Abstract
Tissue engineering has emerged as an important research area that provides numerous research tools for the fabrication of biologically functional constructs that can be used in drug discovery, disease modeling, and the treatment of diseased or injured organs. From a materials point of view, scaffolds have become an important part of tissue engineering activities and are usually used to form an environment supporting cellular growth, differentiation, and maturation. Among various materials used as scaffolds, hydrogels based on natural polymers are considered one of the most suitable groups of materials for creating tissue engineering scaffolds. Natural hydrogels, however, do not always provide the physicochemical and biological characteristics and properties required for optimal cell growth. In this review, we discuss the structure and properties of widely used natural hydrogels. In addition, we present methods of modulation of their physicochemical and biological properties using soft nanoparticles as fillers or reinforcing agents.
Keywords: Nanofunctionalization, Natural Hydrogels, Soft nanoparticles, Tissue engineering
1. Introduction
Tissue engineered constructs have now found various new applications, which are beyond the initial goal of their use for replacing damaged or diseased tissues. Tissue engineered constructs are emerging as research tools that could improve our understanding of biological processes[1–3] and pathophysiology of diseases.[4,5] In addition, the use of patient-specific cells and biological factors is expected to facilitate the development of personalized therapies.[6–8]
More complex, yet functional tissues or organoids can be fabricated by combining the advances in biology, on-chip technologies, biomanufacturing, biomaterials, and drug delivery.[9–15] Despite recent progress, there are still many challenges that remain to be addressed.[16–20] For example, the formation of a niche that supports cellular growth, differentiation, and function is still the subject of many research studies. The natural extracellular environment is comprised of a highly defined microarchitecture formed from various proteins, polysaccharides, and glycosaminoglycans (GAGs); resulting in modulation of cell-level and tissue-level physical and chemical properties.[21–23]
The presence of a cocktail of factors affecting biological processes at different stages of tissue development and maturation combined with proper oxygenation, as well as nutrient transport result in the development and function of different tissues and organs in the human body.[24,25] As discussed extensively by Clegg et al. mimicking these properties in engineered tissue constructs, although desirable, is not trivial.[26]
To facilitate the formation of functional tissues, advanced biomaterials with controlled physical, chemical, biological, and electrical properties should be designed.[2,27–30] Hydrogels are one of the few biomaterials that possess properties required for tissue engineering applications.[25,31] Hydrogels are crosslinked three-dimensional (3D) hydrophilic polymer networks, which form matrices with a high water content of up to a thousand times their dry weight.[32] They possess tunable physical and biological properties, native extracellular matrix (ECM) similarity, high biocompatibility, and robustness in biofabrication.[14,33,34] With these combined characteristics, hydrogels are excellent candidates for biomedical applications,[35–37] drug delivery,[38–41] as well as tissue engineering and regenerative medicine.[33,42–44]
Nonetheless, the majority of existing hydrogels cannot properly mimic all the physical, chemical, and biological properties of native ECM at the same time. Thus, the idea of developing hybrid and composite systems in which nano/micro-features are incorporated to modulate some of these properties have drawn noticeable attention.[2] Soft and hard nanoparticles can be incorporated as fillers in the hydrogel matrix, to yield nanofunctionalized hydrogels with tailored properties.[45,46] Here, we review hybrid and composite hydrogel systems produced from natural polymers that are functionalized with soft nanoparticles. We initially discuss the properties of different types of natural hydrogels. The effects of soft nanoparticles on the physical and biological properties of natural hydrogels will also be highlighted. The challenges and potential opportunities in the field are also outlined.
2. Hydrogels from Natural Polymers
Hydrogels are formed from natural or synthetic polymers or their mixtures. Each of the two classes offers a set of advantages and disadvantages, listed in Table 1. In this section, we briefly introduce some of the natural hydrogels frequently used in tissue engineering. The readers are referred to several reviews for a more comprehensive overview of natural hydrogels.[47–53]
Table 1.
Advantages and disadvantages of using natural or synthetic polymers for the preparation of hydrogels.[54,55]
| Natural Hydrogels | Synthetic Hydrogels | |
|---|---|---|
| Advantages | • Non-toxic • Biocompatible • Biodegradable • Promote cells adhesion • Promote cells growth • Promote cells proliferation • Promote cells differentiation • Promote cells ECM secretion |
• Controllable microstructure • Controllable degradation • Long shelf life • Tailored functionality • Strong mechanical properties • Wide varieties of raw chemical resources |
| Disadvantages | • Low mechanical strength • Batch variation • Risk of disease transmission (ECM-based hydrogel) |
• Low biocompatibility • Risk of inflammatory response • Risk of immunological response |
2.1. Polysaccharide-based hydrogels
Polysaccharides are carbohydrate polymers which can break into physiological breakdown upon degradation. Polysaccharides are biocompatible, usually degradable, and possess tunable mechanical properties. In addition, polysaccharides are a major constituent of native ECM. Thus, hydrogels formed from different polysaccharides have been widely used in tissue engineering, regenerative medicine, and drug delivery. The most common polysaccharide-based hydrogels that have been utilized for tissue engineering scaffolds are alginate,[56] chitosan,[57] hyaluronic acid,[58] and cellulose.[59] Although, hydrogels formed from other polysaccharides such as chitin, gellan gum, etc. have also been utilized for tissue engineering applications.[60,61]
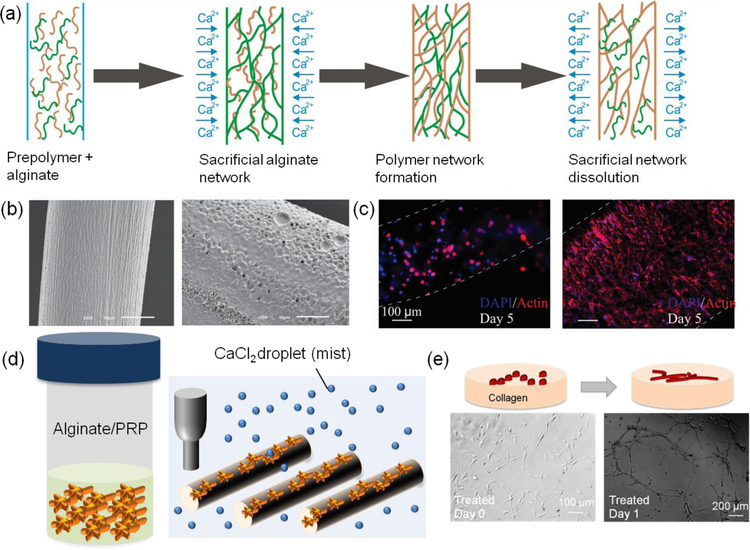
In a recent study, alginate was used to carry platelet-rich plasma (PRP) that can release a cocktail of biological factors essential for tissue healing and growth (Figure 1d, e). The utilized hydrogels could facilitate vascularization and stem cell migration. In addition, the fabrication of an interpenetrating network of polymers from alginate and a protein-based hydrogel with cell binding sequences has also been shown to significantly improve the biological activity of the hydrogels (Figure 1a–c). Key reasons for the popularity of alginate-based hydrogels in tissue engineering applications are its stability, ease-of-handle, and fast crosslinking process.
Figure 1.
Alginate-based hydrogels in tissue engineering applications. (a) The use of alginate for engineering IPN hydrogels with various materials or its use as a sacrificial network for creating fibers from polymers and protein-based hydrogels. (b) SEM image of IPN fibers of GelMA and alginate (left) and the removal of alginate from the construct to fabricate pure GelMA fibers (right). (c) Cellular morphology shown by F-actin staining in IPN fibers of GelMA and alginate (left) and GelMA fibers after the removal alginate from the network (right). Reproduced with permission.[62] Copyright 2015, John Wiley and Sons. (d) The use of alginate for carrying PRP as a source of biological factors in tissue engineering. The hydrogel fibers could be printed in the presence of CaCl2 mist on dry substrates. (e) The effect of PRP encapsulated in alginate in releasing angiogenic factors facilitating vascularization. Reproduced with permission.[6] Copyright 2018, John Wiley and Sons.
2.2. Protein-based hydrogels
Proteins are a major constituent of ECM and they are responsible for the biological and mechanical characteristics of native tissues. Collagen by far is the most abundant protein in the human body. However, other proteins such as fibronectin, elastin, and laminin are also found in noticeable quantities in specific tissues. It is now widely accepted that the composition and physical properties of the environment significantly affect cellular fate and function. Thus, to mimic the native ECM, many research studies have focused on engineering scaffolds from various proteins or a combination of them in ECM. In comparison to different natural protein-based hydrogels, those that are formed from collagen or its denatured form (gelatin) have been extremely popular in tissue engineering.[53,63] Fibronectin-, elastin-, laminin-based hydrogels have also been utilized in tissue engineering. Each of these hydrogels offers unique and interesting properties, which make them suitable for engineering specific tissues.
In one study, the effect of hydrogel composition on the growth pattern of endothelial cells was studied (Figure 2a–d).[64] In this study, fractal-like dense cultures of endothelial cells with different dimensions were generated and covered by a layer of hydrogel. The results showed a distinct growth and migration pattern within the fabricated protein-based hydrogels. In that study, only hydrogels formed from Type I collagen could support the formation of vessel-like structures. Fibronectin-based gels have also shown superior support in the growth of endothelial cells and are being utilized for engineering constructs which require rapid vascularization.[65] In another noticeable example, an elastin-based hydrogel was extremely resilient against mechanical stretch and torsion (Figure 2e,f).[66] As the presence of each protein is expected to affect the function of cells, efforts have been made to utilize the whole ECM to form hydrogels.
Figure 2.
