Hydroxystilbene glucosides, particularly isorhapontin and astringin, are incorporated into Norway spruce bark lignin and participate in radical coupling reactions during lignification.
Abstract
Recent investigations have revealed that, in addition to monolignols, some phenolic compounds derived from the flavonoid and hydroxystilbene biosynthetic pathways can also function as true lignin monomers in some plants. In this study, we found that the hydroxystilbene glucosides isorhapontin (isorhapontigenin-O-glucoside) and, at lower levels, astringin (piceatannol-O-glucoside) and piceid (resveratrol-O-glucoside) are incorporated into the lignin polymer in Norway spruce (Picea abies) bark. The corresponding aglycones isorhapontigenin, piceatannol, and resveratrol, along with glucose, were released by derivatization followed by reductive cleavage, a chemical degradative method that cleaves β-ether bonds in lignin, indicating that the hydroxystilbene glucosides are (partially) incorporated into the lignin structure through β-ether bonds. Two-dimensional NMR analysis confirmed the occurrence of hydroxystilbene glucosides in this lignin, and provided additional information regarding their modes of incorporation into the polymer. The hydroxystilbene glucosides, particularly isorhapontin and astringin, can therefore be considered genuine lignin monomers that participate in coupling and cross-coupling reactions during lignification in Norway spruce bark.
Lignin is a complex aromatic polymer derived essentially from the oxidative coupling of three monolignols, p-coumaryl, coniferyl, and sinapyl alcohols (Boerjan et al., 2003; Ralph et al., 2004). Other phenolic compounds, including monolignol ester conjugates (with acetates, p-hydroxybenzoates, p-coumarates, or ferulates) or compounds derived from the truncated biosynthesis of monolignols (such as caffeyl alcohol or 5-hydroxyconiferyl alcohol and the hydroxycinnamaldehydes), have also been widely found to act as true lignin monomers in the lignins of many plants (Ralph et al., 1994, 2019; del Río et al., 2007, 2008, 2015; Chen et al., 2012, 2013; Rencoret et al., 2013; Tobimatsu et al., 2013; Lu et al., 2015; Karlen et al., 2016).
Recent investigations have shown that phenolic compounds derived from other biosynthetic pathways can also behave as true lignin monomers participating in radical coupling reactions and becoming integrally incorporated into the lignin polymer. The flavone tricin was the first phenolic derived from outside the monolignol biosynthetic pathway to be implicated in lignification (del Río et al., 2012; Lan et al., 2015). Tricin was first discovered in lignin preparations from wheat (Triticum durum) straw (del Río et al., 2012), and further studies indicated that it is widely present in the lignins of all grasses as well as in other monocots (Rencoret et al., 2013; del Río et al., 2015; Lan et al., 2016a, 2016b) and can be manipulated biogenetically (Eloy et al., 2017; Lam et al., 2017). More recently, we also reported the occurrence in macaúba (Acrocomia aculeata), carnauba (Copernicia prunifera), and coconut (Cocos nucifera) palm fruit endocarps of a second class of polyphenolic compounds, hydroxystilbenes (piceatannol, resveratrol, and isorhapontigenin), that behave as authentic lignin monomers participating in coupling and cross-coupling reactions during lignification and becoming integrally incorporated into the lignin structure (del Río et al., 2017; Rencoret et al., 2018). Flavonoids and hydroxystilbenes, unlike the monolignols that derive from the shikimate biosynthetic pathway, are metabolic hybrids deriving from a combination of the shikimate and acetate/malonate-derived polyketide pathways. These phenolic metabolites are known to participate in oxidative radical cross-coupling reactions with monolignols to produce ‘nonconventional’ lignans (termed flavonolignans and stilbenolignans) that have two phenylpropanoid units linked together through a diversity of linkages (Begum et al., 2010; Chambers et al., 2015). The wide natural occurrence in plants of these types of hybrid compounds arising from the cross-coupling with monolignols is an indication that at least some members of the flavonoids and hydroxystilbenes are also compatible with lignification.
In this paper, we provide evidence that hydroxystilbene glucosides are present in the lignin of Norway spruce (Picea abies) bark, participate in radical coupling reactions, and are integrally incorporated into the lignin structure. Part of the importance of this finding lies in their being the first examples of the authenticated incorporation of phenolic glycosides into lignins. Glucosides have been purported to exist in certain lignins, but it was not clear how they could be present as such. After all, if a phenol is protected with a glucoside, it simply cannot be oxidized to a radical and therefore cannot participate in lignification. In principle, it is possible for phenolic end-groups in lignin to be glucosylated after polymerization. However, this possibility is regarded with skepticism, as it is not easy for a large enzyme to penetrate the hydrophobic lignin polymer domain, and it is unclear what the purpose of phenolic glucosylation of the polymer would be. In this study, we propose an elegant solution to this conundrum by demonstrating that the glucosylated phenolic group is not one that would normally participate in radical coupling, and that another phenolic-OH participates in radical coupling in these novel monomers.
RESULTS AND DISCUSSION
Release of Hydroxystilbenes by Derivatization Followed by Reductive Cleavage
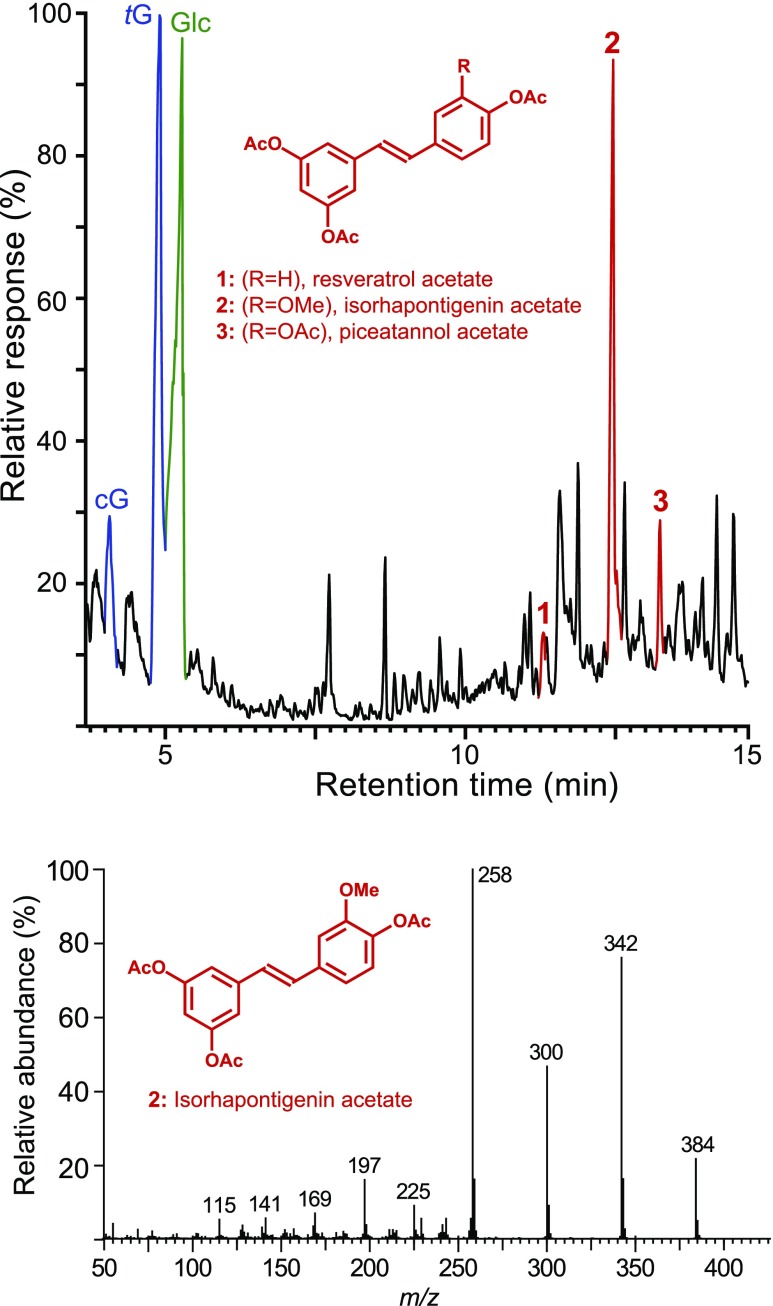
The ‘milled wood lignin’ (MWL) preparation obtained from Norway spruce bark was first analyzed by derivatization followed by reductive cleavage (DFRC), a chemical degradative method that selectively cleaves β-ether linkages in lignin, releasing the corresponding lignin monomers involved in these linkages (Fig. 1). The lignin released the cis- and trans-isomers of the guaiacyl (cG and tG) lignin monomers (as their acetylated derivatives), as is typical from a conifer lignin. More importantly, the chromatogram also indicated the occurrence of substantial amounts of a peak that was identified as isorhapontigenin 2 by comparison with the retention time and mass spectrum of an authentic standard. Minor amounts of the related resveratrol 1 and piceatannol 3 were also identified among the DFRC degradation products. The release of these compounds indicates that hydroxystilbenes are present in the lignin of Norway spruce bark. Hydroxystilbenes, particularly piceatannol, have been recently found incorporated into the lignins of other plant tissues, such as palm fruit endocarps, where they behave as true lignin monomers (del Río et al., 2017; Rencoret et al., 2018). Interestingly, the chromatogram also showed the release of important amounts of a compound that was identified as glucose (as its acetate), which seemed to indicate that the hydroxystilbenes were incorporated into the lignin as the corresponding hydroxystilbene glucosides, the so-called isorhapontin (isorhapontigenin-O-glucoside), astringin (piceatannol-O-glucoside), and piceid (resveratrol-O-glucoside; Fig. 2); it is evident that the phenolic glucosides were cleaved under the acidic conditions of DFRC releasing glucose and the corresponding hydroxystilbene aglycones. The DFRC data, therefore, indicated that at least a fraction of the hydroxystilbene glucosides, particularly isorhapontin, were incorporated into the lignin of Norway spruce bark as β-ether linked structures, the ones cleaved by the DFRC degradation method.
