Background:
Ustekinumab for the treatment of psoriasis is currently administered in a standard dosing regimen. However, some patients tend to benefit from alternative dosing regimens, a step toward personalized medicine.
Methods:
To investigate the role of ustekinumab serum concentrations, anti-ustekinumab antibodies [AUA] and HLA-Cw6 status as tools for optimizing ustekinumab treatment, a multicenter prospective cohort study was conducted at an academic hospital with affiliated nonacademic hospitals in Belgium (cohort 1) and 2 academic hospitals in the Netherlands (cohort 2 and 3). Patients with plaque-type psoriasis were eligible if treated with ustekinumab for ≥16 weeks. Serum samples and Psoriasis Area and Severity Index scores were obtained at baseline, week 16, 28, 40, 52, and/or ≥64 of ustekinumab treatment.
Results:
A total of 137 patients with 229 observations for serum concentrations and AUA and 61 observations for HLA-Cw6 status were included. Presence of AUA (prevalence of 8.7%) was significantly associated with a diminished clinical response (P = 0.032). The median ustekinumab trough concentration was 0.3 mcg/mL (<0.02–3.80). No differences in serum concentrations were observed between moderate to good responders and nonresponders (P = 0.948). Serum trough concentrations were not affected by methotrexate comedication. Prevalence of HLA-Cw6 positivity was 41% with no statistically significant difference in clinical response between HLA-Cw6–positive and HLA-Cw6–negative patients (P = 0.164).
Conclusions:
The presence of AUA was associated with treatment failure in this patient population; measurement of AUA may therefore be a candidate marker for personalized pharmacotherapy. The clinical utility of ustekinumab serum trough concentrations or HLA-Cw6 status determination remains less clear. Further exploration on the potential of measuring ustekinumab serum concentrations and other biomarkers in predicting therapy outcomes should be encouraged.
Key Words: psoriasis, immunogenicity, drug monitoring, ustekinumab
INTRODUCTION
Improved knowledge of the underlying molecular mechanisms involved in the pathogenesis of immune-mediated inflammatory diseases resulted in novel-targeted biologic therapies, which greatly advanced psoriasis care over the last decade. Currently registered biologics for psoriasis include the tumor necrosis factor inhibitors adalimumab, infliximab and etanercept, the interleukin (IL)-17 inhibitors secukinumab, ixekizumab, and brodalumab, the IL-12/IL-23 inhibitor ustekinumab, and p19/IL-23 inhibitor guselkumab. Although they all inhibit proinflammatory cytokines, they differ in composition, efficacy, and their pharmacokinetic and pharmacodynamic behavior. For more than a decade now, physicians have used standard recommended dosing regimens to treat psoriasis patients resulting in remarkable improvements in psoriasis treatment outcomes.1–3 However, some patients fail to achieve desired outcomes or fail to maintain improvement over time.4 Various theories have been investigated elaborately to address this problem, including immunogenicity.5–7 Available evidence demonstrates that antiadalimumab and anti-infliximab antibodies are associated with a decreased treatment response. However, the significance of antiustekinumab antibodies (AUA) is less clear.8,9
Other factors that might contribute to loss of response include patient or disease factors (such as extent and severity or body weight) that lead to variable serum concentrations among patients over time.10 Recently, we have proposed a therapeutic range for adalimumab, based on a significant association between clinical response [Psoriasis Area and Severity Index (PASI)] and serum trough concentrations.11 The defined therapeutic range suggested that a third of the patients were actually overtreated, which may lead to unnecessary higher costs and increased risk of adverse events.12,13
Ustekinumab is generally administered in a standard weight-dependent treatment regimen at week 0, 4, and every 12 weeks thereafter. Currently, limited evidence on therapeutic drug monitoring is available. With this study, we aim to determine whether ustekinumab serum trough concentrations, AUA and HLA-Cw6 status are associated with clinical response to identify potential tools for ustekinumab drug monitoring.
