Abstract
Background
Overall survival (OS) in glioblastoma (GBM) is poor at an average of 14 to 18 months, and long-term survivors (LTS) of GBM are rare. LTS of GBM, defined as surviving >5 years postdiagnosis, represent only 2% to 10% of all GBM patients. LTS of cancer are at high risk of developing second primary neoplasms. This study looks at occurrences of second primary neoplasms in LTS of GBM.
Methods
Records from adult patients newly diagnosed with GBM between January 1, 1998 and February 8, 2010, were retrospectively reviewed to identify LTS, defined as patients who survived ≥5 years. We focused on the identification of a new diagnosis of cancer occurring at least 2 years after the initial GBM diagnosis.
Results
We identified 155 LTS of GBM, with a median OS of 11.0 years (95% CI: 9.0 to 13.1 years) and a median follow-up of 9.6 years (95% CI: 8.7 to 10.7 years). In this cohort of patients, 13 (8.4%) LTS of GBM developed 17 secondary cancers. Eight could potentially be attributed to previous radiation and chemotherapy (skin cancer in radiation field [n = 4], leukemia [n = 2], low-grade glioma [n = 1], and sarcoma of the scalp [n = 1]). The other 9 cases included melanoma (n = 2), prostate cancer (n = 2), bladder cancer (n = 1), endometrioid adenocarcinoma (n = 1), basal cell carcinoma (n = 1), and renal cell carcinoma (n = 1).
Conclusions
Although second primary cancers are rare in GBM LTS, providers should continue close monitoring with appropriate oncologic care. Moreover, this highlights the need for survivorship care of patients with GBM.
Keywords: chemotherapy, glioblastoma, long-term survivors, radiation therapy, second primary neoplasm
Primary brain tumors represent 1% of all diagnosed cancers. Glioblastoma (GBM), the most aggressive and malignant form, is the most common primary glial tumor diagnosed in adults.1 The survival rate of patients with GBM (WHO grade IV) continues to be poor and ranges from 1.9% to 9.8% (in the landmark study of concomitant/adjuvant temozolomide with radiotherapy vs radiotherapy alone) of patients surviving at 5 years postdiagnosis.1,2 However, advances in imaging and treatment have resulted in a gradual improvement of survival outcomes.3,4
The standard of care for newly diagnosed GBM involves surgical resection followed by temozolomide concurrent with radiation therapy and adjuvant temozolomide.5 A recent update to the National Comprehensive Cancer Network (NCCN) guidelines for newly diagnosed GBM also included the use of tumor-treating fields during adjuvant temozolomide. Radiation therapy is a key component in the treatment of GBM at initial diagnosis and at recurrence as stereotactic single-dose radiosurgery and/or fractionated stereotactic radiotherapy.6,7 While radiation therapy is deemed as essential component of GBM treatment, cancer can develop from exposure to radiation therapy and this is well documented and described.8–10 This risk is also well described in the childhood cancer population, notably development of meningiomas in the CNS of survivors of acute leukemias who received craniospinal radiation.11 Even with low-dose radiation, children treated for Hodgkin lymphoma remained at high risk for second primary malignant neoplasms.8
Furthermore, the development of second primary cancer is linked to exposure to chemotherapy. Patients who undergo allogeneic hematopoietic stem cell transplantation with high-dose chemotherapy are at risk for the development of second primary cancers. These can occur approximately 10 years after treatment with common second primary malignancies including breast cancer, lung cancer, skin cancer, oral cancer, and cancers of the genitourinary tract.12,13 While high-dose chemotherapy and stem cell transplantation are not standard treatments for GBM, second primary hematological malignancies such as myelodysplasia, leukemia, or non-Hodgkin lymphoma can developed in association with extended exposure to the alkylating agent temozolomide.14–17 This observation that long-term alkylating chemotherapy can lead to second primary malignancies is well documented in patients with cancers including lymphoma, brain tumors, and gynecological cancers.18
Similar to LTS of other primary cancers, there is a probable risk of second primary neoplasms in adult LTS of GBM. Understanding the prevalence of second primary cancers in this unique and rare population is critical to determine the need for ongoing patient monitoring and medical and psychosocial support. Understanding the possibility of the development of second primary cancers provides a more in-depth education of the benefits and risks of chemotherapy in the GBM population. Furthermore, these characteristics inform physician and patient expectations in the case of long-term survivorship of these very malignant tumors. This study investigates an understudied situation, the risk of second primary neoplasms in the unique cohort of GBM LTS.
