Abstract
Adult specimens of Echinochasmus caninus n. comb. (Verma, 1935) (Trematoda: Echinostomatidae) (syn. Episthmium caninum Yamaguti, 1958) were recovered from 11 riparian people who resided along the Mekong River in Khammouane Province, Lao PDR. In fecal examinations done by the Kato-Katz technique, the cases revealed eggs of Opisthorchis viverrini/minute intestinal flukes, hookworms, and in 2 cases echinostome eggs. To recover the adult helminths, praziquantel 30–40 mg/kg and pyrantel pamoate 10–15 mg/kg in a single dose were given and purged with magnesium salts. Various species of trematodes (including O. viverrini and Haplorchis spp.), cestodes, and nematodes were recovered from their diarrheic stools. Among the trematodes, small echinostome flukes (n=42; av. 3.8 specimens per case) of 0.7–1.2 mm in length are subjected in this study. They are morphologically characterized by having 24 collar spines interrupted dorsally and anterior extension of vitellaria from the cirrus sac or genital pore level to the posterior end of the body. Particularly based on this extensive distribution of vitellaria, the specific diagnosis was made as Echinochasmus caninus. The cases were co-infected with various other helminth parasites; thus, clinical manifestations specific for this echinostome infection were difficult to determine. The present paper describes for the first time human E. caninus infections in Lao PDR. Our cases marked the 4–14th human infections with this echinostome around the world following the 3 previous cases reported from Thailand.
Keywords: Echinchasmus caninus (syn. Episthmium caninum), echinostome, Khammouane Province, Lao PDR
Echinostomes (the family Echinostomatidae) are most harmful among the zoonotic human intestinal flukes [1,2]. They can cause severe gastrointestinal symptoms, including epigastric or abdominal pain accompanied by diarrhea, easy fatigue, malnutrition, and sometimes ulcer and bleeding [1,2]. In particular, heavy worm loads of Artyfechinostomum spp. infection may lead even to death of humans and pigs due to marked malnutrition, anemia, and intestinal perforation [3,4]. A total of 23 echinostome species worldwide which belong to Echinostoma, Isthmiophora, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Echinoparyphium, Himasthla, and Hypoderaeum genera are currently known to infect humans [1,2].
In Lao People’s Democratic Republic (Lao PDR), foodborne trematodes, such as Opisthorchis viverrini, Haplorchis spp. (H. taichui, H. pumilio, and H. yokogawai), and lecithodendriid-like flukes (Phaneropsolus bonnei and Caprimolgorchis molenkampi) are common together with soil-transmitted nematodes, including hookworms [5–13]. With regard to echinostomes, only 3 reports have been available. The first was Echinochasmus japonicus infection in 3 people in Savannakhet Province [14]. The second was echinostome fluke (Echinostoma revolutum, Artyfechinostomum malayanum, E. japonicus, and Euparyphium sp.) infections among the residents of Khammouane Province [8]. The third was 2 human infections with Echinostoma ilocanum in Savannakhet Province [15]. We recently confirmed 11 human cases infected with Echinochasmus caninus n. comb. (syn. Episthmium caninum Yamaguti, 1958) in riparian villages along the Mekong River in Khammouane Province, Lao PDR. These 11 cases included 7 previous cases reported erroneously under the name E. japonicus infection [8] which are here revised as E. caninus infection.
The Korea Association of Health Promotion, Seoul, Korea, and the Department of Hygiene and Prevention, Ministry of Health, Lao PDR conducted a collaborative project to control intestinal helminthiases in Lao PDR (2000–2004 and 2007–2011). During this project, we conducted fecal surveys using the Kato-Katz technique in 5 localities of Khammouane Province (Nong Bone, Mahaxay, Phova, Bone Som, and Thongmay villages) in March 2003 and June 2009, 2 times. We tried to collect adult flukes after anthelmintic treatment followed by purging and finally detected 11 cases infected with E. caninus.