MATERIALS AND METHODS
Participants
Patients were eligible if they were ≥18 years of age, suffering from plaque-type psoriasis, and treated with subcutaneous ustekinumab for a minimum of 16 weeks. Ustekinumab dosing and interval were generally administered in a standard weight-dependent treatment regimen (dose of 45 mg for patients <100 kg and 90 mg for patients ≥100 kg) every 12 weeks. However, dosing and interval could be adjusted in case of treatment failure (based on clinical response, not on pharmacokinetic outcomes) according to daily clinical practice. Patients were recruited from different centers: The Ghent University Hospital and nonacademic affiliated hospitals in Belgium (cohort 1) and the Academic Medical Center and Radboud University Medical Center in the Netherlands (cohort 2 and 3). Patient recruitment started in January 2011 and ended in August 2015. Patient demographics [age, sex, body mass index, disease duration, diagnosis of psoriatic arthritis, previous biologic treatment, disease severity at initiation of ustekinumab therapy (PASI baseline)] and treatment characteristics [ustekinumab dosing and concomitant use of methotrexate (MTX)] were collected at study entry.
Serum Trough Concentrations, AUA, HLA-Cw6 Status, and Determination of Clinical Response
At baseline, week 16, week 28, week 40, week 52, and/or ≥week 64, serum samples were collected to determine ustekinumab trough concentrations, AUA and HLA-Cw6 status, and PASI assessment was performed to determine clinical response.
The serum samples, obtained within 3 days before ustekinumab administration, were each centrifuged for 10 minutes at 1500g. Serum samples from cohort 1 and 3 were stored at −80 degrees, whereas samples of cohort 2 were kept at −20 degrees, until they were sent batchwise to the Laboratory for Monoclonal Therapeutics, Sanquin Diagnostic Services, Amsterdam, the Netherlands. Ustekinumab trough concentrations were determined using an enzyme-linked immunosorbent assay (ELISA). This assay is based on the principle that ustekinumab is captured through its ability to bind IL-12 and rabbit antiustekinumab for the detection of ustekinumab.14,15 Results were reported in micrograms per milliliter (mcg/mL). Detection of AUA was performed through a radioimmunoassay, which measures specific high-avidity IgG antibodies against ustekinumab by an antigen-binding test. These results were converted into arbitrary units (AUs) per milliliter, with a cutoff value for positivity set at 12 AU/mL.16,17
Serum samples to determine ustekinumab trough concentrations and the presence of AUA were collected from all study patients. In cohort 1, HLA-Cw6 status was determined additionally. Samples for HLA-Cw6 allele genotyping were stored at −80°C, and DNA was extracted from leukocytes with the Promega Kit (ReliaPrep Large Volume HT gDNA Isolation System; Promega, Madison, WI). Polymerase chain reaction was performed with allele-specific primers: 5′-TACTACAACCAGAGCGAGGA-3′ and 5′-GGTCGAGCCATACATCCA-3′. Results were interpreted as either positive or negative. All methods were performed according to the manufacturer's instructions.
To assess clinical response, PASI and mean change in PASI (ΔPASI) were obtained, and patients were classified as nonresponder (ΔPASI <50.00%), moderate responder (ΔPASI 50.00%–74.99%), or good responder (ΔPASI 75.00%–100.00%).
Statistical Analysis
SPSS Statistics 22.0 (IBM, Armonk, NY) was used for the statistical analysis of all data. To compare baseline characteristics between the 3 cohorts and between subgroups of patients, a Fisher exact test was used for categorical variables and Mann–Whitney U or Kruskal–Wallis tests were used for continuous variables. The associations between serum trough concentrations, AUA, and clinical response were evaluated using a linear mixed model. To ensure results would not be influenced by transitioning from another biologic or concomitant use of MTX, these factors were accounted for. A Fisher exact test was used to compare clinical response between HLA-Cw6–positive and HLA-Cw6–negative patients. For each test, a P value <0.05 was considered statistically significant.
Ethics
Approval for this multicenter cohort study was obtained from the medical ethics committees of all participating hospitals, and all patients gave their written informed consent before participation. The study is being conducted according to the principles of the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act (WMO) and other relevant guidelines, regulations, and acts.
