Abstract
Aims: The visceral adiposity index (VAI) has been proposed as an estimate of visceral adipose tissue (VAT) mass and as an indicator of VAT dysfunction. Both parameters are associated with cardiometabolic risk, including insulin resistance. In this study, we investigated whether VAI is associated with subclinical atherosclerosis in subjects who were free of cardiovascular disease but were at risk of developing diabetes mellitus.
Methods: A total of 731 adults with a median age of 47 years old without diabetes mellitus were included in this cross-sectional study. The anthropometric data, blood pressure, and lipid profiles of 398 women and 333 men were measured. All subjects underwent an oral glucose tolerance test, and carotid intima–media thickness (cIMT) was evaluated by ultrasound. Insulin resistance was estimated using the homeostatic model assessment of insulin resistance (HOMA-IR).
Results: VAI and HOMA-IR (βst = 0.44, p < 0.0001), VAI and cIMT (βst = 0.17, p < 0.0001), and HOMA-IR and cIMT (βst = 0.09, p = 0.0127) were correlated with each other. After adjusting for cofounding variables, VAI is still correlated with HOMA-IR (βst = 0.42, p < 0.0001). Furthermore, VAI (βst = 0.07, p = 0.0392) but not HOMA-IR (βst = 0.03, p = 0.37) was correlated with cIMT independently of other established cardiovascular risk factors.
Conclusion: The calculation of VAI may provide a better estimation of subclinical atherosclerosis than the calculation of HOMA-IR.
Keywords: Carotid intima–media thickness, Visceral adiposity index, Insulin resistance, Diabetes mellitus, Metabolic syndrome
Introduction
Obesity is not only a cause for a broad range of chronic diseases but also an established risk factor for the development and progression of cardiovascular disease (CVD), which still remains the leading cause of death worldwide1–4). The deposition of adipose tissue determines cardiometabolic risk and CVD-related death rather than the extent of obesity, which is mainly determined by body mass index (BMI)5). Different body fat compartments exist and may contribute to obesity-mediated metabolic disorders and CVD6–8). Among these fat compartments, increased visceral adipose tissue (VAT) mass is strongly associated with insulin resistance, which itself is an important determinant of the inflammatory and atherothrombotic pathways9–11). Increased liver fat content and increased VAT mass are among the strongest phenotypic determinants of subclinical atherosclerosis in subjects with prediabetes12). The aforementioned types of adipose tissue have different biologies and functions13, 14). In particular, the accumulation of VAT appears to be more likely to contribute to CVD13). Currently, VAT measurements are mostly performed by computed tomography or magnetic resonance tomography4, 15). However, these imaging techniques are unsuitable in the routine examination of the general population because of their temporal expenditure, lack of availability, and radiation hazard. By contrast, the waist circumference (WC) can be easily determined and is a widely used estimate of abdominal obesity4, 16, 17). WC can be used to estimate not only VAT mass but also subcutaneous abdominal fat mass. Amato et al.18–20) developed the visceral adiposity index (VAI), which is considered a useful indicator of both VAT mass and VAT function. VAI is associated with cardiometabolic risk parameters, including insulin resistance18, 21–23), and is a simple mathematic model that includes anthropometric parameters (BMI and WC) and biochemical data (triglycerides and high-density lipoprotein cholesterol [HDL-cholesterol]).
The identification of individuals who are at increased cardiometabolic risk, particularly individuals prone to diabetes mellitus, is important in implementing preventive strategies early in the natural history of the cardiometabolic disease. VAI could serve as an interesting candidate for this purpose. Early alterations in vascular morphology can be assessed by the high-resolution B-mode ultrasound of the carotid intima–media thickness (cIMT), which is an accepted indicator of subclinical atherosclerosis24, 25). In the current study, we investigated the relative contributions of VAI and insulin resistance, which was estimated by the calculation of the homeostatic model assessment of insulin resistance (HOMA-IR), as determinants of subclinical atherosclerosis in middle-aged subjects who are free of CVD and diabetes.
Methods
Participants and Study Design
In this cross-sectional analysis, data from the Tübingen intima–media thickness cohort for type 2 diabetes were analyzed. The methodology of the study has been described previously26, 27). Subjects had to be at least 18 years old, and individuals with a history of CVD, such as coronary heart disease or peripheral arterial disease, who have acute disease, or who are pregnant were not included in the study. Participation was allowed when at least one of the following criteria was fulfilled: a family history of type 2 diabetes mellitus, a BMI > 27 kg/m2, previous diagnosis of impaired glucose tolerance, or current diagnosis of gestational diabetes. The participants underwent a 75 g oral glucose tolerance test to measure glucose and insulin. A high-resolution ultrasound of the carotid artery was performed to determine the cIMT. Referring to the calculation of VAI, subjects with serum triglycerides > 3.15 mmol/l and BMI ≥ 40 kg/m2 were not included in the analyses20, 28). After the nature of the study was explained, all study subjects gave their written informed consent. The Ethics Committee of the University of Tübingen approved all protocols.
Anthropometric and Biochemical Data
Blood pressure was measured according to the Riva-Rocci method in sitting position on the upper arm. BMI was calculated using height and weight and is shown in kg/m2. WC was performed undressed and was determined as described previously29).
All blood samples were taken from the cubital vein, and each individual underwent a standardized 75 g oral glucose tolerance test after a 10-hour fasting period. We obtained plasma samples at 0, 30, 60, 90, and 120 minutes to determine plasma glucose and insulin levels. By using these samples, HOMA-IR was calculated according to the Homeostasis Model Assessment Test of insulin resistance30). Glycated hemoglobin was measured by HPLC (Tosoh 2.2 HLC-723, Tokyo, Japan), and total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), and serum triglycerides were measured by the ADVIA 1650 Chemistry System (BAYER Health Care). Low-density lipoprotein cholesterol (LDL-cholesterol) was determined in the participants by using the formula of Friedewald31). High-sensitivity C-reactive protein (hsCRP) was also measured by the ADVIA 1650 Chemistry System.
