1998, with the first evidence of a rapid increase in Bd prevalence being in 2004 (Fig 4). We also detected several early positives from animals collected in 1976 in southwest Inyo County in the most extreme southern reaches of the Sierra Nevada, far south of the Sequoia-Kings National Parks area (Fig 3).
Fig 4. Chronology of Bd infected amphibians collected in and around Yosemite National Park (central Sierra Nevada) and Sequoia-Kings Canyon National Parks (southern Sierra Nevada).
The best model for the stepwise binomial logistic regressions, using Bd presence as a response variable, (Table 2) shows differences in which scaled coefficients were significant at predicting Bd-positive individuals between pre-1970’s and all time periods. The best model for all decades shows both human factors (human footprint, croplands, built environment, human population density, roads, and railways), and climatic variables (precipitation and mean temperature) as significant predictors of Bd infections (Table 3). For the pre-1970s time period (before Bd emergence), anthropogenic factors (built environment, human footprint, and railways) are significant predictors of Bd infection (Table 4). Models that included amphibian species as a factor did not suggest that that factor was significant in the context of this study. Our k-fold cross validation for all time periods and pre-1970s models show a cross-validation estimation of accuracy of 88.7% and 75.5%; respectively.
Table 2. Lowest AIC models for stepwise binomial logistic regression.
| All Time Periods | Pre-1970s | |||
|---|---|---|---|---|
| Predictors | Odds Ratios | p | Odds Ratios | p |
| (Intercept) | 0.11 | <0.001 | 0.31 | <0.001 |
| ppt | 1.63 | <0.001 | 1.15 | 0.111 |
| tmean | 1.57 | <0.001 | ||
| HFP 2009 | 5.57 | 0.001 | 3.43 | 0.003 |
| croplands 2005 | 0.81 | 0.086 | 0.82 | 0.134 |
| Built 2009 | 0.26 | <0.001 | 0.37 | 0.011 |
| Popdensity 2010 | 0.62 | 0.009 | ||
| Roads | 0.48 | 0.016 | 0.66 | 0.087 |
| Railways | 0.71 | 0.034 | 0.76 | 0.041 |
| distwater | 1.19 | 0.108 | ||
| Pasture 2009 | 0.84 | 0.135 | ||
| AIC | 783.788 | 564.465 | ||
Table 3. Selection for the lowest AIC model for all time periods sampled.
| Predictors | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Precipitation | X | X | X | X |
| Mean Temperature | X | X | X | X |
| Human Footprint Index | X | X | X | X |
| Croplands | X | X | X | X |
| Built Environment | X | X | X | X |
| Population Density | X | X | X | X |
| Roads | X | X | X | X |
| Railways | X | X | X | X |
| Category of Closest Water Body | X | |||
| Distance to Closest Water Body | X | X | X | |
| Pasturelands | X | X | ||
| AIC | 783.79 | 785.02 | 786.70 | 792.79 |
| Resid. Dev | 765.79 | 765.02 | 764.70 | 756.79 |
| Resid. Df | 1155.00 | 1154.00 | 1153.00 | 1146.00 |
| Deviance | 0.77 | 0.31 | 7.91 |
Table 4. Selection for the lowest AIC model for pre-1970s.
| Predictors | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Precipitation | X | X | X | X |
| Mean Temperature | X | X | ||
| Human Footprint Index | X | X | X | X |
| Croplands | X | X | X | X |
| Built Environment | X | X | X | X |
| Population Density | 4 | X | X | X |
| Roads | X | X | X | X |
| Railways | X | X | X | X |
| Category of Closest Water Body | X | |||
| Distance to Closest Water Body | X | X | X | X |
| Pasturelands | X | X | X | X |
| AIC | 564.47 | 566.08 | 568.06 | 575.02 |
| Resid. Dev | 546.47 | 546.08 | 546.06 | 541.02 |
| Resid. Df | 498.00 | 497.00 | 496.00 | 490.00 |
| Deviance | 0.39 | 0.02 | 5.04 |
Discussion
The global collapse of amphibian species caused, in part, by the Bd panzootic, stands as an example that we have entered a sixth mass extinction [2]. Because the pathogen was discovered and described after causing epizootics in Central America and Australia [4, 9], retrospective studies are vital to improve our understanding of Bd’s pathogen invasion and spread. Despite sampling biases of museum specimens that were collected for reasons unrelated to disease ecology, retrospective studies, like this one, can provide insight regarding pathogen invasion history and disease dynamics.
