Abstract
Background.
SLCO-encoded transporters have been associated with progression to castration resistant prostate cancer (CRPC) after initiation of androgen deprivation therapy (ADT). Although expressed at lower levels than in CRPC tissues, SLCO-encoded transporters may also play a role in response of primary prostate cancer (PCa) to ADT and biochemical recurrence.
Methods.
We systematically explored expression of the 11 human SLCO genes in a large sample of untreated and ADT-treated normal prostate (NP) and primary PCa tissues, including tumors treated with neoadjuvant abiraterone.
Results.
Transporters with the most recognized role in steroid uptake in PCa, including SLCO2B1 (DHEAS) and 1B3 (testosterone), were consistently detected in primary PCa. SLCO1B3 was nearly 5-fold higher in PCa vs NP with no difference in Gleason 3 vs 4 and no change with ADT. SLCO2B1 was detected at 3-fold lower levels in PCa than NP but was nearly 7-fold higher in Gleason 4 vs Gleason 3 and increased 3-fold following ADT (p<0.05 for all).
Conclusions.
We observed clear differences in SLCO expression in PCa vs NP samples, in Gleason 4 vs Gleason 3 tumors, and in ADT-treated vs untreated tissues. These findings are hypothesis generating due to small sample size, but suggest that baseline and ADT-induced changes in PCa OATP expression may influence steroid uptake and response to ADT, as well as uptake and response to drugs such as abiraterone and docetaxel which are also subject to OATP-mediated transport and are now being routinely combined with ADT in the metastatic castration sensitive setting.
Keywords: OATP, SLCO, androgen transport, biochemical recurrence, genetic variation, primary prostate cancer
INTRODUCTION
Androgen deprivation therapy (ADT) is standard of care for men with advanced prostate cancer (PCa) but is inevitably followed by castration-resistant prostate cancer (CRPC). Despite suppression of circulating androgens, prostatic androgens following castration remain well above levels capable of engaging AR, and CRPC metastases contain testosterone levels four times higher than prostate tissue of eugonadal men.1–3
The organic anion transporting polypeptides (OATP) are SLCO-encoded proteins that transport bile acids, xenobiotics, steroid conjugates, and important drugs including taxanes. 4–6 Several OATPs (e.g. OATP1A2, 1B1, 1B3, and 2B1) mediate uptake of steroids into PCa cells in vitro and in vivo,7–11 and single nucleotide polymorphisms (SNPs) of SLCO1B3 and SLCO2B1 that demonstrate enhanced androgen uptake are associated with more rapid disease progression in men with metastatic disease treated with ADT.8,12,13 CRPC metastases express transcripts encoding SLCO transporters at significantly higher levels than in primary PCa.14 These data strongly support a role for OATP-mediated steroid transport in moderating the tissue response to ADT and promoting disease progression in men with advanced disease.
In contrast, in primary PCa the extent to which SLCO genes influence disease or become altered in response to hormonal therapy is not well-explored. Pressler et al examined SLCO1B1, SLCO1B3 and SLCO2B1 in a five normal prostate and 21 PCa samples and observed higher SLCO1B3 in primary PCa vs normal prostate, and an association of SLCO1B3 with Gleason score.15 Wright et al evaluated SLCO1B1, SLCO1B3, SLCO2A1, SLCO2B1, SLCO3A1, and SLCO4A1 levels in eight benign and eight PCa samples without significant differences in tumor vs normal tissue, likely due to limited number of samples.14 They also found no association of genetic differences in SLCO1B3 or SLCO2B1 with PCa recurrence in a case control sample of 469 men with localized PCa (no hormone therapy). However, an impact of SLCO expression on steroid transport in primary PCa may be most relevant in the setting of ADT (e.g. ADT combined with definitive radiation therapy). In particular, the extent to which expression of SLCO genes in primary PCa is altered by ADT is unknown but may provide insight into induction of SLCO transporters as a mechanism of resistance to ADT.
We evaluated the eleven human SLCO genes in microdissected benign (n=20) and cancer (n=35) tissues from untreated men with localized PCa, including separately microdissected foci of Gleason grade 3 vs grade 4 tumors. We profiled SLCO expression in a cohort of matched benign (n=20) and cancer (n=18) prostate samples from men enrolled in a trial of neoadjuvant ADT prior to radical prostatectomy (RP), and in primary PCa samples (n=13) from men enrolled in a trial of neoadjuvant ADT plus abiraterone acetate (ABI) prior to RP.16,17 Finally, we evaluated the impact of 12 SNPS in 7 SLCO genes on risk of biochemical recurrence in a cohort of 147 men with localized PCa treated with radical prostatectomy (RP).