Determination of VAI
As proposed by Amato et al.18) when using anthropometric and biochemical data, VAI was calculated by separating the participants by gender:
women: VAI =
men: VAI =
Measurement of cIMT
By using high-resolution ultrasound with a linear ultrasound transducer (10–13 MHz; AU5 Harmonic, ESAOTE BIOMEDICA, Hallbergmoos, Germany), the intima–media thickness of the common carotid artery was measured. A trained physician performed ultrasound of the left and right common carotid arteries in B-mode according to the European Mannheim cIMT consensus32). cIMT was assessed on the far wall of the artery approximately 10 mm proximal to the carotid bulb in supine position with the head slightly extended and turned 45° to the contralateral side of the scanning. Three measurements were performed on each side for reproducibility, and a mean of each side was calculated33, 34). Finally, from the left and right means, one mean cIMT was determined by considering the known side differences35).
Statistical Analysis
If not otherwise stated, normally distributed data are presented as means and standard deviations. Prior to linear regression analyses, we logarithmically transformed data that were not normally distributed (Shapiro–Wilk W-test) to approximate a normal distribution. The two-tailed two-sample t-test was performed to compare the groups. We performed multivariate linear regression analyses to adjust for covariates and to identify independent associations. Effect sizes were given as standardized beta coefficients (βst). Furthermore, multivariate linear regression analysis was performed stepwise after a fivefold cross validation, and the results were given as k-fold r2. All numerical variables were used as a continuous variable, and all t-tests were performed as two-tailed Student's t-test. A p-value < 0.05 was considered statistically significant. JMP® 13.0 statistical software (SAS Institute, Cary, NC) was used for the analyses.
Results
Characteristics of the Participants
Data were collected from 2003 to 2006, including laboratory values and cIMT measurements, and a total of 844 individuals were examined. A total of 20 subjects were excluded from the study owing to incomplete data records. In 23 cases, prior unknown diabetes (glycated hemoglobin ≥ 6.5%, fasting blood glucose ≥ 7.0 mmol/l, or 2-hour postload glucose ≥ 11.1 mmo/l) was diagnosed and led to exclusion. A total of 70 participants were excluded owing to triglycerides > 3.15 mmol/l or BMI ≥ 40 kg/m2. Therefore, data from 731 study subjects were available for further analysis. Fig. 1 summarizes the individuals who were recruited and excluded. Table 1 shows the anthropometric data of these subjects, including cIMT and biochemical values, by gender.
Fig. 1.
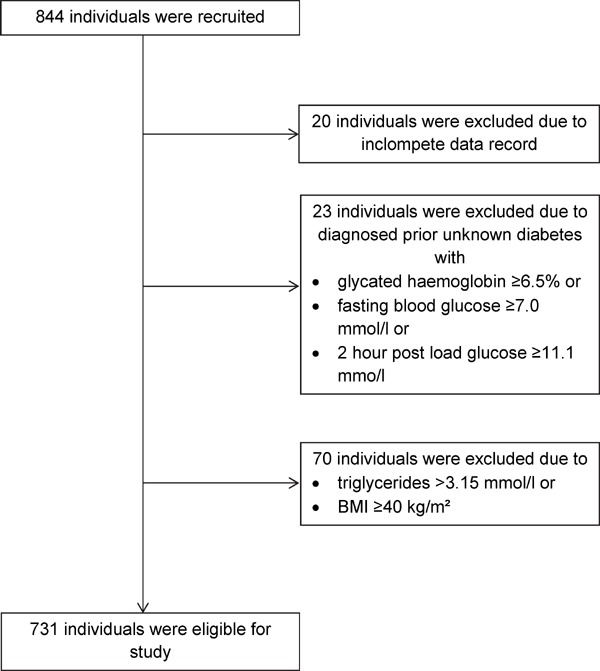
Recruitment and exclusion of participants of the study group
Table 1. Anthropometric data, metabolic characteristics including visceral adiposity index and carotid intima-media thickness of 731 study subjects.
| Total | Women | Men | p value* | |
|---|---|---|---|---|
| (n = 398; 54.4%) | (n = 333; 45.6%) | |||
| Age (years) | 46.0 ± 12.9 | 45.6 ± 12.5 | 46.6 ± 13.2 | 0.42 |
| Body mass index (kg/m2) | 27.0 ± 4.3 | 26.8 ± 4.8 | 27.2 ± 3.7 | 0.06 |
| Waist circumference (cm) | 92.0 ± 12.8 | 87.5 ± 12.3 | 97.3 ± 11.2 | < 0.0001 |
| Systolic blood pressure (mmHg) | 133.4 ± 21.5 | 129.5 ± 22.3 | 138 ± 19.6 | < 0.0001 |
| Diastolic blood pressure (mmHg) | 77.2 ± 11.6 | 75.6 ± 11.8 | 79.1 ± 11 | < 0.0001 |
| Fasting blood glucose (mmol/l) | 5.0 ± 0.5 | 5.0 ± 0.5 | 5.0 ± 0.5 | 0.21 |
| 2 hour post load glucose (mmol/l) | 5.6 ± 1.7 | 5.8 ± 1.7 | 5.4 ± 1.6 | 0.0004 |
| Fasting insulin (pmol/l) | 52.8 ± 30.7 | 53.7 ± 3 | 51.8 ± 31.5 | 0.11 |
| 2 hour post load insulin (pmol/l) | 314.1 ± 297.1 | 340 ± 296.3 | 283.1 ± 295.4 | < 0.0001 |
| Homeostatic model assessment of insulin resistance | 2.0 ± 1.3 | 2.0 ± 1.2 | 2.0 ± 1.3 | 0.21 |
| Glycated haemoglobin (%) | 5.5 ± 0.4 | 5.5 ± 0.4 | 5.5 ± 0.4 | 0.51 |
| Total cholesterol (mmol/l) | 5.3 ± 1.0 | 5.3 ± 0.9 | 5.3 ± 1.0 | 0.97 |
| Triglycerides (mmol/l) | 1.3 ± 0.6 | 1.2 ± 0.5 | 1.4 ± 0.6 | < 0.0001 |
| Low density lipoprotein-cholesterol (mmol/l) | 3.2 ± 0.9 | 3.1 ± 0.8 | 3.3 ± 0.9 | 0.0016 |
| High density lipoprotein-cholesterol (mmol/l) | 1.5 ± 0.4 | 1.7 ± 0.4 | 1.4 ± 0.3 | < 0.0001 |
| High-sensitive C-reactive protein (mg/dl) | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.2 | 0.0020 |
| Visceral adiposity index | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.5 ± 0.8 | 0.94 |
| Carotid intima-media thickness (mm) | 0.56 ± 0.12 | 0.54 ± 0.12 | 0.58 ± 0.13 | < 0.0001 |
Data shown as mean ± standard deviation
=p-level for difference between women and men (two-sample t test)
The study population consisted of 398 women (54.4%) and 333 men (45.6%) and had a mean age of 46.0 ± 12.9 years old (range: 18–69 years old). The mean BMI was 27.0 ± 12.9 kg/m2 (range: 15.8–39.3 kg/m2); this result showed that no difference existed between women and men (p = 0.06). A total of 257 (77.7%) subjects had normal weight or were overweight (n = 257 with a BMI 20.0–24.9 kg/m2; n = 311 with a BMI 25.0–29.9 kg/m2). Obesity (BMI ≥ 30 kg/m2) was present in 163 (22.3%) participants. According to the criteria of the International Diabetes Federation, increased WC (> 80 cm in women and > 94 cm in men) was present in 289 (72.6%) women and 206 (61.9%) men4). Men had significantly higher WC than women (97.3 ± 11.2 vs. 87.5 ± 12.3; p < 0.0001). On the contrary, VAI did not differ between women and men (p = 0.94) and amounted to a mean of 1.5 ± 0.9 (range: 0.27–5.44. The mean cIMT was 0.56 ± 0.12 mm (range: 0.28–1.05 mm), and men had significantly greater cIMT than women (p < 0.0001). A total of 120 study subjects (14.9%) were smokers (55 [12.9%] women, 66 [17.4%] men).