Our survey of museum specimens collected over a 100-year period in the Sierra Nevada found no evidence of Bd before 1939. For almost the next four decades, we detected only a few Bd-infected frogs and no sign of spread or an increase in infection prevalence until the late 1970’s. These results are consistent with a growing body of evidence showing that Bd may have invaded and spread in California and the west coast of North America approximately a decade before mass die offs were discovered [21, 22, 23, 36]. Unfortunately, those studies did not provide disease data from the Sierra Nevada, where studies first described pathogen host dynamics during epizootics that resulted in local host extinctions [7, 19]. However, one previous retrospective study did discover a Bd-positive Yosemite toad (Anaxyrus canorus) collected at Tioga Pass Meadow in 1978, the year before a mass population die-off at the site (Green & Sherman 2001). We also detected Bd-infected R. sierrae in the vicinity of Tioga Pass before the population collapse, suggesting that a Bd epizootic may have swept through the Yosemite area in the mid to late 1970s.
We found evidence of Bd invasion and spread that varies geographically and temporally across the Sierra Nevada mountain range. Unlike previous historical studies that occurred over very large (continental) or limited (1–2 Km) geographic scales [5, 7], we did not detect a directional wave-like spread of Bd across the entire mountain range. Instead, we found Bd invaded host populations asynchronously in separate regions across the 400 km2 area, but specimens were collected for purposes not related to this study, and this limited our ability to detect a wave. In the central Sierra Nevada (Yosemite area), Bd was first detected in the 1970s (Fig 3), and epizootics are suggested to have occurred soon thereafter (Sherman and Morton 1993). In the southern Sierra Nevada (Sequoia-Kings Canyon area), Bd invaded more recently in the 2000s (Fig 3), and epizootics have been documented in 2004–2008 [7]. Interestingly, these areas currently have different Bd-host dynamics, with frog populations in the central Sierra Nevada persisting in an enzootic state with Bd [19, 39] while frog populations in the southern Sierra Nevada are now experiencing epizootics and collapse [7]. Although the invasion patterns could be an artifact of the biased spatial-temporal spread of the museum specimens, our results indicate that Bd may have invaded these separate geographical areas at different times, and that difference in timing could account for the varying present-day Bd-host dynamics at these locations.
There could be other explanations for the differing present-day dynamics of these frog populations. It could be a result of differences in the pathogen, differences in the host or host community, and/or differences in habitat (abiotic factors). The virulence hypothesis states that pathogens gradually lose their highly virulent invasive qualities in order to maintain themselves in host populations [40, 41]. Bd may have undergone selection for lower virulence, or the host populations might have undergone selection for higher resistance to Bd. Host genotype, such as MHC II, have been associated with Bd-resistance [42, 43]. The arrival of a known Bd reservoir, such as American bullfrogs (L. catesbeiana) into hosts populations could also explain the contrasting dynamics [21], along with changes in temperature which could limit Bd growth [44]. However, a recent study in the Sierra Nevada found that neither differences in Bd strains from Yosemite and Sequoia-Kings Canyon nor abiotic factors explained differences in host susceptibility [39]. Instead, differences in host-pathogen dynamics were explained by the geographic location of the frog populations; frogs from the Yosemite area were less susceptible to Bd than frogs from the Sequoia-Kings area [39]. This further supports the hypothesis that the timing of pathogen invasion may explain present day differences in Bd host dynamics in the contemporary populations in these areas.
Infected frogs found prior to the 1970s may represent failed invasions, a pathogen invasion that took decades to establish, or may be evidence of the presence of non-virulent, endemic lineages of Bd. Currently, the only known lineage of Bd in the Sierra Nevada is the Global Panzootic Lineage (Bd-GPL) [12,14], but in other areas (e.g. Brazil) virulent and non-virulent lineages of Bd have been found in the same populations of hosts [45]. More studies are needed to determine which Bd lineages are present in the Sierra Nevada.
