Abstract
Purpose
Few efforts have been made to integrate a next generation sequencing (NGS) panel into standard clinical treatment of ovarian cancer. The aim of this study was to investigate the clinical utility of NGS and to identify clinically impactful information beyond targetable alterations.
Materials and Methods
We conducted a retrospective review of 84 patients with ovarian cancer who underwent NGS between March 1, 2017, and July 31, 2018, at the Yonsei Cancer Hospital. We extracted DNA from formalin-fixed, paraffin-embedded tissue samples of ovarian cancer. The TruSight Tumor 170 gene panel was used to prepare libraries, and the MiSeq instrument was used for NGS.
Results
Of the 84 patients, 55 (65.1%) had high-grade serous carcinomas. Seventy-three (86.7%) patients underwent NGS at the time of diagnosis, and 11 (13.3%) underwent NGS upon relapse. The most common genetic alterations were in TP53 (64%), PIK3CA (15%), and BRCA1/2 (13%), arising as single nucleotide variants and indels. MYC amplification (27%) was the most common copy number variation and fusion. Fifty-seven (67.9%) patients had more than one actionable alteration other than TP53. Seven (8.3%) cases received matched-target therapy based on the following sequencing results: BRCA1 or 2 mutation, poly ADP ribose polymerase inhibitor (n=5); PIK3CA mutation, AKT inhibitor (n=1); and MLH1 mutation, PD-1 inhibitor (n=1). Fifty-three (63.0%) patients had a possibility of treatment change, and 8 (9.5%) patients received genetic counseling.
Conclusion
Implementation of NGS may help in identifying patients who might benefit from targeted treatment therapies and genetic counseling.
Keywords: Next generation sequencing, ovarian cancer, targetable alterations
INTRODUCTION
Epithelial ovarian cancer is the leading cause of disease-related deaths from gynecologic malignancies, and its incidence and mortality rates in Korea are increasing.1,2 In addition to well-known prognostic factors, such as stage, histology, grade, and residual disease after surgery,3 clinical studies are underway to identify potentially actionable mutations in ovarian cancer through a greater understanding of molecular mechanisms and to evaluate therapeutic agents of these mutations.4,5
Next generation sequencing (NGS) is able to reveal genomic aberrations by harnessing its massively parallel sequencing capability to analyze multiple genes simultaneously in a single assay. Moreover, this technology has recently become more affordable, leading to large collaborative studies on whole genomes that have been able to document targetable genes and predictive biomarkers in cancer.6,7 As of March 2017, the National Health Insurance system in Korea has paid the cost of NGS panels for several types of solid tumors, including ovarian cancer, and the number of NGS tests has increased exponentially.
We reviewed retrospective data of 84 patients who underwent NGS and reported our experiences with integrating an NGS panel into clinical practice in ovarian cancer. We identified potentially actionable genomic alterations and used them to evaluate the therapeutic utility of individual treatment options.
MATERIALS AND METHODS
Patient samples
Between March 1, 2017 and July 31, 2018, 84 tumor samples from ovarian cancer patients treated at Yonsei Cancer Center were subjected to NGS. The tumor samples were prepared from formalin-fixed, paraffin-embedded (FFPE) tissues. An expert pathologist (H.S.K.) reviewed hematoxylin and eosinstained slides to ensure that ≥20% of the nucleated cells in the sample were derived from the tumor. Tumor specimens were macrodissected after a hematoxylin-eosin reference slide check to ensure the proportion of tumor content. For DNA and RNA extraction, two to five slides of resected specimens of a thickness of 5 µm were needed. A board-certified gynecological pathologist diagnosed all cases. We performed a retrospective review of patient medical records, including age, histologic type, stage as defined by the International Federation of Gynecology and Obstetrics, and the timing of the NGS test.
NGS
We performed NGS analysis of 84 FFPE cancers with sufficiently high tumor cellularity (>30%). Genomic DNA was extracted using a Maxwell CSC DNA FFPE Kit (Promega, Madison, WI, USA), according to the manufacturer's instructions. The products were sequenced on a MiSeq System (Illumina, San Diego, CA, USA). Mutational and copy number analyses were performed using a TruSight Tumor 170 panel (Illumina) that covers, respectively, 170 genes and 59 genes for mutational and copy number analyses (Supplementary Table 1, only online). For mutational analysis, FASTQ files were uploaded on the Illumina BaseSpace software (Illumina) for variant interpretation. Only variants in coding regions and promoter regions or splice variants were retained. In addition, we retained only variants present in <1% of the population, according to ExAC and 1000 genomes, and also present in >5% of reads with a minimum read depth of 250. All retained variants were reviewed against reference websites [Catalogue of Somatic Mutations in Cancer (http://evs.gs.washington.edu/EVS/), Precision Oncology Knowledge Base (http://oncokb.org), and dbSNP (https://www.ncbi.nlm.nih.gov/snp)]. Only pathogenic variants were selected. In copy number analysis, only genes with a more than two-fold change relative to the average level were considered for amplification. We also performed total nucleic acid extraction to obtain ribonucleic acid (RNA). An Archer FusionPlex Solid Tumor Kit (ArcherDx, Boulder, CO, USA) was used to analyze the RNA for fusions and splice variants: the kit covers 55 genes.8 Specimens yielded more than 40 ng of DNA and RNA. DNA fragment sizes of at least 79 bp and RNA fragment sizes of at least 63 bp were selected for targeted sequencing. Our goal in this study was to assess the feasibility and utility of using the Illumina MiSeq platform to integrate a NGS panel into a real-world setting of ovarian cancer clinical practice.