The mean HOMA-IR was 2 ± 1.3 (range: 0.2–9.9) with no gender difference (p = 0.21), and 177 (24.2%) subjects had HOMA-IR > 2.5. Laboratory values revealed a mean glycated hemoglobin of 5.5% ± 0.4 (range 4.1%–6.4%), with 233 (31.9%) of the study subjects having a glycated hemoglobin between 5.7%–6.4%. According to the criteria of the American Diabetes Association, 104 (14.2%) had impaired fasting glucose (5.6–6.9 mmol/l) and 82 (11.2%) had impaired glucose tolerance (7.8–11 mmol/l in a 2-hour postload glucose)36). In summary, 297 (40.6%) participants had prediabetes and either had impaired fasting glucose, impaired glucose tolerance, or elevated glycated hemoglobin.
Relationships of Anthropometric and Biochemical Parameters with HOMA-IR and cIMT
HOMA-IR was correlated positively with BMI, and BMI showed the strongest correlation with HOMA-IR compared with WC, blood pressure, glycated hemoglobin, triglycerides, hsCRP, VAI, and cIMT, which were also associated positively with HOMA-IR (Table 2). After adjusting for other covariates including age, systolic and diastolic blood pressures, smoking, and hsCRP in a multivariate regression analysis (Table 3), VAI was found to be an independent determinant of HOMA-IR. hsCRP and smoking status were the only other independent (albeit weaker) determinants of HOMA-IR. LDL-cholesterol was included as a covariate in multivariate analysis despite a marginal lack of significance in univariate analysis.
Table 2. Univariate relationships of carotid intima-media thickness and homeostatic model assessment of insulin resistance with age, blood pressure and metabolic parameters.
| Variable | Homeostatic model assessment of insulin resistance |
Carotid intima-media thickness |
||
|---|---|---|---|---|
| βst | p value | βst | p value | |
| Age | 0.03 | 0.49 | 0.67 | < 0.0001 |
| Body mass index | 0.51 | < 0.0001 | 0.24 | < 0.0001 |
| Waist circumference | 0.48 | < 0.0001 | 0.34 | < 0.0001 |
| Systolic blood pressure | 0.17 | < 0.0001 | 0.28 | < 0.0001 |
| Diastolic blood pressure | 0.2 | < 0.0001 | 0.21 | < 0.0001 |
| Fasting blood glucose | 0.46 | < 0.0001 | 0.24 | < 0.0001 |
| 2 hour post load glucose | 0.35 | < 0.0001 | 0.15 | < 0.0001 |
| Fasting insulin | 0.98 | < 0.0001 | 0.05 | 0.16 |
| 2 hour post load insulin | 0.64 | < 0.0001 | 0.06 | 0.09 |
| Homeostatic model assessment of insulin resistance | – | – | 0.09 | 0.0127 |
| Glycated haemoglobin | 0.24 | < 0.0001 | 0.31 | < 0.0001 |
| Total cholesterol | 0.02 | 0.63 | 0.22 | < 0.0001 |
| Triglycerides | 0.33 | < 0.0001 | 0.17 | < 0.0001 |
| Low density lipoprotein-cholesterol | 0.07 | 0.05 | 0.24 | < 0.0001 |
| High density lipoprotein-cholesterol | −0.31 | < 0.0001 | −0.1 | 0.0061 |
| High-sensitive C-reactive protein | 0.22 | < 0.0001 | 0.1 | 0.0053 |
| Visceral adiposity index | 0.44 | < 0.0001 | 0.17 | < 0.0001 |
| Carotid intima-media thickness | 0.09 | 0.0127 | – | – |
Table 3. Multiple linear regression analysis of variables including visceral adiposity index influencing homeostatic model assessment of insulin resistance*.
| Variable | Effect size (βst) | Standard error | p value |
|---|---|---|---|
| Age | −0.07 | 0.06 | 0.07 |
| Systolic blood pressure | 0.07 | 0.17 | 0.16 |
| Diastolic blood pressure | 0.07 | 0.18 | 0.13 |
| Low density lipoprotein-cholesterol | −0.05 | 0.07 | 0.18 |
| High-sensitive C-reactive protein | 0.12 | 0.02 | 0.0006 |
| Visceral adiposity index | 0.42 | 0.03 | < 0.0001 |
| Smoking | −0.08 | 0.03 | 0.0176 |
=not adjusted for gender, BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Thereafter, the correlations between cIMT and the aforementioned variables were investigated in univariate and multivariate regression analyses. Age was the most strongly correlated variable with cIMT (Table 1). Furthermore, a positive correlation was found for cIMT with BMI, WC, blood pressure, blood glucose, glycated hemoglobin, triglycerides, hsCRP, VAI, and cholesterol (except HDL-cholesterol, which showed a negative correlation). No correlation could be detected between cIMT and fasting insulin or 2-hour postload insulin, but a positive correlation between cIMT and HOMA-IR was found. In multivariate regression analyses, only age, systolic blood pressure, and VAI were found to be independent determinants of cIMT (Table 4). We then performed another multivariate regression analysis with variables related to cIMT and included glycated hemoglobin instead of HOMA-IR in the statistical model. Only age, systolic blood pressure, and VAI were independent determinants of cIMT (Table 5). Both VAI and HOMA-IR were correlated with cIMT, and we demonstrated the collinearity between VAI and HOMA-IR in the previous section. To achieve a more precise analysis of covariates that influence cIMT, we performed a stepwise analysis with HOMAIR, VAI, and glycated hemoglobin. After a fivefold cross validation, a correlation with cIMT could only be found for age (rank 1), systolic blood pressure (rank 2), and VAI (rank 3) (Table 6).
