Specific ARGONAUTE proteins function to protect Arabidopsis against Turnip Crinkle Virus infection in a modular mode.
Abstract
RNA-based silencing functions as an important antiviral immunity mechanism in plants. Plant viruses evolved to encode viral suppressors of RNA silencing (VSRs) that interfere with the function of key components in the silencing pathway. As effectors in the RNA silencing pathway, ARGONAUTE (AGO) proteins are targeted by some VSRs, such as that encoded by Turnip crinkle virus (TCV). A VSR-deficient TCV mutant was used to identify AGO proteins with antiviral activities during infection. A quantitative phenotyping protocol using an image-based color trait analysis pipeline on the PlantCV platform, with temporal red, green, and blue imaging and a computational segmentation algorithm, was used to measure plant disease after TCV inoculation. This process captured and analyzed growth and leaf color of Arabidopsis (Arabidopsis thaliana) plants in response to virus infection over time. By combining this quantitative phenotypic data with molecular assays to detect local and systemic virus accumulation, AGO2, AGO3, and AGO7 were shown to play antiviral roles during TCV infection. In leaves, AGO2 and AGO7 functioned as prominent nonadditive, anti-TCV effectors, whereas AGO3 played a minor role. Other AGOs were required to protect inflorescence tissues against TCV. Overall, these results indicate that distinct AGO proteins have specialized, modular roles in antiviral defense across different tissues, and demonstrate the effectiveness of image-based phenotyping to quantify disease progression.
Plants can protect themselves against invasive virus infection through RNA silencing by targeting viral RNA for degradation (Agius et al., 2012). This host silencing machinery is triggered by viral double-stranded RNAs, which are cleaved by Dicer-like (DCL) nucleases associated with double-stranded RNA binding proteins into 21–24-nucleotide RNA duplexes called “viral small interfering RNAs” (vsiRNAs). The vsiRNAs are then methylated and stabilized by HUA enhancer1. One strand of these stabilized vsiRNAs is recruited into the RNA-induced silencing complex containing ARGONAUTE (AGO) proteins, and then serves as the sequence-specific guide for specific AGOs to slice cognate viral RNAs (Bologna and Voinnet, 2014; Fang and Qi, 2016).
Most plant viruses have evolved to encode viral suppressors of RNA silencing (VSRs) that use varied mechanisms to target components in the silencing pathway (Incarbone and Dunoyer, 2013). One such mechanism is interference of AGOs that mediate antiviral silencing. Accumulating evidence indicates that various VSRs use diverse modes of action on AGO proteins, such as promoting AGO degradation (Pazhouhandeh et al., 2006; Baumberger et al., 2007; Bortolamiol et al., 2007; Chiu et al., 2010), inhibiting the slicing activities of AGOs (Zhang et al., 2006), or interfering with factors upstream of AGO activity such as RNA-dependent RNA polymerase-dependent silencing (Fang et al., 2016), obstructing siRNA-loaded RNA-induced silencing complex activity (Giner et al., 2010; Kenesi et al., 2017), or indirectly repressing AGO protein level (Várallyay et al., 2010). Because functional VSRs can mask host antiviral silencing effects, VSR-defective mutant viruses that can only successfully infect immunocompromised plants have been constructed to identify antiviral roles of key components in the silencing pathway during virus infection (Qu et al., 2008; Garcia-Ruiz et al., 2015).
The Arabidopsis (Arabidopsis thaliana) genome encodes 10 AGO proteins, several of which have demonstrated antiviral roles (Carbonell and Carrington, 2015). AGO1 (Qu et al., 2008; Wang et al., 2011; Dzianott et al., 2012; Kontra et al., 2016) and AGO2 (Harvey et al., 2011; Jaubert et al., 2011; Scholthof et al., 2011; Garcia-Ruiz et al., 2015) have been identified as prominent antiviral AGOs against several RNA viruses, whereas other AGOs, including AGO5, AGO7, and AGO10, have limited antiviral roles in some cases (Qu et al., 2008; Garcia-Ruiz et al., 2015). For DNA viruses, AGO4 is the main effector protein in the antiviral silencing machinery (Raja et al., 2008, 2014). The full complement of AGOs with roles in antiviral defense, and how distinct antiviral AGOs are coordinated to silence different viruses in different tissues, remains to be fully determined.
Turnip crinkle virus (TCV) is a positive single-stranded RNA virus belonging to the Carmovirus genus of the Tombusviridae family. The TCV genome encodes five proteins, including two replicase proteins (P28 and P88), two movement proteins (P8 and P9), and the coat protein (CP; P38). The CP is multifunctional, as it has roles in virus movement (Cohen et al., 2000; Cao et al., 2010), serves as a virulence factor (Donze et al., 2014), and functions as a VSR to suppress antiviral silencing (Thomas et al., 2003; Chen et al., 2014). Other virus groups in the Tombusviridae encode separate VSR proteins, such as P19 from Tomato bushy stunt virus (Kontra et al., 2016). TCV systemically infects the susceptible Arabidopsis ecotype Columbia-0 (Col-0), and causes disease symptoms that include severe chlorosis in leaves, stunted bolts, and reduced biomass. A previous study reported that replacement of a single amino acid residue in the TCV CP P38 (R130T) disrupts its VSR function without affecting other functions (Cao et al., 2010). The VSR-deficient TCV is unable to suppress the host antiviral silencing machinery, leading to a lack of disease symptoms in wild-type plants postinoculation.
In this study, the roles of Arabidopsis AGO proteins in anti-TCV silencing were analyzed using genetic and image-based quantitative phenotyping approaches. Most previous pathological studies have relied on qualitative and subjective visual scoring systems to identify and assess disease phenotypes in plants (Bock et al., 2010). A machine learning method (Abbasi and Fahlgren, 2016) and other analysis tools in the open-source Plant Computer Vision (PlantCV) platform (Fahlgren et al., 2015; Gehan et al., 2017) were used to detect subtle, reproducible differences in disease symptoms over time.
RESULTS
Suppressor-Deficient TCV Is Not Able To Elicit Disease Symptoms in Wild-Type Host Plants
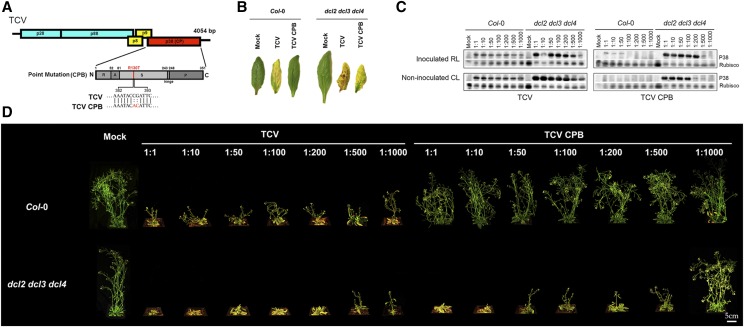
TCV and other carmoviruses use their CPs as VSRs to suppress host antiviral silencing (Meng et al., 2006; Martínez-Turiño and Hernández, 2009). By replacing a single amino acid in TCV CP (P38) with its counterpart residue in Tomato bushy stunt virus CP (R130T; Fig. 1A), the VSR function of TCV CP (P38) is abolished (Cao et al., 2010). Introducing this mutation (R130T) in the CP did not affect its ability to assemble functional virion particles (Supplemental Fig. S1). TCV bearing R130T mutation in its CP was named as TCV CPB. We confirmed that this mutant virus (TCV CPB) lost its capacity to suppress host antiviral silencing by quantifying and comparing the effects of TCV CPB in wild-type (Col-0) and dcl2-1 dcl3-2 dcl4-2 (dcl2 dcl3 dcl4) triple mutant plants. In the dcl triple mutant, three DCL genes with roles in antiviral defense are dysfunctional, so it serves as a hypersusceptible control genotype. Parental TCV infection caused severe chlorosis in Arabidopsis leaves in both wild-type control (Col-0) and the dcl triple mutant plants, whereas TCV CPB caused similar chlorosis symptoms only in the dcl triple mutant (Fig. 1B).
Figure 1.
TCV infection-caused disease symptoms in Arabidopsis. A, Schematic representation of the TCV genome showing the CPB point mutation on the CP (P38). Top diagram shows the genomic RNA of TCV. The bottom diagram shows the CP (P38) region of single-amino acid mutant TCV CPB. The single amino acid change in TCV CPB mutant is marked beneath the bottom diagram. The bottom diagram represents the full-length CP, with the sizes and the relative positions of the five structural domains: N, N terminal; R, RNA-binding domain; A, arm domain; S, surface domain; hinge; P, protruding domain; C, C terminal. B, TCV infection-caused chlorosis in noninoculated cauline leaves. Left: wild-type control (Col-0); right: hypersusceptible control (dcl2 dcl3 dcl4). Photographs were taken at 14 dpi. C, Local and systemic accumulation of CP (P38) caused by TCV (left) and TCV CPB (right) infection was assayed by immunoblotting. P38 was detected using anti-P38 antibody. Rubisco protein was detected by anti-Rubisco antibody as a control. The virion inoculum was in different dilutions. RL, rosette leaf; CL, cauline leaf. RL samples were collected at 7 dpi; CL samples were collected at 14 dpi. D, TCV infection-caused stunt bolt phenotype in Arabidopsis. Upper: wild-type control (Col-0); bottom: hypersusceptible control (dcl2 dcl3 dcl4). The virion inoculum was in different dilutions. Photographs were taken individually at 14 dpi and digitally extracted and aligned for comparison.