Data interpretation
Actionable somatic alterations are defined as those that could be targeted by a drug available for on-label, off-label, or in clinical trials. These alterations were selected based on a literature search of the MD Anderson Knowledge Base for Precision Medicine (http://PCT.MDAnderson.org), The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/), and genes related to homologous recombination repair (HRR) (Supplementary Table 2, only online).
We used the following guidelines to classify these alterations into four tiers.9 Tier 1 comprised known tumor type-specific actionable somatic mutations of confirmed clinical utility in predicting responses to U.S. Food and Drug Administration (FDA)-approved therapies, prognoses, diagnoses, or increased risk of inherited cancer. Tier 2 included actionable somatic mutations in other tumor types or somatic mutations in targetable pathways with potential clinical significance, such as susceptibility to FDA-approved therapies, prognoses, diagnoses, or increased risk of inherited cancer. Alterations of unknown clinical significance were classified as Tier 3, and those considered benign or likely so were grouped in Tier 4.
Clinical implications
We defined the term clinical implication as the capability of NGS results to provide useful information about patients and their family members that could be used to diagnose, monitor, predict the occurrence of disease and to create informed choices about treatment options.10 These clinical implications were categorized into three categories to evaluate the clinical impact of NGS results: 1) those who received targeted therapy; 2) identification of potential candidates for targeted therapy; and 3) genetic counseling for the patient and other at-risk family members.
Ethical statement
This study was approved by the Institutional Review Board of Severance Hospital at Yonsei University College of Medicine (IRB No. 4-2018-0518).
RESULTS
Patient clinicopathologic characteristics
A total of 227 ovarian cancer patients were treated in our institution between March 1, 2017 and July 31, 2018, and 84 (37%) patients underwent NGS analysis. Table 1 illustrates the baseline clinicopathological characteristics of the 84 patients who underwent NGS. The median age of this group was 54 years (range, 34–77). The most common histologic type was high-grade serous carcinoma (65.1%). Sixty-eight (80.7%) patients had advanced-stage disease (Stages III/IV). Forty-nine (57.8%) patients underwent primary debulking surgery (PDS), and 35 (42.2%) underwent neoadjuvant chemotherapy (NAC). Thirty-nine (45.8%) patients underwent NGS during PDS: 8 (9.6%) during pre-NAC, 26 (31.3%) during interval debulking surgery (IDS), and 11 (13.3%) patients at the time of relapse.
Table 1. Clinicopathological Characteristics (n=84).
| Characteristic | Values |
|---|---|
| Age (yr) [median (range)] | 54 (34–77) |
| Histologic type | |
| High grade serous | 55 (65.1) |
| Low grade serous | 4 (4.8) |
| Clear | 10 (12.0) |
| Endometrioid | 6 (7.3) |
| Mucinous | 2 (2.4) |
| Other | 7 (8.4) |
| FIGO stage | |
| I | 12 (14.5) |
| II | 4 (4.8) |
| III | 33 (39.8) |
| IV | 35 (40.9) |
| Treatment type | |
| PDS | 49 (57.8) |
| NAC | 35 (42.2) |
| Tissue tested | |
| PDS | 39 (45.8) |
| Pre-NAC | 8 (9.6) |
| IDS | 26 (31.3) |
| Relapse | 11 (13.3) |
FIGO, International Federation of Gynecology and Obstetrics; PDS, primary debulking surgery; NAC, neoadjuvant chemotherapy; IDS, interval debulking surgery.
Values are presented as n (%) or median (range) unless otherwise indicated.
