Abstract
Background: To evaluate the diagnostic value of Epstein–Barr virus (EBV) DNA in nasopharyngeal carcinoma (NPC) patients with locoregional or distant recurrence.
Methods: Articles related to the diagnosis of recurrent or metastatic NPC by the detection of EBV DNA in plasma or serum were retrieved from different databases. Sensitivity, specificity, summary receiver operating characteristic (SROC) curves, and likelihood ratios were pooled to assess the diagnostic value of individual diagnostic tests.
Results: This meta-analysis pooled 25 eligible studies including 2496 patients with NPC. The sensitivity, specificity, positive likelihood ratio (+LR), and negative likelihood ratio (−LR) of EBV DNA in the diagnosis of NPC were 0.858 (95% confidence interval (CI): 0.801–0.901), 0.890 (95% CI: 0.866–0.909), 7.782 (95% CI: 6.423–9.429) and 0.159 (95% CI: 0.112–0.226), respectively. The diagnostic odds ratio (DOR) was 48.865 (95% CI: 31.903–74.845). The SROC for EBV DNA detection was 0.93 (95% CI: 0.90–0.95).
Conclusion: The detection of EBV DNA for the diagnosis of recurrent or metastatic NPC has good sensitivity and specificity and might be helpful in monitoring recurrent or metastatic NPC.
Keywords: Epstein-Barr Virus DNA, Meta-Analysis, metastatic, nasopharyngeal carcinoma, recurrent
Introduction
Nasopharyngeal carcinoma (NPC) is a type of cancer with a particularly high incidence in Southern China and Southeast Asian countries, affecting 10–50 per 100000 people per year [1–4]. The standard therapy for NPC is radiotherapy and concurrent chemoradiotherapy (CCRT) depending on the stage of disease during presentation [5]. Despite significant improvements in survival and local control due to advances in radiotherapy and combined modality treatments, local recurrence and distant metastasis remain difficult to avoid in patients with advanced NPC [6]. It was reported that the rate of local recurrence and distant metastasis after 5 years of the initial treatment for NPC is 8.2–22.0% [7]. Currently, the main diagnostic method for the recurrence or metastasis of NPC patients is clinical imaging examination combined with endoscopic biopsy. However, a series of abnormal changes, including local edema, tissue disorder, fibrosis, mucositis, and scar formation, always occur in the post-treatment of NPC patients, which significantly interferes with the accuracy of an imaging examination [8–10]. In addition, with computed tomography (CT) and magnetic resonance imaging (MRI), it is difficult to detect distant metastases early and specifically when the diameter of the lesion is less than 5 mm [11], which will delay the discovery of the tumor. Meanwhile, the high cost of PET/CT examinations and the general application during follow-up are inconsistent with the economic development level of China. Pathological examination is the gold standard for the diagnosis of NPC recurrence and metastasis. Generally, it is difficult to obtain pathological sections of recurrent or metastatic lesions, especially those that occur under the mucosa or deep in the nasopharynx. More importantly, invasive procedures used to obtain pathological diagnoses are an important poor prognostic factor for the disease [12]. Therefore, seeking a more concise and effective diagnostic method would be of great importance for NPC patients.
NPC is strongly associated with Epstein–Barr virus (EBV). Plasma EBV DNA level has been used as a tumor marker for NPC and is widely used in clinical screening and diagnosis of NPC [13]. However, the value of plasma-free EBV DNA in the diagnosis of recurrence and metastasis of NPC is not clear currently. Different studies have found that there is a large difference in the critical value of free EBV DNA expression after treatment [14–17]. These differences may be due to experimental methods used by different investigators and geographical differences in NPC itself.
Due to insufficient research and inconsistent reports, there is no uniform and accurate conclusion on whether plasma EBV DNA can effectively detect the recurrence and metastasis of NPC. Therefore, we performed this meta-analysis to better assess the diagnostic value of plasma EBV DNA in recurrent or metastatic NPC patients.
Methods
We conducted this meta-analysis on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. All analyses were conducted based on previously published studies; thus, no ethical approval or patient consent were required.
Search strategy
Two reviewers (Haiqin Peng and Zhanzhan Li) independently completed a search. There was no restriction on the language of the studies. The search strategy combined the following key words: (‘Epstein–Barr Virus’ [All Fields]) OR (‘EBV’ [All Fields]) OR (‘DNA’ [All Fields]) OR (‘EBV-DNA’ [All Fields]) OR (‘EBV DNA’ [All Fields]) OR (‘Epstein–Barr Virus DNA’ [All Fields]) AND (‘nasopharyngeal carcinoma’ [All Fields]) OR (‘nasopharyngeal cancer’ [All Fields]) OR (‘carcinoma of nasopharynx’ [All Fields]) OR (‘NPC’ [All Fields]) AND (‘sensitivity’ [All Fields]) OR (‘specificity’ [All Fields]) OR (‘false-negative’ [All Fields]) OR (‘false-positive’ [All Fields]) OR (‘diagnosis’ [All Fields]) OR (‘detection’ [All Fields]) OR (‘accuracy’ [All Fields]) AND (‘plasma’ [All Fields]) OR (‘serum’ [All Fields]) AND (‘relapse’ [All Fields]) OR (‘recurrence’ [All Fields]) OR (‘metastasis’ [All Fields]). We used this search strategy to search PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Web of Science (https://www.webofknowledge.com), EMBASE (https://www.embase.com), the Chinese Biomedical Database (http://www.sinomed.ac.cn/zh/), and the China National Knowledge Infrastructure (http://www.cnki.net/) website for articles published from January 1998 to July 2018. References cited in the retrieved studies were reviewed for more eligible studies.
Inclusion/exclusion criteria
Studies were considered eligible only when they met all of the following inclusion criteria: (1) the purpose of study was to evaluate the clinical value of EBV DNA in the diagnosis of NPC recurrence or metastasis; (2) identification of NPC was confirmed by histology or pathology; (3) the study clearly identified negative controls; and (4) the article provided data that can calculate true positive value (TP), false positive value (FP), true negative value (TN), false negative value (FN), directly or indirectly. If the data were repeatedly published, the most detailed data or the most recently published article were selected. The exclusion criteria were as follows: (1) studies that were published as review articles or letters; (2) the article lacking important information to calculate TP, FP, TN, and FN directly or indirectly; and (3) studies not clearly identifying negative controls.