TCV CP was detected in inoculated rosette leaves and noninoculated cauline leaves using immunoblot assays with CP antisera (anti-P38). High levels of CP were detected in rosette leaves at 7 d postinoculation (dpi) and in cauline leaves at 14 dpi of both Col-0 and dcl2 dcl3 dcl4 plants infected with parental TCV, even at 1:1,000 dilution (Fig. 1C). In contrast, low levels of CP were detected in rosette leaves of wild-type control plants inoculated with TCV CPB at lower dilutions (1:1–1:50) but not at higher dilutions (1:100–1:1,000; Fig. 1C). In noninoculated cauline leaves of Col-0 plants, no observable CPB-P38 signal was detected at any dilution (Fig. 1C). However, TCV CPB inoculation of dcl2 dcl3 dcl4 mutant led to high levels of CP protein accumulation in both rosette and cauline leaves at each dilution ranging from 1:1 to 1:500 (Fig. 1C). Notably, in dcl2 dcl3 dcl4 mutants, no observable local or systemic CPB CP protein was detected when the TCV CPB inoculum was highly diluted (1:1,000; Fig. 1C).
Parental TCV infection at any dilution had negative effects on the growth of both Col-0 and dcl2 dcl3 dcl4 plants (Fig. 1D). Morphologically, plants infected with TCV were shorter compared to noninfected plants (Fig. 1D). In contrast, the TCV CPB mutant affected growth of only dcl2 dcl3 dcl4 plants (Fig. 1D). These results confirmed that the CPB (R130T) mutation in TCV CP attenuates VSR functions, and suggested that TCV CPB could be used in a genetic analysis to identify components of the silencing machinery necessary for antiviral defense.
Image-Based Analysis of Disease Symptoms
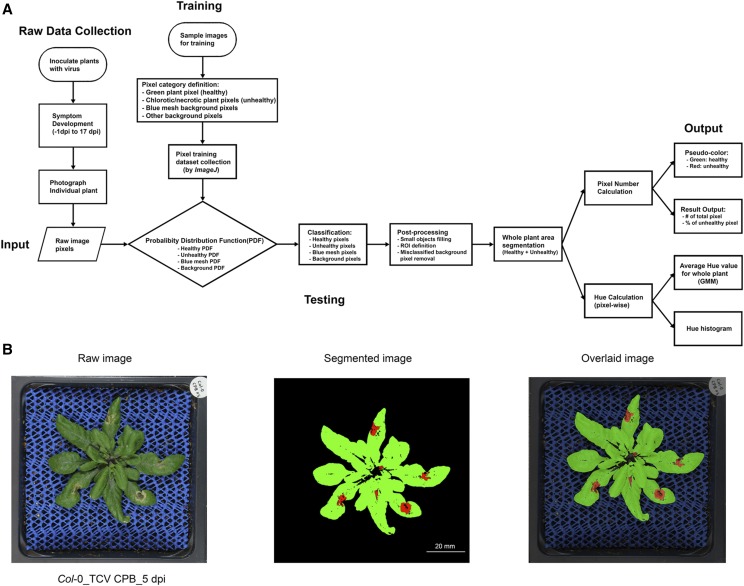
Before initiating a systematic screen of Arabidopsis ago mutants using TCV and TCV CPB, a nondestructive, computer vision-based system using the PlantCV platform (Fahlgren et al., 2015) was developed for high-resolution, quantitative assessment of disease symptom phenotypes over time (Fig. 2A). The system was designed to identify individual plant leaves, measure their areas, lengths, and pixel color characteristics, and distinguish subtle differences in responses of plants with different genotypes over time. Top-down red-green-blue (RGB) images of individual plants were captured every other day, from 1 d preinoculation to 17 dpi. The images were analyzed using a machine learning approach to segment plant from background and to classify plant pixels as “healthy” pixels (green color) or “unhealthy” chlorotic/necrotic pixels (any nongreen color; Fig. 2B). The ratio of chlorotic/necrotic to total plant pixels was used to calculate the extent and severity of symptomatic rosette leaf tissue.
Figure 2.
Image-based traits analysis workflow outline. A, Workflow chart. Plants were inoculated with virus or mock solution. In the raw data collection section, individual plant was photographed from 1 d preinoculation to 17 dpi. In the training session, representative sample images were chosen. Four pixel-classifiers were defined and RGB information of 1,891 pixels in each class were collected to build up the training dataset. The Probability Distribution Function for each category was calculated based on the training dataset. In the testing session, each pixel from an input image was calculated and classified into each class. After postprocessing, the pipeline produced a summary of pixel number in each class and the hue value for each pixel in each raw image. Then appropriate statistics was applied to quantify and compare the output results among different groups. B, One example of original raw plant image, segmented pseudo-color image, and overlaid image of Col-0 plant inoculated with TCV CPB at 5 dpi.
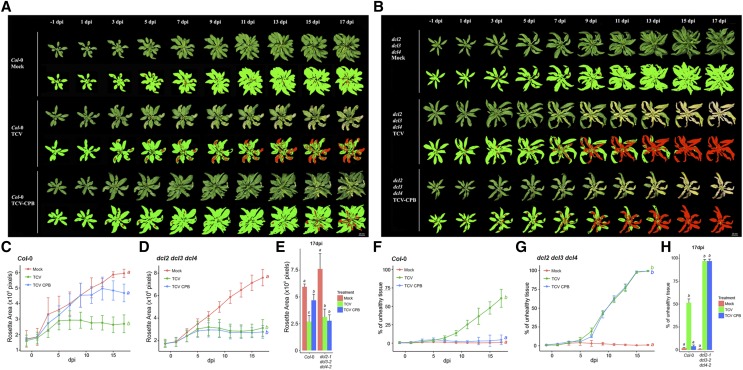
To validate the approach, wild-type (Col-0) and hypersusceptible dcl2 dcl3 dcl4 mutant plants were inoculated with parental TCV or TCV CPB mutant virus, or were mock-inoculated (four replicates per genotype and treatment combination). In the mock-inoculated groups, the majority of rosette area remained healthy (green) over time in both Col-0 and dcl2 dcl3 dcl4 plants (Fig. 3, A and B). Infection of both plant genotypes with parental TCV elicited local chlorosis at 7 dpi and nearly complete chlorosis/necrosis by 17 dpi (Fig. 3, A and B). Discoloration caused by parental TCV infection appeared to be more severe in the dcl2 dcl3 dcl4 mutant plants than in wild-type controls (Fig. 3B). TCV CPB inoculation led to parental virus-like discoloration symptoms in the dcl triple mutant plants (Fig. 3B) but did not cause strong chlorosis symptoms in Col-0 plants (Fig. 3A). Although an increased number of chlorotic pixels was observed in Col-0 rosettes at 15- and 17-dpi TCV CPB inoculation, the increase was visually less than that caused by parental TCV (Fig. 3A).
Figure 3.
TCV-infection–caused temporal changes in rosette size and the percentage of unhealthy tissues in control plants. A and B, Temporal visualization of rosette leaves and the corresponding pseudo-color images of Col-0 (A) and dcl2 dcl3 dcl4 (B) plants inoculated by TCV, TCV CPB inoculum, or mock solution. Photos were taken individually from 1 d preinoculation to 17 dpi, and digitally extracted and aligned for comparison. In the pseudo-color images, green color referred to healthy plant pixels; red color referred to chlorotic/necrotic (unhealthy) plant pixels. C and D, The growth curves show the temporal change of rosette area (total pixel number, averaged by four plants, ±se) in Col-0 (C) and dcl2 dcl3 dcl4 (D) plants from 1 d preinoculation to 17 dpi (K-S test with α = 0.05). E, The box plot shows the rosette size at 17 dpi. Statistical analysis was calculated between treatments in each genotype. Boxes with different letters are statistically different (n = 4, Tukey post hoc test with α = 0.05). F and G, The curves show the percentage of unhealthy tissue change over time (red pixels/total pixels, averaged by four plants, ±se) in Col-0 (F) and dcl2 dcl3 dcl4 (G) plants from 1 d preinoculation to 17 dpi (K-S test with α = 0.05). H, The box plot shows the percentage of unhealthy pixels at 17 dpi. Statistical analysis was calculated between treatments in each genotype. Boxes with different letters are statistically different (n = 4, Tukey post hoc test with α = 0.05).
Parental TCV infection led to reduced rosette area, based on total number of pixels (healthy plus chlorotic), indicating a decrease in biomass. Parental TCV significantly inhibited rosette growth over time in both wild-type and hypersusceptible controls (Fig. 3, C and D; Kolmogorov-Smirnov [K-S] test, P < 0.05). Similarly, TCV CPB led to a comparable decrease in rosette area in dcl2 dcl3 dcl4 mutants after 7 dpi, but not in Col-0 (Fig. 3, B and D). At 17 dpi, a significant difference in rosette area was detected between the two virus-treatments in Col-0, but not in dcl2 dcl3 dcl4 plants (Fig. 3E).
