Abstract
Background:
Hybrid revascularization, combining percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), may be used differently across hospitals. How outcomes compare with multivessel PCI is unknown.
Methods:
We studied hybrid revascularization use in patients in the National Cardiovascular Data Registry from 2009–2017 who underwent PCI for multivessel coronary artery disease (CAD) at 711 hospitals, excluding patients with prior CABG, acute ST-elevation myocardial infarction, emergency/salvage CABG, or PCI without stent placement. In-hospital mortality associated with hybrid revascularization versus multivessel PCI was compared using a multivariable logistic model.
Results:
Among 775,000 patients with multivessel CAD, 1,126 (0.2%) underwent hybrid revascularization and 256,865 (33%) were treated with multivessel PCI. While 358 (50.4%) hospitals performed hybrid revascularizations, most (97.3%) performed <1 per year. Most patients (68.7%) treated with hybrid revascularization underwent CABG after PCI; only 79.4% of these patients were discharged on P2Y12 inhibitors. Patients who underwent hybrid revascularization were younger and more likely to have significant left main or proximal left anterior descending disease. Unadjusted in-hospital mortality rates were higher among patients treated with hybrid revascularization than multivessel PCI (1.5% vs 0.9%, p=0.02), a difference that was not significant after multivariable adjustment (OR=1.54, 95% CI=0.92–2.59).
Conclusions:
Hybrid revascularization remains an infrequently utilized treatment modality for multivessel CAD. Risk-adjusted in-hospital mortality was no different between hybrid revascularization and multivessel PCI, however patients who underwent hybrid revascularization were less likely to be discharged on P2Y12 inhibitor therapy despite stent implantation.
Keywords: Hybrid Coronary Revascularization, Percutaneous Coronary Intervention, Coronary Artery Bypass Grafting
Introduction
The concept of a hybrid revascularization approach to multivessel coronary artery disease (CAD), which combines coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), was first described in 1996 (1). Prior to this, multivessel coronary artery disease was generally managed with either multivessel PCI or multi-graft CABG, the latter typically through a median sternotomy (2). Hybrid coronary revascularization was intended to incorporate the principal benefits of both procedures, specifically decreasing the risk of surgery by utilizing a limited left thoracotomy approach for durable anterior wall revascularization, with a left internal mammary artery graft, while treating disease in other coronary territories with PCI. Since the initial description of the hybrid approach, small studies have evaluated the safety, feasibility and outcomes of patients treated in this manner, as compared to CABG (3). However, data comparing hybrid revascularization to multivessel PCI remain scarce.
The National Cardiovascular Data Registry (NCDR) is a large, nationwide registry capturing consecutive PCI procedures performed in US hospitals, and thus offers a unique perspective on the use of hybrid coronary revascularization in patients with multivessel CAD. The purpose of this study was to evaluate: 1) temporal trends in hybrid coronary revascularization use, 2) clinical and angiographic characteristics of patients treated with hybrid coronary revascularization versus multivessel PCI, and 3) outcomes of these patient cohorts as the next step in understanding the current and future role of hybrid coronary revascularization in the care of patients with multivessel CAD.
Methods
Study Population
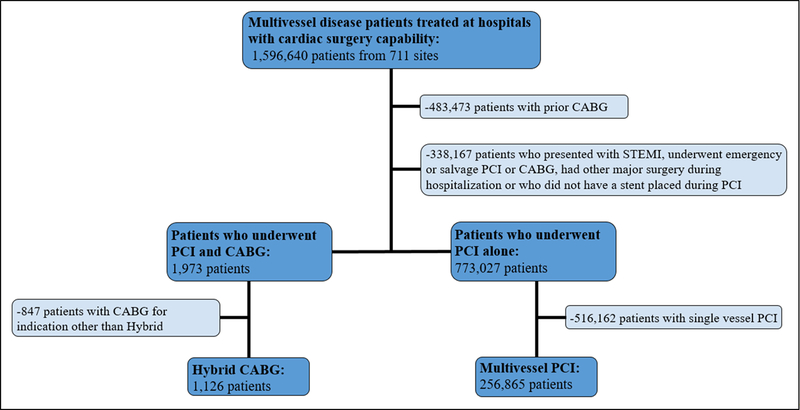
For the period July 1, 2009 through March 31, 2017, 1,596,640 patients with multivessel CAD treated with PCI at 711 hospitals with cardiac surgery capability were identified in the NCDR CathPCI Registry. From this cohort we sought to identify patients where hybrid revascularization might have been considered as the primary strategy. We therefore excluded patients with prior CABG (N=483,473), patients who underwent emergency or salvage PCI or CABG (n=253,763), had other major surgery during the hospitalization (n=14,345), presented with ST-elevation myocardial infarction (n=15,942), or who did not have a stent placed during PCI (n=54,117), as these patients would have been unlikely to have been considered as candidates. This yielded a final study population of 775,000 patients treated at 711 hospitals (Figure 1).
Figure 1. Patient Cohort.
Identification of final patient cohort after application of step-wise exclusion criteria.