Table 4. Multiple linear regression analysis of variables including homeostatic model assessment of insulin resistance influencing carotid intima-media thickness*.
| Variable | Effect size (sst) | Standard error | p value |
|---|---|---|---|
| Age | 0.64 | 0.02 | < 0.0001 |
| Systolic blood pressure | 0.13 | 0.05 | 0.0010 |
| Diastolic blood pressure | −0.06 | 0.06 | 0.13 |
| Low density lipoprotein-cholesterol | 0.01 | 0.02 | 0.72 |
| High-sensitive C-reactive protein | 0.04 | 0.01 | 0.17 |
| Visceral adiposity index | 0.06 | 0.01 | 0.0465 |
| Homeostatic model assessment of insulin resistance | 0.03 | 0.01 | 0.34 |
| Smoking | 0.02 | 0.01 | 0.47 |
=not adjusted for gender, BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Table 5. Multiple linear regression analysis of variables including glycated haemoglobin influencing carotid intima-media thickness*.
| Variable | Effect size (βst) | Standard error | p value |
|---|---|---|---|
| Age | 0.62 | 0.02 | < 0.0001 |
| Systolic blood pressure | 0.13 | 0.05 | 0.0007 |
| Diastolic blood pressure | −0.06 | 0.06 | 0.15 |
| Low density lipoprotein-cholesterol | 0.01 | 0.02 | 0.85 |
| High-sensitive C-reactive protein | 0.04 | 0.01 | 0.14 |
| Visceral adiposity index | 0.07 | 0.01 | 0.0148 |
| Glycated haemoglobin | 0.03 | 0.09 | 0.32 |
| Smoking | 0.02 | 0.01 | 0.53 |
=not adjusted for gender, BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Table 6. Variables influencing carotid intima-media thickness (stepwise analyses after fivefold cross validation)*.
| Rank | Variable | k-fold r2 | p value |
|---|---|---|---|
| 1 | Age | 0.44 | < 0.0001 |
| 2 | Systolic blood pressure | 0.45 | 0.0001 |
| 3 | Visceral adiposity index | 0.45 | 0.0035 |
| 4 | High-sensitive C-reactive protein | 0.46 | 0.14 |
| 5 | Diastolic blood pressure | 0.46 | 0.12 |
| 6 | Glycated haemoglobin | 0.46 | 0.31 |
| 7 | Insulin resistance | 0.46 | 0.50 |
| 8 | Smoking | 0.45 | 0.49 |
| 9 | Low density lipoprotein-cholesterol | 0.45 | 0.80 |
=not adjusted for gender, BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
We performed analyses to test independence from gender and test the models in male/female subgroups. The addition of gender to each multiple linear regression model did not change the results (given in effect size βst and p-value of the variable VAI; the tables are not shown in the manuscript): independent of gender and other cofounding variables the VAI was still correlated with 1) the HOMA-IR model (βst = 0.42; p < 0.0001), 2) the HOMAR-IR influencing the cIMT model (βst = 0.06; p = 0.0466), 3) the glycated hemoglobin influencing the cIMT model (βst = 0.07; p = 0.0112).
We also performed further univariate and multivariate gender-specific analyses, as shown in the supplementary tables. In univariate analyses, VAI was correlated in both women and men, with HOMA-IR (βst = 0.40; p < 0.0001 and βst = 0.49; p < 0.0001) and cIMT (βst = 0.20; p < 0.0001 and βst = 0.14, p = 0.0083) (Table 2.1 in the Supplementary Tables). In multiple linear regression analysis fitted on HOMA-IR, VAI was an independent determinant of HOMA-IR in women and men (Table 3.1 in the Supplementary Tables).
Table 2.1. Univariate relationships of carotid intima-media thickness and homeostatic model assessment of insulin resistance with age, blood pressure and metabolic parameters separated by gender.