Figure 1.
Release of hydroxystilbenes from the lignin of Norway spruce bark by reductive cleavage. Top: Total-ion chromatogram of the DFRC degradation products released from the MWL lignin isolated from Norway spruce bark, showing the presence of the hydroxystilbenes resveratrol 1, isorhapontigenin 2, and piceatannol 3, as their acetate derivatives. cG and tG are the cis- and transconiferyl alcohol monomers (as their acetate derivatives). Note the occurrence of a peak from glucose Glc (as its peracetylated derivative). Bottom: Electron-impact mass spectrum of isorhapontigenin acetate 2.
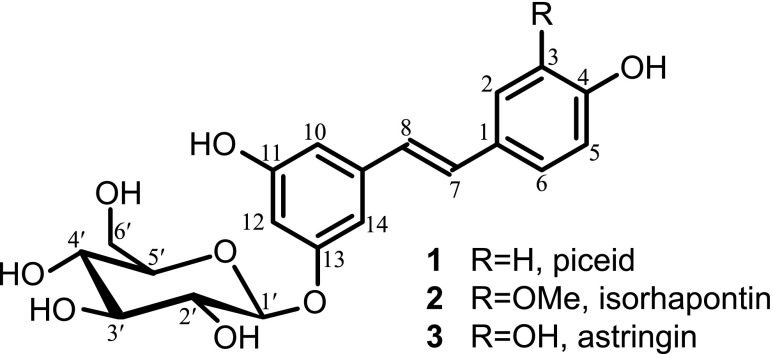
Figure 2.
Structure of the hydroxystilbene glucosides piceid 1, isorhapontin 2, and astringin 3; for convenience, glucose has been drawn linked to C-13 of the hydroxystilbene instead of C-11, as the latter OH participates in some coupling structures.
The hydroxystilbene glucosides piceid, astringin, and isorhapontin are known to occur in the extracts of Norway spruce bark (Hammerbacher et al., 2011; Latva-Mäenpää et al., 2013; Mulat et al., 2014). However, these compounds are highly soluble in water, and because the bark was subjected to exhaustive extraction with various solvents (dichloromethane, ethanol, and water) aimed at removing all of these hydroxystilbene glucosides and other extractives before lignin isolation (and the MWL preparation was additionally exhaustively washed with different organic solvents), it is reasonable to contend that the hydroxystilbene glucosides observed are linked to the lignin by covalent bonds and do not contaminate the lignin as free compounds.
Mode of Incorporation of Hydroxystilbene Glucosides into the Lignin as Determined by Two-dimensional NMR
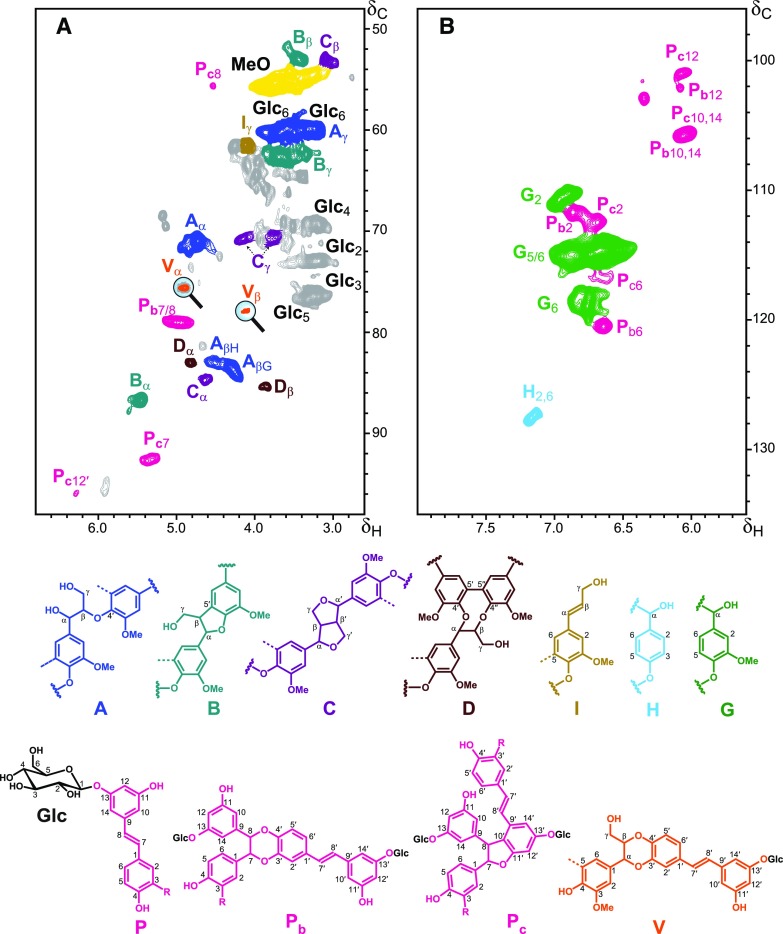
Additional information regarding the composition and structure of the lignin isolated from Norway spruce bark, including the mode of incorporation of the hydroxystilbene glucosides into the lignin polymer, was obtained from two-dimensional heteronuclear single-quantum coherence NMR (2D-HSQC-NMR; Fig. 3). The aromatic region of the spectrum (Fig. 3B) gave information regarding the different lignin and hydroxystilbene units present in the lignin preparation. The signals for the C2/H2, C5/H5, and C6/H6 correlations from G-lignin units were observed in this region of the spectrum, clearly indicating the occurrence of a G-rich lignin in Norway spruce bark, as corresponds to a lignin from a conifer. Minor amounts of signals from C2,6/H2,6 and C3,5/H3,5 correlations of H-lignin units were also observed. Signals from hydroxystilbenes were also clearly present in this part of the spectrum in the region at δC/δH ∼ 100-108/5.9-6.5, and were similar to those from the hydroxystilbenes observed in the 2D-HSQC-NMR spectra of lignins from palm fruit endocarps (del Río et al., 2017; Rencoret et al., 2018), including signals from isorhapontigenin and piceatannol, and confirming the results observed by DFRC.
