Abstract
This study compared outcomes following total (TS) or partial splenectomy (PS) among patients with hereditary spherocytosis. Seventy-nine patients (TS=33, PS=46) were identified. The follow-up period was longer after PS (59.6 vs. 24.9 months, p<0.001). Long-term adverse events occurred more frequently following PS (50% vs 29%, p=0.001). Anemia, jaundice, and fatigue recurred in 6 PS patients, leading to 5 completion splenectomies. Hemoglobin was not different between PS and TS by 5 years post-procedure (12.3 vs 13.4 g/dL, p=0.25). Both PS and TS ameliorate symptoms and improve hematologic parameters. The rate of secondary surgery following PS should be considered when planning the initial surgical procedure.
Keywords: hereditary spherocytosis, splenectomy, health outcomes
Introduction
The hereditary spherocytosis (HS) syndromes are a group of genetic disorders associated with defects in erythrocyte membrane proteins, including ankyrin, α- and β-spectrin, band 3, and protein 4.2.1 Membrane loss results in spherically shaped erythrocytes with decreased deformability, leading to hemolysis, increased splenic clearance, and anemia.1,2 Clinical manifestations of HS vary, including chronic hemolytic anemia, jaundice, gallstones, and splenomegaly.3 Patients with HS are susceptible to bilirubin gallstones and hemolytic crises associated with viral infections. HS is the most common inherited anemia in individuals of northern European ancestry where it affects about 1:2000.1
Splenectomy is important for management of HS. Splenectomy is recommended in severe HS, where severe is defined as hemoglobin <8 g/dl or transfusion-dependence and reticulocyte count >10%.1 Splenectomy should be considered in moderate HS, where moderate is defined by hemoglobin 8–12 g/dl, reticulocyte count between 6 and 10%1,4 Regardless of disease severity, the optimal procedure is unclear.
Total splenectomy (TS) is associated with increased susceptibility to overwhelming sepsis from encapsulated bacteria.5,6 Limited data indicate a higher risk of thromboembolic events7,8, increased incidence of atherosclerosis9 and increased mortality due to parasitic red cell infections (e.g. babesiosis, malaria)10 in patients following TS. Alternatively, partial splenectomy (PS) reduces erythrocyte destruction while maintaining residual splenic phagocytic function.11–17 One limitation of PS is possible splenic regrowth, leading to recurrent symptoms and need for a second surgery.11,15 Both procedures can be performed laparoscopically or by open laparotomy. Long-term follow-up data on these patients may aid in decision-making regarding the extent of spleen removed in HS. Here, we describe the clinical outcomes of 79 patients with HS who underwent TS or PS at a single institution.
Methods
For this retrospective review, patients were identified using ICD-9 and ICD-10 procedure codes for surgical splenectomy (e.g., 38120, 38101, and 38100) between 1998–2016 at Boston Children’s Hospital. The review included the earliest documented pre-operative visit to the Hematology Program at Boston Children’s Hospital through January 2017. PS was defined as < 90% splenic removal. Subjects were excluded if data for the analysis were absent or a significant comorbidity was present. We analyzed demographic characteristics, adverse events, administration of immunizations, use of antibiotic prophylaxis, laboratory data, and spleen size by ultrasound. Statistical analysis was performed using GraphPad Prism. Fisher’s exact test was used to test for differences between groups. Institutional Review Board approval was obtained.
Results
Eighty patients with HS underwent splenectomy during the review period. One patient with end-stage renal failure was excluded. The remaining TS (n=33) and PS (n=46) were included in the analysis. Age ranged from 2–22 years at time of surgery. The mean age was 9.7 (±4.4) years. There was no difference in age between groups. Maximum follow-up period was 16 years, with a mean duration of 45 months. Mean length of follow-up was longer for PS than TS (59.6 vs. 24.9 months, p<0.001).