| Variable | Homeostatic model assessment of insulin resistance |
Carotid intima-media thickness |
||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 398) |
Men (n = 333) |
Women (n = 398) |
Men (n = 333) |
|||||
| βst | p value | βst | p value | βst | p value | βst | p value | |
| Age | −0.04 | 0.40 | 0.10 | 0.08 | 0.68 | < 0.0001 | 0.66 | < 0.0001 |
| Body mass index | 0.51 | < 0.0001 | 0.54 | < 0.0001 | 0.26 | < 0.0001 | 0.20 | 0.0002 |
| Waist circumference | 0.51 | < 0.0001 | 0.57 | < 0.0001 | 0.34 | < 0.0001 | 0.28 | < 0.0001 |
| Systolic blood pressure | 0.21 | < 0.0001 | 0.16 | 0.01 | 0.29 | < 0.0001 | 0.22 | < 0.0001 |
| Diastolic blood pressure | 0.25 | < 0.0001 | 0.17 | 0.06 | 0.17 | 0.0005 | 0.21 | 0.0002 |
| Fasting blood glucose | 0.41 | < 0.0001 | 0.52 | < 0.0001 | 0.24 | < 0.0001 | 0.23 | < 0.0001 |
| 2 hour post load glucose | 0.29 | < 0.0001 | 0.40 | < 0.0001 | 0.14 | 0.0039 | 0.20 | 0.0002 |
| Fasting insulin | 0.98 | < 0.0001 | 0.98 | < 0.0001 | 0.04 | 0.46 | −0.02 | 0.11 |
| 2 hour post load insulin | 0.59 | < 0.0001 | 0.70 | < 0.0001 | 0.05 | 0.31 | 0.14 | 0.0102 |
| Homeostatic model assessment of insulin resistance | – | – | – | – | 0.08 | 0.10 | 0.12 | 0.0280 |
| Glycated haemoglobin | 0.28 | < 0.0001 | 0.20 | 0.0029 | 0.34 | < 0.0001 | 0.30 | < 0.0001 |
| Total cholesterol | 0.02 | 0.71 | 0.02 | 0.76 | 0.26 | < 0.0001 | 0.18 | 0.0009 |
| Triglycerides | 0.30 | < 0.0001 | 0.39 | < 0.0001 | 0.14 | 0.0039 | 0.16 | 0.0030 |
| Low density lipoprotein-cholesterol | 0.11 | 0.0260 | 0.04 | 0.44 | 0.30 | < 0.0001 | 0.14 | 0.0085 |
| High density lipoprotein-cholesterol | −0.32 | < 0.0001 | −0.39 | < 0.0001 | −0.11 | 0.0286 | 0.03 | 0.60 |
| High-sensitive C-reactive protein | 0.29 | < 0.0001 | 0.13 | 0.01 | 0.14 | 0.0052 | 0.10 | 0.07 |
| Visceral adiposity index | 0.40 | < 0.0001 | 0.49 | < 0.0001 | 0.20 | < 0.0001 | 0.14 | 0.0083 |
| Carotid intima-media thickness | 0.08 | 0.10 | 0.12 | 0.0280 | – | – | – | – |
Table 3.1. Multiple linear regression analysis of variables including visceral adiposity index influencing homeostatic model assessment of insulin resistance separated by gender*.
| Variable | Women (n = 398) |
Men (n = 333) |
||||
|---|---|---|---|---|---|---|
| Effect size (βst) | Standard error | p value | Effect size (βst) | Standard error | p value | |
| Age | −0.17 | 0.09 | 0.0008 | 0.03 | 0.10 | 0.56 |
| Systolic blood pressure | 0.10 | 0.20 | 0.11 | 0.07 | 0.30 | 0.31 |
| Diastolic blood pressure | 0.10 | 0.22 | 0.112 | 0.07 | 0.31 | 0.78 |
| Low density lipoprotein-cholesterol | 0.01 | 0.09 | 0.90 | 0.02 | 0.11 | 0.25 |
| High-sensitive C-reactive protein | 0.14 | 0.02 | 0.0028 | 0.08 | 0.03 | 0.10 |
| Visceral adiposity index | 0.36 | 0.05 | < 0.0001 | 0.48 | 0.05 | < 0.0001 |
| Smoking | −0.08 | 0.04 | 0.06 | −0.06 | 0.04 | 0.24 |
=not adjusted for BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
In the multiple linear regression analysis of variables, including HOMA-IR fitted on cIMT and stratified by gender, VAI was no longer associated with cIMT in women (p = 0.35) (Table 4.1 in the Supplementary Tables). In men, there was a trend for an association with cIMT, which barely missed statistical significance (p = 0.08). Regarding gender-specific issues, we performed an additional analysis and found no interaction of VAI and gender on cIMT in multivariate linear regression analysis (Table 7 in the Supplementary Tables). In the multiple linear regression analyses of variables, including glycated hemoglobin fitted on cIMT and stratified by gender, VAI was not associated with cIMT in women (p = 0.17), but an association was found in men (βst = 0.08; p = 0.0481) (Table 5.1 in the Supplementary Tables). We again performed an additional analysis and found no interaction of VAI and gender on cIMT in multivariate linear regression analysis (Table 8 in the Supplementary Tables).
Table 4.1. Multiple linear regression analysis of variables including homeostatic model assessment of insulin resistance influencing carotid intima-media thickness separated by gender*.
| Variable | Women (n = 398) |
Men (n = 333) |
||||
|---|---|---|---|---|---|---|
| Effect size (βst) | Standard error | p value | Effect size (βst) | Standard error | p value | |
| Age | 0.66 | 0.03 | < 0.0001 | 0.65 | 0.03 | < 0.0001 |
| Systolic blood pressure | 0.08 | 0.07 | 0.11 | 0.11 | 0.09 | 0.06 |
| Diastolic blood pressure | −0.05 | 0.07 | 0.30 | −0.08 | 0.10 | 0.18 |
| Low density lipoprotein-cholesterol | 0.02 | 0.03 | 0.68 | −0.02 | 0.03 | 0.60 |
| High-sensitive C-reactive protein | 0.08 | 0.01 | 0.06 | 0.05 | 0.01 | 0.28 |
| Visceral adiposity index | 0.04 | 0.02 | 0.35 | 0.08 | 0.02 | 0.08 |
| Homeostatic model assessment of insulin resistance | 0.07 | 0.02 | 0.10 | 0.01 | 0.02 | 0.89 |
| Smoking | 0.02 | 0.01 | 0.68 | 0.01 | 0.01 | 0.82 |
=not adjusted for BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Table 7. Multiple linear regression analysis of variables including homeostatic model assessment of insulin resistance and interaction of VAI and gender influencing carotid intima-media thickness*.
| Variable | Effect size (βst) | Standard error | p value |
|---|---|---|---|
| Age | 0.64 | 0.02 | < 0.0001 |
| Systolic blood pressure | 0.10 | 0.05 | 0.0016 |
| Diastolic blood pressure | −0.07 | 0.06 | 0.09 |
| Low density lipoprotein-cholesterol | −0.01 | 0.02 | 0.96 |
| High-sensitive C-reactive protein | 0.06 | 0.01 | 0.0324 |
| Visceral adiposity index | 0.06 | 0.01 | 0.0473 |
| Homeostatic model assessment of insulin resistance | 0.04 | 0.01 | 0.22 |
| Smoking | 0.01 | 0.01 | 0.69 |
| Gender | −0.13 | 0.01 | < 0.0001 |
| Gender-Visceral adiposity index (interaction term) | 0.01 | 0.01 | 0.94 |
=not adjusted for BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Table 5.1. Multiple linear regression analysis of variables including glycated haemoglobin influencing carotid intima-media thickness separated by gender*.