RESULTS
Study Population
Of 141 eligible patients, 2 patients refused participation, and 2 patients were excluded as these patients had isolated nail psoriasis. Consequently, 137 patients were included in the cohort (respectively, 62, 48, and 27 patients in cohort 1, 2, and 3). Table 1 demonstrates demographic characteristics, which were comparable between cohorts. Patients (69.1% male) had a high body mass index [28.9 (± 6.1) kg/m2], a mean disease duration of 23.6 ± 12.9 years, and a mean PASI of 14.2 ± 7.6 at initiation of ustekinumab treatment. Thirty patients (21.9%) were diagnosed with psoriatic arthritis, and most patients (76.8%) received other biologic treatment(s) before ustekinumab. Twenty-five of 137 patients (18.2%) did not receive the standard ustekinumab dosing and schedule. In these patients dosing was adjusted based on clinical response. Seven patients with a body weight of 93–99 kg received 90 mg, and 6 patients with a body weight of 101–105 kg received 45 mg. Twelve patients received ustekinumab every 10 weeks instead of every 12 (due to insufficient response).
TABLE 1.
Patient Demographics
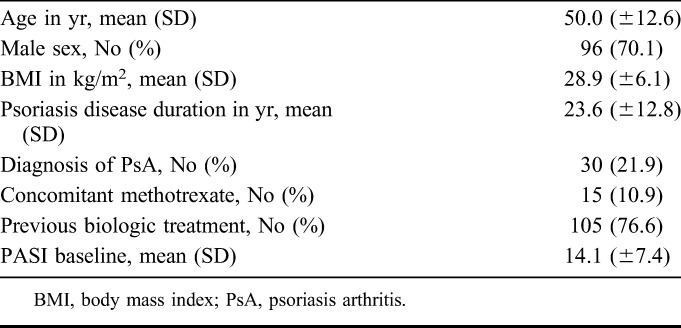
In total, 43 patients (31.2%) were treated with ustekinumab 90 mg and 15 patients (10.9%) used MTX comedication.
Data on serum trough concentrations, AUA, and clinical response were collected in all study patients. Data were collected at a single time point in 77 patients and at repeated time points in 60 patients (in 28 patients at 2 time points and in 32 patients at 3 time points). Data were collected in 75 patients at week 16, in 64 patients at week 28, in 10 patients at week 40, in 42 patients at week 52, and in 38 patients after ≥64 weeks. Subsequently, during this study, 229 observations for serum trough concentrations, AUA, and clinical response were obtained. At week 16, week 28, week 40–52, and ≥64, 34.7, 32.8, 48.1, and 65.8% of patients achieved PASI 75, respectively.
Ustekinumab Serum Trough Concentrations
The median (range) serum trough concentration was 0.3 mcg/mL (<0.02–3.80). Four patients had undetectable serum trough concentrations (<0.02) at week 16. At week 28, 40, 52, and ≥64 no undetectable serum trough concentrations were observed. No statistically significant difference in trough concentrations was observed for patients receiving 45 mg versus 90 mg, with median (range) values of 0.30 mcg/mL (<0.02–3.60) and 0.40 (<0.02–3.80) mcg/mL, respectively (P = 0.14). Patients who used MTX comedication (n = 15, 10.9% of study population; 26 observations) demonstrated ustekinumab trough concentrations similar to patients on ustekinumab monotherapy: 0.30 mcg/mL (<0.02–3.60) and 0.30 mcg/mL (<0.02–3.80), respectively (P = 0.95).
No statistically significant difference was found in serum trough concentrations between moderate to good responders and nonresponders [median (range); 0.3 (<0.02–3.80), 0.3 (<0.02–3.60), and (<0.02–1.96), respectively, P = 0.948; Fig 1]. In addition, no significant correlation was found between ustekinumab trough levels and ∆PASI (P = 0.302).
FIGURE 1.
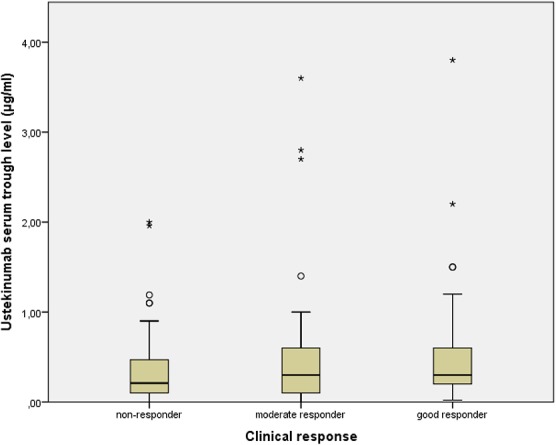
Box-and-whisker plot showing ustekinumab trough concentrations across response groups. No statistically significant difference in serum trough concentrations was found between nonresponders, moderate responders, and good responders (P = 0.948). The limits of the boxes represent the interquartile (IQ) range. The line in the boxes is the median. The whiskers extend from the upper and lower edge of the box to the highest and lowest values, which are no greater than 1.5 times the IQ range. The circles and asterisks indicate outliers (values between 1.5 and 3 times the IQ range) and extreme outliers (values more than 3 times the IQ range), respectively.