Each patient had multiple indications for surgery as documented by the referring Hematologist. The most common were anemia (n=54), jaundice (n=43), abdominal pain (n=36), gallstones (n=35), splenomegaly (n= 27) and fatigue (n=19; Table 1). Anemia, jaundice, and splenomegaly were common indications for TS and PS (p>0.05). Laparoscopy was used for 56% of patients overall, and TS more than PS (94% vs 28%, p<0.01). The surgical approach for PS was related to the technical expertise: the earliest procedures were performed open and, as experience grew a higher proportion were performed laparoscopically. Cholecystectomy was performed in 47 patients (60%), including 7 after splenectomy (Table 1). PS patients had a longer mean length of stay than TS (5.8 vs 4.2 days, p<0.01).
Table 1:
Demographics, operative characteristics, indications for surgery
| DEMOGRAPHICS | Overall | TS (%) | PS (%) | p-value |
|---|---|---|---|---|
| 79 | 33 (42) | 46 (58) | ||
| Gender (%) | ||||
| Female | 34 (43) | 15 (45) | 19 (41) | 0.82 |
| Mean Age at Surgery (y) | 9.7 | 9.6 | 9.8 | 0.8 |
| FOLLOW-UP | ||||
| Average (m) | 45.1 | 24.9 | 59.6 | <0.001 |
| OPERATIVE TECHNIQUES | ||||
| Mean Length of Stay (d) | 5.2 | 4.2 | 5.8 | <0.001 |
| Surgery Type (n, %) | ||||
| Laparoscopic surgery | 44 (56) | 31 (94) | 13 (28) | <0.001 |
| Open surgery | 35 (44) | 2 (6) | 33 (72) | |
| Cholecystectomy | 47 (60) | 21 (64) | 26 (57) | 0.2 |
| Prior to splenectomy | 6 | 5 | 1 | |
| After splenectomy | 7 | 2 | 5 | |
| No data | 6 | 3 | 3 | |
| INDICATIONS FOR SURGERY | ||||
| Anemia/ aplastic crisis | 54 (68) | 23 (70) | 31 (67) | 1 |
| Jaundice | 43 (54) | 22 (67) | 21 (46) | 0.07 |
| Abdominal pain | 36 (46) | 15 (45) | 21 (46) | 1 |
| Gallstones | 34 (43) | 15 (45) | 19 (41) | 0.82 |
| Splenomegaly | 27 (34) | 10 (30) | 17 (37) | 0.63 |
| Fatigue | 19 (24) | 7 (21) | 12 (26) | 0.79 |
| High hemolytic rate | 9 (11) | 3 (9) | 6 (13) | 0.73 |
| Prevention | 5 (6) | 1 (3) | 4 (9) | 0.39 |
| Growth delay | 3 (4) | 2 (6) | 1 (2) | 0.57 |
| Hypersplenism | 2 (3) | 1 (3) | 1 (2) | 1 |
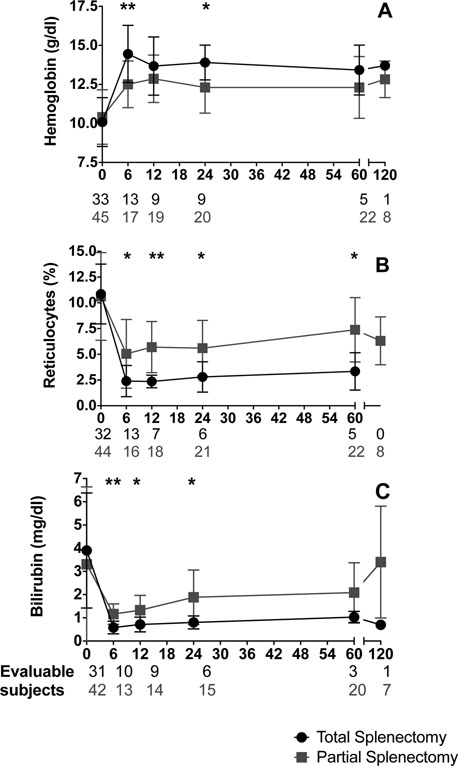
We found no significant difference in the severity of anemia or the rate of hemolysis at baseline between PS and TS cohorts, but post-procedure laboratory values differed by extent of spleen removal (Figure 1). Among PS subjects who required completion splenectomy, there was a trend toward lower baseline hemoglobin compared to PS subjects who did not require completion splenectomy (8.8 vs 10.6 g/dL, p=0.06). Increased hemoglobin, reduced reticulocyte count, and reduced bilirubin were observed after both procedures. At 6 months post-procedure, hemoglobin was higher after TS than PS (14.5 vs 12.5 g/dL, p<0.01). However, 5 years after surgery, hemoglobin was not statistically different (13.4 vs 12.3 g/dL, p=0.25) between groups. All patients became transfusion independent after surgery. Bilirubin after TS was lower than after PS at 6 months (0.6 vs 1.2 mg/dL, p=0.001); and was not significantly different at 60 months (1.0 vs 2.2 mg/dL, p=0.12). The reticulocyte count decreased after both procedures and was lower in TS at 6 months (2.4 vs 5%, p=0.01); this persisted up to 60 months (3.3 vs 7.4%, p=0.01). Thus, TS was associated with less evidence of erythrocyte turnover throughout the follow-up period.
