Abstract
Purpose
Many studies have reported that pollen-food allergy syndrome (PFAS) can cause anaphylaxis. No comprehensive investigations into anaphylaxis in PFAS have been conducted, however. In this study, we investigated the clinical manifestations and risk factors for anaphylaxis in PFAS in Korean patients with pollinosis.
Materials and Methods
Data were obtained from a nationwide cross-sectional study that previously reported on PFAS in Korean patients with pollinosis. Data from 273 patients with PFAS were collected, including demographics, list of culprit fruits and vegetables, and clinical manifestations of food allergy. We analyzed 27 anaphylaxis patients and compared them with patients with PFAS with oropharyngeal symptoms only (n=130).
Results
The most common cause of anaphylaxis in PFAS was peanut (33.3%), apple (22.2%), walnut (22.2%), pine nut (18.5%), peach (14.8%), and ginseng (14.8%). Anaphylaxis was significantly associated with the strength of sensitization to alder, hazel, willow, poplar, timothy, and ragweed (p<0.05, respectively). Multivariable analysis revealed that the presence of atopic dermatitis [odds ratio (OR), 3.58; 95% confidence interval (CI), 1.25–10.23; p=0.017]; sensitization to hazel (OR, 5.27; 95% CI, 1.79–15.53; p=0.003), timothy (OR, 11.8; 95% CI, 2.70–51.64; p=0.001), or ragweed (OR, 3.18; 95% CI, 1.03–9.87; p=0.045); and the number of culprit foods (OR, 1.25; 95% CI, 1.15–1.37; p<0.001) were related to the development of anaphylaxis in PFAS.
Conclusion
The most common culprit foods causing anaphylaxis in PFAS were peanut and apple. The presence of atopic dermatitis; sensitization to hazel, timothy, or ragweed; and a greater number of culprit foods were risk factors for anaphylaxis in PFAS.
Keywords: Pollen-food allergy syndrome, pollen, food allergy, anaphylaxis
INTRODUCTION
Pollen-food allergy syndrome (PFAS) is an immunoglobulin E (IgE)-mediated allergic manifestation to fruits and vegetables due to cross-reactivity with prior sensitization to plant inhalant allergens. PFAS is an emerging public health issue, with a number of studies reporting an increased prevalence of pollen allergies over the last decade due to changes in atmospheric CO2, climate, and pollen counts.1,2,3 PFAS is the most common food allergy in adults, and the prevalence of PFAS varies in the literature from 5–8%.4,5
PFAS, called oral allergy syndrome previously, was thought to be restricted to oropharyngeal symptoms; however, extr-aoral symptoms and systemic symptoms in PFAS have been reported.6,7,8 Moreover, one study reported that 3% of patients experienced systemic reactions without oral symptoms, and 1.7% experienced anaphylaxis.6,9 Because the systemic symptoms reported by researchers have increased and because symptoms are not limited to the oral cavity, the use of the term PFAS is more relevant than oral allergy syndrome:10 This historical background has led to confusion among allergists concerning the diagnosis of PFAS. In a US study investigating the perception of PFAS, allergists estimated that 5–8% of patients with pollinosis had PFAS.4 However, PFAS has been reported in 20–70% of patients with a pollen allergy, and anaphylaxis was reported in 1–2%, which is more prevalent than what allergists had estimated.7,8,11
Although the importance of PFAS is increasing and triggering foods differ from geographic regions and dietary habits, there are only a few studies on PFAS in Korea.12,13 The first nationwide study of PFAS in Korea recently reported that the prevalence of PFAS is 41.7% in Korean patients with pollinosis and that 8.9% of patients with PFAS manifest with anaphylaxis, which is a substantial proportion among patients with PFAS.14
Several allergen components from plant foods are known to contribute to the development of anaphylaxis. Lipid transfer protein and cross-reactive carbohydrate determinants are known as pan-allergens of the plant, and they exhibit thermostability and resistance to proteolysis, which enables the food allergen to reach intestinal absorption in its intact form.15 Although several studies on anaphylaxis-inducing allergenic components have been investigated, studies on detailed clinical manifestations and risk factor analysis of anaphylaxis in PFAS have been rarely conducted worldwide. Therefore, in this study, we investigated the clinical characteristics and risk factors of anaphylaxis in PFAS among Korean patients with pollinosis using data from a previous nationwide survey of PFAS in 2016.14
MATERIALS AND METHODS
Study design and participants
The nationwide, cross-sectional study on PFAS was conducted in South Korea between March and December 2016.14 Data were collected from patients diagnosed with pollinosis at 21 institutes (19 university hospitals and two allergy clinics). The protocol was reviewed and approved by the institutional review boards of each institute (Hallym University Dongtan Sacred Heart Hospital, HDT-2016-04-155-003, etc.). Written informed consent was obtained from each patient or their parents.