At the larger spatial scale of the Sierra Nevada range, our regression analyses show that anthropogenic factors likely played a role in the arrival and spread of Bd, which is similar to previous studies in California [21–24]. We found that, human footprint index in general, and railways, and built environment specifically, were significant predictors of frog specimen being a Bd positive (Table 2). These results support the idea that Bd invaded the high country (areas above 2500m) of the Sierra Nevada earliest in areas with the most human influence, such as Tioga Pass road in Yosemite National Park. The last area of the Sierra Nevada to become infected by Bd, almost four decades later, appears to be the Sequoia-Kings Canyon National Parks area, where the largest wilderness areas are located and access by humans is limited.
Archived amphibian museum specimens provide important historical insights that help increase our understanding of the host-pathogen dynamics of Bd at a local, regional, and global scale. For example, a study in Central America showed Bd invasion coincident with collapse of amphibian communities [32], and this helped explain the timing and losses of amphibian species that had been proposed as Bd-epizootics [5]. Other studies have found a 100-year history of amphibians co-occurring with Bd and no evidence of Bd invasion in areas with no known history of Bd epizootics [38, 46]. Here we show an increase in Bd prevalence before collapse and disappearance of many amphibians in two geographically separate locations within the Sierra Nevada four decades apart. We found that Bd became established and began to increase in prevalence in the late 1970’s in the central Sierra Nevada (Yosemite area), which is coincident with declines in those areas [26, 29], and is consistent with the hypothesis that chytridiomycosis was associated with declines recorded in several Sierra Nevada amphibian species [2, 26, 29]. We also found Bd invasion prior to documented epizootics in the 2000s in the southern Sierra Nevada [7]. Last, we detected early presence (before the 1970s) of Bd at low prevalence in spatially spread out locations that apparently did not lead to epizootics, which may be indicative of either an endemic but less virulent lineage of Bd or failed historic invasions. Further retrospective studies in conjunction with current field and lab studies are needed to improve our understanding of pathogen invasion history and host-pathogen dynamics.
Supporting information
(PDF)
Acknowledgments
We thank the collections managers of the respective museums for allowing access to specimens: J. Vindum (CAS), C. Spencer (MVZ), N. Camacho (LACM), S. Rogers (CM), and G. Shugart (PSM). H. Archer, G. Geiselman, T. Goulding, S. Prado-Irwin, and A. Fredericks assisted in data collection.
Data Availability
Data are freely available through AmphibiaWeb's amphibian disease portal: Butler, H. 2017 "Sierra Nevada Retrospective Analysis" AmphibiaWeb: Amphibian Disease Portal https://n2t.net/ark:/21547/Ars2.
Funding Statement
This work, as part of the People, Pollution, and Pathogens Project (P3 project), was funded partly through the call "Mountains as Sentinels of Change" by the Belmont-Forum (ANR-15-MASC-0001-P3, DFG-SCHM 3059/6-1, NERC-1633948, and NSFC-41661144004). This work was also funded in part by National Science Foundation (Briggs, NSF LTREB DEB-1557190; Vredenburg, Belmont Forum project NSF 1633948; Vredenburg NSF 0728279), the Axa Chair for Functional Mountain Ecology by Axa Research Fund awarded to Schmeller, and the National Institutes of Health (Briggs, NIH 5R01GM109499). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004. December 3;306(5702):1783–6. 10.1126/science.1103538 [DOI] [PubMed] [Google Scholar]
- 2.Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences. 2008. August 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daszak P, Scott DE, Kilpatrick AM, Faggioni C, Gibbons JW, Porter D. Amphibian population declines at Savannah River site are linked to climate, not chytridiomycosis. Ecology. 2005. December 1; 86(12):3232–7. [Google Scholar]
- 4.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid patho- genic to amphibians. Mycologia. 1999. March 1:219–27. [Google Scholar]