The percentage of unhealthy chlorotic/necrotic pixels in the whole rosette was also calculated. Both parental TCV and TCV CPB infection caused a significant increase in the percentage of unhealthy tissues over time in dcl2 dcl3 dcl4 plants (Fig. 3G), from ∼0% at 5 dpi to nearly 100% at 17 dpi (Fig. 3G). In Col-0 plants, parental TCV infection also led to a gradual increase in chlorotic tissue, from ∼0% at 5 dpi to nearly 60% at 17 dpi (Fig. 3F). In contrast, TCV CPB did not significantly change the percentage of chlorotic tissue in wild-type control plants over time (Fig. 3F; K-S test, P < 0.05). At 17 dpi, no significant difference in the percentage of unhealthy tissues was detected in TCV CPB-inoculated and mock treatment Col-0 plants (Fig. 3H).
Measurement of hue value of leaf color has been used to estimate chlorophyll loss caused by biotic and abiotic stress (Sass et al., 2012; Liang et al., 2017; Veley et al., 2017). Hue value information extracted from the RGB images was used as a parameter to quantify chlorosis in rosette leaves. The hue value quantifies color in terms of angle around a circle, with values ranging from 0° to 359° (Gonzalez and Eddins, 2009), starting in the red color range. Yellow and green hue ranges span from ∼51° to 80° and ∼81° to 140°, respectively. For each genotype and treatment combination, the mean proportion of pixels at each degree was plotted for each day. One primary peak centered at ∼100°, representing a green hue, was found in the histograms of mock-inoculated Col-0 and dcl2 dcl3 dcl4 plants over time (Fig. 4, A and B, left). After 7 dpi, parental TCV infection caused a shift from a unimodal distribution of green hue values to a bimodal distribution that included a peak of yellow hue values in both genotypes (Fig. 4, A and B, middle). Notably, this green to yellow shift observed in dcl2 dcl3 dcl4 mutants was more complete than that in Col-0 plants at 17 dpi, suggesting the hypersusceptible control leaves were more yellow than wild-type leaves (Fig. 4, A and B, middle). Similar to parental virus, TCV CPB induced a gradual shift from green to yellow in dcl2 dcl3 dcl4 plants (Fig. 4B, right). In contrast, this green-to-yellow shift was not observed in Col-0 plants inoculated with TCV CPB (Fig. 4A, right).
Figure 4.
TCV infection-caused temporal color changes in control plants. A and B, The histogram illustrates the temporal changes (1 d preinoculation to 17 dpi, from bottom to above) of pixel distribution at each hue value (degree) in Col-0 (A) and dcl2 dcl3 dcl4 (B) plant rosette images. Pixels were collected and summed up from four individual images in the same treatment group: mock (left), TCV (middle), or TCV CPB (right) inoculum. The color chart illustrates the relationship between the hue value (degree) with the corresponding RGB coordinates. C and D, The plots show the average hue value (degree) change over time (averaged by four plants, ±se) in Col-0 (C) and dcl2 dcl3 dcl4 (D) plants from 1 d preinoculation to 17 dpi (K-S test with α = 0.05). E, The box plot shows the average hue value at 17 dpi in Col-0 and dcl2 dcl3 dcl4 plants. Statistical analysis was calculated between treatments in each genotype. Boxes with different letters are statistically different (n = 4, Tukey post hoc test with α = 0.05).
The average hue value for whole plants in each treatment group was calculated. Parental TCV infection caused a temporal decrease in hue value in both Col-0 (from 100° to 75°) and dcl2 dcl3 dcl4 plants (from 100° to 40°; Fig. 4, C and D). TCV CPB induced a similar decrease in average hue value over time in dcl2 dcl3 dcl4 mutants, but did not significantly affect hue value in Col-0 plants (Fig. 4, C and E). These results were consistent with the healthy/chlorotic classification-based results (Fig. 3). These data indicate that the image-based growth and color trait measurement protocols were effective in quantifying virus-induced symptoms in Arabidopsis, and in distinguishing plant susceptibility or virus virulence phenotypes.
Image-Based Analysis of TCV and TCV CPB Infection in ago Mutants
As with the dcl2 dcl3 dcl4 plants, we hypothesized that loss of ago genes with a function in anti-TCV silencing would be revealed by gain of susceptibility to the VSR-deficient TCV CPB mutant. A set of 10 mutant plants with defects in each of the 10 Arabidopsis AGO genes was inoculated with mock solution, parental TCV or TCV CPB, and image-based traits and virus protein levels over an infection time-course were measured. Visually, the images of rosette of each ago mutant and the corresponding pseudo-colored images were digitally extracted and aligned for comparison over time (Supplemental Figs. S2–S11). Statistically, pairwise K-S tests were done to compare time-series data.
Parental TCV infection caused a significant decrease in rosette size over time in all genotypes (Fig. 5A). In contrast, TCV CPB inoculation led to three different effects, depending on genotype: (1) no significant effect on rosette size overtime in ago4-2, ago5-2, ago8-1, ago9-5, and ago10-5; (2) significant negative effects on rosette size change over time in ago2-1, ago3-2, zip-1 (ago7 mutant), and dcl2 dcl3 dcl4 control; or (3) observable, but not significant, negative effects on rosette size change over time in ago1-27, ago6-3, and Col-0 plants (Fig. 5A). After 7 dpi, a significant increase in the percentage of chlorotic/necrotic tissue was observed in all genotypes infected with parental TCV (Fig. 5B), though the degree of increase varied in different genotypes (Fig. 6C). In the dcl2 dcl3 dcl4 mutant, both parental and mutant TCV caused a comparable increase in the percentage of unhealthy tissue (Fig. 5B). Among the 10 ago mutants, TCV CPB significantly increased the percentage of unhealthy tissue only in ago2-1 and zip-1 mutants over time (Fig. 5B). The ago1-27, ago3-2, ago4-2, ago5-2, ago6-3, ago8-1, ago9-5, and ago10-5 mutants were not responsive to TCV CPB inoculation (Fig. 5B). Focusing on the data at 17 dpi, the effects caused by TCV CPB on rosette chlorosis in ago2-1 and zip-1 mutant could be directly displayed (Fig. 6A) and statistically tested (Fig. 6C; Tukey post hoc test with α = 0.05). Similarly, by using this statistic test, we further confirmed that TCV CPB caused a significant decrease in ago2-1, ago3-2, and zip-1 rosette size at 17 dpi (Fig. 6B).
Figure 5.
TCV infection-caused temporal changes in rosette size and the percentage of unhealthy tissues in 10 single ago mutant plants. A, The growth curves show the temporal change of rosette area (total pixel number, averaged by four plants, ±se) in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants from 1 d preinoculation to 17 dpi (K-S test with α = 0.05, detailed D value, and P value are listed in Supplemental Table S1). B, The curves show the percentage of unhealthy tissue change overtime (red pixels/total pixels, averaged by four plants, ±se) in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants from 1 d preinoculation to 17 dpi. (K-S test with α = 0.05, detailed D value, and P value are listed in Supplemental Table S2). Curves with different letters are statistically different.
Figure 6.
Rosette size and the percentage of unhealthy tissue in 10 single ago mutant plants infected with TCV at 17 dpi. A, Representative visualization of rosette leaves and the corresponding pseudo-color images in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants, which were separately inoculated by mock solution, TCV, or TCV CPB inoculum. Photos were taken individually at 17 dpi, and digitally extracted and aligned for comparison. In the pseudo-color images, green color referred to healthy plant pixels; red color referred to chlorotic/necrotic (unhealthy) plant pixels. B, The box plot shows the rosette area (total pixel number) in different genotypes at 17 dpi. C, The box plot shows the percentage of unhealthy tissue (red pixels/total pixels) in different genotypes at 17 dpi. Statistical analysis was calculated between treatments in each genotype. Boxes with different letters are statistically different (n = 4, Tukey post hoc test with α = 0.05).