Pollinosis was diagnosed according to 1) one or more allergic disease, including allergic rhinitis, allergic conjunctivitis, and/or bronchial asthma; 2) sensitization to the pollen of ≥one tree, grass, and/or weed; and 3) aggravated allergic symptoms when exposed to sensitized pollens. Sensitization to pollen was diagnosed by positive results to allergy skin tests and/or high serum-specific IgE levels using multiple allergen simultaneous tests [class ≥2+, Polycheck Allergy (Biocheck Co., Munster, Germany), AdvanSure Allergy Screen (LG Life Science, Seoul, Korea), AllergyScreen (Mediwiss Analytic GmbH, Moers, Germany)] or the ImmunoCAP® system (≥0.35 kU/L, ThermoFisher Scientific, Uppsala, Sweden). A positive skin prick test was defined as wheal size equal or greater to that of histamine [allergen/histamine (A/H) ratio ≥3+, Allergopharma (Reinbek, Germany), Lofarma (Milan, Italy), Bencard (Brefod, UK)] or a mean allergen wheal diameter of at least 3 mm.16 The investigated pollens included tree pollens (birch, alder, hazel, beech, oak, willow, poplar, pine, and tree mix), grass pollens (bermuda, meadow, orchard, rye, timothy, and grass mix), and weed pollens (mugwort, ragweed, and Hop Japanicus). Data were collected using a questionnaire and medical record review, including demographic characteristics, underlying allergic diseases, and allergy test results. For further analysis, we categorized subjects according to the number of systemic symptoms: 1) group 0 (G0), only oropharyngeal symptoms; 2) group 1 (G1), patients with any one systemic symptom; and 3) group 2 (G2) patients with more than two systemic symptoms, that is anaphylaxis.
Questionnaires about PFAS
The list of culprit foods included apple, pear, peach, apricot, plum, cherry, watermelon, melon, Korean melon, banana, kiwi, orange, mandarin, pineapple, strawberry, mango, avocado, grape, carrot, potato, sweet potato, celery, crown daisy, perilla leaf, lettuce, kale, chicory, taro/taro stem, ginseng, deodeok (Codonopsis lanceolata), bellflower root, kudzu, lotus root, Chinese yam, eggplant, zucchini, cucumber, tomato, jujube, chestnut, peanut, walnut, pine nut, and soy.
In addition to oropharyngeal symptoms (tingling/itching sense or edema of the lips, oral cavity, and/or throat), systemic symptoms were categorized according to the organ in which the symptoms developed: dermatologic symptoms (itching, urticaria, or angioedema), respiratory symptoms (rhinorrhea, cough, dyspnea, wheezing, cyanosis, or hypoxia), cardiovascular symptoms (chest pain, hypotension, pale, sweating, or cardiac arrest), gastrointestinal symptoms (nausea or vomiting, diarrhea, or abdominal pain), neurologic and systemic symptoms (dizziness, unconsciousness, anxiety, change of sense, or death), and anaphylaxis. The diagnosis of anaphylaxis was confirmed by a physician using the criteria proposed in the second symposium on the definition and management of anaphylaxis by the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network.17 Anaphylaxis was defined by two or more of the following symptoms occur after exposure to a likely allergen, including involvement of skin tissue, respiratory compromise, cardiovascular symptoms, gastrointestinal symptoms, and neurologic symptoms.
Statistical analysis
All statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria). Comparison between groups was performed using chi-squared analysis and Kruskal-Wallis test (>two independent groups) for discrete and continuous variables, respectively. Because the use of separate univariate tests leads to an inflated type 1 error, Bonferroni correction was applied to each pollen analysis. Multivariate logistic regression was utilized to determine potential predictors of anaphylaxis in PFAS. p<0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study subjects
Of 273 patients with PFAS, 130 (47.6%) reported oropharyngeal symptoms only, 88 (32.2%) showed oropharyngeal symptoms and one systemic symptom, and 27 (9.9%) showed anaphylaxis. Of 27 patients with anaphylaxis, 21 (77.8%) had oropharyngeal symptoms, and 6 (22.2%) experienced systemic reactions without oral symptoms. The patients showed cutaneous manifestations (84.0%), such as pruritus, urticaria and angioedema; respiratory (84.0%); gastrointestinal (48.0%); neurologic (36.0%); and cardiovascular (36.0%) symptoms. Twelve patients (44.4%) were younger than 18 years, and 15 were older than 18 years (Supplementary Table 1, only online).
