Abstract
Background
The availability of image guidance and intensity modulation has led to the increasing use of hypofractionated stereotactic radiotherapy (hSRT) as an alternative to conventionally fractionated radiotherapy or radiosurgery for intracranial meningiomas (ICMs). As the safety and efficacy of this approach is not well characterized, we conducted a systematic review of the literature to assess the clinical outcomes of hSRT in the setting of ICMs.
Methods
A systematic review of Medline and EMBASE databases was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Included studies were retrospective or prospective series that examined an ICM population of ≥10 patients, delivered >1 fraction of photon hSRT (≥2.5 Gy per fraction), and had a median follow-up of ≥2 years. Descriptive statistics were generated for included studies.
Results
Of 1480 initial studies, 14 met eligibility criteria for inclusion, reporting on 630 patients (age range, 18-90) treated for 638 tumors. Primary radiotherapy was delivered in 37% of patients, 36% had radiation following surgery, and surgical details were unavailable for 27%. In 474 tumors assessed for radiologic response, 78% remained stable, 18% decreased in size, and 4% increased in size. Crude local control was 90%-100% as reported in 10 studies. The median late toxicity rate was 10%. The most common significant late toxicities were decreased visual acuity and new cranial neuropathy.
Conclusions
With limited follow-up, the available literature suggests hSRT for ICMs has local control and toxicity profiles comparable to other radiotherapy approaches. Confirmation in larger patient cohorts with a longer duration of follow-up is required.
Keywords: hypofractionation, meningioma, radiosurgery, radiotherapy, stereotactic
Intracranial meningiomas (ICMs) are the most common central nervous system tumors in adults, comprising 24%-30% of all primary intracranial tumors with an annual incidence of 13 per 100000.1 They are typically found at the skull base or over the convexity of the brain and are thought to develop from arachnoid cap cells in the dura. These tumors are classified based on the WHO grading system, which divides meningiomas based on tumor cell type, mitotic activity, necrosis, and brain invasion. Grade I meningiomas are considered benign tumors (65%-80%), Grade II are atypical and more aggressive (20%-35%), while Grade III anaplastic meningiomas (<3%) are malignant.2–4
Treatment options include observation, surgery, radiotherapy, or a combination and are determined both by patient and tumor factors. With benign ICM in asymptomatic patients, observation with routine serial imaging may be considered, particularly if the tumor is small and/or the patient is elderly. For tumors causing symptoms, growing rapidly, or encroaching onto critical structures, definitive management is warranted.3 Standard of care currently consists of complete surgical resection, with consideration for postoperative radiotherapy depending on tumor grade and the completeness of resection. However, certain patients are poor surgical candidates because of other comorbidities or because the tumors are situated in surgically inaccessible or risky locations. In these cases, radiotherapy is an effective option.
External beam radiotherapy can be delivered as single-fraction stereotactic radiosurgery (SRS), hypofractionated stereotactic radiotherapy (hSRT) in a few fractions, or conventionally fractionated radiotherapy (cRT). Recent practice guidelines have defined radiosurgery as “radiation therapy delivered via stereotactic guidance with approximately 1 mm targeting accuracy to intracranial targets in 1 to 5 fractions.”5 However, the term hSRT is still commonly used to denote SRS schedules typically ranging in 2-5 fractions and is an important distinction given the differences in radiobiology and treatment delivery between single- and multifraction schedules. In the present review, SRS strictly denotes single-fraction radiosurgery while hSRT refers to nonconventional fractionated schedules delivered in >1 fraction of >2 Gy/fraction.
Radiotherapy has an established role for meningiomas after subtotal resection, recurrence, and as definitive primary treatment.3 Historically, cRT has been the standard radiotherapy approach for these patients. More recently, SRS has emerged as an effective and more convenient alternative for select patients, taking into account tumor location and proximity to sensitive neuroanatomy. SRS appears to be most effective for smaller ICMs that are situated a safe distance from optic pathways and other critical structures.3,4
hSRT has only recently emerged as an alternative to cRT or SRS for ICMs, but nonetheless there have been accumulating series reporting on outcomes with this approach. As a middle ground between SRS and cRT, hSRT retains the radiobiological advantages of fractionation, while achieving higher doses per fraction and a shorter overall treatment time for patients than cRT. The comparative safety and efficacy of a hypofractionated schedule in the treatment of meningioma patients has not been characterized in a randomized prospective trial. In the context of limited high-level evidence supporting its use, we conducted a systematic review to assess the clinical outcomes and toxicities of hSRT for ICMs.