Genomic alterations
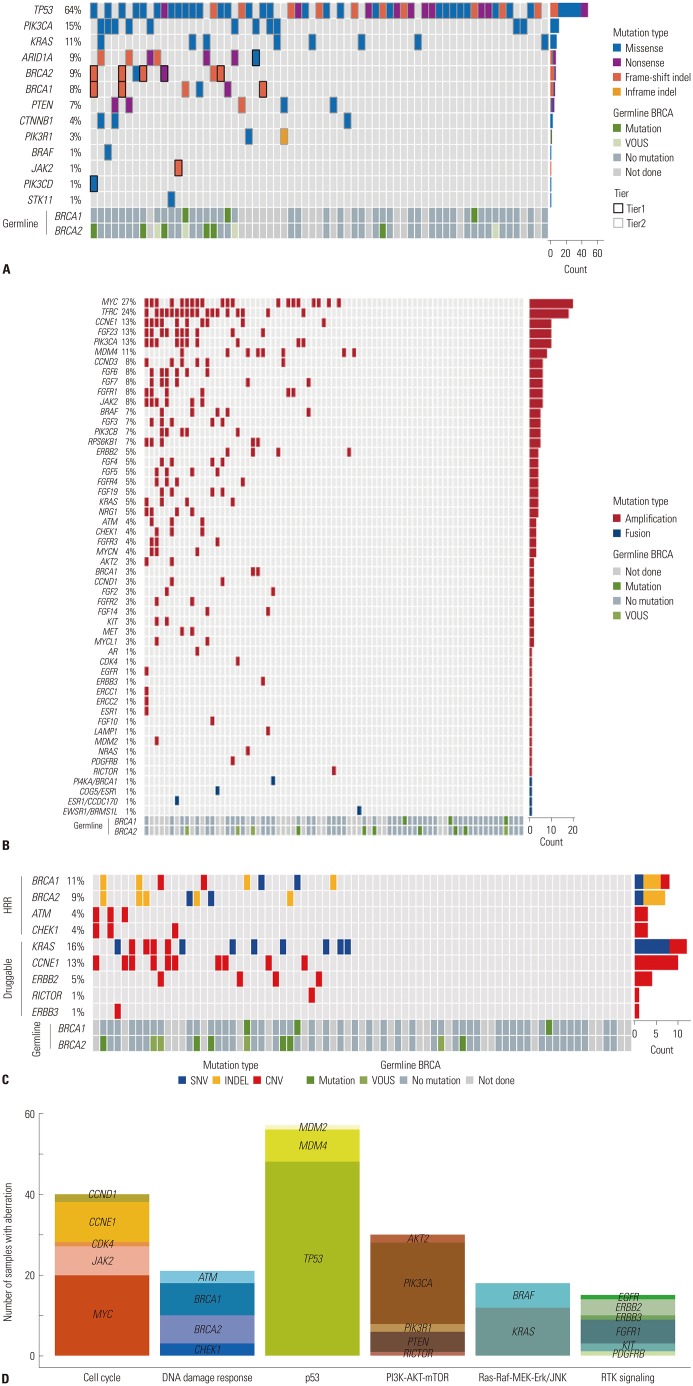
All patients had at least one genomic alteration. The mean number of mutations per patient was 10.5. Fifty-seven (67.9%) patients had more than one actionable alteration other than TP53. Of the 57 patients, 16 (28.6%) had a mutation in HRR-related genes (Fig. 1). In addition, we analyzed the distribution of patients with somatic BRCA mutations at each time point of NGS analysis. Of the 11 patients with somatic BRCA mutations, there were 2 patients (2/8, 25.0%) in the Pre-NAC group, 5 patients (6/39, 15.4%) in the PDS group, 2 patients (2/26, 7.7%) in the IDS group, and 2 patients (1/11, 9.1%) in the relapse group. The chemo-naive group (pre-NAC, PDS) and the chemotherapy group (IDS, relapse) comprised 8/47 (17.0%) and 3/37 (8.1%), respectively. We also reviewed the Germline BRCA status for the patients (Supplementary Table 3, only online). Of the 84 patients, 12 (14.3%) had germline BRCA1/2 mutation, 50 (59.5%) had no germline BRCA1/2 mutation, and 26 (31.0%) did not undergo the germline BRCA test. Table 2 shows the tumor molecular profiles and clinical utility of actionable somatic mutations. Among single nucleotide variants and indel in tiers 1 or 2, the most frequently identified mutations were in TP53 (64%), PIK3CA (15%), and BRCA1/2 (17%) (Fig. 2A). Among copy number variations and fusions, the most frequently identified mutations were in MYC (27%), TFRC (24%), and CCNE1 (13%) (Fig. 2B). The most commonly mutated genes among the HRR-related genes in tiers 1 or 2 included BRCA1 (11%), BRCA2 (9%), ATM (4%), and CHEK1 (4%) (Fig. 2C). The most frequently mutated genes among the TCGA druggable genes in tiers 1 and 2 were KRAS (16%), CCNE1 (13%), ERBB2 (5%), RICTOR (1%), and ERBB3 (1%) (Fig. 2C). Identified mutations were categorized into six pathways or functional groups: cell cycle (RB1, CCNE1, CDK2, CCND1, CDK4, CDK6, CDKN2A, MYC, SRC, JAK1, JAK2, STAT1, STAT3), DNA damage response (CHEK1, CHEK2, BRCA1, BRCA2, MLH1, MSH2, ATM, ATR), p53 (CDKN2A, MDM2, MDM4, TP53), PI3K-AKT-mTOR signaling (PI3KCA, PIK3RA, PTEN, AKT1, AKT2, MTOR, RICTOR, TSC1, TSC2), Ras-Raf, MEK-Erk/JNK signaling (KRAS, HRAS, BRAF, RAF1, MAP2K1, MAP2K2, MAP2K4, MAPK1, MAPK3), and the RTK signaling family (EGFR, ERBB2, ERBB3, ERBB4, PDGFRA, PDGFRB, KIT, FGFR1, KDR). We analyzed the numbers of mutations with six functional and targetable pathways (Fig. 2D). The most frequently identified mutations were MYC in the cell cycle pathway, BRCA1 in the DNA damage response pathway, TP53 in the p53 pathway, PIK3CA in the PI3K-AKT-mTOR pathway, KRAS in the Ras-Raf-MEK-Erk/JNK pathway, and FGFR1 in the RTK signaling pathway.