Clinical characteristics of anaphylaxis patients with PFAS
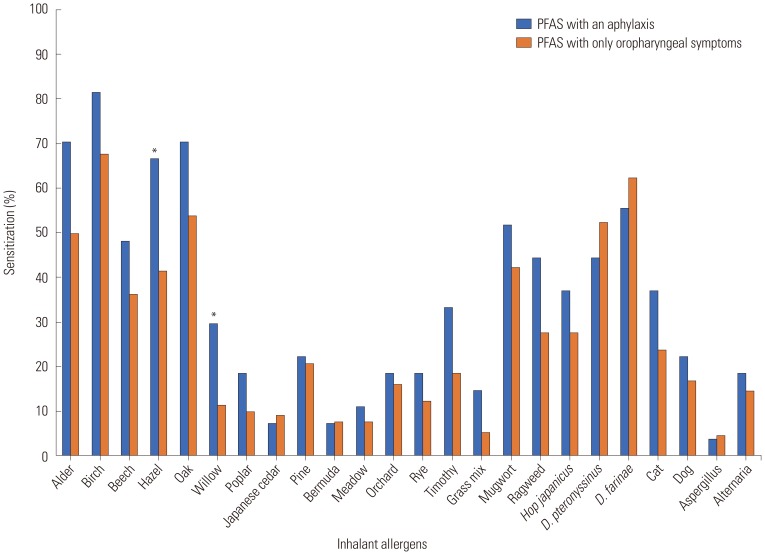
The clinical characteristics of the patients with anaphylaxis in PFAS are described in Table 1. No differences were found between PFAS with anaphylaxis and only oropharyngeal symptoms groups in terms of sex and age (p>0.05, respectively). The anaphylaxis group had a higher prevalence of developing chronic urticaria than those in the only oropharyngeal symptom group (18.5% vs. 4.6%, p=0.023), as well as a higher prevalence of atopic dermatitis (AD) (48.1% vs. 26.2%, p=0.041). Patients with PFAS with anaphylaxis showed no significant differences in the presence of other allergic diseases, such as bronchial asthma, allergic rhinitis, allergic conjunctivitis, or drug allergy, and family history of allergic diseases, compared with only oropharyngeal symptoms (p>0.05, respectively). The severity and duration of allergic rhinitis symptoms were also not significantly different between the anaphylaxis and only oropharyngeal symptom groups. The patients with PFAS with anaphylaxis had significantly higher sensitization rates to hazel (66.7% vs. 41.5%, p=0.030) and willow (29.6% vs. 11.5%, p=0.031) than those with only oropharyngeal symptoms (Fig. 1).
Table 1. Clinical Characteristics of the Study Subjects with Anaphylaxis in PFAS.
| PFAS with anaphylaxis (n=27) | PFAS with only oropharyngeal symptoms (n=130) | p value | |
|---|---|---|---|
| Sex (male) | 14 (51.9) | 69 (53.1) | 1.000 |
| Age (yr) | 27.2±18.7 | 26.2±16.7 | 0.767 |
| Allergic diseases | |||
| Bronchial asthma | 9 (33.3) | 52 (40) | 0.667 |
| Allergic rhinitis | 27 (100) | 126 (96.9) | 1.000 |
| Allergic conjunctivitis | 17 (63.0) | 75 (57.7) | 0.770 |
| Atopic dermatitis | 13 (48.1) | 34 (26.2) | 0.041 |
| Chronic urticaria | 5 (18.5) | 6 (4.6) | 0.023 |
| Drug allergy | 3 (11.1) | 11 (8.5) | 0.710 |
| Family history of allergic diseases | 21 (77.8) | 98 (75.4) | 0.986 |
| Severity of allergic rhinitis | 0.613 | ||
| Mild | 13 (48.1) | 52 (40.6) | |
| Moderate/severe | 14 (51.9) | 76 (59.4) | |
| Duration of allergic rhinitis | 0.723 | ||
| Intermittent | 13 (48.1) | 54 (42.2) | |
| Persistent | 14 (51.9) | 74 (57.8) |
PFAS, pollen-food allergy syndrome.
Data are presented as mean±standard deviation or number (%) unless otherwise indicated.
Fig. 1. Comparison of allergen sensitization profiles between anaphylaxis (n=27) and only oropharyngeal symptom patients (n=130) with pollen-food allergy syndrome (PFAS). More patients with anaphylaxis were sensitized to hazel and willow than patients with only oropharyngeal symptoms. *p<0.05. D, Dermatophagoides.
Causative foods of anaphylaxis in PFAS
Twenty-seven anaphylaxis patients had 84 cases with 44 culprit foods (Table 2). Peanut (33.3%) was the most common offending food, followed by apple (22.2%), walnut (22.2%), pine nut (18.5%), peach (14.8%), ginseng (14.8%), soy (11.1%), etc. Most of the anaphylaxis patients (n=18, 66.7%) developed anaphylaxis to one food item. Two patients (7.4%) experienced anaphylactic reactions to two food items, three patients to three (11.1%), one patient to four (3.7%), and three patients to more than five food items (11.1%).