Methods
Database Search
A systematic review of the literature for studies evaluating the use of hSRT for ICMs was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.6 The electronic databases PubMed and EMBASE were queried from inception to December 2016. Conference proceedings and reference lists from included studies were also hand-searched for additional reports eligible for inclusion. Please see Appendix 1 for the complete search strategy.
Study Selection
Included studies were retrospective or prospective series that examined an ICM population of at least 10 patients, either receiving hSRT as primary treatment or following subtotal resection. We defined hSRT as delivering multifractionated photon radiotherapy with a prescription dose of ≥ 2.5 Gy per fraction, using either a linear accelerator-based platform, CyberKnife (CK) (Accuray Incorporated, CA, USA), or multisession Gamma Knife (Elekta AB, Stockholm, Sweden). All studies reported on clinical outcomes such as local control (LC) and toxicity and had a median follow-up of at least 2 years. Reviews, editorials, and planning studies without reported clinical outcomes were excluded. Studies that combined data from different non-meningioma histologies or treatment modalities—precluding the evaluation of hSRT in the meningioma subset—were also excluded. Two reviewers (EKN and TKN) screened the abstracts of all studies to select articles for further analysis based on these inclusion and exclusion criteria. Full-text review of these selected studies was performed by the same 2 reviewers with discrepancies settled by a third investigator (GSB).
Data Abstraction
Data abstraction was completed using a standardized form, which was reviewed and approved by all authors. The following study characteristics were abstracted: first author, year of publication, country of study, type of study, number of patients, and patient age. Abstracted treatment details included type of radiotherapy, dose and fractionation, and treatment volumes while tumor variables included grade, location, and size. Abstracted outcomes included progression-free survival (PFS), LC, clinical response, radiologic tumor response, follow-up time, and toxicities.
Results
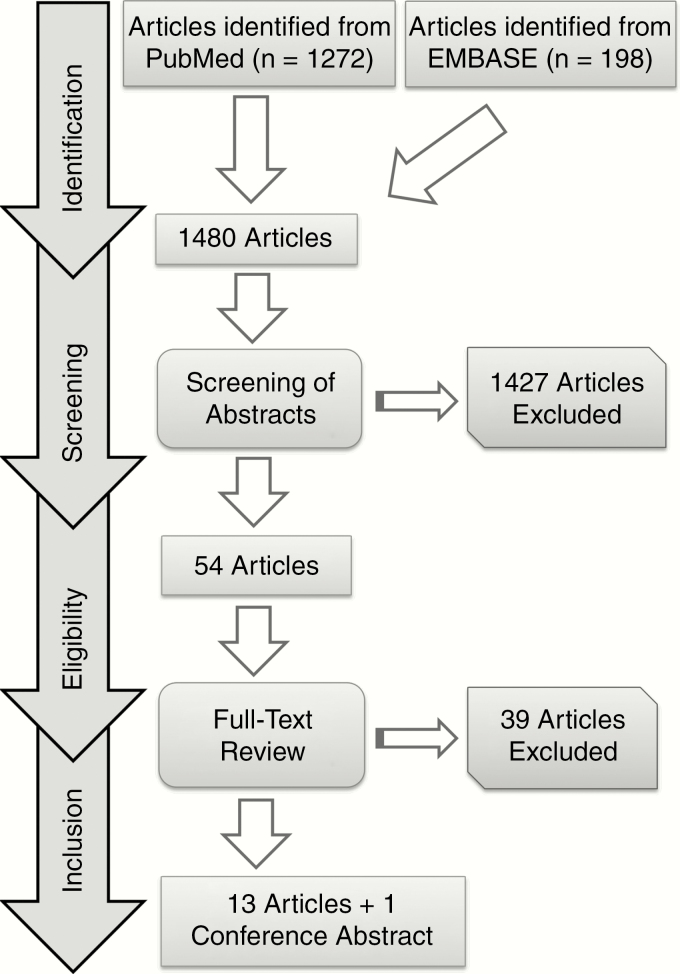
Our search strategy yielded 1480 articles. Subsequent screening of abstracts based on the defined inclusion/exclusion criteria resulted in 53 potentially eligible articles. Following full-text review, 13 articles remained. Hand-searching of conference proceedings resulted in 1 abstract meeting inclusion criteria. In total, 13 full-length publications and 1 abstract were included in our final analysis (Fig. 1).
