Abstract
Objective:
Conventional, breath-holding magnetic resonance imaging (MRI) assesses body composition by measuring fat volumes and proton density fat fraction (PDFF). However, breath-holding MRI is not always feasible in children. This study’s objective was to use free-breathing MRI to quantify visceral and subcutaneous fat volumes and PDFFs and correlate these measurements with hepatic PDFF.
Methods:
This was an observational, hypothesis-forming study that enrolled two groups of children (ages 6–17 years), healthy children and overweight children with presumed non-alcoholic fatty liver disease. Free-breathing MRI was used to measure visceral and subcutaneous fat volumes and PDFFs, and hepatic PDFF. Imaging biomarkers were compared between groups, and correlations coefficients (r) and coefficients of determination (R2) were calculated.
Results:
When compared to the control group (n=10), the overweight group (n=9) had greater mean visceral (1843 vs. 329 cm3, p<0.001) and subcutaneous fat volumes (7663 vs. 893 cm3, p<0.001), as well as greater visceral (80 vs. 45%, p<0.001) and subcutaneous fat PDFFs (89 vs. 75%, p=0.003). Visceral fat volume (r=0.79, p<0.001) and PDFF (r=0.92, p<0.001) correlated with hepatic PDFF. In overweight subjects, for each unit increase in visceral fat PDFF, hepatic PDFF increased by 2.64%; visceral fat PDFF explained 54% of hepatic PDFF variation (R²=0.54, p=0.02).
Conclusion:
In this study, we used free-breathing MRI to measure body composition in children. Future studies are needed to investigate the possible value of subcutaneous and visceral fat PDFFs, and validate free-breathing MRI body composition biomarkers.
Keywords: children, obesity, body composition, liver fat, magnetic resonance imaging
Introduction
Pediatric obesity is linked to non-alcoholic fatty liver disease (NAFLD), cardiovascular disease, and insulin resistance (1–4). Body composition, specifically visceral, subcutaneous, and ectopic fat, are critical to understanding metabolic diseases (4, 5). Overweight children with NAFLD have large amounts of visceral fat; visceral fat secretes fatty acids and adipokines that stimulate hepatic triglyceride synthesis causing fat accumulation (or steatosis) (1, 4). Body mass index and waist circumference are correlated with obesity and NAFLD (3, 5, 6). However, these anthropometrics fail to accurately capture body composition (7). Ultrasound can assess body composition, but it is operator dependent and lacks standardization (6, 8–9). Since ultrasound is unreliable in obese patients and deep body regions, the sensitivity and specificity for quantifying hepatic fat is low (9). Other imaging modalities expose children to radiation (i.e., dual-energy X-ray absorptiometry and computerized tomography), or do not measure hepatic fat (i.e., dual-energy X-ray absorptiometry).
Conventional magnetic resonance imaging (MRI) separates visceral and subcutaneous fat volumes and quantifies hepatic proton density fat fraction (PDFF), a well-known MR-biomarker for steatosis that correlates with liver histology (9–14). PDFF reflects tissue fat content by quantifying the ratio of mobile protons from fat to total mobile protons from fat and water. To avoid motion artifacts, conventional abdominal MRI requires suspension of respiration. Because some children cannot breath-hold, sedation is prescribed. To eliminate breath-holding or sedation, we developed a free-breathing MRI technique to quantify hepatic PDFF. When compared to standard breath-holding techniques, this new technique accurately (concordance coefficient >0.99) and consistently (mean difference within repeated scans=0.25%) measured hepatic PDFF with high image quality (14). Our reseach goal was to further extend the capabilities of free-breathing MRI to assess not only hepatic PDFF and visceral and subcutaneous fat volumes, but also visceral and subcutaneous fat PDFFs, complementary, but distinct measurements to fat volumes that have not been throughly explored. Accordingly, this study’s objective was to determine the feasibility of using free-breathing MRI to quantify visceral and subcutaneous fat volumes and PDFFs, and correlate these measurements with hepatic PDFF and anthropometrics. We hypothesized that visceral fat volume and PDFF would positively correlate with hepatic PDFF, and explain some variation in hepatic PDFF.