Definitions and Data Collection
Data collected for the CathPCI Registry include patient demographic and clinical information, detailed coronary anatomy, PCI procedure details, and in-hospital clinical outcomes. The CathPCI Registry specifically prompts for the performance of hybrid revascularization, with the data dictionary defining hybrid revascularization as follows: “Hybrid therapy occurs when both surgical and percutaneous coronary revascularization are planned, with different lesions treated with the different techniques. Examples include LIMA-LAD followed by PCI of the circumflex or RCA; or primary PCI of the infarct culprit RCA followed by CABG for the severe LMCA stenosis. Unplanned revascularization as a result of a complication (e.g., CABG for PCI-related dissection, PCI for acute graft closure) are NOT considered hybrid procedures because these sequential interventions were not part of a considered treatment strategy.” Other indications for CABG which are available but mutually exclusive from hybrid and thus were not included in our study were “PCI complication”, “PCI failure without clinical deterioration” and “Treatment of CAD without immediately preceding CABG”.
Non-fatal adverse outcomes captured in the CathPCI Registry included myocardial infarction, heart failure, stroke, renal failure requiring new dialysis, bleeding events and vascular complications. Peri-procedural myocardial infarction is defined as myocardial infarction within 24 hours post-PCI, indicated by elevation of cardiac biomarkers above 3 times the upper limit of normal for the local laboratory. Heart failure is defined as the new onset or acute recurrence of heart failure, which necessitates new or increased pharmacologic therapy. Stroke is defined as loss of neurologic function caused by an ischemic or hemorrhagic event with residual symptoms lasting at least 24 hours after onset or leading to death. Renal failure is defined as acute or worsening renal function necessitating the initiation of dialysis. Bleeding events are defined as a suspected or confirmed bleeding event observed and documented in the medical record within 72 hours of the procedure associated with any of the following: 1) hemoglobin drop of ≥ 3g/dL; 2) transfusion of whole blood or packed red blood cells; or 3) percutaneous or surgical intervention at the bleeding site to manage the bleeding. Vascular complications are defined as a hematoma requiring specific treatment or intervention or the occurrence of a vascular complication other than hemostasis-related external bleeding.
Statistical Analysis
We first examined temporal trends in hybrid revascularization use, including total procedures over time and the timing of PCI compared with CABG. We next evaluated the characteristics of the hospitals that performed ≥ 1 hybrid revascularization versus those that did not perform any during the study period. Hospitals that performed hybrid coronary revascularization procedures were then divided into tertiles based on number of hybrid procedures during the study period. Hospital characteristics, including number of beds, location (urban, suburban or rural), category (university, private/community or government), presence of a training program and PCI volume were described based on tertiles of hybrid revascularization use. Categorical variables were compared using Pearson chi-square tests and continuous variables were compared using chi-square rank based group means score statistics.
We compared baseline patient characteristics between patients who underwent hybrid revascularization versus multivessel PCI using Pearson Chi-square tests for categorical variables, and a Chi-square rank-based group means score statistic for continuous variables. In-hospital treatments were described based on timing of PCI relative to CABG for patients undergoing hybrid revascularization. In order to assess independent patient characteristics associated with hybrid revascularization use, we used multivariable logistic regression with generalized estimating equations (GEE) to account for clustering observations. Variables entered into the model included patient age, insurance status, diagnosis of acute coronary syndrome (unstable angina, non-ST elevation myocardial infarction) at admission, prior heart failure, prior PCI, prior cardiac arrest within 24 hours, and lesion characteristics including TIMI flow, presence of a chronic total occlusion, and left main or proximal left anterior descending stenosis of ≥ 50%. Unadjusted mortality was compared between patients undergoing hybrid revascularization versus multivessel PCI using a GEE logistic model. The adjusted odds ratios were also obtained by GEE model but included the following covariates for adjustment: age, body mass index, cerebrovascular disease, peripheral vascular disease, chronic lung disease, prior PCI, diabetes, renal failure, glomerular filtration rate, ejection fraction, cardiogenic shock, heart failure class IV in last two weeks, cardiac arrest within 24 hours, in-stent thrombosis previously treated within one month, lesion segment, number of diseased vessels and chronically occluded vessel. Non-fatal adverse outcomes of interest included peri-procedural myocardial infarction, heart failure exacerbation, cerebrovascular accident, renal failure requiring new dialysis, vascular complications, and bleeding events within 72 hours of the procedure. Each individual event was compared based on type of revascularization using Pearson Chi-square tests for categorical variables, and a Chi-square rank-based group means score statistic for continuous variables. We then evaluated whether there was a difference in the composite of all non-fatal adverse outcomes (myocardial infarction, stroke, heart failure, renal failure requiring new dialysis, bleeding or vascular complications) between patients treated with hybrid revascularization versus multivessel PCI. Adjusted ORs were calculated using the same covariates utilized for mortality (described above). We also compared rates of post-PCI events based on tertile of hospital hybrid volume. Finally, we evaluated hospital length of stay and patient discharge medications based on type of revascularization (multivessel PCI versus hybrid coronary revascularization) using chi-square rank based group means score statistics and Pearson chi-square tests respectively. Patients undergoing hybrid revascularization were further evaluated based on timing of PCI (number of days) relative to CABG.