Methods
Inclusion Criteria and Data Collection
In a retrospective analysis that was approved by the institutional review board (Pro00021750) at the Preston Robert Tisch Brain Tumor Center at Duke, adult (ages >18 years at time of diagnosis) GBM LTS were defined as achieving survival greater than or equal to 5 years from initial GBM diagnosis. In addition to the aforementioned criteria, other inclusion criteria included a histologically confirmed diagnosis of GBM and treatment between January 1, 1998 and August 31, 2011. The histopathology was centrally reviewed at Duke and confirmed in neuropathology consensus conference. Of note, analysis and use of the mutational status of isocitrate dehydrogenase and methylation of methylguanine methyltransferase were not actively being performed at this time so these were not included in the histopathology determination. Clinical characteristics that were collected on eligible GBM LTS included treatment of GBM with surgery, radiation, and/or chemotherapy and types of therapies used. Second primary cancers were identified by searching for a second primary cancer that developed at least 2 years after the initial diagnosis of GBM in the GBM LTS population. Demographic data were obtained for the GBM LTS including the date of second primary cancer diagnosis, as well as the location, pathology, and treatment of the second primary cancer.
Statistical Analysis
Descriptive statistics were performed on the available demographic characteristics of GBM LTS with second primary cancers. Kaplan–Meier methods were used to estimate median overall survival (OS) in GBM LTS. OS was defined as the time from initial GBM diagnosis to the time of death, or until last contact if the patient was still alive. Time to the diagnosis of second primary cancer was defined as the date of initial diagnosis of GBM until the date of diagnosis of the second primary cancer.
Results
As of January 28, 2014, we identified 155 LTS of GBM with a median OS of 11.0 years (95% CI: 9.0 to 13.1 years) and a median follow-up of 9.6 years (95% CI: 8.7 to 10.7 years). In this cohort, there were 17 new and/or second primary cancers in 13 GBM LTS (Table 1). Of these 17 cancers, 8 (47%) could potentially be attributed to previous treatment with radiation and chemotherapy, including skin cancer (n = 4 [24%]), leukemia (n = 2 [12%]), low-grade glioma (n = 1 [6%]), and sarcoma of the scalp (n = 1 [6%]). The other 9 (53%) cases included melanoma (n = 3), prostate cancer (n = 2), bladder cancer (n = 1 [6%]), endometrioid adenocarcinoma (n = 1 [6%]), basal cell carcinoma (BCC) (n = 1 [6%]), and renal cell carcinoma (n = 1 [6%]). For the 13 LTS with new/second primary cancer, the mean age at GBM diagnosis was 52.9 ± 11.6 years and the mean age at new/second primary cancer diagnosis was 60.0 ± 11.0 years. Median time from GBM diagnosis to new/second primary cancer diagnosis was 7.8 years (range, 2.7 to 11.5 years). Out of the 13 patients who had second primary neoplasms, 8 died from GBM, 2 died from leukemia, and 3 were still living as of December 19, 2017. Below, we include a description of selected GBM LTS patients who developed second primary cancers.
Table 1.