Figure 3.
2D-HSQC-NMR spectrum (in dimethyl sulfoxide-d6) of the MWL preparation isolated from Norway spruce bark. A, Aliphatic-oxygenated (δC/δH 48−98/2.6−6.8) and aromatic [δC/δH 96−135/5.6−8.0; (B)] regions. The main structures found are as follows: A: β–O–4′ alkyl-aryl ethers; B: β–5′ phenylcoumarans; C: β–β′ resinols; D: 5–5′ dibenzodioxocins; I: cinnamyl alcohol end-groups; H: p-hydroxyphenyl units; G: guaiacyl units; P: hydroxystilbene glucosides (isorhapontin, R = OCH3; astringin, R = OH; piceid, R = H); Glc: glucose units; Pb: 8–O–4′/3′–O–7 benzodioxane structures involving isorhapontin (R = OCH3), astringin (R = OH), or piceid (R = H) units; Pc: 8–10′/11′–7 phenylcoumaran structures involving isorhapontin (R = OCH3), astringin (R = OH), or piceid (R = H) units (please note that other phenylcoumaran structures arising from other linkages, such as 8–5′ and 8–12′, may also occur, but only the 8–10′ linkage has been authenticated due to the occurrence of signal Pc12′); V: β–O–4′/3′–O–α benzodioxane structure formed by cross-coupling of astringin and coniferyl alcohol.
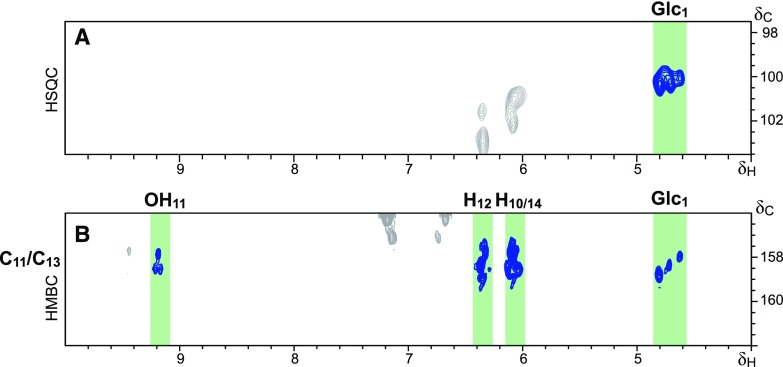
The aliphatic-oxygenated region of the spectra (Fig. 3A) gave information about the different substructures, characterized by their diagnostic interunit linkages, present in the lignin. In this region, typical signals from lignin substructures, including the correlation signals from β–O–4′ alkyl-aryl ethers A, β–5′ phenylcoumarans B, β–β′ resinols C, 5–5′ dibenzodioxocins D, and cinnamyl alcohol end-groups I, were clearly observed. Moreover, the signals for the C2/H2 (Glc2), C3/H3(Glc3), C4/H4(Glc4), C5/H5 (Glc5), and C6/H6 (Glc6) correlations of glucose units were also clearly visible in this region of the spectrum, confirming the occurrence of the hydroxystilbene glucosides, already advanced by DFRC. The linkage between the hydroxystilbenes and the glucose moiety was definitively proved by long-range correlation experiments; the heteronuclear multiple-bond correlation (HMBC) spectrum (Fig. 4) clearly demonstrated that the glucose moiety is linked to a phenolic unit of the resorcinol part of the hydroxystilbenes, indicating the occurrence of isorhapontin, astringin, and piceid, the respective glucosides of isorhapontigenin, piceatannol, and resveratrol. As noted above, we can discard the occurrence of free hydroxystilbene glucosides in the lignin preparation as these compounds are highly soluble in water and other solvents, and have been removed during the exhaustive solvent extraction before the MWL isolation protocol. This contention is also compellingly supported by the absence of signals from double bonds between 7- and 8-position (or 7′- and 8′-position) in the 2D-HSQC-NMR spectrum, a feature evidencing their participation in radical coupling reactions.
Figure 4.
Proof of the linkage between Glc and the C-13 of the hydroxystilbene unit. A, Section of the HSQC spectrum of the lignin of Norway spruce bark showing the correlation of the anomeric carbon of glucose (Glc1); B, Section of the long range 1H–13C correlation HMBC spectrum of the lignin from Norway spruce bark, showing the correlations within 2–3 atoms of C-13, and demonstrating that glucose is linked to the C-13 of hydroxystilbenes. Note that the correlations of C-11 overlap with those of C-13.
Most importantly, signals from structures involving the coupling of hydroxystilbene glucosides were found in the HSQC spectrum (Fig. 3). Signals for benzodioxane (Pb) and phenylcoumaran structures (Pc) involving coupling of two hydroxystilbene glucosides, and signals for benzodioxane structures (V) formed by cross-coupling of astringin and coniferyl alcohol, presented similar correlations to those observed for the incorporation of the hydroxystilbene piceatannol into the lignins of palm fruit endocarps (del Río et al., 2017). Thus, the strong signal present at δC/δH 79.0/5.00 is similar to the signal for the C7/H7 and C8/H8 correlations of the benzodioxane structure (Pb7/8) formed by 8–O–4′ coupling of two piceatannol units observed in the HSQC spectrum of the lignin of palm fruit endocarps; in the case of the lignin from Norway spruce bark, with isorhapontin being the major hydroxystilbene glucoside incorporated into the lignin structure according to the DFRC results, this benzodioxane structure would be formed by the coupling of an isorhapontin (or an astringin) unit at its 8-position to the 4–O-position of an astringin unit. Therefore, two different benzodioxane substructures would be expected to be produced, one comprising two astringin units and another comprising isorhapontin and astringin units. These types of structures are similar to the hydroxystilbene glucoside dimers, piceasides G and H (the RR and SS isomers, respectively, formed by 8–O–4′ coupling of two astringin units), and piceasides E and F (the RR and SS isomers, respectively, formed by 8–O–4′ coupling of an isorhapontin and an astringin unit), that have been identified among the ethanol-water and acetone-water extractives of Norway spruce bark (Li et al., 2008; Gabaston et al., 2017). Because isorhapontin is the major hydroxystilbene glucoside present in the lignin of Norway spruce bark, and the benzodioxane structure requires an astringin unit to form the benzodioxane skeleton, the predominant benzodioxane structure would be that formed by 8–O–4′ coupling of an isorhapontin unit at its 8-position with the 4′–O- of an astringin unit, in structures analogous to those of piceasides E and F.
