The dynamic modifications of O-GlcNAcylation and phosphorylation on the key proteins mediate vernalization for winter wheat flowering.
Abstract
O-GlcNAcylation and phosphorylation are two posttranslational modifications that antagonistically regulate protein function. However, the regulation of and the cross talk between these two protein modifications are poorly understood in plants. Here we investigated the role of O-GlcNAcylation during vernalization, a process whereby prolonged cold exposure promotes flowering in winter wheat (Triticum aestivum), and analyzed the dynamic profile of O-GlcNAcylated and phosphorylated proteins in response to vernalization. Altering O-GlcNAc signaling by chemical inhibitors affected the vernalization response, modifying the expression of VRN genes and subsequently affecting flowering transition. Over a vernalization time-course, O-GlcNAcylated and phosphorylated peptides were enriched from winter wheat plumules by Lectin weak affinity chromatography and iTRAQ-TiO2, respectively. Subsequent mass spectrometry and gene ontology term enrichment analysis identified 168 O-GlcNAcylated proteins that are mainly involved in responses to abiotic stimulus and hormones, metabolic processing, and gene expression; and 124 differentially expressed phosphorylated proteins that participate in translation, transcription, and metabolic processing. Of note, 31 vernalization-associated proteins were identified that carried both phosphorylation and O-GlcNAcylation modifications, of which the majority (97%) exhibited the coexisting module and the remainder exhibited the potential competitive module. Among these, TaGRP2 was decorated with dynamic O-GlcNAcylation (S87) and phosphorylation (S152) modifications, and the mutation of S87 and S152 affected the binding of TaGRP2 to the RIP3 motif of TaVRN1 in vitro. Our data suggest that a dynamic network of O-GlcNAcylation and phosphorylation at key pathway nodes regulate the vernalization response and mediate flowering in wheat.
O-linked GlcNAc (O-GlcNAc) is a simple monosaccharide modification on the side chain of Ser and Thr that is involved in the regulation of multiple biological processes. It cycles rapidly during cellular activity (Wells et al., 2001; Wang et al., 2008; Shimoji et al., 2010; Zeidan and Hart, 2010; Hart et al., 2011). Uridine diphosphate-GlcNAc (UDP-GlcNAc), generated from the hexosamine biosynthesis pathway derived from Glc catabolism, is the direct donor of O-GlcNAc (Hanover et al., 2010). Two conserved enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), mediate the reversible addition and removal of O-GlcNAc (Macauley and Vocadlo, 2010; Wang, 2013; Nagel and Ball, 2014). Their activity can be inhibited specifically by chemical inhibitors such as alloxon and PUGNAc, respectively (Macauley and Vocadlo, 2010; Trapannone et al., 2016). Many proteins have been identified that carry this modification, including transcription factors, cytoskeletal proteins, and nuclear pore proteins. O-GlcNAc modification can affect protein phosphorylation status, stability, localization, and/or interaction with other partners (Wells and Hart, 2003; Slawson et al., 2006; Zachara and Hart, 2006; Ozcan et al., 2010; Liu et al., 2015). O-GlcNAc signaling is implicated in human diseases such as cancer, diabetes, and neurodegeneration (Copeland et al., 2008; Singh et al., 2015; Banerjee et al., 2016). In Arabidopsis (Arabidopsis thaliana), there are two putative OGTs, SECRET AGENT (SEC) and SPINDLY. O-GlcNAc signaling is reported to function in response to hormones (such as GA and CK), environmental signals, circadian rhythms, and developmental stage (Silverstone et al., 2007; Olszewski et al., 2010). A recent study suggested that SEC catalyzes the DELLA protein REPRESSOR OF ga1-3 O-GlcNAcylation, regulating its activity and impacting multiple signaling pathways in Arabidopsis (Zentella et al., 2016). O-GlcNAcylation on histone methytransferase ARABIDOPSIS HOMOLOG OF TRITHORAX1 by SEC impacted transcription of FLOWERING LOCUS C (FLC) involved in flowering (Xing et al., 2018). However, the big challenge for studying O-GlcNAcylation signaling is the technical difficulty of monitoring its dynamics due to its instability. Recently, some strategies have being developed, such as LWAC (lectin weak affinity chromatography) and chemical derivatization approaches (Xu et al., 2012; Kim, 2015), which provide a possibility for exploring the global O-GlcNAcylation map, particularly in plants.
Protein phosphorylation is a major posttranslational modification that regulates diverse cellular processes and functions in various signaling transduction in cells. Protein kinases catalyze the addition of a phosphate group to three amino acids: Ser, Thr, and Tyr, and the added phosphate group can be subsequently removed by protein phosphatases (Olsen et al., 2006; Thingholm et al., 2009). The dynamic phosphorylation status of proteins plays an important role in endogenous hormone perception and transduction, and environmental stress sensing and response (Osakabe et al., 2013; Yu et al., 2014). There are hundreds of predicted protein kinases and phosphatases in both plants and animals, suggesting complicated phosphorylation networks in signaling transduction. By contrast, only one or two genes encode O-GlcNAc transferases in cells (Singh et al., 2015). O-GlcNAcylation and O-phosphorylation can both modify Ser and Thr residues, leading to a “yin-yang” model with antagonistic effects at the global proteome level and on specific amino acids of particular proteins (Butkinaree et al., 2010). However, so far, little is known about their collaborative function in cellular processes in response to environmental cues, especially in plants.
Winter annual plants from temperate regions are sown in autumn, but flower in spring of the next year only after experiencing prolonged exposure to low temperatures during the winter, a process termed vernalization (Dennis et al., 1996; Wilson and Dean, 1996; Xu and Chong, 2018; Gauley and Boden, 2019; Koppolu and Schnurbusch, 2019). Many genes involved in the vernalization response have been identified in cereal crops, such as wheat (Triticum spp.) and barley (Hordeum vulgare), as well as in Arabidopsis (Dubcovsky et al., 1998; Minorsky, 2002; Shindo and Sasakuma, 2002; Henderson et al., 2003). In Arabidopsis, vernalization promotes flowering through epigenetic silencing of FLC, a key flowering repressor. Long noncoding RNA and PHD-Polycomb repressive complex 2 mediate the silencing of FLC through the switching of chromatin states and accumulation of H3K27me3 at the nucleation region during cold exposure (Bastow et al., 2004; Qüesta et al., 2016; Yuan et al., 2016; Zhou et al., 2018). However, in temperate crops such as wheat, a central regulator is TaVRN1, which promotes flowering and is activated during vernalization through a complex transcriptional regulation network (Yan et al., 2003; Trevaskis et al., 2006; Distelfeld et al., 2009; Trevaskis, 2010; Kippes et al., 2015). Several putative vernalization memory-related genes were identified at the transcriptional level through transcriptome analysis in Brachypodium (Huan et al., 2013). Our previous studies have reported that diverse metabolic changes occur sequentially during the different stages of vernalization, and Suc addition at an early stage of cold exposure can promote flowering, substituting the requirement, to some extent, of vernalization treatment (Zhao et al., 1998; Yong et al., 1999). This may be linked to the accumulation of metabolic intermediates from Suc, such as UDP-GlcNAc (Hanover et al., 2010).
We previously cloned the vernalization-induced gene VER2, which encodes a Jacalin-like lectin in winter wheat (Zhao et al., 1998; Yong et al., 1999; Xu et al., 2004). Knockdown of VER2 caused delayed flowering, whereas its overexpression partly replaced the necessity of vernalization for winter wheat to flower (Zhong et al., 1995; Chong et al., 1998; Yong et al., 2003). VER2 can specifically bind to GlcNAc, and vernalization induces an increase in O-GlcNAcylated proteins at the global level (Xing et al., 2009). VER2 interacts with a Gly-rich RNA-binding protein TaGRP2, which directly binds to TaVRN1 precursor mRNA to repress its expression. During vernalization, gradually increased O-GlcNAc modification was detected for TaGRP2, thus allowing phosphorylated VER2 to recognize O-GlcNAcylated TaGRP2 and repress its accumulation in the nucleus and attenuate its binding to TaVRN1, thereby releasing the repression of TaVRN1, ultimately promoting flowering (Xiao et al., 2014). These results illustrate the involvement of O-GlcNAc signaling in vernalization-promoted flowering in winter wheat.
In this study, we investigated dynamic O-GlcNAcylation during the process of vernalization and further explored the importance of O-GlcNAcylation signaling in mediating the vernalization response by using a chemical inhibitor that modifies the enzymatic activity of OGA. In addition, we identified hundreds of proteins with dynamic O-GlcNAcylation or phosphorylation during vernalization. These proteins are mainly involved in metabolic processing, cellular processing, and response to stimulus. We also identified 31 proteins with both O-GlcNAc and phosphorylation, of which some may indeed play an important role in mediating the vernalization response. Our data suggests that O-GlcNAcylation and phosphorylation protein modifications may act in the vernalization response and regulate the transcriptional network of VRNs for flowering in wheat.