For all analyses, a p-value of <0.05 was considered significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Hybrid Coronary Revascularization Use
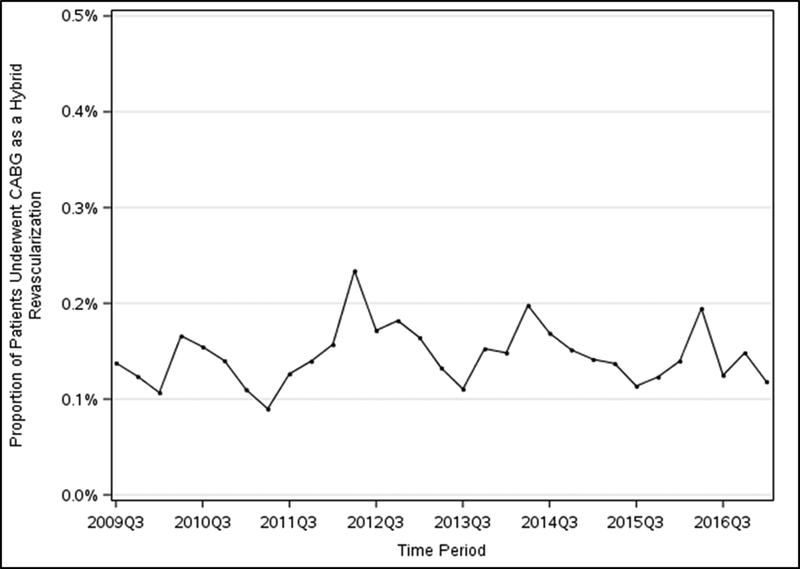
Between July 2009 and March 2017, 775,000 patients with multivessel coronary artery disease underwent non-emergency PCI at 711 hospitals. Of these, 1,973 patients underwent both PCI and CABG and 1,126 of these (57.1% of all PCI-CABG patients, 0.2% of overall multivessel disease patients) were denoted specifically in the CathPCI Registry as hybrid coronary revascularizations. There was no overall change in the proportion of hybrid coronary revascularizations performed between 2009 and 2017 (Figure 2). Of the patients who received PCI alone, 256,865 (33.1%) underwent multivessel PCI alone and the remaining 516,162 underwent single vessel PCI (Figure 1).
Figure 2. Trends of Hybrid CABG/PCI Procedures.
Proportion of patients who underwent hybrid CABG/PCI revascularization for multivessel coronary artery disease between 2009 and 2017.
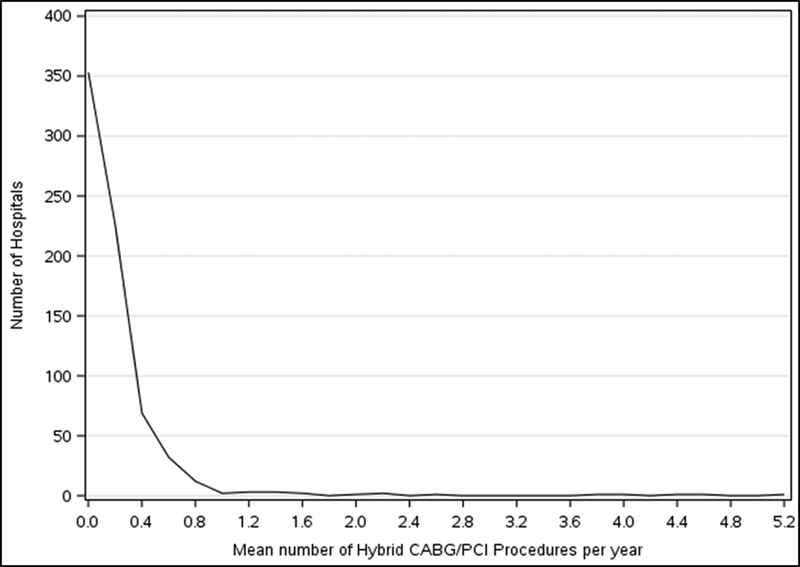
A total of 358 hospitals (50.4%) performed at least one hybrid revascularization over the study period; increasing from 47 hospitals in 2009 to 97 hospitals in 2016. Hospitals performing hybrid revascularizations were generally larger, had higher PCI volumes, and were more likely to be teaching hospitals compared with those that did not perform hybrid revascularization during the study period (Table 1). Among hospitals performing hybrid revascularization procedures, the median number of cases per year was 0.2 (0.1, 0.3); the majority of these hospitals (n=692, 97.3%) performed <1 hybrid coronary revascularization procedure per year and no hospitals performed >6 hybrid revascularization procedures per year (Figure 3).
Table I.
Characteristics of Hospitals based on Hybrid Revascularization Volume.