Data extraction
Two investigators reviewed the titles and abstracts of all records searched above to extract literature information that met the inclusion criteria. General information included study publication date and country, number of subjects, sample source, and study design. Any disagreements were discussed until a final form was agreed upon. For records that could not be evaluated by title and abstract, the full text was retrieved for detailed evaluation according to the inclusion and exclusion criteria. The data extracted from each study included basic characteristics of the studies and outcomes. Basic characteristics of the studies included the first author, year of the publication, country of origin, and sample size. Outcomes included the TP, FP, TN, and FN results calculated from each study.
Quality assessment
The methodological quality of the selected studies was evaluated independently by two reviewers (Haiqin Peng and Zhanzhan Li) using the quality assessment of diagnostic accuracy studies (QUADAS) checklist [14–18]. This checklist includes 14 items: a representative spectrum (item 1), a clear selection criteria (item 2), an acceptable reference standard (item 3), an acceptable delay between tests (item 4), partial verification (item 5), the same reference test regardless of the index test result (item 6), incorporation bias (item 7), the execution of the index test in detail (item 8), the reference standard in detail (item 9), the index test results were blinded to the reference test results (item 10), the reference standard was blinded to the index test results (item 11), the availability of clinical data that would be available in clinical practice when using the index test (item 12), reporting of uninterpretable results (item 13), and an explanation of withdrawals from the study (item 14). The 14 items were assessed in all included articles, each of which was assessed as ‘yes’, ‘no’, or ‘unclear’. Disagreements were resolved by a third reviewer (Rongrong Zhou).
Statistical analysis
Statistical analysis was conducted by using Review Manager 5.3.5 (Cochrane Collaboration, Oxford, U.K.) and STATA 12.0 software (Stata Corp, College Station, TX). The accuracy indexes of EBV DNA were pooled by meta-analysis, including sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR–), diagnostic odds ratio (DOR), and their 95% confidence interval (CI). A summary receiver operating characteristic (SROC) curve was used to evaluate the global summary of test performance, and the area under the SROC curve presents the overall performance of the detection method. An area under the SROC curve of 1.0 (100%) indicates perfect discriminatory ability. Heterogeneity across studies was assessed using Cochran’s Q test and I2 statistics [19]. Heterogeneity was considered statistically significant when P<0.05 or I2 > 50%. A fixed-effect model was used when there was no evidence of significant heterogeneity. Otherwise, a random-effect model was applied. Subgroup analysis was conducted to explore the possible sources of heterogeneity. All P-values were two-sided, and P<0.05 was considered statistically significant.
Subgroup analysis
Subgroup analyses were performed based on the total number of subjects (sample <30 vs ≥30), study design (case–control vs cohort study), and test specimens (serum vs plasma) to investigate differences in sensitivity and specificity between subgroups.
Results
Literature selection
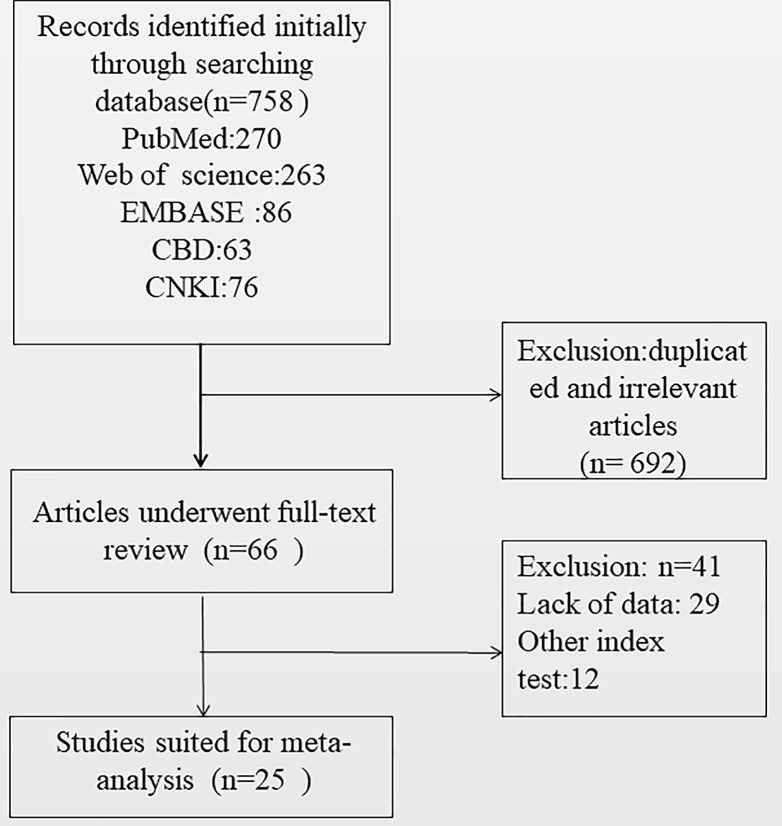
The results of the literature research are presented in Figure 1. A total of 758 records were selected by searching the databases. After reviewing the titles and abstracts of studies, we excluded 733 studies. Finally, 25 studies [20–44] with 2496 samples were included in the final analysis. Among them, 20 papers were from China, which is consistent with the high incidence of NPC in China. The sample size of the studies ranged from 20 to 385. In addition, the gender ratio of the patients was reported in 13 studies, with a total of 1369 patients, of which 946 were males and 423 were females.
Figure 1. Flow chart of the selection process for eligible studies.