RESULTS
O-GlcNAc Signaling Accelerates Vernalization-Promoted Flowering by Regulating VRNs
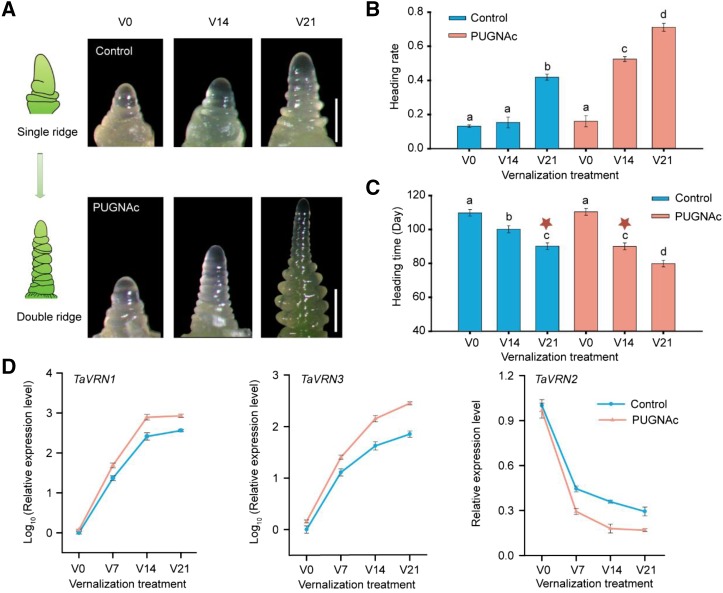
To address the physiological function of O-GlcNAc signaling, PUGNAc [O-(2-acetamido-2-deoxy-d-glucopyranosy lidenamino) N-phenylcarbamate], the inhibitor of OGA, was used to treat V0 (nonvernalization), V14 (vernalization for 14 d), and V21 (vernalization for 21 d) winter wheat cultivar Jingdong1 (JD1). As expected, the global O-GlcNAcylated proteins increased as detected by antiO-GlcNAc antibody in the different vernalized wheat following PUGNAc treatment (Supplemental Fig. S1). Morphologically, the appearance of a double ridge at the shoot apex is a clear marker for initiation of flowering (Fig. 1A). PUGNAc-treated winter wheat flowers earlier than control plants under V14 and V21 conditions, but such effect was not observed in wheat without vernalization (V0; Fig. 1, A–C; Supplemental Fig. S2). Winter wheat treated with PUGNAc under V14 flowers at a similar time as the control plant under V21 (Fig. 1C), with an even higher heading rate (Fig. 1B), suggesting that PUGNAc treatment could partly substitute cold exposure. In addition, the transcription level of the key flowering genes TaVRN1, TaVRN2, and TaFT1 were monitored at different cold exposure durations with or without PUGNAc treatment. The expression of two flowering promoting genes TaVRN1 and TaFT was increased when treated with PUGNAc at V7, V14, and V21 as compared with that in nontreated wheat, but no difference was seen at V0 (Fig. 1D). The expression of TaVRN2, a repressor of flowering, was decreased in wheat treated with PUGNAc at V7, V14, and V21, but no change was observed at V0 (Fig. 1D). Therefore, O-GlcNAc signaling possibly modulates wheat vernalization to impact flowering through regulating vernalization response genes such as TaVRN1, TaVRN2, and TaFT. Of note, the effects of O-GlcNAc signaling only apply at specific periods of the vernalization process, which fits the previous report that Glc addition accelerates flowering but only at certain time windows during vernalization (Yong et al., 2003).
Figure 1.
O-GlcNAc signaling accelerates vernalization-promoted flowering transition in winter wheat. A, Shoot apex morphology of winter wheat JD1 at V0, V14, and V21 with nontreatment (control) and PUGNAc (OGA inhibitor) treatment, respectively. Bar = 0.5 mm. The diagram on the left shows different stages of wheat apex; the double ridge is a clear marker to indicate the initiation of flowering. B and C, Quantification of the heading rate (the percentage of the wheat reaches double ridge when observation; B) and heading time (C) of wheat with different treatment. In (C), stars emphasize that winter wheat with PUGNAc addition under V14 flowers is at similar time as the control plant with V21. Data are means ± sd of 20 plants for each line. Different letters indicate the significant treatment difference at P < 0.05, and one-way ANOVA was used for statistical analysis. D, Relative expression of key flowering genes TaVRN1, TaVRN2, and TaFT1 in JD1 wheat with nontreatment (control) and PUGNAc treatment (data were normalized to housekeeping gene Actin first, and then normalized to nontreated V0 plant). Data shown are means ± sd; n = 3.
A Global Map of Proteins with O-GlcNAcylation and Phosphorylation during Vernalization
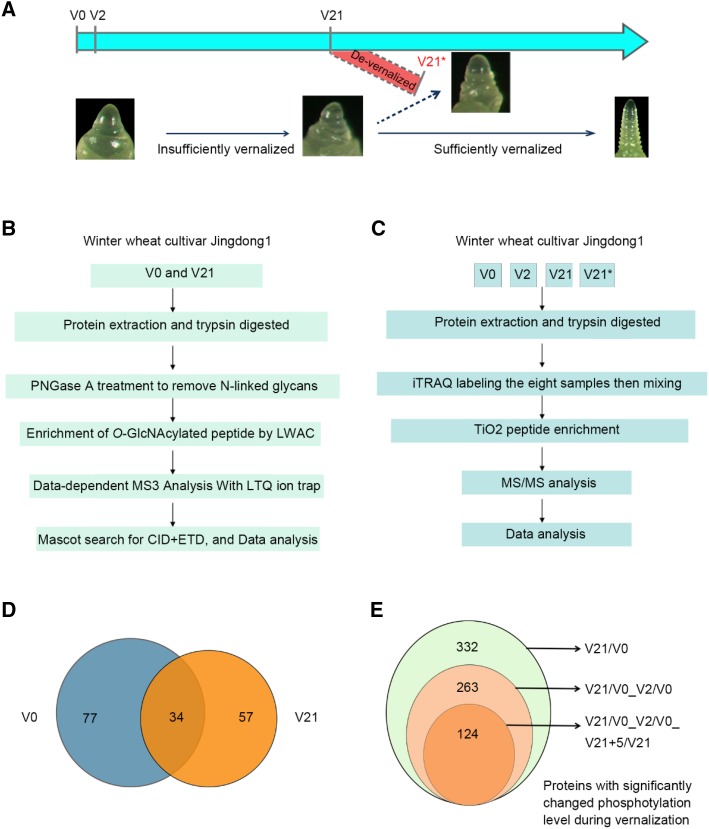
Our previous study shows that the global levels of O-GlcNAcylated proteins are significantly different before and after vernalization treatment (Xing et al., 2009). Here, phosphorylated proteins at different stages of the vernalization process were monitored by immunoblotting using an antibody recognizing Phos-tag-Biotin. As expected, a dynamic phosphoprotein pattern was detected during vernalization (Supplemental Fig. S3A). To understand the global profile of O-GlcNAcylated proteins and phosphorylated proteins participating in vernalization, a proteomic approach was used to enrich and identify such proteins. Wheat plumules at nonvernalized (V0), vernalized for 2 d (V2), vernalized for 21 d (V21), and de-vernalized (V21+5, abbreviated as V21*), of which the seedlings vernalized for 21 d were subsequently exposed at 35°C for 5 d leading to elimination of vernalization, were used for comparison (Fig. 2A). An approach with LWAC and liquid chromatography-tandum mass spectrometry (LC-MS/MS) was used to enrich and identify O-GlcNAcylated peptides (Fig. 2B). As a result, a total of 201 O-GlcNAcylated peptides representing 111 O-GlcNAcylated proteins were identified in the nonvernalized wheat sample, and 143 O-GlcNAcylated peptides from 91 proteins were found in vernalized samples (Supplemental Tables S1 and S2). Among these O-GlcNAcylated proteins, only 34 proteins were present in both nonvernalized and vernalized wheat plumules (Fig. 2D). As for phosphorylated proteins, after protein extraction and digestion with Trypsin, iTRAQ labeling was used for quantification of the different samples, and then TiO2 column was used to enrich phosphorylated peptides, which was followed by LC-MS/MS analysis (Fig. 2C). The 332 proteins that were significantly changed in phosphorylation level (SCPL) between V21 and V0 were identified. There were still 263 SCPL proteins after deducting the SCPL proteins that arose in response to cold stress (V2/V0). A total of 205 unique phosphopeptides, representing 124 SCPL proteins (44 up-regulated proteins and 80 down-regulated proteins, respectively), were found to arise in response to vernalization after subtracting that of V2/V0 (SCPL in response to cold stress) and V21+5/V0 (SCPL in response to devernalization; Fig. 2E; Supplemental Fig. S3B; Supplemental Table S3). This suggests that O-GlcNAcylation and phosphorylation dynamically modify numerous proteins during the process of vernalization.
Figure 2.
Experiments design to enrich and identify proteins with O-GlcNAcylation or phosphorylation at different stage of vernalization and overview of identified proteins. A, Diagram of tissue sampling at different time point during vernalization and the corresponding developmental stages of shoot apex. B and C, Strategies used for isolation, enrichment, and identification of O-GlcNAcylated peptide/protein (B) and phosphorylated peptide/protein with two biologic replications (C). D, Venn diagram showing general and unique O-GlcNAcylated proteins identified at V0 and V21. E, Venn diagram showing alternatively changed phosphorylated proteins identified in response to vernalization. V21/V0, protein of SCPL between V21 and V0; V21/V0_V2/V0, SCPL protein between V21 and V0 deduct that of V2 and V0; V21/V0_V2/V0_V21+5/V0, SCPL protein between V21 and V0 subtract that of V2/V0 and V21+5/V0. V0: no cold exposure; V2: vernalization for 2 d; V21: vernalization for 21 d; and V21*: vernalization for 21 d followed by high temperature (35°C) growth for 5 d (V21+5). CID, collisional induced dissociation; ETD, electron transfer dissociation.