- 5.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proceedings of the national academy of sci- ences of the United States of America. 2006. February 28; 103(9):3165–70. 10.1073/pnas.0506889103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachowicz LJ, Knapp RA, Morgan JA, Stice MJ, Vredenburg VT, Parker JM, et al. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology. 2006. July 1; 87(7):1671–83. 10.1890/0012-9658(2006)87[1671:eidaap]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 7.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences. 2010. May 25; 107 (21):9689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catenazzi A, Lehr E, Rodriguez LO, Vredenburg VT. Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conservation Biology. 2011. April;25(2):382–91. 10.1111/j.1523-1739.2010.01604.x [DOI] [PubMed] [Google Scholar]
- 9.Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of aquatic organisms. 2005. December 30; 68(1):51–63. 10.3354/dao068051 [DOI] [PubMed] [Google Scholar]
- 10.Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009. October 23; 326(5952):582–5. 10.1126/science.1176765 [DOI] [PubMed] [Google Scholar]
- 11.Voyles J, Vredenburg VT, Tunstall TS, Parker JM, Briggs CJ, Rosenblum EB. Pathophysiology in mountain yellow-legged frogs (Rana muscosa) during a chytridiomycosis outbreak. PLoS One. 2012. April 25; 7(4):e35374 10.1371/journal.pone.0035374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrer RA, Weinert LA, Bielby J, Garner TW, Balloux F, Clare F, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proceedings of the National Academy of Sciences. 2011. November 15;108(46):18732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, Lee M, et al. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Molecular Ecology. 2012. November;21(21):5162–77. 10.1111/j.1365-294X.2012.05710.x [DOI] [PubMed] [Google Scholar]
- 14.Rosenblum EB, James TY, Zamudio KR, Poorten TJ, Ilut D, Rodriguez D, et al. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proceedings of the National Academy of Sciences. 2013. June 4;110(23):9385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bataille A, Fong JJ, Cha M, Wogan GO, Baek HJ, Lee H, et al. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Molecular ecology. 2013. August;22(16):4196–209. 10.1111/mec.12385 [DOI] [PubMed] [Google Scholar]
- 16.O’hanlon SJ, Rieux A, Farrer RA, Rosa GM, Waldman B, Bataille A, et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science. 2018. May 11;360(6389):621–7. 10.1126/science.aar1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lips KR, Diffendorfer J, Mendelson JR III, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS biology. 2008. March 25;6(3):e72 10.1371/journal.pbio.0060072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins JP, Storfer A. Global amphibian declines: sorting the hypotheses. Diversity and distributions. 2003. March;9(2):89–98. [Google Scholar]
- 19.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences. 2010. May 3:200912886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huss M, Huntley L, Vredenburg V, Johns J, Green S. Prevalence of Batrachochytrium dendrobatidis in 120 archived specimens of Lithobates catesbeianus (American bullfrog) collected in California, 1924–2007. EcoHealth. 2013. December 1;10(4):339–43. 10.1007/s10393-013-0895-6 [DOI] [PubMed] [Google Scholar]
- 21.Yap TA, Koo MS, Ambrose RF, Vredenburg VT. Introduced bullfrog facilitates pathogen invasion in the western United States. PloS one. 2018. April 16;13(4):e0188384 10.1371/journal.pone.0188384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette CM, Vredenburg VT, Zink AG. Reconstructing historical and contemporary disease dynamics: A case study using the California slender salamander. Biological Conservation. 2015. December 31; 192:20–9. [Google Scholar]
- 23.Yap TA, Gillespie L, Ellison S, Flechas SV, Koo MS, Martinez AE, et al. Invasion of the fungal pathogen Batrachochytrium dendrobatidis on California islands. EcoHealth. 2016. March 1;13(1):145–50. 10.1007/s10393-015-1071-y [DOI] [PubMed] [Google Scholar]
- 24.Chaukulkar S, Sulaeman H, Zink AG, Vredenburg VT. Pathogen invasion and non-epizootic dynamics in Pacific newts in California over the last century. PloS one. 2018. July 2;13(7):e0197710 10.1371/journal.pone.0197710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradford DF. Mass mortality and extinction in a high-elevation population of Rana muscosa. Journal of Herpetology. 1991. June 1:174–7. [Google Scholar]
- 26.Drost CA, Fellers GM. Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada, USA. Conservation biology. 1996. April 1;10(2):414–25. [Google Scholar]