The procedure of worm collection was as described previously [6–10]. Briefly, the egg-positive cases of O. viverrini/minute intestinal flukes, or echinostomes, were given orally 30–40 mg/kg praziquantel (Shinpoong Pharm. Co., Seoul, Korea) and 10–15 mg/kg of pyrantel pamoate (Hangzhou Minsheng Pharm. Group, Hangzhou, China) in a single dose and purged with 20–30 g MgSO4 an hour later. Adult flukes were collected from the diarrheic stools and washed several times in water. They were fixed in 10% formalin under a cover slip pressure, stained with acetocarmine, and morphologically examined. Fecal examination of the village residents and worm recovery from the 11 cases were officially and ethically approved by the Ministry of Health, Lao PDR, under the agreement of the Korea-Laos International Collaboration on Intestinal Parasite Control in Lao PDR (2000–2004 and 2007–2011). The worm recovery was performed after obtaining informed consent from each person.
A total of 42 specimens of E. caninus were recovered from the 11 people (Table 1). The cases were 21–46 years of age, consisting of 6 men and 5 women, and the worm load was relatively low, from 1 to 21 specimens per case. Some people complained of variable degrees of abdominal pain, epigastric pain, and indigestion. However, they appeared to be co-infected with other helminth parasites, such as O. viverrini, Haplorchis spp., lecithodendriid-like flukes, hookworms, whipworms, pinworms, and Taenia species (reported in [7,8]). Therefore, specific symptoms due to E. caninus infection was difficult to determine.
Table 1.
Recovery of Echinochasmus caninus specimens from villagers in Khammouane Province, Lao PDR
| Villager no.a | Age | Sex | Village name | Year surveyed | No. of worms recoveredb |
|---|---|---|---|---|---|
| 1 | 42 | F | Nong Bone | 2003 | 21c |
| 2 | 21 | M | Mahaxay | 2009 | 8 |
| 3 | 30 | F | Nong Bone | 2003 | 4c |
| 4 | 32 | M | Nong Bone | 2003 | 2c |
| 5 | 34 | M | Nong Bone | 2003 | 1c |
| 6 | 43 | M | Nong Bone | 2003 | 1c |
| 7 | 25 | M | Phova | 2003 | 1c |
| 8 | 40 | F | Phova | 2003 | 1c |
| 9 | 46 | F | Bone Som | 2009 | 1 |
| 10 | 45 | F | Bone Som | 2009 | 1 |
| 11 | 30 | M | Thongmay | 2009 | 1 |
| Total | 42 |
Some villagers complained of gastrointestinal symptoms of variable degrees, including abdominal pain, epigastric pain, and indigestion.
Other helminth parasites recovered included Opisthorchis viverrini, Haplorchis spp., lecithodendriid-like flukes, hookworms, Trichuris trichiura, Trichostrongylus sp., Enterobius vermicularis, Taenia sp., Echinostoma revolutum, Artyfechinostomum malayanum, and Euparyphium sp. [7,8].
These specimens were reported erroneously as Echinochasmus japonicus in our previous report [8].