| Hybrid (N=358) |
No Hybrid (N=353) |
p-value | Hybrid 1st Tertile (N=119) |
Hybrid 2nd Tertile (N=120) |
Hybrid 3rd Tertile (N=119) |
p-value | |
|---|---|---|---|---|---|---|---|
| Number of hybrid revascularizations per year | 0.2 (0.1, 0.3) |
--- | <0.001 | 0.1 (0.1, 0.3) |
0.2 (0.1, 0.3) |
0.3 (0.2, 0.7) |
<0.001 |
| Number of CMS Certified Beds | 382 (255–567) |
328 (219 – 450) |
<0.001 | 451 (335 – 700) |
347 (252 – 515) |
343 (199 – 527) |
<0.001 |
| Urban | 55.9% | 48.4% | 0.15 | 55.5% | 60.8% | 51.3% | 0.03 |
| Suburban | 33.8% | 36.5% | 37.8% | 25.8% | 29.4% | ||
| Rural | 13.9% | 14.7% | 6.7% | 13.3% | 19.3% | ||
| University | 13.1% | 9.3% | 0.03 | 14.3% | 8.3% | 16.8% | 0.07 |
| Private/Community | 86.3% | 87.8% | 84.9% | 91.7% | 82.4% | ||
| Government | 0.6% | 2.5% | 0.8% | 0.0% | 0.8% | ||
| South | 42.2% | 34.3% | 0.10 | 39.5% | 47.5% | 39.5% | 0.29 |
| Midwest | 26.0% | 27.8% | 30.3% | 22.5% | 25.2% | ||
| Northeast | 12.8% | 12.2% | 13.4% | 13.3% | 11.8% | ||
| West | 19.0% | 25.2% | 16.8% | 16.7% | 23.5% | ||
| Training Program | 51.4% | 45.3% | 0.11 | 56.3% | 48.3% | 49.6% | 0.23 |
| Average Annual PCI Volume | 573.8 (381.7–857.8) |
335.8 (211.9–538.5) |
<0.001 | 720.7 (514.5–1104.6) |
572.0 (351.5–759.2) |
450.1 (249.7–724.8) |
0.26 |
Figure 3. Mean Number of CABG/PCI Procedures at Each Hospital.
Hospital level data showing the mean number of hybrid CABG/PCI revascularization procedures performed per year during the study period.
Comparison Between Hybrid PCI then CABG and Hybrid CABG then PCI
Of the 1,126 hybrid revascularization procedures, CABG was performed after PCI in 774 (68.7%), at a median of 3.0 (1.7 – 5.0) days later. Patients treated with CABG prior to PCI (n=324, 31.1%) underwent surgery at a median of 2.9 (1.1 – 4.8) days prior to PCI. Out of the entire cohort, only 2 patients underwent simultaneous CABG and PCI. Among hybrid revascularizations, patients who underwent CABG first then PCI were older, less likely to be admitted with a non-ST elevation acute coronary syndrome, and more likely to have comorbidities including prior heart failure, diabetes, cerebrovascular disease, peripheral vascular disease, hypertension, dyslipidemia and renal failure requiring dialysis than patients who underwent PCI first then CABG (Table 2).
Table II.
Hybrid Revascularization Patient Characteristics by Relative Timing of CABG and PCI.
| Hybrid Revascularizations | p-value | ||
|---|---|---|---|
| PCI Then CABG N=774 |
CABG Then PCI N=324 |
||
| Demographics | |||
| Age | 64.0 (56.0, 71.0) | 67.0 (61.0, 74.0) | <0.001 |
| Male Gender | 71.1% | 68.8% | 0.46 |
| White Race | 84.8% | 85.8% | 0.66 |
| Clinical Characteristics | |||
| ACS Admission | 90.3% | 63.9% | <0.001 |
| Previous MI | 24.7% | 29.6% | 0.09 |
| Previous CHF | 9.2% | 19.8% | <0.001 |
| Diabetes | 37.5% | 50.6% | <0.001 |
| Cerebrovascular Disease | 10.7% | 21.0% | <0.001 |
| Peripheral Vascular Disease | 9.7% | 19.1% | <0.001 |
| Chronic Lung Disease | 15.0% | 17.9% | 0.23 |
| Hypertension | 82.3% | 87.7% | 0.03 |
| Dyslipidemia | 75.7% | 83.3% | 0.006 |
| Previous PCI | 24.9% | 27.5% | 0.38 |
| Currently on Dialysis | 1.9% | 8.3% | <0.001 |
| Procedure Characteristics | |||
| Multivessel PCI | 11.2% | 35.8% | <0.001 |
| Lesion Treated with PCI | |||
| RCA territory | 49.4% | 41.0% |
<0.001 |
| Circumflex territory | 31.5% | 40.1% | |
| LAD territory | 17.4% | 6.2% | |
| Drug Eluting Stent Use | 60.1% | 85.5% | <0.001 |
| Discharge Medications | |||
| Aspirin | 97.1% | 96.8% | 0.44 |
| P2Y12 Inhibitor | 79.4% | 96.8% | <0.001 |
| Clopidogrel | 75.6% | 88.8% | <0.01 |
| Prasugrel | 2.6% | 3.2% | 0.63 |
| Ticagrelor | 1.2% | 5.1% | <0.001 |
| Statin | 93.9% | 94.8% | 0.17 |
| Beta Blocker | 94.0% | 94.6% | 0.30 |
| ACE Inhibitor or Angiotensin Receptor Blocker | 47.7% | 40.0% | 0.04 |
Among hybrid coronary revascularization patients treated with PCI before CABG, the majority (88.4%) underwent single vessel PCI. The lesion(s) treated by PCI most often was in the right coronary artery distribution (51.2%) followed by the left circumflex artery (31.3%, Table 2). Drug-eluting stents were implanted in 60.1% of patients. Only 79.4% of these patients were discharged on P2Y12 inhibitor therapy despite stent implantation during their index hospitalization. In comparison, hybrid revascularization patients treated with PCI after CABG were more likely to undergo multivessel PCI. The lesion(s) treated by PCI in these patients were again mostly in the right coronary artery (50.0%) followed by the circumflex artery (44.7%) territory. Drug-eluting stents were implanted in 85.5% of patients, and 96.8% of these patients were discharged on P2Y12 inhibitor therapy.