Methods
Study Population
This was an observational, case-control, hypothesis-forming study. The primary outcome was visceral fat volume. Secondary outcomes were subcutaneous fat volume, visceral and subcutaneous fat PDFFs, and hepatic PDFF. Two cohorts were recruited; healthy children and overweight children. Inclusion criteria for the control group included 6–17 years of age and a normal weight (body mass index (BMI) 5th-85th percentile). Inclusion criteria for the overweight group included 6–17 years of age, overweight (BMI ≥85th percentile), and presumed NAFLD. The presence of NAFLD was assumed by a serum alanine aminotransferase concentration > 22 IU/L for girls and >26 IU/L for boys when other liver diseases were excluded (3). Subjects were recruited from the Pediatric Continuity Clinic and Fit for Healthy Weight Program at the University of California Los Angeles. Exclusion criteria for both cohorts included major congenital anomalies, diseases or infections known to affect the liver, inborn errors of metabolism, metallic materials in the body, claustrophobia, and pregnancy. Written informed consent from a parent/legal guardian and assent from subjects, if appropriate, was obtained. The University of California Los Angeles institutional review board approved the study.
Prior to the MRI exam, a research coordinator measured subjects’ weight, height, waist circumference, and triceps and subscapular skinfold thickness twice. Z-scores were calculated using Center for Disease Control means and standard deviations for weight, height, and BMI (15). Triceps and subscapular measurements were plotted using the anthropometric reference data from the Center for Disease Control (15). Demographics, co-morbidities associated with being overweight (3), and laboratory values (alanine and aspartate aminotransferases, hemoglobin A1C, lipid panels) were collected from the medical chart.
Free-Breathing Radial MRI Acquisition
Our outpatient Magnetic Resonance Research Center has two 3 T scanners (MAGNETOM Skyra or Prisma, Software version VE11, Siemens Healthineers, Erlangen, Germany). MRI exams were performed on these scanners. Subjects were given hearing protection. Our free-breathing MRI fat quantification technique used a 3D stack-of-radial sampling trajectory with golden-angle ordering to suppress motion artifacts and enable liver imaging in a fixed scan time of <5 min (14, 16, 17). Free-breathing radial MR images were acquired fully-sampled without respiratory-gating or self-navigation using an RF-spoiled bipolar multi-echo gradient echo sequence with six echo times, initial echo time of 1.23ms, echo spacing of 1.23ms, repetition time of 8.85ms, isotropic in-plane resolution of 1.67×1.67 mm2 to 1.94×1.94 mm2, slice thickness of 5mm, bandwidth of 1080–1160Hz/pixel, and flip angle of 5° (14). This was a research prototype sequence, which operated in accordance with the Food and Drug Administration guidelines. All images were acquired using spine array and body matrix array coils. All data were used for image reconstruction.
To enable 3D segmentation for abdominal body composition without interpolation or extrapolation, contiguous abdominal MR images were acquired in the axial orientation to encompass the 3D liver volume. Images were reconstructed and used to calculate 3D maps of PDFF (0–100%) based on a seven-peak fat model and single effective R2* per voxel (17–21).
Free-Breathing Radial MRI Quantification of Tissue Volumes and PDFF Values to Characterize Body Composition
To measure visceral and subcutaneous fat volumes (cm3) and PDFFs (%) for each subject, 3D PDFF maps of the abdomen were analyzed in Horos version 3 (The Horos Project, horosproject.org; Horos is a free and open source code software program that is distributed free of charge under the LGPL license at Horosproject.org and sponsored by Nimble Co LLC d/b/a Purview in Annapolis, MD USA). The “2D segmentation grow region” tool was used on each axial MRI slice between the level of the diaphragm and iliac crest to assist with the delineation of visceral and subcutaneous fat. Visceral fat volume was defined as accumulation of fat in the intra-abdominal area excluding blood vessels, bones, bowels, intramuscular fat, and organs (22). When segmenting visceral fat, the reference anatomical images (T2-weighted MRI) and 3D PDFF maps were used together to include voxels with >0% PDFF. Subcutaneous fat volume was defined as fat beneath the skin and above the muscle fascia including mammary fat (22). For each subject, the visceral and subcutaneous fat PDFFs was reported as mean ± standard deviation of the PDFF of all voxels in the compartment of interest as segmented across all slices in the 3D MRI data. An experienced pediatric radiologist (> 10 years experience) reviewed the segmented regions of interest for visceral and subcutaneous fat and requested changes if required. The volume was computed by summing the pixel counts of the segmented regions and multiplying by the pixel resolution with equal weight for any fat accumulation.