Table 2. Causative Foods of Anaphylaxis in Pollen-Food Allergy Syndrome (n=27).
| Foods | No. (%) |
|---|---|
| Peanut | 9 (33.3) |
| Apple | 6 (22.2) |
| Walnut | 6 (22.2) |
| Pine nut | 5 (18.5) |
| Peach | 4 (14.8) |
| Ginseng | 4 (14.8) |
| Soy | 3 (11.1) |
| Eggplant | 2 (7.4) |
| Jujube | 2 (7.4) |
| Chinese yam | 2 (7.4) |
| Buckwheat | 2 (7.4) |
| Chestnut | 2 (7.4) |
| Lotus root | 2 (7.4) |
| Plum | 2 (7.4) |
| Kiwi | 2 (7.4) |
| Taro/Taro stem | 2 (7.4) |
| Pineapple | 2 (7.4) |
| Potato | 1 (3.7) |
| Sweet potato | 1 (3.7) |
| Mandarin | 1 (3.7) |
| Perilla leaf | 1 (3.7) |
| Carrot | 1 (3.7) |
| Deodeok (Codonopsis lanceolata) | 1 (3.7) |
| Bellflower root | 1 (3.7) |
| Perilla | 1 (3.7) |
| Strawberry | 1 (3.7) |
| Garlic | 1 (3.7) |
| Melon | 1 (3.7) |
| Fig | 1 (3.7) |
| Banana | 1 (3.7) |
| Pear | 1 (3.7) |
| Apricot | 1 (3.7) |
| Celery | 1 (3.7) |
| Watermelon | 1 (3.7) |
| Crown daisy | 1 (3.7) |
| Yacon | 1 (3.7) |
| Mulberry | 1 (3.7) |
| Grape fruit | 1 (3.7) |
| Korean melon | 1 (3.7) |
| Cherry | 1 (3.7) |
| Tomato | 1 (3.7) |
| Grape fruit | 1 (3.7) |
| Pistachio | 1 (3.7) |
| Rye | 1 (3.7) |
Data are presented as number (%).
Risk factors for the development of anaphylaxis in PFAS
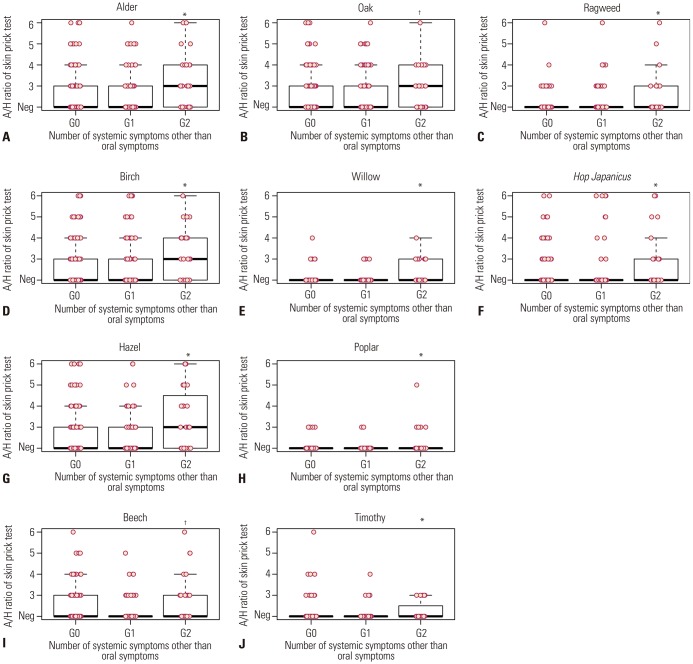
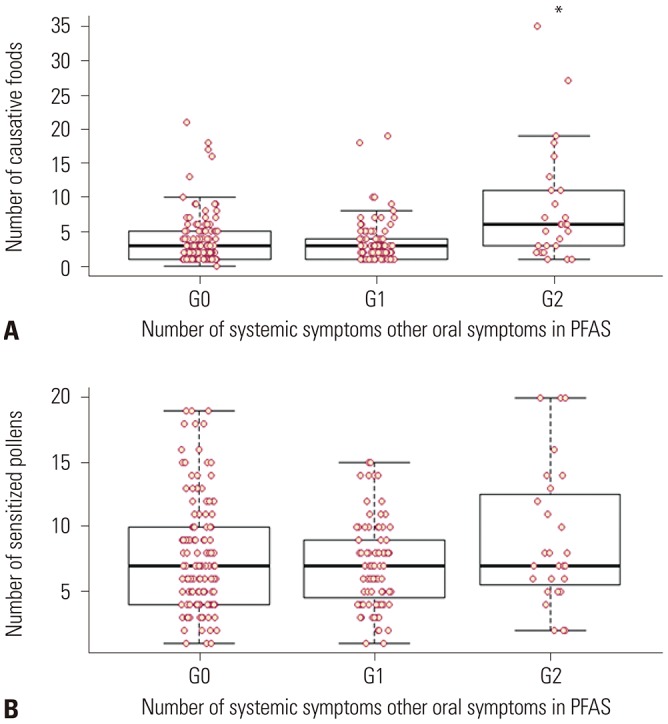
After analysis of the association of systemic symptoms in PFAS with the strength of sensitization to pollen (A/H ratio by skin prick test), a significant association was found between anaphylaxis (G2) and strength of sensitization to alder, birch, hazel, beech, oak, willow, poplar, timothy, ragweed, and Hop Japanicus (Fig. 2). Moreover, the anaphylaxis patients (G2) had a significantly higher number of causative foods (p<0.05), whereas the number of sensitized pollens was not associated with anaphylaxis in PFAS (Fig. 3).