Among all patients who underwent hybrid revascularization, 85.8% were discharged with P2Y12 therapy. Those patients who did not receive a P2Y12 inhibitor at discharge were more likely to present with ACS, to have diabetes and to currently be on dialysis (Supplemental Table 1). Multivessel PCI and drug eluting stent implantation were less common among patients who were not discharged with a P2Y12 inhibitor. Patients discharged without a P2Y12 inhibitor were similarly less likely to be discharged with aspirin and a statin.
Comparison Between Hybrid Coronary Revascularization and Multivessel PCI
Compared with patients treated with multivessel PCI, patients treated with hybrid coronary revascularization were younger, more likely to be uninsured, and less likely to have prior MI or prior PCI compared with patients who underwent multivessel PCI. (Table 3). Additionally, patients who were treated with hybrid coronary revascularization were more likely to present with an acute coronary syndrome, to have cardiogenic shock present at the start of the PCI procedure and to require intra-aortic balloon pump placement. On coronary angiography, patients treated with hybrid coronary revascularization were more likely to have a significant left main or proximal left anterior descending artery lesion than patients who received multivessel PCI (69.8% vs. 42.9%, p<0.001).
Table III.
Baseline Patient Characteristics based on Type of Revascularization.
| Multivessel PCI N=256,865 |
Hybrid PCI/CABG N=1,126 |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 66 (58–75) | 65 (58–72) | <0.001 |
| Female | 32.0% | 29.6% | 0.08 |
| Non-white race | 14.1% | 14.8% | 0.47 |
| Uninsured | 4.5% | 7.3% | <0.001 |
| History and Risk Factors | |||
| BMI | 29.1 (25.7–33.3) | 29.0 (25.8–33.1) | 0.95 |
| Prior MI | 29.0% | 25.9% | 0.02 |
| Previous PCI | 38.9% | 25.7% | <0.001 |
| Diabetes | 41.2% | 41.5% | 0.87 |
| Cerebrovascular disease | 13.3% | 14.0% | 0.45 |
| Hypertension | 85.4% | 84.2% | 0.23 |
| Dyslipidemia | 81.6% | 80.0% | 0.002 |
| Creatinine clearance (mL/min) | 65.0 (46.4–84.8) | 68.4 (49.6–87.0) | 0.002 |
| Presentation | |||
| ACS: NSTEMI | 26.0% | 42.9% | <0.001 |
| ACS: Unstable Angina | 45.7% | 40.0% | |
| Stable Angina | 17.6% | 8.7% | |
| Atypical Chest Pain | 2.7% | 1.1% | |
| No Angina | 8.0% | 7.4% | |
| Cardiac arrest* | 0.4% | 1.4% | <0.001 |
| Ejection Fraction <40% | 11.7% | 12.3% | 0.45 |
| LM or Prox LAD Lesion | 42.9% | 69.8% | <0.001 |
| 3VD (vs. 2VD) | 31.6% | 60.0% | <0.001 |
| PCI Complexity | |||
| Number of lesions treated | 2.0 (2.0, 3.0) |
1.0 (1.0, 2.0) |
<0.001 |
| Lesion length | 16.0 (12.0–24.0) | 18.0 (12.0–26.0) | 0.007 |
| CTO | 36.3% | 11.9% | <0.001 |
| Pre-procedure TIMI 0/1 | 13.2% | 32.2% | <0.001 |
| High/Type C lesion | 50.8% | 58.1% | <0.001 |
| Bifurcation lesion | 11.6% | 9.9% | 0.09 |
| Drug-eluting stent | 89.9% | 67.1% | <0.001 |
| Discharge P2Y12 inhibitor | |||
| Overall | 98.0% | 83.1% | <0.001 |
| Clopidogrel | 73.1% | 78.1% | <0.001 |
| Prasugrel | 15.4% | 2.7% | <0.001 |
| Ticagrelor | 10.2% | 6.3% | <0.001 |
BMI: Body Mass Index
MI: Myocardial Infarction
PCI: Percutaneous Coronary Intervention
ACS: Acute Coronary Syndrome
LM: Left Main
LAD: Left Anterior Descending
IABP: Intra-aortic Balloon Pump
Cardiac arrest within 24 hours of presentation to cardiac catheterization lab
Missing data excluded; all fields with <1% missing data with exception of LM/LAD stenosis
The median number of lesions treated with PCI were 1.0 (1.0, 2.0) and 2.0 (2.0, 3.0) (p<0.001) for patients undergoing hybrid revascularization versus multivessel PCI respectively. Lesion length was longer in patients treated with hybrid revascularization (18.0 mm vs 16.0 mm, p=0.007) and lesions were also more likely to be high risk/type C lesions (58.1% vs 50.8%, p<0.001). Patients undergoing hybrid coronary revascularization were less likely to receive a P2Y12 inhibitor at discharge than patients treated with multivessel PCI (83.1% vs. 98.0%, p<0.001).