Free-Breathing Radial MRI Quantification of Hepatic Fat
To quantify hepatic steatosis, the same 3D PDFF maps were analyzed in OsiriX version 6.0 (Pixmeo Sarl, Bernex, Switzerland). Using five 5 mm slices, a 25 mm × 25 mm × 25 mm cubic region of interest was delineated in the right liver lobe that avoided blood vessels and bile ducts to obtain a single hepatic PDFF measurement for each subject.
Statistical Analysis
Baseline characteristics and MRI measurements were presented as number of observations (%) or means ± standard deviations. For categorical variables, differences between groups were determined by Fisher’s exact test. For continuous variables, differences between groups were determined by Student’s t-test or Mann-Whitney U test depending on normality. Pearson correlations (r) were used to estimate associations between MRI measurements and risk factors and predictors of obesity/NAFLD (anthropometric measurements and laboratory studies) (2–4). Coefficients of determination (R2) were used to measure how close the data are fitted to the regression line. Specifically, R2 was used to quantify the proportion of variation in hepatic PDFF that could be explained by visceral or subcutaneous fat volumes and PDFFs. Statistical analysis was performed with Stata Software (version 14.1 College Station, Texas). A p-value < 0.05 was considered statistically significant.
Results
Demographics
From November 30, 2016 to May 19, 2017, ten control and ten overweight subjects were enrolled. One overweight subject (BMI > 99th percentile) was unable to complete the study because the MRI field-of-view was not large enough to accommodate his/her abdominal girth despite a body weight less than the MRI table weight limit. Thus, data from 19 subjects were included in the analysis. When compared to the control group, the overweight group was older (14.4±2.2 years vs. 11.5±2.7 years, p=0.02). Eight of the nine overweight subjects were obese (BMI >95th percentile) as indicated by greater anthropometric measurements when compared to the control group (p<0.001 for all) (Table I). Overweight subjects had elevated serum alanine and aspartate aminotransferase concentrations at 82.7±60.5 IU/L and 44.4±25.7 IU/L, respectively. In the overweight group, hemoglobin A1C was within normal range (5.6±0.4%).
Table I.
Subject Characteristics
| Control (n=10) | Overweight (n=9) | p-value | |
|---|---|---|---|
| Age, years | 11.5 ± 2.7 | 14.4 ± 2.2 | 0.02 |
| Race | |||
| White | 4 (40%) | 9 (100%) | 0.01 |
| Asian | 4 (40%) | 0 (0%) | |
| More than one race | 2 (20%) | 0 (0%) | |
| Ethnicity – Hispanic | 2 (20%) | 6 (67%) | 0.07 |
| Sex – Male | 6 (60%) | 7 (78%) | 0.63 |
| Body Mass Index z-score | −0.21 ± 0.47 | 3.74 ± 1.35 | <0.001 |
| Weight z-score | −0.04 ± 0.74 | 3.42 ± 1.55 | <0.001 |
| Height z-score | 0.21 ± 1.42 | 0.37 ± 1.01 | 0.87 |
| Subscapular skinfold percentile | |||
| < 10% | 1 (10%) | 0 (0%) | 0.001 |
| ≥10% and ≤90% | 9 (90%) | 2 (22%) | |
| > 90% | 0 (0%) | 7 (78%) | |
| Tricep skinfold percentile | |||
| < 10% | 0 (0%) | 0 (0%) | <0.001 |
| ≥10% and ≤90% | 10 (100%) | 2 (22%) | |
| > 90% | 0 (0%) | 7 (78%) | |
| Waist circumference (cm) | 64 ± 8 | 109 ± 16 | <0.001 |
| Waist-to-height ratio | 0.43 ± 0.04 | 0.66 ± 0.05 | <0.001 |
| Hypertension | 0 (0%) | 0 (0%) | 1 |
| Insulin resistance | 0 (0%) | 5 (56%) | 0.01 |
| Dyslipidemia | 1 (10%) | 0 (0%) | 1 |
| Family history of metabolic diseasesa | 4 (40%) | 7 (78%) | 0.17 |
| Subcutaneous Fat Volume, cm3 | 893 ± 397 | 7663 ± 3325 | p<0.001 |
| Visceral Fat Volume, cm3 | 329 ± 96 | 1843 ± 937 | p<0.001 |
| Subcutaneous PDFF, % | 75.3 ± 14.9 | 89.0 ± 3.9 | 0.003 |
| Visceral PDFF, % | 44.8 ± 11.2 | 80.2 ± 3.6 | p<0.001 |
| Hepatic PDFF, % | 2.6 ± 0.8 | 21.9 ± 13.0 | p<0.001 |
Data are presented as mean ± SD or n (%).