Fig. 2. Associations between the number of systemic symptoms other than oral symptoms in pollen-food allergy syndrome and the strength of sensitization to pollen by allergy skin tests. Significant association was found between anaphylaxis and strength of pollen sensitization (A, C, D, E, F, G, H, J: p<0.05 in G2 vs. G0, G1) (B, I: p<0.05 in G2 vs. G1). X-axis, number of systemic symptoms other than oropharyngeal symptoms, G0: only oropharyngeal symptoms, G1: one systemic symptom, G2: anaphylaxis, Y-axis, strength of sensitization (A/H ratio: Allergen/Histamine ratio). *p<0.05 in G2 vs. G0, G1, respectively; †p<0.05 in G2 vs. G1. Neg, negative skin prick test.
Fig. 3. Associations between the number of systemic symptoms other than oropharyngeal symptoms in pollen-food allergy syndrome (PFAS) and the number of causative pollen-related foods (A) and between the number of systemic symptoms other than oropharyngeal symptoms and the number of sensitized pollen (B). Anaphylaxis (G2) was significantly associated with a higher number of pollen-related foods (p<0.05), but was not associated with the number of sensitized pollens (p>0.05). X-axis, number of systemic symptoms other than oropharyngeal symptoms, G0: only oropharyngeal symptom, G1: one systemic symptom, G2: anaphylaxis. *p<0.05 in G2 vs. G0, G1, respectively.
Multivariate regression analysis revealed that the presence of AD [odds ratio (OR), 3.58; 95% confidence interval (CI), 1.25–10.23; p=0.017], sensitization to hazel (OR, 5.27; 95% CI, 1.79–15.53; p=0.002), sensitization to timothy (OR, 11.8; 95% CI, 2.70–51.64; p=0.001), sensitization to ragweed (OR, 3.18; 95% CI, 1.03–9.87; p=0.045), and number of culprit foods of PFAS (OR, 1.25; 95% CI, 1.15–1.37; p<0.001) were potential risk factors for the development of anaphylaxis in PFAS (Table 3).
Table 3. Multivariate Analysis of Risk Factors for the Development of Anaphylaxis in Pollen-Food Allergy Syndrome.
| Predictor | OR | 95% CI | p value |
|---|---|---|---|
| Presence of atopic dermatitis | 3.58 | 1.25–10.23 | 0.017 |
| Sensitization to hazel | 5.27 | 1.79–15.53 | 0.002 |
| Sensitization to timothy | 11.8 | 2.70–51.64 | 0.001 |
| Sensitization to ragweed | 3.18 | 1.03–9.87 | 0.045 |
| The number of culprit foods | 1.25 | 1.15–1.37 | <0.001 |
OR, odds ratio; CI, confidence interval.
Sensitization was diagnosed by allergy skin tests.
DISCUSSION
In this study, the most commonly offending foods of anaphylaxis in PFAS were peanut, followed by apple, walnut, pine nut, peach, ginseng, and soy in order of frequency. Anaphylaxis was significantly associated with the strength of sensitization to alder, birch, hazel, willow, timothy, and ragweed pollen. Presence of AD; sensitization to hazel, timothy, and ragweed; and the increased number of culprit foods for PFAS would more likely lead to the development of anaphylaxis in PFAS.