The HSQC spectrum of the lignin from Norway spruce bark also showed two signals at δC/δH 92.6/5.36 (Pc7) and at δC/δH 55.6/4.52 (Pc8) that are similar to the signals for the C7/H7 and C8/H8 correlations of the phenylcoumaran structure formed by 8–10′ coupling of two piceatannol units, similar to the stilbene dimer scirpusin B, observed in the HSQC spectra of the lignins from palm fruit endocarps (del Río et al., 2017; Rencoret et al., 2018). In the case of Norway spruce bark, this phenylcoumaran structure would predominantly be formed by coupling of two isorhapontin units, with a skeleton similar to stilbene dimer bisisorhapontigenin A, and with smaller amounts of isorhapontin and astringin cross-coupled structures. However, it is important to note that, besides the 8–10′ coupling, other phenylcoumaran structures can also be formed by other types of coupling between two hydroxystilbenes, including the 8–5′ and the 8–12′ coupling structures that have been found in other plants, including Norway spruce (Iliya et al., 2002; Yao and Lin, 2005; Li et al., 2008; Francezon et al., 2017; Gabaston et al., 2017). Differentiating among the different phenylcoumaran structures, however, is a difficult task as the C7/H7 and C8/H8 correlation signals are very similar for all types of phenylcoumaran structures involving two hydroxystilbenes, regardless the type of coupling (8–5′, 8–10′, or 8–12′). In the case of the lignins from palm fruit endocarps, the differentiation among the different types of linkages and phenylcoumaran structures involving two hydroxystilbenes was possible via the occurrence of a characteristic and intense signal at δC/δH 95.9/6.28 for the C12′/H12′ correlations of the phenylcoumaran structure formed by 8–10′ coupling of two piceatannol units (as in the dimeric stilbene scirpusin B), and that was clearly apparent in the HSQC spectra. In the case of the lignin from Norway spruce bark, this signal (Pc12′) was also present in the HSQC spectrum, indicating the occurrence of a phenylcoumaran structure involving 8–10′ coupling of two hydroxystilbene glucosides (principally two isorhapontin units, having the skeleton of the dimeric stilbene bisisorhapontigenin A); however, the intensity of this signal was somewhat weak in comparison with the signals of Pc7 and Pc8, suggesting that other phenylcoumaran structures arising from other coupling structures (possibly 8–5′ or 8–12′) might also be present in this lignin. In fact, different phenylcoumaran dehydrodimeric structures arising from 8–5′ homocoupling and cross-coupling of isorhapontin and astringin, such as the diastereomer pairs piceasides A and B (astringin dimers), piceasides C and D (astringin-isorhapontin dimers), and piceasides O and P (isorhapontin dimers), have been found among the extractives of Norway spruce and black spruce (Picea mariana) barks (Li et al., 2008; Francezon et al., 2017; Gabaston et al., 2017). In addition, the dimeric phenylcoumaran structure formed by 8–12′ coupling of two isorhapontigenin units, termed bisisorhapontigenin B, has been identified in species of the Gnetum genus (Iliya et al., 2002; Yao and Lin, 2005). However, it is important to note that these are coupled substructures occurring into the lignin polymer, and that we have to discard the occurrence of simple dimeric stilbenes in the lignin isolated from Norway spruce bark due to the absence of the characteristic signals for the double bonds in the HSQC, a feature that indicates the stilbene’s participation in radical coupling reactions; apparently radical coupling at the 8-position occurs first, as we hope to examine using biomimetic coupling reactions, and possible molecular modeling studies, in due course. Moreover, and as already noted above, actual dimeric structures would have been removed during the exhaustive extraction with dichloromethane, ethanol, and water before the lignin isolation process.
A key finding in the HSQC spectrum (Fig. 3) was two signals at δC/δH 75.8/4.90 (Vα) and δC/δH 78.0/4.17 (Vβ) that are similar to the Cα/Hα and Cβ/Hβ correlation signals of the benzodioxane structure formed by cross-coupling of piceatannol and monolignols observed in the spectra of the lignins from palm fruit endocarps (del Río et al., 2017). In the lignin from Norway spruce bark, the benzodioxane structure (V) would uniquely be formed by cross-coupling of coniferyl alcohol and the catechol moiety of astringin; isorhapontin, with a guaiacyl ring, cannot form this type of benzodioxane structure. The occurrence of all of these coupled and cross-coupled structures involving hydroxystilbene glucosides indicates that these phenolic compounds behave as authentic lignin monomers participating in radical coupling reactions during lignification to become integrally incorporated into the lignin polymer of Norway spruce bark. Zhang and Gellerstedt (2008) also found hydroxystilbene glucosides in a ‘milled bark tannin-lignin’ fraction that was isolated from Norway spruce bark after exhaustive removal of extractives with different solvents, and proposed that they might be linked to the condensed tannins. However, we can rule out this type of linkage as condensed tannins are not present in the MWL preparation studied here; in addition, the occurrence of the cross-coupled benzodioxane structure V provides evidence of the direct linkage between hydroxystilbene glucosides and the lignin polymer.
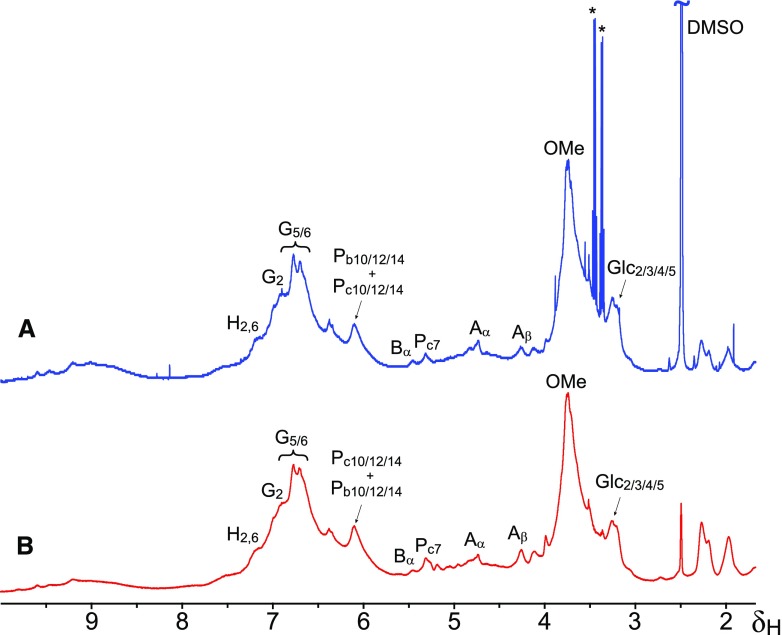
Another approach for demonstrating that hydroxystilbene glucosides are incorporated into the lignin polymer and are not present as free molecules or dimeric structures is by diffusion-edited 1H-NMR, also termed Diffusion-ordered Spectroscopy. Diffusion NMR experiments can resolve compounds of different molecular size spectroscopically in a mixture based on their differing diffusion coefficients. Analysis of the MWL from Norway spruce bark by diffusion-edited 1H-NMR (Fig. 5) indicated that the aromatic protons at δH ∼6.10 assigned to the H10, and H12,14 of isorhapontin, as well as the protons at δH ∼3.23 of the H2/3/4/5 of the glucose moiety, must have similar translational diffusion coefficients as the aromatic protons of G-lignin units (δH ∼6.50–7.00), also providing additional evidence that hydroxystilbene glucosides are incorporated into the lignin polymer. It can be clearly noted that the sharp signals from contaminants present in the MWL that are seen in the normal 1H-NMR are completely absent in the diffusion-edited spectrum (Fig. 5).
Figure 5.
Analysis of the MWL isolated from Norway spruce bark by diffusion-edited 1H-NMR. Comparison of standard 1H-NMR (A) and diffusion-edited 1H-NMR (B) of the MWL preparation isolated from Norway spruce bark indicated that the hydroxystilbene glucosides have translational diffusion coefficients similar to those of the lignin, and providing further evidence of its incorporation into the polymer. *Major contaminants. DMSO, dimethyl sulfoxide.