Functional Categorization of O-GlcNAcylated Proteins and SCPL Proteins
To explore the functions of the identified O-GlcNAcylated and SCPL proteins, gene ontology (GO) analysis was performed using agriGO (http://bioinfo.cau.edu.cn/agriGO/) with Arabidopsis and rice homologue proteins (Supplememtal Table S4). The identified O-GlcNAcylated proteins in either nonvernalized or vernalized wheat plumules are involved in a series of biological processes, such as response to abiotic stimulus, response to hormone, gene expression, nucleoside metabolic process, and developmental process (Fig. 3A). The O-GlcNAcylated proteins identified in nonvernalized (V0) wheat were enriched in GO terms such as shoot system development, plant organ development, and response to abscisic acid (Fig. 3B), whereas the vernalization-specific (V21) O-GlcNAcylated proteins were enriched in response to cytokinin, signal transduction, and reproductive process (Fig. 3C). Of note, the enrichment of GO terms is different in V0- and V21-specific O-GlcNAcylated proteins. This suggests that O-GlcNAcylated proteins may mediate vernalization through integrative processes such as hormone signaling, shoot and reproductive development, and gene expression.
Figure 3.
Enrichment of biological process of the identified O-GlcNAcylated proteins. A–C, Enriched GO terms of all identified O-GlcNAcylated proteins in either vernalized or nonvernalized wheat (A); specifically in nonvernalized (V0) wheat (B); or in vernalized (V21) wheat (C). D, Four major processes with phosphorylation or O-GlcNAcylation modification involved in vernalization response. The red small dots mean O-GlcNAc modification, and the blue dots mean phosphorylation modification. ABCG37/40, ATP-BINDING CASSETTE G 37; ABCG40, ATP-BINDING CASSETTE G 40; ARR10, ARABIDOPSIS RESPONSE REGULATOR 10; GSTF6, GLUTATHIONE TRANSFERASES F 6; LTP3, LIPID TRANSFER PROTEIN 3; GH17, GLYCOSYL HYDROLASE 17; NPAP, NUCLEAR POLY (A) POLYMERASE; RPL4, RIBOSOMAL LARGE SUBUNIT 4; PER64, PEROXIDASE 64; CPN60B, CHAPERONIN 60 BETA; ETR2, ETHYLENE RESPONSE 2; RD22, RESPONSIVE TO DESICCATION 22; RZFP, RZFP, RING/FYVE/PHD zinc finger superfamily protein; PTR3, PEPTIDE TRANSPORTER 3; UPL3, UBIQUITIN-PROTEIN LIGASE 3; APG9, AUTOPHAGY 9; GAPC, GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE OF PLASTID 2; BGLU13, BETA GLUCOSIDASE 13; UXS, UDP-XYLOSE SYNTHASE; AAC2, ADP/ATP CARRIER 2; GRP2, GLYCINE-RICH RNA-BINDING PROTEIN 2; GBF4, G-BOX BINDING FACTOR 4; H1.2, HISTONE 1.2.
Further analysis showed that these O-GlcNAcylated or phosphorylated proteins in response to vernalization belong to four clusters as follow (Fig. 3D): The first cluster is hormone response, including several hormones response factors such as ARF3 (AUXIN RESPONSE TRANSCRIPTION FACTOR 3), ARABIDOPSIS RESPONSE REGULATOR 10, PIN5 (PIN-FORMED 5), ETHYLENE RESPONSE 2, and ATP-BINDING CASSETTE G 37/40, which are O-GlcNAcylated after vernalization. The second cluster is stress response, comprising PEPTIDE TRANSPORTER 3, RESPONSIVE TO DESICCATION 22, UBIQUITIN-PROTEIN LIGASE 3, PEROXIDASE 64, AUTOPHAGY 9, and RING/FYVE/PHD zinc finger superfamily protein with changeable O-GlcNAcylation or phosphorylation status during vernalization. The third cluster is involved in energy and carbohydrate metabolism such as FBA5/6 (FRU-BISPHOSPHATE ALDOLASE 5/6), GLYCERALDEHYDE-3-PHOSPHATE DEHYDRONASE OF PLASTID 2, BETA GLUCOSIDASE 13, UDP-XYL SYNTHASE, and ADP/ATP CARRIER 2. This may explain how prolonged low temperature changes the cellular nutrient status by shaping the metabolic patterns of energy and carbohydrate metabolites through dynamic Yin-Yang modification of the related enzymes. The fourth cluster is enriched in genetic information processing factors, such as proteins related to RNA splicing, epigenetic modification, translation, and transcription, with O-GlcNAcylation and/or phosphorylation modification during vernalization. Such proteins are GRP2 (GLY-RICH RNA-BINDING PROTEIN 2), PABP8 (POLY(A) BINDING PROTEIN 8), G-BOX BINDING FACTOR 4, CHAPERONIN 60 BETA, and H1.2 (HISTONE 1.). Most of the dynamic phosphoproteins identified during different vernalization treatment were involved in several processes such as protein folding, nucleosome and chromatin assembly, translation, protein metabolic process, regulation of RNA biosynthetic process, transcription, and primary metabolic process (Supplemental Fig. S4). In the modification profiling, there were 31 proteins carrying both O-GlcNAcylation and phosphorylation modifications (Table 1). Most of these 31 proteins are involved in metabolic processing, and some are involved in response to stress (Table 1). These proteins may play an important role in mediating flowering during vernalization through multiple processes.
Table 1. The proteins with O-GlcNAcylation and phophorylation modification.
| No. | GeneBank ID | Protein Descriptions |
|---|---|---|
| Metabolic process (32.3%) | ||
| 1 | 525291 | ATP synthase beta subunit [T. aestivum] |
| 2 | 474210338 | Fru-bisphosphatealdolase cytoplasmic isozyme [Triticum urartu] |
| 3 | 148508784 | Glyceraldehyde-3-phosphate dehydrogenase [T. aestivum] |
| 4 | 474433294 | Peptidyl-prolylisomerase PASTICCINO1 [T. urartu] |
| 5 | 473990310 | Peroxidase 64 [T. urartu] |
| 6 | 474111415 | Polyadenylate-binding protein 2 [T. urartu] |
| 7 | 474305843 | DEAD-box ATP-dependent RNA helicase 24 [T. urartu] |
| 8 | 473923422 | Elongation factor 1-alpha [T. urartu] |
| 9 | 461744056 | Enolase [T. aestivum] |
| 10 | 474137978 | Rubisco large subunit-binding protein subunit beta, chloroplastic [T. urartu] |
| Response to stress (29%) | ||
| 11 | 473949239 | E3 ubiquitin-protein ligase TRIM33 [T. urartu] |
| 12 | 474267869 | Ethylene insensitive 3-like 5 protein [T. urartu] |
| 13 | 474173714 | Heat shock 70 kD protein, mitochondrial [T. urartu] |
| 14 | 474378056 | Heat shock protein 83 [T. urartu] |
| 15 | 294717808 | Heat shock protein 90 [T. aestivum] |
| 16 | 25989705 | LEA1 protein [T. aestivum] |
| 17 | 300681479 | bZIP transcription factor domain containing protein, expressed [T. aestivum] |
| 18 | 474425093 | Zinc finger protein VAR3, chloroplastic [T. urartu] |
| 19 | 474302864 | Putative calcium-binding protein CML7 [T. urartu] |
| Kinase and phosphatase (12.9%) | ||
| 20 | 262192761 | LRR receptor-like kinase [T. aestivum] |
| 21 | 474016289 | Ser/Thr-protein kinase PBS1 [T. urartu] |
| 22 | 473996388 | Ser/Thr protein phosphatase 2A 57 kD regulatory subunit B ∼ iota isoform [T. urartu] |
| 23 | 114145394 | Gly-rich RNA-binding protein [T. aestivum] |
| Others (25.8%) | ||
| 24 | 4098272 | Alpha-tubulin [T. aestivum] |
| 25 | 215398470 | Globulin 3 [T. aestivum] |
| 26 | 2980891 | Histone H1 [T. aestivum] |
| 27 | 474360395 | Nuclear-pore anchor [T. urartu] |
| 28 | 473956884 | Patatin group A-3 [T. urartu] |
| 29 | 474241959 | Tetratricopeptide repeat protein 7B [T. urartu] |
| 30 | 473889537 | 40S ribosomal protein S3-3 [T. urartu] |
| 31 | 474323352 | 60S ribosomal protein L22-2 [T. urartu] |
The Possible Correlation between Phosphorylation and O-GlcNAcylation Modification
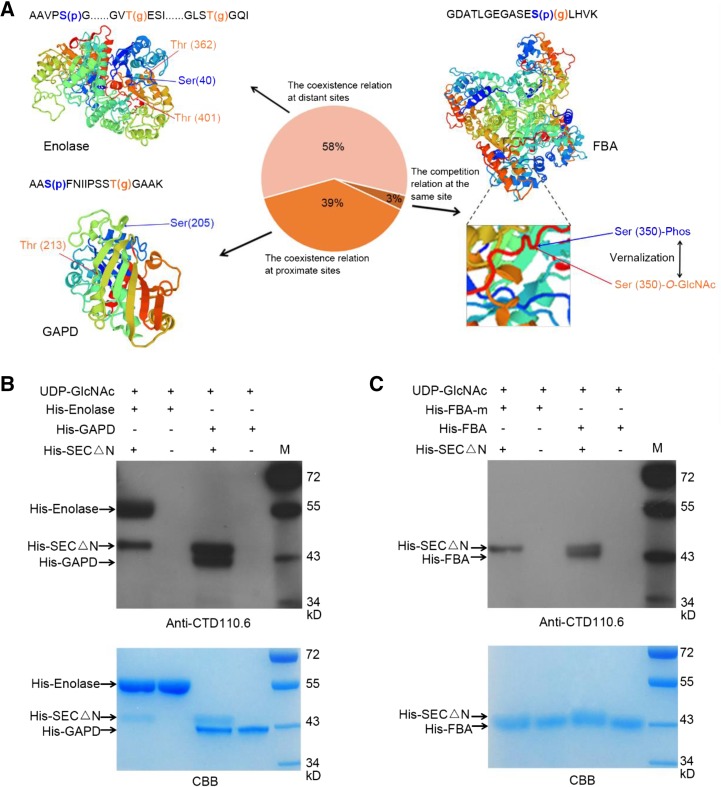
Considering the cross talk modification module proposed in animal studies (Gupta and Brunak, 2002; Leney et al., 2017), we further explored the possible correlation between the modified sites and the computationally defined structures of the 31 proteins with both O-GlcNAcylation and phosphorylation. The results suggested that there were two major patterns between the O-GlcNAcylation and phosphorylation modification: coexisting or competitive (Fig. 4A). For example, the phosphorylation modified site (S205) and the O-GlcNAc modified site (T213) of glyceraldehydes-3-phosphate dehydrogenase (GAPD) were coexisting in nonvernalization samples and erased (O-GlcNAcylation on T213) or reduced (phosphorylation on S205) during vernalization. This suggests that both modifications are coexisting and coordinately regulated. Although the linear distance between S205 and T213 is very close, they are spatially far away from each other (located at the two terminals of β-sheet), representing 39% of the identified proteins with O-GlcNAcylation and phosphorylation (Fig. 4A). Meanwhile, the coexisting relation was also found between the long linear distant sites on Enolase protein, which are physically close to each other in the three-dimensional structure. This represents more than 58% of such bivalent modified proteins (Fig. 4A). Interestingly, we found the Fru-bisphosphatealdolase (FBA) with the O-GlcNAcylation and phosphorylation at the same site S350, which existed in only 3% of the proteins with both modifications identified here (Fig. 4A). The level of phosphorylation at S350 was decreased during vernalization, whereas the O-GlcNAcylation at S350 was present after vernalization. This illustrates the potential competitive relation (Yin-Yang model) of O-GlcNAcylation and phosphorylation during vernalization. These predicted results will help guide further studies in the crosstalk of O-GlcNAcylation and phosphorylation modifications on key proteins that regulate vernalization response in the future.