Protein-based hydrogels as scaffolding materials for tissue engineering. (a-c) The growth of endothelial cells cultured in vascular-like organizations in different protein-based hydrogels including Matrigel (a), GelMA (b), Collagen (c). The patterns of cellular migration into the hydrogel constructs were fundamentally different. Among them, only collagen supported the formation of tubular sprouts. Reproduced with permission.[64] Copyright 2016, John Wiley and Sons. (d) The density of cells within the original patterns was also dependent on the material. (e,f) The fabrication of highly elastic hydrogel networks from photocrosslinkable methacrylated tropoelastin (MeTro). The fabricated hydrogel showed excellent torsional resilience. Reproduced with permission.[66] Copyright 2015, John Wiley and Sons.
While hydrogels offer high porosity, favorable transport properties, and tunable mechanical properties, they do have various limitations affecting the possibility of creating a tissue biomimetic environment. For example, hydrogel systems lack factors that can initiate or facilitate physiological and biological processes crucial for tissue formation and maturation. Thus, modulating their properties to become more biomimetic has been the subject of numerous researches. Engineering nanocomposite hydrogels with the use of suitable nanomaterials have shown to be effective in addressing these challenges and will be discussed in the following sections.
3. Nanofunctionalized Hydrogels
The physicochemical properties of biomaterials and their biological activity affect the response of cells or tissues embedded within or interfaced with them. The physical properties of natural hydrogels including their mechanical and electrical properties can deviate from those observed in native tissues. In addition, the presence of various biological factors and proteins play a key role in the growth and maturation of the engineered tissue-like constructs. There have been various ways for modulating the physical and biological properties of the environment of natural hydrogels; this includes creating composite fibers and constructs, forming double or multiple interpenetrating polymer networks,[67] and incorporation of nano/micro-features. These strategies have been used for modulation of the mechanical and electrical properties at the cellular or tissue level providing topography for guiding cellular morphology and growth, as well as the slow release of various growth factors.[53]
Among different strategies, the incorporation of nanoparticles has drawn conspicuous attention due to their high surface-area-to-volume ratio, ease-of-delivery, and the ability to target cellular components rather than an entire cell or tissue.[68] Additionally, the use of nanoscale particles enables a bottom-up approach for engineering the hydrogel niche. For example, similar to the formation of reinforced bricks from mud and straw, nanoparticle incorporation in the hydrogel matrix will strengthen and reinforce the hydrogel structure, while adding new functionalities.
These properties have led to the widespread use of nanoparticles of various biomaterials in biomedical applications, such as controlled drug and gene delivery,[69] tissue engineering and regenerative medicine,[70] biosensors,[71,72] bioimaging,[73] and bioseparation.[74]
Nanoparticles can be generally classified as hard and soft nanoparticles.[75] Hard nanoparticles typically are referred to those with a compressive modulus that is significantly different from the value for natural hydrogels. Hard nanoparticles can be fabricated from materials such as silica and gold, quantum dots, carbon nanotubes, graphene sheets, and polymeric nanoparticles. Whereas soft nanoparticles with mechanical properties comparable to hydrogels include liposomes, dendrimers, polymeric micelles, and nanogels.[76–78]
Hard nanoparticles possess various intrinsic functionalities, highly ordered structures, and are usually stable during their circulation and storage. On the contrary, they can cause an adverse inflammatory response and can induce toxicity upon their accumulation in different tissues.[79,80] Another major concern associated with the use of inorganic hard nanoparticles has been their carcinogenicity. On the other hand, soft nanoparticles (Table 2) manifest non-conducting, insulator-type properties associated with covalent, flexible, organic-like structures and assemblies.[75] Moreover, these nanoparticles are typically biodegradable and are considered more biocompatible in comparison to their hard inorganic counterparts. Thus, in biomedical applications especially in tissue engineering, the use of soft organic nanoparticles should be prioritized. In this review, we mainly focus on soft nanoparticles and how they can modulate various properties of natural hydrogels. A number of reviews on the use of hard nanoparticles in tissue engineering and drug delivery have recently been published.[2,81]
Table 2.
Comparing the characteristics of four soft nanoparticles: nanoliposomes, dendrimers, polymeric micelles, and nanogels. Images reproduced with permission.[126,127]
| Type | Nanoliposomes
|
Dendrimers
|
Polymeric Micelles
|
Nanogels
|
|---|---|---|---|---|
| Nature | • Natural | • Synthetic | • Synthetic | • Natural • Synthetic |
| Size | • ~50 nm | • 2–15 nm | • 10–100 nm | • <100 nm |
| Preparation methods | • Microfluidization • Extrusion • Sonication |
• Convergent • Divergent |
• Direct dissolution • Film casting • Dialysis • Oil in water emulsion |
• Physical self-assembly of interactive polymers • Chemical synthesis in colloidal environments • Chemical crosslinking of preformed polymers • Template-assisted nanofabrication |
| Adv. | • Biocompatible • Biodegradable • Non-toxic • Non-immunogenic • Controlled and targeted drug delivery • Sustained release • Increase drug efficacy and stability • Many administration routes |
• Biodegradable • Very small size • Well-defined and flexible structure • Precise controllability • High deformability • Stimuli-responsiveness • Surface functionality |
• Small size • Narrow distribution • Easy sterilization • High structural stability • Low toxicity • Excellent blood stability • High water solubility • Controlled release functions |
• Biocompatible • Large surface area • Stimuli sensitivity • High water content/swellability and hydrophilicity • Tunable nanoparticle size • Site targeting • Controllable release • Increased drug stability |
| Disadv. | • Low solubility • Short half-life • High production cost • Difficult sterilization • Lysosomal degradation • Low efficacy active targeting |
• Low biocompatibility • Significant liver accumulation • Material’s homogeneity deterioration • Great batch-to-batch variability |
• Low biocompatibility • Difficult synthesis • Difficult to scale-up • Limited choice of monomers • Concerns over nanotoxicity and storage stability |
• Challenging optimization of degradation mechanism, biodistribution, and component toxicity • Drug instability and rapid degradation in the bloodstream |
| REF. | [92,96,128] | [101,129] | [128,130–132] | [114,127,133] |
3.1. Nanoliposomes
Liposomes are continuous, closed, and round-shaped vesicles formed from one or several phospholipid bilayers dispersed in an aqueous medium.[82] Phospholipids are amphiphilic molecules, composed of a hydrophobic lipid soluble tail segment and a hydrophilic water-soluble head segment. Liposomes have been widely used in drug and gene delivery,[83–86] and in tissue engineering.[87,88] Liposomes with submicron sizes are called nanoliposomes. They are around 50–200 nm in size and are extensively used for the encapsulation and controlled release systems of bioactive agents in the food, cosmetic, and pharmaceutical industries.[89–92] Nanoliposomes are natural soft nanoparticles, biocompatible, biodegradable, easy-to-fabricate, easy-to-decorate, and possess low toxicity.[93–95] Nanoliposomes can be prepared by sonication, extrusion, or microfluidization method.[92,96,97] Nanoliposomes provide more surface area than liposomes and have the potential to significantly improve the controlled release, enhanced bioavailability, increased solubility, and enabled precision targeting of the encapsulated material.[92] Being amphiphilic, nanoliposomes are able to increase the in vivo and in vitro stability of hydrophobic drugs or molecules by embedding them in the lipid bilayer or encapsulating them in the central aqueous cavity.[94,98]
3.2. Dendrimers
Medical and pharmaceutical properties of the various star and star-shaped polymers have attracted considerable attention by researchers interested in the development of various polymeric systems with architectures other than linear systems. Polymeric systems with such architectures include ladder, star and comb polymers, which have some degree of a three-dimensional character. Star and star-shaped polymers are molecules of hyperbranched structures that emanate from a central core and consist of a large number of terminal groups with a definite geometrical growth.
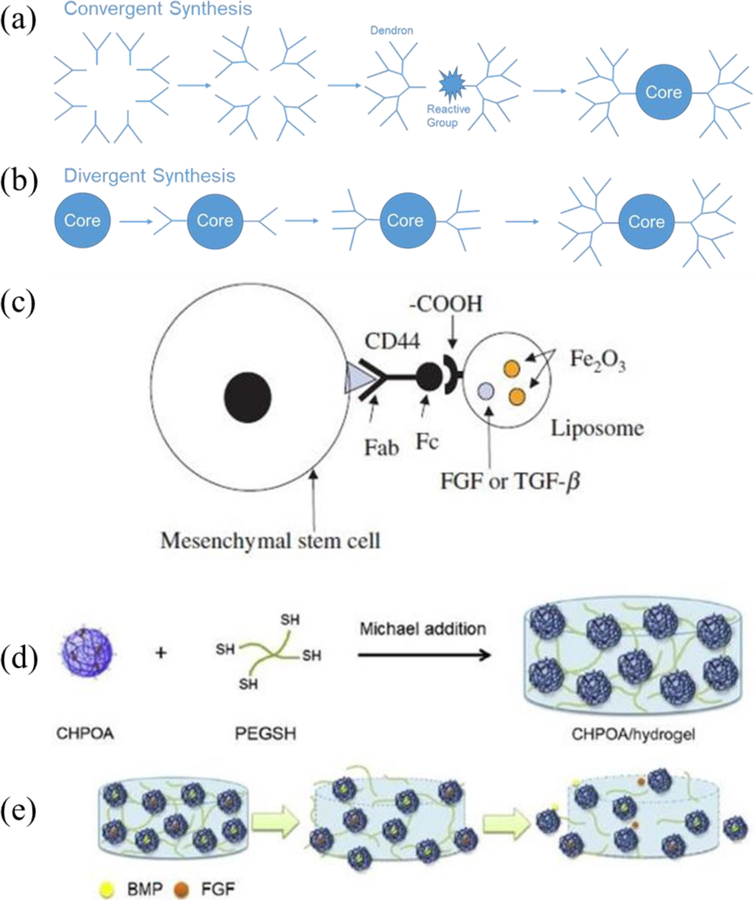
Dendrimers are highly branched and symmetrical polymeric molecules composed of numerous perfectly branched monomers that originate from a central core.[99] A dendrimer molecule is composed of an interior core, several layers composed of repeating units called dendrons, and multiple active terminal groups.[100] Dendrimers are generally synthesized by the divergent and convergent methods (Figure 4a, b). In the first approach, synthesis is initiated at the center of the star polymer, whereas in the second approach, synthesis starts in the outside of the dendrimer.