GBM LTS Patient and Second Primary Cancer Characteristics
| Patient No. | Sex | Age at GBM Diagnosis, y | OS from GBM Diagnosis, y | GBM Treatment | Age at Second Primary Cancer Diagnosis, y | Second Primary Cancer Pathology | Second Primary Cancer Location | Second Primary Cancer Treatment | Outcome (at Time of Analysis) |
| 1 | F | 36 | 14.3 | GTR, standard chemoradiation | 39 | Melanoma | Left fifth toe | Resection, amputation of toe | Died from GBM |
| 2 | M | 61 | 8.3 | GTR, standard chemoradiation | 69 | Leukemia | Systemic | High-dose chemotherapy | Died from leukemia |
| 3 | M | 63 | 6.2 | GTR, standard chemoradiation | 67 | Prostate cancer | Prostate | Radiation therapy and chemotherapy | Died from GBM |
| 4 | M | 54 | 6.5 | STR, upfront 4 cycles of 5-day TMZ, standard radiation alone | 61 | Squamous cell carcinoma | Right eyebrow | Biopsy | Died from pulmonary embolism |
| 61 | Basal cell carcinoma | Right earlobe | Biopsy | ||||||
| 61 | Basal cell carcinoma | Right posterior auricular | Biopsy | ||||||
| 5 | M | 60 | 7 | GTR, standard chemoradiation | 63 | Prostate cancer | Prostate | Cryotherapy | Died from GBM |
| 6 | F | 26 | NA | GTR, standard chemoradiation | 38 | Undifferentiated sarcoma | Scalp | Resection | Alive |
| 30 | Malignant melanoma | Right forearm | Resection | ||||||
| 7 | M | 48 | 6.6 | GTR with carmustine wafer, standard chemoradiation | 54 | Oligodendroglioma (WHO grade II) | Brain | Chemotherapy (daily TMZ) | Died from GBM |
| 8 | M | 44 | NA | GTR, standard radiation | 54 | Basal cell carcinoma | Right ear | Resection | Alive |
| 9 | F | 59 | NA | GTR, standard chemoradiation | 66 | Breast cancer | Breast | Lumpectomy and anastrozole | Alive |
| 69 | Basal cell carcinoma | Left cheek | Resection | ||||||
| 10 | M | 62 | 11.7 | GTR, clinical trial followed by standard radiation | 71 | Renal cell carcinoma | Kidney | Nephrectomy | Died from GBM |
| 11 | M | 54 | 7.8 | GTR, radiation alone followed by chemotherapy | 61 | B cell acute lymphocytic leukemia | Systemic | Chemotherapy | Died from leukemia |
| 12 | M | 66 | 7.4 | GTR, radiation alone followed by chemotherapy | 70 | Bladder cancer | Bladder | Resection | Died from GBM |
| 13 | F | 55 | 12.2 | GTR with carmustine wafer, standard chemoradiation | 66 | Grade I endometrioid adenocarcinoma | Endometrium | Hysterectomy | Died from GBM |
Abbreviations: F, female; GBM, glioblastoma; GTR, gross total resection; LTS, long-term survivors; M, male; NA, not available; OS, overall survival; standard chemoradiation, concurrent temozolomide and radiation; STR, subtotal resection; TMZ, temozolomide.
Patient 2 was diagnosed with GBM (WHO grade IV) at the age of 61. The patient underwent a gross total resection followed by external-beam radiation with concurrent temozolomide. The patient completed 12 months of adjuvant temozolomide followed by serial imaging. The patient developed leukemia 8 years after the initial diagnosis of GBM. The patient received systemic chemotherapy to treat the leukemia and died from leukemia about 3 months after the diagnosis.
Patient 11 developed leukemia after having a gross total resection and standard chemoradiation followed by 1 year of adjuvant temozolomide for treatment of GBM. The leukemia was diagnosed 7 years after the initial diagnosis of GBM. The patient received systemic chemotherapy to treat the leukemia and died from complications of neutropenic sepsis second primary to treatment of leukemia.
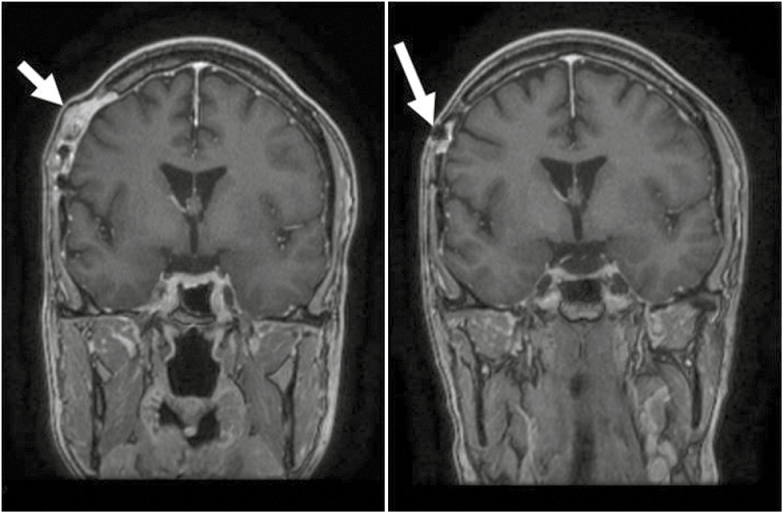
Patient 6 was diagnosed with right parietal GBM at the age of 26. The patient had a resection and subsequently received a full course of radiation therapy with concurrent low-dose temozolomide, followed by 16 cycles of temozolomide and occasional use of isotretinoin. The patient was followed with serial MRIs until diagnosed with high-grade sarcoma of the scalp, at which point the patient underwent surgical resection (positive margin on the dura) followed by postoperative radiotherapy (Fig. 1). This patient was still alive as of the last follow-up, December 19, 2017.
