Abstract
Serum and tissue miR-21 expression in patients with breast cancer (BC) is a useful biomarker for cancer diagnosis, progression, and treatment. Matrix metalloproteinase-1 (MMP-1) is also important in breast cancer carcinogenesis. However, miR-21 and MMP-1/CD63 in urine exosomes in these patients have not been examined. Urine samples were collected from patients with BC and 26 healthy females. Urinary exosomes were isolated and confirmed by western blotting with anti-CD63 antibody and electron microscopy observation. MiR-21 and MMP-1/CD63 expression was examined by quantitative RT-PCR and western blotting, respectively. Patients with very early stage breast cancer were evaluated. MiR-21 expression in the patients was 0.26 [95% CI: 0.20–0.78], which was significant lower than in the 26 controls (1.00 [95% CI: 1.01–3.37], p = 0.0947). MMP-1/CD63 expression in patients was significantly higher than in controls (1.74 [95% CI: 0.86–5.08] vs 0.535 [95% CI: −0.01–2.81], p = 0.0001). Sensitivity and specificity were 0.708 and 0.783 for miR-21 and 0.792 and 0.840 for MMP-1/CD63, respectively. Sensitivity and specificity of combined expression were 95% and 79%, respectively. The sensitivity of MMP-1/CD63 expression in urinary exosomes was better than that of miR-21 expression. Thus, miR-21 and MMP/CD63 may be useful markers for BC screening.
Subject terms: Breast cancer, Predictive markers
Introduction
Breast cancer (BC) is prevalent worldwide. In the United States, the incidence of BC was high in the 1990s, but has somewhat decreased1. In Japan, however, the prevalence of BC has gradually increased, with the highest incidence rate and the third leading cause of mortality among all cancers2. Early detection of BC is associated with a good prognosis, and thus BC screening has been widely promoted for the past 30 years in Japan. However, mammography and/or echography is time-consuming and costly, as well as inconvenient.
We previously reported urinary cancer screening tests for measuring urinary 3-hdroxyproline3–5. However, the reliability of these tests was low. Screening methods for BC that are simple, reliable, easy to access, and inexpensive are needed. Molecular biology studies showed that exosomes secreted from cancer cells can migrate to distant organs to form niches, leading to metastasis6,7. Exosomes contain both microRNAs (miRs) and oncogenes6,7. The expression levels of miR-21 in the tissue and serum derived from patients with BC have been reported as useful biomarkers for diagnosis, cancer progression, and treatment selection8–16. MiR-21 inhibits the function of several tumour suppressor genes17,18. The expression of miR-21 in urinary exosomes of patients with BC has not been reported because they had not confirmed their samples by CD63 antibody nor by the electron microscopy observation16.
We also previously investigated the mechanism of matrix metalloproteinase-1 (MMP-1) in the formation of liver cirrhosis19–23 and development of hepatocellular carcinoma24–26. MMP-1 is a key proteinase in matrix metabolism27. MMP-1 not only attacks collagen molecules at the three quarters from N-terminals27 but also is involved in cancer development including BC27–29.
This study was performed to determine the expression levels of both miR-21 and MMP-1/CD63 in urinary exosomes and evaluate these biomarkers for screening of early stage BC.
Results
Clinical characteristics and histopathological findings of 22 patients with BC
The clinical characteristics and histopathological findings of the 22 patients with BC are shown in Table 1. Cases were not advanced at surgical resection; one case showed metastasis at 6 months after surgical resection. These cases were detected relatively early because most patients visited the Department of Preventive Medicine of Sanno Medical Center for a health check which included BC screening, and suspicious lesions were detected by breast echography and mammography. Two cases were under 40 years old, 11 cases were between 40 and 49 years old, 5 cases were between 50 and 59 years old, one case was 61 years old, and 3 cases were 72, 74, and 74 years old. Therefore, 16 cases (72%) were between 40 and 59 years old. Among the 22 patients, two patients were in Stage 0, 7 patients were in Stage I, 7 patients were in Stage II, and the remaining 6 patients were in Stage III. Regarding tumour size, 11 cases were 1–2 cm, 6 cases were 2–5 cm, and 5 cases were >5 cm. Seven cases were positive in the lymph nodes. Ten cases showed scirrhous carcinoma, 3 cases showed mucinous carcinoma, 2 cases showed solid tubular carcinoma, 2 cases showed papillary tubular carcinoma, 2 cases showed invasive lobular carcinoma, and 3 cases were microinvasion, invasive ductular carcinoma, and ductular carcinoma in situ.
Table 1.
Breast Cancer Cases, TNM Classification, and Characteristics.
