Abstract
Despite early differences in orienting to sounds, no study to date has investigated whether children with ASD demonstrate impairments in attentional disengagement in the auditory modality. Twenty-one nine- to fifteen year old children with ASD and 20 age- and IQ-matched TD children were presented with an auditory gap-overlap paradigm. Evidence of impaired disengagement in ASD was mixed. Differences in saccadic reaction time for overlap and gap conditions did not differ between groups. However, children with ASD did show increased no-shift trials in the overlap condition, as well as reduced disengagement efficiency compared to their TD peers. These results provide further support for disengagement impairments in ASD, and suggest that these deficits include disengaging from and shifting to unimodal auditory information.
Keywords: autism spectrum disorder, attention, auditory, disengagement, eye movements, EOG
Introduction
Attentional orienting - defined as disengaging, shifting, and re-engaging attention (Posner et al., 1984) - is important for cognitive (Colombo et al., 2001), social (Salley et al., 2016), and affective (Rothbart et al., 1994) development. Overt shifts of visual attention (i.e., shifts associated with head and/or eye movements) allow individuals, from infancy onward, to extract critical information from their environment, participate in joint attention with communicative partners, and adopt and maintain self-regulatory strategies. Results from an extensive literature of non-social attentional paradigms provide evidence of dysfunctional disengagement and shifting of attention in individuals with autism spectrum disorder (ASD) (see O. Landry & Parker, 2013; Sacrey et al., 2014, for reviews). Further, as these attentional impairments are present early and are associated with a subsequent diagnosis of ASD (Elison et al., 2013; Elsabbagh et al., 2013; Zwaigenbaum et al., 2005), they have been hypothesized to play a role in the emergence of the ASD phenotype (Keehn et al., 2013).
Evidence of impaired attentional disengagement in ASD has been primarily provided by gap-overlap paradigms, which examine differences in saccadic reaction times (SRT) to peripheral visual targets appearing with, and without, a central fixation (Sacrey et al., 2014). Latency to execute saccadic eye movements is reduced when a fixated visual stimulus is removed simultaneously with or prior to (e.g., 200ms) the onset of a peripheral target (Saslow, 1967). Attentional disengagement is often measured by examining SRT to targets appearing when the fixation cross remains on the screen (i.e., overlap condition) compared to when the cross disappears prior to (i.e., gap condition) or simultaneously with (i.e., baseline/step condition) the target onset. This effect (i.e., the gap effect) results from two separate sources: 1) a generalized warning effect as a consequence of fixation offset, which cues participants to the impending target, and 2) the release of ocular inhibition due to a) the disappearance of a foveal stimulus, and b) the top-down preparation of a saccadic response (Kingstone & Klein, 1993; Taylor et al., 1998). Although experimental parameters vary widely across previous reports (see Sacrey et al., 2014, for discussion), infants, children, and adults with ASD exhibit disproportionally longer SRT to overlap trials relative to gap trials compared to their typically developing (TD) peers (although see, for example Fischer et al., 2014; 2016; Schmitt et al., 2014, for evidence of unimpaired disengagement).
Additionally, identifying the source of gap-effect differences between ASD and TD groups has the potential to highlight network-specific oculo-motor and/or attentional factors associated with the task. For example, differences in latencies between overlap and baseline conditions result primarily from the release of oculo-motor inhibition in the superior colliculus due to removal of the central stimulus (Dorris & Munoz, 1995) and/or pre-target saccadic preparation associated with bilateral activation of frontal parietal regions (Connolly et al., 2005; Curtis & Connolly, 2008). Alternatively, faster SRTs to gap compared to overlap trials may be associated with phasic alerting response (i.e., fixation offset), which is associated with a right-lateralized attentional network (Sturm & Willmes, 2001).
To date, experimental studies that have investigated attentional disengagement in ASD have almost exclusively used visual stimuli (with exception of Sabatos-DeVito et al., 2016, who included an audio-visual fixation condition). However, auditory processing and attention to sounds may be atypical in ASD (see O’Connor, 2012, for review). For example, retrospective (Baranek, 1999; Osterling & Dawson, 1994; Osterling et al., 2002; Werner et al., 2000) and prospective (Nadig et al., 2007) reports have shown that 12-month-old infants later diagnosed with ASD often fail to respond to their name. Further, these deficits in orienting to social information persist into early childhood, as 3- to 5-year-olds with ASD exhibit reduced orienting to social sounds (Dawson et al., 1998; Dawson et al., 2004). Additionally, studies using event-related potentials (ERP) to examine sensory and attentional responses to auditory stimuli have shown that arousal and re-orienting of attention may be atypical in ASD (Orekhova & Stroganova, 2014; Stroganova et al., 2013). Thus, atypical orienting to auditory information is present in ASD; however, it is unclear whether these impairments in orienting to social sounds reflect a deficit in social orienting or a more fundamental impairment in non-social attention function.
Similar to gap-overlap tasks using purely visual stimuli, prior studies in TD individuals have demonstrated gap effects using visual fixations and auditory targets (Fendrich et al., 1991) as well as auditory fixations and targets (Shafiq et al., 1998; Taylor et al., 1999). However, no study has investigated whether impaired attentional disengagement in individuals with ASD is present to sounds in the absence of visual information. Thus, the objective of the current study was to investigate differences in unimodal auditory attentional disengagement in children with ASD. Evidence of impaired auditory attentional disengagement would extend prior findings from the visual domain, and suggest that disengagement deficits are present across multiple modalities. Given prior observational evidence of impaired orienting in ASD in the auditory domain (e.g., to one’s name) and presence of atypical attentional disengagement in the visual domain, we hypothesized that children with ASD would show impaired attentional disengagement in the context of a unimodal auditory gap-overlap paradigm. Furthermore, given prior evidence of impaired orienting to environmental sounds (e.g., name call) reviewed above, which may occur well outside the current locus of attention, and that ASD may be associated with impaired zooming out of attention (Mann & Walker, 2003; Ronconi et al., 2018; Ronconi et al., 2013), we examined disengagement and shifting attention to targets occurring both near and far from central fixation. We hypothesized that these impairments in attention al disengagement may be greater for targets occurring at larger distances from the central fixation.