Characteristics of studies
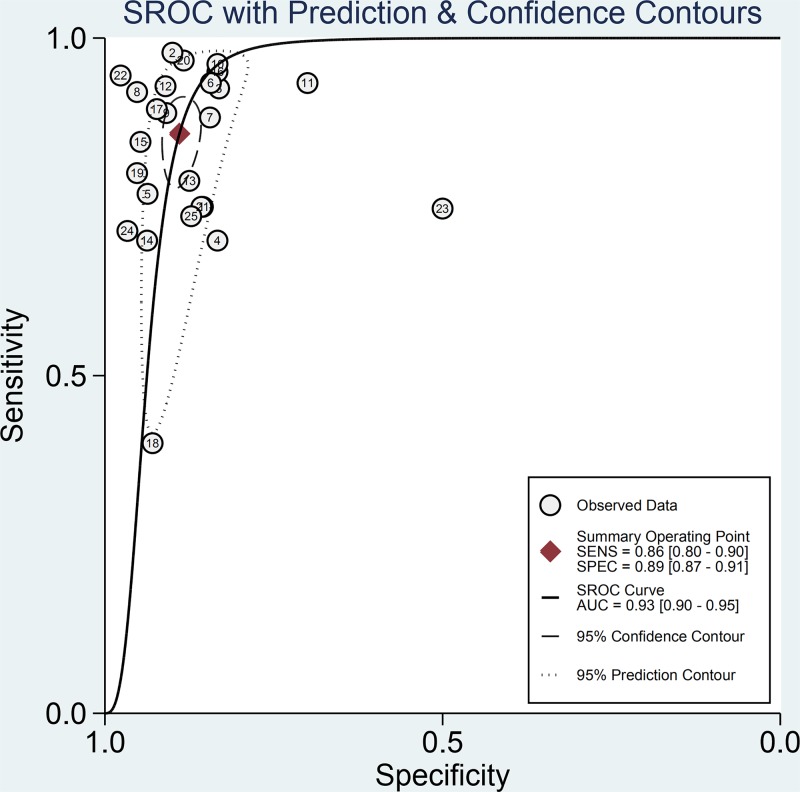
The main characteristics of the studies included in the meta-analysis are shown in Table 1. Among them, 22 study samples were from patients’ plasma and 3 study samples were from patients’ serum. In addition, 9 studies were case–control and 16 were cohort studies. The value of the plasma EBV DNA in diagnosing recurrent or metastatic NPC and the basic characteristics (TP, FP, TN, and FN values for serum EBV DNA) are shown in Table 2. We analyzed the pooled sensitivity, specificity, DOR, positive likelihood (+LR) and likelihood negative (−LR) of EBV DNA. Summary of meta-analysis results are shown in Table 3. The pooled results for sensitivity and specificity were 0.858 (95% CI: 0.801–0.901, Figure 2) and 0.890 (95% CI: 0.866–0.909, Figure 3), respectively. The highest sensitivity was 0.98 (95% CI: 0.88–1.0), which came from Zhu et al.’s study [21]. The lowest sensitivity was 0.40 (95% CI: 0.21–0.61), which came from Hsiao et al.’s study [37]. The highest specificity was 0.98 (95% CI: 0.88–1.0), which came from Chan et al.’s study [41]. The lowest specificity was 0.50 (95% CI: 0.01–0.99), which came from Shen et al.’s study [42]. The value of the DOR was 48.865 (95% CI: 31.903–74.845, Figure 4), which reflects the extent of the association between the results of diagnostic tests and diseases. Fagan diagram (Supplementary Figure S1.) also indicated the plasma EBV DNA had a high diagnostic ability in detecting recurrence or metastasis NPC. In addition, we also calculated LR+ and LR−, which are considered to be more clinically meaningful than sensitivity or specificity, to measure the diagnostic performance of the plasma EBV DNA in NPC with recurrence or metastasis. The pooled results of LR+ and LR− were 7.782 (95% CI: 6.423–9.429) and 0.159 (95% CI: 0.112 –0.226), respectively. The largest area of diagnosis under the summary receiver operator curve (AUC) for NPC by overall EBV DNA detection was 0.93 (95% CI: 0.90–0.95, Figure 5), indicating a relatively high accuracy. According to the QUADS scale, green stands for low risk, red stands for high risk, and yellow stands for risk unclear. Risk of bias and applicability concerns graph is presented in Figure 6. Risk of bias and applicability concerns summary is presented in Figure 7.
Table 1. Characteristics of studies included in the meta-analysis.
| Study ID | Year | Region | Number | Sample source | Sampling consecutive | Data collection retrospective | Study design |
|---|---|---|---|---|---|---|---|
| Tan et al. [20] | 2010 | China | 78 | Plasma | Yes | No | Cohort study |
| Zhu et al. [21] | 2006 | China | 106 | Plasma | Yes | No | Cohort study |
| Ma et al. [22] | 2011 | China | 274 | Plasma | Yes | No | Cohort study |
| Liao et al. [23] | 2008 | China | 22 | Plasma | Yes | No | Case–control study |
| Gong et al. [24] | 2007 | China | 360 | Plasma | Yes | No | Cohort study |
| Li et al. [25] | 2008 | China | 81 | Plasma | Yes | No | Cohort study |
| Sun et al. [26] | 2011 | China | 62 | Plasma | Yes | No | Cohort study |
| Peng et al. [27] | 2009 | China | 46 | Plasma | Yes | No | Case–control study |
| Zhang et al. [28] | 2004 | China | 20 | Plasma | Yes | No | Case–control study |
| Sun et al. [29] | 2009 | China | 68 | Plasma | Yes | No | Cohort study |
| Sun et al. [30] | 2008 | China | 25 | Plasma | Yes | No | Cohort study |
| Ozyar et al. [31] | 2008 | Turkey | 92 | Plasma | Yes | No | Case–control study |
| Cao et al. [32] | 2003 | China | 76 | Plasma | Yes | No | Cohort study |
| Lo et al. [33] | 1999 | China | 26 | Plasma | Yes | No | Case–control study |
| Fan et al. [34] | 2004 | China | 32 | Serum | yes | No | Case–control study |
| Jiang et al. [35] | 2005 | China | 26 | Plasma | Yes | No | Cohort study |
| Kondo et al. [36] | 2004 | Japan | 45 | Serum | Yes | No | Case–control study |
| Hsiao et al. [37] | 2002 | China | 110 | Serum | Yes | No | Cohort study |
| Chang et al. [38] | 2008 | China | 31 | Plasma | Yes | No | Cohort study |
| Shao et al. [39] | 2003 | China | 90 | Plasma | Yes | No | Cohort study |
| Stoker et al. [40] | 2016 | Netherlands | 147 | Plasma | Yes | No | Cohort study |
| Chan et al. [41] | 2014 | China | 61 | Plasma | Yes | No | Cohort study |
| Shen et al. [42] | 2014 | China | 196 | Plasma | Yes | No | Case–control study |
| Ferrari et al. [43] | 2012 | Italy | 37 | Plasma | Yes | No | Cohort study |
| Li et al. [44] | 2017 | China | 385 | Plasma | Yes | No | Case–control study |
Table 2. Summary measures of test accuracy from the studies included.