MATERIALS AND METHODS
Patient Samples
All procedures were approved by Institutional Review Boards of University of Washington and Fred Hutchinson Cancer Research Center. Frozen prostate tissue was collected from men with Gleason grade 3 and/or grade 4 PCa under an approved protocol for use of excess tissue after RP, and from men with intermediate to high risk PCa (Gleason score >7) treated on a previously published trial of ADT prior to RP (NCT00298155).17 (Greater than 95% of men for whom data was available self-identified as Caucasian). Hormonal regimens included 1) goserelin with bicalutamide, 2) goserelin with dutasteride (3.5 mg), 3) goserelin with bicalutamide and dutasteride, and 4) goserelin with bicalutamide, dutasteride and ketoconazole (200 mg three times daily; with prednisone 5 mg daily). Formalin fixed prostate tissue (FFPE) was obtained from men with intermediate to high risk PCa (Gleason score >7) treated on a previously published trial of lupron plus ABI prior to RP (NCT00924469).16 Genomic DNA was isolated via macrodissection of benign prostate tissue from hematoxylin and eosin (H&E) stained FFPE tissue sections from 147 patients who underwent RP between 1995 and 2010 at the University of Washington for whom recurrence data was available.
Laser Capture Microdissection and RNA Isolation
Microdissection and RNA isolation was performed from frozen prostate tissue as epreviously described.3 In untreated tissues, foci of Gleason 3 and Gleason 4 cancer were separately microdissected. All areas to be micro-dissected were selected reviewed by a pathologist (X.Z., L.T. or H.Y). Microdissection and RNA isolation of FFPE samples from ABI treated samples was performed as described.18
SLCO Transcript Profiling
Quantitative reverse transcription (qPCR) was performed as described.14 The mean Ct for each gene was normalized to the housekeeping gene RPL13A (delta Ct or dCt). No consistent differences in RPL13A expression itself were observed in the normal vs prostate samples (Supplementary Fig S1). Samples with undetectable expression of a given gene were assigned dCt value of 33 for purposes of calculation. Fold change was calculated from the difference in mean dCt between sample groups (ddCt method; fold = 2^ddCt). Primer sequences are as published.14
SLCO Genotyping Analysis
Genomic DNA was purified using the QIAamp® DNA FFPE Tissue Kit. Twelve single nucleotide polymorphisms (SNPs) with minor allelic frequency (MAF) > 10% in 6 SLCO genes (SLCO1B1, 1B3, 2B1, 2A1, 3A1, and 5A1) were genotyped using TaqMan SNP assays as described.13 The SNP assay numbers, minor allele frequency and potential role in PCa are shown in Supplementary Table 1.
Statistical Analysis
Comparisons of SLCO gene expression in PCa vs NP, Gleason grade 3 vs 4 samples, and treated vs untreated PCa were performed with un-paired two-tailed t tests. The variance between groups was compared using an F test and if significantly different (p<0.05) Welch’s correction was applied. Time to recurrence was number of months from RP to the first measurement of biochemical relapse (BCR; PSA ≥ 0.2), death, loss to follow-up or 10 years. Binary recurrence within 10 years was compared across different genotypes using Fisher’s Exact Test. Cox proportional hazards models were used to compare time to recurrence by genotype, adjusted for tumor volume, TNM staging, pre-prostatectomy PSA, Gleason grade, and margin status. Heterozygous and rare homozygous variants were combined if total number of cases in any genotype was fewer than 10.
RESULTS
Expression of SLCO genes in untreated prostate tissue
We evaluated SLCO expression in normal prostate (NP; n=20) and separately microdissected foci of Gleason grade 3 and grade 4 PCa (n=35) from men with localized PCa undergoing RP. In untreated tissues (cancer or benign), the percentage of samples that were undetectable for a given gene varied and did not represent a unique population (0–3% samples undetectable for SLCO2B1, 3A1, and 5A1; 10–20% for 1B3 and 4A1; 40% for 1C1 and 4C1; 60–80% for 1A2 and 1B1; and 93% for 6A1). Transcript data in NP vs PCa (comprising both Gleason grade 3 and 4 tumors) are shown in Fig 1 and summarized in Table 1.
Figure 1. Expression of SLCO genes, ranked by abundance in primary prostate tissues.