Figure 1. Hematologic outcomes after total and partial splenectomy.
Serial laboratory evaluations of hemoglobin (A), reticulocyte percentage (B), and bilirubin (C) are summarized for all evaluable patients over 10 years (mean ± S.D.). Total splenectomy = black; partial splenectomy = gray. * = p 0.05, ** = p 0.005.
Short-term (<30 days) postsurgical complications included fever, abdominal and chest pain, infections, blood transfusion and left pleural effusion (Table 2). These occurred more frequently after PS than TS. Short-term events occurred in 22% of patients after TS and 63% of patients after PS (p<0.001). Specifically, fever occurred more frequently in PS than in TS (44 vs 9%, p=0.01). Blood transfusions were significantly more likely in the laparoscopic PS group than the laparoscopic TS group (23 vs 0%, p=0.02). Documented infections (e.g. pneumonia, UTI and diarrhea) were more common in the PS group, but the difference was not statistically significant. No infections observed in the short term were caused by encapsulated organisms.
Table 2.
Adverse Events
| SHORT-TERM ADVERSE EVENTS (<30 days) | Overall (%) | TS (%) | PS (%) | p-value | Lap TS (%) | Lap PS (%) | p-value |
|---|---|---|---|---|---|---|---|
| None | 42 (54) | 25 (78) | 17 (37) | <0.001 | 24 (80) | 7 (54) | 0.14 |
| Fever | 23 (30) | 3 (9) | 20 (44) | 0.001 | 3 (10) | 3 (23) | 0.35 |
| Abdominal / chest pain | 13 (17) | 4 (13) | 9 (20) | 0.54 | 3 (10) | 3 (23) | 0.35 |
| Infections | 6 (8) | 0 | 6 (13) | 0.08 | 0 | 1 (8) | 0.30 |
| Blood transfusion | 5 (6) | 0 | 5 (11) | 0.07 | 0 | 3 (23) | 0.02 |
| Left pleural effusion | 5 (6) | 0 | 5 (11) | 0.07 | 0 | 1 (8) | 0.30 |
| Hemorrhage | 1 (1) | 0 | 1 (2) | 1 | 0 | 1 (8) | 0.30 |
| Re-operation1 | 1 (1) | 0 | 1 (2) | 1 | |||
| Subcutaneous hematoma | 1 (1) | 1 (3) | 0 | 0.41 | 1 (3) | 0 | 1 |
| Jaundice | 1 (1) | 0 | 1 (2) | 1 | 0 | 0 | |
| Perisplenic fluid collection | 1 (1) | 0 | 1 (2) | 1 | 1 | 0 | |
| Death | 0 | 0 | 0 |
| LONG-TERM ADVERSE EVENTS (>30 days) | ||||
|---|---|---|---|---|
| None | 35 (57) | 15 (71) | 20 (50) | 0.001 |
| Recurrent abdominal pain2 | 10 (16) | 1 (5) | 9 (23) | 0.14 |
| Gallstones | 11 (18) | 2 (10) | 9 (23) | 0.15 |
| Re-operation3 | 10 (16) | 2 (10) | 8 (20) | 0.47 |
| Recurrence of symptoms | 7 (11) | 0 | 7 (18) | 0.08 |
| Radiologic spleen alterations4 | 5 (8) | 0 | 5 (13) | 0.15 |
| Infections | 2 (3) | 2 (10) | 0 | 0.11 |
| Thromboembolism | 1 (2) | 1 (5) | 0 | 0.34 |
| Thrombocytosis5 | 1(2) | 1 (5) | 0 | 0.34 |
| Death | 0 | 0 | 0 | |
| Not available | 18 (23) | 12 (36) | 6 (13) |
Notes:
Short-term re-operation were thoracentesis and abdominal fluid aspiration due to perisplenic fluid collection and left pleural effusion.