Fig. 1.
Literature Search Strategy
In total, 13 retrospective studies and 1 prospective study met criteria for this review, reporting on a total of 638 tumors across 630 patients, with ages ranging from 18 to 90 (Table 1).7–20 Nearly an equal number of patients received primary upfront radiotherapy compared with adjuvant radiotherapy following surgery. Out of 307 tumors for which there was pathological histology, 91% were WHO Grade I meningiomas. Most of the tumors were located in the base of skull (66%) and 8% involved optic nerves or chiasm. Approximately one-third of patients received radiotherapy upfront, one-third received postoperative radiotherapy, and the timing of radiotherapy was not specified for the remainder. A narrow majority of patients were treated on a linear accelerator (57%) and the remainder were treated with CK. There were 7 patients who were previously irradiated. Complete summary of included studies can be found in Table 1.
Table 1.
Summary of Included Studies
| Study ID | No. Patients (No. Tumors) | Median Age | Tumor size (cc) Median (Range) |
Tumor Grade | Treatment Platform | Dose and Fractionation (Gy/fractions) |
Median Dose (Gy) | IDL Prescribed To | Crude Local Control (%) | Late Toxicity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Marchetti 201614 |
143 (143) | 52 | 8 (0.1-126) |
50% Grade I 50% presumed Grade I |
CK | 14-25/3-5 | 25 | 65%-86% | 95 | 4.9 |
| Demiral 201615 |
19 (19) | 40 | 26 (4-103) |
100% Grade I | LINAC | 25/5 | 25 | 85%-95% | N/R | 21.1 |
| Conti 201512 |
25 (25) | N/R | 5 (0.3-19) |
N/R | CK | 18/2 18-21/3 20-22/4 23-25/5 |
N/R | 63%-80% | 100 | 8 |
| Haghighi 201511 |
57 (57) | N/R | 7 (N/R) |
38% Grade I 4% Grade II 58% presumed Grade I |
LINAC | 37.5-40/15 | N/R | 75%-100% | 93 | 14 |
| Maranzano 201520 |
77 (80) | 65 | 23 (1-110) |
46% Grade I 15% Grade II 39% presumed Grade I |
LINAC | 45/15 42/14 |
43.5 | 90% (median) |
N/R | 0 |
| Navarria 20159 | 26 (27) | 67 | 13 (2-93) |
N/R | LINAC | 30/5 | N/R | N/R | 100 | N/R |
| Han 201419 |
22 (22) | 62 | N/R | N/R | LINAC | 25/5 | N/R | 90% (median) | 100 | 0 |
| Kaul 201410 |
92 (92) | N/R | N/R | 89% Grade I or presumed Grade I 11% Grade II or III |
LINAC | 37.6 ± 4.4 Gy total dose; 2.2-5 Gy/fraction | N/R | 80% (median) |
N/R | N/R |
| Smith 2014 (abstract)18 |
24 (26) | 60 (mean) |
N/R | N/R | CK | 22.5-30/5 | N/R | N/R | 100 | 8.3 |
| Marchetti 201113 |
21 (21) | 58 | 3 (0.3-23) |
N/R | CK | 25/5 | 25 | 75%-85% | 100 | 9.5 |
| Morimoto 201117 |
31 (32) | 68 | N/R | 100% Grade I or presumed Grade I | CK | 21-36/3-5 | 28 | 90%-100% | 91 | 9.7 |
| Trippa 200916 |
35 (35) | 59 | 23 (4-58) |
94% Grade I or presumed Grade I 6% Grade II |
LINAC | 45/15 42/14 |
N/R | N/R | N/R | 0 |
| Gorman 20087 |
38 (39) | 56 | N/R | 96% Grade I or presumed Grade I 4% Grade II |
LINAC | 35-45/15 | 37.5 | 75%-90% 80% (median) |
100 | 15.8 |
| Pham 20048 |
20 (20) | 53 | 6 (2-19) |
N/R | CK | 15-30/2-5 | 20 | 67%-95% | 90 | 0 |
Abbreviations: CK, CyberKnife; IDL, isodose level; LINAC, linear accelerator; N/R, not reported.