Family history of metabolic diseases was defined as having at least one of the following: diabetes, hypertension, obesity, dyslipidemia, coronary artery disease, or NAFLD.
Mean Visceral and Subcutaneous Fat Volume and PDFF Values
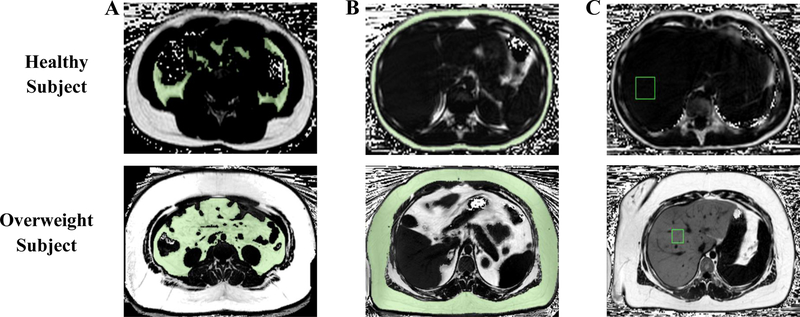
Representative free-breathing MRIs are provided in Figure 1. As expected, visceral and subcutaneous fat volumes were 460% and 758% greater in the overweight group vs. the control group (p<0.001 for all). Similarly, visceral and subcutaneous fat PDFFs were 80% and 18% greater in the overweight group when compared to the control group (p<0.05 for all). Last, hepatic PDFF for the overweight group was 742% greater than the control group (p<0.001) (Table 1). The supplemental figure depicts each subjects mean visceral and subcutaneous fat PDFFs.
Figure I.
Representative free-breathing MRIs used to measure A) visceral fat volume, B) subcutaneous fat volume and C) hepatic proton density fat fraction (PDFF). White pixels represent fat (PDFF=100%); black pixels represent water (PDFF=0%); grey pixels represent a mix of water and fat. The green contour represents the regions included in the visceral and subcutaneous fat volume quantifications. The regions of interest are shown for hepatic PDFF with a green box as an example of how liver regions were used to calculate hepatic PDFF.
Body Composition and Hepatic PDFF Correlations
Visceral and subcutaneous fat volumes (r=0.79 and r=0.75, p<0.001 for both, respectively) and PDFFs (r=0.92 and 0.66, p<0.001 and 0.05, respectively) showed significant, positive correlations with hepatic PDFF. Visceral fat PDFF demonstrated the highest correlation with hepatic PDFF (r=0.92, p<0.001) when compared to other MRI biomarkers (r=0.66–0.79, p<0.001). Visceral and subcutaneous fat volumes correlated with waist circumference (r=0.97, p<0.001 and r=0.98, p<0.001, respectively) (Table II).
Table II.
Correlation Coefficients (r) for Visceral and Subcutaneous Fat Volumes and PDFFs with Hepatic PDFF and Anthropometric Measurements
| Visceral Fat Volume | Subcutaneous Fat Volume | Hepatic PDFF | |
|---|---|---|---|
| Hepatic PDFF, % | 0.79** | 0.75** | - |
| Visceral fat PDFF, % | 0.75** | 0.76** | 0.92** |
| Subcutaneous fat PDFF, % | 0.64* | 0.71* | 0.66* |
| Alanine aminotransferase, IU/La | 0.03 | −0.43 | 0.06 |
| Aspartate aminotransferase, IU/La | −0.02 | −0.3 | 0.13 |
| Hemoglobin A1C, %a | 0.54 | 0.44 | 0.43 |
| Total cholesterol, mg/dLa | −0.62 | −0.12 | −0.23 |
| Low-density lipoprotein, mg/dLa | −0.30 | 0.22 | −0.37 |
| High-density lipoprotein, mg/dLa | −0.72* | −0.1 | −0.23 |
| Body mass index z-score | 0.88** | 0.94** | 0.75** |
| Waist circumference (cm) | 0.97** | 0.98** | 0.74** |
| Waist circumference percentile | 0.94** | 0.96** | 0.84** |
| Waist-to-height ratio | 0.89** | 0.92** | 0.79** |
| Subscapular skinfold percentile | 0.89** | 0.92** | 0.79** |
| Tricep skinfold percentile | 0.84** | 0.86** | 0.75** |
Laboratory values were only obtained from the overweight group (n=9 for all laboratory values, except n=8 for hemoglobinA1C).
p<0.05,
p<0.001.