Figure 4.
Occupancy patterns between phosphorylation and O-GlcNAcylation modification in response to vernalization and SEC O-GlcNAcylates GAPD, Enolase, and FBA in vitro. A, Three-dimensional structures of the proteins (such as Enolase, GAPD, and FBA) with O-GlcNAcylation and phosphorylation modification predicted by Swiss-model (https://swissmodel.expasy.org/) and the sequences of identified peptides. The amino acid with O-GlcNAc modification (g) was orange, and the one with phosphorylation modification (p) was blue. There were two states between the O-GlcNAcylation and phosphorylation modifications from the results. One was the competition relation at the same site (3%), and the other was the coexistence relationship of the two modifications at proximate sites (58%) or distant sites (39%). B, Detection of O-GlcNAc modification of His-GAPD and His-Enolase, catalyzed by His-SECΔN in vitro. His-GAPD and His-Enolase were recombinantly expressed and affinity purified separately; His-SECΔN (expressing residues 801–1,062 of the C terminus) exhibited OGT activity [23]. O-GlcNAcylation of His-GAPD and His-Enolase were detected by anti-CTD110.6 antibody. C, Detection of O-GlcNAc modification of His-FBA and His-FBA-m catalyzed by His-SEC△N in vitro. His-FBA-m means the mutation of the three identified O-GlcNAcylated sites (T35, T320, and S350) of FBA.
To validate the identified O-GlcNAcylation on these proteins by MS, an in vitro O-GlcNAcylation assay was used. His-tagged GAPD, Enolase, FBA, and FBA-m (mutation of the three identified O-GlcNAcylated sites T35, T320, and S350) were expressed in Escherichia coli and affinity purified, as well as the truncated version of SECΔN with proofed OGT activity in vitro (Xing et al., 2018). Incubation with SECΔN, GAPD, Enolase, and FBA could be recognized by the O-GlcNAcylation-specific antibody CTD110.6, whereas FBA-m was not recognized (Fig. 4, B and C). This further verifies our observation by MS.
Mutations of the O-GlcNAcylation and Phosphorylation Modified Sites on TaGRP2 May Impact Its Binding to TaVRN1-RIP3
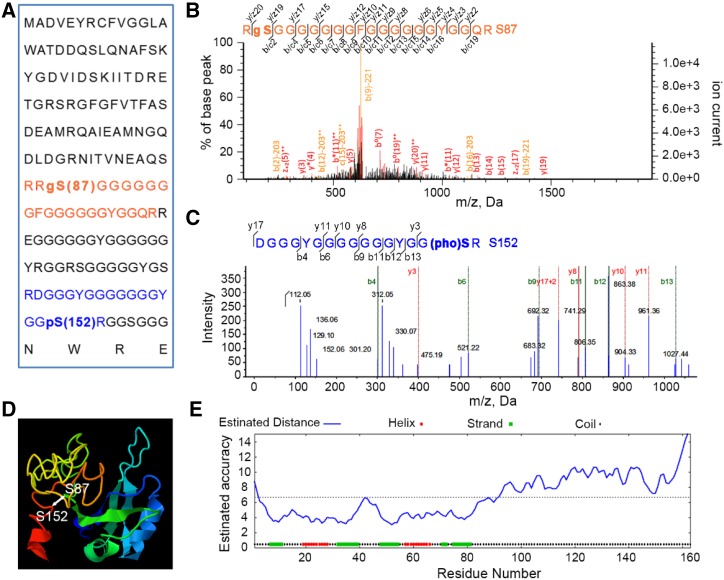
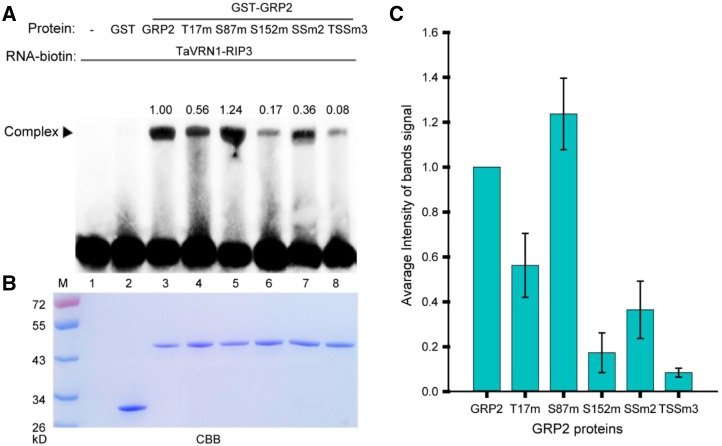
Among the 31 proteins with both O-GlcNAcylation and phosphorylation, TaGRP2, a RNA binding protein, was chosen for further study to test that the identified proteins with posttranslational modifications may play vital roles in vernalization (Table 1). From the MS data, Ser-87 of TaGRP2 was identified as an O-GlcNAcylation site, whereas Ser-152 was a phosphorylation site (Fig. 5, A–C). The linear distance between Ser-87 and Ser-152 was far, but they are close to each other in the higher structure (Fig. 5, A, D, and E). This suggests the possibility of cross talk between O-GlcNAcylation and phosphorylation. Sequence alignment showed that the S87 of TaGRP2 is relatively conserved, and is either Ser (50%) or Gly (50%) in different species (Supplemental Figs. S5 and S6). However, the residues corresponding to S152 of TaGRP2 are variable (e.g. S, G, R, and I) in different species (Supplemental Fig. S5). This pattern indicates that the conserved S87 might be important in regulating TaGRP2 function. To test the effect of O-GlcNAcylated and phosphorylated sites on the function of TaGRP2, RNA Electrophoretic Mobility Shift Assay (RNA-EMSA) was used to analyze the binding of TaGRP2 and TaGRP2 mutants to the TaVRN1-RIP3. TaVRN1-RIP3 was the target binding-motif of TaGRP2 (Xiao et al., 2014). Glutathione S-transferase (GST)-tagged TaGRP2, GRP2-T17m (T17 of TaGRP2 is detected to be modified by O-GlcNAcylation in the previous study Xiao et al. [2014]), TaGRP2-S87m, TaGRP2-S152m, TaGRP2-SS2m (mutation of the identified O-GlcNAcylated sites S87 and the phosphorylated site S152), and TaGRP2-TSS3m (mutation of the two identified O-GlcNAcylated sites T17 and S87, the identified phosphorylated site S152) were expressed in Escherichia coli and affinity purified. The RNA-EMSA results showed that mutation of the O-GlcNAcylated sites (T17 and S87) or the phosphorylated site (S152) of TaGRP2 changed the signal density of the TaGRP2-TaVRN1-RIP3 complex bands (Fig. 6; Supplemental Fig. S7). Changing S87 to A87 enhanced the signal, whereas mutant S152 weakened it. Mutating both S87 and S152 reduced the signal slightly, but mutating the three sites T17, S87, and S152 clearly abated the signal. These results suggest that the three modified sites may be important for the function of TaGRP2 to bind RNA. The TaGRP2-OE transgenic wheat lines and wild type were vernalized for 0, 21, and 28 d to test the function of TaGRP2 during vernalization. The results showed that the shift from the single ridge to double ridge stage of apex development in TaGRP2-OE transgenic wheat lines was slower than that in wild type in either V21 or V28 treatment (Supplemental Fig. S8), which is consistent with the previous report that TaGRP2 represses flowering transition (Xiao et al., 2014). The O-GlcNAcylated and phosphorylated sites of TaGRP2 may be involved in vernalization regulation in wheat.
Figure 5.
The O-GlcNAcylated and phosphorylated peptides of TaGRP2 identified by MS. A, The amino acid sequences of TaGRP2 with identification of phosphorylation and O-GlcNAcylation modification sites. B, The representative MS/MS spectra of O-GlcNAcylated peptide. C, The representative MS/MS spectra of phosphorylated peptide. D, Three-dimensional structure map of TaGRP2 predicted by Phyre; the phosphorylated site is S152, and the O-GlcNAcylated site is S87 (http://www.sbg.bio.ic.ac.uk/phyre2/). E, The estimated accuracy of the predicted 3D structure of TaGRP2.