| No. | Age (years) |
Body Weight (kg) |
BMI (kg/m2) |
TNM classification* | Stage | Patho† | Expression Levels of miR-21 (2ΔCt)# | Expression Levels of MMP-1/CD63## |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 110 | 40.4 | T2N0M0 | II | scirrhous ca. | 0.11 | 1.78 |
| 2 | 59 | 53 | 19.5 | TisN0M0 | 0 | microinvasive- | 0.26 | 1.02 |
| 3 | 36 | 67 | 24.3 | T2N0M0 | II | solid tub. ca. | 0.09 | 1.70 |
| 4 | 56 | 49 | 19.1 | T1N0M0 | II | scirrhous ca. | 1.04 | 2.00 |
| 5 | 46 | 53 | 21.5 | T2N0M0 | I | solid tub. ca. | 0.37 | 1.08 |
| 6 | 40 | 68 | 26.9 | T3N1M0 | IIIA | pap-tub.ca. | 0.18 | 0.76 |
| 7 | 52 | 52 | 20.8 | T1N1M0 | IIA | scirrhous ca. | 0.32 | 1.09 |
| 8 | 52 | 63 | 26.1 | T2N1M0 | IIB | scirrhous ca. | 0.22 | 0.85 |
| 9 | 48 | 54 | 20.4 | T1N0M0 | I | scirrhous ca. | 0.30 | 1.84 |
| 10 | 74 | 69 | 25.3 | T2N0M0 | II | mucinous ca. | 0.08 | 3.04 |
| 11 | 49 | 56 | 22.4 | T1N0M0 | I | scirrhous ca. | 0.00 | 23.20 |
| 12 | 48 | 62 | 22.5 | T1N0M0 | I | pap-tub. ca. | 0.06 | 3.56 |
| 13 | 46 | 65 | 22.8 | T1 N0 M0 | I | ductal ca. in situ | 0.01 | 2.25 |
| 14 | 47 | 54 | 20.1 | T3 N3 M0 | IIIC | scirrhous ca | 0.01 | 3.91 |
| 15 | 47 | 70 | 27.0 | T1 N0 M0 | I | scirrhous ca | 0.36 | 2.23 |
| 16 | 45 | 57 | 22.7 | Tis N0 M0 | 0 | inv lob ca | 0.26 | 6.78 |
| 17 | 74 | 57 | 24.3 | T3 N0 M0 | II | scirrhous ca | 0.23 | 3.90 |
| 18 | 52 | 50 | 16.7 | T3N0M0 | IIIA | inv. lobular ca | 0.49 | 1.53 |
| 19 | 72 | 61 | 26.8 | T3N1M0 | IIIA | mucinous ca | 0.41 | 0.78 |
| 20 | 45 | 50 | 19.8 | T1cN3M0 | IIIA | inv. ductal ca | 2.27 | 1.02 |
| 21 | 46 | 52 | 20.6 | T1N3M0 | IIIA | scirrhous ca | 1.48 | 0.92 |
| 22 | 35 | 58 | 20.1 | T2N0M0 | I | mucinous ca | 2.22 | 0.11 |
|
Median (95% CI) |
0.26 (0.20–0.73) |
1.74 (0.86–5.08) |
*TNM classification; †Pathological diagnosis; ca: carcinoma; tub: tubular; pap: papillary.
#Number indicates the relative expression levels, that is microRNA copies by RT-PCR in patients divided the mean number of microRNA copies in healthy controls.
##Number indicates the relative expression levels of MMP-1/CD63, that was measured by western blotting with both antibodies.
Isolation and confirmation of urinary exosomes
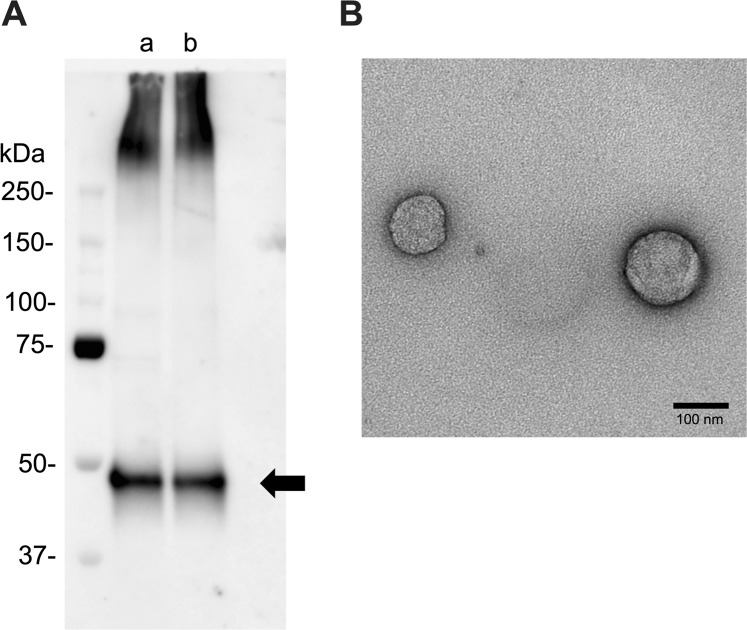
Urinary exosomes isolated with a Miltenyi Biotec isolation kit (Bergisch Gladbach, Germany) were confirmed to be exosomes by western blotting with anti-CD63 antibody (Fig. 1A) and the size of isolated exosomes was determined by electron microscopy (Fig. 1B).
Figure 1.
(A) Western blot analysis of urinary exosome in patients with BC and healthy controls. (a) Patients with BC (No. 1) and (b) Healthy control (No. 1). One-sixth of the urinary exosome extracted from 2 mL urine was applied to the wells. anti-CD63 antibody biotin conjugated (1:1000). The blot is full-length and no trimming. (B) Electron microscopy of urinary exosomes. The size of exosomes was nearly 100 nm and the EM features were identified.