Radical Coupling of Hydroxystilbene Glucosides and Cross-Coupling with Monolignols and the Growing Lignin Polymer
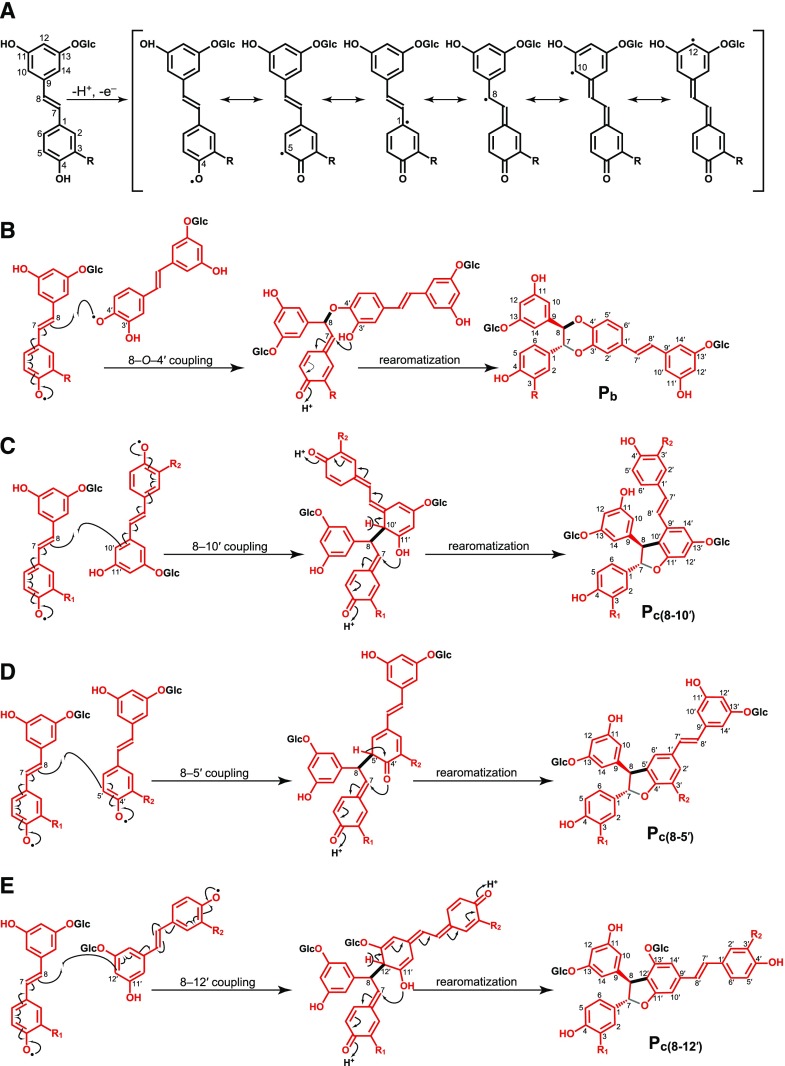
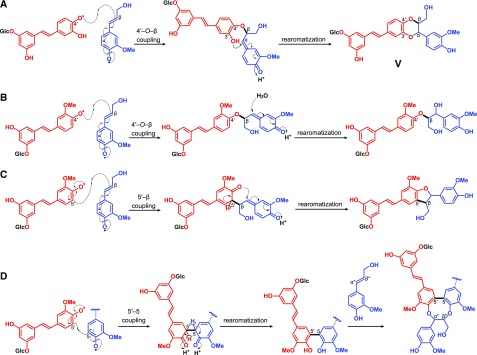
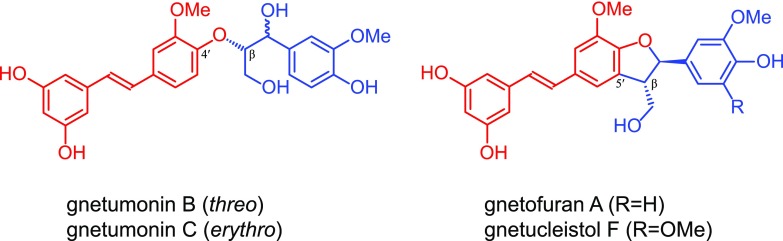
Hydroxystilbene glucosides, as for the hydroxystilbenes, and because the crucial phenolic-OH (4-position) remains free (Fig. 6), are compatible with the radical coupling reactions that typify lignification, and are therefore expected to participate in radical coupling reactions with other hydroxystilbenes glucosides as well as with monolignols and become integrally incorporated into the lignin polymer. As occurs with monolignols and the corresponding hydroxystilbenes isorhapontigenin and piceatannol, the hydroxystilbene glucosides isorhapontin and astringin can be oxidized by peroxidases and/or laccases to form radicals that are stabilized by resonance (Fig. 6A). These radicals can eventually couple and cross-couple with another hydroxystilbene glucoside molecule forming a variety of dehydrodimers and higher dehydro-oligomers (Fig. 6, B–E), similar to the structures with benzodioxane and phenylcoumaran skeletons observed by 2D-NMR in the lignin of Norway spruce bark. In addition, the hydroxystilbene glucosides (and their dimers and higher oligomers) can also cross-couple with monolignols and the growing lignin polymer via radical coupling reactions to become integrally incorporated into the lignin structure, as shown in Figure 7. As indicated above, the hydroxystilbene glucoside astringin, with its catechol ring, can cross-couple with coniferyl alcohol forming the benzodioxane structure V, that is formed via β–O–4′ radical coupling of coniferyl alcohol (at its β-position) and astringin (at its 4′–O position) followed by internal trapping of the quinone methide intermediate by the 3′-OH and forming the benzodioxane bridge (Fig. 7A). On the other hand, the hydroxystilbene glucoside isorhapontin, with its guaiacyl ring, can easily cross-couple with coniferyl alcohol in different ways, including via 4–O–β or 5–β forming the β–O–4′ alkyl-aryl ether or β–5′ phenylcoumaran structures (Fig. 7, B–C); it can also couple with lignin oligomers (or the polymer) to form the 5–5′/4–O–β′′-linked dibenzodioxocin structures shown in Figure 7D. In fact, several stilbenolignans formed by cross-coupling of isorhapontigenin with monolignols through different linkage types (Fig. 8) have been found in other gymnosperms, such as the gnetumonins B and C, the threo and erythro forms of the aryl-alkyl ethers arising from 4′–O–β cross-coupling of isorhapontigenin and coniferyl alcohol, or the phenylcoumarans gnetofuran A and gnetucleistol F arising from the 5′–β cross-coupling of isorhapontigenin and coniferyl or sinapyl alcohol, respectively (Ma et al., 2017). The occurrence of all of these stilbenolignans, not to be confused with stilbenolignins, reveal the compatibility of hydroxystilbenes, and more particularly of isorhapontigenin, with lignification. Although the occurrence of stilbenolignans involving hydroxystilbene glucosides have not been reported so far, the cross-coupling of hydroxystilbene glucosides, and more particularly isorhapontin that presents a guaiacyl unit, with monolignols is feasible and can be anticipated. However, unambiguous identification of these cross-coupled structures of isorhapontin with monolignols is not a trivial task as the NMR signals overlap with those of the typical β–O–4′ alkyl aryl ether A, β–5′ phenylcoumaran B, and the 5–5′ dibenzodioxocin D lignin structures. In any case, the release of hydroxystilbenes upon DFRC and the inherent polymeric nature of the lignin seem to suggest the existence of such linkages between the hydroxystilbene glucosides and the lignin polymer. Additional work with authentic models and detailed NMR studies are needed to unambiguously confirm this contention.
Figure 6.
Hydroxystilbene glucoside radicals and dehydrodimeric radical coupling reactions. A, Oxidative radicalization resulting from one-electron oxidation of isorhapontin (R = OCH3, isorhapontigenin-O-glucoside), astringin (R = OH, piceatannol-O-glucoside), and piceid (R = H, resveratrol-O-glucoside) stabilized by delocalization; resonance forms are displayed, in which the single-electron density is shown to localize at the 4–O-, 5-, 1-, 8-, 10-, and 12-positions. B, Dehydrodimerization products arising from 8–O–4′ coupling of isorhapontin (R = OCH3) or astringin (R = OH) with another astringin unit producing a benzodioxane structure; this structure is similar to the stilbene glucoside dimers piceasides E and F (R = OCH3), and piceasides G and H (R = OH) referred to in the text. C, Phenylcoumaran structures arising from the 8–10′ coupling of isorhapontin (R1,R2 = OCH3) and astringin (R1,R2 = OH) units; this structure has the same skeleton as the stilbene dimers bisisorhapontigenin A (R1 = R2 = OCH3) and scirpusin B (R1 = R2 = OH), referred to in the text. D, Phenylcoumaran structures arising from the 8–5′ coupling of isorhapontin (R1,R2 = OCH3) and astringin (R1,R2 = OH) units; this structure is similar to that of the stilbene glucoside dimers piceasides A and B (R1 = R2 = OH), piceasides C and D (R1 = OCH3, R2 = OH), and piceasides O and P (R1 = R2 = OCH3), referred to in the text. E, Phenylcoumaran structures arising from the 8–12′ coupling of isorhapontin (R1,R2 = OCH3) and astringin (R1,R2 = OH) units; this structure presents the same skeleton as that of the stilbene dimer bisisorhapontigenin B (R1 = R2 = OCH3).