Recurrent abdominal pain was associated with the presence of gallstones, spleen alterations, spleen regrowth or no other signs/symptoms (4 patients).
Long-term re-operations were spleen regrowth, splenic infarction, and gallstones with/without pancreatitis.
The radiologic spleen alterations were postoperative seroma in splenic bed; spleen cysts; intrasplenic calcifications; functional asplenia.
Platelet count > 1,000,000/mmc
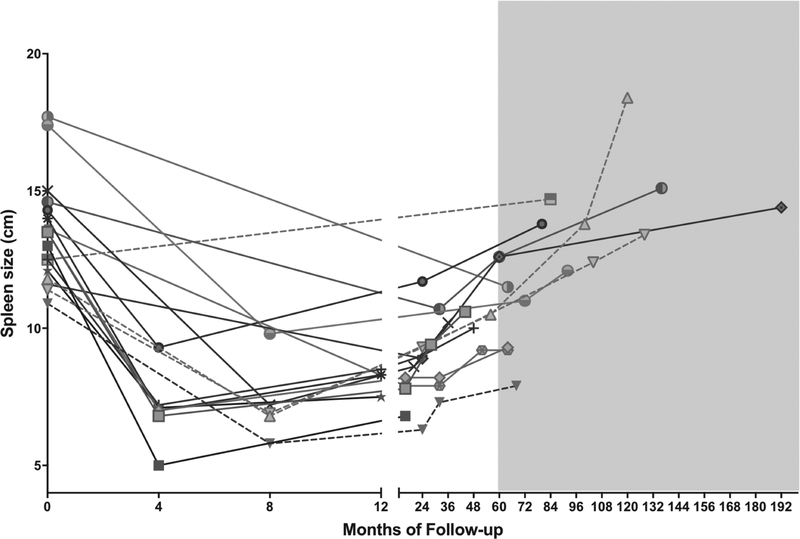
Patients undergoing PS experienced more long-term (>30 days) adverse events, but the difference was not statistically significant (Table 2). Re-operations after PS were an important long-term adverse event, where 5 subjects required completion splenectomy. In the PS group, the change in spleen length from baseline to 5 years after PS was evaluable in 11 subjects, including 4 of those requiring completion splenectomy. At baseline, the mean anteroposterior diameter of the spleen for the PS cohort was 14.2 cm. The spleen regrew to larger than baseline by 5 years in 45.5% of evaluable patients. The mean spleen size was 96% of baseline (n=11) at 5.2–11.5 years after PS (Figure 2). This is consistent with regrowth and led to completion splenectomy.
Figure 2. Spleen size before surgery and during follow-up for partial splenectomy.
Serial ultrasound measurements of spleen size were available in a subset of subjects. Included in this figure are subjects for whom a pre-operative spleen size measurement was available and at least two post-operative measurements were available. The shaded area highlights subjects with more than 5 years of follow-up. Dashed lines denote subjects who underwent completion splenectomy.