Expression levels of miR-21 and MMP-1/CD63 in 22 patients with BC and 26 healthy females
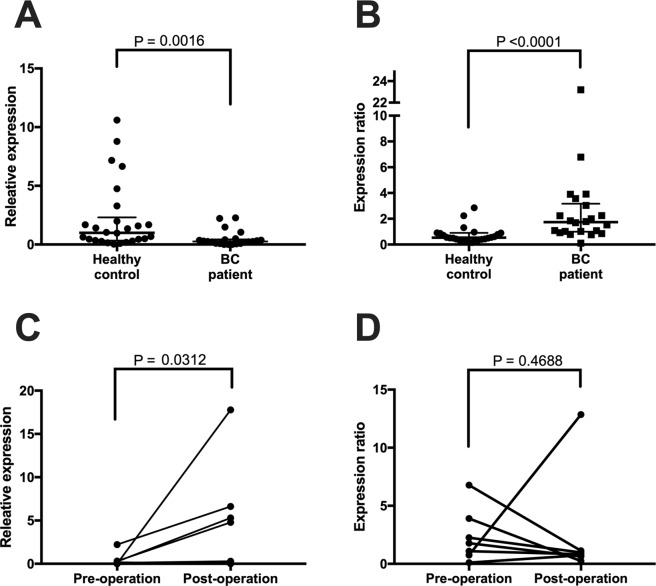
The relative expression levels of miR-21 in 22 patients with BC based on the expression levels of healthy controls are shown in Table 1, and the individual data of 26 healthy controls are listed in Supplementary Table 2. The mean expression level of 22 patients with BC was 0.26 [95% CI: 0.20–0.78], which was significantly lower than that in the 26 healthy controls (1.00 [95% CI: 1.01–3.37], p = 0.0947), as shown in Fig. 2A. The mean expression levels of miR-21 in healthy controls in the 60–79 years group was 1.84, which was higher than that in the 30–39 years group (0.68), but the difference was not significant (Table 2).
Figure 2.
(A) Comparison of miR-21 expression urinary exosomes between patients with BC and healthy controls. Exosomal miR-21 expression in BC patients is shown as relative expression based on CT value of miR-21 in 26 healthy controls. BC, breast cancer; CT, threshold cycle. (B) Comparison of MMP-1/CD63 expression in urinary exosomes between BC patients and healthy controls. (C) Comparison of miR-21 expression pre- and post-operation. (D) Comparison of MMP-1/CD63 expression pre- and post-operation. Exosomal expression of MMP-1/CD63 was based on the ratio of the expression of western blotting.
Table 2.
Age group of Healthy Controls and Expression Levels of miR-21 and MMP-1.
| Age group (years) |
Number of healthy controls | Expression Levels of miR-21 (2ΔCt)* [Median (95% CI)] |
Expression Levels of MMP-1/CD63** [Median (95% CI)] |
|---|---|---|---|
| 30–39 | 5 |
0.68 (0.18–1.34) |
0.30 (0.14–1.31) |
| 40–49 | 12 |
1.02 (0.27–4.75) |
0.64 (0.50–2.24) |
| 50–59 | 5 |
1.03 (0.45–8.78) |
0.43 (0.29–0.96) |
| 60–79 | 4 |
1.84 (0.09–10.6) |
0.49 (0.35–0.78) |
| Median (95% CI) |
1.00 (1.01–3.37) |
0.54 (−0.01–2.81) |
|
*Number indicates the relative expression levels, that is microRNA copies by RT-PCR in patients divided the mean number of microRNA copies in healthy controls.
**Number indicates the relative expression levels of MMP-1/CD63, that was measured by western blotting by both antibodies.
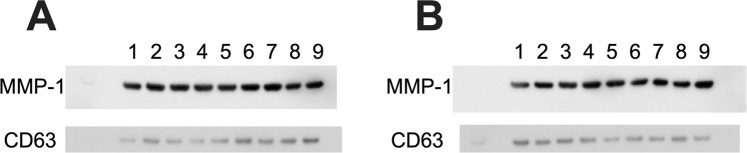
The expression levels of MMP-1/CD63 in 22 patients are shown in Table 1 and those in the 26 healthy controls are shown in Supplementary Table 2. Figure 3 shows the process of evaluation of MMP-1/CD 63 expression by western blotting. The mean expression level in the 22 patients was 1.74 [95% CI: 0.86–5.08], which was significantly higher than that in the 26 healthy controls (0.54 [95% CI: −0.01–2.81], p = 0.0001), as shown in Fig. 2B. There was no significant difference between the 4 age groups of healthy controls in the expression levels of MMP-1/CD 63 (Table 2).
Figure 3.
Western blot analysis of MMP-1 and CD63 in urinary exosomes in patients with BC and healthy controls. (A) BC patients (n = 9); No. 1–9, and (B) Healthy control (n = 9); No. 1–9 (shown in Table 1). One-sixth of the urinary exosome extracted from 2 mL urine was applied to the wells. Anti-MMP-1 antibody (1:1000) and anti-CD63 antibody (1:1000) were used. Samples derived from the same experiment with blots processed in parallel. Strict loading control was performed on all blots. Full-length blots are presented in Supplementary Fig. 1. BC, breast cancer.
ROC Analysis: Sensitivity and specificity of miR-21 and MMP-1/CD63 in primary screening for BC
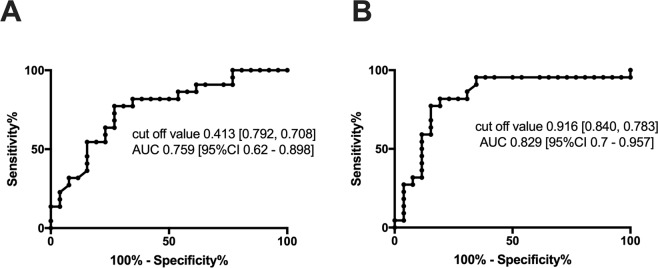
The sensitivity of miR-21 in BC patients was 0.708, and the specificity of miR-21 was 0.792 in primary screening for BC against the 26 healthy controls, as shown in Fig. 4A. The sensitivity and specificity of MMP-1/CD63 expression were 0.783 and 0.840, respectively, as shown in Fig. 4B. When the expression of miR-21 and MMP-1/CD63 were combined, the final sensitivity and specificity in BC screening were 95% and 79%, respectively.