Outcomes
Patients who underwent hybrid coronary revascularization had significantly higher unadjusted in-hospital mortality (1.5% vs 0.9%, p=0.02, unadjusted OR = 1.78, 95% CI=1.09–2.90) compared with patients who were treated with multivessel PCI. However, upon adjusting for patient comorbidities and angiographic data, this was no longer significant (adjusted OR= 1.54, 95% CI = 0.92–2.59). There was no difference in raw mortality between patients with hybrid coronary revascularization who underwent PCI before or after CABG (1.3% vs. 2.2%, p=0.29).
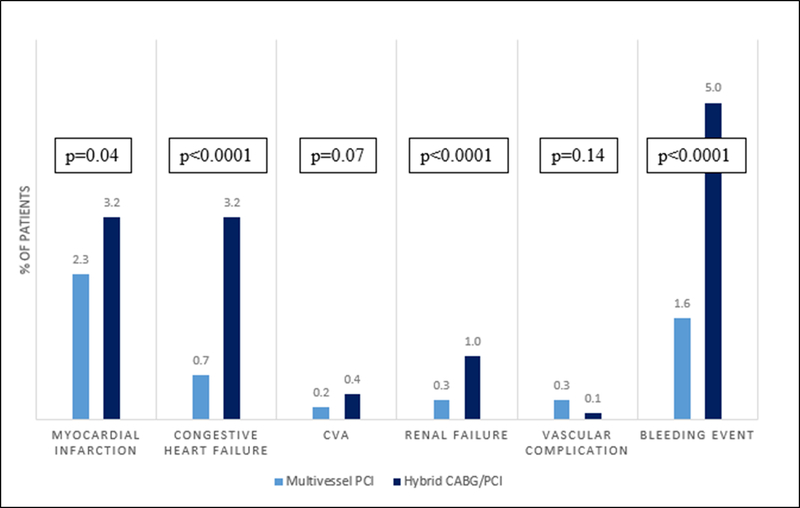
Patients treated with hybrid coronary revascularization were more likely to have post-PCI non-fatal adverse outcomes than patients who underwent multivessel PCI (individual event rates shown in Figure 4). After multivariable adjustment, patients treated with hybrid revascularization remained more likely to experience the composite of non-fatal adverse outcomes (adj OR 2.57, 95% CI 2.03, 3.26; p<0.001) compared with those treated with multivessel PCI. Among hybrid coronary revascularization patients only, those who underwent PCI then CABG were more likely to have periprocedural myocardial infarction (3.9% vs. 1.2%, p=0.02), HF (3.9% vs. 1.5%, p=0.04) and bleeding (6.6% vs. 1.5%, p<0.001) than patients who underwent CABG then PCI. When post-PCI complications were evaluated based on the tertile of hospital hybrid procedural volume, there was no significant differences in the rates of myocardial infarction (1.1% 1st tertile vs 1.0% 2nd tertile vs 1.4% 3rd tertile, p=0.11), heart failure (1.0% vs 0.9% vs 1.0%, p=0.32) or bleeding events (1.9% vs 1.8% vs 1.8%, p=0.08).
Figure 4. Adverse Events in Patients Undergoing Hybrid CABG/PCI versus Multivessel PCI.
Proportion of patients with adverse events during the index hospitalization based on revascularization technique.
Myocardial infarction: Indicates peri-procedural myocardial infarction
CVA: Cerebral vascular accident
Patients treated with hybrid coronary revascularization had significantly longer lengths of stay (9.0 vs. 2.0 days, p<0.001) with 85.9% of patients staying ≥4 days post-cardiac catheterization procedure. At the time of discharge, those treated with hybrid CABG/PCI were significantly less likely to be discharged to home (78.2% vs. 95.3%, p<0.001) compared with those who underwent multivessel PCI. Among patients who underwent hybrid coronary revascularization, those treated with PCI before CABG had longer hospital lengths of stay (10.0 vs. 8.0 days, p<0.001) but were equally likely to be discharged to home (79.8% vs. 79.2%, p=0.16) compared with patients who underwent PCI after CABG.
Discussion
We studied the use of hybrid coronary revascularization across the United States between 2009 and 2017 and observed the following: 1) hybrid coronary revascularization procedures were performed in only a subset of PCI- and CABG-capable hospitals, with the majority of these hospitals performing <1 case per year; 2) two thirds of hybrid revascularizations were performed as CABG following PCI with the procedures separated by a median of 3 days; 3) risk-adjusted mortality was not significantly different between patients treated with hybrid revascularization versus multivessel PCI, and also not different among hybrid revascularization patients regardless of order of revascularization, and 4) patients who underwent hybrid revascularization were less likely to be discharged on P2Y12 inhibitor therapy despite stenting, particularly among patients who underwent PCI before CABG.