| Study ID | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | +LR (95% CI) | −LR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Tan et al. (2010) | 27 | 6 | 9 | 36 | 0.75 (0.58–0.88) | 0.86 (0.71–0.95) | 5.25 (2.44–11.2) | 0.29 (0.16–0.52) |
| Zhu et al. (2006) | 45 | 6 | 1 | 54 | 0.98 (0.88–1.00) | 0.9 (0.79–0.96) | 9.78 (4.57–20.9) | 0.02 (0.00–0.17) |
| Ma et al. (2011) | 62 | 35 | 5 | 172 | 0.93 (0.83–0.98) | 0.8 (0.77–0.88) | 5.47 (4.02–7.46) | 0.09 (0.04–0.21) |
| Liao et al. (2008) | 7 | 2 | 3 | 10 | 0.7 (0.35–0.93) | 0.83 (0.52–0.98) | 4.2 (1.11–15.8) | 0.36 (0.14–0.96) |
| Gong et al. (2007) | 70 | 17 | 21 | 252 | 0.77 (0.67–0.85) | 0.94 (0.90–0.96) | 12.17 (7.58–19.5) | 0.25 (0.17–0.36) |
| Li et al. (2008) | 28 | 8 | 2 | 43 | 0.93 (0.78–0.99) | 0.84 (0.71–0.93) | 5.95 (3.13–11.3) | 0.08 (0.02–0.30) |
| Sun et al. (2011) | 15 | 7 | 2 | 38 | 0.88 (0.64–0.99) | 0.84 (0.71–0.94) | 5.67 (2.81–11.4) | 0.14 (0.04–0.52) |
| Peng et al. (2009) | 23 | 1 | 2 | 20 | 0.92 (0.74–0.99) | 0.95 (0.76–1.00) | 19.32 (2.84–131.3) | 0.08 (0.02–0.32) |
| Zhang et al. (2004) | 8 | 1 | 1 | 10 | 0.89 (0.52–1.00) | 0.91 (0.59–1.00) | 9.78 (1.49–64.2) | 0.12 (0.02–0.78) |
| Sun et al. (2009) | 25 | 7 | 1 | 35 | 0.96 (0.80–1.00) | 0.83 (0.69–0.93) | 5.77 (2.92–11.3) | 0.05 (0.01–0.32) |
| Sun et al. (2008) | 14 | 3 | 1 | 7 | 0.93 (0.68–1.00) | 0.7 (0.35–0.93) | 3.11 (1.20–8.10) | 0.10 (0.01–0.66) |
| Ozyar et al. (2008) | 13 | 7 | 1 | 71 | 0.93 (0.66–1.00) | 0.91 (0.82–0.96) | 10.35 (5.03–21.2) | 0.08 (0.01–0.52) |
| Cao et al. (2003) | 41 | 3 | 11 | 21 | 0.79 (0.65–0.89) | 0.88 (0.68–0.97) | 6.31 (2.17–18.3) | 0.24 (0.14–0.42) |
| Lo et al. (1999) | 7 | 1 | 3 | 15 | 0.7 (0.35–0.93) | 0.94 (0.70–1.00) | 11.2 (1.61–77.9) | 0.32 (0.12–0.83) |
| Fan et al. (2004) | 11 | 1 | 2 | 18 | 0.85 (0.55–0.98) | 0.95 (0.74–1.00) | 16.08 (2.35–109.6) | 0.16 (0.05–0.58) |
| Jiang et al. (2005) | 19 | 1 | 1 | 5 | 0.95 (0.75–1.00) | 0.83 (0.36–1.00) | 5.7 (0.95–34.2) | 0.06 (0.01–0.42) |
| Kondo et al. (2004) | 17 | 2 | 2 | 24 | 0.89 (0.67–0.99) | 0.92 (0.75–0.99) | 11.63 (3.04–44.4) | 0.11 (0.03–0.43) |
| Hsiao et al. (2002) | 10 | 6 | 15 | 79 | 0.4 (0.21–0.61) | 0.93 (0.85–0.97) | 5.67 (2.28–14.0) | 0.65 (0.47–0.89) |
| Chang et al. (2008) | 8 | 1 | 2 | 20 | 0.8 (0.44–0.97) | 0.95 (0.76–1.00) | 16.8 (2.42–116.1) | 0.21 (0.06–0.73) |
| Shao et al. (2003) | 29 | 7 | 1 | 53 | 0.97 (0.83–1.00) | 0.88 (0.77–0.95) | 8.29 (4.12–16.6) | 0.04 (0.01–0.26) |
| Stoker et al. (2016) | 12 | 19 | 4 | 112 | 0.75 (0.48–0.93) | 0.85 (0.78–0.91) | 5.17 (3.13–8.55) | 0.29 (0.12–0.69) |
| Chan et al. (2014) | 17 | 1 | 1 | 42 | 0.94 (0.73–1.00) | 0.98 (0.88–1.00) | 40.61 (5.83–282.3) | 0.06 (0.01–0.38) |
| Shen et al. (2014) | 145 | 1 | 49 | 1 | 0.75 (0.68–0.81) | 0.5 (0.01–0.99) | 1.49 (0.37–5.99) | 0.51 (0.12–2.06) |
| Ferrari et al. (2012) | 5 | 1 | 2 | 29 | 0.71 (0.29–0.9) | 0.97 (0.83–1.00) | 21.43 (2.95–155.6) | 0.30 (0.09–0.96) |
| Li et al. (2017) | 53 | 40 | 19 | 273 | 0.74 (0.62–0.83) | 0.87 (0.83–0.91) | 5.76 (4.18–7.94) | 0.30 (0.21–0.45) |
Table 3. Summary of meta-analysis results.
| Parameter | Estimate (95% CI) |
|---|---|
| Sensitivity | 0.858 (0.801-0.901) |
| Specificity | 0.890 (0.866-0.909) |
| Positive Likelihood Ratio | 7.782 (6.423-9.429) |
| Negative Likelihood Ratio | 0.159 (0.112-0.226) |
| Diagnostic Score | 3.889 (3.463-4.315) |
| DOR | 48.865(31.903-74.845) |
Figure 2. Forest plots of pooled sensitivity for EBV DNA assay in the recurrence or/and metastasis of NPC.