Fig. 1. Pie chart of the distribution of actionable somatic alterations. HRR, homologous recombination repair.
Table 2. Tumor Molecular Profiles and Clinical Utility of Targetable Somatic Mutations.
| Patient | Cell type | Alterations | Clinical implication | ||
|---|---|---|---|---|---|
| Actual change | Potential change | Genetic counseling | |||
| 1 | High grade serous | BRCA1_c.1961delA, BRCA2_c.1806delA | No | Consider future PARP inhibitor, consider future PARP inhibitor | Yes |
| 2 | High grade serous | BRCA1_c.1961delA, BRCA2_c.1806delA | No | Consider future PARP inhibitor, consider future PARP inhibitor | No |
| 3 | High grade serous | BRCA1_c.3548A>G | No | Consider future PARP inhibitor | No |
| 4 | High grade serous | BRCA2_c.3896A>T | No | Consider future PARP inhibitor | No |
| 5 | Mucinous | ERBB2_Amplification | No | Consider future ERBB inhibitor | No |
| 6 | Seromucinous | BRCA2_c.2795_2796delAC KRAS_C.38G>A | No | Consider future PARP inhibitor, consider future MAPK inhibitor | No |
| 7 | Clear cell | PTEN_G850A | No | Consider future PTEN inhibitor | No |
| 8 | Endometrioid | PIK3CA_241G>A PTEN_19G>T | No | Consider future AKT inhibitor, consider future PTEN inhibitor | No |
| 9 | High grade serous | BRAF_Amplification | No | Consider future BRAF inhibitor | No |
| 10 | High grade serous | BRCA2_c.1805_1806insA KRAS_Amplification | No | Consider future PARP inhibitor, consider future MAPK inhibitor | No |
| 11 | Low grade serous | KRAS_c.G38A MLH1_c440_44insT | Enrolled in NCT02628067 (pembrolizumab) | Consider future MAPK inhibitor | Yes |
| 12 | High grade serous | ATM_Amplification CCNE1_Amplification | No | Consider future PARP inhibitor, consider future CDK inhibitor | No |
| 13 | High grade serous | BRCA2_c.1399A>T | Treated with Olaparib | No | Yes |
| 14 | High grade serous | MET_Amplification PIK3CA_Amplification | No | Consider future MET inhibitor, consider future AKT inhibitor | No |
| 15 | High grade serous | CCNE1_Amplification | No | Consider future CDK inhibitor | No |
| 16 | High grade serous | CCNE1_Amplification PIK3CA_Amplification | No | Consider future CDK inhibitor, consider future AKT inhibitor | No |
| 17 | Mixed (clear+endometrioid) | CCNE1_Amplification | No | Consider future CDK inhibitor | No |
| 18 | High grade serous | CCNE1_Amplification KRAS_Amplification PIK3CA_Amplification | No | Consider future CDK inhibitor, consider future MAPK inhibitor, consider future AKT inhibitor | No |
| 19 | High grade serous | KRAS_c.35G>T NRAS_Amplification | No | Consider future MAPK inhibitor, consider future MAPK inhibitor | No |
| 20 | High grade serous | CDK4_Amplification PIK3CA_Amplification | No | Consider future CDK4 inhibitor, consider future AKT inhibitor | No |
| 21 | High grade serous | PIK3CA_Amplification | No | Consider future AKT inhibitor | No |
| 22 | Carcinosarcoma | FGFR1_Amplification KIT_Amplification | No | Consider future FGFR inhibitor, consider future KIT inhibitor | No |
| 23 | High grade serous | BRCA1_c.3991C>T | No | Consider future PARP inhibitor | Yes |
| 24 | High grade serous | BRCA2_c.2798del_2799delCA | Treated with Olaparib | No | Yes |
| 25 | High grade serous | PIK3CA_c.1035T>A ERBB2_Amplification | No | Consider future AKT inhibitor, consider future ERBB inhibitor | No |
| 26 | High grade serous | BRCA2_c.2808_2811del | No | Consider future PARP inhibitor | Yes |
| 27 | Clear cell | PIK3CA_c.3140A>T | No | Consider future AKT inhibitor | No |
| 28 | High grade serous | RICTOR_Amplification | No | Consider future RICTOR inhibitor | No |
| 29 | Mucinous | BRAF_c.1799T>A PIK3CA_c.1357G>A | No | Consider future BRAF inhibitor, consider future AKT inhibitor | No |
| 30 | Clear cell | PIK3CA_c.3140A>G FGFR1_Amplification | No | Consider future AKT inhibitor, consider future FGFR inhibitor | No |
| 31 | Clear cell | PIK3CA_c.3140A>G | No | Consider future AKT inhibitor | No |