Figure 6.
Mutant of S87 or S152 affects the TaGRP2’s binding to TaVRN1-RIP3. A, RNA-EMSA assay to analyze the binding of GRP2 and GRP2 mutants to the TaVRN1-RIP3. T17m, S87m, and S152m mean the GRP2 protein with mutation of T17, S87, and S152, respectively; SSm2 means GRP2 protein with mutation of S87 and S152; TSSm3 means GRP2 protein with mutation of T17, S87, and S152. The numbers above indicate the average band intensity of three replicates as quantified using Image J. The Coomassie brilliant blue (CBB) signal was normalized by EMSA signal. B, CBB staining result of the EMSA samples in (A). C, The quantitative data of three replicates for the binding-affinity comparisons among wild-type and mutant GRP2 proteins. Data shown are means ± sd; n = 3.
DISCUSSION
O-GlcNAc Is a Protein Modification that Regulates Vernalization
As sessile organisms, plants are constantly exposed to various environmental stresses (Qi et al., 2018; Wang et al., 2018). Low temperature constitutes a key factor influencing plant growth, development, crop productivity, and geographic distribution (Guo et al., 2018; Liu et al., 2018). In responding to cold, plants could rapidly change the metabolism in existing tissue, and metabolome analyses revealed that the levels of monosaccharides such as Glc and Fru from the starch degradation and Suc metabolism were significantly higher in cold-treated plants (Maruyama et al., 2014; Zhang et al., 2016), and the Glc addition can reduce a requirement of winter wheat for vernalization (Yong et al., 2003). However, little is known about which metabolite of Glc participates in the transduction of signaling during vernalization.
O-GlcNAcylation is an abundant nutrient-driven modification linked to cellular signaling and regulation of gene expression (Zachara and Hart, 2004; Butkinaree et al., 2010; Lewis and Hanover, 2014). In Drosophila, O-GlcNAc signaling is extremely important in developmental regulation, stem cell maintenance, circadian regulation, and responses to ambient temperature (Gambetta et al., 2009; Sinclair et al., 2009; Kaasik et al., 2013; Radermacher et al., 2014). Our results showed that the addition of an inhibitor of OGA can reduce the requirement of vernalization in winter wheat, which suggests that elevation of O-GlcNAcylation level can partly mimic vernalization treatment to promote wheat flowering and regulate the expression of VRNs (Fig. 1; Supplemental Fig. S2), thus indicating that O-GlcNAc signaling plays an important role in regulating vernalization response.
There were 168 O-GlcNAc modified proteins identified in our data (Fig. 2; Supplemental Tables S1 and S2). Many of them shared the expression patterns observed in animal cells, such as H4, H2B, PFK, and Heat Shock Protein 70 (Guinez et al., 2006; Singh et al., 2015). Histones are subject to posttranslational modification, and these modifications are important parts of regulatory circuits that control chromatin dynamics and the activities of DNA. Numerous reports have shown that histones possess lots of posttranslational modifications such as methylation, phosphorylation, ubiquitination, and acetylation (Yun et al., 2011). Recent research also reported that histone H3 Lys 4 trimethylation (H3K4me3) and histone H3 Lys 27 trimethylation (H3K27me3) at VRN3 regulated the epigenetic memory of vernalization in Brachypodium distachyon (Huan et al., 2018). However, there is a poor understanding of the O-GlcNAcylation on histones that are involved in regulating vernalization; the O-GlcNAcylation modification of histones will be an attractive research direction in the future. About 15% of the identified O-GlcNAcylated proteins in wheat plumules have close homologs as SEC interactors in Arabidopsis (Supplemental Table S5). Although SEC is highly conserved between monocotyledons and dicotyledons, the O-GlcNAcylated target proteins were very diverse. Recently, a report has showed the profile of the O-GlcNAcylated proteins in Arabidopsis (Xu et al., 2017); however, only a few proteins were identified as O-GlcNAcylated proteins in our data, such as T-complex protein, ARF, Time for coffee, 60S, and PAB8. A possible explanation for this is that the samples and treatments were very different between the Arabidopsis inflorescence tissues used in the previous study and the wheat plumules used here. However, the cellular processes related to O-GlcNAc-modified proteins were similar among wheat, Arabidopsis, and animals, and mainly involved in signal transduction, translation, transcription, and metabolic process (Liu et al., 2015; Xu et al., 2017). In our study, the O-GlcNAc–modified proteins from vernalized samples were associated with amino acid and nucleotide metabolism and translation (Supplemental Fig. S9). O-GlcNAc may be a protein modification that regulates vernalization in winter wheat.
The Possible Correlation between O-GlcNAcylation and Phosphorylation Modifications during Vernalization
O-GlcNAcylation and O-phosphorylation both modify Ser and Thr residues, which leads to the “yin-yang” regulatory theory (Hu et al., 2010). Based on our data, 31 proteins were detected to have both O-GlcNAc modification and phosphorylation modification at the same time (Table 1), but co-occurrence of the two modifications in the same peptide was rare. The FBA was identified to have O-GlcNAcylation and phosphorylation modification at the same site S350. In addition, the two modifications existed competitively during vernalization (Fig. 4A). The Yin-Yang relationship may regulate flowering through mediating vernalization response. TaGRP2 was gradually O-GlcNAcylated during vernalization (Xiao et al., 2014). Meanwhile, TaGRP2 can be phosphorylated before vernalization, and the spatial distance of the two modifications on TaGRP2 protein was very close (Fig. 5). The O-GlcNAc and phosphorylation modification of TaGRP2 may antagonistically mediate the function of TaGRP2 to bind RNA (such as TaVRN1-RIP3) through changing GRP2’s structure (Fig. 6). According to a previous study (Leney et al., 2017; van der Laarse et al., 2018), the correlation of the two modifications also existed between the function of phosphorylation and O-GlcNAcylation on different proteins during vernalization. Vernalization increases the O-GlcNAc modification of TaGRP2 (a repressor in vernalization) in the nucleus and the phosphorylation of VER2 (an activator in vernalization) in cytoplasm, which antagonistically regulated the expression of TaVRN1 to mediate flowering in winter wheat (Xiao et al., 2014). The study of vernalization has mainly been focused on the regulation and function of VRNs so far. But it is unclear how wheat transduces the vernalization signaling, which is of vital importance for vernalization. Our data here suggest that the O-GlcNAc signaling plays a role in transducing vernalization signal, and the possible correlation between O-GlcNAcylation and phosphorylation modifications may participate in regulation of wheat flowering through affecting vernalization, which will attract us to continue the follow up research in future. In summary, the approaches of LWAC and iTRAQ-TiO2 were used to detect and identify a series of O-GlcNAcylated and alternatively changed phosphorylated proteins in different vernalized wheat. Functional analysis showed that the O-GlcNAcylated proteins identified in vernalized wheat mediated the vernalization response through the four main processes, such as hormone response (such as ARF3, ARABIDOPSIS RESPONSE REGULATOR 10, PIN5, and ETHYLENE RESPONSE 2), response to stress (such as PEPTIDE TRANSPORTER 3, RESPONSIVE TO DESICCATION 22, UBIQUITIN-PROTEIN LIGASE 3, and PEROXIDASE 64), energy and carbohydrate metabolism (such as ADP/ATP CARRIER 2, FBA5, UDP-XYL SYNTHASE, and BETA GLUCOSIDASE 13), and genetic information processing (such as GRP2, PABP8, G-BOX BINDING FACTOR 4, and CHAPERONIN 60 BETA), and some of the O-GlcNAcylated proteins also were modified by phosphorylation, which indicated that the cross talk of the two modifications may involve in vernalization regulation (Fig. 3D). The results of OGA inhibitor treatment showed that O-GlcNAc signaling during vernalization accelerated flowering transition in winter wheat. O-GlcNAc as an abundant nutrient-driven modification may measure the time horizon to initiate vernalization in winter wheat. Taken together, O-GlcNAcylation and phosphorylation modification may act as signals to mediate vernalization response and regulate the network of VRNs for flowering in wheat.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
JD1 and JH9 were Chinese winter wheat (Triticum aestivum) cultivars. TaGRP2 overexpression (TaGRP2-OE) and RNA interference (TaGRP2- RNAi) transgenic wheat were generated in JH9 accession by microprojectile bombardment-mediated transformation. Seeds of winter wheat (JD1, JH9, and TaGRP2 transgenic lines) were surface sterilized in 2% (v/v) NaClO for 20 min, then rinsed overnight with flowing water. After that, the seeds were germinated on moist filter paper under gradient time (14, 21, and 28 d, as V14, V21, V28) of 4°C treatment in the dark (V), or grown at 25°C for 3 d (V0). Twenty μm PUGNAc (the inhibitor of OGA) was used to treat JD1 during the vernalization, then transferred to soil, and grown in greenhouse (20–22°C, 16-h light/8-h dark) for 70 d. Finally, we used a dissecting mirror to dissect the wheat to observe the flowering phenotype.
The Methods of Inhibitor PUGNAc of OGA-Treated Plant Materials
The seeds were germinated on moist filter paper under gradient time 14 and 21 d (as V14, V21) of 4°C treatment in the dark, or grown at 25°C for 3 d as nonvernalization (V0), and then transferred to soil and grown in greenhouse (20–22°C, 16-h light/8-h dark) for 70 d. The dissecting mirror was then used to dissect the wheat to observe the phenotype of apex development; 14 to 16 seedlings of each treatment were dissected. The one showed in Figure 1 was the representative image in each treatment.