Figure 3. Forest plots of pooled specificity for EBV DNA assay in the recurrence or/and metastasis of NPC.
Figure 4. Forest plots of pooled DOR for EBV DNA assay in the recurrence or/and metastasis of NPC.
Figure 5. Summary ROC curve of all included articles with 95% CIs for pooled sensitivity and pooled specificity and the 95% prediction interval.
Figure 6. Risk of bias and applicability concerns graph.
Figure 7. Risk of bias and applicability concerns summary.
In addition, we used Cochran’s Q test and I2 statistic to assess heterogeneity between studies. Heterogeneity was considered statistically significant when P<0.05 or I2 > 50%. According to the results in the Figures, there is a large heterogeneity in the study.
Publication bias
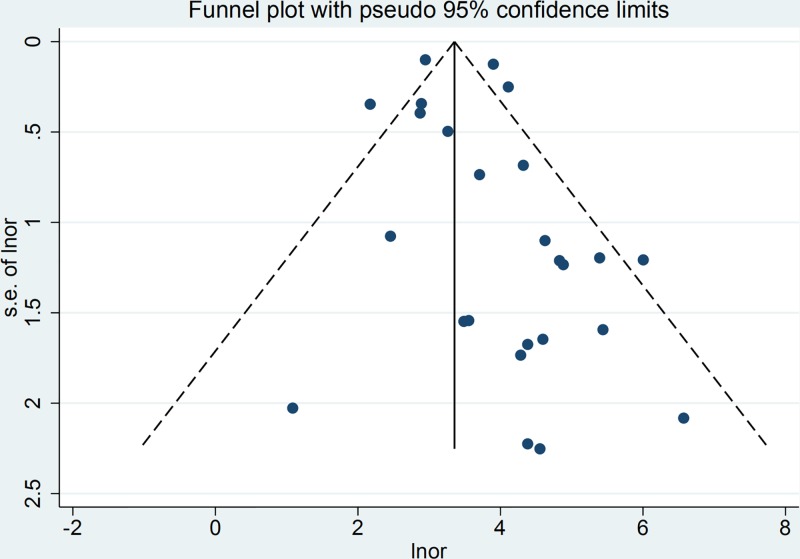
Funnel plots were performed to evaluate publication bias. Figure 8 shows the asymmetry of the funnel plot of publication bias, indicating the presence of publication bias in the meta-analysis.
Figure 8. Funnel plot with pseudo 95% confidence limits for EBV DNA assay in the recurrence or/and metastasis of NPC.
Subgroup analysis
The results of the subgroup analysis are shown in Table 4. The results showed that the source of heterogeneity between studies was independent of the total number of subjects (n<30 vs ≥30), study design (case–control vs cohort study), and test specimens (serum vs plasma) (P>0.05).
Table 4. Subgroup analysis.
| Grouping situation | Number | Sensitivity | Specificity |
|---|---|---|---|
| Sample | |||
| <30 | 5 | 0.83 ± 0.12 | 0.84 ± 0.09 |
| >30 | 20 | 0.83 ± 0.14 | 0.87 ± 0.10 |
| P-value | 0.976 | 0.489 | |
| Sample source | |||
| Plasma | 22 | 0.85 ± 0.10 | 0.86 ± 0.10 |
| Serum | 3 | 0.71 ± 0.27 | 0.93 ± 0.02 |
| P-value | 0.481 | 0.253 | |
| Study design | |||
| Cohort study | 16 | 0.84 ± 0.15 | 0.87 ± 0.07 |
| Case–control study | 9 | 0.82 ± 0.10 | 0.86 ± 0.14 |
| P-value | 0.707 | 0.082 |
Discussion
The local recurrence or distant metastasis rate of NPC after 5 years of first-course treatment was 8.2–22.0%, and mainly occurred within 1–3 years after treatment [45,46]. Studies have shown that the survival period for the distant metastasis of NPC is only 12–20 months [47]. During follow-up of patients with NPC, diagnosis of recurrence or metastasis is based on a basic medical history, physical examination, appropriate imaging studies (MRI and/or enhanced CT), and histological examination. PET/CT, as a functional imaging examination, can reflect local tissue metabolism and identify abnormal changes after radiotherapy such as scarring, fibrosis, or tumor recurrence in the diagnosis of the recurrence or metastasis of NPC in patients [48,49]. In addition, PET can be used to determine the correlation between the differentiation degree of NPC through SUV, which could help to confirm the pathological classification of patients who cannot obtain a pathological diagnosis [50]. Nonetheless, local chronic mucosal ulcers, granulomatous tissue, inflammatory changes, and radioactive osteomyelitis formed after radiotherapy inevitably lead to FP results [51]. FN results were also difficult to avoid, as the activity of tumor cells decreases after treatment, and the SUV value decreases accordingly. Furthermore, for lesions less than 1 cm in diameter, the diagnostic sensitivity will be further reduced if 18f-fdg uptake is insufficient [51]. One of the most realistic and important problems is the high cost of PET/CT examination, and the general application during follow-up is inconsistent with the economic development level of China. Hence, it is urgent to find a simple and convenient, economical, highly sensitive, and specific detection method for the early diagnosis of recurrence or metastasis of NPC, and to give more active and appropriate treatment accordingly to guide clinical work.
Mutirangura et al. [52] first detected serum-free EBV DNA by conventional PCR in 1998. Later, Shotelersuk et al. [53] further confirmed the above conclusions by nested-PCR and proposed that free EBV DNA in peripheral blood was obtained from tumor cells. Lo et al. [33] were the first to use RT-PCR technology to study the relationship between the level of plasma EBV DNA and tumor recurrence. The results showed that the level of plasma EBV DNA (median copy number: 32350 copies/ml) in 10 patients with recurrence was significantly higher than that in 15 patients with continuous remission for 2 years (median copy number: zero copies/ml), P=0.01. These results indicated that the level of plasma EBV DNA could be used as a reliable indicator for the diagnosis of NPC recurrence or metastasis. Moreover, the value of free EBV DNA in the diagnosis of NPC recurrence or metastasis is also supported by imaging examination. Makitie et al. [54] reported that the detection effects of the copy number of plasma EBV DNA with NPC patients were consistent with the PET/CT examination, both of which were superior to MRI, in the monitoring of NPC local recurrence or distant metastasis.