PDFF; proton density fat fraction.
When the overweight cohort was analyzed alone, visceral fat PDFF positively correlated with hepatic PDFF (r=0.72, p=0.03). For each unit increase in visceral fat PDFF, hepatic PDFF increased by 2.64%; 54% of the observed variation in hepatic PDFF was explained by visceral fat PDFF (R2=0.54, p=0.02) (Figure II). Hepatic PDFF could not be explained by visceral fat (R2=0.03, p=0.66) and subcutaneous fat volumes (R2=0.02, p=0.71). In the control group, visceral fat PDFF (R2=0.25, p=0.14) and visceral (R2=0.22, p=0.17) and subcutaneous fat volumes (R2=0.02, p=0.70) did not explain the range of hepatic PDFF measurements.
Figure II.
Coefficient of determinations (R2) for mean visceral fat PDFF and hepatic PDFF for the healthy cohort (represented by circles) and overweight cohort (represented by triangles). PDFF=proton density fat fraction.
Discussion
In this study, we used free-breathing MRI to quantify body composition in healthy and overweight/obese children, and correlate these measurements with hepatic PDFF and well-known risk factors for obesity and NAFLD (2–4). As to be expected, visceral and subcutaneous fat volumes and PDFFs and hepatic PDFF were greater in the overweight group vs. the control group. MRI adiposity measurements and hepatic PDFF were strongly correlated with anthropometrics, particularly waist circumference. While waist circumference is a well-known surrogate for visceral fat, it measures visceral and subcutaneous fat and does not provide any information about fat PDFF (5, 6). The PDFF findings in our study are of interest. Specifically, visceral fat PDFF demonstrated the strongest correlation with hepatic PDFF (r=0.92, p<0.001) in comparison to visceral fat volume (r=0.79, p<0.001) and waist circumference (r=0.74, p<0.001). For each unit increase in visceral fat PDFF, hepatic PDFF increased by 2.64%. Moreover, 54% of the observed variation in hepatic PDFF was explained by visceral fat PDFF.
Some of our study’s results add further insight to the research of pediatric body composition (23, 24). In one study, 2D chemical-shift-encoded 1.5 T MRI with 2 echo times was used to estimate liver fat content, but this technique did not achieve 3D coverage and measured hepatic signal fat fraction, which is not the reference standard for hepatic fat, (i.e., PDFF) (9–14). Moreover, this study only measured subcutaneous and visceral fat area with a single MRI slice (23). In another study, investigators used 3 T MRI to quantify abdominal fat in adolescents using a single T1-weighted MRI slice, but did not achieve 3D coverage or measure hepatic PDFF (24). Potential advantages of our study are that we achieved 3D quantification of visceral and subcutaneous fat volumes and PDFFs and measured hepatic PDFF using multiple slices. This technique may provide more accurate information about body composition and may be used to better understand obesity.
Free-breathing MRI provides information (fat volume and PDFF) that may complement serum liver function tests, the standard screening tests for NAFLD, and liver biopsy, the diagnostic gold standard for NAFLD (3, 25–27). In our study, despite a mean hepatic PDFF of 29% (normal <5.5%), hepatic PDFF did not correlate with alanine aminotransferase concentrations (r=0.06, p=0.08). Alanine aminotransferase has a low sensitivity leading to an underestimation of NAFLD (3, 25, 26). Liver biopsy is an invasive procedure, requires anesthesia, and can be associated with rare, but serious complications. Biopsies are subject to sampling error and observer variability (27–29).