Approximately 20% to 70% of patients with pollinosis reported symptoms of PFAS after ingesting causative foods,7,8,11 and the prevalence of anaphylaxis in patients with PFAS is estimated to be 1–2%.6 In our previous reports, the prevalences of PFAS and anaphylaxis in PFAS were 41.7% and 8.9% in Korea, respectively.14 The prevalence of PFAS in our country was similar to that in previous reports for other countries, while the prevalence of anaphylaxis was much higher. This might be due to several reasons, such as 1) anaphylaxis in PFAS might be overlooked as class I food allergy by physicians; 2) the definition of anaphylaxis was broadly defined, including more than two organ involvements; and 3) the number of patients with PFAS might be increased when considering the increasing pollen allergic populations due to global climate changes and increases in pollen counts.17,18
In the present study, the most common anaphylaxis-triggering foods in PFAS were peanut, followed by apple, walnut, pine nut, peach, ginseng, and soy, whereas the most common causative foods of PFAS in Korea were peach, apple, kiwi, peanut, and plum.14 Although common foods that are causative of anaphylaxis in PFAS have not been reported in other countries, apple and hazelnut are the most common causative foods of PFAS because of the high rate of birch sensitization in Europe, and apple and peach are known as the most common causative foods in relation to oak sensitization in Japan.19,20,21 In Korea, the most important sensitized pollens are oak, followed by birch and alder, which have cross-allergenicity with the Rosaceae family (apple, peach, plum, pear, cherry, apricot, almond, etc.), Apiaceae family (cantaloupe, honeydew, watermelon, zucchini, and cucumber), Fabaceae family (soybean and peanut), Juglans family (walnut), and Betulaceae family (hazelnut).9,18,22,23 Accordingly, cross-reactivity patterns between pollens and foods vary depending on regional distribution of inhaled pollens and dietary habits. Relatedly, hazelnut anaphylaxis has not been reported in Korea, potentially in reflection of dietary customs of not consuming hazelnut, whereas ginseng is a frequently consumed herbal medication in Far-East Asia. Lim, et al.24 reported a case of anaphylaxis after ingestion of fresh ginseng, wherein they found 17-kDa common allergens between birch pollen and ginseng using immunoblot analysis. Furthermore, Kim, et al.25 reported that 45% of birch pollen-sensitized patients with allergic rhinitis show positive responses to skin tests with raw Korean ginseng extracts, suggesting cross-allergenicity between birch and ginseng. Interestingly, all of the anaphylaxis cases to ginseng were sensitized to birch pollen in our study. However, there has been no study about cross-allergenicity between pollens and other local foods, such as jujube, lotus root, crown daisy, yacon, and mulberry. Further research will be needed.
In this study, the most frequent causative food of anaphylaxis in PFAS was peanut. Sensitization to seed storage allergenic components (Ara h 1, 2, 3, and 6) in peanut suggests the possibility of class I food allergy, whereas isolated specific IgE to pathogenesis-related protein PR-10 family members, such as Bet v1 (birch), Mal d 1 (apple), Cor a 1 (hazelnut), Gly m 1(soybean), or Ara h 8 (peanut), usually causes PFAS without systemic symptoms.26 Lipid transfer proteins, including Art v 3 (mugwort), Ara h 9 (peanut), Pru p 3 (peach), Mal d 3 (apple), and Gly m 1 (soybean), frequently are attributed to the development of anaphylaxis in PFAS.27 However, this was a questionnaire-based epidemiological study, and allergenic components analysis was not performed. Ara h 9 (peanut) or other unknown allergenic components might contribute to the development of anaphylaxis in our study subjects. Further studies will be needed to elucidate which allergenic components of pollens and foods are related to the development of anaphylaxis in PFAS in these study subjects.
The risk factors of anaphylactic reactions in PFAS have been previously reported, such as systemic reactions to one of the associated foods and positive skin test results with commercial extracts.28 In our study, the strength of sensitization to skin tests to alder, birch, hazel, willow, timothy, and ragweed was associated with development of anaphylaxis in PFAS. Furthermore, the sensitization to specific pollens, such as hazel, timothy, and ragweed, was an independent risk factor of anaphylaxis in PFAS. Eriksson, et al.29 have reported a positive correlation between the size of the skin reaction to birch pollen and the incidence of hypersensitivity to PFAS; however, the association between the strength of pollen sensitization and anaphylaxis in PFAS has not been reported to date. In this study, the presence of AD and a higher number of culprit foods were also important risk factors for anaphylaxis in PFAS. AD has been reported as an important risk factor in a study with recurrent anaphylaxis patients.30 Although the exact mechanism has not been elucidated, researchers have suggested that patients with AD have more activated mast cells and basophil cells.31
Currently, many clinicians maintain that PFAS does not cause anaphylaxis.4 Considering our study results with a higher prevalence of anaphylaxis in PFAS than that which has been reported, clinicians must be aware of the potential risks generated by PFAS and consider this when making a decision for risk management. If pollinosis patients have multiple pollen-related food allergies and strong positive responses on skin tests to hazel, timothy, or ragweed, the culprit foods may be related to the development of anaphylaxis. In addition, if patients have systemic symptoms or an anaphylactic reaction, allergists should educate the patients to avoid potential culprit foods, and they should not hesitate to prescribe self-injectable epinephrine.