The present meta-analysis included 25 studies with 2496 patients to evaluate the effectiveness and identify the value of EBV DNA levels as a tool to diagnose the recurrence or metastasis of NPC. Our statistical analysis shows that the pooled sensitivity, specificity, and AUG values achieved 0.858, 0.890, and 0.93, respectively, indicating a very high level of overall accuracy. For post-treatment NPC patients, the specificity value of diagnostic tests during follow-up should be as high as possible to exclude FP diagnosis. According to the results of subgroup analysis, the specific value of serum-derived EBV DNA was the highest, which was 0.933 (95% CI: 0.91–0.95). This means that during follow-up, NPC patients should be selected for serum-derived EBV DNA as much as possible. In statistics, DOR reflects the extent of association between the results of diagnostic tests and diseases. The DOR value was 48.865, indicating a very high discriminant effect in the diagnostic test. Overall, our results suggested that plasma EBV DNA has a high enough accuracy in diagnosing the recurrence or metastasis of NPC.
However, there are several limitations to our study. First, there are publication biases and heterogeneity in the results. After ensuring that the raw data were entered correctly, we conducted a subgroup analysis to explore the sources of heterogeneity and publication bias. Subgroup analysis of possible factors (sample size, sample source, study design) showed that these factors have little impact on the results. This may be related to incomplete information in the selected studies, including disease stage, age distribution, gender distribution etc., which led to the inability of the present study to adequately assess the impact of these variables. In addition, because most of the studies considered recurrence or metastasis as a whole and did not provide specific case data for recurrence or metastasis, the diagnostic efficacy of detecting EBV DNA in the diagnosis of NPC recurrence or metastasis could not be obtained, respectively. Finally, follow-up times are key factors for the accurate diagnosis of the posttreatment clinical remission period. A sufficiently long follow-up time will contribute to distinguishing cases in clinical remission. However, only five of the included studies provided follow-up time data (2–145 months), while most of the studies lacked data on the specific follow-up time, which would affect the accuracy of diagnosis to some extent.
In conclusion, our meta-analysis of currently available data provided reliable evidence that the level of plasma EBV DNA, as a tumor marker for NPC with high sensitivity and good specificity, has a high diagnostic efficacy in the diagnosis of the recurrence or metastasis of NPC. However, it is important to note that the level of plasma EBV DNA cannot replace nasopharyngeal endoscopy and imaging examination as the gold standard for the diagnosis of the recurrence or metastasis of NPC. In the clinic, the frequency of nasopharyngeal endoscopy and imaging examination can be appropriately reduced according to the level of plasma EBV DNA, and the interval time of the above examination can be extended, which is a simple, effective and economical diagnostic method during the follow-up of NPC. These measures are of great significance in improving the therapeutic effect and prognosis of patients with malignant NPC.
Supporting information
Supplementary Figure S1.
Abbreviations
- CI
confidence interval
- CT
computed tomography
- DOR
diagnostic odds ratio
- EBV
Epstein–Barr virus
- FN
false negative
- FP
false positive
- MRI
magnetic resonance imaging
- NPC
nasopharyngeal carcinoma
- PET
positron emission tomography
- QUADS
quality assessment of diagnostic accuracy studies
- SROC
summary receiver operating characteristic
- SUV
standard uptake value
- TN
true negative
- TP
true postive
Funding
This work was supported by the National Nature Science Foundation of China [grant number 8177041157].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
R.Z. and H.P. designed the study. H.P., Z.z.L., Y.L., J.L. and Z.y.L. performed experiments and analyzed data. H.P. and R.Z. prepared the manuscript. All authors have read and approved the manuscript.
References
- 1.Marks J.E., Phillips J.L. and Menck H.R. (1998) The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 83, 582–588 [DOI] [PubMed] [Google Scholar]
- 2.Wei W.I. and Sham J.S. (2005) Nasopharyngeal carcinoma. Lancet 365, 2041–2054 10.1016/S0140-6736(05)66698-6 [DOI] [PubMed] [Google Scholar]
- 3.Parkin D.M., Bray F., Ferlay J. and Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 10.3322/canjclin.55.2.74 [DOI] [PubMed] [Google Scholar]
- 4.Jiang G.M., Wang H.S., Du J., Ma W.F., Wang H., Qiu Y.. et al. (2017) Bortezomib relieves immune tolerance in nasopharyngeal carcinoma via STAT1 suppression and indoleamine 2,3-dioxygenase downregulation. Cancer Immunol. Res. 5, 42–51 10.1158/2326-6066.CIR-16-0102 [DOI] [PubMed] [Google Scholar]
- 5.Tan W.L., Tan E.H., Lim D.W., Ng Q.S., Tan D.S., Jain A.. et al. (2016) Advances in systemic treatment for nasopharyngeal carcinoma. Chin. Clin. Oncol. 5, 21 10.21037/cco.2016.03.03 [DOI] [PubMed] [Google Scholar]
- 6.Kam M.K., Teo P.M., Chau R.M., Cheung K.Y., Choi P.H., Kwan W.H.. et al. (2004) Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 60, 1440–1450 10.1016/j.ijrobp.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 7.Lu T.X. (2004) Advance in diagnosis and management of local recurrent nasopharyngeal carcinoma. Ai Zheng 23, 230–234 [PubMed] [Google Scholar]