| Variable | Women (n = 398) |
Men (n = 333) |
||||
|---|---|---|---|---|---|---|
| Effect size (βst) | Standard error | p value | Effect size (βst) | Standard error | p value | |
| Age | 0.63 | 0.03 | < 0.001 | 0.64 | 0.03 | < 0.0001 |
| Systolic blood pressure | 0.09 | 0.07 | 0.07 | 0.11 | 0.09 | 0.06 |
| Diastolic blood pressure | −0.05 | 0.07 | 0.36 | −0.08 | 0.09 | 0.18 |
| Low density lipoprotein-cholesterol | 0.01 | 0.03 | 0.77 | −0.02 | 0.03 | 0.56 |
| High-sensitive C-reactive protein | 0.09 | 0.01 | 0.0305 | 0.05 | 0.01 | 0.28 |
| Visceral adiposity index | 0.06 | 0.02 | 0.17 | 0.08 | 0.02 | 0.0481 |
| Glycated haemoglobin | 0.04 | 0.12 | 0.41 | 0.03 | 0.15 | 0.47 |
| Smoking | 0.01 | 0.01 | 0.77 | 0.01 | 0.01 | 0.86 |
=not adjusted for BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Table 8. Multiple linear regression analysis of variables including glycated haemoglobin and interaction of VAI and gender influencing carotid intima-media thickness*.
| Variable | Effect size (sst) | Standard error | p value |
|---|---|---|---|
| Age | 0.63 | 0.02 | < 0.0001 |
| Systolic blood pressure | 0.11 | 0.05 | 0.0074 |
| Diastolic blood pressure | −0.06 | 0.06 | 0.11 |
| Low density lipoprotein-cholesterol | −0.01 | 0.02 | 0.81 |
| High-sensitive C-reactive protein | 0.07 | 0.01 | 0.0218 |
| Visceral adiposity index | 0.07 | 0.01 | 0.0119 |
| Glycated haemoglobin | 0.03 | 0.09 | 0.28 |
| Smoking | 0.01 | 0.01 | 0.77 |
| Gender | −0.12 | 0.01 | < 0.0001 |
| Gender-Visceral adiposity index (interaction term) | 0.01 | 0.08 | 0.85 |
=not adjusted for BMI, waist circumference, triglycerides and HDL-cholesterol (already included in visceral adiposity index)
Discussion
In this cross-sectional study, which included nondiabetic individuals who were free of overt CVD but were at risk for developing diabetes mellitus, we found that HOMA-IR increased with VAT dysfunction, which was presented by increased VAI. Even after adjusting for other cardiovascular risk factors in multivariate analyses, VAI was independently related to HOMA-IR. However, by referring to subclinical atherosclerosis, our present study provides novel evidence that VAT dysfunction, which was estimated by VAI, is associated with cIMT. In line with the observation that VAI is associated with both the HOMA-IR and cIMT, these results emphasize the different effects of VAT accumulation and dysfunction in the development of cardiometabolic disorders.
To our knowledge, this study is the first study to investigate the relationship of VAI and HOMA-IR as independent determinants of subclinical atherosclerosis. Our results are consistent with other studies that demonstrated an association between VAT mass and HOMA-IR18, 23, 37). Recently, an association between VAI and HOMA-IR was found even in subjects without central obesity; central obesity was determined by low WC38). These data suggest that VAI could be a useful indicator of both VAT mass and dysfunction. Nevertheless, there are several risk calculators and surrogate indices such as BMI or WC, which are commonly used for the classification of adipose tissue content and dysfunction. Nevertheless, the aforementioned indices have limitations, and the accuracy of these indices remains controversial in routine clinical practice. BMI is an attempt to quantify the amount of total tissue mass. WC, which is an excellent marker of central localized obesity and is effective for predicting metabolic disease, is sensitive to height and weight. Furthermore, WC is not helpful in distinguishing between subcutaneous and visceral fat mas. Both BMI and WC are based on anthropometric measurements and present the amount of adipose tissue but do not reflect adipose tissue-derived metabolic disorders. In the present study, only age was associated with cIMT in a multivariate analysis that included both anthropometric and metabolic parameters. Consequently, more reliable indicators are necessary, and VAI takes into account the physical and metabolic parameters of particularly central localized obesity. In detail, the advantage of gender-adjusted VAI may be explained by its close correlation with visceral adiposity. Given that VAT is known to be metabolically active regardless of the amount of adipose tissue, the combination of both anthropometric and metabolic parameters may be more advantageous in evaluating an individual's cardiometabolic risk. Therefore, VAI, which is easy to calculate, is a surrogate marker of visceral adipose dysfunction and may reflect directly the proatherogenic processes leading to arteriosclerosis.
In respect to the associations between established cardiometabolic risk parameters and cIMT, increased age was repeatedly confirmed as an independent risk factor for early atherosclerosis and was most strongly associated with cIMT in our study39, 40). Furthermore, systolic blood pressure was an independent risk factor for cIMT, which is in accordance with recently published data41). VAI and HOMA-IR were associated with cIMT in our study, thus indicating that both variables may play a role in the development of atherosclerosis. Compared with HOMA-IR, VAI was strongly associated with cIMT. Even after adjusting for age and other cardiovascular risk factors, a higher VAI was independently associated with a higher cIMT. There was also an association between fasting glucose, 2-hour postload glucose, and glycated hemoglobin with cIMT; this result is in line with previous data showing a link between hyperglycemia and increased risk of atherosclerosis42–44). In our subjects without diabetes, glycated hemoglobin showed a more pronounced relationship with cIMT than both fasting and 2-hour postload glucose levels. These results indicate that long-term hyperglycemia rather than acutely elevated glucose levels may contribute to the development of subclinical atherosclerosis. However, the association between glycated hemoglobin and cIMT was no longer present after adjusting for age, VAI, and other cardiovascular risk factors. In this statistical model, VAI was still associated with cIMT. This finding implied that VAT accumulation and dysfunction seems to have a stronger atherogenic potential than hyperglycemia.