Methods
Participants
Twenty-one school-aged children and adolescents with ASD (M = 11.5 years; SD =1.3); and 20 age- and IQ-matched TD children and adolescents (M = 11.2 years; SD = 1.5) participated in the study (see Table 1). Clinical diagnoses were confirmed using the Autism Diagnostic Observation Schedule, Second Edition, (ADOS-2; Lord et al., 2012), Social Communication Questionnaire (SCQ; Rutter et al., 2003), and expert clinical judgment according to DSM-5 criteria (RMK). Children with ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) were excluded. Participants in the TD group had no reported family history of ASD and were confirmed via parent report to be free of ASD-related symptoms. In addition, children in both groups were confirmed to have no uncorrected vision or hearing impairments based on parent report. Informed assent and consent was obtained from all participants and their caregivers in accordance with the Purdue University Institutional Review Board.
Table 1.
Participant Characteristics
| ASD | TD | t-value | P | |
|---|---|---|---|---|
| n (M:F) | 21 (17:4) | 20 (15:5) | - | |
| Age (years) | 11.5 (1.3); 9.2–14.5 |
11.2 (1.5); 9.3–15.0 |
0.6 | 0.57 |
| Verbal IQ | 102 (19); 69–154 |
110 (11); 95–127 |
−1.5 | 0.14 |
| Nonverbal IQ | 101 (20); 52–132 |
111 (13); 87–134 |
−1.9 | 0.07 |
| SRS-2 Total score (t-score) | 75 (11); 57–90 |
44 (5); 37–55 |
11.6 | < .001 |
| ADOS-2 Social Affect | 11 (4); 5–17 |
- | - | - |
| Repetitive Behavior | 2 (1); 0–5 |
- | - | - |
| Comparison Score | 7 (2); 4–10 |
- | - | - |
IQ determined using the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II; Wechsler, 2011). Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012).Mean (SD); range.
Apparatus and Stimuli
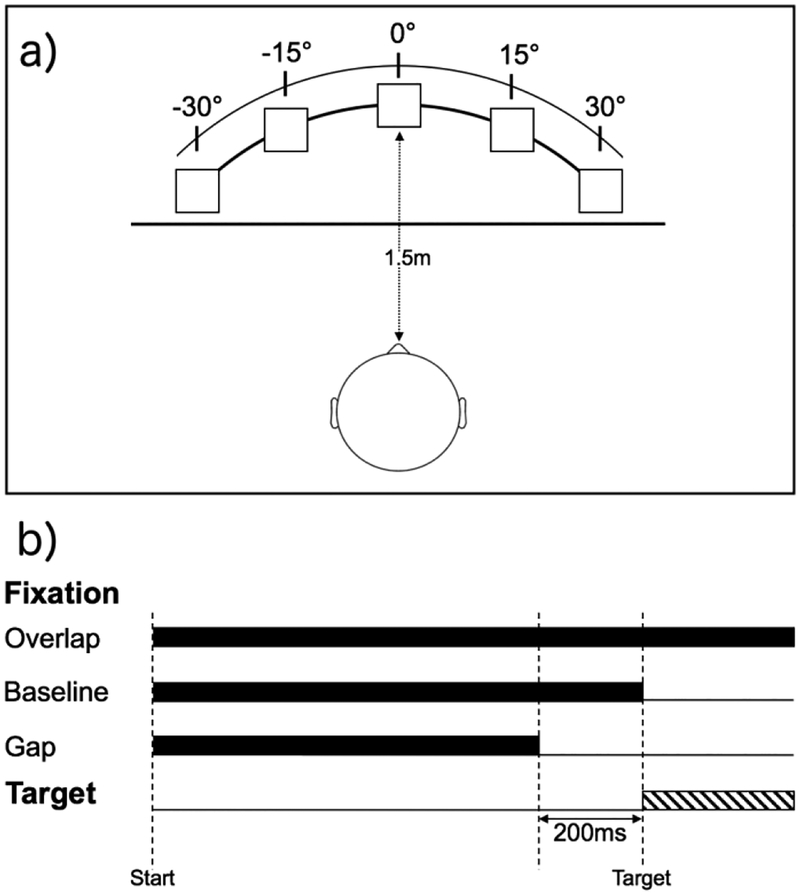
The experiment was presented using Presentation software (http://nbs.neuro-bs.com; version 11.8). Participants were tested in a sound attenuated, darkened room, and seated comfortably approximately 1.5m directly in front of five speakers (Hafler M5 Reference) positioned on stands at approximately eye-level. Speakers were positioned in a semi-circular array at 0° (i.e., directly in front of) and at 15° and 30° to the left and right of participant (see Figure 1a). Auditory fixation at the central location was a 500Hz pure tone, whereas peripheral targets emitted from side speakers were white noise (similar to Shafiq et al., 1998). Unique fixation and target sounds were selected in order to reduce potential masking effects (Taylor et al., 1999), and, further, noise was selected for targets and tone as the fixation due to prior research demonstrating that saccadic accuracy is reduced for tone compared to noise stimuli (Frens et al., 1995). All stimuli were played at a comfortable listening level (approximately 60 dBA measured at the location of the seated participant’s head).
Figure 1.