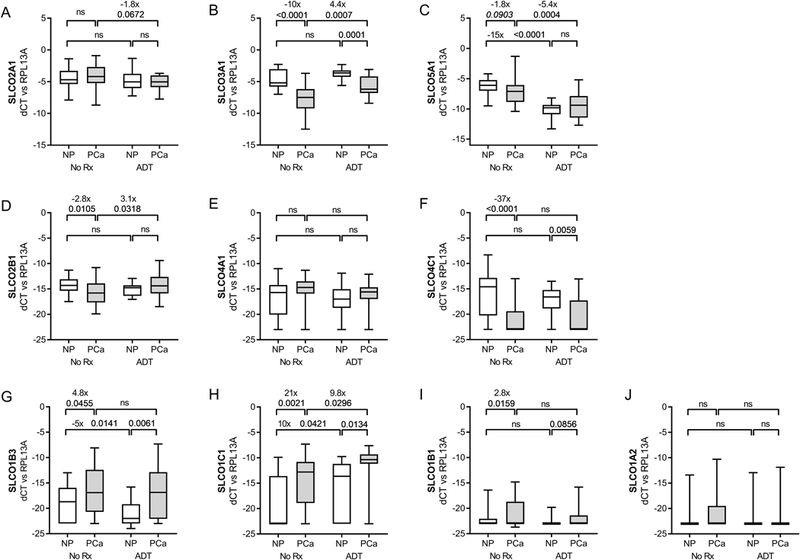
Transcript levels of the indicated SLCO genes (normalized to the housekeeping gene RPL13A) were evaluated in microdissected normal prostate (NP) and prostate cancer (PCa) epithelium from untreated men (No Rx) or from men treated with neoadjuvant androgen deprivation therapy (ADT) for three months prior to prostatectomy. Data are shown as box-and-whisker plots, where horizontal lines indicate median values; white boxes denote the 75th (upper margin) and 25th percentiles (lower margin), and upper and lower bars indicate minimum and maximum values, respectively. Fold changes and p values for the indicated comparisons are given. P-values calculated using a two-sided t test (P values <0.05 were considered significant; and those < 0.01 as trending toward significance - italicized).
Table 1.
Expression of SLCO Genes in Hormone Naive Primary Prostate Cancer
| Normal Prostate (NP) | Prostate Cancer (PCa) | PCa vs NP | Gleason 4 vs 3 | |||
|---|---|---|---|---|---|---|
| avg dCt* | avg dCt* | fold | P value** | fold | P value** | |
| SLCO1A2 | −22 | −21 | 2.0 | ns | −1.5 | ns |
| SLCO1B1 | −22 | −21 | 2.7 | 0.016 | 1.0 | ns |
| SLCO1B3 | −19 | −16 | 4.8 | 0.045 | −1.3 | ns |
| SLCO1C1 | −19 | −15 | 21 | 0.002 | 24 | 0.007 |
| SLCO2A1 | −5 | −4 | 1.7 | 0.055 | 1.8 | ns |
| SLCO2B1 | −14 | −16 | −2.8 | 0.011 | 6.7 | 0.0001 |
| SLCO3A1 | −4 | −8 | −10 | <0.0001 | 3.5 | 0.014 |
| SLCO4A1 | −17 | −15 | 2.8 | ns | 1.6 | ns |
| SLCO4C1 | −16 | −21 | −37 | <0.0001 | 2.3 | ns |
| SLCO5A1 | −6 | −7 | −1.8 | 0.09 | 1.5 | ns |
normalized to RPL13A, dCt of −23 considered negative
p values from two-sided t tests, p<0.05 significant, p<0.10 (italicized) trending toward significance
In NP, levels of SLCO2A1, 3A1 and 5A1 (Fig 1A-C) were most abundant (average dCt vs RPL13A of −4 to −6), while SLCO2B1, 4A1 and 4C1 (Fig 1D–F) were several orders of magnitude lower (dCt −14 to −17), but still easily detectable. Average levels of SLCO1B3, 1C1 and 1B1 were very low in NP (dCt of −19–22), and largely undetectable for SLCO1A2 (Fig 1G-J). SLCO6A1 was only detected in a few samples (not shown) and was not analyzed further.
Multiple SLCO genes showed differential expression in PCa vs NP. PCa expressed higher SLCO1B3 (4.8 fold, p=0.045; Fig 1G), SLCO1C1 (21 fold, p=0.002; Fig 1H), and 1B1 (2.7 fold, p=0.016; Fig 1I), and lower SLCO4C1 (−37 fold, p<0.0001; Fig 1E), 3A1 (−10 fold, p=<0.0001; Fig 1B) and 2B1 (−2.8 fold, p=0.011; Fig 1D). Thus, SLCO2A1, 3A1 and 5A1 remain the most highly expressed genes in tumor (dCt −4 to −8), while SLCO1B3 and 1C1 move up to join SLCO2B1 and 4A1 as the next most abundant (dCt −15 to −16), and SLCO4C1 moves down with SLCO1A2 and 1B1 as the least abundant (dCt-21 to −22). Consistent with a prior report, we detected minimal to no expression of the truncated SLCO1B3 splice variant in primary PCa (not shown).11
Figure 3. Expression of SLCO genes in primary prostate cancer after neoadjuvant treatment with abiraterone.