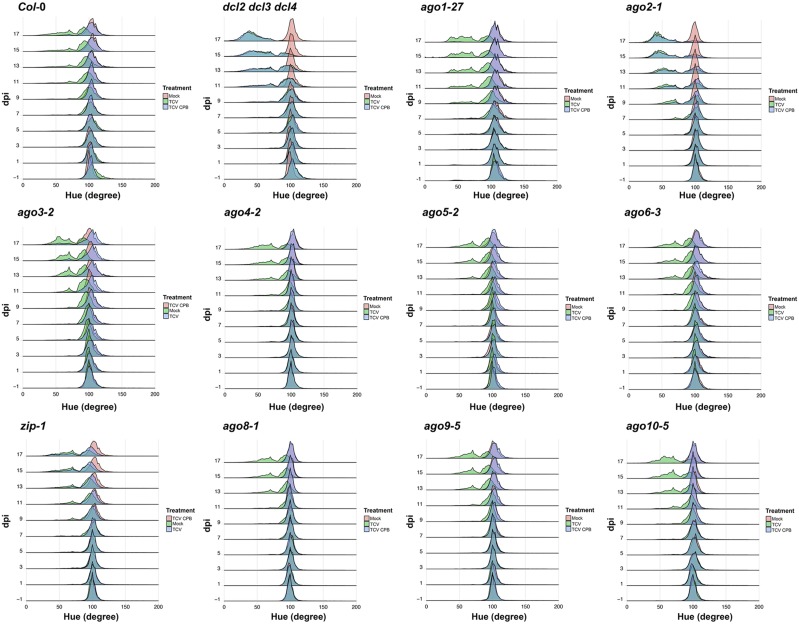
Another output of the phenotyping pipeline to evaluate leaf color is hue value (Fig. 2A). The temporal changes in hue value in the 10 inoculated ago mutants and control plants were measured over time (Fig. 7). One primary peak at ∼100° (green) was observed in early stages for all genotype and inoculation combinations (Fig. 7, −1 to 5 dpi). In the mock-inoculated group, the primary peak remained at ∼100° in all genotypes throughout the time course. The green peak gradually shifted to yellow in each ago mutant and the two controls infected with parental TCV (Fig. 7, 7–17 dpi). As expected, dcl2 dcl3 dcl4 mutant plants infected with TCV CPB turned from green (peak at 100°) to yellow over time (Fig. 7, blue area in dcl2/3/4). Similarly, a complete green-to-yellow shift was measured in ago2-1 mutant plants infected with TCV CPB (Fig. 7, blue area in ago2-1). Gradual, partial shift from green to yellow was measured in zip-1 plants infected with TCV CPB (Fig. 7, blue area in zip-1). All other ago mutants (ago1-27, ago3-2, ago4-2, ago5-2, ago6-3, ago8-1, ago9-5, and ago10-5) inoculated with TCV CPB were similar to mock-inoculated plants (Fig. 7, blue area).
Figure 7.
TCV infection-caused temporal pixel distribution changes in 10 single ago mutant plants. Each histogram illustrates the temporal changes (1 d preinoculation to 17 dpi, from bottom to above) of pixel distribution at each hue value (degree) in Col-0, dcl2 dcl3 dcl4, or 10 single ago mutant rosette images. Pixels were collected and summed up from four individual images in the same treatment group. For the separated distribution histogram in each genotype and treatment combination, refer to Supplemental Figure S12.
To quantify the color shift observed above, average hue value was calculated. Pairwise K-S tests were applied to compare time-series data. Parental TCV infection led to a gradual decrease in average hue value in all genotypes (Fig. 8A), whereas TCV CPB caused a similar decrease only in ago2-1 and zip-1 mutants (Fig. 8A) In addition, data at 17 dpi further confirmed the negative effects of TCV CPB on average hue value in ago2-1 and zip-1 mutants (Fig. 8B; Tukey post hoc test with α = 0.05).
Figure 8.
TCV infection-caused temporal average hue value changes in 10 single ago mutant plants. A, The plots show the average hue value (degree) change over time (averaged by four images, ±se) in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants from 1 d preinoculation to 17 dpi (K-S test with α = 0.05, detailed D value and P value are listed in Supplemental Table S3). B, The box plot shows the average hue value in different genotypes at 17 dpi. Statistical analysis was calculated between treatments in each genotype. Bars and curves with different letters are statistically different (n = 4. Tukey post hoc test with α = 0.05). The color chart illustrates the relationship between the hue value (degree) with the corresponding RGB coordinates.
Analysis of Viral CP Levels in ago Mutants
To further investigate the roles of anti-TCV AGO proteins identified above in different tissues, immunoblotting assay was used to analyze viral CP (P38) levels in inoculated, noninoculated (systemic) tissues, and inflorescence tissues of ago mutants and control plants. High levels of P38 were detected in leaves and inflorescence tissues of all 10 single ago mutants and two control plants inoculated with parental TCV (Fig. 9A, left; Fig. 9, B–D, blue bars). Local accumulation of CPB-P38 was only detected in ago1-27, ago2-1, and zip-1 mutant rosette leaves (Fig. 9A, right; Fig. 9B, red bars), at a comparable level with that in dcl2 dcl3 dcl4 leaves (Fig. 9B, red bars). In noninoculated cauline leaves, systemic accumulation of CPB-P38 was only detected in ago2-1 and zip-1 mutants (Fig. 9C), and at levels that were approximately half of that in dcl2 dcl3 dcl4 mutants (Fig. 9C). CPB-P38 in local and systemic leaves was also detected in two of four biological replicates of ago3-2 mutants (Fig. 9A, right), though at a significantly lower level than that in ago2-1 mutants (Fig. 9, B and C). Notably, CPB-P38 was not detected in inflorescence tissues of any of the 10 ago single mutant and Col-0 plants (Fig. 9, A and C, right).
Figure 9.
Local and systemic accumulation of TCV CP in 10 single ago mutant plants. A, TCV viral CP (P38) accumulation in different tissues of Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants infected with TCV (left) or TCV CPB (right). RL: rosette leaves; CL: cauline leaves; Inflorescence. Rubisco protein: internal control. B, Summary data: the local level of parental or CPB mutant P38 accumulation in inoculated rosette leaves of Col-0, dcl2 dcl3 dcl4, and the 10 single ago mutant plants at 7 dpi. C, Summary data: the systemic level of parental or CPB mutant P38 accumulation in noninoculated cauline leaves of Col-0, dcl2 dcl3 dcl4, and the 10 single ago mutant plants at 14 dpi. D, Summary data: the systemic level of parental or CPB mutant P38 accumulation in inflorescence tissues of Col-0, dcl2 dcl3 dcl4, and the 10 single ago mutant plants at 14 dpi. All bar plots show average (±se) of four biological replicates, expressed relative to dcl2 dcl3 dcl4. Bars with different letters are statistically different (Tukey post hoc test with α = 0.05).
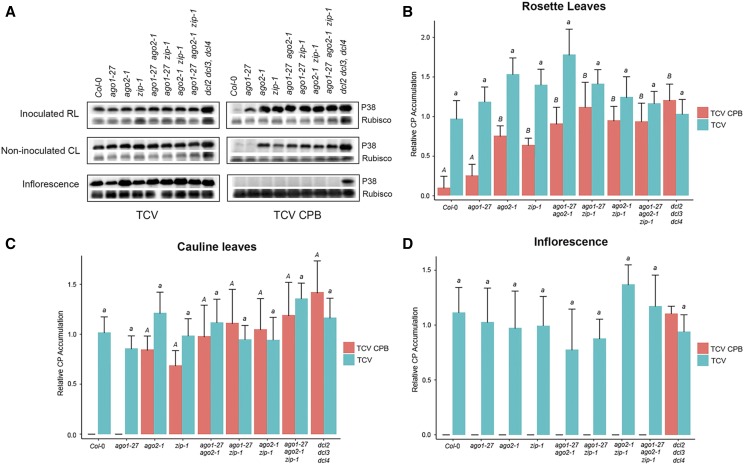
Next, to investigate if AGO2 and AGO7 had additive antiviral effects and also to test if the antiviral activities of AGO1, the previously reported antiviral AGO protein against TCV (Qu et al., 2008), was masked by AGO2 or AGO7, we performed immunoblotting experiments as described above in single, double, or triple mutants combining ago1-27, ago2-1, and/or zip-1 mutant allele (Fig. 10). First, parental TCV CPs accumulated at comparable levels in inoculated rosette, noninoculated cauline leaves, and inflorescence tissue in the single, double, and triple ago mutants (Fig. 10, A–C). In local and systemic leaves, no significant differences in P38 level were observed among ago2-1, zip-1, and ago2-1 zip-1 double mutant (Fig. 10, B and C). These results suggested that AGO2 and AGO7 play nonadditive antiviral roles in Arabidopsis leaves during TCV infection. To examine if AGO1, AGO2, or AGO7 play redundant antiviral roles, we checked if down-regulating ago1 had any enhancing effects on P38 accumulation in ago2 or ago7 mutants. We found no significant differences in local P38 level in rosette leaves among ago2-1 single, zip-1 single, ago1-27 ago2-1 double, ago1-27 zip-1 double, and ago1-27, ago2-1 zip-1 triple mutant (Fig. 10B). Similarly, combining the ago1-27 allele with an ago2-1 or zip-1 allele, or ago2-1 and zip-1, did not significantly affect the CPB-P38 accumulation level in systemic cauline leaves (Fig. 10C). These results suggested that introducing the ago1-27 allele does not enhance or suppress the antiviral activities of AGO2 and AGO7 in Arabidopsis leaves. Similar to the observation in ago single mutants, no TCV CPB CPs were detected in the inflorescence clusters of the double and triple mutants tested (Fig. 10D).
Figure 10.