- 27.Vredenburg VT, Koo MS, Wake DB. Declines of amphibians in California. Threatened Amphibians of the World. 2008:126. [Google Scholar]
- 28.Vredenburg VT. Reversing introduced species effects: experimental removal of introduced fish leads to rapid recovery of a declining frog. Proceedings of the National Academy of Sciences. 2004. May 18;101(20):7646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman CK, Morton ML. Population declines of Yosemite toads in the eastern Sierra Nevada of California. Journal of Herpetology. 1993. June 1:186–98. [Google Scholar]
- 30.Green DE, Sherman CK. Diagnostic histological findings in Yosemite toads (Bufo canorus) from a die-off in the 1970s. Journal of Herpetology. 2001. March 1:92–103. [Google Scholar]
- 31.Vredenburg VT, Bingham R, Knapp R, Morgan JA, Moritz C, Wake D. Concordant molecular and phenotypic data delineate new taxonomy and conservation priorities for the endangered mountain yellow‐legged frog. Journal of Zoology. 2007. April;271(4):361–74. [Google Scholar]
- 32.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences. 2011. June 7; 108(23):9502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler H. 2017. "Sierra Nevada Retrospective Analysis " AmphibiaWeb: Amphibian Disease Portal. <https://n2t.net/ark:/21547/Ars2>. [Google Scholar]
- 34.Becker RA, Chambers JM, Wilks AR. The New S Language Pacific Grove CA: Wadsworth & Brooks/Cole. BeckerThe New S Language; 1988. 1988. [Google Scholar]
- 35.Venter O, Sanderson EW, Magrach A, Allan JR, Beher J, Jones KR, et al. Global terrestrial Human Footprint maps for 1993 and 2009. Scientific data. 2016. August 23; 3:sdata201667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Leon ME, Vredenburg VT, Piovia-Scott J. Recent emergence of a chytrid fungal pathogen in California Cascades frogs (Rana Cascadae). EcoHealth. 2017. March 1; 14(1):155–61. 10.1007/s10393-016-1201-1 [DOI] [PubMed] [Google Scholar]
- 37.Phillips BL, Puschendorf R. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proceedings of the Royal Society of London B: Biological Sciences. 2013. September 7; 280(1766):20131290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talley BL, Muletz CR, Vredenburg VT, Fleischer RC, Lips KR. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989). Biological Conservation. 2015. February 28; 182:254–61. [Google Scholar]
- 39.Knapp RA, Fellers GM, Kleeman PM, Miller DA, Vredenburg VT, Rosenblum EB, et al. Large-scale recovery of an endangered amphibian despite ongoing exposure to multiple stressors. Proceedings of the National Academy of Sciences. 2016. October 18;113(42):11889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alizon S, Hurford A, Mideo N, Van Baalen M. Virulence evolution and the trade‐off hypothesis: history, current state of affairs and the future. Journal of evolutionary biology. 2009. February;22(2):245–59. 10.1111/j.1420-9101.2008.01658.x [DOI] [PubMed] [Google Scholar]
- 41.May RM, Anderson RM. Epidemiology and genetics in the coevolution of parasites and hosts. Proc. R. Soc. Lond. B. 1983. October 22;219(1216):281–313. 10.1098/rspb.1983.0075 [DOI] [PubMed] [Google Scholar]
- 42.Savage AE, Zamudio KR. MHC genotypes associate with resistance to a frog-killing fungus. Proceedings of the National Academy of Sciences. 2011. September 19:201106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bataille A, Cashins SD, Grogan L, Skerratt LF, Hunter D, McFadden M, et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proceedings of the Royal Society B: Biological Sciences. 2015. April 22;282(1805):20143127 10.1098/rspb.2014.3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004. January 1;96(1):9–15. [PubMed] [Google Scholar]
- 45.Becker CG, Greenspan SE, Tracy KE, Dash JA, Lambertini C, Jenkinson TS, et al. Variation in phenotype and virulence among enzootic and panzootic amphibian chytrid lineages. Fungal Ecology. 2017. April 30;26:45–50. [Google Scholar]
- 46.Rodriguez D, Becker CG, Pupin NC, Haddad CF, Zamudio KR. Long‐term endemism of two highly divergent lineages of the amphibian‐killing fungus in the Atlantic Forest of Brazil. Molecular Ecology. 2014. February;23(4):774–87. 10.1111/mec.12615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Data are freely available through AmphibiaWeb's amphibian disease portal: Butler, H. 2017 "Sierra Nevada Retrospective Analysis" AmphibiaWeb: Amphibian Disease Portal https://n2t.net/ark:/21547/Ars2.