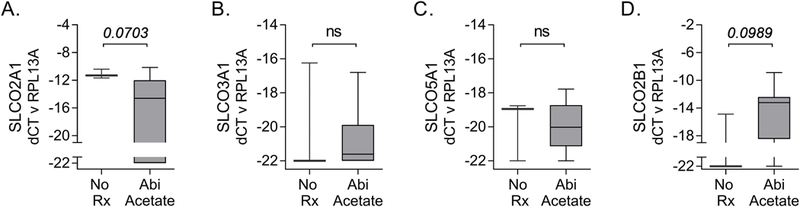
Transcript levels of the indicated SLCO genes (normalized to the housekeeping gene RPL13A) were evaluated in microdissected foci of cancer from untreated men or those treated with neoadjuvant abiraterone for 3–6 months prior to prostatectomy. Presentation of data as box and whisker plots and statistical analyses are as described in the legend for Figure 1.
Figure 2. SLCO gene expression in Gleason 3 vs Gleason 4 prostate tumors.
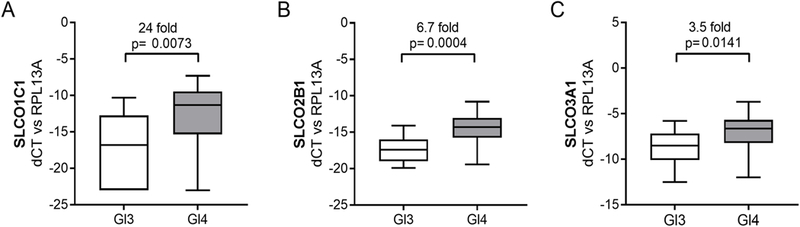
Transcript levels of the indicated SLCO genes were evaluated in microdissected foci of Gleason 3 (Gl3, n=18) or Gleason 4 (Gl4, n=17) PCa from untreated men. Presentation of data as box and whisker plots and statistical analyses are as described in the legend for Figure 1.
Association of Gleason grade with SLCO gene expression
Differences in tumor androgen levels have been reported in high vs low grade PCa.19 Therefore, we sought to determine whether differential expression of SLCO genes (and thereby differences in androgen uptake) might plausibly contribute to this difference. Gleason 4 tumors (n=18) had significantly higher SLCO1C1 (24-fold, p= 0.007, Fig 2A), 2B1 (6.7-fold, p=0.0001; Fig 2B) and 3A1 (3.5-fold, p=0.014; Fig 2C) than Gleason 3 tumors (n=17; Table 1 and Fig 2). No differences were observed in the remaining SLCO genes (Supplementary Fig S2).
Impact of androgen deprivation on SLCO gene expression
To determine whether expression of SLCO genes is altered by hormonal therapy, we microdissected PCa and NP in frozen prostate tissue from 20 men with high risk localized PCa treated with various combinations of goserelin (Zoladex, Z), bicalutamide (Casodex, C), dutasteride (D) and ketoconazole (K) in a previously completed trial of neoadjuvant ADT for three months prior to surgery.17 Due to morphologic changes caused by ADT, Gleason grade was not assessed. Specimen numbers in each subset do not enable a statistically rigorous assessment Specimen numbers in each subset do not enable a statistically rigorous assessment among the different regimens (ZC, n=2; ZD n=4; ZCD n=6; and ZCDK n=8; Supplementary Fig S3), and samples were grouped into treated NP or treated PCa sets for subsequent analysis.
Compared to untreated tissues, ADT induced differential expression of SLCO genes in both NP and PCa (Fig 1 and Table 2). ADT-treated tumor samples had higher SLCO3A1 (4.4 fold, p=0.0007; Fig 1B), 2B1 (3.6 fold, p=0.032; Fig 1D), and 1C1 (9.8 fold, p=0.03; Fig 1H), but lower 2A1 (−2.3 fold, p=0004; Fig 1A) and 5A1 (−4.2 fold, p=0.0004; Fig 1C). The direction and magnitude of change was generally similar for PCa and NP except for SLCO5A1, in which NP showed a significantly larger decrease in expression (−15 fold, p<0.0001; Fig 1C) than that in tumor; SLCO1B3, in which NP showed a decrease after ADT (−5 fold, p=014; Fig 1G) while tumor did not; and SLCO3A1 and 2B1, in which NP did not show the increase in expression observed in tumor (Fig 1B and 1D).
Table 2.