Protein Sample Preparation and iTRAQ Labeling
Total proteins from the wheat plumules (V0, V2, V21, and V21+5) were extracted in homogenization buffer (20 mm Tris-HCl [pH 8.0], 150 mm NaCl, 1 mm EDTA, 10% [v/v] glycerol, 0.2% [v/v] Triton X-100, 1 mm phenylmethylsulfonyl fluoride, Protease inhibitor cocktail, Phosphatase Inhibitor Cocktail). The mixture was thoroughly vortexed for 1 min and centrifugated at 16,000 g and 4°C for 30 min. The supernatant was pipetted into fresh 10-mL tubes, and 3-fold volumes of cold TCA-acetone were added, −20°C to precipitate 2 h, and then centrifugated at 16,000 g and 4°C for 30 min. The supernatant was carefully discarded, and the precipitated proteins were washed twice with cold acetone. Finally, the precipitated proteins were dissolved in lysis buffer (8 m urea, 30 mm HEPES, 2 mm Na3VO4, 2 mm NaF, and 2 mm β-sodium glycerophosphate) and then centrifugated at 16,000 g and 4°C for 30 min. The supernatant was pipetted into fresh 1.5-mL tubes and then quantified of protein by Bradford method, with bovine serum albumin (BSA; 1 mg/mL) as the standard. Protein (200 µg) from each sample was digested by 6.6 µg trypsin (m/m 1:30), incubated at 37°C, 16 h. Then each vial of iTRAQ reagent required was allowed to reach room temperature and spun to bring the solution to the bottom of the tube. Add 70 µL of ethanol to each room-temperature iTRAQ reagent vial. Vortex each vial to mix, then spin. Transfer the contents of one iTRAQ reagent vial to one sample tube. iTRAQ reagent 113 vial to the sample V0-1 protein digest tube, 114 to V0-2, 115 toV2-1, 116 to V2-2, 117 to V21-1, 118 to V21-2, 119 to V21+5-1, and 121 to V21+5-2 (followed the protocol of Applied Biosystems iTRAQ Reagents).
Phosphopeptide Enrichment using TiO2 Microcolumns and Identification using Q-Exactive
The peptides labeling with the iTRAQ reagents were merged using 1-ml loading buffer (60% [v/v] Acetonitrile [ACN], 2% [v/v] trifluoroacetic acid [TFA; pH 2.0]), saturated with Glu, and then incubated with 3.2 mg TiO2 beads (GL Sciences), which were incubated in 500 μL loading buffer containing 60% (v/v) CAN and 2% (v/v) TFA (pH 2.0), saturated with Glu, 30 min at room temperature. After being washed twice with 500 μL wash buffer I (60% [v/v] ACN and 0.5% [v/v] TFA [pH 2.5]) and 500 μL wash buffer II (60% [v/v] ACN and 0.1% [v/v] TFA [pH 3]), the phosphopeptides were eluted twice with 500 μL elution buffer I (50% [v/v] CAN and 300 mm NH4OH [pH 11]) and 500 μL elution buffer II (50% [v/v] ACN and 500 mm NH4OH [pH 11]). The eluates were dried and reconstituted in 30 μL 50% (v/v) triethylamine-carbonate buffer for MS analysis. The enriched phosphopeptides were identified using Q-Exactive, separated on a C18 chromatographic column (5 μm I.D., 100 mm length). Pump flow was split to obtain a flow rate of 1 mL/min for sample loading and 400 nL/min for the MS analysis. The mobile phases consisted of 0.1% (v/v) FA (formic acid; A), and 0.1% (v/v) FA and 80% (v/v) ACN (B). A five-step linear gradient of 3% to 30% B in 70 min, 30% to 80% B in 8 min, 80% B in 7 min, 80% to 5% B for 3 min, and 5% B for 7 min was used. The spray voltage was set to 1.8 kV, and the temperature of the heated capillary was set to 320°C. For data acquisition, each MS scan was acquired at a resolution of 17,500, with the lock mass option being enabled, and was followed by data-dependent top 10 MS/MS scans using higher energy collisional dissociation. The threshold for precursor ion selection was 500, and the mass window for precursor ion selection was set to 350–2000 D. The raw files were processed using Mascot (version 2.4.1) and were then searched against the uniprot_triticeae database. The fixed modification is carbamidomethyl (C), and the variable modifications are oxidation (M), Gln (N-termQ), phospho (ST), phospho (Y), iTRAQ8plex (K), iTRAQ 8 plex(Y), and iTRAQ 8 plex (N-term). One missing cleavage point was allowed. Proteome Discoverer 1.3 (Thermo) was used to extract the peak intensity within 15 ppm of each expected iTRAQ reporter ion from each fragmentation spectrum. Only spectra in which all the expected iTRAQ reporter ions were detected were used for quantification. The phosphopeptide ratios were normalized by dividing the average value of all peptides identified. The false discovery rate was set to < 1.0% for the identification of both peptides and proteins and with PhosphoRS probability ≥ 0.75. Significant changes in a phosphopeptide’s abundance were inferred where its abundance ratio was > 1.2 or < 0.83, and p value < 0.05, which was derived from the Student’s t test. The mass spectrometry proteomics data obtained in this study have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the Proteomics Identifications (PRIDE) partner repository with the dataset identifier PXD008298.
O-GlcNAcylated Peptides Enrichment and Identification
Total proteins from the wheat plumules (V0 and V21) were extracted with NitroExtraTM (Cat. PEX-001-250ML, N-Cell Technology) added 20 μm the inhibitor PUGNAc of OGA. The mixture was thoroughly vortexed for 1 min and centrifugated at 16,000 g and 4°C for 1 h. After centrifugation, protein was precipitated with 1:3 (sample to acetone) cold acetone at −20°C overnight. Precipitated proteins were washed twice with cold acetone and finally resuspend in 8M urea after protein precipitate has been air dried, and quantified of protein by Bradford method, with BSA (1 mg/mL) as the standard. Appropriate amount of trypsin is then added to the sample in an enzyme-to-substrate ratio of 1:30, and incubated at 37°C, 16 h. Digested proteins were desalted by C18 column and dried in spin vacuum. N-glycopepetides were de-glycosylated with 20 μm PNGase F (P7367-50UN, Sigma) and 10 μm PNGase A (G0535-0.005UN, Sigma-Aldrich) in 50 mm ammonium bicarbonate (pH 8.0) and 50 mm citrate-phosphate buffer (pH 5.0), respectively, for 24 h. The GlcNAcylated peptides were enriched from the sample with a Wheat Germ Agglutinin Column. Enriched glycopeptides were dried in spin vacuum. Each dried peptide sample is dissolved in 25 μL of 0.1% (v/v) FA. The sample was analyzed by nanoLC-MS/MS using an UltiMate 3000 RSLCnano System (Thermo Fisher Scientific/ Dionex) coupled to LTQ Velos Dual-Pressure Ion Trap (Thermo Fisher Scientific). After sample was loaded onto a reversed-phase 25 cm C18 PicoFrit column (New Objective) a linear gradient of acetonitrile (3–36%, v/v) in 0.1% (v/v) FA was used. The elution duration was 120 min at a flow rate of 0.3 μL/min. Eluted peptides from the PicoFrit column were ionized and sprayed into the mass spectrometer, using a Nanospray Flex Ion Source ES071 (Thermo Fisher Scientific) under the following settings: spray voltage, 1.6 kV, capillary temperature 250°C. The LTQ instrument was operated in the data dependent mode to automatically switch between full scan MS and MS/MS acquisition. The 12 most intense multiply charged ions (z ≥ 2) were sequentially isolated and fragmented with collisional induced dissociation. The presence of HexNAc oxonium ions (mass to charge ratio 203, 101.5, and 67.67) will trigger the acquisition of an electron transfer dissociation fragmentation spectrum MS3 of the precursor ions. Raw data files were converted to Mascot Generic Format. The Mascot Generic Format files were searched against the UniProt, National Center for Biotechnology Information, and common MS contaminant database using Mascot Software (version 2.4.1). The tolerance for MS1 and MS2 error is 1 D and 0.5 D, respectively. Caramidomethylation (+57 D) was added as fixed modification, whereas Oxidation (M) and O-GlcNAc (S/T) were added as variable modification. A maximum of 2 trypsin miss cleavages was allowed. The mass spectrometry proteomics data obtained in this study have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD008285.
Determination of UDP-GlcNAc by Ultra High Performance LC-MS
Seedlings (500 mg) of winter wheat (JD1) vernalized for 0, 3, 7, 10, 14, 21, 28, and 35 d respectively were frozen in liquid nitrogen and stored at −70°C. Frozen samples were ground into power, and the resultant powder was transferred to 0.5 ml of 75% (v/v) ice-cold ethanol. The extract was vortexed and centrifuged at 16,000g for 10 min at 4°C to remove large chunks of debris. The supernatant collected was filtered through a 0.44 μm filter (Millipore). Extracts (20 μl injections) were separated using an Agilent 1290 Infinity ultra high performance LC system consisting of a binary pump, an autosampler, and a thermostatted column compartment. The chromatography was performed using an X BridgeTM HILIC column from Agilent Technologies (2.1 × 150 mm, 5 μm). The mobile phases consisted of H2O (A) and acetonitrile (B). The UHPLC eluting conditions were optimized as follows: 95% B (0–5 min), 75% B (5–10 min), 55% B (10–15 min), and 95% B (15–20 min). The flow rate was 0.4 mL/min. The column was maintained at 30°C. The UDP-GlcNAc standard (Sigma) was used to determine UDP-GlcNAc concentration and composition in seedlings extracts. Mass spectrometry was performed using an Agilent 6540 Q-TOF equipped with electrospray ionization (Rotini et al., 2018) source operating in negative ion mode. The nebulization gas was set to 35 psi. The drying gas was set to 10 L/min at temperature of 350°C; the sheath gas was set to 11 L/min at temperature of 350°C. The capillary voltage was set to 3500 V. The Q-TOF acquisition rate was set to 0.5 s.