| 32 | High grade serous | PTEN_c.604dupA BRAF_Amplification CCNE1_Amplification KRAS_Amplification | No | Consider future PTEN inhibitor, consider future BRAF inhibitor, consider future CDK inhibitor, consider future MAPK inhibitor | No |
| 33 | High grade serous | CCNE1_Amplification EGFR_Amplification FGFR1_Amplification KRAS_Amplification PIK3CA_Amplification | No | Consider future CDK inhibitor, consider future EGFR inhibitor, consider future FGFR inhibitor, consider future MAPK inhibitor, consider future AKT inhibitor | No |
| 34 | Seromucinous | PIK3CA_c.1624G>A KRAS_c.35G>T | No | Consider future AKT inhibitor, consider future MAPK inhibitor | No |
| 35 | Carcinosarcoma | KRAS_c.35G>T | No | Consider future MAPK inhibitor | No |
| 36 | High grade serous | BRCA1_c.5093_5096del | Treated with Olaparib | No | No |
| 37 | High grade serous | BRAF_Amplification ERBB2_Amplification | No | Consider future BRAF inhibitor, consider future ERBB inhibitor. | No |
| 38 | High grade serous | ATM_Amplification CCNE1_Amplification FGFR1_Amplification PIK3CA_Amplification | No | Consider future PARP inhibitor, consider future CDK inhibitor, consider future FGFR inhibitor, consider future AKT inhibitor | No |
| 39 | High grade serous | BRAF_Amplification FGFR2_Amplification MET_Amplification | No | Consider future BRAF inhibitor, consider future FGFR inhibitor, consider future MET inhibitor | No |
| 40 | Endometrioid | PIK3CA_c.113G>A PTEN_c.540C>G | No | Consider future AKT inhibitor, consider future PTEN inhibitor | No |
| 41 | High grade serous | KRAS_c.35G>T ERBB3_Amplification | No | Consider future MAPK inhibitor, consider future ERBB inhibitor | No |
| 42 | Clear cell | ERBB2_Amplification | No | Consider future ERBB inhibitor | No |
| 43 | Clear cell | PIK3CA_c.3140A>G FGFR1_Amplification | No | Consider future AKT inhibitor, consider future FGFR inhibitor | No |
| 44 | High grade serous | CCNE1_Amplification | No | Consider future CDK inhibitor | No |
| 45 | High grade serous | ATM_Amplification CHEK1_Amplification | No | Consider future PARP inhibitor, consider future CHEK1 inhibitor | No |
| 46 | High grade serous | BRCA2_c.6952C>T PIK3CA_Amplification | Treated with Olaparib | Consider future AKT inhibitor | Yes |
| 47 | Endometrioid | PIK3CA_Amplification | Treated with Olaparib based on germline BRCA mutation | Consider future AKT inhibitor | Yes |
| 48 | Seromucionous | KRAS_c.35G>T PIK3CA_c.1810T>C | No | Consider future MAPK inhibitor, consider future AKT inhibitor | No |
| 49 | Clear cell | PTEN_c.810G>T | No | Consider future PTEN inhibitor | No |
| 50 | Endometrioid | PIK3CA_c.1624G>A CCNE1_Amplification CHEK1_Amplification FGFR2_Amplification KIT_Amplification MDM2_Amplification PIK3CA_Amplification | No | Consider future AKT inhibitor, consider future CDK inhibitor, consider future PARP inhibitor, consider future FGFR inhibitor, consider future KIT inhibitor, consider future MDM2 inhibitor, consider future AKT inhibitor | No |
| 51 | Low grade serous | KRAS_c.35G>A BRAF_Amplification | No | Consider future MAPK inhibitor, consider future BRAF inhibitor | No |
| 52 | High grade serous | KRAS_c.38G>A CCNE1_Amplification | No | Consider future MAPK inhibitor, consider future CDK inhibitor | No |
| 53 | High grade serous | BRCA1_c.1399A>T | No | Consider future PARP inhibitor | No |
| 54 | High grade serous | BRAF_Amplification CCNE1_Amplification PTEN_Amplification | No | Consider future BRAF inhibitor, consider future CDK inhibitor, consider future PTEN inhibitor | No |
| 55 | High grade serous | KRAS_Amplification | No | Consider future MAPK inhibitor | No |
| 56 | Clear cell | PIK3CA_c.1624G>A | No | Consider future ATK inhibitor | No |
| 57 | High grade serous | PIK3CA_c.1633G>A | Enrolled in NCT03017521 (ATK inhibitor) | No | No |
PARP, poly ADP ribose polymerase.