Change in SLCO Gene Expression in Normal and Cancer Prostate Epithelium after Androgen Deprivation
| Normal Prostate (NP) | Prostate Cancer (PCa) | |||
|---|---|---|---|---|
| fold* | p value** | fold* | p value** | |
| SLCO1A2 | −1.3 | ns | −1.9 | ns |
| SLCO1B1 | −1.4 | ns | −1.7 | ns |
| SLCO1B3 | −4.9 | 0.014 | −1.2 | ns |
| SLCO1C1 | 10.2 | 0.042 | 9.8 | 0.030 |
| SLCO2A1 | −1.3 | ns | −1.8 | 0.07 |
| SLCO2B1 | −1.8 | 0.07 | 3.1 | 0.032 |
| SLCO3A1 | 1.7 | 0.06 | 4.4 | 0.0007 |
| SLCO4A1 | −1.2 | ns | −1.4 | ns |
| SLCO4C1 | −2.8 | ns | 1.5 | ns |
| SLCO5A1 | −15.3 | <0.0001 | −5.4 | 0.0004 |
fold change relative to untreated tissue
p values calculated as in Table 1
To assess the impact of more potent androgen suppression we microdissected FFPE PCa samples from a trial of Lupron plus 3 to 6 months of ABI prior to RP.16 While absolute transcript levels were lower than those in frozen tissue, the four SLCO genes most highly expressed in PCa in the studies above (SLCO2A1, 3A1, 5A1 and 2B1) were also detected in the FFPE samples (Fig 3). Moreover, there was a near statistically significant decrease in SLCO2A1 (p=0.07; Fig 3A) and increase in SLCO2B1 (p=0.09; Fig 3D) in the ABI plus ADT-treated samples, similar to our findings in the ADT-treated tumor samples. While the changes in SLCO3A1 and SLCO5A1 were not statistically significant, the direction of changes in the tumor samples following treatment (increased for SLCO3A1, decreased for SLCO5A1) were also consistent with the findings above.
Association of SLCO genotype with prostate cancer recurrence after prostatectomy
Genomic DNA was obtained from 147 patients with PCa who underwent RP between 1995 and 2010. Longitudinal follow up (median 60 months) yielded 67 patients with and 80 without evidence of BCR. Demographics and clinical characteristics of patients are shown in Supplementary Table 2. PCa patients with BCR had more aggressive features on final prostate pathology; higher stage disease (30% had pT3 N0 vs. only 8.8% in non-BCR group), higher Gleason scores (80.6% Gleason 7 vs. 42.5 % in non-BCR), more positive margins and larger tumor volumes. Samples were genotyped for 12 SNPS with MAF>10%, selected based a published role in hormone transport/metabolism and/or significance in PCa.13 Binary recurrence within 10 years showed no statistically significant difference across the different SNP genotypes except for SLCO2A1 SNP rs34550074 (p= 0.025). However, after adjustment for clinical variables the resultant model was no longer significant (Supplementary Table 3).
DISCUSSION
Although expressed at lower levels than in CRPC, SLCO-encoded transporters may also play a role in response of primary PCa to neoadjuvant/adjuvant ADT and in driving BR following definitive therapy. We systematically explored expression of SLCO genes in a large sample of untreated and ADT-treated primary PCa tissues. Transporters with the most recognized role in steroid uptake in PCa, including SLCO2B1 (DHEAS) and 1B3 (testosterone), were consistently detected in primary PCa tissue.8–11 We observed clear differences in PCa vs NP samples, in Gleason 4 vs Gleason 3 tumors, and in ADT-treated vs untreated tissues.
The increased expression of SLCO1B3 observed in PCa vs NP is consistent with prior reports showing higher SLCO1B3 in PCa vs NP.8,15 While Pressler et al noted an association of Gleason grade with SLCO1B3, we observed an association of Gleason grade with SLCO2B1 (this may reflect differences in sample preparation as we report microdissected foci of Gleason 3 and 4 tumors whereas Pressler et al reported tumor grade as the Gleason sum). However, the increased expression of SLCO2B1 in higher Gleason grade tumors is consistent with findings recently reported in primary PCa samples from The Cancer Genome Atlas (TCGA).20
SLCO-encoded genes may influence primary PCa in multiple ways. While the higher SLCO1B3 expression in cancer, or the higher SLCO2B1 levels in Gleason 4 vs Gleason 3 tumors might associate with higher tissue androgen levels and more aggressive cancer, the association of tissue androgens and PCa risk is not well understood. In one study of 196 patients, higher tissue testosterone levels were significantly related to higher Gleason scores, but no association was noted with DHT, whereas in a different study of 81 patients, DHT levels were lower in patients with Gleason 7–10 disease vs Gleason 6 (testosterone not measured), actually suggesting an inverse association of androgen uptake with tumor aggressiveness.19,21
Importantly, testosterone interferes with OATP2B1-mediated transport of DHEAS, and thus OATP-mediated steroid uptake in the eugonadal setting may not follow the same paradigm as in ADT.22 This is consistent with our data showing no association of SLCO genotype with PCa recurrence in 147 men not treated with ADT. Our recurrence data set clearly has limitations due to small cohort size, but is consistent with the larger study of Wright et al showing no association of SLCO genotype with recurrence in 489 patients.14
However, in a study of 494 primary PCa cases from TCGA, high SLCO2B1 was associated with worse disease free survival (DFS) after RP (no association was noted for SLCO1B3). 20 Notably, this study reported higher SLCO2B1 in higher Gleason grade tumors (consistent with our data), and found the association with DFS was only significant for Gleason score >8. This suggests that any impact of SLCO2B1 expression (or genotype) on recurrence or progression is most important in high grade disease in which it is more highly expressed. In this regard, our study and that of Wright et al may have been negative because the populations were low risk (81% and 85% with Gleason score 6 or 3+4, respectively), whereas >40% of patients in the TCGA study had a Gleason score >8.