Western Blot Analysis
Total proteins were extracted from wheat plumules in homogenization buffer (20 mm Tris-HCl [pH 8.0], 150 mm NaCl, 1 mm EDTA, 10% [v/v] glycerol, 0.2% [v/v] Triton X-100, 1 mm phenylmethylsulfonyl fluoride, Protease inhibitor cocktail) and quantified by Bradford assay, then separated by denaturing polyacrylamide electrophoresis on 4–12% SDS-PAGE gels and electro-blotted onto polyvinylidene difluoride membranes. Phosphorylated proteins were detected using antibody Phos-tag-Biotin (BTL-111S1, wako) in Tris-buffered saline with Tween 20 buffer with 5% (v/v) BSA at 1/1,000 dilution, and O-GlcNAcylated proteins were detected using antibody CTD110.6 (9875S, CST) in Tris-buffered saline with Tween 20 buffer with 5% (v/v) BSA at 1/2,000 dilution. Stabilized streptandin-horseradish peroxidase Conjugate (Thermo Fisher Scientific) and horseradish peroxidase-Goat anti Mouse IgM (Proteintech) were used for secondary detection at 1/10,000 and 1/1,000 dilution, respectively, and Supersignal West Dura Substrate was used for signal detection.
Total RNA Extraction and Real Time-Quantitative PCR
Total RNA of V0, V7, V14, V21, and V28 wheat plumules was extracted using a TRIzol RNA extraction kit according to the user manual (Invitrogen). Total RNA was treated with DNase I (Fermentas), and then 2 μg RNA was used to synthesize complementary DNA using Avian Myeloblastoma Virus (AMV) Reverse Transcriptase (Promega). Complementary DNA was diluted 30-fold to be used as template for real time-quantitative PCR (RT-qPCR) analysis. RT-qPCR analyses were performed on an Mx3000P (Stratagene) Real-Time PCR System using the SYBR Green Master Mix (TOYOBO) according to the manufacturer’s instructions. The expression levels of the samples were normalized to that of Actin. The gene-specific primers used for RT-qPCR are described in Supplemental Table S6.
O-GlcNAcylation Assay in Vitro
The O-GlcNAcylation assay in vitro with some modification was used as previously described (Xing et al., 2018). Recombinant expressed His-SEC△N (2 μg) was incubated with 8 μg His-GAPD, 20 μg His-Enolase, 5 μg His-FBA, and 5 μg His-FBA-m, respectively, and 50 μm UDP-GlcNAc in 50 μL of reaction system for 1 h at 37°C. The reaction buffer contained 12.5 mm MgCl2, 50 mm Tris–HCl (pH 7.5), and 1 mm dithiothreitol (pH 7.5). After reaction, the mix were denatured at 95°C for 15 min in 5× loading buffer (100 mm Tris–HCl [pH 6.8], 4% [w/v] SDS, 20% [v/v] glycerol, 200 mm dithiothreitol, and 0.2% [w/v] bromophenol blue) and electrophoresed by SDS–PAGE. The antibody CTD110.6 specific to O-GlcNAc sites was used to detect O-GlcNAc modification of proteins in immunoblot analysis.
RNA-EMSA
Biotin-tagged RNA probe was synthesized by Invitrogen. RNA-EMSA was performed according to the kit instructions (Pierce). Purified RNase-free GST-TaGRP2 and GST-TaGRP2m were used. The probe sequence is listed in Supplemental Table S6.
Statistical Analyses
Statistical differences were assessed by one-way ANOVA. Different letters in graphs indicate the significant treatment difference at p-value < 0.05.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Enolase (AGH20062.1), FBA (EMS58841.1), GAPD (EMS68847.1), and TaGRP2 (BAF30986.1).
Supplemental Data
The following supplemental information is available:
Supplemental Figure S1. Detection of global O-GlcNAcylated proteins in different vernalized-winter-wheat with or without PUGNAc treatment by immunoprecipitation, Actin serves as loading control.
Supplemental Figure S2. Alteration of O-GlcNAc signaling affects vernalization accelerated flowering transition in winter wheat JD1, bar = 0.5 mm.
Supplemental Figure S3. Dynamic phosphorylated proteins during vernalization.
Supplemental Figure S4. Biological function enrichment of alternative phosphorylated proteins during vernailization.
Supplemental Figure S5. Sequence alignment of GRP2 protein in wheat and its homologs in other species.
Supplemental Figure S6. Phylogenetic tree of GRP2 proteins based on the alignment analysis of TaGRP2 and its homologs in these species.
Supplemental Figure S7. Sequencing identification of mutated nucleotide to cause an encoding amino acid change (T17 to A17, S87 to A87 and S152 to A152) in GRP2 used in the RNA-EMSA.
Supplemental Figure S8. TaGRP2 regulated vernalization inhibited flowering transition in winter wheat.
Supplemental Figure S9. KEGG analysis of O-GlcNAcylated proteins in metabolism processing.
Supplemental Table S1. Details of O-GlcNAcylated peptides in nonvernalized wheat.
Supplemental Table S2. Details of O-GlcNAcylated peptides in vernalized wheat.
Supplemental Table S3. 124 phosphoproteins which are significant changes in phosphorylation level (SCPL) between vernalization and nonvernalization.
Supplemental Table S4. Details of O-GlcNAcylated proteins homologous in rice and Arabidopsis.
Supplemental Table S5. The O-GlcNAc modified proteins are consistent with the potential interactors of SEC (O-GlcNActransferase) detected in Arabidopsis.
Supplemental Table S6. The list of the primers used in RT-qPCR and RNA-EMSA.
Acknowledgments
We thank Zhuang Lu for her MS analysis.
Footnotes
This work was supported by The National Key Research and Development Program of China (2016YFD0101004) and the China Postdoctoral Science Foundation.
Articles can be viewed without a subscription.
References
- Banerjee PS, Lagerlöf O, Hart GW (2016) Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med 51: 1–15 [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167 [DOI] [PubMed] [Google Scholar]
- Butkinaree C, Park K, Hart GW (2010) O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K, Bao SL, Xu T, Tan KH, Liang TB, Zeng JZ, Huang HL, Xu J, Xu ZH (1998) Functional analysis of the ver gene using antisense transgenic wheat. Physiol Plant 102: 87–92 [DOI] [PubMed] [Google Scholar]
- Copeland RJ, Bullen JW, Hart GW (2008) Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab 295: E17–E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ES, Finnegan EJ, Bilodeau P, Chaudhury A, Genger R, Helliwell CA, Sheldon CC, Bagnall DJ, Peacock WJ (1996) Vernalization and the initiation of flowering. Semin Cell Dev Biol 7: 441–448 [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97: 968–975 [Google Scholar]
- Gambetta MC, Oktaba K, Müller J (2009) Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science 325: 93–96 [DOI] [PubMed] [Google Scholar]
- Gauley A, Boden SA (2019) Genetic pathways controlling inflorescence architecture and development in wheat and barley. J Integr Plant Biol 61: 296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinez C, Losfeld ME, Cacan R, Michalski JC, Lefebvre T (2006) Modulation of HSP70 GlcNAc-directed lectin activity by glucose availability and utilization. Glycobiology 16: 22–28 [DOI] [PubMed] [Google Scholar]
- Guo X, Liu D, Chong K (2018) Cold signaling in plants: Insights into mechanisms and regulation. J Integr Plant Biol 60: 745–756 [DOI] [PubMed] [Google Scholar]
- Gupta R, Brunak S (2002) Prediction of glycosylation across the human proteome and the correlation to protein function. Pac Symp Biocomput 7: 310–322 [PubMed] [Google Scholar]
- Hanover JA, Krause MW, Love DC (2010) The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta 1800: 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80: 825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Shindo C, Dean C (2003) The need for winter in the switch to flowering. Annu Rev Genet 37: 371–392 [DOI] [PubMed] [Google Scholar]
- Hu P, Shimoji S, Hart GW (2010) Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett 584: 2526–2538 [DOI] [PubMed] [Google Scholar]
- Huan Q, Mao Z, Zhang J, Xu Y, Chong K (2013) Transcriptome-wide analysis of vernalization reveals conserved and species-specific mechanisms in Brachypodium. J Integr Plant Biol 55: 696–709 [DOI] [PubMed] [Google Scholar]
- Huan Q, Mao Z, Chong K, Zhang J (2018) Global analysis of H3K4me3/H3K27me3 in Brachypodium distachyon reveals VRN3 as critical epigenetic regulation point in vernalization and provides insights into epigenetic memory. New Phytol 219: 1373–1387 [DOI] [PubMed] [Google Scholar]
- Kaasik K, Kivimäe S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptáček LJ, Fu Y-H (2013) Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab 17: 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ. (2015) The utilities of chemical reactions and molecular tools for O-GlcNAc proteomic studies. ChemBioChem 16: 1397–1409 [DOI] [PubMed] [Google Scholar]
- Kippes N, Debernardi JM, Vasquez-Gross HA, Akpinar BA, Budak H, Kato K, Chao S, Akhunov E, Dubcovsky J (2015) Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci USA 112: E5401–E5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppolu R, Schnurbusch T (2019) Developmental pathways for shaping spike inflorescence architecture in barley and wheat. J Integr Plant Biol 61: 278–295 [DOI] [PubMed] [Google Scholar]
- Leney AC, El Atmioui D, Wu W, Ovaa H, Heck AJR (2017) Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc Natl Acad Sci USA 114: E7255–E7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BA, Hanover JA (2014) O-GlcNAc and the epigenetic regulation of gene expression. J Biol Chem 289: 34440–34448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shi Y, Yang S (2018) Insights into the regulation of C-repeat binding factors in plant cold signaling. J Integr Plant Biol 60: 780–795 [DOI] [PubMed] [Google Scholar]