Local and systemic accumulation of P38 in a selected group of double and triple ago mutant plants. A, TCV viral CP (P38) accumulation in different tissues of Col-0, dcl2 dcl3 dcl4, ago1-27, ago2-1, zip-1 single, double, or triple mutant plants infected with TCV (left) or TCV CPB (right). RL: rosette leaves; CL: cauline leaves; Inflorescence. Rubisco protein: internal control. B, Summary data: the local level of parental or CPB mutant P38 accumulation in inoculated rosette leaves of Col-0, dcl2 dcl3 dcl4, ago1-27, ago2-1, zip-1 single, double, or triple mutant plants at 7 dpi. C, Summary data: the systemic level of parental or CPB mutant P38 accumulation in noninoculated cauline leaves of Col-0, dcl2 dcl3 dcl4, ago1-27, ago2-1, zip-1 single, double, or triple mutant plants at 14 dpi. D, Summary data: the systemic level of parental or CPB mutant P38 accumulation in inflorescence tissues of Col-0, dcl2 dcl3 dcl4, ago1-27, ago2-1, zip-1 single, double, or triple mutant plants at 14 dpi. All bar plots show average (±se) of four biological replicates, expressed relative to dcl2 dcl3 dcl4. Bars with different letters are statistically different (Tukey post hoc test with α = 0.05).
Analysis of Plant Height during TCV Infection in ago Mutants
The effects of TCV infection on plant height in 10 ago mutants, the dcl triple mutant, and wild-type plants inoculated with parental and VSR-deficient TCV were measured. Growth curves were plotted from 1 to 21 dpi as described in Boyes et al. (2001). Pairwise K-S tests were done to compare time-series data.
Under mock treatment, plant height was >30 cm in most genotypes at 21 dpi, except in ago1-27 and dcl2 dcl3 dcl4 mutants (Fig. 11, A and C). TCV infection led to reduced plant height (<10 cm) starting at ∼7 dpi in all genotypes (Fig. 11A). The TCV CPB mutant virus had only minor effects on height of inoculated Col-0 plants over time, but strongly affected height in dcl2 dcl3 dcl4 mutant plants (Fig. 11). Mild inhibition of height caused by TCV CPB was observed at intermediate time points (Tukey post hoc test, α = 0.05, Fig. 11B), though no effects on plant height were detected at 21 dpi (Fig. 11C).
Figure 11.
The effects of TCV infection on plant growth in 10 single ago mutant plants. A, The histograms show the plant growth curve (averaged by six plants, ±se) in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants inoculated with mock solution, TCV, or TCV CPB inoculum from 1 d preinoculation to 21 dpi (K-S test with α = 0.05, detailed D value, and P value are listed in Supplemental Table S4). B and C, The bar plot shows the average plant height (±se, n = 6) in Col-0, dcl2 dcl3 dcl4, and 10 single ago mutant plants at 13 dpi (B) or 21 dpi (C). Statistical analysis was calculated between treatments in each genotype. Bars and curves with different letters are statistically different (Tukey post hoc test with α = 0.05).
The TCV CPB mutant also strongly inhibited plant height in ago2-1 and zip-1 mutants (Fig. 11A). In ago2-1 mutants, TCV CPB infection had parental virus-like effects on plant height at 13 and 21 dpi (Fig. 11, B and C). In zip-1 mutants, the suppressor-deficient virus led to an ∼60% decrease in plant height compared with mock-inoculated control plants at both time points (Fig. 11, B and C). In ago3-2 mutant plants, TCV CPB caused modest reduction of reduced height at both 13 dpi and 21 dpi (by Tukey post hoc test with α = 0.05; Fig. 11C). TCV CPB had little or no effects on plant height in ago1-27, ago4-2, ago5-2, ago6-3, ago8-1, ago9-5, or ago10-5 mutants.
DISCUSSION
Precisely measuring biotic and abiotic stress traits is important to understand the impact of the environment, pathogens, and pests on plant growth and development, and to assist breeders to improve crops in modern agriculture. Objective, reproducible, and accurate quantification of disease severity is important for development and testing of disease-resistant crops. Various methods to estimate disease severity in plants are in use (Bock et al., 2010; Nguyen et al., 2010; Lloyd et al., 2014; Arend et al., 2016), though many of these are dependent on qualitative or subjective observations, or require destructive sampling of plant material. Recently, some nondestructive, image-based analysis methods have been developed to measure plant morphology (Mutka and Bart, 2015; Pethybridge and Nelson, 2015; Laflamme et al., 2016). Machine learning techniques have been applied to efficiently process color trait data from large numbers of images, and to link the inputs to the outputs mathematically (Singh et al., 2016; Lee et al., 2018). Here, we integrated a machine learning algorithm and analysis tools in the open-source phenotyping platform PlantCV (Fahlgren et al., 2015; Gehan et al., 2017) to develop an image-based disease trait analysis pipeline to objectively measure disease severity in Arabidopsis plants infected by viruses.
In this pipeline, the naïve Bayes machine learning method (Abbasi and Fahlgren, 2016; Gehan et al., 2017) was used for segmenting plant tissue from background in an image at pixel-level. First, four naïve Bayes classifiers were defined: (1) green plant pixels (“healthy”), (2) chlorotic/necrotic plant pixels (“unhealthy”), (3) blue mesh background pixels, and (4) other background pixels. Because the light intensity condition was relatively constant, the color information of pixels from a small number of sample images was sufficient for generating the training dataset to cover the range of variation of all images. In the training session, four classes of pixels could be segmented simultaneously. In addition, the naïve Bayes segmentation process is robust across large number of images due to its probabilistic nature. This pipeline is also simple and computationally expedient. The output of the pipeline could be statistically analyzed to quantify plant size, the proportion of unhealthy tissue, and leaf color. This nondestructive phenotyping pipeline enables visualization and quantification of disease symptom development over time from large numbers of plants.
In this study, the image-based disease analysis method was put to the test in wild-type and mutant Arabidopsis plants with defects in AGO genes, infected by parental and VSR-defective TCV. Detailed phenotyping of inoculated Arabidopsis ago mutant plants was carried out. Ten AGO genes are encoded in the Arabidopsis genome and they are grouped into three major clades: AGO1/AGO5/AGO10, AGO2/AGO3/AGO7, and AGO4/AGO6/AGO8/AGO9 (Fang and Qi, 2016). Combining disease phenotyping and biochemical results, AGO2 and AGO7 were identified as prominent factors during anti-TCV defense, along with minor antiviral roles of AGO3. The results with AGO2 and AGO7 are consistent with previous studies (Qu et al., 2008; Harvey et al., 2011). Using the VSR-defective virus, the antiviral role of AGO7 was relatively minor compared to that of AGO2. AGO2 and AGO7 were nonadditive in leaves (Fig. 10). However, movement of the TCV mutant to inflorescence tissues was still inhibited in ago2 ago7 double mutant, but not in dcl2 dcl3 dcl4 mutants (Fig. 10). These results implied that other AGOs not tested in these genetic combinations are involved to restrict TCV movement to, or accumulation in, inflorescence tissues.
AGO1 was previously considered as a prominent antiviral factor against different viruses (Qu et al., 2008; Wang et al., 2011; Dzianott et al., 2012; Garcia-Ruiz et al., 2015). We observed that the ago1-27 mutant was not susceptible to the VSR-defective TCV. Because AGO1 is critical for regulating gene expression in numerous developmental and physiological pathways (Fang and Qi, 2016), ago1 null alleles are lethal and difficult to test. Thus, in the hypo-morphic ago1-27 mutant used in our genetic analysis, it is possible that enough functional AGO1 is still available to repress the virus. It should be noted that disruption of AGO1 function leads to upregulation of AGO2 expression (Harvey et al., 2011; Wang et al., 2011). It is also possible that elevated AGO2 levels in ago1-27 mutant affected its susceptibility against the mutant TCV. Therefore, our results did not exclude possible direct or indirect antiviral roles for AGO1 against TCV. Beside the imaging analysis data, more molecular evidence is needed to further clarify the roles of AGO1 during TCV infection.
Our finding that ago2 mutants were hypersusceptible to the VSR-defective TCV provided additional evidence that AGO2 functions as an important antiviral effector against a broad spectrum of plant viruses (Harvey et al., 2011; Jaubert et al., 2011; Scholthof et al., 2011; Garcia-Ruiz et al., 2015). Our results also implied minor antiviral effects of AGO3 against TCV. AGO3 was reported to bind 24-nt siRNAs in Arabidopsis (Zhang et al., 2016), but was suggested to have no antiviral activities against Cucumber mosaic virus (Wang et al., 2011; Zhang et al., 2016). AGO3 has slicer activity against viral RNAs in vitro (Schuck et al., 2013) and binds siRNAs derived from Potato Spindle Tuber viroid in vivo (Minoia et al., 2014). In addition, AGO3 was shown to play antiviral roles against Bamboo Mosaic Virus via the abscisic acid pathway (Alazem et al., 2017). AGO3 and AGO2 belong to the same phylogenetic clade within the AGO gene family, and they are closely linked in the Arabidopsis genome. Therefore, mild antiviral activities of AGO3 against TCV might not be surprising. However, to further investigate the roles of AGO2 and AGO3 during TCV infection, an ago2 ago3 double mutant would be informative. Because viruses use different strategies to suppress the host antiviral machinery, it is possible that AGO3 has distinct functions during infection by different viruses.