Alternatively, SLCO-encoded transporters may mediate uptake of other OATP substrates relevant to prostate carcinogenesis and/or progression, including prostaglandins (PGs), thyroid hormones, green tea catechins, taxanes, statins, cardiac glycosides, glitazones, and metformin.23–33 In this regard, several of the transporters most abundantly expressed in the prostate do not have a recognized role in androgen transport. Of these, SLCO3A1 (estrone-sulfate, PGs), and 4C1 (estrone-sulfate, cAMP, thyroid hormones) were expressed at significantly lower levels in CP vs NP, possibly suggesting transport of a substrate with anti-tumor activity. In contrast, increased expression of the thyroid hormone transporter SLCO1C1 may represent a substrate with tumor promoting properties as thyroid hormones have been shown to promote PCa cell proliferation in vitro, and several studies have suggested an association between increased thyroid hormone levels and PCa risk.24,29,34–41
As in advanced PCa, OATP-mediated uptake of androgens in primary PCa may be most relevant in modifying response to ADT. The most substantive evidence for OATP-mediated androgen uptake in PCa is that of DHEAS by OATP2B1. DHEAS uptake is dependent on the expression of SLCO2B1 and greater expression of SLCO2B1 resulted in increased DHEAS transport into cells.9 Thus, the increased SLCO2B1 observed with standard and ABI containing neoadjuvant ADT may adversely influence response to therapy, such as in patients with localized PCa receiving neoadjuvant/adjuvant ADT in context of definitive radiation, or patients with newly diagnosed metastatic disease being treated with ADT and ABI. This may be particularly relevant for SLCO2B1 genotypes associated with increased transport efficiency or higher protein expression.9,12 Additionally, the 6.7 fold higher SLCO2B1 in Gl4 vs Gl3 tumors may render Gl4 disease more resistant to ADT, which would be consistent with data from a small study of 28 patients which showed that the decrease in tissue DHT following 6 months of ADT was less in Gleason 7–10 disease than Gleason 6 disease.42 As OATP2B1 transports both DHEAS and ABI, any net impact of the increase in SLCO2B1 expression induced by ADT and ABI remains to be empirically determined from clinical studies.43
OATP proteins are critical mediators of hepatic taxane uptake and primary determinants of systemic taxane exposure.29,40 A pharmacokinetic study reported that docetaxel clearance increased by approximately 100% in castrate compared to non-castrate men with PCa, and rat studies found that castration increased docetaxel clearance and was associated with increased hepatic expression of rOat2 (Slc22a7), a mouse Oatp shown to transport docetaxel.44 OATPs also modify intratumoral accumulation of docetaxel and cabazitaxel, strongly influencing response of PCa xenografts to treatment28,45, and loss of tumor SLCO1B3 expression is a mechanism of resistance in taxane-refractory prostate tumors.41 Thus, an increase in tumor SLCO expression following ADT could potentially influence docetaxel transport and enhance treatment response.
Regulation of OATP expression and function occurs at both the transcriptional and post-transcriptional level and is, at least in part, tissue-specific.46,47 While androgen regulation of rodent renal Oatp expression has been demonstrated in male and female rodents, androgen regulation of SLCO genes in prostate tissue has not been explored.48,49
In summary, clear differences in SLCO transcript expression are present in primary PCa compared to NP in Gleason 4 vs Gleason 3 tumors, and in ADT-treated vs untreated tissues. These findings are hypothesis generating due to small sample size, but suggest that baseline and ADT-induced changes in PCa OATP expression may influence steroid uptake and response to ADT, as well as uptake and response to critical PCa drugs such as ABI and docetaxel, which are subject to OATP-mediated transport and are now being routinely combined with ADT in the metastatic castration sensitive setting. Future work may identify how targeting the induction, repression or inhibition of these transporters might be exploited for therapeutic benefit.50,51 Supplementary information is available at PCAN’s website.
Supplementary Material
Acknowledgements
The authors wish to acknowledge technical assistance from Jennifer Noteboom and Lori Kollath who assisted with provision of clinical outcome data, as well as Drs. Daniel Lin, William Ellis, and Jonathan Wright for supporting collection of excess frozen prostate tissue at time of radical prostatectomy.
Financial Support: This work was supported by NIH grants (Pacific Northwest Prostate Cancer SPORE P50 CA097186 to LT, PSN, RBM, EAM; FHCRC Cancer Center Support Grant 5P30 CA015704–40 (PSN, EAM); DF/HCC SPORE P50 CA090381 to AGS, HY, MET, and SPB; P01 CA163227 to EAM, PSN, SPB); Prostate Cancer Foundation (Challenge Awards to EAM, PSN, MET, SPB; Young Investigator Awards to AGS, HE, EAM); Department of Defense Prostate Cancer Research Program (W81XWH-11–2-0154 to EAM; W81XWH-13–1-0267 and W81XWH-16–1-0433 to AGS; W81XWH-16–1-0431 to SPB and W81XWH-16–1-0432 to MET), the Intramural Research Program of the NIH, National Cancer Institute (AGS), and the Department of Veterans Affairs Puget Sound Health Care System (EAM).