Figure 4.
(A) ROC curves of miR-21 expression in urinary exosomes. When the cut-off value was 0.413, the sensitivity was 0.708 and specificity was 0.792. PPR: 0.773; NPR: 0.731. (B) ROC curves of MMP-1/CD 63 expression in urinary exosomes. When the cut-off value was 0.916, the sensitivity was 0.783 and specificity was 0.840. PPR: 0.818; NPR: 0.808.
Correlation between expression levels and each parameter of TNM classification, stage classification and histopathological findings
For miR-21, values less than the cut-off level value (0.413) were considered as positive in screening by receiver operating characteristics (ROC) analysis. For MMP-1/CD63, values higher than the cut-off level value (0.916) were considered as positive in screening. Table 3 shows the numbers of patients with positive and negative levels of miR-21 and MMP-1/CD63 in each parameter of TNM classification, stage classification, and histological findings. Using the combined expression, 95% of BC cases were detected. Table 3 shows that analysis of the expression of both miR-21 and MMP-1/CD63 detected nearly all patients with BC with Tis and T1, very small cancer, and Stage 0 and Stage I, which are very early cancers.
Table 3.
Correlation between Expression of Both Markers and TNM Classification, Stage Classification and Characteristics in 22 Patients with Breast Cancer. (Patient Numbers).
| miR-21 | MMP-1/CD63 | Both | Both | Using both | |||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Final positive | |
| Tumour Size | |||||||
| Tis (2 cases) | 2 | 0 | 2 | 0 | 2 | 0 | 2/2 (100%) |
| T1 (9 cases) | 6 | 3 | 9 | 0 | 6 | 0 | 9/9 (100%) |
| T2 (6 cases) | 5 | 1 | 4 | 2 | 4 | 1 | 5/6 (83%) |
| T3 (5 cases) | 4 | 1 | 3 | 2 | 2 | 0 | 5/5 (100%) |
| Total 21/22 (95%) | |||||||
| Lymph Node Metastasis | |||||||
| N0 (15 cases) | 12 | 3 | 14 | 1 | 12 | 1 | 14/15 (93%) |
| N1 (4 cases) | 4 | 0 | 1 | 3 | 1 | 0 | 4/4 (100%) |
| N2 (0 cases) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N3 (3 cases) | 1 | 2 | 3 | 0 | 1 | 0 | 3/3 (100%) |
| Total 21/22 (95%) | |||||||
| Stage | |||||||
| 0 (2 cases) | 2 | 0 | 2 | 0 | 2 | 0 | 2/2 (100%) |
| I (7 cases) | 6 | 1 | 6 | 1 | 6 | 1 | 6/7 (93%) |
| II (7 cases) | 6 | 1 | 6 | 1 | 5 | 0 | 7/7(100%) |
| III (6 cases) | 3 | 3 | 4 | 2 | 1 | 0 | 6/6 (100%) |
| Total 21/22 (95%) | |||||||
| Pathological Findings | |||||||
| Scirrhous (10) | 8 | 2 | 9 | 1 | 7 | 0 | 10/10 (100%) |
| Mucinous (3) | 2 | 1 | 1 | 2 | 1 | 1 | 2/3 (67%) |
| Solid (2 cases) | 2 | 0 | 2 | 0 | 2 | 0 | 2/2 |
| Pap-tub (2) | 2 | 0 | 1 | 1 | 1 | 0 | 2/2 |
| Inv lob (2 cases) | 1 | 1 | 2 | 0 | 1 | 0 | 2/2 |
| Microinvasion (1) | 1 | 0 | 1 | 0 | 0 | 0 | 1/1 |
| Inv duct (1 case) | 0 | 1 | 1 | 0 | 0 | 0 | 1/1 |
| DCIS (1 case) | 1 | 0 | 1 | 0 | 1 | 0 | 1/1 |
| Total 21/22 (95%) | |||||||
Changes in expression levels in seven BC cases
Seven cases provided urine samples at 20–136 weeks after surgical treatments (Table 4). Post-operative miR-21 expression was significantly increased compared to pre-operative expression (Fig. 2C). The expression levels of miR-21 were recovered to a normal range in four of seven patients after surgical intervention, whereas three cases showed still values lower than the cut-off level (0.413), as shown in Table 4 and Fig. 2C. A patient with a markedly high level of miR-21 after operation (No. 14) showed multiple bone metastasis 6 months later.
Table 4.
Comparison of miR-21 and MMP-1/CD63 expression between pre- and post-surgical operation in seven patients with breast cancer.
| No. | Pre-operation | Post-operation | |||||
|---|---|---|---|---|---|---|---|
| Expression Levels of miR-21 (2ΔCt)# | Expression Levels of MMP-1/CD63## | Weeks since operation | Expression Levels of miR-21 (2ΔCt)# | Expression Levels of MMP-1/CD63## | Adjuvant chemotherapy | Metastasis/Recurrence | |
| 1 | 0.11 | 1.78 | 20 | 0.3 | 0.63 | TC | — |
| 6 | 0.18 | 0.76 | 71 | 0 | 12.86 | after Trastuzumab, TC | — |
| 7 | 0.32 | 1.09 | 136 | 4.79 | 0.83 | Anastrozole | — |
| 13 | 0.01 | 2.25 | 63 | 0.22 | 0.98 | Tamoxifen | — |
| 14 | 0.01 | 3.91 | 61 | 17.78 | 0.24 | S-1 | multiple bone metastasis |
| 16 | 0.26 | 6.78 | 59 | 5.29 | 1.13 | Tamoxifen | — |
| 22 | 2.22 | 0.11 | 48 | 6.62 | 0.75 | Tamoxifen | — |
|
Median (95% CI) |
0.18 (0.01–0.32) |
4.79 (0.22–6.62) |
1.78 (0.76–3.91) |
0.83 (0.63–1.13) |
|||
#Number indicates the relative expression levels, that is microRNA copies by RT-PCR in patients divided the mean number of microRNA copies in healthy controls.