Fig. 1.
(Left) MRI T1+ contrast coronal of scalp sarcoma adjacent to original craniotomy site (Patient 6); (right) MRI T1+ contrast sagittal 1 year before scalp sarcoma diagnosis (Patient 6); white arrow indicates location of scalp sarcoma.
In addition to the specific aforementioned cases, numerous skin cancers including BCC, squamous cell carcinoma, and melanoma were noted among the patients who had second primary cancers after diagnosis and treatments of GBM.
Discussion
Our study indicates that second primary cancers can occur in LTS of GBM. GBM LTS are rare, with 2% to 10% of patients surviving 5 years postdiagnosis.1,2,5,19 Recent advances in imaging to detect recurrence before excessive symptomology have modestly improved survival in GBM by increasing the timely use of therapies such as oral chemotherapies and antiangiogenic agents and allowing for well-timed involvement in clinical trials. With this increase in survival, providers and patients are confronted with the late toxicities of treatment such as cognitive dysfunction, functional decline, and on rare occasions, second primary cancers.
As mentioned above, providers and patients have not contended with second primary cancer in this particular population because, as a whole, GBM patients have not lived long enough to be diagnosed with a second primary cancer. However, second primary cancers are more common and well studied in many childhood cancer LTS and adult LTS of other cancer types for which long-term follow-up and observations have been possible. Survivors of childhood cancers have experienced second primary cancer presumably due to the exposure to radiation therapy. A cohort study with more than 30 years of follow-up by Dörffel and colleagues found that 85% of patients who had radiation therapy for treatment of Hodgkin lymphoma in childhood and adolescence developed second primary solid tumors in the irradiated region. This study suggested that the patients must be informed about the risk of second primary neoplasms in the irradiated lesion and should undergo regular follow-ups.20 Given that the first-line therapy of GBM is radiation, GBM LTS could be at significant risk of developing second primary neoplasms in the irradiated regions of their brain. With increased LTS in GBM and knowledge of the risk of second primary neoplasm in patients who receive radiation, a study identifying the prevalence of second primary neoplasms in GBM LTS is a useful tool in guiding future care of LTS. Moreover, providers for GBM patients should discuss second primary cancers as part of a survivorship program.21 Other primary cancer specialists have developed survivorship programmatic guidelines and/or recommendations that aid providers.22
Out of 155 GBM LTS identified, there were 17 second primary cancers among 13 GBM LTS. Thirteen out of 17 cases were skin cancers including melanoma, squamous cell carcinoma, and BCC. These 13 cases could be second primary to previous chemotherapy and/or radiation therapy as reported in other adult and childhood cancer survivors. Watt and colleagues performed a study to find the association between radiation therapy and BCC in childhood cancer survivors. Among case individuals, radiation therapy alone or in combination with chemotherapy was associated with an increased risk of BCC compared with no chemotherapy or radiation.23 Given that there is an associated risk of BCC with previous radiation and chemotherapy, it is possible that this risk can extend to other cancer types depending on where the radiation is applied and what type of chemotherapy was previously used. Additionally, with this association, it is reasonable to assume that patients with GBM who live longer are at risk of developing cancers as they age, including but not limited to skin cancers, breast cancer, and prostate cancer. Etiology of the second primary cancers from this study could not be determined; however, the detailed case descriptions that were included in the text do suggest that cancers developed in the treatment field of radiation. These included a sarcoma of the scalp in the radiation field and skin cancers within the radiation field. The number of cases is too small to suggest any direct association between exposure to systemic chemotherapy and the development of leukemia. However, this study showed that 2 LTS patients who received systemic chemotherapy developed leukemia and died from leukemia, not from GBM.