Figure 4.
The schematic representation of (a) convergent and (b) divergent synthesis of dendrimers. (c) Schematic of autologous bone marrow mesenchymal stem cell–liposome complex. Reproduced with permission.[167] (d) Synthesis of the CHPOA/hydrogel block by Michael addition. (e) Schematic representation of nanogels releasing FGF18 and BMP2 after disintegration. Reproduced with permission.[168] Copyright 2009, American Chemical Society.
Dendrimers are widely used in the biomedical field, especially in nanomedicine because it is possible to control their molecular weight and chemical composition.[99,101] Thereby, it is possible to control their polyvalency properties, biocompatibility, bioactivity, and pharmacokinetics. Dendrimers provide the ability to develop drug-loaded biomaterials by simple functionalization of their external groups, and increase the efficiency of drug loading or increase electrostatic interaction with the anionic bioactive agent.[102] Moreover, they provide sustained drug release, and the ability to enhance the solubility of hydrophobic drugs. Polyamidoamine (PAMAM) dendrimers, the most common class of dendrimers, were used for bioimaging,[103] as drugs,[104] drug carriers,[105] and gene carriers.[106] Polypeptide and polyester dendrimers were used as scaffolds for tissue repair,[107,108] as well as drug carriers.[109,110]
3.3. Polymeric micelles
Polymeric micelles are self-assemblies (10–200 nm in diameter) of amphiphilic polymers (hydrophobic core and hydrophilic shell) in an aqueous environment with remarkable therapeutic potential. They are formed through self-assembly into a core-shell micellar structure (hydrophilic shell and hydrophobic core) of block copolymers comprising two or more polymeric chains with different hydrophobicity.[111] These polymers are chemically different and covalently attached to each other. Since one of the polymers is hydrophobic and the other is hydrophilic, when they are present in an aqueous solution, many unfavorable interactions between the hydrophobic polymer and water molecules will occur. Thus, at a specific and narrow concentration range of amphiphilic polymers in aqueous solution, called the critical micelle concentration, the amphiphilic block copolymers will self-assemble into colloidal-sized particles or micelles. Resulting in the removal of the hydrophobic polymer from solution.
Preparation methods for drug-loaded polymeric micelles are dependent on the solubility of the copolymer being used. Direct dissolution or film casting methods may be employed if the copolymer is relatively water soluble, whereas dialysis method or oil in water emulsion procedure may be employed if the copolymer is not readily soluble in water.[112] On account of their small size, controlled release of drugs, aqueous solubility enhancement of carried drugs, and simple sterilization; polymeric micelles are an ideal carrier for hydrophobic drugs.[113]
3.4. Nanogels
Nanogels are nanometer-sized (<100 nm) crosslinked colloidal particles that may also respond to environmental changes (pH, temperature, ionic strength, presence of molecules or ions, light) and to external fields (magnetic and electric) by changing their volume significantly.[114] Nanogels can be chemically crosslinked using covalent bonds or physically crosslinked using non-covalent bonds.[115–117] This response can be used to control the release of encapsulated bioactive compounds such as drugs, proteins, DNA, and RNA [115]. Nanogels preparation methods can be divided into four categories: template-assisted nanofabrication of nanogels particles, polymerization of monomers in homogeneous or heterogeneous environments, physical self-assembly of interactive polymers, and chemical crosslinking of preformed polymers [118]. Due to their encapsulation stability, biocompatibility, water solubility, control of drug release rate, and reduction of toxicity; nanogels have been used in drug delivery as drug delivery vehicles[119] and in tissue engineering as scaffolds.[120–122]
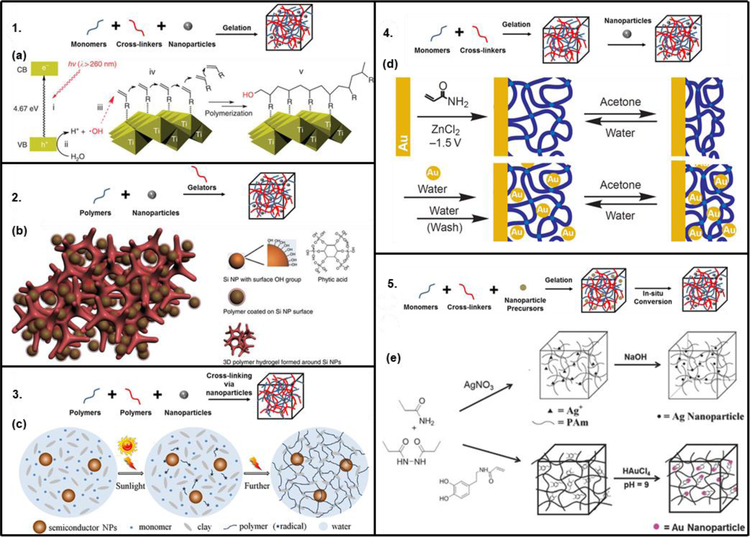
In general, among all soft nanoparticles, nanoliposomes offer superior biocompatibility, ease of surface modification, favorable pharmacokinetic profile, and long circulation time.[123] However, liposomes suffer from the disadvantages of fast elimination from the blood and the capture by the cells of the reticuloendothelial system.[124] On the other hand, polymeric nanoparticles (dendrimers, micelles, and nanogels) are superior in terms of controlled release capability, versatile drug loading, improved stability in biological fluids, desired pharmacokinetics, and high cellular internalization efficiency.[125] Overall, different techniques, presented in Figure 3, can be used to incorporate nanoparticles into the hydrogel matrix and they can be divided into 5 groups.[46] Even though the examples presented are for hard nanoparticles, the same techniques can be used to incorporate soft nanoparticles.
Figure 3.
Five main nanofunctionalization techniques with their relative examples (Adapted from [46]): 1. hydrogel formation in a nanoparticle suspension, 2. gel formation using nanoparticles, polymers, and distinct gelator molecules, 3. cross-linking using nanoparticles to form hydrogels, 4. physical incorporation after gelation of nanoparticles into the hydrogel matrix, and 5. formation of reactive nanoparticle within a preformed gel. (a) Schematic of the usage of the photo catalyst, titania nano sheets, for gelation. Reproduced with permission.[136] (b) Schematic illustration of 3D porous silicon-nanoparticles/conductive polymer hydrogel. Reproduced with permission.[137] (c) Cross-linking using semiconductor nanoparticles, monomer, and clay nanostructure to form nanoparticle-hydrogel composites with enhanced mechanical properties. Reproduced with permission.[139] (d) The switch between its swollen and shrunken states resulting in the construction of a gold-nanoparticle/hydrogel composite. Reproduced with permission.[140] (e) Preparation of Ag/PAAm hydrogel composite without using thiols. Reproduced with permission.[143] Hydrogel nanofunctionalization with gold nanoparticles resulting in catalytic hydrogels. Reproduced with permission.[144] Copyright 2014, American Chemical Society.
The gelation of a hydrogel-forming monomer solution, in which pre-formed nanoparticles are suspended, is the easiest method. However, if the crosslink density is low, the risk of the leaching nanoparticles out of the hydrogel matrix may exist.[134,135] Liu et al. synthesized photo-modulable thermo-responsive hydrogels using unilamellar titania nanosheets as photocatalytic crosslinkers (Figure 3a).[136]
Distinct gelator molecules can be used to crosslink polymers incorporating nanoparticles into a hydrogel matrix. This method has been used to create 3D porous silicon nanoparticles/conductive polymer hydrogel composite electrodes by encapsulating the silicon nanoparticles within a conductive polymer surface coating and connecting them to a highly porous hydrogel framework (Figure 3b).[137]
Crosslinking groups present on the nanoparticle surface can be used to form a hydrogel matrix.[138] One recent example of this approach is the synthesis, by Zhang et al., of semiconductor nanoparticle-based hydrogels by self-initiated polymerization using light irradiation (Figure 3c).[139] Semiconductor nanoparticles were used here to initiate monomer polymerization under sunlight and to crosslink it to form nanocomposite hydrogels with the help of clay nanosheets.