The median follow-up time ranged from 24.5 to 57.5 months. In 474 tumors assessed for radiologic response, 78% remained stable, 18% decreased in size and 4% increased in size (Table 2). Of 327 patients who were symptomatic prior to treatment, 17% had a complete resolution of symptoms, 41% had improvement, 36% had stability, and 6% had deterioration after radiotherapy. Crude LC was 90%-100% as reported in 10 studies. Actuarial LC rates, reported in 2 studies, were 89% and 92 ± 3% at 3 years. Median 5-year PFS was 88% as reported in 4 studies. Recognizing the popularity of hSRT dose schedules of ≤ 5 fractions, treatment response and crude LC rates were also summarized for studies with these prescriptions (Table 2).
Table 2.
Summary of Treatment Outcomes for all Included Studies and for Studies that Delivered hSRT in 2-5 Fractions
| Outcome | All Included Studies (n = 14; Pts = 630) | Studies with hSRT in 2-5 Fractions (n = 9; Pts = 331) | ||||
|---|---|---|---|---|---|---|
| % | n | Pts | % | n | Pts | |
| Clinical Response | ||||||
| Median | 45 | 43 | ||||
| Range | 18-87 | 11 | 482 | 18-87 | 7 | 275 |
| Mean | 51 | 46 | ||||
| Radiographic Response | ||||||
| Median | 14 | 22 | ||||
| Range | 6-41 | 10 | 469 | 9-41 | 6 | 262 |
| Mean | 19 | 23 | ||||
| Radiographic Stability | ||||||
| Median | 78 | 70 | ||||
| Range | 59-92 | 10 | 469 | 59-90 | 6 | 262 |
| Mean | 76 | 71 | ||||
| Crude Local Control | ||||||
| Median | 100 | 100 | ||||
| Range | 90-100 | 10 | 407 | 90-100 | 8 | 312 |
| Mean | 97 | 97 | ||||
| 3-Year PFS | ||||||
| Median | 92 | 94 | ||||
| Range | 89-100 | 3 | 254 | 89-100 | 2 | 162 |
| Mean | 94 | 94 | ||||
| 5-Year PFS | ||||||
| Median | 88 | 90 | ||||
| Range | 81-98 | 4 | 323 | 87-93 | 2 | 174 |
| Mean | 89 | 90 | ||||
Abbreviations: hSRT, hypofractionated stereotactic radiotherapy; PFS, progression-free survival; Pts, patients.
Overall, the median late toxicity rate was 8% with a range of 0% to 21% (Table 3). The median late toxicity rates for tumors involving the skull base and optic structures were 5% and 9%, respectively. The majority of the reported toxicities were Grades 1–2, and the most common late toxicities were decreased visual acuity in 8 patients and new cranial neuropathy in 11 patients. Grade 3 or higher toxicity was observed in 1 study, which reported 3 patients who developed Grade 3 or 4 deficits. These were seizure, gait disturbance, and bilateral vision loss. As above, for patients receiving hSRT in 5 fractions or less, the late toxicities were also summarized (Table 3).
Table 3.
Summary of Toxicities for All Included Studies and for Studies that Delivered hSRT in 2-5 Fractions
| Late Toxicity | All Included Studies (n = 14; Pts = 630) | Studies With hSRT in 2-5 Fractions (n = 9; Pts = 331) | ||||
|---|---|---|---|---|---|---|
| % | n | Pts | % | n | Pts | |
| Overall Late Toxicity | ||||||
| Median | 8 | 8 | ||||
| Range | 0-21 | 12 | 512 | 0-21 | 8 | 305 |
| Mean | 8 | 8 | ||||
| Overall > Grade 3 Toxicitys | ||||||
| Median | 0 | 0 | ||||
| Range | 0-10 | 12 | 581 | 0-10 | 9 | 331 |
| Mean | 1 | 1 | ||||
| Skull Base Tumors | ||||||
| Median | 5 | 2 | ||||
| Range | 0-21 | 5 | 261 | 0-21 | 4 | 204 |
| Mean | 8 | 6 | ||||
| Optic Tumors | ||||||
| Median | 9 | 9 | ||||
| Range | 8-10 | 2 | 46 | 8-10 | 2 | 46 |
| Mean | 9 | 9 | ||||
Abbreviations: hSRT, hypofractionated stereotactic radiotherapy; Pts, patients.