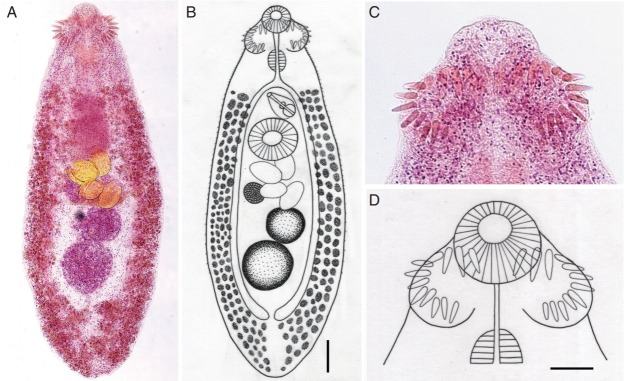
Adult flukes of E. caninus were leaf-like, plump, or slightly elongated (Fig. 1A, B), being averaged 0.99 (0.67–1.18) mm in length and 0.36 (0.27–0.45) mm in width (n=10) (Table 2). The anterior and posterior ends were attenuated, and the anterior end showed the characteristic features of an echinostome fluke, equipped with a head collar and collar spines around the oral sucker (Fig. 1A–D). The number of collar spines was 24 in total, 12 on each side, interrupted dorsally on the oral sucker, arranged in a single row for the dorsal spines (2 dorsal spines on each side overlapping the oral sucker) and 2 alternating rows for the lateral and ventral ones (Fig. 1C, D). The collar spines were relatively long (0.032–0.045 mm) and broad (1/3 of the length), larger for the dorsal spines and slightly smaller for ventral spines, with sharply pointed ends (Fig. 1C, D). The oral sucker was small and about a half the size of the ventral sucker. The cirrus sac containing the seminal vesicle and ejaculatory duct was located near the anterior edge of the ventral sucker but sometimes passed posteriorly toward the middle level of the ventral sucker. The uterine tubule was broad but short only with a few number of eggs, 1–4 (mean; 2.7) per specimen. The ovary was usually submedian and rarely median. The distribution of the vitellaria was extensive; they extended laterally from the level of the intestinal bifurcation near the cirrus sac or genital pore down to the posterior end of the body. These 2 lateral groups of vitellaria merged near the posterior end of the body. Two testes were tandem in the posterior field of the body; their shape was almost round or elliptical without any lobulations. The eggs in the uterus were large and oval, with a relatively narrow operculum and small abopercular wrinkles at the posterior end; they measured 98 (84–121) μm long and 68 (66–80) μm wide (n=20). All these morphological characters were compatible with previous descriptions of E. caninus [16–18].
Fig. 1.
Adult specimens of Echinochasmus caninus n. comb. (syn. Episthmium caninum) recovered from riparian residents in Khammouane Province, Lao PDR. (A) Adult E. caninus specimen (1.0 mm long and 0.37 mm wide) showing its most characteristic feature for differentiation from other species of Echinochasmus, i.e., the extensive distribution of the vitellaria from the level of the cirrus sac or genital pore to the posterior end of the body. The head collar, collar spines, uterine eggs (n=4), ovary, and 2 tandem testes are also characteristically seen. Semichon’s acetocarmine stained. (B) Line drawing of the specimen in Fig. 1A. Scale bar=90 μm. (C) The head collar bearing 24 collar spines interrupted dorsally, 12 on each side, in a single row in dorsal spines and 2 alternative rows in lateral and ventral ones. The dorsal spines are a little larger than the lateral and ventral ones. (D) Line drawing of the head collar and collar spines in Fig. 1C. Scale bar=50 μm.
Table 2.
Measurements of Echinochasmus caninus adult specimens recovered from riparian people in Khammouane Province, Lao PDR in comparison with those in a previous report
| Item | Measurements in mm | |
|---|---|---|
| The present study (n=10) (mean) | Verma [16] (n=3) | |
| Body | 0.67–1.18 (0.99)×0.27–0.45 (0.36) | 0.88–1.21×0.36–0.46 |
| Head collar | 0.096–0.15 (0.12)×0.15–0.20 (0.18) | 0.13–0.17×0.20–0.22 |
| No. collar spines | 24 | 24 |
| Oral sucker | 0.053–0.091 (0.076)×0.068–0.096 (0.080) | 0.060–0.076×0.070–0.084 |
| Pharynx | 0.052–0.081 (0.067)×0.042–0.078 (0.056) | 0.050–0.076×0.050–0.067 |
| Esophagus (length) | 0.11–0.23 | 0.050–0.11 |
| Cirrus sac | 0.057–0.17 (0.11)×0.054–0.12 (0.093) | 0.10–0.13×0.084–0.09 |
| Ventral sucker | 0.076–0.16 (0.13)×0.084–0.18 (0.16) | 0.11–0.12×0.13–0.14 |
| Ovary | 0.057–0.19 (0.098)×0.047–0.15 (0.084) | 0.09–0.13×0.07–0.084 |
| Anterior testes | 0.058–0.15 (0.10)×0.094–0.21 (0.15) | 0.13×0.084–0.11 |
| Posterior testes | 0.083–0.17 (0.12)×0.057–0.17 (0.13) | 0.05–0.24×0.14–0.19 |
| No. of uterine eggs | 1–4 | 0–3 |