Physical incorporation of nanoparticles can occur after the polymerization formation of the hydrogel. The physical incorporation can be achieved by either the “breathing” method or by centrifugation/thermal annealing method. The “breathing” consists of placing the swollen hydrogel into a solvent which causes it to get rid of entrapped water and shrink, then in an aqueous solution containing nanoparticles where it causes the hydrogel to swell and “breath in” the nanoparticles. Even with extra “breathing out” cycles the nanoparticles concentration in the hydrogel remains intact due to physical entanglement and to hydrogen bond interactions. This method was used to construct a gold-nanoparticle/hydrogel composite at the electrode interface (Figure 3d).[140] The other approach consists on incorporating the nanoparticles into the hydrogel matrix by repeated heating, centrifugation and re-dispersion followed by annealing. This was used by Jones and Lyon to co-assemble poly-N-isopropylacrylamide hydrogel particles and nanosized colloidal Au into colloidal crystals.[141]
In-situ formation of nanoparticles, in which nanoparticle precursors can be loaded into the matrix before gelation, followed by nanoparticles formation supported by the hydrogel network, was developed by Langer’s group.[142] This method produces mechanically strong composite hydrogels without an external reducing agent. Saravanan et al. used this method to synthesize silver nanoparticles containing polyacrylamide hydrogel composites by free-radical cross-linking polymerization of acrylamide monomer in an aqueous medium containing Ag+ ions (Figure 3e).[143] In a more recent example, Marcelo et al. used redox active catechol side chain in acrylamide-NIPAAm hydrogels to produce gold nanoparticles from precursors already incorporated and in the absence of any external reducing agent (Figure 3e).[144]
4. Properties of Soft Nanoparticle-Functionalized Hydrogels
The effectiveness of the scaffolds in tissue engineering applications depends on how closely they can mimic native tissues. It has been shown that physical properties (mechanical and electrical), chemical composition, pore size distribution, and biological activity affect cellular growth, alignment, differentiation, and function. On the contrary, some natural hydrogels resembling the structure and in some cases the composition of native ECM fail to offer suitable mechanical and electrical properties, both at cellular and tissue levels. In addition, biological factors essential for cellular functions including phenotyping, differentiation, and maturation are typically not available in natural hydrogels. The incorporation of soft nanoparticles is a promising method for modulating the properties of scaffolds made of natural hydrogels, inherently affecting the response of incorporated cells. As the evaluation of biomaterial biocompatibility/biological response continues to be a challenge,[145] the use of biocompatible soft nanoparticles is favored in comparison to their hard counterparts. In this section, we highlight how the incorporation of soft nanoparticles within natural hydrogels can improve the physical and biological outcome for various tissue engineering applications.
4.1. Hydrogels with modulated physical properties
Physical specifications of hydrogel constructs, such as mechanical properties and electric conductivity, play a vital role in maintaining their 3D architecture, in mechanical interaction with cells, and in inducing cell-to-cell signaling.[146] Providing high tensile strength to the fragile hydrogels while mimicking the electrical stimulations that occur naturally in the body, is extremely important when engineering load-bearing or electrophysiologically active tissues.[147] While imitating the electrical conductivity in tissues remains work in progress, functionalizing hydrogels to reinforce their soft structures to mimic the elastic moduli of native tissues is essential. The elastic modulus of bone has been found to be directly correlated to specimen size, however, it has been previously reported that trabecular and cortical bone have an averaged modulus around 4.59 and 5.44 GPa, respectively.[148] In comparison, muscle and connective tissue can range on average from 12 – 134 kPa.[149] Myocardial tissue also has a wide range of elastic modulus, which is dependent on the beginning and end of diastole; ranging from tens of kPa to several hundred.[150] More recently, dynamic loading was used to determine the shear and bulk modulus of soft tissues: liver (37 – 340 kPa, 0.28 GPa), heart (60 – 148 kPa, 0.49 GPa), stomach (8 – 45 kPa, 0.48 GPa), and lung (10 – 54 kPa, 0.15 GPa).[151] Physical properties can be improved with the incorporation of predominantly hard nanoparticles within the hydrogel network,[152] however, there are a few examples that have demonstrated changes in mechanical properties of hydrogel constructs with the incorporation of soft nanoparticles.
For example, Xiao et al. created an amphiphilic block copolymer that self-assembled into micelles of 21 nm diameter and incorporated them in poly(acrylamide) hydrogels.[153] By varying the block copolymer micelle concentration, the mechanical properties of these highly elastomeric block copolymer micelle crosslinked hydrogels were able to be controlled. The increase in micelle concentration from 7.5 to 15 mg.mL−1 achieved a 4-fold increase in Young’s modulus and a 2-fold increase in tensile stress.
Soft nanoparticles can interact with the polymer chains or can help with the further crosslinking of the hydrogel network to improve its mechanical properties. In one study, Duan and Sheardown used polypropyleneimine octaamine dendrimers to crosslink a highly concentrated collagen solution (2–4%) using the water-soluble carbodiimide EDC.[154] When compared with the natural human cornea, as well as EDC and glutaraldehyde cross-linked collagen, the dendrimer crosslinked collagen showed better optical transparency, mechanical properties, adhesion ability, and glucose permeability. This was due to an increase in free amine groups that react with activated carboxylic acid groups to crosslink the collagen hydrogel. The presence of dendrimers did not adversely affect the biocompatibility of the gels, which was confirmed by the in vitro culture of human corneal epithelial cells on dendrimer cross-linked collagen gels. Human corneal epithelial cell growth and adhesion were supported in these dendrimer crosslinked collagen gels with no cell toxicity; implying that they might be suitable scaffolds for corneal tissue engineering.
Rahali et al. functionalized GelMA hydrogels with naturally derived nanoliposomes and reported that the incorporation of nanoliposomes enhanced the mechanical stability of the functionalized GelMA hydrogels; proven by their higher resistance to twist and shear.[155] Similarly, it was also demonstrated that the incorporation of nanoliposomes within alginate/GelMA hydrogels affected the rheological properties of the formed hydrogels, as well as their mechanical properties.[156] This observation was related to the ability of the alginate-gelatin mixture to tune the mechanical properties of refined architectures.
Overall, modulating the physical properties of hydrogels is important in optimizing the cellular response. The incorporation of soft nanoparticles can moderately modulate the local and global mechanical properties; however, they are generally electrically non-conductive and cannot positively affect global conductivity. Despite their moderate effects in comparison to hard inorganic particles; soft nanoparticles offer better biocompatibility, along with superior drug transportation abilities, and factors further assisting the modulation of the cellular environment.
4.2. Hydrogels with enhanced availability of biological factors and drugs
It is very important for tissue engineering scaffolds to possess strong mechanical properties similar to native tissues, a well-defined 3D microstructure with interconnected pores, and a suitable biodegradability rate. Another key factor is the presence of biological factors within the scaffolds to facilitate tissue regeneration and growth.[157] Be that as it may, usually natural hydrogels lack sufficient growth factors essential for cellular functions. To address this challenge, the field of drug delivery goes hand-in-hand with tissue engineering to design an ideal scaffold. Scaffolds have evolved from releasing single molecules within simple hydrogel systems to releasing multiple molecules in a sequential manner from advanced systems.[158–162] Many drug delivery and tissue engineering applications, summarized in Table 3, are based on soft nanoparticles. These nanoparticles have also been incorporated within hydrogels as carriers for controlled or on-demand release of necessary drugs and growth factors. As a result, scaffolds produced from hydrogels formed from natural polymers functionalized with natural soft nanoparticles are highly desirable.
Table 3.
Biomedical applications of functionalized natural hydrogels by soft nanoparticles. Images reproduced with permission.[126,127]
| Nanoparticle | Natural Hydrogel | Application | Properties | REF. |
|---|---|---|---|---|
Nanoliposomes
|
• Alginate | • Bone tissue engineering • siRNA delivery • Protein delivery system |
• Physical • Biological • Biological |
• [182] • [183] • [184] |
| • Collagen | • Cartilage tissue engineering • Bone tissue engineering • Wound healing |
• Biological • Biological • Biological |
• [167] • [165] • [185] |
|
| • Chitosan | • Cancer | • Biological | • [186] | |
| • Hyaluronic acid | • Bone tissue engineering • Ocular pathologies |
• Physical • Physical |
• [166] • [187] |
|
Dendrimers
|
• Collagen | • Corneal tissue engineering • Gene delivery system |
• Physical • Biological |
• [154] • [188] |
Polymeric Micelles
|
• Hyaluronic acid | • Cartilage tissue engineering | • Physical | • [189] |
Nanogels
|
• Cholesterol-bearing pullulan | • Bone tissue engineering | • Biological | • [168] |
| • Chitosan | • Bone tissue engineering | • Biological | • [174] |
The addition of bioactive molecules to biomaterial scaffolds, such as growth factors, can significantly enhance regenerative scaffolds.[163] However, larger pore sizes of natural hydrogels in comparison to the size of active compounds reduce their residence time within the hydrogel limiting their effectiveness. Thus, nanoparticles that can prolong the release time of active compounds could potentially overcome this challenge. Liposomes could be used as carriers for delivery systems by covalent conjugation of carboxyl groups of HA to amine groups of liposomes. This has the potential to increase the circulation time in the body and accumulation of encapsulated drugs in the intended site. Taetz et al. used conjugated liposome as a siRNA carrier to lung cancer cells.[164] Furthermore, one example of this involves a minimally invasive bone reconstruction system was created by Pederson et al., which consisted of a combination of calcium and phosphate-loaded liposomes with an acid-soluble collagen solution.[165] This liposome/collagen precursor fluid forms a mineralized collagen gel when heated from room temperature to body temperature (37 °C). Self-assembling collagen contained within a liposome-encompassing suspension was then shown to be integrated into an injectable mineral/collagen biomaterial. In another instance, Samadikuchaksaraei et al. employed nanoliposomes as the nucleation site for the synthesis of nano-hydroxyapatite particles under hydrothermal conditions.[166] A nano-hydroxyapatite/gelatin nanocomposite scaffold was conditioned with osteoblasts and showed an increase in biocompatibility, biodegradation, and osteoinduction. These findings suggest that this method can be used to develop a variety of bone tissue engineering scaffolds.