Discussion
To our knowledge, this is the first systematic review examining the use of hSRT in ICMs. A previous systematic review by Chung et al4 compared the outcomes achieved with cRT vs SRS; however, apart from 2 studies that included patients receiving staged radiosurgery, the review predominantly assessed single-fraction SRS. Our study demonstrates excellent local control rates with hSRT, ranging from 90% to 100%, and 5-year PFS of 81%-98%. In comparison to single-fraction SRS and cRT, hSRT is well tolerated, with Grade 3 or greater late toxicity rates ranging from 0% to 9.7%.
Studies with a larger median tumor volume (>20 cc) appeared to have lower LC rates. Maranzano and colleagues 20 had the lowest reported LC at 5 years with a median tumor volume of 23 cc, while a study by Demiral et al,15 which had the largest median tumor size of 26 cc, showed an LC rate of only 89%, as well as the highest late toxicity rate of 21.1%. This trend is in keeping with SRS studies that have shown worse outcomes with larger ICMs.21,22 All included studies primarily examined WHO Grade I ICMs based on histology or imaging alone. For tumors in which histology was available, only 2 studies had more than 2 patients with Grade II or greater ICMs.10,20 One of these did find lower LC and disease-specific survival in their grade II cohort, while the other study, which included a mixture both of Grade II and III ICMs, showed significantly worse 3-year and 5-year PFS.
The outcomes for ICMs treated with hSRT compare favorably to the published literature for single-fraction SRS and cRT. In the review by Chung and colleagues,4 28 articles were reviewed, including 3683 patients treated with SRS or cRT. The mean 5-year PFS was 93.2% for SRS and 91.8% for cRT. Both modalities had a similar mean complication rate of approximately 10%. These data are very similar to the outcomes we observed for patients treated with hSRT. Further to this point, one of our included series by Han et al19 reported no significant differences in clinical response, late toxicities, radiographic tumor control, or PFS among hSRT, SRS, and cRT. Albert and colleagues23 demonstrated a trend toward improved 3-year overall survival in postoperative patients receiving SRS or hSRT compared to cRT, but this difference was not statistically significant. The Response Assessment in Neuro-Oncology working group also conducted a review of 70 studies comparing radiotherapy modalities in low-grade ICMs.3 For SRS and cRT, median clinical responses to treatment were 29% and 43.2%, respectively, and median radiographic responses were 49% and 23%, respectively. The higher radiographic regression following SRS has been observed in other studies as well and is postulated to be an effect of high-dose radiation overcoming radioresistance in targeted tissue.4 Median radiologic response across the included hSRT studies was only 14% but ranged widely from 6% to 41%. Generally, patients are counseled to expect tumor stability rather than shrinkage as the most common outcome following radiotherapy, which is supported by our finding that radiographic stability ranged from 52% to 98% across included studies. There was excellent clinical response in the studies we examined with a median of 59%.
While SRS provides favorable benefits to some patients, there are limitations to its widespread application. Larger tumors or those in non–skull-base locations typically have a higher risk of adverse radiation reactions, compromising the utility of SRS as alternative therapy in patients who are not operable because of tumor size or location.24 In one series, 38.2% of patients receiving SRS for parafalcine or parasagittal ICM developed new or worsened perilesional swelling, with tumor size and venous sinus invasion being predictive factors for post-SRS edema.25,26 In addition, Patil et al27 found that patients with parasagittal meningiomas were more than 4 times as likely to develop symptomatic edema after SRS than nonparasagittal ICMs. Finally, Pollock and colleagues26,28 reported higher complication rates when treating larger ICMs (>10 cc) with SRS.
As an alternative to single-fraction SRS, hSRT potentially provides a broader scope of application. Rates of peritumoral edema appear to be low, as Morimoto et al17 found 19% of patients receiving hSRT had treatment-associated edema.Unger and colleagues29 reported lower rates of symptomatic edema with hSRT compared to SRS with 2-year rates of 3.2% and 12.5%, respectively. Additionally, hypofractionation has fewer restrictions with regards to tumor location. A study by Columbo et al30 found that patients with ICMs close to critical structures had low rates of toxicities when treated using hSRT and included 63 patients who could not have been treated by SRS otherwise. Further support for the relative safety of hSRT was demonstrated in a report31 that described hSRT symptomatic edema rates of 6.3%, compared to 43% with SRS when treating ICMs in convexity or parasagittal locations.