Fig. 2. Mutation, copy number variation profiling. (A) Single nucleotide variants and indels. Color legend of the variations represented, including frameshift indel, inframe indel missense, and nonsense. Vertical lines indicate gene names; horizontal lines indicate cases with germline mutations. (B) Copy number variations and fusions. Color legend of the variations represented, including amplification, fusion. Vertical lines indicate gene names; horizontal lines indicate cases with germline mutations. (C) Homologous recombination repair (HRR)-related genes and The Cancer Genome Atlas druggable genes. Color legend of the variations represented, including single nucleotide variants, indel, and copy number variations. Vertical lines indicate gene names; horizontal lines indicate cases with germline mutations. (D) Number of mutations with six functional and targetable pathways. Vertical lines indicate the number of cases with mutations; horizontal lines indicate specific genes grouped according to pathways.
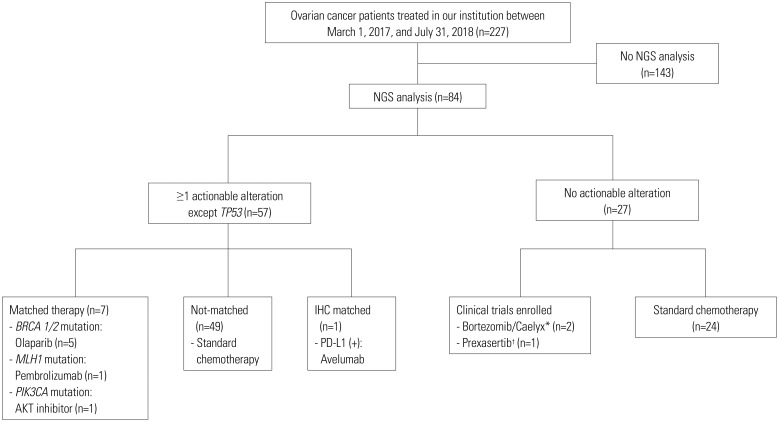
Targeted therapies
Of 57 patients with more than one actionable alteration, 7 (8.3%) were treated with matched therapies; 49 underwent standard chemotherapy without matched therapy; and 1 patient was treated with immunohistochemistry matched therapy (Fig. 3). Among the patients treated with matched therapies, five were treated with biomarker-driven therapy, and two were enrolled in biomarker matched clinical trials. BRCA1/2 mutations (n=5) were treated with poly ADP ribose polymerase (PARP) inhibitor; a MLH1 mutation with high microsatellite instability (n=1) was treated with a programmed cell death-1 (PD-1) inhibitor (ClinicalTrials.Gov Identifier: NCT02628067), and a PIK3CA mutation (n=1) was treated with an AKT inhibitor (ClinicalTrials.Gov Identifier: NCT03017521).
Fig. 3. CONSORT diagram. *ClinicalTrials. Gov Identifier: NCT03509246; †ClinicalTrials.Gov Identifier: NCT03414047. NGS, next generation sequencing; IHC, immunohistochemistry; PD-L1, programmed cell death-ligand 1.
Outcomes of matched therapy patients
A total of 7 patients were treated with a targeted agent, and the median value of prior lines of chemotherapy was 2 (range, 2–4). Of the 7 patients, five received PARP inhibitor therapy, one received PD-1 inhibitor, and one received AKT inhibitor therapy. Among patients who were treated with PARP inhibitor, four were treated with a PARP inhibitor for maintenance therapy, and one was treated with a PARP inhibitor as a 4th-line monotherapy. Among patients who underwent maintenance therapy with a PARP inhibitor, three were on follow-up without recurrence for more than 5 months; one experienced disease progression at 7 months after initiation of maintenance therapy. One patient treated with a PARP inhibitor as a 4th-line monotherapy had stable disease at the time of the analysis and had been undergoing treatment for 7 months. A patient treated with a PD-1 inhibitor experienced disease progression at 5 months after initiation of therapy. One patient treated with an AKT inhibitor had stable disease and had been undergoing treatment for 2 months.