Ochi et al. introduced a new technique for tissue-engineered cartilage transplantation, illustrated in Figure 4c, with a minimally invasive procedure.[167] The novel scaffold was composed of a collagen hydrogel (Atelocollagen) embedded with human chondrocytes. The group also suggested that integrating magnetic liposomes for controlled release of cytokines or growth factors (FGF or TGF-α) can ameliorate cell proliferation and ECM synthesis during cultivation. In this study, magnetic liposomes were successfully concentrated and maintained within the defect area to further improve the bioavailability of the incorporated factors.
Stem cells are able to generate mature cells of a particular tissue upon proper differentiation.[169] Transforming growth factor-beta 1, TGF-β1, is a mammalian protein that plays a role in stem cells differentiation,[170] proliferation,[171] and metabolic activities. [172] In one study, Dostert et al. tested the effect of TGF-β1 encapsulated in salmon-derived nanoliposomes on human mesenchymal stem cells (hMSCs).[173] The group reported that TGF-β1 encapsulated in nanoliposomes had a higher impact on cellular proliferation in comparison to free TGF-β1. In addition, the studied concentrations of nanoliposomes, free TGF-β1, and TGF-β1 encapsulated in nanoliposomes did not induce any inhibitory effects.
Nanogels-functionalized hydrogels have also been utilized as drug carriers. Cholesterol-bearing pullulan nanogel-crosslinking hydrogel (CHPA/hydrogel), prepared by Michael addition, were used as a scaffold to deliver low amounts of bone morphogenetic proteins (BMP), which stimulated osteoblasts and induced bone formation.[158] Fujioka-Kobayashi et al. used cholesteryl and acryloyl group-bearing pullulan (CHPOA) nanogels to prepare fast-degradable hydrogels (CHPOA/hydrogels) for the controlled delivery of two growth factors: recombinant human bone morphogenetic protein 2 (BMP2) and recombinant human fibroblast growth factor 18 (FGF18), as seen in Figure 4d, e.[168] This study concluded that the CHPOA/hydrogel system was able to efficiently deliver BMP2 and FGF18 to a bone defect site and induce effective bone repair, which suggests that this system can be successfully used for bone tissue engineering. Joo et al. created a novel formulation of a hybrid liposome-based hyaluronic acid-chitosan nanogel using a self-assembly process.[174] This hydrogel was successfully used to encapsulate and release recombinant human bone morphogenetic protein 7 (BMP7), also known as osteogenic protein-1.
The addition of soft nanoparticles to natural hydrogels addresses the need for growth factors that natural hydrogels alone cannot provide. Tissue regeneration and growth are key concerns for current and future development of bioengineered scaffolds in bone, cartilage, and muscle tissues. Considering that it is essential for tissue-engineered scaffolds to mimic native tissues, the ability to have controlled the release of growth factors and drugs encapsulated in soft nanoparticles is pertinent.
4.3. Hydrogels with enhanced cell-cell signaling
Intercellular communication can occur via extracellular vesicles known as exosomes. They behave as a vectorized signaling method that originates within a donor cell and is received at the periphery, cytosol, or nucleus of a target cell.[175] Exosomes derived from stem cells provide an alternative approach to culturing cells in vitro. Scaffolds employing exosomes have the advantage of providing the extracellular signaling needed without the difficulty of retaining the multipotent properties of mesenchymal stem cell.[176,177] In one experiment, led by Liu et al., exosomes were isolated from stem cells and integrated into a photoinduced imine crosslinking hydrogel glue.[176] The result was an acellular tissue patch used to regenerate articular cartilage. In another experiment, conducted by Shi et al., exosomes were isolated from GMSCs and combined with a chitosan/silk hydrogel, for the treatment of diabetic ulcers in rat models.[178] Nonetheless, further research is still required on how these mechanisms work.[179]
4.4. Scaffolds capable of gene and plasmid delivery
Biological factors can direct cellular growth, differentiation, and maturation. However, biological factors typically possess low half-lives and can get deactivated, both in the presence of other chemokines or cytokines. Nevertheless, recent advancements in biology have enabled cellular reprogramming through the use of plasmids and gene editing tools. Moreover, adverse side effects caused by the burst release of supraphysiological quantities of recombinant proteins can be prevented by the delivery of genes-encoding growth factors, rather than the protein itself. As soft nanoparticles possess excellent cell affinity and can be internalized once interfaced with cells, they have emerged as attractive tools for plasmid delivery and cell transfection.
Gene-activated matrix (GAM) is a gene transfer technology that also provides a structural template for cell proliferation and ECM synthesis. Peng et al. coupled this technology with soft nanoparticles to create a porous scaffold for periodontal tissue regeneration.[180] The GAM was composed of chitosan/plasmid DNA nanoparticles, encoding platelet-derived growth factors, embedded in a porous chitosan/collagen composite scaffold. This GAM was used to culture periodontal ligament cells, which achieved high proliferation, formed a periodontal connective tissue-like structure after 2 weeks, and maintained a fibroblast figure. In another study, Raftery et al. developed and optimized chitosan–pDNA nanoparticles that facilitated MSC transfection via incorporation into collagen-based scaffolds.[181]
The incorporation of soft nanoparticles in many studies has shown that functionalized natural hydrogels offer the necessary biodegradability, biocompatibility and support native tissue proliferation. Consequently, soft nanofunctionalized hydrogels are the preferred choice for biomaterial scaffolds, that can perform enhanced functions of native ECM.
5. Conclusions and Future Directions
Conventional and novel applications of tissue engineering require the design of scaffolds that are biocompatible and biodegradable, facilitate cellular growth and nutrient transport, and mimic the architecture and physical properties of native tissues.[47] The utilized biomaterial in the fabrication of scaffolds plays a key role in achieving this goal. Natural hydrogels have been widely used as scaffolds for tissue engineering due to their excellent biocompatibility, tunable biodegradability, and low cytotoxicity.
To improve their biological activity, these hydrogels can be functionalized by soft nanoparticles. Although soft nanoparticles are highly biocompatible and do not negatively impact cellular functions, they cannot significantly modulate the physical properties of the functionalized scaffolds. Thus, the development of soft nanoparticles that can improve the electrical and ionic conductivity of the hydrogels would be an important step towards engineering scaffolds for the culture of neural, muscular, and cardiac tissues.
An emerging area in the field of drug delivery is the development of smart systems for on-demand administration of active compounds.[190] This concept is also important in tissue engineering applications where different spatial and temporal concentrations of biological factors are needed at different stages of tissue formation. Towards this end, engineering soft nanoparticles that can respond to external and internal stimuli would be a major step forward. The use of bioresorbable electronics for forming electrically enabled scaffolds in which the drug delivery can be triggered using embedded electronics is another possibility.
One of the areas that are expected to achieve significant attention is the development of scaffolds that can direct cellular fate during tissue growth. Soft nanoparticles are excellent choices for delivering plasmids or factors into cells and thus can be used for engineering such biologically active scaffolds. The biofabrication of hydrogels functionalized with different soft nanoparticles and controlling their spatial distribution could potentially enable directing the spatial organization of cells during tissue maturation.
It has become evident that hydrogels for wound care applications and drug delivery systems have substantial potential to be utilized in pharmaceutical applications. In recent years, a number of materials attributed to hydrogels have been approved by the Food and Drug Administration (FDA).[191] Hydrogels can be considered a Class I, II, or III medical devices dependent upon added biologics and drugs.[192] Their approval process, therefore, begins with a 501(k) premarket notification. Commercialization of hydrogel products has a bright future, the demand for patient-specific treatments and healing processes continues to grow.
Acknowledgments
A.T. acknowledge financial support from the National Institutes of Health (GM126831, AR073822), the University of Nebraska-Lincoln, and Nebraska Tobacco Settlement Biomedical Research Enhancement Funds. K.E. acknowledge financial support from the Ministry of Higher Education, Research and Innovation. N. A. P. acknowledges support in part by grant U54-143837-02 and R01-EB022025 from the National Institutes of Health (NIH), the Cockrell Family Regents Chair, and the Pratt Foundation.
Footnotes
Authors declare no conflict of interests in this work.
Contributor Information
Kamil Elkhoury, LIBio, Université de Lorraine, F-54000 Nancy, France.
Carina Russell, Department of Mechanical and Materials Engineering, University of Nebraska, Lincoln, NE, 68508, USA.
Laura Sanchez-Gonzalez, LIBio, Université de Lorraine, F-54000 Nancy, France.
Tyrell Williams, Department of Mechanical and Materials Engineering, University of Nebraska, Lincoln, NE, 68508, USA.
Cyril Kahn, LIBio, Université de Lorraine, F-54000 Nancy, France.
Nicholas A. Peppas, Departments of Biomedical and Chemical Engineering, Departments of Pediatrics and Surgery, Dell Medical School, University of Texas at Austin, Austin, TX, 78712, USA.
Elmira Arab-Tehrany, LIBio, Université de Lorraine, F-54000 Nancy, France.
Ali Tamayol, Department of Mechanical and Materials Engineering, University of Nebraska, Lincoln, NE, 68508, USA; Mary and Dick Holland Regenerative Medicine Program University of Nebraska-Medical Center, Omaha, NE, 68198.