From a radiobiological perspective, fractionation allows clinicians to take advantage of differences in alpha-beta ratios between tumor and normal tissue to potentially improve the therapeutic ratio. As described in an example outlined by Kirkpatrick and colleagues,24 compared to single-fraction SRS, a hypofractionated regimen can maintain the biological effective dose (BED) to tumor while reducing normal tissue toxicity. In addition, the higher dose per fraction may theoretically improve LC compared to cRT, although no prospective study has shown superiority of one modality over another.23 While most of the studies included in the present report delivered ≥5 Gy per fraction, 5 studies included patients receiving 14 to 15 fractions with less than or equal to 3 Gy per fraction.7,10,11,16,20 This moderately hypofractionated cohort may differ from the more common regimen of 5 fractions or less. In particular, one of these studies reported the lowest 5-year LC rate of 84%, one had a relatively low median LC of 24 months, and another had a 5-year PFS of only 80.9%.10,16,20 While these findings are in support of the theory that higher doses per fraction offer superior control, no conclusions can be drawn based on the limited evidence at the present time. Conversely, extreme hypofractionation can be potentially harmful. The only study that reported Grade III-IV late toxicity had a higher average dose per fraction. The patient who experienced Grade IV toxicity received the largest dose of 36 Gy in 3 fractions.17
BED allows dose fractionation schedules to be compared, and these values can be estimated for benign meningiomas based on a previously reported32 alpha-beta ratio of 3. For all included studies, the BED ranged from 37.33 to 180 Gy3, with outcomes and toxicities summarized in Tables 2 and 3. The most common prescription was 25 Gy in 5 fractions, which was associated with a BED of 66.67 Gy3. Three studies that exclusively used this fractionation achieved local control rates of 89%, 100%, and 100%.13,15,19 Two studies that delivered lower BED dose fractionations (<40 Gy3) had slightly inferior local control rates of 90% and 95%, but the number of patients receiving these schedules was relatively low.8,14 Studies that delivered moderately hypofractionated dose regimens in 14-15 fractions had a BED of 57.2-90 Gy3, which is comparable to the 2-5 fraction regimens. A significant caveat that limits our ability to truly compare these dose schedules based on BED alone is the variability in which isodose level (IDL) was prescribed to, with most studies reporting this information only as a median or range. A course of 25 Gy in 5 fractions prescribed to the 80% IDL would result in a higher dose within the tumor as the same course prescribed to the 100% IDL. Taken together the heterogeneity in dose fractionations across studies and uncertainties in which IDL each dose regimen was prescribed to prohibits any strong conclusions or recommendations based on these data.
There are several limitations to this review worth noting. First, the study is retrospective in nature and only descriptive analyses were completed given the substantial heterogeneity between studies, most notably in dose fractionation delivered and outcomes reported. Second, there was a relatively short follow-up duration across a number of studies. Longer follow-up will be essential to confirm an acceptable toxicity profile but also to ascertain the true LC rates with hSRT given the indolent biology of this mostly Grade I meningioma population. Third, studies with a mixed cohort of hSRT with either SRS or cRT were excluded in an effort to obtain a dataset of only meningiomas treated with hSRT. The reports omitted through this approach may have had an impact on our results. Finally, there were several studies that did not meet the minimum median follow-up time of 2 years that were also excluded.
Conclusion
Larger, prospective studies with extended follow-up are required to fully characterize the tolerability and long-term control of hSRT for ICMs and assess how it truly compares to SRS and cRT. Until then, this systematic review supports the application of hSRT as an effective and safe therapeutic option for ICMs. It remains a reasonable alternative to SRS for tumors inappropriate for single-fraction radiotherapy, provides the radiobiological advantages of fractionation, and allows for a shorter and more convenient treatment course for patients.Supplementary MaterialSupplementary material is available at Neuro-Oncology Practice online.
Funding
The authors have no sources of funding to disclose.
Conflict of interest statement
AVL has previously accepted an honorarium from Varian Medical Systems Inc. The other authors have nothing to declare.