Development of AUA
AUA were detected in 12 of 137 patients (8.7%). In 3 of these patients, AUA were cleared during the study. Two of these patients remained nonresponders, and 1 patient achieved a good clinical response when AUA were cleared. In the other patients AUA persisted during the study (n = 5) or the evolution of AUA status remained unknown (n = 4), out of which AUA status was only collected at a single time point (n = 3), or AUA were only detected at the final study observation (n = 1).
AUA were detected in 6 of 75 observations (8.0%) at week 16, in 7 of 64 observations (10.9%) at week 28, in 1 of 10 observations (10%) at week 40, in 4 of 42 observations (9.5%) at week 52, and in 0 of 38 observations (0.0%) after ≥64 weeks. The AUA titer in AUA-positive patients ranged from 22 to 320 AU/mL. Median (range) serum trough concentrations were significantly lower in antibody-positive patients compared with antibody-negative patients; 0.02 mcg/mL (<0.02–0.20) versus 0.30 mcg/mL (<0.02–3.80), respectively, (P < 0.001). A good response was significantly more frequently achieved in AUA-negative patients compared with AUA-positive patients (44.1% versus 22.2%, P = 0.032), Figure 2.
FIGURE 2.
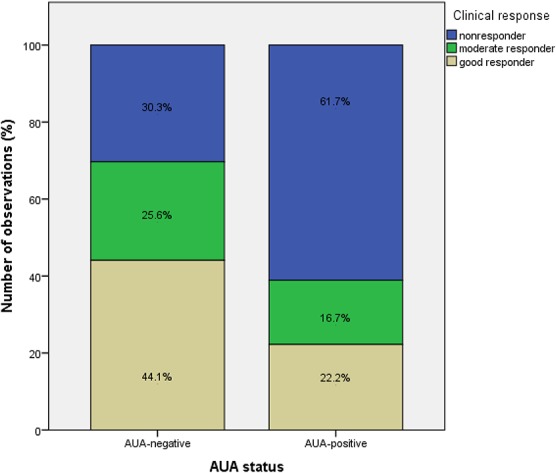
Clinical response according to AUA status. A good response was significantly more frequent achieved in AUA-negative patients compared with AUA-positive patients (44.1% versus 22.2%, P = 0.032).
In patients on MTX comedication, 1 of 15 patients (6.7%) developed AUA compared with 11 of 122 patients (9.0%) on ustekinumab monotherapy, with no statistically significant differences between groups (P = 0.77).
HLA-Cw6 Genotyping
HLA-Cw6 status was determined in 61 of 137 patients (44.5% of study population). Prevalence of HLA-Cw6 positivity was 41%. Most of the demographic characteristics were comparable between HLA-Cw6–positive and HLA-Cw6–negative patients, except for age at onset of psoriasis and prevalence of psoriatic arthritis. HLA-Cw6–positive patients developed psoriasis at an earlier age (21.4 versus 31.6 years, P = 0.012), and the prevalence of psoriatic arthritis was higher (although not significant) in HLA-Cw6–negative patients (28.6 versus 8.3%, P = 0.098). No statistically significant difference in clinical response (assessed ≥16 weeks) between Cw6-positive and Cw6-negative patients could be demonstrated (P = 0.164), although on average, slightly better response rates in Cw6-positive patients were observed (Fig. 3).
FIGURE 3.
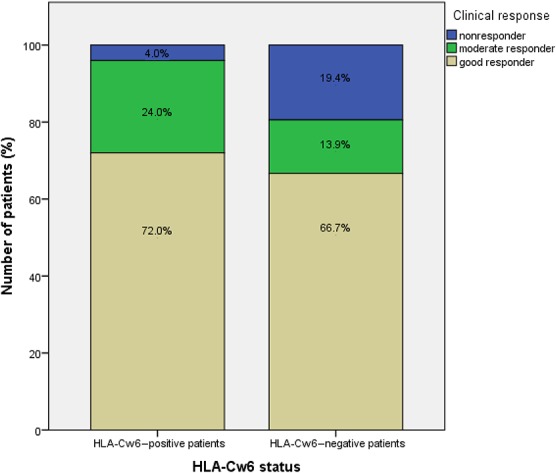
Clinical response according to HLA-Cw6 status. There was no statistically significant difference in response between HLA-Cw6–positive and HLA-Cw6–negative patients (P = 0.164).