- Liu Y, Dai S, Xing L, Xu Y, Chong K (2015) O-linked beta-N-acetylglucosamine modification and its biological functions. Sci Bull (Beijing) 60: 1055–1061 [Google Scholar]
- Macauley MS, Vocadlo DJ (2010) Increasing O-GlcNAc levels: An overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta 1800: 107–121 [DOI] [PubMed] [Google Scholar]
- Maruyama K, Urano K, Yoshiwara K, Morishita Y, Sakurai N, Suzuki H, Kojima M, Sakakibara H, Shibata D, Saito K, Shinozaki K, Yamaguchi-Shinozaki K (2014) Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol 164: 1759–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minorsky PV. (2002) Vernalization: The flower school. J Biosci 27: 79–83 [DOI] [PubMed] [Google Scholar]
- Nagel AK, Ball LE (2014) O-GlcNAc transferase and O-GlcNAcase: achieving target substrate specificity. Amino Acids 46: 2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635–648 [DOI] [PubMed] [Google Scholar]
- Olszewski NE, West CM, Sassi SO, Hartweck LM (2010) O-GlcNAc protein modification in plants: Evolution and function. Biochim Biophys Acta 1800: 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2013) Sensing the environment: Key roles of membrane-localized kinases in plant perception and response to abiotic stress. J Exp Bot 64: 445–458 [DOI] [PubMed] [Google Scholar]
- Ozcan S, Andrali SS, Cantrell JEL (2010) Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta 1799: 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Song C-P, Wang B, Zhou J, Kangasjärvi J, Zhu J-K, Gong Z (2018) Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60: 805–826 [DOI] [PubMed] [Google Scholar]
- Qüesta JI, Song J, Geraldo N, An H, Dean C (2016) Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science 353: 485–488 [DOI] [PubMed] [Google Scholar]
- Radermacher PT, Myachina F, Bosshardt F, Pandey R, Mariappa D, Müller HA, Lehner CF (2014) O-GlcNAc reports ambient temperature and confers heat resistance on ectotherm development. Proc Natl Acad Sci USA 111: 5592–5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotini A, Martínez-Sarrà E, Pozzo E, Sampaolesi M (2018) Interactions between microRNAs and long non-coding RNAs in cardiac development and repair. Pharmacol Res 127: 58–66 [DOI] [PubMed] [Google Scholar]
- Shimoji S, Park K, Hart GW (2010) Dynamic ccrosstalk between GlcNAcylation and phosphorylation: roles in signaling, transcription and human disease. Curr Signal Transduct Ther 5: 25–40 [Google Scholar]
- Shindo C, Sasakuma T (2002) Genes responding to vernalization in hexaploid wheat. Theor Appl Genet 104: 1003–1010 [DOI] [PubMed] [Google Scholar]
- Silverstone AL, Tseng T-S, Swain SM, Dill A, Jeong SY, Olszewski NE, Sun TP (2007) Functional analysis of SPINDLY in gibberellin signaling in Arabidopsis. Plant Physiol 143: 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DAR, Syrzycka M, Macauley MS, Rastgardani T, Komljenovic I, Vocadlo DJ, Brock HW, Honda BM (2009) Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc Natl Acad Sci USA 106: 13427–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Zhang K, Wu J, Yang X (2015) O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett 356(2 Pt A): 244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson C, Housley MP, Hart GW (2006) O-GlcNAc cycling: How a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem 97: 71–83 [DOI] [PubMed] [Google Scholar]
- Thingholm TE, Jensen ON, Larsen MR (2009) Analytical strategies for phosphoproteomics. Proteomics 9: 1451–1468 [DOI] [PubMed] [Google Scholar]
- Trapannone R, Rafie K, van Aalten DM (2016) O-GlcNAc transferase inhibitors: current tools and future challenges. Biochem Soc Trans 44: 88–93 [DOI] [PubMed] [Google Scholar]
- Trevaskis B. (2010) The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct Plant Biol 37: 479–487 [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laarse SAM, Leney AC, Heck AJR (2018) Crosstalk between phosphorylation and O-GlcNAcylation: Friend or foe. FEBS J 285: 3152–3167 [DOI] [PubMed] [Google Scholar]
- Wang Y. (2013) O-GlcNAc transferase and its inhibitors. Acta Chimi Sin 71: 1477–1487 [Google Scholar]
- Wang B, Wei J, Song N, Wang N, Zhao J, Kang Z (2018) A novel wheat NAC transcription factor, TaNAC30, negatively regulates resistance of wheat to stripe rust. J Integr Plant Biol 60: 432–443 [DOI] [PubMed] [Google Scholar]
- Wang Z, Gucek M, Hart GW (2008) Cross-talk between GlcNAcylation and phosphorylation: Site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA 105: 13793–13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Hart GW (2003) O-GlcNAc turns twenty: Functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett 546: 154–158 [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291: 2376–2378 [DOI] [PubMed] [Google Scholar]
- Wilson A, Dean C (1996) Analysis of the molecular basis of vernalization in Arabidopsis thaliana. Semin Cell Dev Biol 7: 435–440 [Google Scholar]
- Xiao J, Xu S, Li C, Xu Y, Xing L, Niu Y, Huan Q, Tang Y, Zhao C, Wagner D, Gao C, Chong K (2014) O-GlcNAc-mediated interaction between VER2 and TaGRP2 elicits TaVRN1 mRNA accumulation during vernalization in winter wheat. Nat Commun 5: 4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Li J, Xu Y, Xu Z, Chong K (2009) Phosphorylation modification of wheat lectin VER2 is associated with vernalization-induced O-GlcNAc signaling and intracellular motility. PLoS One 4: e4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Liu Y, Xu S, Xiao J, Wang B, Deng H, Lu Z, Xu Y, Chong K (2018) Arabidopsis O-GlcNAc transferase SEC activates histone methyltransferase ATX1 to regulate flowering. EMBO J 37: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chong K (2018) Remembering winter through vernalisation. Nat Plants 4: 997–1009 [DOI] [PubMed] [Google Scholar]
- Xu S-L, Chalkley RJ, Wang Z-Y, Burlingame AL (2012) Identification of O-linked β-D-N-acetylglucosamine-modified proteins from Arabidopsis. Methods Mol Biol 876: 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S-L, Chalkley RJ, Maynard JC, Wang W, Ni W, Jiang X, Shin K, Cheng L, Savage D, Huhmer AFR, Burlingame AL, Wang Z-Y (2017) Proteomic analysis reveals O-GlcNAc modification on proteins with key regulatory functions in Arabidopsis. Proc Natl Acad Sci U S A 114: E1536–E1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WZ, Wang X, Feng Q, Zhang L, Liu YG, Han B, Chong K, Xu ZH, Tan KH (2004) TheVER2 promoter contains repeated sequences and requires vernalization for its activity in winter wheat (Triticum aestivum L.). Chin Sci Bull 49: 355–362 [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong WD, Chong K, Liang TB, Xu ZH, Tan KH, Zhu ZQ (1999) Cloning and characterization of vernalization-related gene (ver203F) cDNA 3 ' end. Chin Sci Bull 44: 1289–1294 [Google Scholar]
- Yong WD, Xu YY, Xu WZ, Wang X, Li N, Wu JS, Liang TB, Chong K, Xu ZH, Tan KH, Zhu ZQ (2003) Vernalization-induced flowering in wheat is mediated by a lectin-like gene VER2. Planta 217: 261–270 [DOI] [PubMed] [Google Scholar]
- Yu Q, An L, Li W (2014) The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep 33: 203–214 [DOI] [PubMed] [Google Scholar]
- Yuan W, Luo X, Li Z, Yang W, Wang Y, Liu R, Du J, He Y (2016) A cis cold memory element and a trans epigenome reader mediate Polycomb silencing of FLC by vernalization in Arabidopsis. Nat Genet 48: 1527–1534 [DOI] [PubMed] [Google Scholar]
- Yun M, Wu J, Workman JL, Li B (2011) Readers of histone modifications. Cell Res 21: 564–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW (2004) O-GlcNAc modification: A nutritional sensor that modulates proteasome function. Trends Cell Biol 14: 218–221 [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW (2006) Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761: 599–617 [DOI] [PubMed] [Google Scholar]
- Zeidan Q, Hart GW (2010) The intersections between O-GlcNAcylation and phosphorylation: Implications for multiple signaling pathways. J Cell Sci 123: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R, Hu J, Hsieh W-P, Matsumoto PA, Dawdy A, Barnhill B, Oldenhof H, Hartweck LM, Maitra S, Thomas SG, et al. (2016) O-GlcNAcylation of master growth repressor DELLA by SECRET AGENT modulates multiple signaling pathways in Arabidopsis. Genes Dev 30: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo W, Zhao Y, Xu Y, Song S, Chong K (2016) Comparative metabolomic analysis reveals a reactive oxygen species-dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol 211: 1295–1310 [DOI] [PubMed] [Google Scholar]
- Zhao DZ, Chen M, Chong K, Wan L, Huang HL, Tan KH (1998) Isolation of a vernalization-related cDNA clone (VRC) using mRNA differential display in winter wheat. Chin Sci Bull 43: 1201–1205 [Google Scholar]
- Zhong K, Tan KH, Huang HL, Liang HG (1995) Molecular-cloning of a cDNA related to vernalization (VERC203) in winter-wheat. Sci China B Chem Life Sci Earth Sci 38: 799–806 [Google Scholar]
- Zhou J-X, Liu Z-W, Li Y-Q, Li L, Wang B, Chen S, He X-J (2018) Arabidopsis PWWP domain proteins mediate H3K27 trimethylation on FLC and regulate flowering time. J Integr Plant Biol 60: 362–368 [DOI] [PubMed] [Google Scholar]