(a) Experimental arrangement of speakers (squares) positioned directly in front of and 15 and 30 degrees to right and left of participants with black curtain (horizontal line) placed between participant and speakers, (b) Different stimulus presentation for each trial type with central auditory fixation (500Hz tone; black bars) conditions and peripheral target (white noise; striped bar).
Saccadic eye movements were recorded using electrooculography (EOG) via a Biopac EOG100C amplifier at a sampling rate of 500Hz. Two 4mm reusable Ag/AgCL shielded electrodes (Biopac EL254S) filled with conductive gel (5% NaCl, 0.85 molar NaCl) were applied at the lateral canthi of the left and right eye. Hardware gain was set to 5000 (corresponding to an input gain of ±2 mV), and filter bandwidth was set to 0.05 – 35Hz prior to digitization. Data were acquired using AcqKnowledge 4.3 software (Biopac Systems, Inc.).
Procedure
Participants first completed an EOG calibration, and were instructed to keep their head still and to move their eyes to each speaker, which were visible, when a sound was presented. During calibration, white noise was played from each speaker separately beginning at the far left (−30°) and then proceeding to near left (−15°), center (0°), near right (15°), and far right (30°) speakers. This process (−30,−15, 0, 15, 30°) was repeated five times.
Prior to the start of the gap-overlap task, a black curtain was drawn in front of the speaker array approximately 1.2m from the participant, thus visually occluding speakers. As the present study included children, a black curtain was used to remove visual stimulation rather than total darkness, which was used in previous studies of TD adults (Shafiq et al., 1998; Taylor et al., 1999). Thus, rather than fixate on a specific object (e.g., central crosshair/LED in a visual gap-overlap task), participants fixated on a specific location (i.e., the source of the sound). Each trial began with a tone presented alone from the center speaker for a random duration between 1300 and 1500ms. Next, a peripheral noise was played from one of the side speakers for 1200ms either: 1) with the tone continuing to play from the center speaker (overlap condition), 2) 200ms after the central tone stopped (gap condition), or 3) with the simultaneous offset of the central tone (baseline condition). Finally, there was a 2000ms inter-stimulus interval during which time no sound was presented (see Figure 1b). Prior to beginning the experiment, participants were told they were going to play the ‘find the noise’ game. They were instructed to look at the center location when the tone was playing, then to move only their eyes to location of the sound once the noise played, and then to look back towards the central location to wait for the tone. Finally, participants completed a series of six practice trials.
Design
The experiment consisted of 108 trials, divided into three blocks of 36 trials. Within each block, condition (gap, baseline, overlap), location (near, far), and side (left, right) were varied in pseudorandom order. All trial types were presented an equal number of times within each block and across the experiment.
Analysis
Horizontal saccadic eye movements to the target locations were detected as abrupt changes in the EOG with a peak velocity greater than 50°/s for a duration of at least 20ms for the duration of the peripheral noise (1200ms). In addition, data from each trial were visually inspected by trained research assistants blind to group membership. To be included, initial saccadic eye movements were required to follow a steady fixation at the central location for at least 200ms prior to target presentation. Trials in which there was no stable fixation at the central location (due to movements or noise) or trials in which the initial saccade was directed towards the incorrect side (e.g., saccade to left with target on right) were excluded (incorrect saccades: ASD = 1.2%; TD = 0.8%, t(39) = .656, p = .516). Saccadic reaction time (SRT) was defined as duration between the presentation of the peripheral noise and the onset of the first saccadic eye movement directed toward the side of the noise. Trials with SRTs less than 80ms were considered anticipatory and excluded. Finally, trials on which no saccade occurred but where fixation was maintained at the central location were coded as no-shift trials.
Medians were used m all SRT analyses to reduce the influence of outliers. Saccadic reaction time and the variability of SRT (standard deviation of SRT for each individual), percentage of no-shift trials (number of no-shift trials divided by total number of usable trials), and disengagement efficiency (SRT/[1-no-shift percentage]) were analyzed using mixed-model repeated-measures ANOVA with between-subject factor group (ASD, TD) and within-subjects factors condition (gap, baseline, overlap) and location (near, far). In addition, the gap effect and step effect were calculated by subtracting SRT for gap from overlap (overlap-gap) and baseline from overlap (overlap-baseline) conditions, respectively, and compared using independent-samples t-tests.
Results
There was no main effect of group, F(l, 39) < 1, nor were there any significant interactions between group and other experimental factors for number of usable trials (all p >.2; see Table 2).
Table 2.