##Number indicates the relative expression levels of MMP-1/CD63, that was measured by western blotting by both antibodies.
S-1, Tegafur/Gimeracil/Oteracil therapy; TC, Paclitaxel and Carboplatin therapy.
The expression levels of MMP-1/CD63 were recovered to a normal range in four of seven patients after surgical intervention, although postoperative MMP-1/CD63 expression was not significant but showed a decreasing tendency (Table 4 and Fig. 2D). Dramatically decreases were detected in four cases (No. 1, 13, 14, and 16). Three cases showed still higher levels than the cut-off level value (0.916).
Combined expression analysis revealed normal ranges in four of seven cases (Table 4). Case No. 14 showed bone metastasis with recovered levels of miR-21 and a normal range for MMP-1/CD63. Although the interaction mechanism of these markers with the clinical course including chemotherapy remains unclear, more than 50% of cases showed improved marker levels with good clinical courses after surgical treatment and chemotherapy.
Correlation between expression levels and oestrogen receptor, progesterone receptor, and HER2
According to recent clinical classification for treatment selection, Luminal A, Luminal B, Her2, and triple-negative were analysed to detect oestrogen receptor (ER), progesterone receptor (PR), and Her2 levels (Supplementary Table 1). Table 5 shows Luminal B, Her2, and triple-negative (TN) cases were detected 100% although BC patients with Luminal A 75%.
Table 5.
Correlation between Expression of Both Markers and Luminal A, Luminal B, Her2, and Triple Negative in 22 Patients with Breast Cancer. (Patient Numbers).
| miR-21 | MMP-1/CD63 | both | both | using both | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Final positive |
| Luminal A (4 cases) | ||||||
| 2 | 2 | 2 | 2 | 1 | 1 | 3/4 (75%) |
| Luminal B (12 cases) | ||||||
| 9 | 3 | 11 | 1 | 8 | 1 | 12/12 (100%) |
| Her2 (2 cases) | ||||||
| 2 | 0 | 1 | 1 | 0 | 0 | 2/2 (100%) |
| Triple Negative (4 cases) | ||||||
| 4 | 0 | 4 | 0 | 4 | 0 | 4/4 (100%) |
| Total 21/22 (95%) | ||||||
Correlation between expression levels and clinical data in 22 patients with BC
An inverse correlation between miR-21 and MMP-1/CD63 was observed in the patients with BC (r = −0.62; p = 0.002). The correlations between both expression levels and age (less than 49 years old vs more than 50 years old), body weight, creatinine, carcinoembryonic antigen (CEA), CA15-3, Ki67, alcohol drinking habit, and smoking were analysed. A significant correlation was detected between miR-21 and body weight (r = −0.49; p = 0.02), Ki67 (r = −0.45; p = 0.02) and creatinine (r = −0.53; p = 0.01), while MMP-1/CD63 showed no correlation.
Discussion
Several excellent screening methods for BC are available, including physical examination, mammography, ultrasonography, PET-CT, and MRI. Although these examinations are widely performed, they are costly and time-consuming. We developed a novel primary screening test based on urine collection that is inexpensive and convenient. The present study revealed that the combined expression levels of miR-21 and MMP-1/CD63 in urine exosomes can detect 95% of early BC without metastasis. However, there were some limitations. This was a pilot study performed in a single centre and few patients were available for analysis. Additional patients should be evaluated in further studies.
Detection of the expression of both miR-21 and MMP-1/CD63 in urine exosomes as a BC screening has not been reported previously. miR-21 and MMP-1 play very different biological roles, and thus their expression levels differed. miR-21 and MMP-1 are not specific to BC27–30. When only miR-21 is positive, other cancers (ovary, lung, liver, brain, leukemia, colorectal, pancreas, head and neck, thyroid)30 and liver fibrosis should be considered31. When only MMP-1 is positive, although lung cancer, prostate carcinoma, hepatocellular carcinoma, and other malignancies are considered27,28, BC should be suspected, particularly in younger women. The expression mechanism of MMP-1 in BC was recently clarified29. Ma et al.29 reported that miR-361-5p inhibited the proliferation of BC cells by suppressing glycolysis and inhibited BC cell invasion and metastasis by targeting MMP-1. Patients with bone disease, skin disease, and rheumatoid arthritis may also show positive results in this test.
For miR-21, the results of the present study were consistent with those of numerous previous reports of plasma or serum miR-21 without exosome isolation, which has been reported as a useful biomarker for the diagnosis, treatment selection, and future prediction of BC8–16. Erbes, et al.16 noted that their method was equivalent to using exosome isolation, but they did not confirm the exosomes themselves. Their results for the expression of miR-21 were consistent with those determined in this study.