References
- [1].Khademhosseini A, Langer R, Borenstein J, Vacanti JP, Proc. Natl. Acad. Sci 2006, 103, 2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gaharwar AK, Peppas NA, Khademhosseini A, Biotechnol. Bioeng 2014, 111, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Uto K, Tsui JH, DeForest CA, Kim D-H, Prog. Polym. Sci 2017, 65, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatia SN, Underhill GH, Zaret KS, Fox IJ, Sci. Transl. Med 2014, 6, 245sr2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, et al. , Proc. Natl. Acad. Sci 2016, 113, 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Faramarzi N, Yazdi IK, Nabavinia M, Gemma A, Fanelli A, Caizzone A, Ptaszek LM, Sinha I, Khademhosseini A, Ruskin JN, et al. , Adv. Healthc. Mater 2018, 7, 1701347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].L’Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T, Nat. Clin. Pract. Cardiovasc. Med 2007, 4, 389. [DOI] [PubMed] [Google Scholar]
- [8].Neves LS, Rodrigues MT, Reis RL, Gomes ME, Expert Rev. Precis. Med. Drug Dev 2016, 1, 93. [Google Scholar]
- [9].Bhatia SN, Ingber DE, Nat. Biotechnol 2014, 32, 760. [DOI] [PubMed] [Google Scholar]
- [10].Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A, Adv. Healthc. Mater 2017, 6, 1700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caldorera-Moore M, Peppas NA, Adv. Drug Deliv. Rev 2009, 61, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Culver HR, Daily AM, Khademhosseini A, Peppas NA, Curr. Opin. Chem. Eng 2014, 4, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huh D, Hamilton GA, Ingber DE, Trends Cell Biol 2011, 21, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khademhosseini A, Peppas NA, Adv. Healthc. Mater 2013, 2, 10. [DOI] [PubMed] [Google Scholar]
- [15].Liechty WB, Caldorera-Moore M, Phillips MA, Schoener C, Peppas NA, J. Controlled Release 2011, 155, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Amini AR, Laurencin CT, Nukavarapu SP, Crit. Rev. Biomed. Eng 2012, 40, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Onoe H, Takeuchi S, Drug Discov. Today 2015, 20, 236. [DOI] [PubMed] [Google Scholar]
- [18].Peppas NA, Hoffman AS, in Biomater. Sci Third Ed., Academic Press, 2013, pp. 166–179. [Google Scholar]
- [19].Peppas NA, Slaughter BV, Kanzelberger MA, in Polym. Sci. Compr. Ref, Elsevier, 2012, pp. 385–395. [Google Scholar]
- [20].Vacanti JP, Vacanti CA, in Princ. Tissue Eng, Elsevier, 2014, pp. 3–8. [Google Scholar]
- [21].Hay ED, Cell Biology of Extracellular Matrix, Springer Science & Business Media, 2013. [Google Scholar]
- [22].Hynes RO, Science 2009, 326, 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yannas IV, Regen. Biomater 2018, 10.1093/rb/rby012. [DOI] [PMC free article] [PubMed]
- [24].Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, Khademhosseini A, Sci. Transl. Med 2012, 4, 160ps23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].De Witte T-M, Fratila-Apachitei LE, Zadpoor AA, Peppas NA, Regen. Biomater 2018, 5, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clegg JR, Wechsler ME, Peppas NA, Regen. Eng. Transl. Med 2017, 3, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Culver HR, Clegg JR, Peppas NA, Acc. Chem. Res 2017, 50, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Culver HR, Peppas NA, Chem. Mater 2017, 29, 5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wagner AM, Gran MP, Peppas NA, Acta Pharm. Sin. B 2018, 8, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wagner AM, Spencer DS, Peppas NA, J. Appl. Polym. Sci 2018, 135, 46154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peppas NA, Vela Ramirez J, Beijing, 2018.
- [32].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, 18, 1345. [Google Scholar]
- [33].Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, Adv. Mater 2014, 26, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peppas NA, Van Blarcom DS, J. Controlled Release 2016, 240, 142. [DOI] [PubMed] [Google Scholar]
- [35].Hoffman AS, Adv. Drug Deliv. Rev 2012, 64, 18. [Google Scholar]
- [36].Klouda L, Mikos AG, Eur. J. Pharm. Biopharm 2008, 68, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koetting MC, Peters JT, Steichen SD, Peppas NA, Mater. Sci. Eng. R Rep 2015, 93, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Caldorera-Moore ME, Liechty WB, Peppas NA, Acc. Chem. Res 2011, 44, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoare TR, Kohane DS, Polymer 2008, 49, 1993. [Google Scholar]
- [40].Peppas NA, Curr. Opin. Colloid Interface Sci 1997, 2, 531. [Google Scholar]
- [41].Qiu Y, Park K, Adv. Drug Deliv. Rev 2001, 53, 321. [DOI] [PubMed] [Google Scholar]
- [42].Drury JL, Mooney DJ, Biomaterials 2003, 24, 4337. [DOI] [PubMed] [Google Scholar]
- [43].Lee KY, Mooney DJ, Chem. Rev 2001, 101, 1869. [DOI] [PubMed] [Google Scholar]
- [44].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA, Adv. Mater 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H, et al. , Adv. Drug Deliv. Rev 2018, 127, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ, Adv. Sci 2015, 2, 1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Brien FJ, Mater. Today 2011, 14, 88. [Google Scholar]
- [48].Lee KY, Mooney DJ, Prog. Polym. Sci 2012, 37, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Croisier F, Jérôme C, Eur. Polym. J 2013, 49, 780. [Google Scholar]
- [50].Chang C, Zhang L, Carbohydr. Polym 2011, 84, 40. [Google Scholar]
- [51].Burdick JA, Prestwich GD, Adv. Mater 2011, 23, H41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Antoine EE, Vlachos PP, Rylander MN, Tissue Eng. Part B Rev 2014, 20, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Biomaterials 2015, 73, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ahmed EM, J. Adv. Res 2015, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhao W, Jin X, Cong Y, Liu Y, Fu J, J. Chem. Technol. Biotechnol 2013, 88, 327. [Google Scholar]
- [56].Venkatesan J, Bhatnagar I, Manivasagan P, Kang K-H, Kim S-K, Int. J. Biol. Macromol 2015, 72, 269. [DOI] [PubMed] [Google Scholar]
- [57].Madihally SV, Matthew HWT, Biomaterials 1999, 20, 1133. [DOI] [PubMed] [Google Scholar]
- [58].Collins MN, Birkinshaw C, Carbohydr. Polym 2013, 92, 1262. [DOI] [PubMed] [Google Scholar]
- [59].Dugan JM, Gough JE, Eichhorn SJ, Nanomed 2013, 8, 287. [DOI] [PubMed] [Google Scholar]
- [60].Freier T, Montenegro R, Shan Koh H, Shoichet MS, Biomaterials 2005, 26, 4624. [DOI] [PubMed] [Google Scholar]
- [61].Oliveira JT, Martins L, Picciochi R, Malafaya PB, Sousa RA, Neves NM, Mano JF, Reis RL, J. Biomed. Mater. Res. A 2009, 9999A, NA. [DOI] [PubMed] [Google Scholar]
- [62].Tamayol A, Najafabadi AH, Aliakbarian B, Arab-Tehrany E, Akbari M, Annabi N, Juncker D, Khademhosseini A, Adv. Healthc. Mater 2015, 4, 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cen L, Liu W, Cui L, Zhang W, Cao Y, Pediatr. Res 2008, 63, 492. [DOI] [PubMed] [Google Scholar]
- [64].Rezaei Nejad H, Goli Malekabadi Z, Kazemzadeh Narbat M, Annabi N, Mostafalu P, Tarlan F, Zhang YS, Hoorfar M, Tamayol A, Khademhosseini A, Small 2016, 12, 5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, Lin Y, Friedrich CC, Daheron L, Lin CP, et al. , Proc. Natl. Acad. Sci 2010, 107, 3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Annabi N, Shin SR, Tamayol A, Miscuglio M, Bakooshli MA, Assmann A, Mostafalu P, Sun J-Y, Mithieux S, Cheung L, et al. , Adv. Mater 2016, 28, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Xiao W, He J, Nichol JW, Wang L, Hutson CB, Wang B, Du Y, Fan H, Khademhosseini A, Acta Biomater 2011, 7, 2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J, Nanomaterials 2015, 5, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nitta S, Numata K, Int. J. Mol. Sci 2013, 14, 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smith IO, Liu XH, Smith LA, Ma PX, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2009, 1, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Luo X, Morrin A, Killard AJ, Smyth MR, Electroanalysis 2006, 18, 319. [Google Scholar]