DISCUSSION
The prevalence of AUA development was 8.7%, which is comparable with the prevalence rates reported in the current literature (1%–11%)9,18–20 but much lower than observed for other biologics such as adalimumab and infliximab. AUA-positivity significantly reduced serum trough concentrations and impaired clinical response. These data are supported by previously reported findings suggesting a trend toward decreased treatment response with the formation of AUA.18,21,22
In this cohort, AUA did develop during the first 52 weeks of ustekinumab treatment. However, the number of observations obtained in the first year of ustekinumab treatment (n = 191) was higher compared with the number of observations obtained in patients >1 year on ustekinumab treatment (n = 38), which might underestimate the prevalence of AUA formation after long-term ustekinumab treatment.
We did not find a significant association between ustekinumab serum trough concentrations and clinical response. This observation is in line with the findings of Menting et al.19 An optimal ustekinumab threshold trough concentration as suggested in other inflammatory diseases can therefore not yet be recommended. In a cohort study by Toro-Montecinos et al,23 maintenance trough concentrations of ustekinumab in Crohn disease above 4.5 mcg/mL at 26 weeks or later were identified to correspond to an optimal clinical effect.
In a small number of patients (1.7%) in our cohort, undetectable ustekinumab serum trough concentrations were measured. This could be partly explained by the presence of antidrug antibodies. Other factors that could have contributed to this include patient's nonadherence, total clearance of ustekinumab at the time of serum sample collection, or inadequate detection of serum trough concentrations in patients with severe and active psoriasis in which all ustekinumab is bound to IL-12 and IL-23.
For anti–tumor necrosis factor inhibitors, a significant association between immunomodulatory comedication (eg, MTX) and serum trough concentrations has been demonstrated in several studies.24–26 In our study, MTX comedication did not significantly impact ustekinumab serum concentrations, which might be partly due to the low incidence of AUA. However, results should be interpreted with caution due to the small number of patients on MTX comedication. The therapeutic impact of MTX in patients on ustekinumab needs to be confirmed by future studies with sufficient power.
In this cohort, we included patients treated with ustekinumab for a minimum of 16 weeks. More and more data arise showing that early pharmacokinetic drug measurements, that is, during induction phase, might be predictive for drug concentrations and clinical response on maintenance treatment. Recently, Wilkinson et al27 reported on a large adalimumab cohort of 544 psoriasis patients and demonstrated that early drug concentration measurements (obtained between 1 and 12 weeks) were predictive for clinical response at 6 months. Whether measurement of ustekinumab early in treatment will help to make strategic treatment decisions is currently unknown and will be a valuable topic for future research.
With regard to pharmacogenetic markers, HLA-Cw6 has been suggested to potentially predict clinical response in psoriasis patients on ustekinumab.28–31 We observed a slight increase in response to ustekinumab in HLA-Cw6–positive patients compared with HLA-Cw6–negative patients, but the differences were small and not statistically significant. Several studies have elaborated on single-nucleotide polymorphisms other than HLA-Cw6 and detected an association between some of these polymorphisms and response to ustekinumab treatment.31–33 These results will have to be further explored to assess whether pharmacogenetic markers can play a role in the prediction of response to ustekinumab.
Our study has some limitations. First, it is still not an extensive patient population, especially to draw conclusions on whether HLA-Cw6 status can predict clinical response. Second, not all possible factors that might influence treatment response to ustekinumab (eg, topical therapy and adherence) were considered. Third, the data were collected at different time points, and serial measurements were collected only in part of the patients (43.8%), which might impact the detection of, for example, AUA formation.
CONCLUSIONS
Based on available evidence and our study results, we conclude that there is currently overall insufficient evidence to support the use of serum trough concentrations or HLA-Cw6 status to guide treatment decisions in ustekinumab patients. Measurement of AUA in ustekinumab patients should be considered if treatment response is unsatisfactory.