AGO7 binds miR390, which targets TAS3 transcripts for biogenesis of trans-acting small interfering RNAs (Axtell et al., 2006, 2007; Montgomery et al., 2008; Jouannet et al., 2012). AGO7 has also been shown to affect antiviral function (Qu et al., 2008; Garcia-Ruiz et al., 2015), although its role is not clear. AGO7 has slicer activity (Carbonell et al., 2012), but it is not clear if AGO7 directly interacts with vsiRNAs or genomic (or subgenomic) RNAs during infection.
Our findings partly agreed with a previously reported modular, tissue-specific mode of antiviral AGO function against Turnip Mosaic Virus (TuMV), with AGO2 possessing a major role and AGO10, AGO5, and AGO7 having minor effects (Garcia-Ruiz et al., 2015). With TCV, both AGO2 and AGO7 are influential in protecting leaves, along with minor contributions from AGO3. This may reflect the fact that the VSRs encoded by different viruses use different molecular strategies to affect antiviral effector functions (Burgyán and Havelda, 2011). The helper component proteinase from TuMV was found to sequester vsiRNAs away from multiple AGO proteins (Garcia-Ruiz et al., 2015). Previous reports suggested that TCV infection led to expression changes of many endogenous genes, including AGO genes (Wu et al., 2016). AGO2 expression was induced by TCV infection (Harvey et al., 2011). In contrast, TuMV did not affect AGO accumulation (Garcia-Ruiz et al., 2015). Therefore, the availability and coordination among antiviral AGOs against different viruses could possibly be virus-dependent.
Furthermore, the capacity to physically interact with AGO protein was considered to be critical for the VSR functions of TCV CPs (P38; Azevedo et al., 2010). However, the VSR function of P37, CP of Pelargonium Line Pattern virus, was shown to be dependent on its sRNA binding capacity instead of its physical interactions with AGO protein (Pérez-Cañamás and Hernández, 2015). Here, we showed that multiple AGOs participate in anti-TCV silencing. More molecular evidence is needed to understand how P38 interacts with these AGOs to suppress antiviral silencing machinery.
Regardless of precise antiviral mechanisms in play against TCV, the image-based phenotyping system developed here was shown to be useful in delineating both major and minor contributions of AGOs during virus infection over time. This should encourage development and refinement of additional tools and readouts to measure more effects and responses, even before visible symptoms of disease are detectable. Closing the gap between knowledge at the genetic and molecular levels with phenotypic effects during pathogen infection and nonpathogen colonization should yield considerable new insights into host-microbe interactions.
CONCLUSION
Precise phenotyping methods to measure biotic and abiotic effects on plant health are important to develop. A high-throughput, image-based disease trait phenotyping pipeline to quantify virus-induced symptoms in Arabidopsis was developed. Combined with infectivity experiments using both parental and VSR-defective TCV variants, AGO2 and AGO7 were identified as the most prominent antiviral AGO proteins against TCV, whereas AGO3 was found to have a minor effect on antiviral silencing. These and previous data support the idea that multiple AGOs are recruited and programmed during antiviral defense in unique ways against different virus species.
MATERIALS AND METHODS
Plant Materials
All Arabidopsis (Arabidopsis thaliana) plants used in this study (including all mutant lines) were in the Columbia-0 (Col-0) background and were grown in growth chambers under long day (16-h light/8-h dark) conditions, at 22°C and 75% relative humidity. The 10 ago mutants used in this study were described in Garcia-Ruiz et al. (2015): ago1-27, ago2-1, ago3-2, ago4-2, ago5-2, ago6-3, zip-1, ago8-1, ago9-5, and ago10-5. Double and triple ago mutants were generated by crossing between single ago mutants. The dcl2-1 dcl3-1 dcl4-2 triple mutant was described in Deleris et al. (2006).
Nicotiana benthamiana plants were grown in growth chambers under long day (16-h light/8-h dark) conditions, at 22°C and 75% relative humidity.
DNA Plasmids
The pSW-TCV plasmid is equivalent to pPZP212-TCV described in Cao et al. (2010); pSW is derived from the pPZP212 plasmid by deleting most of the restriction sites with its multiple cloning site). The pSW-TCV CPB construct incorporates the mutation (R130T) in the pSW-TCV CP region (Cao et al., 2010). The TCV CP fragment was amplified from pSW-TCV and pSW-TCV CPB using one set of primers: P38_L (ATGGAAAATGATCCTAGAGTCCG) and P38_R (CTAAATTCTGAGTGCTTGCCATTT). P38 PCR segments were sent to GENEWIZ (https://www.genewiz.com/en) to confirm the CPB mutation by using sequencing primer P38_L (ATGGAAAATGATCCTAGAGTCCG).
Virus Infection Assays
Plasmids carrying TCV DNA clones were transformed into Agrobacterium tumefaciens (GV3101) and then infiltration was done on N. benthamiana leaves for viral inoculum preparation. The infected N. benthamiana leaves were grinded in 200-mm NaOAc solution (pH 5.2) at 4°C. The grinded mixtures were centrifuged at 4°C to collect the supernatant. Next, 40% (w/v) polyethylene glycol (MW:8000) in 1-M NaCl solution was added to the supernatant and incubated on ice overnight. Virion pellets were collected by centrifuging and resuspended in 10-mM NaOAc (pH 5.5) solution for inoculum stock. The inoculum stocks were aliquoted into equal volumes and stored at −80°C. The same set of inoculums (1:10 dilution with 10 mM of NaOAc at pH 5.5) were used to inoculate all genotypes in this study. The Arabidopsis plants to be inoculated were ∼2 weeks old. The ago1-27 mutant plant was planted 1 week earlier than the other genotypes. The four largest rosette leaves were inoculated by gently rubbing carborundum dust on the leaf surface. Plants under different treatments were placed on separate benches to avoid possible cross contaminations in the growth chamber.
Protein Blot Assays
To measure local CP accumulation, four inoculated rosette leaves per plant were collected at 7 dpi and pooled into a single sample. For systemic CP accumulation, the four largest noninoculated cauline leaves or five to six inflorescence clusters per plant were collected at 14 dpi and pooled into a single sample. Four biological replicates were randomly collected from each genotype-treatment group. Total protein was extracted and normalized to 0.5 μg/μL. Protein samples (6 μg each) were separated on NuPAGE 4% to 12% Bis-Tris protein gels (Thermo Fisher Scientific) and subsequently transferred to nitrocellulose blotting members (0.45 μm; GE Healthcare Life Science) for protein detection with corresponding antibodies. TCV CP was detected using anti-TCV-P38 serum (F. Qu, personal communication) at a dilution of 1:20,000 and the large subunit of Rubisco protein was detected using anti-Rubisco (plant) antibody in chicken (Sigma-Aldrich) at 1:10,000. The blots were incubated with goat anti-rabbit lgG-HRP conjugate (GE Healthcare) secondary serum at a dilution of 1:10,000 to detect TCV CP and with rabbit anti-chicken lgY (H+L) HRP conjugate secondary antibody (Thermo Fisher Scientific) at a dilution of 1:10,000 to detect Rubisco protein. TCV CP and Rubisco protein were detected on the same blot. Quantification of western-blot images was done by normalizing all other bands relative to dcl2-1 dcl3-1 dcl4-2 (P38 band/loading control band) using ImageJ software (National Institutes of Health).
Manual Plant Height Measurement
Measurement of plant height was done manually using a cubic ruler to measure from the rosette plane to the top of the main plant stem (Boyes et al., 2001). Data were collected every other day from the day of virus inoculation to 21 dpi.
RGB Image Acquisition
The soil in the growth pots was covered by a blue mesh (Con-Tact Brand, Euro Blue, 12 inch x 5 ft), leaving a hole in the center for the plant to grow out (Fig. 2B). Meshed plants were bottom-watered to avoid water splash on plant tissues. Images of plant rosettes were manually acquired using a Digital Rebel XT DSLR camera (Canon) with an EF-S 18-55mm f2.5-5.6 lens (0.60 s exposure, F/14, ISO100; Canon) on a model no. RS1 Copy Stand (Kaiser Fototechnik). The flash function was kept off and fixed ambient light was used in a closed room to minimize illumination discrepancies between images. A ColorChecker Digital SG (X-Rite) was placed next to the plant as a color reference and correction. Images were stored in the native RAW format and also in high-resolution JPG format. Plants were removed from the growth chamber (10 am to 11 am) and any bolts above the rosette plane were removed before imaging. Stationary plant rosettes were imaged every other day from 1 d preinoculation to 17 dpi.
Image Analysis
Color images of individual mock- or virus-infected Arabidopsis plants were processed using the PlantCV (https://plantcv.danforthcenter.org/) package to quantify the progression of disease symptoms (Fahlgren et al., 2015; Gehan et al., 2017). PlantCV v2.1 (commit: d553a2c1c6bd29e734d19898e3e9ac4fcff40aa9) was used. Image analysis was done in parallel using HTCondor v8.6.8 (Thain et al., 2005).