VAT as an endocrine organ plays a key role in the development of both HOMA-IR and atherosclerosis by inflammatory atherothrombotic pathway9, 45). VAT accumulation seems to have a more important role in the development of early atherosclerosis beyond total adipose body tissue owing to its secretion of more proinflammatory adipokines and nonesterified fatty acids, which are atherogenic3, 46, 47). We found that the decrease of VAT mass is related to improved vascular function and an improvement of endothelial dysfunction in the narrow sense48). Considering that adipose tissue and VAT is a source for proinflammatory cytokine expression and secretion, the association between VAT and cIMT may be due to an inflammatory process at the vascular wall, wherein VAT can be indirectly represented by VAI. HOMA-IR was associated with cIMT but was not an independent risk factor for subclinical atherosclerosis. However, a correlation was found for HOMA-IR with VAI in our participants, thus possibly displaying the interrelationship between altered adipose tissue function and insulin resistance in atherosclerosis due to insulin resistance9, 49).
Another important finding of our study was the association between hsCRP levels and cIMT. The hsCRP level is supposed to be a good marker of subclinical inflammation of vascular structure50). In the present study, the hsCRP level was associated with cIMT in the univariate analysis. However, it was not associated with cIMT after adjusting for confounding variables, including VAI. Other studies have confirmed that the hsCRP level can differ substantially in CVD and that there is no causal relationship between hsCRP and manifest macrovascular disease51). Although there is evidence that chronic inflammation is related to CVD, most ischemic vascular diseases depend on conventional risk factors and other markers of chronic inflammation51, 52). Therefore, hsCRP might not be a suitable biochemical marker in the early stage of atherosclerosis. Furthermore, other inflammatory markers, such as interleukin-6 or tumor necrosis factor alpha, may play a more important role.
The strength of our study is the availability of a well-characterized population with measurements of subclinical atherosclerosis, insulin, glucose, and parameters allowing the calculation of VAI. The latter appears to be a suitable index in routine clinical practice. Furthermore, the analysis was performed in individuals without diabetes and who were free of overt CVD. The limitation of this study is its cross-sectional design, which does not allow drawing conclusions about causal relationships. The correlation with cIMT was found for both VAI and HOMA-IR. However, in stepwise analysis, a correlation with cIMT could only be found for age, systolic blood pressure, and VAI. This additional analysis indicates that VAI may be a more reliable indicator of subclinical atherosclerosis than the calculation of HOMA-IR. Despite these results, we cannot exclude the influence of HOMA-IR on cIMT, in addition to VAI, owing to the collinearity between VAI and HOMA-IR. We demonstrated the association of VAI with cIMT in our study population. Additional analyses revealed that no significant interaction of gender and VAI existed on cIMT. However, in gender-stratified tests, which were probably affected by lower statistical power, only the male subgroup had significant or nearly significant associations with the outcome. We did not analyze the association between VAI and manifest cardiovascular events, which would be an interesting issue and is one of the aims of a follow-up study of our individuals. In our study, we focused on a surrogate index that may classify individuals who were free of overt CVD but were at increased cardiometabolic risk and potentially needed an early appropriate treatment tailored to individual risk. To properly apply VAI, we excluded subjects with severe hypertriglyceridemia (> 3.15 mmol/l) as mentioned above. Particularly, high values of triglycerides could affect the validity of VAI. Therefore, the use of the index in this study is not recommended. However, our data set does not allow a distinction between primary and secondary dyslipidemia. Therefore, we cannot exclude the possibility that individuals with primary dyslipidemia were present in our study. Furthermore, no data of medication and family history of CVD were recorded, thus potentially modifying the associations with cIMT. One limitation of our study is that the measurement of cIMT was limited to the wall approximately 10 mm proximal to the carotid bulb, and we did not examine more distal segments (i.e., the internal carotid artery) or plaque burden, which is known to be an important marker of advanced atherosclerosis53).
In conclusion, we found that VAI was associated with cIMT in individuals who were free of CVD but were prone to diabetes mellitus. Therefore, VAI could be a simple and widely usable tool for estimating visceral fat mass and predicting cardiometabolic risk.
Abbreviations
- BMI
Body mass index
- CVD
cardiovascular disease
- cIMT
carotid intima-media thickness
- HDL-cholesterol
high density lipoprotein-cholesterol
- hsCRP
high-sensitive C-reactive protein
- HOMA-IR
insulin resistance
- LDL-cholesterol
low density lipoprotein-cholesterol
- VAT
visceral adipose tissue
- VAI
visceral adiposity index
- WC
waist circumference
Author Contribution
All authors contributed to (1) the conception and design of the study and the acquisition, analysis, or interpretation of data; (2) the drafting of the article or the critical revision of the article for important intellectual content; and (3) the final approval of the version to be published. The authors have read and agreed to the editorial policies.
Declaration of Conflicting Interests
The authors have no conflicts of interest to declare.