Future research is needed to gain a better understanding on ustekinumab pharmacokinetics and pharmacodynamics and to identify additional tools for therapeutic drug monitoring in psoriasis patients on ustekinumab treatment.
ACKNOWLEDGMENTS
The authors thank Lynda Grine for assistance in writing the manuscript. The authors thank A. Costanzo and Dr E. Botti (Sapienza University, Rome, Italy) for genotyping the HLA-Cw6 allele. The authors also thank Dr H. Boonen (H. Hart Hospital, Mol, Belgium), Dr M. Van De Kerckhove (Private Dermatology Practice, Maldegem, Belgium), Dr A. Stockman (Sint-Rembert Hospital, Torhout, Belgium), Dr L. Temmerman (AZ Maria-Middelares Hospital, Ghent, Belgium), and all colleagues of the dermatology department of the Ghent University hospital, the AMC university medical center (Amsterdam, the Netherlands), and the Radboud university medical center (Nijmegen, the Netherlands) for helping with patient recruiting and sample collection.
Footnotes
J. M. van den Reek performs clinical trials for AbbVie and Janssen, received speaking fees from AbbVie and Eli Lilly, and reimbursement for attending a symposium from Janssen, Pfizer, and AbbVie. J. Zweegers performs trials for Abbvie, Janssen, and Sciderm and received research grants for the independent research fund of the department of dermatology of Radboudumc Nijmegen, the Netherlands, from AbbVie. J. Lambert is advisory board member and speaker for, and received grants from Pfizer Inc, AbbVie, Janssen Cilag, Novartis, LEO Pharma, Celgene, Eli Lilly. P. I. Spuls has been a consultant for Abbvie, Amgen, Novartis, and LEO pharma, received unrestricted research grants from MSD and LEO pharma, and performed many studies with pharmaceutical companies. The remaining authors declare no conflict of interest. This manuscript was not supported by any external funding.
E. De Keyser and C. I. Busard have contributed equally.
REFERENCES
- 1.Grine L, Dejager L, Libert C, et al. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. [DOI] [PubMed] [Google Scholar]
- 2.Benson JM, Sachs CW, Treacy G, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29:615–624. [DOI] [PubMed] [Google Scholar]
- 3.Wang YM, Wang J, Hon YY, et al. Evaluating and reporting the immunogenicity impacts for biological products—a clinical pharmacology perspective. AAPS J. 2016;18:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol. 2015;135:2632–2640. [DOI] [PubMed] [Google Scholar]
- 5.de la Brassinne M, Ghislain PD, Lambert JL, et al. Recommendations for managing a suboptimal response to biologics for moderate-to-severe psoriasis: a Belgian perspective. J Dermatolog Treat. 2016;27:128–133. [DOI] [PubMed] [Google Scholar]
- 6.Lambert J, Nast A, Nestle FO, et al. Practical guidance on immunogenicity to biologic agents used in the treatment of psoriasis: what can be learnt from other diseases? J Dermatolog Treat. 2015;26:520–527. [DOI] [PubMed] [Google Scholar]
- 7.Vincent FB, Morand EF, Murphy K, et al. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72:165–178. [DOI] [PubMed] [Google Scholar]
- 8.Bito T, Nishikawa R, Hatakeyama M, et al. Influence of neutralizing antibodies to adalimumab and infliximab on the treatment of psoriasis. Br J Dermatol. 2014;170:922–929. [DOI] [PubMed] [Google Scholar]
- 9.Hsu L, Snodgrass BT, Armstrong AW. Antidrug antibodies in psoriasis: a systematic review. Br J Dermatol. 2014;170:261–273. [DOI] [PubMed] [Google Scholar]
- 10.Jullien D, Prinz JC, Nestle FO. Immunogenicity of biotherapy used in psoriasis: the science behind the scenes. J Invest Dermatol. 2015;135:31–38. [DOI] [PubMed] [Google Scholar]
- 11.Menting SP, Coussens E, Pouw MF, et al. Developing a therapeutic range of adalimumab serum concentrations in management of psoriasis: a step toward personalized treatment. JAMA Dermatol. 2015;151:616–622. [DOI] [PubMed] [Google Scholar]
- 12.Krieckaert CL, Nair SC, Nurmohamed MT, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis. 2015;74:361–368. [DOI] [PubMed] [Google Scholar]