This study has several limitations. First, this study was conducted as a questionnaire-based study depending on the patient's recollection and clinical history. Double-blind, placebo-controlled food challenge (DBPCFC), a gold standard for the diagnosis of food allergy, was not performed for diagnosis of PFAS in this study. Although DBPCFC is the gold standard for diagnosis of food allergy, careful clinical history in patients with pollinosis could replace provocation tests for diagnosis of PFAS because the excipient and taste covering for DBPCFC may cause the loss of the allergenic properties. Moreover, component-resolved diagnosis has yet to be prevalent for the diagnosis of PFAS. Therefore, in this study, PFAS was defined by typical oropharyngeal symptoms, including systemic symptoms, with raw fruits and/or vegetables and positive results of allergy tests to pollens. Second, this study was conducted by voluntarily participating hospitals, and the possibility of selection bias cannot be excluded. Because most of the contributing hospitals are referral hospitals, the prevalence of PFAS and anaphylaxis was possibly higher due to the involvement of more severe pollinosis cases. Third, all investigators did not use same allergy skin test solutions for diagnosis of pollen allergy, which are not standardized between manufacturers. This might affect the results of strength of pollen sensitization. Fourth, the relationship between specific allergenic components and the risk of anaphylaxis has not been studied. Component-resolved diagnosis could also help differentiating class I food allergy and PFAS, especially in case of peanut allergy. Further studies are needed.
In conclusion, the most frequently associated foods with anaphylaxis in PFAS were peanut, apple, walnut, pine nut, peach, ginseng, and soy in Korea. The presence of AD, strong sensitization to specific pollens, such as hazel, timothy, and ragweed, and a higher number of pollen-related foods significantly increased the risk of anaphylaxis in patients with PFAS, compared to patients with only oropharyngeal symptoms. Allergists should inform their patients that pollen-related foods can cause anaphylaxis and prescribe self-injectable epinephrine to high-risk patients.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Academy of Asthma, Allergy, and Clinical Immunology (2016).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jeong-Hee Choi and Dae Hyun Lim.
- Data curation: Dong-Kyu Kim, Young-Il Koh, Jaechun Lee, Sang Min Lee, and Yang Park, Work Group for Rhinitis, the Korean Academy of Asthma, Allergy and Clinical Immunology.
- Formal analysis: Dong-Kyu Kim, Hyo Yeol Kim, and Hyun Jong Lee.
- Funding acquisition: Mi-Ae Kim, Young Joon Jun, and Bong-Seong Kim.
- Investigation: Hyun Jong Lee, Bong-Seong Kim, Yong Min Kim, and Woo Yong Bae.
- Methodology: Dong-Kyu Kim, Woo Yong Bae, Yunsun Kim, Jeong Hee Kim, and Yang Park.
- Project administration: Dong-Kyu Kim and Jaechun Lee.
- Resources: Young-Il Koh, Dae Hyun Lim, and Hyo Yeol Kim.
- Software: Hae-Sim Park, Mi-Ae Kim, and An-Soo Jang.
- Supervision: Hae-Sim Park, Young Yoo, and An-Soo Jang.
- Validation: Hyeon-Jong Yang and Yi Yeong Jeong.
- Visualization: Hyeon-Jong Yang, Yunsun Kim, and Yi Yeong Jeong.
- Writing—original draft: Minji Kim and Youngmin Ahn.
- Writing—review & editing: Young Yoo and Jeong-Hee Choi.
SUPPLEMENTARY MATERIAL
Causative Foods and Sensitized Pollens in Pollen-Food Allergy Syndrome Patients with Anaphylaxis
References
- 1.Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D ISAAC Phase III Study Group. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–124. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 2.Yura A, Kouda K, Iki M, Shimizu T. Trends of allergic symptoms in school children: large-scale long-term consecutive cross-sectional studies in Osaka Prefecture, Japan. Pediatr Allergy Immunol. 2011;22:631–637. doi: 10.1111/j.1399-3038.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Park HS, Jang JY. Impact of meteorological variation on hospital visits of patients with tree pollen allergy. BMC Public Health. 2011;11:890. doi: 10.1186/1471-2458-11-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003;112:784–788. doi: 10.1016/s0091-6749(03)02008-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee SH, Ban GY, Jeong K, Shin YS, Park HS, Lee S, et al. A retrospective study of Korean adults with food allergy: differences in phenotypes and causes. Allergy Asthma Immunol Res. 2017;9:534–539. doi: 10.4168/aair.2017.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortolani C, Pastorello EA, Farioli L, Ispano M, Pravettoni V, Berti C, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy. 1993;71:470–476. [PubMed] [Google Scholar]
- 7.Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014;50:795–800. doi: 10.1111/jpc.12658. [DOI] [PubMed] [Google Scholar]