Among subjects with regrowth and completion splenectomy, all had recurrent symptoms including anemia, jaundice, fatigue and abdominal pain. One additional subject in whom regrowth was not evaluable also reported recurrent symptoms and required completion splenectomy. When compared to the patients who underwent completion splenectomy (n=4), the patients with spleen regrowth and no completion splenectomy (n=7) were significantly older at surgery (10.6 vs 4.3 years, p<0.01), had larger pre-operative spleen size (14.7 vs 11.7 cm, p=0.03), trended toward a higher baseline hemoglobin (10.7 vs 8.8 g/dl, p=0.05), similar baseline reticulocyte percentage (10.2 vs 10.5%, p=0.9), and had a similar bilirubin (1.8 vs 2.3, p=0.7).
Cholecystectomies also represented an important re-operation after PS. Two patients who underwent completion splenectomy had a concurrent cholecystectomy. In total, 2 TS patients and 9 PS patients developed gallstones during follow-up. Of these, 7 patients underwent cholecystectomy (Table 1).
Previously reported long-term complications of TS were uncommon in our cohort. During 15,686 person-years of follow-up, we observed no deaths or cases of bacteremia, but two serious events were recorded in the TS group. One case of Streptococcus pneumoniae meningoencephalitis in a 5 year-old, fully-immunized child occurred one year after TS and one month after cessation of antibiotic prophylaxis. One pulmonary embolus occurred 9 years after TS.
Use of infectious prophylaxis was difficult to assess retrospectively. Data were not available on pre-operative immunization for 7 patients (8.9%), or post-operative antibiotic prophylaxis for 18 patients (22.8%). Among those with available data, 90.3% of patients were vaccinated against Streptococcus pneumoniae and Neisseria meningitidis. Antibiotic prophylaxis was documented for 50% TS patients and 28% PS patients.
Discussion
We have described the clinical and laboratory outcomes of a 79-patient cohort of children with HS who underwent splenectomy. There were no deaths and one severe encapsulated bacterial infection. Both TS and PS addressed the most common indications for surgery. TS was associated with improved markers of hemolysis compared to PS during early follow-up, but not long-term. Our retrospective review was limited by unavailable data for patients with follow-up outside of our institution and by unavailable long-term data for many patients who underwent TS, possibly because their symptoms had not recurred and they did not continue Hematology follow-up. Follow-up was longer for PS likely due to the need to monitor spleen regrowth.
PS was associated with a higher incidence of post-surgical complications. Fever was more common in PS, even after adjusting for surgical approach. Longer length of hospital stay in PS was likely due to the higher incidence of open procedures in this cohort. Prior to 2011, 7% of PS were laparoscopic procedures. After 2011, the proportion increased to 58%. During the period of our review, laparoscopic surgery became the preferred technique for both TS and PS and was associated with shorter length of stay.18 Our data are limited by the high rate of loss to long-term follow-up among TS patients.
When considering TS versus PS, the likelihood of future surgery should be considered. Our finding of continued hemolysis over time after PS explains the recurrence of symptoms and eventual need for completion splenectomy in some patients. Our cohort identified a trend toward completion splenectomy among subjects with lower baseline hemoglobin. A larger cohort should re-examine this as a risk-factor in the future. Our rate of secondary splenectomy (12.5%) is comparable to other series with more than 3 years of follow-up.12,15–17 Furthermore, 28% patients in this review developed gallstones during follow-up and 18% of our cohort who did not have a cholecystectomy with their primary splenectomy required a second surgery for cholecystectomy. These findings support the need for continued follow-up of patients with total or partial splenectomy for symptoms of cholelithiasis/cholecystitis.
The reported estimated incidence of severe encapsulated bacterial infections after splenectomy is 0.23–0.42% per year, with a lifetime risk of 5%.19. Although the mean duration of follow-up in our review was 45 months, there were not enough events to compare the incidence of severe infections and thromboembolic events between groups, even though serious events were recorded only in the TS group. Within our cohort, the rate of preoperative immunization was high, and likely protective. A future study should assess immunization and prophylaxis practices in the post-vaccine era, along with quality of life outcomes and evaluation of residual spleen function.