Number of Valid Trials per Group and Condition
| Gap | Baseline | Overlap | ||||
|---|---|---|---|---|---|---|
| Near | Far | Near | Far | Near | Far | |
| ASD | 12 (3.1) | 13 (3.7) | 13 (3.2) | 13 (3.2) | 13 (3.0) | 13 (2.87) |
| TD | 13 (3.4) | 13 (2.1) | 13 (2.6) | 14 (3.4) | 13 (3.0) | 14 (2.86) |
Mean (SD)
Saccadic Reaction Time (SRT)
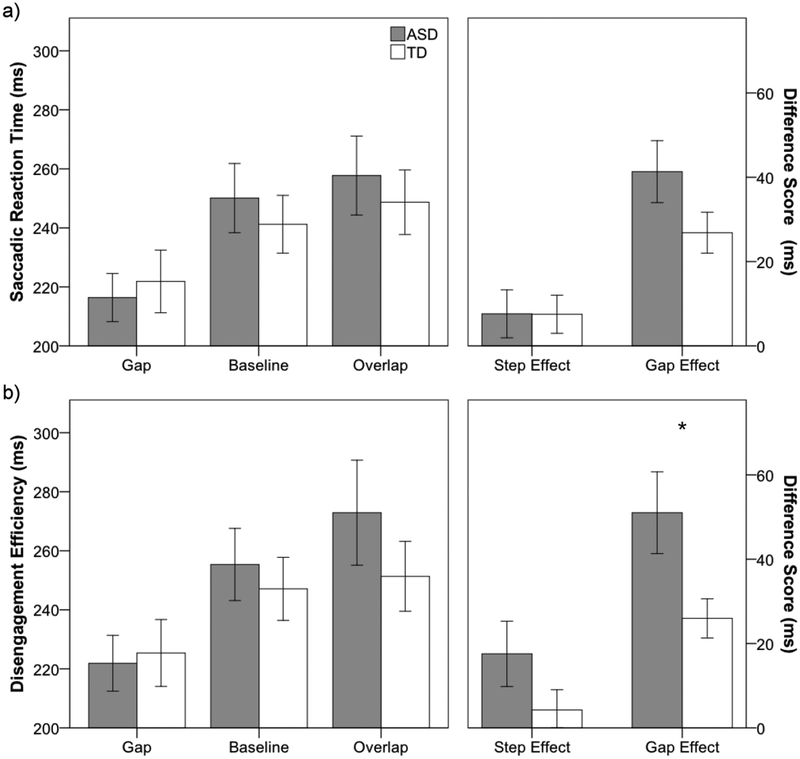
As expected based on previous findings from gap-overlap paradigms, there was a significant within-subjects main effect of condition, F(2, 78) = 37.2,p< .001, ηp2 = 0.49. Saccadic reaction time was faster to gap compared to overlap, t(40) = −7.5,p < .001, d= 1.18, and baseline, t(40) = −6.0, p< .001, d = .93, conditions, and SRT in the baseline condition was faster than the overlap condition, t(40) = −2.0, p = .04, d = .33 (gap < baseline < overlap; see Figure 2a). In addition, there was a significant main effect of location, F(1, 39) = 10.4, p = .003, ηp2= 0.21, as latency to far (M = 234ms) targets was faster compared to near (M = 247ms) targets. There was no significant main effect of group (ASD = 244ms; TD = 238ms), F(1, 39) < 1, nor was there significant interactions between group and any other factor (p > .17). While the gap effect was numerically larger in the ASD group (M = 41.3ms; SD = 33.7) compared to the TD group (M = 26.9ms; SD = 21.7), these did not differ statistically, t(39) = 1.63, p = .11, d = .51. Additionally, groups did not differ for step effect (ASD = 7.6ms; TD = 7.5ms), t(39) = .02, p = .99, d = .01.
Figure 2.
(a) Saccadic reaction time (SRT) to gap, baseline, and overlap conditions for ASD (gray) and TD (white) groups (left), and difference scores for step (overlap - baseline) and gap (overlap - gap) effects (right), (b) Disengagement efficiency scores (SRT/[1-no-shift percentage]) for gap, baseline, and overlap conditions for ASD and TD groups (left), and difference scores for step and gap effects (right). Error bars represent ± 1 SEM. * p < .05
For intra-individual standard deviation of SRT, there was a significant main effect of condition, F(2, 78) = 2=3.8, p = .026, ηp2 = 0.09. Variability was greater for the overlap (M = 108ms; SD = 58)compared to gap (M = 94ms; SD = 48), t(40) = −2.2, p = .037, d = .34, and baseline (M = 89ms; SD 43),t(40) = −2.4, p = .021, d = .37, conditions, but SRT variability did not differ between gap and baseline conditions, t(40) = .63, p = .532, d = .10 (overlap > gap = baseline). There was no significant difference between the groups, F(1, 39) = .7, p = .41, ηp2 = 0.02, nor was there significant interaction between group and any other factor (all p > .2)
No-Shift Trials
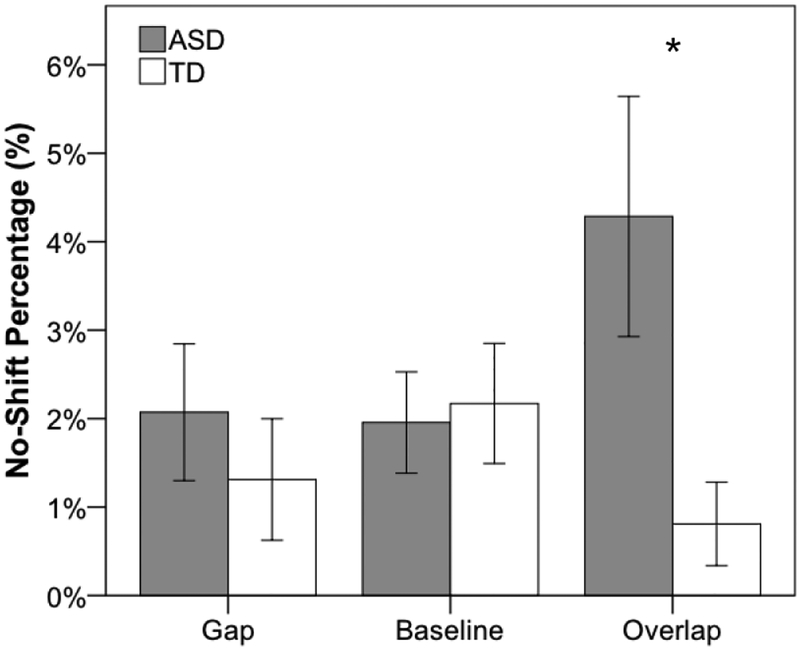
For no-shift trials, there was no main effect of group, F(1, 39) = 2.2, p = .15, ηp2 = 0.05, nor was there a main effect of condition, F(2, 78) = 1.3, p = .29, ηp2 = 0.03. However, no-shift percentage was greater for near compared to far conditions, F(1, 39) = 7.0, p = .01, ηp2 = 0.15. Furthermore, as illustrated in Figure 3, there was a significant interaction between group and condition, F(2, 78) = 5.2, p = .008, ηp2= 0.12. Follow-up independent-samples t-tests showed that while groups did not differ in percentage of no-shift trials collapsed across near and far locations for gap, t(39) = .73, p = .47, d = .23, or baseline trials, t(39) = .73, p = .81, d = .07, the ASD group had a significantly higher no-shift percentage for the overlap condition compared to the TD group, t(39) = 2.37, p = .023, d = .75. No other significant interactions were present (p > .05). Additionally, because no-shift was non-normally distributed (Shapiro-Wilk test: W = .72, p < .001), with skewness of 1.74 (SE = 0.37) and kurtosis of 2.16 (SE = 0.72), data were entered into a generalized linear mixed-effects model with a log link function to examine the likelihood of a no-shift trial with within-subjects variables of location (near, far) and condition (gap, baseline, overlap) and the between-subjects variable of group (ASD, TD), and all main effects and two and three-way interactions were included as fixed factors. Additionally, a random participant intercept and random slopes for location and condition were estimated. There was no longer a significant effect of location (fixed or random), and this was removed from the model. Importantly, the group by condition interaction remained significant, F(2, 3249) = 6.63, p = .0013, and outcomes for follow-up pairwise comparisons were unchanged.