The serum levels of CEA and carbohydrate antigen 153 (CA 153) have been reported as circulating biomarkers for BC. They are not useful for detecting early BC and have been used as prognostic markers for monitoring disease progression or recurrence32. In the present study, no cases showed higher levels of CEA and/or CA 153 (Supplementary Table 1).
Although the expression levels of miR-21 and MMP-1 in urine exosomes were not specific for BC, the present study showed good results. Ninety-five percent of BC cases were detected compared to using combined expression of both biomarkers. This percentage does not differ from those of Cohen33, although the number of cases in the present study was low. Further studies are necessary to confirm the usefulness of both biomarkers.
We could not conclude whether miR-21 from urine exosomes is equal to plasma or serum exosomal miR-21. MiR-21 from urine exosomes may be useful for selecting treatments after pathological diagnosis can reveal the metastasis of axillary lymph node and/or sentinel lymph node. Thus, decision making for the selection of radiation and/or chemotherapy requires miR-21 urine exosomes34. The present study revealed that the sensitivity for Luminal B, Her2, and TN cases was 100%, although Luminal A showed a value of 75%. A clear correlation between the percentages of ER-positive cells, PR-positive cells, and HER2-positive cells and the expression levels of miR-21 and MMP-1 were observed. Blenkiron et al.35 showed that elevated miR-21 was associated with ER-positive tumours, and Huang et al.36 reported that miR-21 was up-regulated via the MAP (ERK1/2) pathway upon stimulation of HER2 signalling in BC cells. A correlation between miR-21 and PR has not been reported37. These issues must be resolved to improve the selection of BC treatment, and measuring the expression of miR-21 in urinary exosomes is a convenient approach.
MiRs in the cancer exosomes inhibit the expression of their respective mRNA targets, PTEN, and HOXD10 in recipient epithelial cells38. Melo et al.38 showed that exosomes from BC cells transfer miRs to normal cells and stimulate them to become cancerous. Higher miR-21 expression may be related to suppression of programmed cell death 417, tumour suppressor gene TPM118, and mapin (a tumour suppressing serpin protein)39. Liu et al. reported that miR-21 induced angiogenesis through AKT and ERK activation and HIF1α expression40. Moreover, Fabbri et al. showed that tumour-secreted miR-21 binds to TLR8 to trigger a Toll-like receptor-mediated pro-metastatic inflammatory response that ultimately may lead to tumour growth and metastasis41. We observed one patient with multiple bone marrow metastasis after surgical treatment who showed very low levels of miR-21 (0.01) before operation, whereas high levels (17.78) were detected after the occurrence of metastasis (Table 4). Further studies are needed to clarify whether the occurrence of metastasis after operation leads to a normal expression range of miR-21 or abnormally high expression is due to metastasis.
The incidence of BC in women aged 65 years and older has increased from 25.3% to 32.9% in the last 10 years2. The risks of BC in Japanese women is thought to have increased because of the consumption of Western-style foods, increased obesity, early age at menarche, late age at first pregnancy, late age at menopause, and an increased aging population2,42–44. BC screening is important in both Japan and worldwide.
Methods
Study population
This study was conducted at Sanno Hospital (Minato-ku, Tokyo, Japan), the Kitasato University Medical Center (Kitamoto City, Saitama, Japan), and the International University of Health and Welfare Hospital (Nasu-Shiobara City, Tochigi Japan) between July 1, 2016 and November 30, 2018. Prior to implementation, the study was approved by the ethics committee of the three institutions (#16-S-8 Sanno Hospital under the authority of IUHW on June 26, 2016 and on March 15, 2017; #28–51 Kitasato University Medical Center on March 27, 2017; #13-B-287 IUHW Hospital on March 22, 2018). All patients provided informed consent by formal letter for this study. Twenty-two patients with BC were diagnosed by mammography, echography, MRI, and subsequent needle biopsy before surgery, and the final pathological diagnosis was delivered by surgical resection at Sanno Hospital (Table 1 and Supplementary Table 1). Urine samples were collected before surgical treatment before breakfast as the first morning urine and stored at −80 °C until the exosomes were separated. All urine samples were collected before the patients had been administered neo-adjuvant chemotherapy or showed evident distant metastasis.
The control urine samples were collected from 26 healthy females who visited the Health Care Center, the International University of Health and Welfare Hospital for a health check. No subjects had complaints, abnormal physical findings including obesity and blood hypertension, abnormal peripheral blood examination and blood chemistry, or abnormal examinations of ECG, abdominal echography, upper GI endoscopic findings, breast mammography, and echography. They also had no history of cancer, signs of dysplasia, inflammatory disease, autoimmune disease, or chronic diseases such as cardiac, liver, or kidney diseases (Supplementary Table 2).
Classification of breast cancer clinical stage
The clinical stage was classified according to the American Joint Committee on Cancer tumour-lymph node-metastasis (TNM) classification system45 as shown in Table 1. The 22 patients with BC showed no metastasis and were in the very early stage of BC.
Classification of Luminal A, Luminal B, Her2, and TN for selecting BC treatment was performed to evaluate sensitivity and specificity46 (Supplementary Table 1).
Isolation of urinary exosomes
All samples were transferred to Kitasato University Medical Center at −80 °C and urinary exosomes were isolated to analyse the expression of miR-21 by RT-PCR and the expression of MMP-1/CD63 by western blotting. Urinary exosomes were isolated with an Exosome Isolation Kit (Miltenyi Biotec) and were confirmed to be exosomes by western blotting with biotin-conjugated anti-CD63 antibody (BioLegend, San Diego, CA, USA). The isolated exosomes were also identified by electron microscopy15. Exosomes were layered on a carbon/Formvar film-coated TEM grid (Okenshoji Co., Ltd., Tokyo Japan) for 10 min. They were stained with 2% uranyl acetate for 2 min. Observation was performed with a transmission electron microscope (Hitachi H-7600, Tokyo, Japan).