Footnotes
The authors declare there are no competing financial interests in relation to the work described.
REFERENCES
- 1.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68(11):4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab 2006;91(10):3850–6. [DOI] [PubMed] [Google Scholar]
- 3.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res 2007;67(10):5033–41. [DOI] [PubMed] [Google Scholar]
- 4.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 2004;447(5):653–65. [DOI] [PubMed] [Google Scholar]
- 5.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. British journal of pharmacology 2009;158(3):693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. British journal of pharmacology 2012;165(5):1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arakawa H, Nakanishi T, Yanagihara C, Nishimoto T, Wakayama T, Mizokami A, et al. Enhanced expression of organic anion transporting polypeptides (OATPs) in androgen receptor-positive prostate cancer cells: possible role of OATP1A2 in adaptive cell growth under androgen-depleted conditions. Biochemical pharmacology 2012;84(8):1070–7. [DOI] [PubMed] [Google Scholar]
- 8.Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res 2008;14(11):3312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Harshman LC, Xie W, Nakabayashi M, Qu F, Pomerantz MM, et al. Association of SLCO2B1 Genotypes With Time to Progression and Overall Survival in Patients Receiving Androgen-Deprivation Therapy for Prostate Cancer. J Clin Oncol 2016;34(4):352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green SM, Kaipainen A, Bullock K, Zhang A, Lucas JM, Matson C, et al. Role of OATP transporters in steroid uptake by prostate cancer cells in vivo. Prostate cancer and prostatic diseases 2017;20(1):20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sissung TM, Ley AM, Strope JD, McCrea EM, Beedie S, Peer CJ, et al. Differential Expression of OATP1B3 Mediates Unconjugated Testosterone Influx. Mol Cancer Res 2017;15(8):1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Xie W, Mostaghel E, Nakabayashi M, Werner L, Sun T, et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2011;29(18):2565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho E, Montgomery RB, Mostaghel EA. Minireview: SLCO and ABC Transporters: A Role for Steroid Transport in Prostate Cancer Progression. Endocrinology 2014;155(11):4124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JL, Kwon EM, Ostrander EA, Montgomery B, Line DW, Vessella RL, et al. Expression of SLCO transport genes in castration resistant prostate cancer and impact of genetic variation in SCLO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2011. [DOI] [PMC free article] [PubMed]
- 15.Pressler H, Sissung TM, Venzon D, Price DK, Figg WD. Expression of OATP family members in hormone-related cancers: potential markers of progression. PLoS ONE 2011;6(5):e20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol 2014;32(33):3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J Clin Oncol 2014;32(3):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowalsky AG, Ye H, Bhasin M, Van Allen EM, Loda M, Lis RT, et al. Neoadjuvant-Intensive Androgen Deprivation Therapy Selects for Prostate Tumor Foci with Diverse Subclonal Oncogenic Alterations. Cancer Res 2018;78(16):4716–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama T, Ikarashi T, Hashimoto Y, Suzuki K, Takahashi K. Association between the dihydrotestosterone level in the prostate and prostate cancer aggressiveness using the Gleason score. J Urol 2006;176(4 Pt 1):1387–91. [DOI] [PubMed] [Google Scholar]
- 20.Terakawa T, Katsuta E, Yan L, Turaga N, McDonald K-A, Fujisawa M, et al. High expression of SLCO2B1 is associated with prostate cancer recurrence after radical prostatectomy 2018. [DOI] [PMC free article] [PubMed]
- 21.Miyoshi Y, Uemura H, Umemoto S, Sakamaki K, Morita S, Suzuki K, et al. High testosterone levels in prostate tissue obtained by needle biopsy correlate with poor-prognosis factors in prostate cancer patients. BMC cancer 2014;14:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G, et al. Modification of OATP2B1-mediated transport by steroid hormones. Molecular pharmacology 2006;70(5):1735–41. [DOI] [PubMed] [Google Scholar]
- 23.Konig J Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. Handbook of experimental pharmacology 2011(201):1–28. [DOI] [PubMed] [Google Scholar]
- 24.Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol 2011;25(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh ML, Juang HH. Cell growth effects of triiodothyronine and expression of thyroid hormone receptor in prostate carcinoma cells. Journal of andrology 2005;26(3):422–8. [DOI] [PubMed] [Google Scholar]
- 26.Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug metabolism and disposition: the biological fate of chemicals 2011;39(5):920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett 1995;96(2):239–43. [DOI] [PubMed] [Google Scholar]