Acknowledgements
VNT received support from the National Institutes of Health (K12HL087164). SIT received support from the Global Health Initiative at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. The authors would like to thank Dr. Samuel E. Lux III for critical review of this manuscript.
Footnotes
Declaration of Interest
The authors have no relevant conflicts of interest to declare.
REFERENCES:
- 1.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372(9647):1411–1426. [DOI] [PubMed] [Google Scholar]
- 2.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher PG. Abnormalities of the erythrocyte membrane. Pediatr Clin North Am. 2013;60(6):1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton-Maggs PH, Langer JC, Iolascon A, Tittensor P, King MJ. Guidelines for the diagnosis and management of hereditary spherocytosis−−2011 update. Brit J Haematol. 2012;156(1):37–49. [DOI] [PubMed] [Google Scholar]
- 5.Guizzetti L Total versus partial splenectomy in pediatric hereditary spherocytosis: A systematic review and meta-analysis. Pediatr Blood Cancer. 2016;63(10):1713–1722. [DOI] [PubMed] [Google Scholar]
- 6.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infection. 2001;43(3):182–186. [DOI] [PubMed] [Google Scholar]
- 7.Schilling RF, Gangnon RE, Traver MI. Delayed adverse vascular events after splenectomy in hereditary spherocytosis. J Thromb Haemost. 2008;6(8):1289–1295. [DOI] [PubMed] [Google Scholar]
- 8.Das A, Bansal D, Ahluwalia J, et al. Risk factors for thromboembolism and pulmonary artery hypertension following splenectomy in children with hereditary spherocytosis. Pediatr Blood Cancer. 2014;61(1):29–33. [DOI] [PubMed] [Google Scholar]
- 9.Robinette CD, Fraumeni JF, Jr. Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet. 1977;2(8029):127–129. [DOI] [PubMed] [Google Scholar]
- 10.Petithory JC, Khelil A, Galeazzi G, Ardoin F. [Malaria in splenectomized patients. Three fatal cases]. Presse Med. 2005;34(7):519–521. [DOI] [PubMed] [Google Scholar]
- 11.Pincez T, Guitton C, Gauthier F, et al. Long-term follow-up of subtotal splenectomy for hereditary spherocytosis: a single-center study. Blood. 2016;127(12):1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice HE, Crary SE, Langer JC, Kemper AR. Comparative effectiveness of different types of splenectomy for children with congenital hemolytic anemias. J Pediatr. 2012;160(4):684–689.e613. [DOI] [PubMed] [Google Scholar]
- 13.Bader-Meunier B, Gauthier F, Archambaud F, et al. Long-term evaluation of the beneficial effect of subtotal splenectomy for management of hereditary spherocytosis. Blood. 2001;97(2):399–403. [DOI] [PubMed] [Google Scholar]
- 14.Tchernia G, Gauthier F, Mielot F, et al. Initial assessment of the beneficial effect of partial splenectomy in hereditary spherocytosis. Blood. 1993;81(8):2014–2020. [PubMed] [Google Scholar]
- 15.Buesing KL, Tracy ET, Kiernan C, et al. Partial splenectomy for hereditary spherocytosis: a multi-institutional review. J Pediatr Surg. 2011;46(1):178–183. [DOI] [PubMed] [Google Scholar]
- 16.Englum BR, Rothman J, Leonard S, et al. Hematologic outcomes after total splenectomy and partial splenectomy for congenital hemolytic anemia. J Pediatr Surg. 2016;51(1):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice HE, Englum BR, Rothman J, et al. Clinical outcomes of splenectomy in children: report of the splenectomy in congenital hemolytic anemia registry. Am J Hematol. 2015;90(3):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng S, Qiu Y, Li X, et al. Laparoscopic versus open splenectomy in children: a systematic review and meta-analysis. Pediatr Surg Int. 2016;32(3):253–259. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infection. 2001;7(12):657–660. [DOI] [PubMed] [Google Scholar]