Figure 7.
Dehydrodimerization products arising from oxidative cross-coupling of hydroxystilbene glucosides with monolignols or the growing lignin polymer. A, β–O–4′ coupling of astringin and coniferyl alcohol, producing the benzodioxane structure V; B, β–O–4′ coupling of isorhapontin and coniferyl alcohol, producing β–O–4′ alkyl-aryl ether structures; C, β–5′ Coupling of isorhapontin and coniferyl alcohol, producing phenylcoumaran structures; D, 5–5′ Coupling of isorhapontin and guaiacyl phenolic polymer end-units, producing dibenzodioxocin structures.
Figure 8.
Examples of stilbenolignans involving cross-coupling of isorhapontigenin and monolignols. Gnetumonins B and C are the threo and erythro aryl-alkyl ether structures arising from 4′–O–β cross-coupling of isorhapontigenin and coniferyl alcohol; gnetofuran A and gnetucleistol F are the phenylcoumaran structures arising from the 5′–β cross-coupling of isorhapontigenin and coniferyl or sinapyl alcohol, respectively.
If the hydroxystilbene glucosides are fully integrated into the lignin polymer in Norway spruce bark, then they should also be considered true lignin monomers participating in radical coupling reactions during lignification in this bark tissue. Thus, the hydroxystilbene glucosides, particularly isorhapontin and astringin, can also be added to the list of ‘nonconventional’ phenolic lignin monomers recently discovered in plants, including phenolics from different biosynthetic pathways such as the corresponding hydroxystilbene aglycones, particularly piceatannol, discovered in the lignins of palm fruit endocarps (del Río et al., 2017; Rencoret et al., 2018), the flavone tricin present in the lignins of all grasses and other monocots (del Río et al., 2012; Lan et al., 2015, 2016a, 2016b), or the hydroxycinnamic amides, such as tyramine ferulate in tobacco and other Solanaceae (Negrel et al., 1996; Ralph et al., 1998), and diferuloylputrescine, recently identified in the lignin of maize grain kernels (del Río et al., 2018). This would be the first report of a phenolic glucoside acting as authentic lignin monomer. All of these discoveries continue to provide further evidence of the plasticity of the lignification process and indicate that, as has been noted early on, “any phenolic transported to the lignifying zone of the cell wall can, subject to simple chemical concerns, be incorporated into the polymer” (Ralph et al., 2008).
Implications for Prior Claims of Lignin Glucosides
Lignin-(poly)saccharide binding has long fascinated plant cell wall researchers (Terrett and Dupree, 2019). Apart from the well-authenticated example in which ferulates on arabinoxylans in commelinid monocots provide a powerful mechanism for extensive cell wall cross-linking (Ralph, 2010), securing definitive evidence for other types of lignin-polysaccharide cross-linking, particularly in noncommelinids, has been a challenge essentially unmet. The proposed benzylic-polysaccharide ethers, for example, have been only weakly ‘observed’ in NMR spectra (Balakshin et al., 2007), but there is one validated account of evidence from degradation and isolation of a lignin-sugar unit from Pinus densiflora (Japanese red pine; Nishimura et al., 2018). Glucosides (or, more generally, glycosides) also have weak evidence from NMR spectra (Balakshin et al., 2007), but the assignments are again unauthenticated and there is the additional conceptual problem as to how they result. Logically, they cannot arise from the phenol-glucosylated monolignols, coniferin and syringin, that are known to be derivatives stored in the vacuole (Dima et al., 2015) and which, in the case of coniferin and in softwoods, may be deglucosylated and the usual monomer used for lignification (Terashima et al., 2016); this is simply because a free-phenol is an absolute requirement to enable the generation of the phenolic radical that is used in the radical coupling reactions that typify lignification. If there truly are glucosides in ‘normal’ lignins, the glucoside must be introduced postpolymerization, and it is difficult to imagine how or why this might happen. For these reasons, the claims of glucosides in lignins, and the weak and tenuous NMR evidence for them, have never been compelling. Here, however, we have a very simple way for explaining how such glucosides might in fact appear in the polymer. If a lignin precursor has more than one phenolic-OH, and at least a single phenolic-OH capable of undergoing radicalization and enabling the radical coupling reactions, then there is a sound mechanism by which phenolic glucosides can be accommodated. The stilbene glycosides here provide one such example (and flavonoid glucosides might also provide another example, although they have not been authenticated as lignin monomers yet). Another in ‘normal lignification’ must be via, for example, a resinol unit in which only one of the phenols is glucosylated. Interestingly, this type of half-glucosylated pinoresinol metabolites has been identified in Arabidopsis (Arabidopsis thaliana; Morreel et al., 2014). The important point here is that there is finally a plausible pathway for creating lignins bearing phenolic glucosides.
Biosynthesis, Role of Hydroxystilbene Glucosides in the Lignin of Norway Spruce Bark, and Prospects for Metabolic Engineering
The biosynthesis of hydroxystilbenes is controlled by the stilbene synthase that catalyzes the formation of the stilbene backbone from three malonyl-CoA units and one CoA-ester of a cinnamate derivative. In the case of Norway spruce, the biosynthesis of the major tetrahydroxystilbene glucosides, astringin and isorhapontin, goes through the formation of resveratrol from p-coumaroyl-CoA by the corresponding stilbene synthase, which is further modified through hydroxylation, O-methylation, and O-glucosidation reactions to produce isorhapontin and astringin (Hammerbacher et al., 2011). The production of isorhapontin and astringin was found to be enhanced by fungal infection, suggesting that these phenolic metabolites have a role in antifungal defense (Hammerbacher et al., 2011). It is therefore evident that the incorporation of hydroxystilbene glucosides into the lignin polymer in bark, which is the outermost layer that covers the wood and gives protection to the tree against external agents, can provide antifungal and antimicrobial properties, contributing to resistance to disease and to pathogenic attack. In addition, and as also occurs in the palm fruit endocarps, the incorporation of hydroxystilbene glucosides into the lignin may allow for the production of higher amounts of lignin by incorporating other phenolic compounds present in the cell wall into the lignin polymer, which indeed will produce more condensed structures, as the phenylcoumarans and benzodioxane structures shown above illustrate, thus reinforcing and strengthening the cell wall.
The identification in recent years of nonconventional lignin monomers, not usually present in the lignins of other plants, as is the case of the hydroxystilbene glucosides described here, can open up new ways to design and engineer the lignin structure to produce polymers with new or improved properties, as already considered with other phenolic compounds (Vanholme et al., 2008, 2012; Grabber et al., 2010; Wilkerson et al., 2014; Mottiar et al., 2016). Metabolic engineering to introduce hydroxystilbene glucosides into the lignin polymer could provide a means to increase disease resistance in plants by adding antifungal and antimicrobial properties, and may provide a way of producing lignins with special properties, including enhanced hydrophilicity, a trait potentially proposed to improve enzymatic wall digestibility (Grabber et al., 2010). The particular structure of the hydroxystilbene glucosides, with a pendant glucose moiety that obviously does not participate in the radical coupling reactions, makes these phenolic metabolites potentially interesting for introducing specific functionalities into the lignin via functionalization of the glucose moiety.
CONCLUSION
Hydroxystilbene glucosides (isorhapontin, astringin, and piceid) have been found incorporated into the lignin of Norway spruce bark, participating in radical coupling reactions during lignification, and should therefore be considered as authentic lignin monomers. The occurrence of hydroxystilbene glucosides in the lignin of Norway spruce bark seems to have a role in plant protection, providing antifungal and antimicrobial properties to the bark; in addition, the incorporation of phenolic metabolites beyond the traditional monolignols allows for the production of higher amounts of lignin, which may be more condensed, thus providing additional protection to the bark.