Figure 3.
Percentage of no-shift trials for ASD (gray) and TD (white) for gap, baseline, and overlap conditions collapsed across near and far locations. Error bars represent ± 1 SEM. * p < .05
Disengagement Efficiency
Similar to SRT, there were significant main effects for condition, F(2, 78) = 35.6,p < .001, ηp2 = 0.48, and location, F(1, 39) = 13.1,p = .001, ηp2 = 0.25, but not group, F(1, 39) = .5, p = .50, ηp2 = 0.01 (see Figure 2b). Again, efficiency was greater in the gap condition compared both to overlap, t(39) = −6.8, p < .001, d = 1.06, and baseline, t(39) = −6.7, p < .001, d = 1.04, conditions, and efficiency in the baseline condition was greater than the overlap condition, t(39) = −2.4, p = .02, d = .37. Additionally, similar to no-shift percentage, the group by condition interaction was significant, F(2, 78) = 3.9,p = .02, ηp2= 0.09. Follow-up independent-samples t-tests showed that efficiency did not differ between groups for gap, baseline, and overlap conditions (all p > .3). However, the disengagement efficiency gap effect was significantly larger in the ASD group compared to the TD group, t(39) = 2.3, p = .03, d = .72, whereas disengagement efficiency step effect did not differ between groups, t(39) = 1.4 p = .16, d = .45. No other interactions were significant (p > .3). Additionally, because disengagement efficiency was non-normally distributed (Shapiro-Wilk test: W = .91, p = .005), with skewness of 1.21 (SE = 0.37) and kurtosis of 2.11(SE = 0.72), data were log transformed to meet assumptions of normality. Results using log-transformed data were unchanged.
Correlations with ASD Symptomatology
We examined the relationship between the gap effect for SRT and disengagement efficiency measures, as well as no-shift percentages for overlap trials and ADOS-2 and SRS scores. There were no significant correlations between disengagement measures and ADOS calibrated severity score or SRS Total scores (all p > .05).
Discussion
The goal of the current study was to investigate non-social auditory attentional disengagement in children with ASD. Consistent with previous reports in TD adults, results from our auditory gap-overlap paradigm demonstrated expected differences in SRT between gap, baseline, and overlap conditions (Shafiq et al., 1998; Taylor et al., 1999) as well as shorter SRT to targets at greater eccentricities (Zambarbieri, 2002). Evidence of hypothesized impaired disengagement in ASD was mixed; although numerically larger, the difference in SRT for overlap and gap conditions (i.e., the gap effect) did not differ statistically between ASD and TD children. However, children with ASD did show an increased percentage of no-shift trials compared to their TD peers, specifically for the overlap condition. Moreover, compared to TD children, children with ASD showed a significantly greater disengagement efficiency gap effect, a measure that integrates SRT with no-shift percentage. Lastly, while within-subject differences in responsivity based on target location were present, the groups did not vary with regard to how the responded to near and far targets. How these discrepant findings fit within the broader context of disengagement literature in ASD is the focus of the discussion below.
When do we see impaired attentional disengagement in ASD? When don’t we?
Findings from gap-overlap paradigms in ASD are mixed with evidence of equivalent (Fischer et al., 2014; Fischer et al., 2016; Schmitt et al., 2014; Zalla et al., 2018), slower (Elison et al., 2013; Elsabbagh et al., 2013; Kleberg et al., 2017; R. Landry & Bryson, 2004; Sabatos-DeVito et al., 2016), and faster (van der Geest et al., 2001) disengagement. Inconsistent or contradictory results in ASD research is not unique to attentional disengagement and gap-overlap paradigms, nor is it surprising given the heterogeneity of ASD, the different ages that have been investigated, and the variability in the tasks and the metrics used to measure attentional disengagement.