The retrospective study design and absence of genetic information of specific patients (genetic cancer syndromes) are the primary limitations of this study. Understanding the genetic makeup of the primary tumor is relevant for anticipating the location/type of any second primary cancer that may present. For example, approximately 5% to 10% of all breast cancer can be accounted for by germline mutations in the breast cancer (BRCA1 and BRCA2) genes responsible for hereditary breast and ovarian cancer syndrome.24 It is critical to note that the development of these second primary cancers could point to underlying germline mutations that could include p53 (Li-Fraumeni syndrome), NF1 (neurofibromatosis type 1) or mismatch repair genes (Lynch syndrome).25 Providers should consider a referral/consultation with cancer genetic providers if there is a suspicion of genetic/germline cause of the multiple malignancies. Advances in understanding genetic characteristics and traits of primary brain tumors will improve identifying the association between the genotype and second primary cancers and anticipating possible second primary cancers.
The finding of second primary cancers in GBM LTS raises several issues concerning management and counseling for these patients. One such issue is the need for continued clinical and radiological surveillance for signs of secondary cancers 5 years or more after initial diagnosis of GBM. Currently, there are no clinical trials that define optimal frequency for follow-up after GBM therapy; however, the NCCN recommends, “a repeat MRI should be obtained four weeks after completion of radiation therapy, then every two to four months for two to three years, and less frequently thereafter.” While ceasing regular evaluation following a protracted disease-free period may be appealing to physicians, patients, and institutions, these data suggest that even with long-term absence of disease following treatment (≥ 2 years), there continues to be a risk for second primary cancers. Protracted GBM LTS surveillance is thus warranted to maximize prompt diagnosis and treatment of second primary cancers.
Understanding the risk of second primary neoplasms in GBM LTS is necessary to determine the need for ongoing patient monitoring and medical support as well as for psychosocial support. GBM LTS may benefit from being better informed of their risk of acquiring second primary cancers even after protracted disease-free periods. Physicians should be prepared to inform their patients to remain vigilant for new symptoms for years after diagnosis, and patients themselves may benefit from support groups catering to the specific needs and challenges of GBM LTS. An ideal GBM LTS support group could address and identify the risk of second primary neoplasms and help patients to adopt behaviors associated with better outcomes such as regular exercise, which has been associated with increased OS following GBM diagnosis.26 To have better understanding and identification of the risk of second primary neoplasms in GBM LTS, future research is warranted.
In conclusion, GBM LTS are at risk of having second primary cancers after cessation of treatment. This study provides new information that second primary cancers in GBM LTS remain a possibility even after years of disease-free status following treatment cessation. Further studies to identify the types of second primary cancers and the associated treatments of the original cancer in GBM LTS are needed. Further studies, across multiple institutions, are warranted to validate the secondary cancer rates that were present at Duke University Medical Center, and to determine the true probability of acquiring second primary cancers following long-term survival of a GBM therapeutic regimen. The continued observation of second primary cancers from a large study as improvements in the treatment of GBM occur would indicate the need for patient surveillance programs and psychosocial survivor support catering to the unique needs of GBM LTS.
Funding
This work was supported by The Preston Robert Tisch Brain Tumor at Duke.
Conflict of interest statement. None declared.