MATERIALS AND METHODS
Samples
The Norway spruce (Picea abies) bark used for this study was obtained from a sawmill located in Jyvaskyla, Finland, after the debarking process, and the chemical composition has been published elsewhere (Neiva et al., 2018). The air-dried samples were milled using a knife-mill (1 mm screen) and successively extracted with dichloromethane (2000 mL) in a Soxhlet apparatus for 24 h, ethanol (2000 mL, 24 h), and hot water (two times 2000 mL, 24 h at 100°C). Klason lignin content was estimated as the residue after sulfuric acid hydrolysis of the pre-extracted material, corrected for ash and protein content, according to the TAPPI method T222 om-88, and presented a value of 30.7% ± 0.8 (three replicate samples were used).
Lignin Isolation and Purification
The MWL preparation was obtained from extractive-free bark according to the classical procedure (Björkman, 1956). Around 40 g of extractive-free material were finely ball-milled in a Retsch PM100 planetary ball mill (Retsch) for 5 h at 400 rpm using a 500 mL agate jar and agate ball-bearings (20 × 20 mm). The ball-milled material was then extracted three times with dioxane-water, 90:10 (v/v; 25 mL of solvent/g of milled bark), and the isolated lignin was exhaustively purified as described elsewhere (del Río et al., 2012). The MWL yield was 37% of the Klason lignin content.
DFRC
DFRC degradation was performed according to the original protocol (Lu and Ralph, 1997), and the detailed explanation can be found elsewhere (del Río et al., 2012). Briefly, around 10 mg of MWL were treated with 2.5 mL acetyl bromide in acetic acid (8:92, v/v) at 50°C for 2 h. The solvents and excess of bromide were removed by rotary evaporation, then dissolved in dioxane/acetic acid/water (5:4:1, v/v/v), and 50 mg of powdered zinc was added. After 40 min stirring at room temperature the mixture was transferred into a separatory funnel with dichloromethane and saturated ammonium chloride. The pH of the aqueous phase was adjusted to less than 3 by adding 3% (v/v) HCl, the mixture vigorously mixed, and the organic layer separated. The water phase was extracted twice more with dichloromethane. The combined dichloromethane fractions were dried over anhydrous NaSO4, and the filtrate was evaporated in a rotary evaporator. The lignin degradation products were acetylated with an acetic anhydride: pyridine solution (1:1 v/v) for 1 h at room temperature and dissolved in dichloromethane for a subsequence gas chromatography/mass spectrometric analysis. A Saturn 4000 (Varian) equipment fitted with a capillary column (DB5-HT, 15 m × 0.25 mm i.d., 0.1 μm film thickness; from J&W Scientific) was used to analyze the lignin degradation products obtained upon DFRC. The samples were injected with an autoinjector (Varian 8200), which was programmed from 120°C (0.1 min) to 330°C (until the end of the analysis) at a rate of 200°C min−1. The oven was programmed from 120°C (1 min) to 380°C (5 min) at a rate of 10°C min−1. The temperature of the transfer line was set at 300°C during the analysis. Helium was used as carrier gas at a rate of 2 mL min−1. The different hydroxystilbenes (resveratrol, isorhapontigenin, piceatannol) were identified by comparison with authentic standards.
NMR Spectroscopy
Multidimensional NMR spectra (2D HSQC, 2D HMBC, 2D HSQC-TOCSY) experiments were recorded on an AVANCE III 500 MHz instrument (Bruker) fitted with a cryogenically cooled 5 mm triple resonance gradient probe with inverse geometry. Around 40 mg of lignin sample were dissolved in 0.75 mL of dimethyl sulfoxide-d6. The central solvent peaks were used as internal references (δC/δH 39.5/2.49). The HSQC experiments used Bruker’s standard “hsqcetgpsisp2.2” pulse program (adiabatic-pulsed version), the HMBC experiments used Bruker’s standard “hmbcgplpndqf” pulse program with long-range J-coupling evolution times of 62.5 ms [JLR = 8 Hz, and/or 80 ms (JLR = 6.25 Hz) when required], and the diffusion-edited 1H-NMR used the pulse program “ledbpgp2s1d”. The detailed NMR experimental conditions have been described elsewhere (del Río et al., 2012).
Acknowledgments
We thank Asko Ojaniemi for providing the Norway spruce bark samples. We also thank Manuel Angulo (University of Seville) for performing the NMR analyses that were acquired on a Bruker Avance III 500 MHz instrument from the NMR facilities of the General Research Services of the University of Seville (SGI-CITIUS).
Footnotes
This work was supported by Ministerio de Economia y Competitividad (Ministry of Economy and Competitiveness) projects (CTQ2014-60764-JIN and AGL2017-83036-R to J. Rencoret., G.M., A.G., and J.C.d.R.); a Ministry of Economy and Competitiveness | Consejo Superior de Investigaciones Cientificas (Spanish National Research Council) project (2017-40E-071); the U.S. Department of Energy (DOE) (DE-SC0018409 to H.K. and J. Ralph); Fundação para a Ciência e a Tecnologia (Foundation for Science and Technology) (UID/AGR/00239/2013 to J.G. and H.P.); and the SUSFOR doctoral program (PD/BD/52697/2014 to D.N.).
Articles can be viewed without a subscription.
References
- Balakshin MY, Capanema EA, Chang HM (2007) MWL fraction with a high concentration of lignin-carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 61: 1–7 [Google Scholar]
- Begum SA, Sahai M, Ray AB (2010) Non-conventional lignans: Coumarinolignans, flavonolignans, and stilbenolignans. Fortschr Chem Org Naturst 93: 1–70 [DOI] [PubMed] [Google Scholar]
- Björkman A. (1956) Studies on finely divided wood. Part I. Extraction of lignin with neutral solvents. Sven Papperstidn 59: 477–485 [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Chambers CS, Valentová K, Křen V (2015) Non-taxifolin derived flavonolignans: Phytochemistry and biology. Curr Pharm Des 21: 5489–5500 [DOI] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Havkin-Frenkel D, Dixon RA, Ralph J (2012) A polymer of caffeyl alcohol in plant seeds. Proc Natl Acad Sci USA 109: 1772–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tobimatsu Y, Jackson L, Nakashima J, Ralph J, Dixon RA (2013) Novel seed coat lignins in the Cactaceae: Structure, distribution and implications for the evolution of lignin diversity. Plant J 73: 201–211 [DOI] [PubMed] [Google Scholar]
- del Río JC, Marques G, Rencoret J, Martínez AT, Gutiérrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55: 5461–5468 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Marques G, Gutiérrez A, Ibarra D, Santos JI, Jiménez-Barbero J, Zhang L, Martínez AT (2008) Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J Agric Food Chem 56: 9525–9534 [DOI] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Prinsen P, Martínez AT, Ralph J, Gutiérrez A (2012) Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J Agric Food Chem 60: 5922–5935 [DOI] [PubMed] [Google Scholar]
- del Río JC, Lino AG, Colodette JL, Lima CF, Gutiérrez A, Martínez AT, Lu F, Ralph J, Rencoret J (2015) Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 81: 322–338 [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Kim H, Ralph J (2017) Hydroxystilbenes are monomers in palm fruit endocarp lignins. Plant Physiol 174: 2072–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río JC, Rencoret J, Gutiérrez A, Kim H, Ralph J (2018) Structural characterization of lignin from maize (Zea mays L.) fibers: Evidences for diferuloylputrescine incorporated into the lignin polymer in maize kernels. J Agric Food Chem 66: 4402–4413 [DOI] [PubMed] [Google Scholar]