Nevertheless, evidence of impaired disengagement in ASD has received the most support from studies of high-risk infant siblings of children with ASD. Prior research has demonstrated that deficits in disengagement may be present as early as 7 months (Elison et al., 2013) or emerge by at least the end of the first year of life (Elsabbagh et al., 2013; Zwaigenbaum et al., 2005). These disengagement deficits have been shown to persist in younger children with ASD (R. Landry & Bryson, 2004), although there is evidence to the contrary in toddlers (Fischer et al., 2016). This age-specific absence of group differences may reflect non-linear trajectories of disengagement abilities in TD children (Colombo, 2002); however, it is also important to note that experimental factors, which potentially contribute to group differences in disengagement, may be confounded by the age of the study participants. For example, as noted by Sacrey et al. (2014), to maintain engagement of younger participants these studies tend to use dynamic stimuli (see also Kleberg et al., 2017; Sabatos-DeVito et al., 2016, for more recent reports of impaired disengagement in young children with ASD using dynamic stimuli), whereas studies of older children, adolescents, and adults with ASD may use more basic stimuli (e.g., fixation cross, LEDs). The present study elected to use basic auditory stimuli (i.e., 500Hz tone, white noise), consistent with prior auditory gap-overlap tasks (Shafiq et al., 1998; Taylor et al., 1999), rather than more engaging sounds (e.g., speech) to reduce any confounds associated with the social nature of the stimuli. While significant group differences were not present for the SRT gap effect, there was a medium effect size (d = .51). Assuming this effect size and 80% power, approximately 62 participants per group would be required to reliably detect group differences (α = .05, two-tailed), suggesting our study may be underpowered. However, the selection of low-interest, simple sounds may have contributed to the absence of significant differences for SRT analysis by reducing the magnitude of the effect due to limited engagement with the fixation stimulus.
A second factor - predictability - may also contribute to the presence or absence of disengagement difficulties in any given gap-overlap paradigm. Recently, impairments in predictive coding have been hypothesized to contribute to the development of the heterogeneous ASD phenotype (e.g., Van de Cruys et al., 2014). At least two key aspects of gap-overlap tasks can be varied to decrease/increase the predictability of the stimulus sequences: 1) whether the fixation stimulus has a fixed or a variable duration, and 2) whether trial types are blocked or randomized. For example, in TD adults, blocking trial types (i.e., presenting only overlap trials, rather than presenting gap, baseline, and/or overlap trials in a pseudorandom sequence) affects SRT for overlap trials but not gap trials (Jin & Reeves, 2009). Of previous studies failing to show significant disengagement differences many have used blocked presentation (Schmitt et al., 2014; van der Geest et al., 2001; Zalla et al., 2018) and fixed fixation durations (Fischer et al., 2016; van der Geest et al., 2001; Wilson & Saldana, 2018), which would be likely to reduce uncertainty and possibly improve performance in individuals with ASD (i.e., so that group differences are no longer present).
Furthermore, across all studies, gap trials provide a predictable sequence of events: 1) fixation cross appears (fixed or variable duration), 2) fixation cross is removed (fixed duration; e.g., 200ms), and 3) target appears. Thus, disappearance of the central fixation, regardless of study paradigm, always provides a fixed cue associated with the appearance of the target, whereas overlap trials, especially those with variable fixation durations and presented within randomized blocks of trials, reflect the most unpredictable trial type. Thus, slower SRT for overlap trials compared to gap trials may reflect delays (or failures) to respond to targets appearing without predictable antecedents. This is consistent with the findings of Robic and colleagues (2015), who showed that individuals with ASD perform more poorly in unstable versus stable contexts compared to their TD peers. The present study included a variable fixation duration and randomized stimulus presentation, which may have provided an unpredictable stimulus sequence resulting in increased percentage of no-shifts for overlap trials and reduced disengagement efficiency in ASD even in the presence of low-interest, simple stimuli.
Lastly, the present study also manipulated proximity of the target to the central fixation. Our rationale was that atypical orienting behavior - disengaging and shifting - to salient environmental stimuli often occurs to information that is remote relative to the child’s current focus. Additionally, prior work in the visual domain has demonstrated that children with ASD have difficulties in broadening or zooming out their attentional focus (Mann & Walker, 2003; Ronconi et al., 2018; Ronconi et al., 2013). We hypothesized that presentation of central fixation (which may facilitate a narrowing or zooming in of attention), may exacerbate disengagement impairment, especially to targets occurring at greater distances from the central location. However, differences in performance to near and far targets did not vary across groups, suggesting that the proximity of the to-be-attended information from the current focus of attention may not be associated with disengagement impairments in ASD.
What does slower saccadic RT on overlap trials, larger gap effects, and increased percentage of no-shift trials in ASD reflect?
“Disengagement” as measured by the gap effect is associated with both oculo-motor and attentional components. Gap effects derived from differences in latencies between overlap and baseline conditions result primarily from the release of oculo-motor inhibition due to removal of the central stimulus (Dorris & Munoz, 1995). The absence of group differences between overlap and baseline conditions for all measures (SRT, no-shift percentage, and disengagement efficiency) in the present study, as well as previous studies (Fischer et al., 2014; Fischer et al., 2016; Goldberg et al., 2002), suggests that impairments in disengagement do not derive from this source (although see Elsabbagh et al., 2013, for evidence of impaired disengagement using baseline trials). Rather, group differences become apparent in comparisons between gap and overlap trials. Thus, as outlined by Sabatos-Devito and colleagues (2016), atypical attentional disengagement, as measured by gap-overlap paradigms, may reflect an attentional rather than an oculomotor deficit. Failure to orient (i.e., no-shift trials), slower SRTs in the overlapay result from a more general ability to detect behaviorally-relevant events (i.e., the target), especially when targets are not preceded by a cue (i.e., the offset of the fixation) and occur in unpredictable situations.