In the MRI Rosetta Stone Project, 174 children had breath-holding MRIs and liver biopsies. Hepatic PDFF correlated with steatosis grade (r=0.73, p<0.01), and the area under the receiver operating characteristic curve for a 5.5% cut-point for grade 0 vs. grade 1 steatosis was 0.81 with a sensitivity and specificity of 74% and 88%, respectively (11). While these results are promising, incomplete breath holds can lead to inaccurate PDFF quantification (14). Conventional MRIs require suspension of respiration for approximately 12–20 seconds. Many young or sick children, or children with disabilities cannot hold their breath or follow instructions. In a recent study by our group, breath-holding MRI exhibited artifacts or misaligned slices from imperfect or failed breath-holds. When breath-holding scans were compared to free-breathing scans in healthy and children with NAFLD, image quality suffered and scans were more likely to be repeated to achieve diagnostic quality. The mean number of repeated breath-held Cartesian scans ± SD was 0.4 ± 0.7 and 0.9 ± 0.9 for healthy subjects and NAFLD subjects, respectively. In contrast, free-breathing radial was only repeated once for a healthy subject and was not repeated for NAFLD subjects (14). For these reasons, sedation, which adds cost and has side effects is prescribed (30). Validation with liver biopsy is required prior to the adoption of free-breathing MRI. As of today, there are very few studies comparing breath-holding MRI to liver biopsy, and no studies comparing free-breathing MRI to biopsy in adults or children (11–13).
While our study did not investigate different types of fat, adipose tissue PDFF may reflect differences in fat content, such as an increase or decrease in brown, beige, and white fat (31). Located in the cervical, supraclavicular, and parasternal areas, brown fat is postulated to protect against obesity and is inversely proportional to BMI (10, 31–34). Uncoupling factor 1, which is located in brown fat’s mitochondria, promotes energy expenditure via lipid metabolism and thermogenesis (32). In contrast, white fat surrounds organs and stores energy. Because brown fat contains smaller lipid droplets than white fat, brown fat is predicted to have a lower PDFF (31). When exposed to cold or other stimulants, white fat can convert to beige fat, which has similar properties to brown fat (32). This process generally occurs in subcutaneous tissue. In this study, we did not measure adiposity volumes or PDFF where brown fat is typically found. Instead, we focused on visceral and subcutaneous fat volumes and PDFFs. Based on our results, we speculate that overweight children with NAFLD may have less beige fat in their visceral and subcutaneous fat. Further research is needed to investigate and validate these potential biomarkers.
We recognize the limitations of our study. MRI machines are not readily accessible by all patients, require children to be stationary in a tightly enclosed space, and generate loud, repetitive noises. Also, quantification of adipose tissue volumes is time consuming. Limitations also include a small sample size, and the overweight cohort was older than the control cohort. Age, sex, and pubertal stage affect body composition (35). There were more Hispanic subjects in the overweight cohort compared to the control cohort. This reflects the well-known epidemiology of obesity and NAFLD in the United States (2). In our study, a single region of interest encompassing a large homogenous region in the right liver lobe was used to measure PDFF. This may not capture potential heterogeneity in hepatic PDFF. Further work should use multiple regions of interest to measure hepatic PDFF. Last, no comparison was made in this preliminary study to conventional MRI techniques. Future studies are needed to assess improvement in image quality and biomarker analyzability of free-breathing MRI vs. conventional methods for body composition analysis.
In conclusion, in this pilot study, we used free-breathing MRI, to not only measure adiposity volumes and hepatic PDFF, but also visceral and subcutaneous fat PDFF. We noted that overweight children with elevated liver function tests have increased visceral and subcutaneous fat PDFF compared to their healthy counterparts, and that visceral fat PDFF was highly correlated with hepatic PDFF, and explained some variation in hepatic PDFF. Additional studies are required to confirm these findings, and decipher how different types of fat (i.e., white, beige, and brown fat) contribute to obesity.
Supplementary Material
Figure, Supplemental Digital Content I. Mean visceral and subcutaneous fat PDFFs (± SD) for each subject in the control cohort and overweight cohort.
What is Known:
Conventional breath-holding magnetic resonance imaging (MRI) can quantify visceral and subcutaneous fat volumes and hepatic proton density fat fraction (PDFF).
Preliminary studies have shown that hepatic PDFF can be quantified with free-breathing MRI.
What is New:
In this observational study, we demonstrated that free-breathing MRI measures visceral and subcutaneous fat PDFFs, along with visceral and subcutaneous fat volumes and hepatic PDFFs.
Visceral and subcutaneous fat volumes and PDFFs were strongly and positively correlated with hepatic PDFF; and visceral fat PDFF explained some variation in hepatic PDFF.