| Size of uterine eggs | 0.084–0.121 (0.098)×0.066–0.080 (0.068) | 0.084×0.05–0.06 |
The genus Episthmium was erected by Lühe in 1909 using Episthmium africanum (Stiles, 1901) as the type, including another species Episthmium bursicola (Braun, 1901) [19]. However, the genus Episthmium was rejected by Odhner in 1910 and the 2 species were renamed as Echinochasmus africanum and Echinochasmus bursicola, respectively [19]. Later, Episthochasmus caninum Verma, 1935 was reported as a new taxon from dogs in Calcutta, India [16]. In 1958, Yamaguti [20] renamed this echinostome parasite as Episthmium caninum synonymizing Episthochasmus Verma, 1935 with Episthmium Lühe, 1909. Thereafter, the name Episthmium caninum had been used for a considerably long time until 2005. In the meantime, 3 human infections with this fluke were first found in Thailand which were reported in 2 papers, 1985 [17] and 1991 [18]. However, in 2001, Kostadinova and Gibson [19] suggested that Episthmium may be a synonym of Echinochasmus, accepting the Odhner’s opinion, and subsequently, in 2005, Kostadinova [21] synonymized the genus Episthmium with Echinochasmus. Following this taxonomic move, in the present report, the name Episthmium caninum has been changed into Echinochasmus caninus as a new combination.
The present specimens of E. caninus most closely resembled E. japonicus and Echinochasmus liliputanus. However, they differed from these 2 and also from other species of Echinochasmus in that the former (under the name Episthmium caninum) has a profusely developed and extensively distributed vitellaria into the forebody, whereas in other species of Echinochasmus the extent of the vitellaria in the forebody is considerably limited [20]. The size, shape, and arrangement of the collar spines are also characteristic by different species of Echinochasmus. In our specimens of E. caninus, the collar spines were relatively large (larger in dorsal spines and smaller in ventral ones), broad, and sharply pointed, with 6 dorsal spines arranged in a single row and 6 lateral and ventral ones arranged in a double row on each side. In addition, in E. caninus, 2 dorsal spines on each side were overlapped with the oral sucker [16]. In E. japonicus, the collar spines were similar in size with our specimens; however, they were arranged in a double row both in dorsal as well as in lateral and ventral spines [22]. Only 1 dorsal spine on each side was overlapped with the oral sucker in E. japonicus [22], whereas 2 dorsal ones on each side were overlapped with the oral sucker in most of our specimens. The collar spines of E. japonicus were almost uniform in size and slender with sharp-pointed ends [22] but those of our specimens differed in size between the dorsal and ventral ones and were relatively broad with sharp-pointed ends. E. liliputanus had a larger body size than E. caninus and smaller collar spines arranged simply in a single row [22].
Echinostome infections are foodborne but have been neglected because there is no much information about this group of parasites [1,2,15]. However, human echinostomiasis is not uncommon in endemic localities, particularly in Southeast Asia and the Far East [1,2,15]. In addition, microscopists may overlook or misinterpret echinostome eggs as other kinds of eggs or even artifacts particularly in routine Kato-Katz fecal smear examinations [1,2,15]. Moreover, eggs of different echinostome species closely resemble one another, and thus, for a specific diagnosis, adult worms should be recovered from each case and identified morphologically or molecularly [15].
In Lao PDR, with the exception of the following 3 reports, i.e., E. japonicus infection in 3 residents of Savannakhet Province [14], E. revolutum, A. malayanum, E. japonicus, and Euparyphium sp. infections in 9 people of Khammouane Province [8], and E. ilocanum infection in 2 residents of Savannakhet Province [15], no other echinostome infections have been documented from humans. In the present study, we detected 11 riparian people infected with E. caninus in Khammouane Province, Lao PDR.