Recently, we have shown that deficits in orienting to behaviorally-relevant information in children with ASD are associated with reduced activation of right temporal parietal junction (TPJ) (Keehn et al., 2016) and decreased alpha-band event-related desynchronization (Keehn et al., 2017). In addition, others (Orekhova & Stroganova, 2014; Stroganova et al., 2013) have also demonstrated atypical right-lateralized responses to auditory information, which may be associated with impaired re-orienting of attention. Activity of the right TPJ (a key node in the ventral attentional network) has been linked to the locus coeruleus - norepinephrine (LC-NE) system (Corbetta et al., 2008), which can be indirectly measured using both pupil dilation (Joshi et al., 2016) and event-related electrophysiological responses (e.g., P3 amplitude; Nieuwenhuis et al., 2005). Prior evidence of increased tonic activation, as evidenced by larger pupil diameter in ASD (Anderson & Colombo, 2009; Anderson et al., 2013), and reduced phasic activity, linked to smaller P3 amplitudes in ASD (see Jeste & Nelson, 2009, for review), suggest that ASD may be associated with atypical tonic and phasic LC-NE activation. Interestingly, this neurotransmitter system has also been hypothesized to play an important role in predictive coding (discussed above as a potential contributor to poorer overlap performance) and responding to unexpected environmental changes (Yu & Dayan, 2005). Thus, increased tonic activation and associated reductions in phasic responsiveness may lead to slower, more variable attentional disengagement in ASD, as the onset of peripheral targets does not result in robust phasic activity without a prior attentional cue, especially in the context of an unpredictable sequence of events.
Conclusion
Atypical orienting to auditory information is one of the earliest and most salient red flags that may indicate risk for ASD. Failure to respond to name and impairments in orienting to social sounds are present early and may contribute to atypical development of joint attention and language abilities in ASD (Dawson et al., 2004). The present study extends the findings of Sabatos-DeVito and colleagues (2016), who showed deficits in disengagement from multimodal (audio-visual) compared to unimodal (visual only) fixations in children with ASD, and suggest that impaired disengagement in ASD is also present for non-social unimodal auditory information. While these may present as subtle deficits in attentional disengagement in well-controlled laboratory settings, it is likely that these differences are exacerbated in real-world contexts, which are multi-modal, dynamic, engaging, and unpredictable. More recently, these disengaging and shifting impairments have been observed in more naturalistic environments as differences in flexibly allocating visual attention during play (Sacrey et al., 2013) and reduced orienting to caregiver touches (Kadlaskar et al., in press). The present results provide further support for the presence of atypical attentional disengagement in ASD, and suggest that these impairments include disengaging from and shifting to unimodal auditory information.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copy edited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed assent and consent was obtained from all individual participants included in the study.
Conflict of Interest
All authors report no conflict of interest.
References
- Anderson CJ, & Colombo J (2009). Larger tonic pupil size in young children with autism spectrum disorder. Dev Psychobiol, 51(2), 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, & Unruh KE (2013). Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev Psychobiol, 55(5), 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT (1999). Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29(3), 213–224. [DOI] [PubMed] [Google Scholar]
- Colombo J (2002). Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science, 11(6), 196–200. [Google Scholar]
- Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, & Maikranz JM (2001). Heart rate-defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Development, 72(6), 1605–1616. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, & Munoz DP (2005). fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol, 94(1), 605–611. [DOI] [PubMed] [Google Scholar]
- Constantino JM, & Gruber CP (2012). Social Responsiveness Scale - Second Edition (SRS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Corbetta M, Patel G, & Shulman GL (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, & Connolly JD (2008). Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol, 99(1), 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, & Brown E (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders, 28(6), 479–485. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, & Liaw J (2004). Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology, 40(2), 271–283. [DOI] [PubMed] [Google Scholar]
- Dorris MC, & Munoz DP (1995). A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol, 73(6), 2558–2562. [DOI] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, … Network I. (2013).White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry, 170(8), 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, & Johnson MH (2013). Disengagement of Visual Attention in Infancy Is Associated with Emerging Autism in Toddlerhood. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich R, Hughes HC, & Reuter-Lorenz PA (1991). Fixation-point offsets reduce the latency of saccades to acoustic targets. Percept Psychophys, 50(4), 383–387. [DOI] [PubMed] [Google Scholar]
- Fischer J, Koldewyn K, Jiang YV, & Kanwisher N (2014). Unimpaired Attentional Disengagement and Social Orienting in Children with Autism. Clin Psychol Sci, 2(2), 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Smith H, Martinez-Pedraza F, Carter AS, Kanwisher N, & Kaldy Z (2016).Unimpaired attentional disengagement in toddlers with autism spectrum disorder. Dev Sci, 19(6), 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ, & Van der Willigen RF (1995). Spatial and temporal factors determine auditory-visual interactions in human saccadic eye movements. Percept Psychophys, 57(6), 802–816. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, & Landa RJ (2002). Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia, 40(12), 2039–2049. [DOI] [PubMed] [Google Scholar]
- Jeste SS, & Nelson CA 3rd. (2009). Event related potentials in the understanding of autism spectrum disorders: an analytical review. Journal of Autism and Developmental Disorders, 39(3), 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, & Reeves A (2009). Attentional release in the saccadic gap effect. Vision Res, 49(16), 2045–2055. [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, & Gold JI (2016). Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron, 89(1), 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlaskar G, Seidl A, Tager-Flusberg H, Nelson C, & Keehn B (in press). Atypical response to caregiver touch in infants at high-risk for autism spectrum disorder Journal of Autism and Developmental Disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Muller RA, & Townsend J (2013). Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev, 37(2), 164–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Nair A, Lincoln AJ, Townsend J, & Muller RA (2016). Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Dev Cogn Neurosci, 17, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Westerfield M, Müller R-A, & Townsend J (2017). Autism, Attention, and Alpha Oscillations: An Electrophysiological Study of Attentional Capture. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingstone A, & Klein RM (1993). Visual offsets facilitate saccadic latency: Does predisengagement of visuospatial attention mediate this gap effect? Journal of Experimental Psychology: Human Perception and Performance, 19(6), 1251–1265. [DOI] [PubMed] [Google Scholar]