- 28.de Morree E, van Soest R, Aghai A, de Ridder C, de Bruijn P, Ghobadi Moghaddam-Helmantel I, et al. Understanding taxanes in prostate cancer; importance of intratumoral drug accumulation. The Prostate 2016;76(10):927–36. [DOI] [PubMed] [Google Scholar]
- 29.Iusuf D, Hendrikx JJ, van Esch A, van de Steeg E, Wagenaar E, Rosing H, et al. Human OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and clearance of docetaxel. Int J Cancer 2015;136(1):225–33. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues AC. Efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol 2010;6(5):621–32. [DOI] [PubMed] [Google Scholar]
- 31.Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nat Rev Urol 2017;14(2):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juang HH, Lin YF, Chang PL, Tsui KH. Cardiac glycosides decrease prostate specific antigen expression by down-regulation of prostate derived Ets factor. J Urol 2010;184(5):2158–64. [DOI] [PubMed] [Google Scholar]
- 33.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol 2011;22(12):2640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoption Cann SA, Qiu Z, van Netten C. A prospective study of iodine status, thyroid function, and prostate cancer risk: follow-up of the First National Health and Nutrition Examination Survey. Nutrition and cancer 2007;58(1):28–34. [DOI] [PubMed] [Google Scholar]
- 35.Mondul AM, Weinstein SJ, Bosworth T, Remaley AT, Virtamo J, Albanes D. Circulating thyroxine, thyroid-stimulating hormone, and hypothyroid status and the risk of prostate cancer. PLoS ONE 2012;7(10):e47730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan YX, Knuiman MW, Divitini ML, Brown SJ, Walsh J, Yeap BB. Lower TSH and higher free thyroxine predict incidence of prostate but not breast, colorectal or lung cancer. European journal of endocrinology 2017;177(4):297–308. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Hsieh ML, Zhu W, Klee GG, Tindall DJ, Young CY. Interactive effects of triiodothyronine and androgens on prostate cell growth and gene expression. Endocrinology 1999;140(4):1665–71. [DOI] [PubMed] [Google Scholar]
- 38.Tsui KH, Hsieh WC, Lin MH, Chang PL, Juang HH. Triiodothyronine modulates cell proliferation of human prostatic carcinoma cells by downregulation of the B-cell translocation gene 2. The Prostate 2008;68(6):610–9. [DOI] [PubMed] [Google Scholar]
- 39.van der Deure WM, Hansen PS, Peeters RP, Kyvik KO, Friesema EC, Hegedus L, et al. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology 2008;149(10):5307–14. [DOI] [PubMed] [Google Scholar]
- 40.Lee HH, Leake BF, Teft W, Tirona RG, Kim RB, Ho RH. Contribution of hepatic organic anion-transporting polypeptides to docetaxel uptake and clearance. Molecular cancer therapeutics 2015;14(4):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Morrée ES, Böttcher R, van Soest RJ, Aghai A, de Ridder CM, Gibson AA, et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. British journal of cancer 2016;115:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiyama T, Ikarashi T, Hashimoto Y, Wako K, Takahashi K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J Urol 2007;178(4 Pt 1):1282–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 43.Mostaghel EA, Cho E, Zhang A, Alyamani M, Kaipainen A, Green S, et al. Association of Tissue Abiraterone Levels and SLCO Genotype with Intraprostatic Steroids and Pathologic Response in Men with High-Risk Localized Prostate Cancer. Clin Cancer Res 2017;23(16):4592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franke RM, Carducci MA, Rudek MA, Baker SD, Sparreboom A. Castration-Dependent Pharmacokinetics of Docetaxel in Patients With Prostate Cancer. Journal of Clinical Oncology 2010;28(30):4562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mout L, de Wit R, Stuurman D, Verhoef E, Mathijssen R, de Ridder C, et al. Testosterone Diminishes Cabazitaxel Efficacy and Intratumoral Accumulation in a Prostate Cancer Xenograft Model. EBioMedicine 2018;27:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 2010;62(1):1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray M, Zhou F. Trafficking and other regulatory mechanisms for organic anion transporting polypeptides and organic anion transporters that modulate cellular drug and xenobiotic influx and that are dysregulated in disease. British journal of pharmacology 2017;174(13):1908–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu R, Kanai N, Bao Y, Wolkoff AW, Schuster VL. Regulation of renal oatp mRNA expression by testosterone. Am J Physiol 1996;270(2 Pt 2):F332–7. [DOI] [PubMed] [Google Scholar]
- 49.Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug metabolism and disposition: the biological fate of chemicals 2012;40(7):1366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakkar N, Lockhart AC, Lee W. Role of Organic Anion-Transporting Polypeptides (OATPs) in Cancer Therapy. The AAPS journal 2015;17(3):535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyquist MD, Prasad B, Mostaghel EA. Harnessing Solute Carrier Transporters for Precision Oncology. Molecules (Basel, Switzerland) 2017;22(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