- 8.Fountzilas G., Tolis C., Kalogera-Fountzila A., Karanikiotis C., Bai M., Misailidou D.. et al. (2005) Induction chemotherapy with cisplatin, epirubicin, and paclitaxel (CEP), followed by concomitant radiotherapy and weekly paclitaxel for the management of locally advanced nasopharyngeal carcinoma. A Hellenic Cooperative Oncology Group phase II study. Strahlenther. Onkol. 181, 223–230 10.1007/s00066-005-1355-1 [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.I., Han M.H., Cha S.H., Sung M.W., Kim K.H. and Chang K.H. (2003) Nasopharyngeal carcinoma: posttreatment changes of imaging findings. Am. J. Otolaryngol. 24, 224–230 10.1016/S0196-0709(03)00052-8 [DOI] [PubMed] [Google Scholar]
- 10.Jeyakumar A., Brickman T.M., Jeyakumar A. and Doerr T. (2006) Review of nasopharyngeal carcinoma. Ear Nose Throat J. 85, 168–184, 10.1177/014556130608500313 [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Shu C., Song Y., Li Q., Huang J. and Ma X. (2016) Epstein-Barr virus DNA level as a novel prognostic factor in nasopharyngeal carcinoma: a meta-analysis. Medicine (Baltimore) 95, e5130 10.1097/MD.0000000000005130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sham J.S., Wei W.I., Kwan W.H., Chan C.W., Kwong W.K. and Choy D. (1990) Nasopharyngeal carcinoma. Pattern of tumor regression after radiotherapy. Cancer 65, 216–220 [DOI] [PubMed] [Google Scholar]
- 13.Chua M., Wee J., Hui E.P. and Chan A. (2016) Nasopharyngeal carcinoma. Lancet 387, 1012–1024 10.1016/S0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
- 14.Lo Y.M., Chan L.Y., Chan A.T., Leung S.F., Lo K.W., Zhang J.. et al. (1999) Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. 59, 5452–5455 [PubMed] [Google Scholar]
- 15.Lo Y.M., Leung S.F., Chan L.Y., Chan A.T., Lo K.W., Johnson P.J.. et al. (2000) Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 60, 2351–2355 [PubMed] [Google Scholar]
- 16.Lin J.C., Wang W.Y., Chen K.Y., Wei Y.H., Liang W.M., Jan J.S.. et al. (2004) Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N. Engl. J. Med. 350, 2461–2470 10.1056/NEJMoa032260 [DOI] [PubMed] [Google Scholar]
- 17.Chan A.T., Lo Y.M., Zee B., Chan L.Y., Ma B.B., Leung S.F.. et al. (2002) Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J. Natl. Cancer Inst. 94, 1614–1619 10.1093/jnci/94.21.1614 [DOI] [PubMed] [Google Scholar]
- 18.Whiting P., Rutjes A.W., Reitsma J.B., Bossuyt P.M. and Kleijnen J. (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 3, 25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J., Wang H., Xiao F. and LIU Y. (2010) The clinical value of plasma EBV DNA, serum cyfra21-1 and vca-iga in monitoring the recurrence and metastasis of nasopharyngeal carcinoma. Chin. Otolaryngol. Head Neck Surg. 2010, 125–127 [Google Scholar]
- 21.Tan J., Liao Y., Cao X., Sun S. and Wu L. (2010) The value of quantitative determination of EB virus in plasma in monitoring recurrence and metastasis of nasopharyngeal carcinoma after radiotherapy. J. Hubei Univ. Nationalities (Medical) 27, 20–21 [Google Scholar]
- 22.Peng J., Liao H., Lin Z. and Xiao M. (2009) Significance of detection of plasma EB virus DNA level in patients with recurrent and metastatic nasopharyngeal carcinoma. Chin. J. Gen. Pract. 2009, 570–571 [Google Scholar]
- 23.Zhu X., Du B., Huang X., Lin S. and Lan Y. (2006) Quantitative detection of EB virus DNA in plasma for the diagnosis and prognosis of nasopharyngeal carcinoma. Chin. J. Otolaryngol. 2006, 73–75 [Google Scholar]
- 24.Sun W. and Jin Y. (2008) Detection and clinical application of plasma EBV-DNA, serum CYFRA21-1 and TSGF in patients with nasopharyngeal carcinoma. J. Modern Oncol. 2008, 537–540 [Google Scholar]
- 25.Ma D., Li Y. and He X. (2011) Clinical value of quantitative detection of EB virus DNA in plasma in the diagnosis and treatment of nasopharyngeal carcinoma. Lab. Med. Clinic 8, 1978–1980 [Google Scholar]
- 26.Liao Y. and Lu Z. (2008) Quantitative determination of EB virus DNA in plasma in patients with nasopharyngeal carcinoma. Chin. Otolaryngol. Head Neck Surg. 2008, 206 [Google Scholar]
- 27.Gong X., Li J., Yuan X., Liao Z., Ao F., Zou X.. et al. (2007) The clinical significance of plasma EBV DNA level in monitoring the recurrence and metastasis of nasopharyngeal carcinoma after radiotherapy. Pract. J. Cancer 2007, 150–153 [Google Scholar]
- 28.Li D., Peng K., Jiang H., Liu J. and Shen X. (2008) The value of plasma EBV DNA quantitative analysis in monitoring the recurrence and metastasis of nasopharyngeal carcinoma. J. Pract. Oncol. 2008, 12–16 [Google Scholar]
- 29.Sun J. and Zheng A. (2008) The clinical significance of plasma EBV DNA level and VCA-IgA in patients with nasopharyngeal carcinoma. J. Modern Oncol. 16, 2086–2087 [Google Scholar]
- 30.Zhang L., Li S., Zhao C., Peng J., Huang P. and Li W. (2004) Relationship between plasma EB virus DNA level and tumor recurrence in patients with nasopharyngeal carcinoma. J. Clin. Oncol. 2004, 122–125 [Google Scholar]
- 31.Ozyar E., Gultekin M., Alp A., Hascelik G., Ugur O. and Atahan I.L. (2007) Use of plasma Epstein-Barr virus DNA monitoring as a tumor marker in follow-up of patients with nasopharyngeal carcinoma: preliminary results and report of two cases. Int. J. Biol. Markers 22, 194–199 10.1177/172460080702200305 [DOI] [PubMed] [Google Scholar]
- 32.Cao S.M., Min H.Q., Gao J.S., Hong M.H., Xiao X.B., Zhang C.Q.. et al. (2003) Significance of cell-free Epstein-Barr virus DNA in monitoring prognosis of nasopharyngeal carcinoma. Ai Zheng 22, 302–306 [PubMed] [Google Scholar]