References
- 1. Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3. Koshy M, Villano JL, Dolecek TA, et al. . Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slotty PJ, Siantidis B, Beez T, Steiger HJ, Sabel M.et al. . The impact of improved treatment strategies on overall survival in glioblastoma patients. Acta Neurochir (Wien). 2013;155(6):959–963; discussion 963. doi: 10.1007/s00701-013-1693-1. [DOI] [PubMed] [Google Scholar]
- 5. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6. Patel M, Siddiqui F, Jin JY, et al. . Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92(2):185–191. doi: 10.1007/s11060-008-9752-9. [DOI] [PubMed] [Google Scholar]
- 7. Bir SC, Connor DE Jr, Ambekar S, Wilden JA, Nanda A.et al. . Factors predictive of improved overall survival following stereotactic radiosurgery for recurrent glioblastoma. Neurosurg Rev. 2015;38(4):705–713. doi: 10.1007/s10143-015-0632-4. [DOI] [PubMed] [Google Scholar]
- 8. O’Brien MM, Donaldson SS, Balise RR, Whittemore AS, Link MP.et al. . Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28(7):1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grantzau T, Mellemkjær L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: a national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother Oncol. 2013;106(1):42–49. doi: 10.1016/j.radonc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 10. Rapiti E, Fioretta G, Verkooijen HM, et al. . Increased risk of colon cancer after external radiation therapy for prostate cancer. Int J Cancer. 2008;123(5):1141–1145. doi: 10.1002/ijc.23601. [DOI] [PubMed] [Google Scholar]
- 11. Goshen Y, Stark B, Kornreich L, Michowiz S, Feinmesser M, Yaniv I.et al. . High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(3):294–297. doi: 10.1002/pbc.21153. [DOI] [PubMed] [Google Scholar]
- 12. Adhikari J, Sharma P, Bhatt VR. Risk of secondary solid malignancies after allogeneic hematopoietic stem cell transplantation and preventive strategies. Future Oncol. 2015;11(23):3175–3185. doi: 10.2217/fon.15.252. [DOI] [PubMed] [Google Scholar]
- 13. Majhail NS, Brazauskas R, Rizzo JD, et al. . Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–322. doi: 10.1182/blood-2010-07-294629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Momota H, Narita Y, Miyakita Y, Shibui S.et al. . Secondary hematological malignancies associated with temozolomide in patients with glioma. Neuro Oncol. 2013;15(10):1445–1450. doi: 10.1093/neuonc/not036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma A, Gupta D, Mohanti BK, et al. . Non-Hodgkin lymphoma following temozolomide. Pediatr Blood Cancer. 2009;53(4):661–662. doi: 10.1002/pbc.22090. [DOI] [PubMed] [Google Scholar]
- 16. Su YW, Chang MC, Chiang MF, Hsieh RK.et al. . Treatment-related myelodysplastic syndrome after temozolomide for recurrent high-grade glioma. J Neurooncol. 2005;71(3):315–318. doi: 10.1007/s11060-004-2028-0. [DOI] [PubMed] [Google Scholar]
- 17. Chamberlain MC, Raizer J. Extended exposure to alkylator chemotherapy: delayed appearance of myelodysplasia. J Neurooncol. 2009;93(2):229–232. doi: 10.1007/s11060-008-9764-5. [DOI] [PubMed] [Google Scholar]
- 18. Vega-Stromberg T. Chemotherapy-induced secondary malignancies. J Infus Nurs. 2003;26(6):353–361. doi: 10.1097/00129804-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 19. Scott JN, Rewcastle NB, Brasher PM, et al. . Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol. 1999;46(2):183–188. doi:10.1002/1531-8249(199908)46:2183::AID-ANA7>3.0.CO;2-7. [PubMed] [Google Scholar]
- 20. Dörffel W, Riepenhausenl M, Lüders H, Brämswig J, Schellong G.et al. . Secondary malignancies following treatment for Hodgkin’s lymphoma in childhood and adolescence. Dtsch Arztebl Int. 2015;112(18):320–327, i. doi: 10.3238/arztebl.2015.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leeper HE, Aquaye AA, Bell S, et al. . Survivorship care planning in neuro-oncology. Neurooncol Pract. 2018;5(1):3–9. doi: 10.1093/nop/npx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilbur J. Surveillance of the adult cancer survivor. Am Fam Physician. 2015;91(1):29–36. [PubMed] [Google Scholar]
- 23. Watt TC, Inskip PD, Stratton K, et al. . Radiation-related risk of basal cell carcinoma: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2012;104(16):1240–1250. doi: 10.1093/jnci/djs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allain DC. Genetic counseling and testing for common hereditary breast cancer syndromes: a paper from the 2007 William Beaumont hospital symposium on molecular pathology. J Mol Diagn. 2008;10(5):383–395. doi: 10.2353/jmoldx.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kyritsis AP, Bondy ML, Rao JS, Sioka C.et al. . Inherited predisposition to glioma. Neuro Oncol. 2010;12(1):104–113. doi: 10.1093/neuonc/nop011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruden E, Reardon DA, Coan AD, et al. . Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29(21):2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]