Supplementary Material
References
- 1. Abbassy M, Woodard TD, Sindwani R Recinos PF. An overview of anterior skull base meningiomas and the endoscopic endonasal approach. Otolaryngol Clin North Am. 2016;49(1):141–152. doi: 10.1016/j.otc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 2. Magill ST, Young JS, Chae R Aghi MK Theodosopoulos PV McDermott MW. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg Focus. 2018;44(4):E4. doi: 10.3171/2018.1.FOCUS17752. [DOI] [PubMed] [Google Scholar]
- 3. Rogers L, Barani I, Chamberlain M et al. . Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung LK, Mathur I, Lagman C et al. . Stereotactic radiosurgery versus fractionated stereotactic radiotherapy in benign meningioma. J Clin Neurosci. 2017;36:1–5. doi: 10.1016/j.jocn.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 5. Seung SK, Larson DA, Galvin JM et al. . American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) practice guideline for the performance of stereotactic radiosurgery (SRS). Am J Clin Oncol. 2013;36(3):310–315. doi: 10.1097/COC.0b013e31826e053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stewart LA, Clarke M, Rovers M et al. ; PRISMA-IPD Development Group Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 7. Gorman L, Ruben J, Myers R Dally M. Role of hypofractionated stereotactic radiotherapy in treatment of skull base meningiomas. J Clin Neurosci. 2008;15(8):856–862. doi: 10.1016/j.jocn.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 8. Pham CJ, Chang SD, Gibbs IC Jones P Heilbrun MP Adler JR Jr.. Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2004;54(4):799–810; discussion 810. doi: 10.1227/01.NEU.0000114261.18723.6A. [DOI] [PubMed] [Google Scholar]
- 9. Navarria P, Pessina F, Cozzi L et al. . Hypofractionated stereotactic radiation therapy in skull base meningiomas. J Neurooncol. 2015;124(2):283–289. doi: 10.1007/s11060-015-1838-6. [DOI] [PubMed] [Google Scholar]
- 10. Kaul D, Budach V, Wurm R et al. . Linac-based stereotactic radiotherapy and radiosurgery in patients with meningioma. Radiat Oncol. 2014;9:78. doi: 10.1186/1748-717X-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haghighi N, Seely A, Paul E Dally M. Hypofractionated stereotactic radiotherapy for benign intracranial tumours of the cavernous sinus. J Clin Neurosci. 2015;22(9):1450–1455. doi: 10.1016/j.jocn.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 12. Conti A, Pontoriero A, Midili F et al. . CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. Springerplus. 2015;4:37. doi: 10.1186/s40064-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchetti M, Bianchi S, Milanesi I et al. . Multisession radiosurgery for optic nerve sheath meningiomas—an effective option: preliminary results of a single-center experience. Neurosurgery. 2011;69(5):1116–1122; discussion 1122. doi: 10.1227/NEU.0b013e31822932fe. [DOI] [PubMed] [Google Scholar]
- 14. Marchetti M, Bianchi S, Pinzi V et al. . Multisession radiosurgery for sellar and parasellar benign meningiomas: long-term tumor growth control and visual outcome. Neurosurgery. 2016;78(5):638–646. doi: 10.1227/NEU.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 15. Demiral S, Dincoglan F, Sager O et al. . Hypofractionated stereotactic radiotherapy (HFSRT) for WHO grade I anterior clinoid meningiomas (ACM). Jpn J Radiol. 2016;34(11):730–737. doi: 10.1007/s11604-016-0581-z. [DOI] [PubMed] [Google Scholar]
- 16. Trippa F, Maranzano E, Costantini S Giorni C. Hypofractionated stereotactic radiotherapy for intracranial meningiomas: preliminary results of a feasible trial. J Neurosurg Sci. 2009;53(1):7–11. [PubMed] [Google Scholar]
- 17. Morimoto M, Yoshioka Y, Shiomi H, et al. . Significance of tumor volume related to peritumoral edema in intracranial meningioma treated with extreme hypofractionated stereotactic radiation therapy in three to five fractions. Jpn J Clin Oncol. 2011;41(5):609–616. doi: 10.1093/jjco/hyr022. [DOI] [PubMed] [Google Scholar]