The PlantCV naive Bayes machine learning method (Abbasi and Fahlgren, 2016; Gehan et al., 2017) was used to segment image pixels into four classes: (1) green plant pixels (“healthy”); (2) chlorotic/necrotic plant pixels (“unhealthy”); (3) background pixels from the blue mesh material used to cover the soil; and (4) all other background pixels. The PlantCV naive Bayes classifier was trained using pixel RGB color values from 1,980 and 3,779 pixels manually sampled from the background and foreground classes, respectively, from multiple images using the ImageJ pixel picking tool (Schneider et al., 2012) as described in Gehan et al. (2017). The training data were used to calculate probability density functions using kernel density estimation for each class in the hue, saturation, and value color space (Gehan et al., 2017). During image analysis, the probability density functions were used to parameterize the naive Bayes classifier to segment images into the four output classes (one binary image per class).
After segmentation, the blue mesh was used to automatically identify the position of the pot within each image. Morphological erosion was used to reduce noise in the classified blue mesh pixels. A universal region of interest was used to keep blue-mesh–connected components in the center area of each image because the pot was consistently centered in each image. The remaining blue-mesh–connected components were flattened into a single object to create a pot binary mask using the bounding rectangle area of the blue mesh. Padding was added to the minimum bounding rectangle area using morphological dilation. The resulting pot binary mask was used to mask the “healthy” and “unhealthy” binary masks to remove misclassified background pixels. The “healthy” and “unhealthy” binary images were further filtered to remove background pixels misclassified as foreground pixels using a size-based filter that removed small connected components (300 and 1,000 pixels for “healthy” and “unhealthy” binary images, respectively). A universal region of interest was used to keep connected components in the center area of each image because the Arabidopsis plants were consistently centered in each image. The resulting cleaned binary images for the “healthy” and “unhealthy” classes were used to measure the area of green and chlorotic/necrotic pixels in each image.
Additionally, the union of the “healthy” and “unhealthy” plant pixels was calculated to create a combined plant mask. The input RGB images were converted to hue, saturation, and value color space and the frequency distribution of hue color values for each plant at each timepoint was extracted from the pixels that overlapped the combined plant mask. The hue color histograms were plotted in “R” v3.4.4 using the libraries “ggplot2,” “ggridges,” “reshape2,” and “dplyr” (Wickham, 2007, 2009; Wickham et al., 2017; R Core Team, 2018; Wilke, 2018). The PlantCV and R analysis code and result files are available at GitHub (https://github.com/carringtonlab/tcv-image-analysis). The raw input images, the analyzed output images, and analysis results are available at Figshare (https://doi.org/10.6084/m9.figshare.7599923).
Statistics
For statistical analysis, the nonparametric two-sample K-S test was used to determine whether two datasets are from the same distribution. The K-S statistic calculates the maximum vertical distance (D value) between the empirical distribution functions of the two datasets. If the D value is greater than the critical D value (at α = 0.05), the two datasets are not from the same distribution. Pairwise K-S tests were applied on the three datasets in the same genotype under different treatments. As mentioned in the figure legends, different letters represent statistically different distributions.
The Tukey post hoc tests in one-way ANOVA was applied to determine whether any statistically significant differences exist between three group means under different treatments in one genotype. As mentioned in the figure legends, boxes with different letters are statistically different (at α = 0.05).
All statistical analysis was performed in “R,” v3.4.4.
Accession Numbers
AGO1, AT1G48410; AGO2, AT1G31280; AGO3, AT1G31290; AGO4, AT2G27040; AGO5, AT2G27880; AGO6, AT2G32940; AGO7, AT1G69440; AGO8, AT5G21030; AGO9, AT5G21150; AGO10, AT5G43810; TCV CP, UniProtKB-Q2V851.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. CPB mutation in TCV CP does not affect its function in virion assembly.
Supplemental Figure S2. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago1-27 mutant.
Supplemental Figure S3. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago2-1 mutant.
Supplemental Figure S4. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago3-2 mutant.
Supplemental Figure S5. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago4-2 mutant.
Supplemental Figure S6. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago5-2 mutant.
Supplemental Figure S7. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago6-3 mutant.
Supplemental Figure S8. Temporal visualization of rosette leaves and the corresponding pseudo-color images in zip-1 mutant.
Supplemental Figure S9. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago8-5 mutant.
Supplemental Figure S10. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago9-5 mutant.
Supplemental Figure S11. Temporal visualization of rosette leaves and the corresponding pseudo-color images in ago10-5 mutant.
Supplemental Figure S12. Temporal color change in rosette leaves in 10 single ago mutant plants infected by TCV.
Supplemental Table S1. Kolmogorov-Smirnov statistical analysis results for rosette size in single ago mutants and control plants..
Supplemental Table S2. Kolmogorov-Smirnov statistical analysis results forthe percentage of unhealthy tissue curve in single ago mutants and control plants.
Supplemental Table S3. Kolmogorov-Smirnov statistical analysis result foraverage hue curve in single ago mutants and control plants.
Supplemental Table S4. Kolmogorov-Smirnovstatistical results for plant height curve in single ago mutants and control plants.
Acknowledgments
We are grateful for Dr. Feng Qufor providing pSW-TCV plasmids and anti-P38 antibody. We thank Kerrigan B. Gilbert, Dr. Dan Lin, Dr. Steen Hoyer, and Dr. Kira Veley for support and critique of the study. We also thank Robyn Allscheid for constructive editorial advices on this manuscript.
Footnotes
This study was supported by the National Institutes of Health (grant no. AI043288 to J.C.C.) and the National Science Foundation (grant no. 1330562 to J.C.C.).
Articles can be viewed without a subscription.
References
- Abbasi A, Fahlgren N (2016) Naïve Bayes pixel-level plant segmentation. In 2016 IEEE Western New York Image and Signal Processing Workshop (WNYISPW), Rochester, NY, pp 1–4 [Google Scholar]
- Agius C, Eamens AL, Millar AA, Watson JM, Wang MB (2012) RNA silencing and antiviral defense in plants. Methods Mol Biol 894: 17–38 [DOI] [PubMed] [Google Scholar]
- Alazem M, He MH, Moffett P, Lin NS (2017) Abscisic acid induces resistance against Bamboo mosaic virus through Argonaute2 and 3. Plant Physiol 174: 339–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend D, Lange M, Pape JM, Weigelt-Fischer K, Arana-Ceballos F, Mücke I, Klukas C, Altmann T, Scholz U, Junker A (2016) Quantitative monitoring of Arabidopsis thaliana growth and development using high-throughput plant phenotyping. Sci Data 3: 160055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Jan C, Rajagopalan R, Bartel DP (2006) A two-hit trigger for siRNA biogenesis in plants. Cell 127: 565–577 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Snyder JA, Bartel DP (2007) Common functions for diverse small RNAs of land plants. Plant Cell 19: 1750–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo J, Garcia D, Pontier D, Ohnesorge S, Yu A, Garcia S, Braun L, Bergdoll M, Hakimi MA, Lagrange T, et al. (2010) Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev 24: 904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Tsai CH, Lie M, Havecker E, Baulcombe DC (2007) The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr Biol 17: 1609–1614 [DOI] [PubMed] [Google Scholar]
- Bock CH, Poole GH, Parker PE, Gottwald TR (2010) Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit Rev Plant Sci 29: 59–107 [Google Scholar]
- Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65: 473–503 [DOI] [PubMed] [Google Scholar]
- Bortolamiol D, Pazhouhandeh M, Marrocco K, Genschik P, Ziegler-Graff V (2007) The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr Biol 17: 1615–1621 [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgyán J, Havelda Z (2011) Viral suppressors of RNA silencing. Trends Plant Sci 16: 265–272 [DOI] [PubMed] [Google Scholar]
- Cao M, Ye X, Willie K, Lin J, Zhang X, Redinbaugh MG, Simon AE, Morris TJ, Qu F (2010) The capsid protein of Turnip crinkle virus overcomes two separate defense barriers to facilitate systemic movement of the virus in Arabidopsis. J Virol 84: 7793–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A, Carrington JC (2015) Antiviral roles of plant ARGONAUTES. Curr Opin Plant Biol 27: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A, Fahlgren N, Garcia-Ruiz H, Gilbert KB, Montgomery TA, Nguyen T, Cuperus JT, Carrington JC (2012) Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 24: 3613–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang J, Liu J, Deng XG, Zhang P, Zhu T, Chen LJ, Bao WK, Xi DH, Lin HH (2014) The capsid protein p38 of Turnip crinkle virus is associated with the suppression of Cucumber mosaic virus in Arabidopsis thaliana co-infected with Cucumber mosaic virus and Turnip crinkle virus. Virology 462-463: 71–80 [DOI] [PubMed] [Google Scholar]