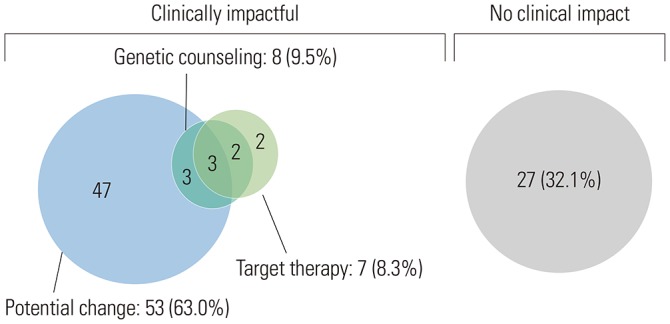
Clinical impact
Clinically meaningful results are shown in Fig. 4. Clinically significant alterations were found in 57 (67.9%) patients. Of these 57, seven (8.3%) had matched targeted therapies, 53 (63.0%) had potentially actionable alterations, and eight (9.5%) and their atrisk family members without potentially actionable alterations received genetic counseling. Currently, the patients with potentially actionable alterations are currently either undergoing standard treatment or are in a state in which no disease is evident after treatment but remain candidates for matched therapy if there is a subsequent disease recurrence or progression. Several patients had multiple actionable alterations. These patients received matched therapy but had other alterations that may later make them potential candidates for matched therapy. Patients with actionable alterations that could make them future potential candidates for matched therapy are shown in Table 2.
Fig. 4. Clinical impact of next generation sequencing panel.
DISCUSSION
In this study, we evaluated the clinical utility of NGS and identified clinically significant information beyond actionable alterations in ovarian cancer patients. In our review of NGS results for the 84 ovarian cancer patients in our institution, we found that 57 (67.9%) of them had one or more actionable alterations other than TP53 and that 16 (28.6%) of them had a mutation in HRR-related genes. Fifty-two (61.9%) patients had clinically significant alterations, seven (8.3%) were treated with matched targeted therapies, 48 (57.1%) had potentially actionable alterations, and eight (9.5%) received genetic counseling. In our study, 12 (14.3%) had germline BRCA1/2 mutations, and 50 (59.5%) had no germline BRCA1/2 mutation. In addition, the distribution of patients with somatic BRCA mutations at each time point of NGS were 8/47 (17.0%) in the chemo-naive group (pre-NAC, PDS) and 3/37 (8.1%) in the post chemotherapy group (IDS, relapse). Incorporation of NGS into standard clinical practice could provide a complementary tool with which to identify patients who might benefit from targeted therapies and genetic counseling.
NGS has been used in several studies to identify the actionable mutations of specific cancers and the clinical impact thereof.11,12 Oberg, et al.11 showed the feasibility of incorporating NGS into pediatric hematology-oncology. They found it was clinically significant in 66% of all cases in which it was used. Its benefits included avoidance of inappropriate treatments, confirmation of definitive diagnoses, and identification of pharmacogenomics modifiers. Heong, et al.12 described the feasibility of a molecular screening program in Asian cancer patients. Eighty-two percent of all patients had at least one reportable genomic alteration. Eight percent of the patients with reportable alterations were treated with matched therapies based on their specific molecular alteration. Nine of these patients (45%; 95% CI, 23.1–68.5%) showed a clinical benefit, including three partial responses and six with stable disease. However, in the SHIVA trial,13 treatment with matched targeted agents based on a patient's actionable molecular alterations did not improve progression-free survival in heavily pretreated cancer patients, compared with their physicians' treatment choices. However, no studies have been conducted to assess the clinical impact, if any, of using NGS to identify actionable mutations in ovarian cancer patients.
In our study, 61.9% of all patients were identified as potential candidates for targeted therapy. This is comparable with other studies and may be the rationale for performing comprehensive genomic analysis in ovarian cancer patients. Despite our findings, only 8.3% of patients received targeted therapy. Even after their detection, matching susceptible mutations with specific efficacious treatment agents is especially difficult with ovarian cancer patients. Ovarian cancers have heterogeneous cell populations, an extremely complex etiology, and any number of mutations can occur as the cancer develops.14 As a further complication, health insurers are not obligated to cover the off-label use of expensive drugs.