Several small studies have evaluated the safety, feasibility and outcomes of patients treated with hybrid revascularization (3–5). The largest scale study of hybrid coronary revascularization to date was an examination of the Society of Thoracic Surgery (STS) database by Harskamp and colleagues which described 950 patients undergoing hybrid revascularization and 197,672 patients treated with CABG at 361 US hospitals between 2011 and 2013 (6). The hybrid coronary revascularization cohort similarly represented a small fraction (<0.5%) of CABG revascularization procedures performed during that time. Contrary to what we observed, CABG preceded PCI in 2/3 of cases, as would be supported by the American College of Cardiology Foundation/American Heart Association guidelines for CABG (7). This may be a reflection of the differing patient populations - our study included all patients undergoing PCI whereas the STS registry included patients undergoing CABG. However, this distinction may also give insight into the definitions used to identify patients undergoing hybrid revascularization. While the literature defines hybrid revascularization as a patient undergoing planned CABG and PCI, generally as a staged procedure, some would note that patients treated with PCI prior to CABG represent an inherently different subset of patients. The CathPCI registry retains the definition of “planned surgical and percutaneous coronary revascularization with different lesions treated with the different techniques” but does not delineate timing of CABG relative to PCI as a criterion for hybrid revascularization. A similar definition has been used previously in analyses completed from the STS database(6). We excluded patients who presented with STEMI, who underwent emergent cardiac catheterization or CABG procedures, or who underwent PCI without stenting, as these are patients do not fit the spirit of hybrid revascularization. Even after these exclusions, we observed patients undergoing CABG then PCI constituted only one third of patients denoted as hybrid revascularization.
The majority of comparative studies examining hybrid coronary revascularization used patients undergoing CABG as the active comparator to hybrid coronary revascularization (3,8–10). A recent meta-analysis of eight studies showed similar risk of major adverse cardiac and cerebrovascular events (MACCE), all-cause mortality, myocardial infarction, stroke and repeat revascularization between hybrid coronary revascularization and CABG (11). Limited data, however, is available for the comparison of patient outcomes between hybrid coronary revascularization and multivessel PCI. An observational study by Puskas et al represents the only prospective data currently available and showed no significant difference in MACCE at 12 months in patients treated with multivessel PCI compared with hybrid coronary revascularization (5). In unadjusted comparisons, our analysis showed an increased risk of mortality, peri-PCI myocardial infarction, heart failure, renal failure and significant bleeding events in patients undergoing hybrid coronary revascularization compared with multivessel PCI. After multivariable adjustment, there was no longer a significant difference in mortality between patients undergoing hybrid coronary revascularization and multivessel PCI. In the era of contemporary drug-eluting stents, where outcomes from PCI continue to improve, this comparison remains a topic of increasing interest. The National Heart, Lung and Blood Institute currently funds a large-scale, prospective, multi-center randomized controlled trial which will compare hybrid coronary revascularization and multivessel PCI in nearly 2,400 patients with follow-up planned for five years (clinicaltrials.gov/) and will provide further insight into the long term outcomes of this patient population.
As expected, length of stay was longer in patients undergoing hybrid coronary revascularization than multivessel PCI- an unsurprising result based on the typical course of the surgical CABG patient. However, there were also significant differences between the groups in terms of treatment regimen at the time of discharge. Despite being more likely to present with an acute coronary syndrome, patients treated with hybrid revascularization were significantly less likely to be discharged on a P2Y12 inhibitor. Based on current guidelines, it is reasonable in patients without contraindication to dual antiplatelet therapy to be treated with aspirin and a P2Y12 inhibitor for up to 12 months after CABG for acute coronary syndrome (12,13). Additionally, the majority of patients in our study underwent percutaneous intervention with placement of a drug eluting stent (DES) for which guidelines recommend at least six months of dual antiplatelet therapy (14,15). However, neither of these guidelines adequately address the patient who undergoes both procedures. That patients treated with hybrid coronary revascularization were less likely to be prescribed dual antiplatelet therapy at discharge, particularly in the group of patients who underwent PCI then CABG, either as an error of omission, or as a consequence of avoidance of post-CABG bleeding, is concerning because of the potential for subsequent stent thrombosis in the absence of dual antiplatelet therapy. Going forward, strategies to address this omission will be important to optimize the secondary prevention of cardiovascular events and potentially reduce admissions and improve outcomes in this patient population.
Hybrid revascularization remains largely unutilized for the vast majority of patients with multivessel coronary artery disease. One concern our study raises is how successful hybrid revascularization procedures can be when performed rarely, as the majority of centers performed <1 hybrid revascularization annually. Much of the hesitancy to recommend hybrid coronary revascularization may lie in the limited data on long-term outcomes. The ongoing clinical trial comparing hybrid coronary revascularization with multivessel PCI may help to clarify some of the uncertainty around hybrid revascularization, to better inform patients of long-term benefits vs. higher short-term surgical risk, and to more fully understand the ideal patient populations who may benefit from the hybrid revascularization approach.
Limitations
Several limitations associated with our study should be acknowledged. First, given the observational nature of our analysis, insight into physician decision making was not available; the inability to account for selection bias is thus a major limitation. While multivariable adjustment was performed, unmeasured confounding cannot be excluded. Determination of the planned nature of a procedure is made via chart abstraction after discharge and thus there is potential for misclassification of hybrid revascularization procedures. Additionally, as a database centered around diagnostic and interventional cardiac catheterization procedures, the CathPCI registry captures data only about clinical events surrounding the timing of the heart catheterization. In the case of bleeding events, by definition, only events within 72 hours of the catheterization procedure are recorded. Events that occurred at the time of CABG and as complications following CABG may also not have been captured and the exact timing of adverse events relative to each revascularization procedure is not available. Additionally, outcomes were only available until the time of discharge from the primary hospitalization and thus evaluation beyond this was not available. In a similar manner, the NCDR does not link across hospitalizations, so information about staged CABG or staged PCI procedures during a subsequent admission at the same or different hospital is not available. There may be hospitals performing hybrid procedures that do not participate in the Cath PCI registry. The CathPCI registry also does not capture the anatomic description or clinical details regarding the CABG procedure for hybrid coronary revascularization patients. Finally, hybrid revascularization was reported by participating hospitals using the standard NCDR definition described above, which does not fully align with other definitions of hybrid coronary revascularization used in practice and further highlights the need for a standardized definition of hybrid coronary revascularization (16).