- Kleberg JL, Thorup E, & Falck-Ytter T (2017). Reduced visual disengagement but intact phasic alerting in young children with autism. Autism Res, 10(3), 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry O, & Parker A (2013). A meta-analysis of visual orienting in autism. Front Hum Neurosci, 7, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry R, & Bryson SE (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, 45(6), 1115–1122. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Obervation Schedule, Second Edition Torrance, CA: Western Psychological Services. [Google Scholar]
- Mann TA, & Walker P (2003). Autism and a deficit in broadening the spread of visual attention. Journal of Child Psychology and Psychiatry, 44(2), 274–284. [DOI] [PubMed] [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, & Rogers SJ (2007). A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med, 161(4), 378–383. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, & Cohen JD (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin, 131(4), 510–532. [DOI] [PubMed] [Google Scholar]
- O’Connor K (2012). Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev, 36(2), 836–854. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, & Stroganova TA (2014). Arousal and attention re-orienting in autism spectrum disorders: evidence from auditory event-related potentials. Front Hum Neurosci, 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling J, & Dawson G (1994). Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders, 24(3), 247–257. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G, & Munson JA (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology, 14(2), 239–251. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, & Rafal RD (1984). Effects of parietal injury on covert orienting of attention. Journal of Neuroscience, 4(7), 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robic S, Sonie S, Fonlupt P, Henaff MA, Touil N, Coricelli G,… Schmitz C (2015). Decision-making in a changing world: a study in autism spectrum disorders. J Autism Dev Disord, 45(6), 1603–1613 [DOI] [PubMed] [Google Scholar]
- Ronconi L, Devita M, Molteni M, Gori S, & Facoetti A (2018). Brief Report: When Large Becomes Slow: Zooming-Out Visual Attention Is Associated to Orienting Deficits in Autism. J Autism Dev Disord, 48(7), 2577–2584. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Gori S, Ruffmo M, Molteni M, & Facoetti A (2013). Zoom-out attentional impairment in children with autism spectrum disorder. Cortex, 49(4), 1025–1033. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, & Rosicky J (1994). Orienting in Normal and Pathological Development. Development and Psychopathology, 6(4), 635–652. [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). Social Communication Questionnaire (SCQ). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Sabatos-DeVito M, Schipul SE, Bulluck JC, Beiger A, & Baranek GT (2016). Eye Tracking Reveals Impaired Attentional Disengagement Associated with Sensory Response Patterns in Children with Autism. J Autism Dev Disord, 46(4), 1319–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LA, Armstrong VL, Bryson SE, & Zwaigenbaum L (2014). Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neurosci Biobehav Rev, 47, 559–577. [DOI] [PubMed] [Google Scholar]
- Sacrey LA, Bryson SE, & Zwaigenbaum L (2013). Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: a longitudinal study from 6 to 36 months of age. Behav Brain Res, 256, 441–450. [DOI] [PubMed] [Google Scholar]
- Salley B, Sheinkopf SJ, Neal-Beevers AR, Tenenbaum EJ, Miller-Loncar CL,T ronick E, … Lester BM (2016). Infants’ early visual attention and social engagement as developmental precursors to joint attention. Dev Psychol, 52(11), 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow MG (1967). Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America, 57(8), 1024–1029. [DOI] [PubMed] [Google Scholar]
- Schmitt LM, Cook EH, Sweeney JA, & Mosconi MW (2014). Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem. Mol Autism, 5(1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq R, Stuart GW, Sandbach J, Maruff P, & Currie J (1998). The gap effect and express saccades in the auditory modality. Exp Brain Res, 118(2), 221–229. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Kozunov VV, Posikera IN, Galuta IA, Gratchev VV, & Orekhova EV(2013). Abnormal pre-attentive arousal in young children with autism spectrum disorder contributes to their atypical auditory behavior: an ERP study. PLoS One, 8(7), e69100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, &Willmes K(2001). On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage, 14(1 Pt2), S76–84. [DOI] [PubMed] [Google Scholar]
- Taylor TL, Kingstone A, & Klein RM (1998). The disapperance of foveal and nonfoveal stimuli: Decomposing the gap effect. Canadian Journal of Experimental Psychology, 52(4), 192–199. [Google Scholar]
- Taylor TL, Klein RM, & Munoz DP (1999). Saccadic performance as a function of the presence and disappearance of auditory and visual fixation stimuli. J Cogn Neurosci, 11(2), 206–213. [DOI] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, & Wagemans J(2014). Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev, 121(4), 649–675. [DOI] [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, & van Engeland H (2001). Eye movements, visual attention, and autism: a saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry, 50(8), 614–619. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler’s Abbreviated Scale of Intelligence - Second Edition (WASI-II). San Antonio, TX: NCS Pearson. [Google Scholar]
- Werner E, Dawson G, Osterling J, & Dinno N (2000). Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J Autism Dev Disord, 30(2), 157–162. [DOI] [PubMed] [Google Scholar]
- Wilson CE, & Saldana D (2018). No evidence of atypical attentional disengagement in autism: A study across the spectrum. Autism, 1362361318768025. [DOI] [PubMed] [Google Scholar]
- Yu AJ, & Dayan P (2005). Uncertainty, neuromodulation, and attention. Neuron, 46(4), 681–692. [DOI] [PubMed] [Google Scholar]
- Zalla T, Seassau M, Cazalis F, Gras D, & Leboyer M (2018). Saccadic eye movements in adults with high-functioning autism spectrum disorder. Autism, 22(2), 195–204. [DOI] [PubMed] [Google Scholar]
- Zambarbieri D (2002). The latency of saccades toward auditory targets in humans. Prog Brain Res, 140, 51–59. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, & Szatmari P (2005) . Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2–3), 143–152. [DOI] [PubMed] [Google Scholar]