- 13.Lebwohl M, Yeilding N, Szapary P, et al. Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol. 2010;63:571–579. [DOI] [PubMed] [Google Scholar]
- 14.van Schouwenburg PA, Bartelds GM, Hart MH, et al. A novel method for the detection of antibodies to adalimumab in the presence of drug reveals “hidden” immunogenicity in rheumatoid arthritis patients. J Immunol Methods. 2010;362:82–88. [DOI] [PubMed] [Google Scholar]
- 15.Rispens T, Leeuwen AV, Vennegoor A, et al. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem. 2011;411:271–276. [DOI] [PubMed] [Google Scholar]
- 16.Rispens T, de Vrieze H, de Groot E, et al. Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J Immunol Methods. 2012;375:93–99. [DOI] [PubMed] [Google Scholar]
- 17.Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715. [DOI] [PubMed] [Google Scholar]
- 18.Chiu HY, Chu TW, Cheng YP, et al. The association between clinical response to ustekinumab and immunogenicity to ustekinumab and prior adalimumab. PLoS One. 2015;10:e0142930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menting SP, van den Reek JM, Baerveldt EM, et al. The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting. Br J Dermatol. 2015;173:855–857. [DOI] [PubMed] [Google Scholar]
- 20.Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154–163. [DOI] [PubMed] [Google Scholar]
- 22.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675–1684. [DOI] [PubMed] [Google Scholar]
- 23.Toro-Montecinos M, Ballesca F, Ferrandiz A, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2017;15:1427–1434. [DOI] [PubMed] [Google Scholar]
- 24.Ternant D, Ducourau E, Perdriger A, et al. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol. 2014;78:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelzang EH, Pouw MF, Nurmohamed M, et al. Adalimumab trough concentrations in patients with rheumatoid arthritis and psoriatic arthritis treated with concomitant disease-modifying antirheumatic drugs. Ann Rheum Dis. 2015;74:474–475. [DOI] [PubMed] [Google Scholar]
- 26.Xu Z, Davis HM, Zhou H. Clinical impact of concomitant immunomodulators on biologic therapy: pharmacokinetics, immunogenicity, efficacy and safety. J Clin Pharmacol. 2015;55(suppl 3):S60–S74. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson N, Tsakok T, Dand N, et al. Defining the therapeutic range for Adalimumab and predicting response in psoriasis: a multicenter prospective observational cohort study. J Invest Dermatol. 2019;139:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talamonti M, Galluzzo M, van den Reek JM, et al. Role of the HLA-C*06 allele in clinical response to ustekinumab: evidence from real life in a large cohort of European patients. Br J Dermatol. 2017;177:489–496. [DOI] [PubMed] [Google Scholar]
- 29.Talamonti M, Galluzo M, Chimenti S, et al. HLA-C*06 and response to ustekinumab in Caucasian patients with psoriasis: outcome and long-term follow-up. J Am Acad Dermatol. 2016;74:374–375. [DOI] [PubMed] [Google Scholar]
- 30.Chiu HY, Wang TS, Chan CC, et al. Human leucocyte antigen-Cw6 as a predictor for clinical response to ustekinumab, an interleukin-12/23 blocker, in Chinese patients with psoriasis: a retrospective analysis. Br J Dermatol. 2014;171:1181–1188. [DOI] [PubMed] [Google Scholar]
- 31.Talamonti M, Botti E, Galluzzo M, et al. Pharmacogenetics of psoriasis: HLA-Cw6 but not LCE3B/3C deletion nor TNFAIP3 polymorphism predisposes to clinical response to interleukin 12/23 blocker ustekinumab. Br J Dermatol. 2013;169:458–463. [DOI] [PubMed] [Google Scholar]
- 32.Prieto-Perez R, Llamas-Velasco M, et al. Pharmacogenetics of ustekinumab in patients with moderate-to-severe plaque psoriasis. Pharmacogenomics. 2017;18:157–164. [DOI] [PubMed] [Google Scholar]
- 33.Prieto-Perez R, Solano-Lopez G, Cabaleiro T, et al. New polymorphisms associated with response to anti-TNF drugs in patients with moderate-to-severe plaque psoriasis. Pharmacogenomics J. 2018;18:70–75. [DOI] [PubMed] [Google Scholar]