Conclusions
In this nationwide cohort of patients with multivessel CAD, we observed limited use of hybrid coronary revascularization in contemporary clinical practice. Approximately one third of hybrid revascularizations are performed as CABG followed by PCI while two thirds have CABG performed following PCI. The latter was associated with lower rates of discharge dual antiplatelet therapy despite recent stent implantation. Adjusted mortality rates were not significantly different between patients treated with hybrid coronary revascularization and multivessel PCI, nor between hybrid revascularization patients, regardless of the order of PCI and CABG. Further work is necessary to fully clarify the comparative effectiveness of hybrid coronary revascularization versus multivessel PCI, the patient populations where hybrid coronary revascularization is most beneficial, and to optimize the post-procedural medical management and outcomes of these patients.
Supplementary Material
Acknowledgments
This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR.
Sources of Funding
This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR.
Disclosures
A Lowenstern - Dr. Lowenstern reports funding through NIH T-32 training grant #5 T32 HL069749–14.
J Wu - None
SM Bradley - None
AC Fanaroff - Dr. Fanaroff reports research support from the American Heart Association.
JE Tcheng - Dr. Tcheng reports membership on the ACC NCDR Management Board
TY Wang - Dr. Wang reports research support from AstraZeneca, Glaxo SmithKline, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Regeneron Pharmaceuticals, Sanofi and Consulting from Eli Lilly, Premier, Inc., Bristol Myers Squibb, AstraZeneca
Abbreviations
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CVA
Cerebrovascular accident
- DES
Drug eluting stent
- GEE
Generalized estimating equation
- MACCE
Major adverse cardiac and cerebrovascular events
- NCDR
National Cardiovascular Data Registry
- PCI
Percutaneous coronary intervention
- STS
Society of Thoracic Surgeons
References
- 1.Angelini GD, Wilde P, Salerno TA, Bosco G, Calafiore AM. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 1996;347:757–8. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Morice MC, Kappetein AP et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. [DOI] [PubMed] [Google Scholar]
- 3.Zhu P, Zhou P, Sun Y, Guo Y, Mai M, Zheng S. Hybrid coronary revascularization versus coronary artery bypass grafting for multivessel coronary artery disease: systematic review and meta-analysis. J Cardiothorac Surg 2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasior M, Zembala MO, Tajstra M et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1277–83. [DOI] [PubMed] [Google Scholar]
- 5.Puskas JD, Halkos ME, DeRose JJ et al. Hybrid Coronary Revascularization for the Treatment of Multivessel Coronary Artery Disease: A Multicenter Observational Study. J Am Coll Cardiol 2016;68:356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harskamp RE, Brennan JM, Xian Y et al. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the society of thoracic surgeons adult cardiac database. Circulation 2014;130:872–9. [DOI] [PubMed] [Google Scholar]
- 7.Hillis LD, Smith PK, Anderson JL et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123–210. [DOI] [PubMed] [Google Scholar]
- 8.Leacche M, Byrne JG, Solenkova NS et al. Comparison of 30-day outcomes of coronary artery bypass grafting surgery verus hybrid coronary revascularization stratified by SYNTAX and euroSCORE. J Thorac Cardiovasc Surg 2013;145:1004–12. [DOI] [PubMed] [Google Scholar]
- 9.Halkos ME, Vassiliades TA, Douglas JS et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting for the treatment of multivessel coronary artery disease. Ann Thorac Surg 2011;92:1695–701; discussion 1701–2. [DOI] [PubMed] [Google Scholar]
- 10.Vassiliades TA, Kilgo PD, Douglas JS et al. Clinical outcomes after hybrid coronary revascularization versus off-pump coronary artery bypass: a prospective evaluation. Innovations (Phila) 2009;4:299–306. [DOI] [PubMed] [Google Scholar]
- 11.Sardar P, Kundu A, Bischoff M et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: A meta-analysis. Catheter Cardiovasc Interv 2018;91:203–212. [DOI] [PubMed] [Google Scholar]
- 12.Amsterdam EA, Wenger NK, Brindis RG et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 13.Kulik A, Ruel M, Jneid H et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131:927–64. [DOI] [PubMed] [Google Scholar]
- 14.Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122. [DOI] [PubMed] [Google Scholar]
- 15.Levine GN, Bates ER, Bittl JA et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016;134:e123–55. [DOI] [PubMed] [Google Scholar]
- 16.Harskamp RE, Bonatti JO, Zhao DX et al. Standardizing definitions for hybrid coronary revascularization. J Thorac Cardiovasc Surg 2014;147:556–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.