Echinostome infections can cause severe clinical manifestations, particularly in cases with heavy worm loads [1–4]. However, in our cases, the worm load was considerably low, i.e., 1–21 worms per case, and the cases were co-infected with other helminth species. Therefore, specific symptoms due to E. caninus were difficult to determine. Possible clinical manifestations that would occur in heavy E. caninus and other Echinochasmus spp. infection cases need to be determined.
Human echinostome infections can be fish-borne, snail-borne, or amphibian-borne [23]. E. caninus is a fish-borne species, although the complete life cycle of E. caninus has not yet been uncovered. In Hainan Island, China, a freshwater fish, Macropodus opercularis, was reported to be the second intermediate host of this echinostome [24]. Thus, the source of human infection in 3 Thai patients was suggested to be the fish M. opercularis [25], although fish surveys were not performed in Thailand. The life cycle of E. caninus has never been studied in Lao PDR, and the source of infection in our cases is unclear. Studies on the life cycle of E. caninus should be investigated in the near future in Lao PDR.
In conclusion, the present study demonstrated for the first time the presence of human E. caninus infection in Lao PDR. This is the third report following the 2 dealing with 3 human cases of E. caninus infection in Thailand [17,18].
ACKNOWLEDGMENTS
We thank the staff of the Center for Laboratory and Epidemiology, Department of Hygiene and Prevention, Vientiane, and Khammouane Provincial Health Department, Khammouane, Lao PDR for their help in this study. We also thank the staff of Korea Association of Health Promotion, Seoul, Korea who kindly cooperated in Korea-Laos International Collaboration on Intestinal Parasite Control in Lao PDR (2000–2004 and 2007–2011).
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
REFERENCES
- 1.Chai JY. Echinostomes in humans. In: Fried B, Toledo R, editors. The Biology of Echinostomes. New York, USA: Springer; 2009. pp. 147–183. [Google Scholar]
- 2.Chai JY. Echinostomes. In: Xiao L, Ryan U, Feng Y, editors. Biology of Foodborne Parasites. Boca Raton, Florida, USA: CRC Press, Taylor & Francis Group; 2015. pp. 351–369. (Food Microbiology Series). [Google Scholar]
- 3.Reddy DB, Ranganaykamma I, Venkataratnam D. Artyfechinostomum mehrai infestation in man. J Trop Med Hyg. 1964;67:58–59. [PubMed] [Google Scholar]
- 4.Bandyopadhyay AK, Maji AK, Manna B, Bera DK, Addy M, Nandy A. Pathogenicity of Artyfechinostomum oraoni in naturally infected pigs. Trop Med Parasitol. 1995;46:138–139. [PubMed] [Google Scholar]
- 5.Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- 6.Chai JY, Park JH, Han ET, Guk SM, Shin EH, Lin A, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay, Rim HJ. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol. 2005;79:283–289. doi: 10.1079/joh2005302. [DOI] [PubMed] [Google Scholar]
- 7.Chai JY, Han ET, Shin EH, Sohn WM, Yong TS, Eom KS, Min DY, Um JY, Park MS, Hoang EH, Phommasack B, Insisiengmay, Lee SH, Rim HJ. High prevalence of Haplorchis taichui, Prosthodendrium molenkampi, and other helminth infections among people in Khammouane Province, Lao PDR. Korean J Parasitol. 2009;47:243–247. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Hoang EH, Phammasack B, Insisiengmay B, Rim HJ. Echinostome flukes recovered from humans in Khammouane Province, Lao PDR. Korean J Parasitol. 2012;50:269–272. doi: 10.3347/kjp.2012.50.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai JY, Yong TS, Eom KS, Min DY, Jeon HK, Kim TY, Jung BK, Sisabath L, Insisiengmay B, Phommasack B, Rim HJ. Hyperendemicity of Haplorchis taichui infection among riparian people in Saravane and Champasak Province, Lao PDR. Korean J Parasitol. 