- 33.Lo Y.M., Chan L.Y., Lo K.W., Leung S.F., Zhang J., Chan A.T.. et al. (1999) Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 59, 1188–1191 [PubMed] [Google Scholar]
- 34.Fan H., Nicholls J., Chua D., Chan K.H., Sham J., Lee S.. et al. (2004) Laboratory markers of tumor burden in nasopharyngeal carcinoma: a comparison of viral load and serologic tests for Epstein-Barr virus. Int. J. Cancer 112, 1036–1041 10.1002/ijc.20520 [DOI] [PubMed] [Google Scholar]
- 35.Jiang W. and Liao Y. (2005) Dynamic study on the relationship between EB virus DNA and nasopharyngeal carcinoma. J. Clin. Otorhinolaryngol. 2005, 920–922 [PubMed] [Google Scholar]
- 36.Kondo S., Horikawa T., Takeshita H., Kanegane C., Kasahara Y., Sheen T.S.. et al. (2004) Diagnostic value of serum EBV-DNA quantification and antibody to viral capsid antigen in nasopharyngeal carcinoma patients. Cancer Sci. 95, 508–513 10.1111/j.1349-7006.2004.tb03241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao J.R., Jin Y.T. and Tsai S.T. (2002) Detection of cell free Epstein-Barr virus DNA in sera from patients with nasopharyngeal carcinoma. Cancer 94, 723–729 [DOI] [PubMed] [Google Scholar]
- 38.Shao J.Y., Li Y.H., Gao H.Y., Wu Q.L., Cui N.J., Zhang L.. et al. (2004) Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 100, 1162–1170 [DOI] [PubMed] [Google Scholar]
- 39.Chang K.P., Hsu C.L., Chang Y.L., Tsang N.M., Chen C.K., Lee T.J.. et al. (2008) Complementary serum test of antibodies to Epstein-Barr virus nuclear antigen-1 and early antigen: a possible alternative for primary screening of nasopharyngeal carcinoma. Oral Oncol. 44, 784–792 10.1016/j.oraloncology.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 40.Chan J.Y. and Wong S.T. (2014) The role of plasma Epstein-Barr virus DNA in the management of recurrent nasopharyngeal carcinoma. Laryngoscope 124, 126–130 10.1002/lary.24193 [DOI] [PubMed] [Google Scholar]
- 41.Ferrari D., Codeca C., Bertuzzi C., Broggio F., Crepaldi F., Luciani A.. et al. (2012) Role of plasma EBV DNA levels in predicting recurrence of nasopharyngeal carcinoma in a western population. BMC Cancer 12, 208. 10.1186/1471-2407-12-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoker S.D., Wildeman M.A., Novalic Z., Fles R., van der Noort V., de Bree R.. et al. (2016) Can Epstein-Barr virus DNA load in nasopharyngeal brushings or whole blood predict recurrent nasopharyngeal carcinoma in a non-endemic region? A prospective nationwide study of the Dutch Head and Neck Oncology Cooperative Group Eur. Arch. Otorhinolaryngol. 273, 1557–1567 10.1007/s00405-015-3620-y [DOI] [PubMed] [Google Scholar]
- 43.Shen T., Tang L.Q., Luo D.H., Chen Q.Y., Li P.J., Mai D.M.. et al. (2015) Different prognostic values of plasma Epstein-Barr virus DNA and maximal standardized uptake value of 18F-FDG PET/CT for nasopharyngeal carcinoma patients with recurrence. PLoS ONE 10, e122756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W.F., Zhang Y., Huang X.B., Du X.J., Tang L.L., Chen L.. et al. (2017) Prognostic value of plasma Epstein-Barr virus DNA level during posttreatment follow-up in the patients with nasopharyngeal carcinoma having undergone intensity-modulated radiotherapy. Chin. J. Cancer 36, 87 10.1186/s40880-017-0256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee A.W., Sze W.M., Au J.S., Leung S.F., Leung T.W., Chua D.T.. et al. (2005) Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 61, 1107–1116 10.1016/j.ijrobp.2004.07.702 [DOI] [PubMed] [Google Scholar]
- 46.Xiao W.W., Han F., Lu T.X., Chen C.Y., Huang Y. and Zhao C. (2009) Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 74, 1070–1076 10.1016/j.ijrobp.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 47.Chan A.T. (2010) Nasopharyngeal carcinoma. Ann. Oncol. 21, i308–i312 10.1093/annonc/mdq277 [DOI] [PubMed] [Google Scholar]
- 48.Ahn P.H. and Garg M.K. (2008) Positron emission tomography/computed tomography for target delineation in head and neck cancers. Semin. Nucl. Med. 38, 141–148 10.1053/j.semnuclmed.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 49.Connell C.A., Corry J., Milner A.D., Hogg A., Hicks R.J., Rischin D.. et al. (2007) Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck 29, 986–995 10.1002/hed.20629 [DOI] [PubMed] [Google Scholar]
- 50.Liu F., Xi X.P., Wang H., Han Y.Q., Xiao F., Hu Y.. et al. (2017) PET/CT-guided dose-painting versus CT-based intensity modulated radiation therapy in locoregional advanced nasopharyngeal carcinoma. Radiat. Oncol. 12, 15 10.1186/s13014-016-0739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S.H., Chang J.T., Ng S.H., Chan S.C. and Yen T.C. (2004) False positive fluorine-18 fluorodeoxy-D-glucose positron emission tomography finding caused by osteoradionecrosis in a nasopharyngeal carcinoma patient. Br. J. Radiol. 77, 257–260 10.1259/bjr/69516821 [DOI] [PubMed] [Google Scholar]
- 52.Mutirangura A., Pornthanakasem W., Theamboonlers A., Sriuranpong V., Lertsanguansinchi P., Yenrudi S.. et al. (1998) Epstein-Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin. Cancer Res. 4, 665–669 [PubMed] [Google Scholar]
- 53.Shotelersuk K., Khorprasert C., Sakdikul S., Pornthanakasem W., Voravud N. and Mutirangura A. (2000) Epstein-Barr virus DNA in serum/plasma as a tumor marker for nasopharyngeal cancer. Clin. Cancer Res. 6, 1046–1051 [PubMed] [Google Scholar]
- 54.Makitie A.A., Reis P.P., Irish J., Zhang T., Chin S.F., Chen X.. et al. (2004) Correlation of Epstein-Barr virus DNA in cell-free plasma, functional imaging and clinical course in locally advanced nasopharyngeal cancer: a pilot study. Head Neck 26, 815–822 10.1002/hed.20028 [DOI] [PubMed] [Google Scholar]