A volume of 2 mL urine was used from all samples collected from patients with BC and healthy controls for exosome isolation. Before exosome isolation, the thawed urine was centrifuged at 3,000 × g for 15 min at 4 °C and passed through 0.22-μm nylons filter. The RNA was extracted from isolated exosomes using a Total Exosome RNA & Protein Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was synthesized from the acquired microRNA template with a reverse transcription kit (TaqMan MicroRNA Reverse Transcription Kit, Thermo Fisher Scientific). We performed miRNA extraction after quantifying the amount of urine exosomes using a PS Capture Exosome ELISA Kit (Wako Pure Chemical Industries, Osaka, Japan). Furthermore, the miRNA amount between numerous samples was corrected by performing a reverse transcription reaction using 5 ng of the collected miRNA for expression analysis.
Analysis of miR-21 extracted from urinary exosomes
Quantitative RT-PCR was carried out in a Step One Plus® (Applied Biosystems, Foster City, CA, USA) for miR extracted from urinary exosomes. An miR-21 assay (Applied Biosystems) was performed for miR expression assays according to the manufacturer’s instructions. Assays were performed in duplicate tubes for each sample.
Comparison of miR-21 expression levels in urine exosomes
Based on the mean number of miR-21 copies in the 26 healthy controls, the copy number of miR-21 in patients with BC was calculated as the relative expression grade. ΔCT was determined by subtracting the mean CT value of miR-21 in healthy controls from the individual CT value of miR-21 in patients with BC. Finally, the copy number of miR-21 was compared as the value of 2ΔCT. Similarly, the value was compared to preoperative and postoperative expression in 7 patients with BC.
Comparison of MMP-1/CD 63 expression levels in urinary exosomes
The expression levels of MMP-1 and CD63 were determined by western blotting. Expression of MMP-1/CD63 was examined using an anti-MMP-1 antibody (abcam plc., Cambridge, UK) and biotin-conjugated anti-CD63 antibody (BioLegend). Their expression were pixeled and the ratio of MMP-1/CD63 in the 22 patients with BC was compared to those in the 26 controls with Image J 1.52a software47. Similarly, preoperative and postoperative expression was compared in 7 patients with BC, which revealed identical results. To validate reproducibility, all experiments were performed twice, and the average or median value was confirmed.
Statistical analysis
All values are expressed as the mean ± standard deviation (SD) or median [95% confidence interval (CI)]. Statistical analysis was performed using PRISM 7 for Mac OS X (GraphPad Software, Inc., La Jolla, CA, USA). Data were tested for normality and equal variance to confirm the appropriateness of parametric tests. Data followed a normal distribution were analysed by Student t test. Mann-Whitney U tests were used to evaluate differences between groups: a p value < 0.05 was regarded as statistically significant.
Ethics approval
All procedures performed in this study were in accordance with the ethical standard of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committees of the three institutions (#16-S-8 Sanno Hospital under the authority of IUHW on June 26, 2016 and on March 15, 2017; #28–51 Kitasato University Medical Center on March 27, 2017; #13-B-287 IUHW Hospital on March 22, 2018). This article does not contain any studies with animals performed by any of the authors.
Conclusions
Measuring the expression of both miR-21 and MMP-1/CD63 in urine exosomes is an effective, reliable, inexpensive, and simple screening test. Further studies are needed to confirm these results in a larger sample size.
Supplementary information
Acknowledgements
The fund (Grant No. IUHW: 2016–64 and -65) by Mr. Takeshi Mori and Mrs. Eiko Mori given to Dr. Okazaki have supported this work. This work was supported by JSPS KAKENHI Grant Number JP 18K08797.
Author Contributions
W.A., K.K., T.U., Y.K., K.O., S.I. and I.O. designed and conducted the study. W.A., T.U., H.Y. and M.S. analysed the data. T.T. performed morphological analysis. W.A., K.K., T.U., Y.K., K.O. and I.O. interpreted and discussed the data. W.A. performed statistical analysis. W.A. and I.O. wrote the draft of this manuscript. All authors read and approved the final manuscript.