- 8.Flores E, Cervera L, Sanz ML, Diaz-Perales A, Fernández J. Plant food allergy in patients with pollinosis from the Mediterranean area. Int Arch Allergy Immunol. 2012;159:346–354. doi: 10.1159/000338282. [DOI] [PubMed] [Google Scholar]
- 9.Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol. 2001;108:881–890. doi: 10.1067/mai.2001.118515. [DOI] [PubMed] [Google Scholar]
- 10.Mari A, Ballmer-Weber BK, Vieths S. The oral allergy syndrome: improved diagnostic and treatment methods. Curr Opin Allergy Clin Immunol. 2005;5:267–273. doi: 10.1097/01.all.0000168793.27948.b0. [DOI] [PubMed] [Google Scholar]
- 11.Osterballe M, Hansen TK, Mortz CG, Høst A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 12.Kong N, Kim S, Lee SC, Park KH, Lee JH, Park JW. Subcutaneous immunotherapy in patients with Fagales pollen-induced oral allergy syndrome. Yonsei Med J. 2019;60:389–394. doi: 10.3349/ymj.2019.60.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Kim SH, Park HW, Cho SH, Chang YS. Oral allergy syndrome in birch pollen-sensitized patients from a Korean University Hospital. J Korean Med Sci. 2018;33:e218. doi: 10.3346/jkms.2018.33.e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim MA, Kim DK, Yang HJ, Yoo Y, Ahn Y, Park HS, et al. Pollenfood allergy syndrome in Korean pollinosis patients: a nationwide survey. Allergy Asthma Immunol Res. 2018;10:648–661. doi: 10.4168/aair.2018.10.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann A, Burks AW. Pollen food syndrome: update on the allergens. Curr Allergy Asthma Rep. 2008;8:413–417. doi: 10.1007/s11882-008-0080-0. [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 18.Park HJ, Lim HS, Park KH, Lee JH, Park JW, Hong CS. Changes in allergen sensitization over the last 30 years in Korea respiratory allergic patients: a single-center. Allergy Asthma Immunol Res. 2014;6:434–443. doi: 10.4168/aair.2014.6.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda N, Inomata N, Morita A, Kirino M, Ikezawa Z. Correlation of oral allergy syndrome due to plant-derived foods with pollen sensitization in Japan. Ann Allergy Asthma Immunol. 2010;104:205–210. doi: 10.1016/j.anai.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 20.Katelaris CH. Food allergy and oral allergy or pollen-food syndrome. Curr Opin Allergy Clin Immunol. 2010;10:246–251. doi: 10.1097/ACI.0b013e32833973fb. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Rivas M, Bolhaar S, González-Mancebo E, Asero R, van Leeuwen A, Bohle B, et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006;118:481–488. doi: 10.1016/j.jaci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Hahm MI, Lee SY, Kim WK, Chae Y, Park YM, et al. Sensitization to aeroallergens in Korean children: a population-based study in 2010. J Korean Med Sci. 2011;26:1165–1172. doi: 10.3346/jkms.2011.26.9.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wangorsch A, Jamin A, Lidholm J, Gräni N, Lang C, Ballmer-Weber B, et al. Identification and implication of an allergenic PR-10 protein from walnut in birch pollen associated walnut allergy. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600902. [DOI] [PubMed] [Google Scholar]
- 24.Lim KH, Kang MK, Kim BK, Kim MH, Park HK, Lim JA, et al. A case of anaphylaxis after ingestion of freshginseng. Korean J Asthma Allergy Clin Immunol. 2010;30:131–134. [Google Scholar]
- 25.Kim M, Choi E, Yoon T. Korean ginseng makes oral allergy syndrome in birch-sensitized respiratory allergy patients. J Allergy Clin Immunol. 2008;121:S187. [Google Scholar]
- 26.Stiefel G, Anagnostou K, Boyle RJ, Brathwaite N, Ewan P, Fox AT, et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin Exp Allergy. 2017;47:719–739. doi: 10.1111/cea.12957. [DOI] [PubMed] [Google Scholar]
- 27.Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106(1 Pt 1):27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- 28.Muluk NB, Cingi C. Oral allergy syndrome. Am J Rhinol Allergy. 2018;32:27–30. doi: 10.2500/ajra.2018.32.4489. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson NE, Formgren H, Svenonius E. Food hypersensitivity in patients with pollen allergy. Allergy. 1982;37:437–443. doi: 10.1111/j.1398-9995.1982.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Bashore C, Lohse CM, Bellolio MF, Chamberlain A, Yuki K, et al. Rate of recurrent anaphylaxis and associated risk factors among Olmsted County, Minnesota, residents: a population-based study. Ann Allergy Asthma Immunol. 2016;117:655–660. doi: 10.1016/j.anai.2016.09.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis. A review of 266 cases. Arch Intern Med. 1995;155:1749–1754. doi: 10.1001/archinte.155.16.1749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Causative Foods and Sensitized Pollens in Pollen-Food Allergy Syndrome Patients with Anaphylaxis