Acknowledgments
Financial Support
Supported by the National Institutes of Health (NIH/NCATS KL2TR000122 [to KLC]. Supported by University of California, Los Angeles Radiology Department Exploratory Research Grant (#16–0002 [to HHW and KLC]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
Dr. Holden Wu and Tess Armstrong receive institutional research support from Siemens Healthineers. However, Siemens did not fund or participate in this study.
References
- 1.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15(4):6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118(4):1388–93. [DOI] [PubMed] [Google Scholar]
- 3.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64(2):319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damaso AR, do Prado WL, de Piano A, et al. Relationship between nonalcoholic fatty liver disease prevalence and visceral fat in obese adolescents. Dig Liver Dis 2008;40(2):132–9. [DOI] [PubMed] [Google Scholar]
- 5.Linge J, Borga M, West J, et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity (Silver Spring) 2018. [DOI] [PMC free article] [PubMed]
- 6.Monteiro PA, Antunes Bde M, Silveira LS, et al. Body composition variables as predictors of NAFLD by ultrasound in obese children and adolescents. BMC Pediatr 2014;14(25):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderwall C, Randall Clark R, Eickhoff J, et al. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr 2017;17(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol 2012;85(1009):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CC, Nguyen P, Hernandez C, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152(3):598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu HH, Chen J, Shen W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. Magma 2016;29(2):259–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015;61(6):1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middleton MS, Heba ER, Hooker CA, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology 2017;153(3):753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton MS, Van Natta ML, Heba ER, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology 2018;67(3):858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong T, Ly KV, Murthy S, et al. Free-breathing quantification of hepatic fat in healthy children and children with nonalcoholic fatty liver disease using a multi-echo 3-D stack-of-radial MRI technique. Pediatric Radiology 2018. [DOI] [PubMed]
- 15.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Health Stat 11 2012252):1–48. [PubMed] [Google Scholar]
- 16.Winkelmann S, Schaeffter T, Koehler T, et al. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging 2007;26(1):68–76. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong T, Dregely I, Stemmer A, et al. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med 2018;79(1):370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong X, Nickel MD, Kannengiesser SA, et al. Liver fat quantification using a multi-step adaptive fitting approach with multi-echo GRE imaging. Magn Reson Med 2014;72(5):1353–65. [DOI] [PubMed] [Google Scholar]
- 19.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging 2012;36(5):1011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoo T, Serai SD, Pirasteh A, et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018;286(2):486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Dimitrov I, Sherry AD, et al. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res 2008;49(9):2055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen W, Liu H, Punyanitya M, et al. Pediatric obesity phenotyping by magnetic resonance methods. Curr Opin Clin Nutr Metab Care 2005;8(6):595–601. [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbein MH, Mogren C, Gleason T, et al. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 2006;42:83–8. [PubMed] [Google Scholar]
- 24.Eloi JC, Epifanio M, de Goncalves MM, et al. Quantification of Abdominal Fat in Obese and Healthy Adolescents Using 3 Tesla Magnetic Resonance Imaging and Free Software for Image Analysis. PLoS One 2017;12(1):e0167625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunde SS, Lazenby AJ, Clements RH, et al. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology 2005;42(3):650–6. [DOI] [PubMed] [Google Scholar]
- 26.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37(6):1286–92. [DOI] [PubMed] [Google Scholar]
- 27.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97(10):2614–8. [DOI] [PubMed] [Google Scholar]
- 28.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20(2):475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ovchinsky N, Moreira RK, Lefkowitch JH, et al. Liver biopsy in modern clinical practice: a pediatric point-of-view. Adv Anat Pathol 2012;19(4):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andropoulos DB, Greene MF. Anesthesia and Developing Brains - Implications of the FDA Warning. N Engl J Med 2017;376(10):905–07. [DOI] [PubMed] [Google Scholar]
- 31.Hu HH, Perkins TG, Chia JM, et al. Characterization of human brown adipose tissue by chemical-shift water-fat MRI. AJR Am J Roentgenol 2013;200(1):177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol 2016;64(5):1176–86. [DOI] [PubMed] [Google Scholar]
- 33.Lowell BB, V SS, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366(6457):740–2. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Enerback S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res 2003;11(10):1182–91. [DOI] [PubMed] [Google Scholar]
- 35.Staiano AE, Broyles ST, Gupta AK, et al. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity (Silver Spring) 2013;21(6):1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content I. Mean visceral and subcutaneous fat PDFFs (± SD) for each subject in the control cohort and overweight cohort.