Data Availability
All data generated or analysed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wataru Ando and Kiyoshi Kikuchi contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50084-5.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Uchida K, et al. Breast cancer screening and the changing population pyramid of Japan. Breast Cancer. 2015;22:172–176. doi: 10.1007/s12282-013-0470-6. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki I, et al. Endogenous urinary 3-hydroxyproline has 96% specificity and 44% sensitivity for cancer screening. J Lab Clin Med. 1992;120:908–920. [PubMed] [Google Scholar]
- 4.Bos A, et al. Effects of aging on urinary secretion of 3-hydroxyproline and its importance for cancer screening in the elderly with ROCF analysis. Jpn J Hyg. 1994;49:797–806. doi: 10.1265/jjh.49.797. [DOI] [PubMed] [Google Scholar]
- 5.Saito J, et al. ELISA measurement for urinary 3-hydroxyproline-containing peptides and its preliminary application to healthy persons and cancer patients. Anticancer Res. 2010;30:1007–1014. [PubMed] [Google Scholar]
- 6.Anastasiadou E, Slack FJ. Cancer. Malicious exosomes. Science. 2014;346:1459–1460. doi: 10.1126/science.aaa4024. [DOI] [PubMed] [Google Scholar]
- 7.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic N, et al. The difference in miR-21 expression levels between invasive and non-invasive breast cancers emphasizes its role in breast cancer invasion. Med Oncol. 2014;31:867. doi: 10.1007/s12032-014-0867-x. [DOI] [PubMed] [Google Scholar]
- 9.Si H, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139:223–229. doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan LX, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. Rna. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volinia S., Galasso M., Sana M. E., Wise T. F., Palatini J., Huebner K., Croce C. M. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proceedings of the National Academy of Sciences. 2012;109(8):3024–3029. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannafon BN, Sebastiani P, de las Morenas A, Lu J, Rosenberg CL. Expression of microRNA and their gene targets are dysregulated in preinvasive breast cancer. Breast Cancer Res. 2011;13:R24. doi: 10.1186/bcr2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iorio MV, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.can-05-1783. [DOI] [PubMed] [Google Scholar]
- 14.Asaga S, et al. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57:84–91. doi: 10.1373/clinchem.2010.151845. [DOI] [PubMed] [Google Scholar]
- 15.Hannafon BN, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90. doi: 10.1186/s13058-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbes T, et al. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer. 2015;15:193. doi: 10.1186/s12885-015-1190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel LB, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 18.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki I, Maruyama K. Collagenase activity in experimental hepatic fibrosis. Nature. 1974;252:49–50. doi: 10.1038/252049a0. [DOI] [PubMed] [Google Scholar]
- 20.Higashiyama R, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 21.Endo H, et al. Matrix metalloproteinase-13 promotes recovery from experimental liver cirrhosis in rats. Pathobiology. 2011;78:239–252. doi: 10.1159/000328841. [DOI] [PubMed] [Google Scholar]
- 22.H Yokomori, Y Inagaki, W Ando, M Hara, T Komiyama, S Kojima, M Oda, H Kuroda, Y Suzuki, I Okazaki. Spatiotemporal expression of matrix metalloproteinase-1 in progression of nonalcoholic steatohepatitis. Journal of Modern Human Pathology. 2016;1(2):11–20. doi: 10.14312/2397-6845.2016-3. [DOI] [Google Scholar]
- 23.Ando W, et al. Serum matrix metalloproteinase-1 level represents disease activity as opposed to fibrosis in patients with histologically proven nonalcoholic steatohepatitis. Clin Mol Hepatol. 2018;24:61–76. doi: 10.3350/cmh.2017.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki I, et al. Difference in gene expression for matrix metalloproteinase-1 between early and advanced hepatocellular carcinomas. Hepatology. 1997;25:580–584. doi: 10.1002/hep.510250315. [DOI] [PubMed] [Google Scholar]
- 25.Sugioka Y, et al. c-Jun NH2-terminal kinase pathway is involved in constitutive matrix metalloproteinase-1 expression in a hepatocellular carcinoma-derived cell line. Int J Cancer. 2004;109:867–874. doi: 10.1002/ijc.20095. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki I, Inagaki Y. Novel strategies for hepatocellular carcinoma based on MMPs science. Anticancer Agents Med Chem. 2012;12:753–763. doi: 10.2174/187152012802650165. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki I, Nabeshima K. Introduction: MMPs, ADAMs/ADAMTSs research products to achieve big dream. Anticancer Agents Med Chem. 2012;12:688–706. doi: 10.2174/187152012802650200. [DOI] [PubMed] [Google Scholar]
- 28.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma F, et al. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J Exp Clin Cancer Res. 2017;36:158. doi: 10.1186/s13046-017-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abba M, Mudduluru G, Allgayer H. MicroRNAs in cancer: small molecules, big chances. Anticancer Agents Med Chem. 2012;12:733–743. doi: 10.2174/187152012802650273. [DOI] [PubMed] [Google Scholar]
- 31.Caviglia JM, et al. MicroRNA-21 and Dicer are dispensable for hepatic stellate cell activation and the development of liver fibrosis. Hepatology. 2018;67:2414–2429. doi: 10.1002/hep.29627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uehara M, et al. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 2008;13:447–451. doi: 10.1007/s10147-008-0773-3. [DOI] [PubMed] [Google Scholar]
- 33.Cohen JD, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong C, et al. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286:19127–19137. doi: 10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blenkiron C, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TH, et al. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem. 2009;284:18515–18524. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilam A, et al. MicroRNA regulation of progesterone receptor in breast cancer. Oncotarget. 2017;8:25963–25976. doi: 10.18632/oncotarget.15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 40.Liu LZ, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawai M, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 2016;5:1328–1340. doi: 10.1002/cam4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buono G, et al. Combined effect of obesity and diabetes on early breast cancer outcome: a prospective observational study. Oncotarget. 2017;8:115709–115717. doi: 10.18632/oncotarget.22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson OC, Quail DF, Joyce JA. Obesity and the tumor microenvironment. Science. 2017;358:1130–1131. doi: 10.1126/science.aao5801. [DOI] [PubMed] [Google Scholar]
- 45.Cserni G, Chmielik E, Cserni B, Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472:697–703. doi: 10.1007/s00428-018-2301-9. [DOI] [PubMed] [Google Scholar]
- 46.Gluck S, de Snoo F, Peeters J, Stork-Sloots L, Somlo G. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;139:759–767. doi: 10.1007/s10549-013-2572-4. [DOI] [PubMed] [Google Scholar]
- 47.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.