- [72].VanBlarcom DS, Peppas NA, Biomed. Microdevices 2011, 13, 829. [DOI] [PubMed] [Google Scholar]
- [73].Sharma P, Brown S, Walter G, Santra S, Moudgil B, Adv. Colloid Interface Sci 2006, 123–126, 471. [DOI] [PubMed] [Google Scholar]
- [74].Yang H-H, Zhang S-Q, Chen X-L, Zhuang Z-X, Xu J-G, Wang X-R, Anal. Chem 2004, 76, 1316. [DOI] [PubMed] [Google Scholar]
- [75].Tomalia DA, J. Nanoparticle Res 2009, 11, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Peters JT, Hutchinson SS, Lizana N, Verma I, Peppas NA, Chem. Eng. J 2018, 340, 58. [Google Scholar]
- [77].Peters JT, Verghese S, Subramanian D, Peppas NA, Regen. Biomater 2017, 4, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Roohani-Esfahani S-I, Zreiqat H, Nanomed 2017, 12, 419. [DOI] [PubMed] [Google Scholar]
- [79].Kim T, Hyeon T, Nanotechnology 2014, 25, 012001. [DOI] [PubMed] [Google Scholar]
- [80].Dykman L, Khlebtsov N, Chem Soc Rev 2012, 41, 2256. [DOI] [PubMed] [Google Scholar]
- [81].Memic A, Alhadrami HA, Hussain MA, Aldhahri M, Al Nowaiser F, Al-Hazmi F, Oklu R, Khademhosseini A, Biomed. Mater 2015, 11, 014104. [DOI] [PubMed] [Google Scholar]
- [82].Bangham AD, Horne RW, J. Mol. Biol 1964, 8, 660. [DOI] [PubMed] [Google Scholar]
- [83].Allen TM, Science 2004, 303, 1818. [DOI] [PubMed] [Google Scholar]
- [84].Allen TM, Cullis PR, Adv. Drug Deliv. Rev 2013, 65, 36. [DOI] [PubMed] [Google Scholar]
- [85].Korsmeyer R, Regen. Biomater 2016, 3, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li L, He Z-Y, Wei X-W, Wei Y-Q, Regen. Biomater 2016, 3, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kulkarni M, Greiser U, O’Brien T, Pandit A, Trends Biotechnol 2010, 28, 28. [DOI] [PubMed] [Google Scholar]
- [88].Monteiro N, Martins A, Reis RL, Neves NM, Soc JR. Interface 2014, 11, 10.1098/rsif.2014.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hasan M, Elkhoury K, Kahn CJF, Arab-Tehrany E, Linder M, Molecules 2019, 24, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hasan M, Latifi S, Kahn C, Tamayol A, Habibey R, Passeri E, Linder M, Arab-Tehrany E, Mar. Drugs 2018, 16, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Reza Mozafari M, Johnson C, Hatziantoniou S, Demetzos C, J. Liposome Res 2008, 18, 309. [DOI] [PubMed] [Google Scholar]
- [92].Mozafari MR, in Liposomes (Ed: Weissig V), Humana Press, Totowa, NJ, 2010, pp. 29–50. [Google Scholar]
- [93].Doll TAPF, Raman S, Dey R, Burkhard P, J. R. Soc. Interface 2013, 10, 20120740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Díaz M, Vivas-Mejia P, Pharmaceuticals 2013, 6, 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cleymand F, Zhang H, Dostert G, Menu P, Arab-Tehrany E, Velot E, Mano JF, RSC Adv 2016, 6, 83626. [Google Scholar]
- [96].Fakhravar Z, Ebrahimnejad P, Daraee H, Akbarzadeh A, J. Cosmet. Laser Ther 2016, 18, 174. [DOI] [PubMed] [Google Scholar]
- [97].Hasan M, Ben Messaoud G, Michaux F, Tamayol A, Kahn CJF, Belhaj N, Linder M, Arab-Tehrany E, RSC Adv 2016, 6, 45290. [Google Scholar]
- [98].Hasan M, Belhaj N, Benachour H, Barberi-Heyob M, Kahn CJF, Jabbari E, Linder M, Arab-Tehrany E, Int. J. Pharm 2014, 461, 519. [DOI] [PubMed] [Google Scholar]
- [99].Lee CC, MacKay JA, Fréchet JMJ, Szoka FC, Nat. Biotechnol 2005, 23, 1517. [DOI] [PubMed] [Google Scholar]
- [100].Kolhatkar R, Sweet D, Ghandehari H, in Multifunct. Pharm. Nanocarriers (Ed: Torchilin V), Springer New York, New York, NY, 2008, pp. 201–232. [Google Scholar]
- [101].Perisé-Barrios AJ, Sepúlveda-Crespo D, Shcharbin D, Rasines B, Gómez R, Klajnert-Maculewicz B, Bryszewska M, de la Mata FJ, Muñoz-Fernández MA, in Nanosci. Nanotechnol. Ser (Eds: Callejas-Fernández J, Estelrich J, Quesada-Pérez M, Forcada J), Royal Society Of Chemistry, Cambridge, 2014, pp. 246–279. [Google Scholar]
- [102].Nguyen CK, Tran NQ, Nguyen TP, Nguyen DH, Adv. Nat. Sci. Nanosci. Nanotechnol 2017, 8, 015001. [Google Scholar]
- [103].Wiener E, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC, Magn. Reson. Med 1994, 31, 1. [DOI] [PubMed] [Google Scholar]
- [104].Supattapone S, Nguyen H-OB, Cohen FE, Prusiner SB, Scott MR, Proc. Natl. Acad. Sci 1999, 96, 14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kojima C, Kono K, Maruyama K, Takagishi T, Bioconjug. Chem 2000, 11, 910. [DOI] [PubMed] [Google Scholar]
- [106].Tang MX, Redemann CT, Szoka FC, Bioconjug. Chem 1996, 7, 703. [DOI] [PubMed] [Google Scholar]
- [107].Wathier M, Jung PJ, Carnahan MA, Kim T, Grinstaff MW, J. Am. Chem. Soc 2004, 126, 12744. [DOI] [PubMed] [Google Scholar]
- [108].Velazquez AJ, Carnahan MA, Kristinsson J, Stinnett S, Grinstaff MW, Kim T, Arch. Ophthalmol 2004, 122, 867. [DOI] [PubMed] [Google Scholar]
- [109].King HD, Dubowchik GM, Mastalerz H, Willner D, Hofstead SJ, Firestone RA, Lasch SJ, Trail PA, J. Med. Chem 2002, 45, 4336. [DOI] [PubMed] [Google Scholar]
- [110].Morgan MT, Carnahan MA, Immoos CE, Ribeiro AA, Finkelstein S, Lee SJ, Grinstaff MW, J. Am. Chem. Soc 2003, 125, 15485. [DOI] [PubMed] [Google Scholar]
- [111].Estelrich J, Quesada-Pérez M, Forcada J, Callejas-Fernández J, in Nanosci. Nanotechnol. Ser (Eds: Callejas-Fernández J, Estelrich J, Quesada-Pérez M, Forcada J), Royal Society Of Chemistry, Cambridge, 2014, pp. 1–18. [Google Scholar]
- [112].Letchford K, Burt H, Eur. J. Pharm. Biopharm 2007, 65, 259. [DOI] [PubMed] [Google Scholar]
- [113].Jones M-C, Leroux J-C, Eur. J. Pharm. Biopharm 1999, 48, 101. [DOI] [PubMed] [Google Scholar]
- [114].Ramos J, Pelaez-Fernandez M, Forcada J, Moncho-Jorda A, in Nanosci. Nanotechnol. Ser (Eds: Callejas-Fernández J, Estelrich J, Quesada-Pérez M, Forcada J), Royal Society Of Chemistry, Cambridge, 2014, pp. 133–156. [Google Scholar]
- [115].Sasaki Y, Akiyoshi K, Chem. Rec 2010, n/a. [DOI] [PubMed]
- [116].Akiyoshi K, Deguchi S, Moriguchi N, Yamaguchi S, Sunamoto J, Macromolecules 1993, 26, 3062. [Google Scholar]
- [117].Vinogradov S, Batrakova E, Kabanov A, Colloids Surf. B Biointerfaces 1999, 16, 291. [Google Scholar]
- [118].Kabanov AV, Vinogradov SV, Angew. Chem. Int. Ed 2009, 48, 5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chacko RT, Ventura J, Zhuang J, Thayumanavan S, Adv. Drug Deliv. Rev 2012, 64, 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Culver HR, Steichen SD, Peppas NA, Biomacromolecules 2016, 17, 4045. [DOI] [PubMed] [Google Scholar]
- [121].Neves MI, Wechsler ME, Gomes ME, Reis RL, Granja PL, Peppas NA, Tissue Eng. Part B Rev 2017, 23, 27. [DOI] [PubMed] [Google Scholar]
- [122].Zhang Q, Chen X, Geng S, Wei L, Miron RJ, Zhao Y, Zhang Y, J. Biomed. Mater. Res. A 2017, 105, 1175. [DOI] [PubMed] [Google Scholar]
- [123].Zhu D, Zhang L, Dong X, Sun H, Song C, Wang C, Kong D, Int. J. Nanomedicine 2015, 2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Karanth H, Murthy RSR, J. Pharm. Pharmacol 2007, 59, 469. [DOI] [PubMed] [Google Scholar]
- [125].Pridgen EM, Alexis F, Farokhzad OC, Clin. Gastroenterol. Hepatol 2014, 12, 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bitounis D, Fanciullino R, Iliadis A, Ciccolini J, ISRN Pharm 2012, 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gowda R, Jones NR, Banerjee S, Robertson GP, J. Nanomedicine Nanotechnol 2013, 4, 10.4172/2157-7439.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Vahabi S, Eatemadi A, Biomed. Pharmacother 2017, 86, 1. [DOI] [PubMed] [Google Scholar]
- [129].Pearson RM, Sunoqrot S, Hsu H, Bae JW, Hong S, Ther. Deliv 2012, 3, 941. [DOI] [PubMed] [Google Scholar]
- [130].Taboada P, Barbosa S, Concheiro A, Alvarez-Lorenzo C, in Nanosci. Nanotechnol. Ser (Eds: Callejas-Fernández J, Estelrich J, Quesada-Pérez M, Forcada J), Royal Society Of Chemistry, Cambridge, 2014, pp. 157–215. [Google Scholar]
- [131].Chen H, Khemtong C, Yang X, Chang X, Gao J, Drug Discov. Today 2011, 16, 354. [DOI] [PubMed] [Google Scholar]