- Dima O, Morreel K, Vanholme B, Kim H, Ralph J, Boerjan W (2015) Small glycosylated lignin oligomers are stored in Arabidopsis leaf vacuoles. Plant Cell 27: 695–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloy NB, Voorend W, Lan W, Saleme ML, Cesarino I, Vanholme R, Smith RA, Goeminne G, Pallidis A, Morreel K, et al. (2017) Silencing CHALCONE SYNTHASE in maize impedes the incorporation of tricin into lignin and increases lignin content. Plant Physiol 173: 998–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francezon N, Meda NR, Stevanovic T (2017) Optimization of bioactive polyphenols extraction from Picea mariana bark. Molecules 22: 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaston J, Richard T, Biais B, Waffo-Teguo P, Pedrot E, Jourdes M, Coio-Costet M-F, Mérillon J-M (2017) Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind Crops Prod 103: 267–273 [Google Scholar]
- Grabber JH, Schatz PF, Kim H, Lu F, Ralph J (2010) Identifying new lignin bioengineering targets: 1. Monolignol-substitute impacts on lignin formation and cell wall fermentability. BMC Plant Biol 10: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerbacher A, Ralph SG, Bohlmann J, Fenning TM, Gershenzon J, Schmidt A (2011) Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol 157: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliya I, Tanaka T, Iinuma M, Ali Z, Furasawa M, Nakaya K-I (2002) Dimeric stilbenes from stem lianas of Gnetum africanum. Heterocycles 57: 1057–1062 [Google Scholar]
- Karlen SD, Zhang C, Peck ML, Smith RA, Padmakshan D, Helmich KE, Free HCA, Lee S, Smith BG, Lu F, et al. (2016) Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci Adv 2: e1600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PY, Tobimatsu Y, Takeda Y, Suzuki S, Yamamura M, Umezawa T, Lo C (2017) Disrupting Flavone Synthase II alters lignin and improves biomass digestibility. Plant Physiol 174: 972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Lu F, Regner M, Zhu Y, Rencoret J, Ralph SA, Zakai UI, Morreel K, Boerjan W, Ralph J (2015) Tricin, a flavonoid monomer in monocot lignification. Plant Physiol 167: 1284–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Morreel K, Lu F, Rencoret J, del Río JC, Voorend W, Vermerris W, Boerjan W, Ralph J (2016a) Maize tricin-oligolignol metabolites and their implications for monocot lignification. Plant Physiol 171: 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Rencoret J, Lu F, Karlen SD, Smith BG, Harris PJ, del Río JC, Ralph J (2016b) Tricin-lignins: Occurrence and quantitation of tricin in relation to phylogeny. Plant J 88: 1046–1057 [DOI] [PubMed] [Google Scholar]
- Latva-Mäenpää H, Laakso T, Sarjala T, Wähälä K, Saranpää P (2013) Root neck of Norway spruce as a source of bioactive lignans and stilbenes. Holzforschung 68: 1–7 [Google Scholar]
- Li S-H, Niu X-M, Zahn S, Gershenzon J, Weston J, Schneider B (2008) Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies). Phytochemistry 69: 772–782 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (1997) Derivatization followed by reductive cleavage (DFRC method), a new method for lignin analysis: Protocol for analysis of DFRC monomers. J Agric Food Chem 45: 2590–2592 [Google Scholar]
- Lu F, Karlen S, Regner M, Kim H, Ralph S, Sun RC, Kuroda K, Augustin M, Mawson R, Sabarez H, et al. (2015) Naturally p-hydroxybenzoylated lignins in palms. BioEnergy Res 8: 934–952 [Google Scholar]
- Ma YQ, Zhai YM, Deng Y, Guo L, Wan YQ, Tan CH (2017) Stilbeno-phenylpropanoids from Gnetum montanum Markgr. Phytochem Lett 21: 42–45 [Google Scholar]
- Morreel K, Saeys Y, Dima O, Lu F, Van de Peer Y, Vanholme R, Ralph J, Vanholme B, Boerjan W (2014) Systematic structural characterization of metabolites in Arabidopsis via candidate substrate-product pair networks. Plant Cell 26: 929–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottiar Y, Vanholme R, Boerjan W, Ralph J, Mansfield SD (2016) Designer lignins: Harnessing the plasticity of lignification. Curr Opin Biotechnol 37: 190–200 [DOI] [PubMed] [Google Scholar]
- Mulat DG, Latva-Mäenpää H, Koskela H, Saranpää P, Wähälä K (2014) Rapid chemical characterisation of stilbenes in the root bark of Norway spruce by off-line HPLC/DAD-NMR. Phytochem Anal 25: 529–536 [DOI] [PubMed] [Google Scholar]
- Negrel J, Pollet B, Lapierre C (1996) Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry 43: 1195–1199 [Google Scholar]
- Neiva DM, Araújo S, Gominho J, Carneiro AC, Pereira H (2018) An integrated characterization of Picea abies industrial bark regarding chemical composition, thermal properties and polar extracts activity. PLoS One 13: e0208270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Kamiya A, Nagata T, Katahira M, Watanabe T (2018) Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci Rep 8: 6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J. (2010) Hydroxycinnamates in lignification. Phytochem Rev 9: 65–83 [Google Scholar]
- Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung H-JG (1994) Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc 116: 9448–9456 [Google Scholar]
- Ralph J, Hatfield RD, Piquemal J, Yahiaoui N, Pean M, Lapierre C, Boudet AM (1998) NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnamylalcohol dehydrogenase and cinnamoyl-CoA reductase. Proc Natl Acad Sci USA 95: 12803–12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, Boerjan W (2004) Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem Rev 3: 29–60 [Google Scholar]
- Ralph J, Brunow G, Harris PJ, Dixon RA, Schatz PF, Boerjan W (2008) Lignification: Are lignins biosynthesized via simple combinatorial chemistry or via proteinaceous control and template replication? In Daayf F, El Hadrami A, Adam L, Ballance GM, eds, Recent Advances in Polyphenol Research, Vol 1 Wiley-Blackwell Publishing, Oxford, pp 36–66 [Google Scholar]
- Ralph J, Lapierre C, Boerjan W (2019) Lignin structure and its engineering. Curr Opin Biotechnol 56: 240–249 [DOI] [PubMed] [Google Scholar]
- Rencoret J, Ralph J, Marques G, Gutiérrez A, Martínez AT, del Río JC (2013) Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J Agric Food Chem 61: 2434–2445 [DOI] [PubMed] [Google Scholar]
- Rencoret J, Kim H, Evaristo AB, Gutiérrez A, Ralph J, del Río JC (2018) Variability in lignin composition and structure in cell walls of different parts of macaúba (Acrocomia aculeata) palm fruit. J Agric Food Chem 66: 138–153 [DOI] [PubMed] [Google Scholar]
- Terashima N, Ko C, Matsushita Y, Westermark U (2016) Monolignol glucosides as intermediate compounds in lignin biosynthesis. Revisiting the cell wall lignification and new 13C-tracer experiments with Ginkgo biloba and Magnolia liliiflora. Holzforschung 70: 801–810 [Google Scholar]
- Terrett OM, Dupree P (2019) Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr Opin Biotechnol 56: 97–104 [DOI] [PubMed] [Google Scholar]
- Tobimatsu Y, Chen F, Nakashima J, Escamilla-Treviño LL, Jackson L, Dixon RA, Ralph J (2013) Coexistence but independent biosynthesis of catechyl and guaiacyl/syringyl lignin polymers in seed coats. Plant Cell 25: 2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Ralph J, Boerjan W (2008) Lignin engineering. Curr Opin Plant Biol 11: 278–285 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Darrah C, Oyarce P, Grabber JH, Ralph J, Boerjan W (2012) Metabolic engineering of novel lignin in biomass crops. New Phytol 196: 978–1000 [DOI] [PubMed] [Google Scholar]
- Wilkerson CG, Mansfield SD, Lu F, Withers S, Park JY, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Rencoret J, Ralph J (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344: 90–93 [DOI] [PubMed] [Google Scholar]
- Yao C-S, Lin M (2005) Bioactive stilbene dimers from Gnetum cleistostachyum. Nat Prod Res 19: 443–448 [DOI] [PubMed] [Google Scholar]
- Zhang L, Gellerstedt G (2008) 2D Heteronuclear (1H–13C) single quantum correlation (HSQC) NMR analysis of Norway spruce bark components. In Hu TQ, ed, Characterization of Lignocellulosic Materials. Blackwell Publishing Ltd, Oxford, pp 3–16 [Google Scholar]