Nevertheless, the incorporation of NGS into the treatment of ovarian cancer may have a significant clinical impact, including success in finding potential candidates for future targeted therapies and genetic counseling. In our study, 57.1% of all patients were potential candidates for future targeted therapy, and 9.5% of all patients received genetic counseling for the patient and their at-risk family members. In addition, the proportion of somatic BRCA mutations varied according to the time point of NGS analysis. Although the number of patients was small, the difference in the proportion of patients with somatic BRCA mutations in the two groups may be due to the effect of BRCA reversion by platinum-based chemotherapy. Reversion mutations in BRCA1/2 have been reported in ovarian cancer as a mechanism of acquired resistance to platinum-based chemotherapies and PARP inhibitors.15,16 Based on these results, further studies are needed to analyze the genetic alterations in serial samples of chemo-naïve and post-chemotherapy patients.
Our study has some limitations. There are still cost-effective issues with NGS that can limit clinical testing and prevent or delay the initiation of targeted therapies, and failures, such as insufficient collection of tissues or improper sequencing, can lead to significantly increased turnaround times. In addition, our follow-up period on patients was too short to demonstrate definitively that matched targeted therapies based on the results of NGS yield better outcomes than empiric treatment choices.
NGS may help guide immediate and future treatment options for patients with ovarian cancer. Implementation of NGS served as a complementary tool to identify patients who may benefit from targeted therapies and genetic counseling. Further large-scale studies are needed to investigate the overall clinical utility and feasibility of NGS in ovarian cancer.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2016R1D1A1B03931916) and by the Bio & Medical Technology Development Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2017M3A9E8029714). This study was also supported by a faculty research grant of Yonsei University College of Medicine (6-2018-0169).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung-Yun Lee.
- Data curation: Yong Jae Lee, Dachan Kim, Hyun-Soo Kim, and Kiyong Na.
- Formal analysis: Yong Jae Lee.
- Funding acquisition: Jung-Yun Lee.
- Investigation: Eun Ji Nam, Sang Wun Kim, Sunghoon Kim, and Young Tae Kim.
- Methodology: Yong Jae Lee.
- Project administration: Jung-Yun Lee.
- Resources: Jung-Yun Lee.
- Software: Yong Jae Lee.
- Supervision: Jung-Yun Lee.
- Validation: Yong Jae Lee and Jung-Yun Lee.
- Visualization: Yong Jae Lee and Dachan Kim.
- Writing—original draft: Yong Jae Lee.
- Writing—review & editing: Yong Jae Lee and Jung-Yun Lee.
SUPPLEMENTARY MATERIALS
Gene Content in the TruSight Tumor 170 Panel
Actionable Somatic Alterations
Germline BRCA1/2 Mutation Status
References
- 1.Lee JY, Kim EY, Jung KW, Shin A, Chan KK, Aoki D, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014;25:174–182. doi: 10.3802/jgo.2014.25.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 4.Groisberg R, Hong DS, Roszik J, Janku F, Tsimberidou AM, Javle M, et al. Clinical next-generation sequencing for precision oncology in rare cancers. Mol Cancer Ther. 2018;17:1595–1601. doi: 10.1158/1535-7163.MCT-17-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr TH, McEwen R, Dougherty B, Johnson JH, Dry JR, Lai Z, et al. Defining actionable mutations for oncology therapeutic development. Nat Rev Cancer. 2016;16:319–329. doi: 10.1038/nrc.2016.35. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher KE, Zhang L, Wang J, Smith GH, Newman S, Schneider TM, et al. Clinical validation and implementation of a targeted next-generation sequencing assay to detect somatic variants in non-small cell lung, melanoma, and gastrointestinal malignancies. J Mol Diagn. 2016;18:299–315. doi: 10.1016/j.jmoldx.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph L, Cankovic M, Caughron S, Chandra P, Emmadi R, Hagenkord J, et al. The spectrum of clinical utilities in molecular pathology testing procedures for inherited conditions and cancer: a report of the Association for Molecular Pathology. J Mol Diagn. 2016;18:605–619. doi: 10.1016/j.jmoldx.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Oberg JA, Glade Bender JL, Sulis ML, Pendrick D, Sireci AN, Hsiao SJ, et al. Implementation of next generation sequencing into pediatric hematology-oncology practice: moving beyond actionable alterations. Genome Med. 2016;8:133. doi: 10.1186/s13073-016-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heong V, Syn NL, Lee XW, Sapari NS, Koh XQ, Adam Isa ZF, et al. Value of a molecular screening program to support clinical trial enrollment in Asian cancer patients: the Integrated Molecular Analysis of Cancer (IMAC) Study. Int J Cancer. 2018;142:1890–1900. doi: 10.1002/ijc.31091. [DOI] [PubMed] [Google Scholar]
- 13.Le Tourneau C, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 14.Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21:379–385. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- 15.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene Content in the TruSight Tumor 170 Panel
Actionable Somatic Alterations
Germline BRCA1/2 Mutation Status