2013;51:305–311. doi: 10.3347/kjp.2013.51.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai JY, Sohn WM, Jung BK, Yong TS, Eom KS, Min DY, Insisiengmay B, Insisiengmay S, Phommasack B, Rim HJ. Intestinal helminths recovered from humans in Xieng Khouang Province, Lao PDR with a particular note on Haplorchis pumilio infection. Korean J Parasitol. 2015;53:439–445. doi: 10.3347/kjp.2015.53.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayasone S, Vonghajak Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, Akkhavong K, Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Sayasone S, Mak TK, Vanmany M, Rasphone O, Vounatsou P, Utzinger J, Akkhavong K, Odermatt P. Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao Peoples’ Democratic Republic. PLoS Negl Trop Dis. 2011;5:e1037. doi: 10.1371/journal.pntd.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laymanivong S, Hongvanthong B, Keokhamphavanh B, Phommasansak M, Phinmaland B, Sanpool O, Maleewong W, Intapan PM. Current status of human hookworm infections, ascariasis, trichuriasis, schistosomiasis mekongi and other trematodiases in Lao Peoples’ Democratic Republic. Am J Trop Med Hyg. 2014;90:667–669. doi: 10.4269/ajtmh.13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayasone S, Tesana S, Utzinger J, Hatz C, Akkhavong K, Odermatt P. Rare human infection with the trematode Echinochasmus japonicus in Lao PDR. Parasitol Int. 2009;58:106–109. doi: 10.1016/j.parint.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Chai JY, Sohn WM, Cho J, Eom KS, Yong TS, Min DY, Hoang EH, Phammasack B, Insisiengmay B, Rim HJ. Echinostoma ilocanum infection in two residents of Savannakhet Province, Lao PDR. Korean J Parasitol. 2018;56:77–81. doi: 10.3347/kjp.2018.56.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma SC. Studies on the Indian species of the genus Echinochasmus, Part I. and on an allied new genus Episthochasmus. Proc Ind Acad Sci. 1935;1:837–856. [Google Scholar]
- 17.Radomyos P, Bunnag D, Harinasuta T. Report of Episthmium caninum (Verma, 1935) Yamaguti, 1958 (Digenea: Echinostomatidae) in man. Southeast Asian J Trop Med Public Health. 1985;16:508–511. [PubMed] [Google Scholar]
- 18.Radomyos P, Charoenlarp P, Harinasuta T. Human Episthmium caninum (Digenea: Echinostomatidae) infection: report of two more cases. J Trop Med Parasitol. 1991;14:48–50. [Google Scholar]
- 19.Kostadinova A, Gibson D. A redescription of Uroproctepisthmium bursicola (Creplin, 1837) n. comb. (Digenea: Echinostomatidae), and re-evaluation of the genera Episthmium Lühe, 1909 and Uroproctepisthmium Fischthal & Kuntz, 1976. Syst Parasitol. 2001;50:63–67. doi: 10.1023/a:1011813107208. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguti S. The Digenetic Trematodes of Vertebrates (Part I) I. New York, USA: Interscience Publishers; 1958. Systema Helminthum; pp. 1–979. [Google Scholar]
- 21.Kostadinova A. Family Echinostomatidae looss, 1899. In: Jones A, Bray RA, Gibson DI, editors. Keys to the Trematoda. Vol. 2. London, UK: Natural History Museum; 2005. pp. 9–64. [Google Scholar]
- 22.Chen HT, Chen S, Chu H, Zheng Z, Tang Z. Echinostomatidae dietz, 1909. In: Chen HT, editor. Fauna Sinica. Platyhelminthes. Trematoda. Digenea (I) Beijing, China: Science Press; 1985. pp. 333–497. [Google Scholar]
- 23.Yu SH, Mott KE. Epidemiology and morbidity of food-borne intestinal trematode infections. Trop Dis Bull. 1994;91:125–152. [Google Scholar]
- 24.Kobayasi H. Studies on the flukes in Hainan Island. III. On the flukes belonging to the Family Echinostomatidae Odhner, 1910, Episthochasmus caninum Verma, 1935. Acta Nippon Med Trop. 1942;4:97–105. [Google Scholar]
- 25.Tungtrongchitr A, Radomyos P. Human intestinal fluke infections in Thailand. Bull Chulalongkorn Med Tech. 1989;3:417–427. (in Thai). [Google Scholar]