- [132].Yokoyama M, Expert Opin. Drug Deliv 2010, 7, 145. [DOI] [PubMed] [Google Scholar]
- [133].Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP, Drug Deliv 2017, 24, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Holtz JH, Asher SA, Nature 1997, 389, 829. [DOI] [PubMed] [Google Scholar]
- [135].Lauten EH, Peppas NA, J. Drug Deliv. Sci. Technol 2009, 19, 391. [Google Scholar]
- [136].Liu M, Ishida Y, Ebina Y, Sasaki T, Aida T, Nat. Commun 2013, 4, 10.1038/ncomms3029. [DOI] [PubMed] [Google Scholar]
- [137].Wu H, Yu G, Pan L, Liu N, McDowell MT, Bao Z, Cui Y, Nat. Commun 2013, 4, 10.1038/ncomms2941. [DOI] [PubMed] [Google Scholar]
- [138].Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL, Miller JH, Arap W, Pasqualini R, Proc. Natl. Acad. Sci 2006, 103, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhang D, Yang J, Bao S, Wu Q, Wang Q, Sci. Rep 2013, 3, 10.1038/srep01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pardo-Yissar V, Gabai R, Shipway AN, Bourenko T, Willner I, Adv. Mater 2001, 13, 1320. [Google Scholar]
- [141].Jones CD, Lyon LA, J. Am. Chem. Soc 2003, 125, 460. [DOI] [PubMed] [Google Scholar]
- [142].Wang C, Flynn NT, Langer R, Adv. Mater 2004, 16, 1074. [Google Scholar]
- [143].Saravanan P, Padmanabha Raju M, Alam S, Mater. Chem. Phys 2007, 103, 278. [Google Scholar]
- [144].Marcelo G, López-González M, Mendicuti F, Tarazona MP, Valiente M, Macromolecules 2014, 47, 6028. [Google Scholar]
- [145].Anderson JM, Regen. Biomater 2016, 3, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz A, et al. , Adv. Mater 2017, 29, 1606061. [DOI] [PubMed] [Google Scholar]
- [147].Leijten J, Seo J, Yue K, Trujillo-de Santiago G, Tamayol A, Ruiz-Esparza GU, Shin SR, Sharifi R, Noshadi I, Álvarez MM, et al. , Mater. Sci. Eng. R Rep 2017, 119, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Choi K, Kuhn JL, Ciarelli MJ, Goldstein SA, J. Biomech 1990, 23, 1103. [DOI] [PubMed] [Google Scholar]
- [149].Kot BCW, Zhang ZJ, Lee AWC, Leung VYF, Fu SN, PLoS ONE 2012, 7, e44348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Chen Q-Z, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, Boccaccini AR, Biomaterials 2008, 29, 47. [DOI] [PubMed] [Google Scholar]
- [151].Saraf H, Ramesh KT, Lennon AM, Merkle AC, Roberts JC, J. Biomech 2007, 40, 1960. [DOI] [PubMed] [Google Scholar]
- [152].Shin SR, Li Y-C, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, Zhang YS, Tamayol A, Khademhosseini A, Adv. Drug Deliv. Rev 2016, 105, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Xiao L, Liu C, Zhu J, Pochan DJ, Jia X, Soft Matter 2010, 6, 5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Duan X, Sheardown H, Biomaterials 2006, 27, 4608. [DOI] [PubMed] [Google Scholar]
- [155].Rahali K, Ben Messaoud G, Kahn C, Sanchez-Gonzalez L, Kaci M, Cleymand F, Fleutot S, Linder M, Desobry S, Arab-Tehrany E, Int. J. Mol. Sci 2017, 18, 2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Kadri R, Ben Messaoud G, Tamayol A, Aliakbarian B, Zhang HY, Hasan M, Sánchez-González L, Arab-Tehrany E, RSC Adv 2016, 6, 27879. [Google Scholar]
- [157].Biondi M, Ungaro F, Quaglia F, Netti PA, Adv. Drug Deliv. Rev 2008, 60, 229. [DOI] [PubMed] [Google Scholar]
- [158].Parker J, Mitrousis N, Shoichet MS, Biomacromolecules 2016, 17, 476. [DOI] [PubMed] [Google Scholar]
- [159].Peng L-H, Xu S-Y, Shan Y-H, Wei W, Liu S, Zhang C-Z, Wu J-H, Liang W-Q, Gao J-Q, Int. J. Nanomedicine 2014, 1897. [DOI] [PMC free article] [PubMed]
- [160].Pulat M, Kahraman AS, Tan N, Gümüşderelioğlu M, J. Biomater. Sci. Polym. Ed 2013, 24, 807. [DOI] [PubMed] [Google Scholar]
- [161].Hao X, Silva E, Manssonbroberg A, Grinnemo K, Siddiqui A, Dellgren G, Wardell E, Brodin L, Mooney D, Sylven C, Cardiovasc. Res 2007, 75, 178. [DOI] [PubMed] [Google Scholar]
- [162].Nelson DM, Ma Z, Leeson CE, Wagner WR, J. Biomed. Mater. Res. A 2012, 100A, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Quinlan E, López-Noriega A, Thompson E, Kelly HM, Cryan SA, O’Brien FJ, J. Controlled Release 2015, 198, 71. [DOI] [PubMed] [Google Scholar]
- [164].Taetz S, Bochot A, Surace C, Arpicco S, Renoir J-M, Schaefer UF, Marsaud V, Kerdine-Roemer S, Lehr C-M, Fattal E, Oligonucleotides 2009, 19, 103. [DOI] [PubMed] [Google Scholar]
- [165].Pederson A, Biomaterials 2003, 24, 4881. [DOI] [PubMed] [Google Scholar]
- [166].Samadikuchaksaraei A, Gholipourmalekabadi M, Erfani Ezadyar E, Azami M, Mozafari M, Johari B, Kargozar S, Jameie SB, Korourian A, Seifalian AM, J. Biomed. Mater. Res. A 2016, 104, 2001. [DOI] [PubMed] [Google Scholar]
- [167].Ochi M, Adachi N, Nobuto H, Yanada S, Ito Y, Agung M, Artif. Organs 2004, 28, 28. [DOI] [PubMed] [Google Scholar]
- [168].Fujioka-Kobayashi M, Ota MS, Shimoda A, Nakahama K, Akiyoshi K, Miyamoto Y, Iseki S, Biomaterials 2012, 33, 7613. [DOI] [PubMed] [Google Scholar]
- [169].Reya T, Morrison SJ, Clarke MF, Weissman IL, nature 2001, 414, 105. [DOI] [PubMed] [Google Scholar]
- [170].Narita Y, Yamawaki A, Kagami H, Ueda M, Ueda Y, Cell Tissue Res 2008, 333, 449. [DOI] [PubMed] [Google Scholar]
- [171].Miller MW, Luo J, Alcohol. Clin. Exp. Res 2002, 26, 1281. [DOI] [PubMed] [Google Scholar]
- [172].Hosseinzadeh Z, Schmid E, Shumilina E, Laufer S, Borst O, Gawaz M, Lang F, Biochem. Biophys. Res. Commun 2014, 452, 537. [DOI] [PubMed] [Google Scholar]
- [173].Dostert G, Kahn CJF, Menu P, Mesure B, Cleymand F, Linder M, é. Velot, E. Arab-Tehrany, J. Biomater. Tissue Eng 2017, 7, 1163. [Google Scholar]
- [174].Joo V, Ramasamy T, Haidar ZS, Polymers 2011, 3, 967. [Google Scholar]
- [175].Record M, Subra C, Silvente-Poirot S, Poirot M, Biochem. Pharmacol 2011, 81, 1171. [DOI] [PubMed] [Google Scholar]
- [176].Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L, Nanoscale 2017, 9, 4430. [DOI] [PubMed] [Google Scholar]
- [177].Siddappa R, Licht R, van Blitterswijk C, de Boer J, J. Orthop. Res 2007, 25, 1029. [DOI] [PubMed] [Google Scholar]
- [178].Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X, Front. Physiol 2017, 8, 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Fisher OZ, Khademhosseini A, Langer R, Peppas NA, Acc. Chem. Res 2010, 43, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Peng L, Cheng X, Zhuo R, Lan J, Wang Y, Shi B, Li S, J. Biomed. Mater. Res. A 2009, 90A, 564. [DOI] [PubMed] [Google Scholar]
- [181].Raftery RM, Tierney EG, Curtin CM, Cryan S-A, O’Brien FJ, J. Controlled Release 2015, 210, 84. [DOI] [PubMed] [Google Scholar]
- [182].Smith AM, Harris JJ, Shelton RM, Perrie Y, J. Controlled Release 2007, 119, 94. [DOI] [PubMed] [Google Scholar]
- [183].Wu SY, Chang H-I, Burgess M, McMillan NAJ, J. Controlled Release 2011, 155, 418. [DOI] [PubMed] [Google Scholar]
- [184].Dai C, Wang B, Zhao H, Li B, Wang J, Colloids Surf. B Biointerfaces 2006, 47, 205. [DOI] [PubMed] [Google Scholar]
- [185].Weiner AL, Carpenter-Green SS, Soehngen EC, Lenk RP, Popescu MC, J. Pharm. Sci 1985, 74, 922. [DOI] [PubMed] [Google Scholar]
- [186].Lee J-H, Oh H, Baxa U, Raghavan SR, Blumenthal R, Biomacromolecules 2012, 13, 3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lajavardi L, Camelo S, Agnely F, Luo W, Goldenberg B, Naud M-C, Behar-Cohen F, de Kozak Y, Bochot A, J. Controlled Release 2009, 139, 22. [DOI] [PubMed] [Google Scholar]
- [188].Walsh DP, Heise A, O’Brien FJ, Cryan S-A, Gene Ther 2017, 24, 681. [DOI] [PubMed] [Google Scholar]
- [189].Xiao L, Tong Z, Chen Y, Pochan DJ, Sabanayagam CR, Jia X, Biomacromolecules 2013, 14, 3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Derakhshandeh H, Kashaf SS, Aghabaglou F, Ghanavati IO, Tamayol A, Trends Biotechnol 2018, 10.1016/j.tibtech.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Patel G, Dalwadi C, Recent Pat. Drug Deliv. Formul 2013, 7, 206. [DOI] [PubMed] [Google Scholar]
- [192].FDA Executive Summary: Classification of Wound Dressings Combined with Drugs, 2016.