- 18. Smith D, Ghosh S, O’Leary M, Chu C. Abstract 40: Meningioma patients treated with hypofractionated radiosurgery—a review of 24 patients. J Radiat Oncol. 2014; 3:17. doi: 10.1007/s13566-014-0140-0. Abstract presented at: 23rd Meeting of the American College of Radiation Oncology; February 14–16, 2013. San Antonio, TX. [DOI] [Google Scholar]
- 19. Han J, Girvigian MR, Chen JC et al. . A comparative study of stereotactic radiosurgery, hypofractionated, and fractionated stereotactic radiotherapy in the treatment of skull base meningioma. Am J Clin Oncol. 2014;37(3):255–260. doi: 10.1097/COC.0b013e318271b36a. [DOI] [PubMed] [Google Scholar]
- 20. Maranzano E, Draghini L, Casale M et al. . Long-term outcome of moderate hypofractionated stereotactic radiotherapy for meningiomas. Strahlenther Onkol. 2015;191(12):953–960. doi: 10.1007/s00066-015-0915-2. [DOI] [PubMed] [Google Scholar]
- 21. DiBiase SJ, Kwok Y, Yovino S et al. . Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60(5):1515–1519. doi: 10.1016/j.ijrobp.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 22. Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43(3):405–413; discussion 413. doi: 10.1097/00006123-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 23. Albert A, Lee A, Vijayakumar S Kanakamedala M Allbright R Schreiber D. Adjuvant treatment of meningioma with stereotactic radiation surgery and hypofractionated stereotactic radiation surgery: patterns of care and survival in a large, hospital database. Adv Radiat Oncol. 2018;3(3):280–287. doi: 10.1016/j.adro.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirkpatrick JP, Soltys SG, Lo SS et al. . The radiosurgery fractionation quandary: single fraction or hypofractionation?Neuro Oncol. 2017;19(suppl 2):ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheehan JP, Cohen-Inbar O, Ruangkanchanasetr R et al. . Post-radiosurgical edema associated with parasagittal and parafalcine meningiomas: a multicenter study. J Neurooncol. 2015;125(2):317–324. doi: 10.1007/s11060-015-1911-1. [DOI] [PubMed] [Google Scholar]
- 26. Pollock BE, Stafford SL, Link MJ. Gamma knife radiosurgery for skull base meningiomas. Neurosurg Clin N Am. 2000;11(4):659–666. doi: 10.1016/S1042-3680(18)30091-3. [DOI] [PubMed] [Google Scholar]
- 27. Patil CG, Hoang S, Borchers DJ 3rd et al. . Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63(3):435–440; discussion 440. doi: 10.1227/01.NEU.0000325257.58684.92. [DOI] [PubMed] [Google Scholar]
- 28. Pollock BE, Stafford SL, Link MJ Brown PD Garces YI Foote RL. Single-fraction radiosurgery of benign intracranial meningiomas. Neurosurgery. 2012;71(3):604–612; discussion 613. doi: 10.1227/NEU.0b013e31825ea557. [DOI] [PubMed] [Google Scholar]
- 29. Unger KR, Lominska CE, Chanyasulkit J et al. . Risk factors for posttreatment edema in patients treated with stereotactic radiosurgery for meningiomas. Neurosurgery. 2012;70(3):639–645. doi: 10.1227/NEU.0b013e3182351ae7. [DOI] [PubMed] [Google Scholar]
- 30. Colombo F, Casentini L, Cavedon C Scalchi P Cora S Francescon P.. Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery. 2009;64(2 suppl):A7–A13. doi: 10.1227/01.NEU.0000338947.84636.A6. [DOI] [PubMed] [Google Scholar]
- 31. Girvigian MR, Chen JC, Rahimian J Miller MJ Tome M. Comparison of early complications for patients with convexity and parasagittal meningiomas treated with either stereotactic radiosurgery or fractionated stereotactic radiotherapy. Neurosurgery. 2008;62(5 suppl):A19–A27; discussion A27–A28. doi: 10.1227/01.neu.0000325933.34154.cb. [DOI] [PubMed] [Google Scholar]
- 32. Kondziolka D, Shin SM, Brunswick A Kim I Silverman JS. The biology of radiosurgery and its clinical applications for brain tumors. Neuro Oncol. 2015;17(1):29–44. doi: 10.1093/neuonc/nou284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.