References
- 1). Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and metaanalysis. BMC Public Heal 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367: 1747-1757 [DOI] [PubMed] [Google Scholar]
- 3). Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006, 444: 881-887 [DOI] [PubMed] [Google Scholar]
- 4). Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23: 469-480 [DOI] [PubMed] [Google Scholar]
- 5). Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2010; 362: 2433. [DOI] [PubMed] [Google Scholar]
- 6). Stefan N, Haring H-U, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? lancet Diabetes Endocrinol. 2017. 10.1016/S2213-8587(17)30292-9 [DOI] [PubMed] [Google Scholar]
- 7). Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol 2015; 11: 90-100 [DOI] [PubMed] [Google Scholar]
- 8). Stefan N, Haring H-U, Hu FB, Schulze MB. Divergent associations of height with cardiometabolic disease and cancer: epidemiology, pathophysiology, and global implications. lancet Diabetes Endocrinol 2016; 4: 457-467 [DOI] [PubMed] [Google Scholar]
- 9). Despres JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol 2012; 28: 642-652 [DOI] [PubMed] [Google Scholar]
- 10). Stefan N, Haring HU. The metabolically benign and malignant fatty liver. Diabetes 2011; 60: 2011-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293-302 [DOI] [PubMed] [Google Scholar]
- 12). Stefan N, Fritsche A, Schick F, Haring H-U. Phenotypes of prediabetes and stratification of cardiometabolic risk. lancet Diabetes Endocrinol. 2016 [DOI] [PubMed] [Google Scholar]
- 13). Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol 2012; 9: 689-702 [DOI] [PubMed] [Google Scholar]
- 14). Stefan N, Schick F, Haring H-U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab 2017; 26: 292-300 [DOI] [PubMed] [Google Scholar]
- 15). Machann J, Thamer C, Stefan N, Schwenzer NF, Kantartzis K, Häring HU, Claussen CD, Fritsche A, Schick F. Follow-up whole-body assessment of adipose tissue compartments during a lifestyle intervention in a large cohort at increased risk for type 2 diabetes. Radiology 2010; 257: 353-363 [DOI] [PubMed] [Google Scholar]
- 16). Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004; 79: 379-384 [DOI] [PubMed] [Google Scholar]
- 17). World Health O. Measuring obesity-classification and description of anthropometric data. Report on a WHO consultation on the epidemiology of obesity. Warsaw 1987; 2-7 [Google Scholar]
- 18). Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A, AlkaMeSy Study Group Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33: 920-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Amato MC, Giordano C, Pitrone M, Galluzzo A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis 2011; 10: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol 2014; 730827. 10.1155/2014/730827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Du T, Yu X, Zhang J, Sun X. Lipid accumulation product and visceral adiposity index are effective markers for identifying the metabolically obese normal-weight phenotype. Acta Diabetol 2015; 52: 855-863 [DOI] [PubMed] [Google Scholar]
- 22). Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 2014; 13: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Stepien M, Stepien A, Wlazel RN, Paradowski M, Rizzo M, Banach M, Rysz J. Predictors of insulin resistance in patients with obesity: a pilot study. Angiology 2014; 65: 22-30 [DOI] [PubMed] [Google Scholar]
- 24). Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and metaanalysis. Circulation 2007; 115: 459-467 [DOI] [PubMed] [Google Scholar]
- 25). Grobbee DE, Bots ML. Carotid artery intima-media thickness as an indicator of generalized atherosclerosis. J Intern Med 1994; 236: 567-573 [DOI] [PubMed] [Google Scholar]
- 26). Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609-1616 [DOI] [PubMed] [Google Scholar]
- 27). Randrianarisoa E, Rietig R, Jacob S, Blumenstock G, Haering HU, Rittig K, Balletshofer B. Normal values for intima-media thickness of the common carotid artery--an update following a novel risk factor profiling. Vasa 2015; 44: 444-450 [DOI] [PubMed] [Google Scholar]
- 28). Amato MC, Giordano C. Clinical indications and proper use of Visceral Adiposity Index. Nutr Metab Cardiovasc Dis 2013; 23: e31-2. 10.1016/j.numecd.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 29). Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Heal Organ Tech Rep Ser 1995; 854: 1-452 [PubMed] [Google Scholar]
- 30). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 31). Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499-502 [PubMed] [Google Scholar]
- 32). Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, B. Cerebrovasc Dis 2012; 34: 290-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: a review. Stroke 1997; 28: 665-671 [DOI] [PubMed] [Google Scholar]
- 34). Schmidt C, Wendelhag I. How can the variability in ultrasound measurement of intima-media thickness be reduced? Studies of interobserver variability in carotid and femoral arteries. Clin Physiol 1999; 19: 45-55 [DOI] [PubMed] [Google Scholar]
- 35). Foerch C, Buehler A, von Kegler S, Sitzer M. Intima-media thickness side differences are limited to the common carotid artery. Hypertension 42: e17. [DOI] [PubMed] [Google Scholar]
- 36). (2016) Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care 2003; 39 Suppl 1: S4-5. 10.2337/dc16-S003 [DOI] [PubMed] [Google Scholar]
- 37). Oh J-Y, Sung Y-A, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring) 2013; 21: 1690-1694 [DOI] [PubMed] [Google Scholar]
- 38). Ji B, Qu H, Wang H, Wei H, Deng H. Association Between the Visceral Adiposity Index and Homeostatic Model Assessment of Insulin Resistance in Participants With Normal Waist Circumference. Angiology 2017; 68: 716-721 [DOI] [PubMed] [Google Scholar]
- 39). O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, Bommer W, Price TR, Gardin JM, Savage PJ. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke 1992; 23: 1752-1760 [DOI] [PubMed] [Google Scholar]
- 40). O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 41). Carpenter M, Sinclair H, Kunadian V. Carotid Intima Media Thickness and Its Utility as a Predictor of Cardiovascular Disease: A Review of Evidence. Cardiol Rev 2016; 24: 70-75 [DOI] [PubMed] [Google Scholar]
- 42). Succurro E, Marini MA, Arturi F, Grembiale A, Lugarà M, Andreozzi F, Sciacqua A, Lauro R, Hribal ML, Perticone F, Sesti G. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009; 207: 245-249 [DOI] [PubMed] [Google Scholar]
- 43). Marini MA, Succurro E, Castaldo E, Cufone S, Arturi F, Sciacqua A, Lauro R, Hribal ML, Perticone F, Sesti G. Cardiometabolic risk profiles and carotid atherosclerosis in individuals with prediabetes identified by fasting glucose, postchallenge glucose, and hemoglobin A1c criteria. Diabetes Care 2012; 35: 1144-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism eObesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation 2006; 113: 898-918 [DOI] [PubMed] [Google Scholar]
- 46). Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res =Horm und Stoffwechselforsch =Horm Metab 2002; 34: 616-621 [DOI] [PubMed] [Google Scholar]
- 47). Gast KB, den Heijer M, Smit JWA, Widya RL, Lamb HJ, de Roos A, Jukema JW, Rosendaal FR, de Mutsert R, NEO study group Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: The NEO study. Atherosclerosis 2015; 241: 547-554 [DOI] [PubMed] [Google Scholar]
- 48). Rittig K, Hieronimus A, Thamer C, Machann J, Peter A, Stock J, Schick F, Fritsche A, Stefan N, Häring HU, Balletshofer B. Reducing visceral adipose tissue mass is essential for improving endothelial function in type 2 diabetes prone individuals. Atherosclerosis 2010. 212: 575-579 [DOI] [PubMed] [Google Scholar]
- 49). Faerch K, Bergman B, Perreault L. Does insulin resistance drive the association between hyperglycemia and cardiovascular risk? PLoS One 2010; 7: e39260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes--Mendelian randomization using CRP haplotypes points upstream. PLoS Med 2008; 5: e155. 10.1371/journal.pmed.0050155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet (London, England) 2010; 375: 132-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 2013; 62: 397-408 [DOI] [PubMed] [Google Scholar]
- 53). Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol 2010; 30: 182-185 [DOI] [PubMed] [Google Scholar]