- Chiu MH, Chen IH, Baulcombe DC, Tsai CH (2010) The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol Plant Pathol 11: 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Gisel A, Zambryski PC (2000) Cell-to-cell and systemic movement of recombinant green fluorescent protein-tagged turnip crinkle viruses. Virology 273: 258–266 [DOI] [PubMed] [Google Scholar]
- Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O (2006) Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313: 68–71 [DOI] [PubMed] [Google Scholar]
- Donze T, Qu F, Twigg P, Morris TJ (2014) Turnip crinkle virus coat protein inhibits the basal immune response to virus invasion in Arabidopsis by binding to the NAC transcription factor TIP. Virology 449: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzianott A, Sztuba-Solińska J, Bujarski JJ (2012) Mutations in the antiviral RNAi defense pathway modify Brome mosaic virus RNA recombinant profiles. Mol Plant Microbe Interact 25: 97–106 [DOI] [PubMed] [Google Scholar]
- Fahlgren N, Feldman M, Gehan MA, Wilson MS, Shyu C, Bryant DW, Hill ST, McEntee CJ, Warnasooriya SN, Kumar I, et al. (2015) A versatile phenotyping system and analytics platform reveals diverse temporal responses to water availability in Setaria. Mol Plant 8: 1520–1535 [DOI] [PubMed] [Google Scholar]
- Fang X, Qi Y (2016) RNAi in plants: An Argonaute-centered view. Plant Cell 28: 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YY, Zhao JH, Liu SW, Wang S, Duan CG, Guo HS (2016) CMV2b-AGO interaction is required for the suppression of RDR-dependent antiviral silencing in Arabidopsis. Front Microbiol 7: 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz H, Carbonell A, Hoyer JS, Fahlgren N, Gilbert KB, Takeda A, Giampetruzzi A, Garcia Ruiz MT, McGinn MG, Lowery N, et al. (2015) Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip mosaic virus infection. PLoS Pathog 11: e1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehan MA, Fahlgren N, Abbasi A, Berry JC, Callen ST, Chavez L, Doust AN, Feldman MJ, Gilbert KB, Hodge JG, et al. (2017) PlantCV v2: Image analysis software for high-throughput plant phenotyping. PeerJ 5: e4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner A, Lakatos L, García-Chapa M, López-Moya JJ, Burgyán J (2010) Viral protein inhibits RISC activity by Argonaute binding through conserved WG/GW motifs. PLoS Pathog 6: e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RCWR, Eddins SL (2009) Digital Image Processing Using MATLAB. Gatesmark Publishing, New York [Google Scholar]
- Harvey JJ, Lewsey MG, Patel K, Westwood J, Heimstädt S, Carr JP, Baulcombe DC (2011) An antiviral defense role of AGO2 in plants. PLoS One 6: e14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incarbone M, Dunoyer P (2013) RNA silencing and its suppression: Novel insights from in planta analyses. Trends Plant Sci 18: 382–392 [DOI] [PubMed] [Google Scholar]
- Jaubert M, Bhattacharjee S, Mello AF, Perry KL, Moffett P (2011) ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol 156: 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouannet V, Moreno AB, Elmayan T, Vaucheret H, Crespi MD, Maizel A (2012) Cytoplasmic Arabidopsis AGO7 accumulates in membrane-associated siRNA bodies and is required for ta-siRNA biogenesis. EMBO J 31: 1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenesi E, Carbonell A, Lózsa R, Vértessy B, Lakatos L (2017) A viral suppressor of RNA silencing inhibits ARGONAUTE 1 function by precluding target RNA binding to pre-assembled RISC. Nucleic Acids Res 45: 7736–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontra L, Csorba T, Tavazza M, Lucioli A, Tavazza R, Moxon S, Tisza V, Medzihradszky A, Turina M, Burgyán J (2016) Distinct effects of p19 RNA silencing suppressor on small RNA mediated pathways in plants. PLoS Pathog 12: e1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme B, Middleton M, Lo T, Desveaux D, Guttman DS (2016) Image-based quantification of plant immunity and disease. Mol Plant Microbe Interact 29: 919–924 [DOI] [PubMed] [Google Scholar]
- Lee U, Chang S, Putra GA, Kim H, Kim DH (2018) An automated, high-throughput plant phenotyping system using machine learning-based plant segmentation and image analysis. PLoS One 13: e0196615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Urano D, Liao KL, Hedrick TL, Gao Y, Jones AM (2017) A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SR, Schoonbeek HJ, Trick M, Zipfel C, Ridout CJ (2014) Methods to study PAMP-triggered immunity in Brassica species. Mol Plant Microbe Interact 27: 286–295 [DOI] [PubMed] [Google Scholar]
- Martínez-Turiño S, Hernández C (2009) Inhibition of RNA silencing by the coat protein of Pelargonium flower break virus: Distinctions from closely related suppressors. J Gen Virol 90: 519–525 [DOI] [PubMed] [Google Scholar]
- Meng C, Chen J, Peng J, Wong SM (2006) Host-induced avirulence of hibiscus chlorotic ringspot virus mutants correlates with reduced gene-silencing suppression activity. J Gen Virol 87: 451–459 [DOI] [PubMed] [Google Scholar]
- Minoia S, Carbonell A, Di Serio F, Gisel A, Carrington JC, Navarro B, Flores R (2014) Specific argonautes selectively bind small RNAs derived from Potato spindle tuber viroid and attenuate viroid accumulation in vivo. J Virol 88: 11933–11945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TA, Howell MD, Cuperus JT, Li D, Hansen JE, Alexander AL, Chapman EJ, Fahlgren N, Allen E, Carrington JC (2008) Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133: 128–141 [DOI] [PubMed] [Google Scholar]
- Mutka AM, Bart RS (2015) Image-based phenotyping of plant disease symptoms. Front Plant Sci 5: 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HP, Chakravarthy S, Velásquez AC, McLane HL, Zeng L, Nakayashiki H, Park DH, Collmer A, Martin GB (2010) Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Mol Plant Microbe Interact 23: 991–999 [DOI] [PubMed] [Google Scholar]
- Pazhouhandeh M, Dieterle M, Marrocco K, Lechner E, Berry B, Brault V, Hemmer O, Kretsch T, Richards KE, Genschik P, et al. (2006) F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proc Natl Acad Sci USA 103: 1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cañamás M, Hernández C (2015) Key importance of small RNA binding for the activity of a glycine-tryptophan (GW) motif-containing viral suppressor of RNA silencing. J Biol Chem 290: 3106–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethybridge SJ, Nelson SC (2015) Leaf Doctor: A new portable application for quantifying plant disease severity. Plant Dis 99: 1310–1316 [DOI] [PubMed] [Google Scholar]
- Qu F, Ye X, Morris TJ (2008) Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci USA 105: 14732–14737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja P, Sanville BC, Buchmann RC, Bisaro DM (2008) Viral genome methylation as an epigenetic defense against geminiviruses. J Virol 82: 8997–9007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja P, Jackel JN, Li S, Heard IM, Bisaro DM (2014) Arabidopsis double-stranded RNA binding protein DRB3 participates in methylation-mediated defense against geminiviruses. J Virol 88: 2611–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ [Google Scholar]
- Sass L, Majer P, Hideg E (2012) Leaf hue measurements: A high-throughput screening of chlorophyll content. Methods Mol Biol 918: 61–69 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof HB, Alvarado VY, Vega-Arreguin JC, Ciomperlik J, Odokonyero D, Brosseau C, Jaubert M, Zamora A, Moffett P (2011) Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana. Plant Physiol 156: 1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck J, Gursinsky T, Pantaleo V, Burgyán J, Behrens SE (2013) AGO/RISC-mediated antiviral RNA silencing in a plant in vitro system. Nucleic Acids Res 41: 5090–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Ganapathysubramanian B, Singh AK, Sarkar S (2016) Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci 21: 110–124 [DOI] [PubMed] [Google Scholar]
- Thain D, Tannenbaum T, Livny M (2005) Distributed computing in practice: The Condor experience. Concurr Comput 17: 323–356 [Google Scholar]
- Thomas CL, Leh V, Lederer C, Maule AJ (2003) Turnip crinkle virus coat protein mediates suppression of RNA silencing in Nicotiana benthamiana. Virology 306: 33–41 [DOI] [PubMed] [Google Scholar]
- Várallyay E, Válóczi A, Agyi A, Burgyán J, Havelda Z (2010) Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J 29: 3507–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veley KM, Berry JC, Fentress SJ, Schachtman DP, Baxter I, Bart R (2017) High-throughput profiling and analysis of plant responses over time to abiotic stress. Plant Direct 1: e00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Jovel J, Udomporn P, Wang Y, Wu Q, Li WX, Gasciolli V, Vaucheret H, Ding SW (2011) The 21-nucleotide, but not 22-nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell 23: 1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2007) Reshaping data with the reshape package. J Stat Softw 21: 1–20 [Google Scholar]
- Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis. Springer, New York [Google Scholar]
- Wickham H, Henry L, Müller K, Francois R (2017) dplyr: A Grammar of Data Manipulation. http://dplyr.tidyverse.org [Google Scholar]
- Wilke CO. (2018) Geoms to make ridgeline plots using ggplots. https://github.com/clauswilke/ggridges [Google Scholar]
- Wu C, Li X, Guo S, Wong SM (2016) Analyses of RNA-Seq and sRNA-Seq data reveal a complex network of anti-viral defense in TCV-infected Arabidopsis thaliana. Sci Rep 6: 36007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH (2006) Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20: 3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Guo X, Wang XJ, Zhang X (2016) Arabidopsis AGO3 predominantly recruits 24-nt small RNAs to regulate epigenetic silencing. Nat Plants 2: 16049. [DOI] [PubMed] [Google Scholar]