Abstract
Background
Using data from a large national stroke registry, we aimed to investigate the incidence and determinants of in-hospital and post-discharge recovery after acute ischemic stroke and the independence of their occurrence.
Methods
In-hospital recovery was defined as an improvement of 4 points or > 40% in the National Institutes of Health Stroke Scale (NIHSS) score from admission to discharge. Post-discharge recovery was defined as any improvement in the modified Rankin Scale (mRS) score from discharge to 3 months after stroke onset. Two analytic methods (multivariate and multivariable logistic regression) were applied to compare the effects of 18 known determinants of 3-month outcome and to verify whether in-hospital and post-discharge recovery occur independently.
Results
During 54 months, 11,088 patients with acute ischemic stroke meeting the eligibility criteria were identified. In-hospital and post-discharge recovery occurred in 36% and 33% of patients, respectively. Multivariate logistic regression with an equality test for odds ratios showed that 7 determinants (age, onset-to-admission time, NIHSS score at admission, blood glucose at admission, systolic blood pressure, smoking, recanalization therapy) had a differential effect on in-hospital and post-discharge recovery in the way of the opposite direction or of the same direction with different degree (all P values < 0.05). Both in-hospital and post-discharge recovery occurred in 12% of the study population and neither of them in 43%. The incidence of post-discharge recovery in those with in-hospital recovery was similar to that in those without (33.8% vs. 32.7%, respectively), but multivariable analysis showed that these 2 types of recovery occurred independently.
Conclusion
Our findings suggest that, in patients with acute ischemic stroke, in-hospital and post-discharge recovery may occur independently and largely in response to different factors.
Keywords: Registries, Stroke, Brain Infarction: Recovery of Function, Prognosis
Graphical Abstract
INTRODUCTION
While stroke recovery may proceed rapidly during the first month after stroke onset, subsequent recovery is often slower, and may continue for over a year.1,2 Thus, stroke recovery can be divided into early and later phases, in-hospital and post-discharge recovery, which may differ in speed, determinants and mechanisms of recovery.
In-hospital recovery after stroke refers to early neurologic improvement and is reportedly influenced by recanalization therapy, age, gender, time from onset to treatment, glucose levels, stroke severity, blood pressure, and history of atrial fibrillation.3,4,5,6 Stroke patients who are stable and ready for being discharged from an acute care hospital are usually discharged to home or transferred to rehabilitation units, where post-discharge recovery may proceed during the following 3 months.1
Functional outcomes of stroke and their determinants have been thoroughly studied, but such investigations were generally based on clinical trials, included a limited sample of patients, or single-center observational studies.7,8,9,10,11,12,13,14,15 With respect to large-scale and nationwide stroke registers, there is limited information on functional outcomes after stroke. Furthermore, few studies have distinguished between recovery during hospitalization and recovery after discharge (e.g., during a subsequent period of up to 3 months, which is when the most significant recovery is expected to occur).16
Using a prospective multicenter nationwide stroke registry comprising over 10,000 acute ischemic stroke patients,17,18 the present study aimed to elucidate 1) the incidence of in-hospital and post-discharge recovery, 2) the differential effect of the known determinants of 3-month functional outcomes on in-hospital and post-discharge recovery, and 3) whether in-hospital and post-discharge recovery occur independently.
METHODS
Study subjects
This study was a retrospective analysis based on data from the Clinical Research Center for Stroke-5th division (CRCS-5) registry database. The CRCS-5 registry is a prospective, web-based registry of ischemic stroke patients admitted to participating centers throughout Korea, including the provinces of Seoul, Busan, Daegu, Gwangju, Daejeon, Gyeonggi, and Jeju, where > 60% of Koreans reside (Supplementary Fig. 1). The CRCS-5 registry was established to monitor the quality of care in stroke patients, and collected clinical information on demographics, vascular risk factors, stroke characteristics including stroke subtype, diagnostic work-up, in-hospital management, laboratory findings, and functional outcomes. A detailed description of the CRCS-5 registry is available elsewhere.17,18
Using this CRCS-5 registry database, we identified consecutive patients with acute ischemic stroke who were admitted at a participating center between April 2008 and September 2012. The following inclusion criteria were applied: hospital admission within 7 days of stroke onset; discharge within 30 days of admission; functional outcomes measured with the modified Rankin Scale (mRS) at discharge and at 3 months from stroke onset.
Data collection
Known determinants of 3-month functional outcomes were selected, based on previous studies5,19 and consensus meetings of stroke experts considering clinical relevance of their associations with stroke recovery, among variables that were available in the CRCS-5 registry database.17 Nineteen variables were chosen: age, gender, body mass index (BMI), onset-to-admission time, stroke subtype according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification with some modifications,20,21 blood glucose levels at admission, systolic blood pressure (SBP) at admission, pre-stroke mRS score, history of stroke, hypertension, diabetes mellitus, hyperlipidemia, smoking, congestive heart failure (CHF), atrial fibrillation, NIHSS score at admission, all kinds of recanalization therapy including thrombolysis and endovascular treatment, prior statin use, and statin use at discharge. Stroke subtype was re-classified as large artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardioembolism (CE), and other (stroke of other determined etiology, or undetermined etiology).
Outcome measures
Post-discharge recovery was defined as any improvement in a mRS score22 from discharge to 3 months after stroke onset, while in-hospital recovery was defined as an improvement of 4 points14 or > 40% in a NIHSS score23 from admission to discharge. In Korea, most of hospitalized acute stroke patients are treated in acute care settings including stroke units, intensive care units or neurological general wards until they are stabilized neurologically and medically. Once stabilized, they are transferred to in-hospital rehabilitation service, subacute care or rehabilitation hospitals or long-term facilities, or are discharged to home. In this study, discharge was defined as discharge from an acute care setting, usually one or two weeks after admission, including being discharged to home or other hospitals or transferred to in-hospital rehabilitation service, as defined in the CRCS-5 registry. The mRS scores were recorded at two distinct time points via direct examination or telephone interviews performed by trained stroke coordinators.
Statistical analysis
Summary statistics are presented as mean ± standard deviation or median (interquartile range [IQR]) for continuous variables, and as number of participants (percentage) for categorical variables. For each variable, median or mode imputation was used to account for missing values of < 5%. The effects on the incidence of in-hospital and post-discharge recovery were compared for each of the 19 determinants of 3-month outcome.
Because in-hospital and post-discharge recovery occur in succession and may affect each other, we adopted an analytic method that allowed us to investigate the effects of covariates simultaneously on two different but potentially correlated outcomes. Multivariate logistic regression analysis represents a statistical means for analyzing the dependence of multiple binary outcomes on a set of covariates while considering the correlation among these outcomes. We applied Gumbel logistic regression to model the dependence of in-hospital and post-discharge recovery on the same set of covariates while estimating the degree of association among the two recovery phases and the correlation coefficients of the responses.24 The analysis was performed using the generalized estimating equation (GEE) method, which takes into account the correlation structure between two correlated outcomes. For each variable, the equality of odds ratios (ORs) between the two correlated outcomes was examined using the Wald test. Among the 19 selected covariates, hyperlipidemia was excluded from the final analysis because of its collinearity with prior statin use.
Meanwhile, the dependency of post-discharge recovery on in-hospital recovery was examined using multivariable logistic regression models in which the above-described 18 covariates together with in-hospital recovery were entered as independent variables, whereas post-discharge recovery was entered as a dependent variable.
Furthermore, reflecting the observed differences in duration of hospitalization by center and year of admission (as described in the first paragraph of the Results section), we adopted the GEE method for center effect and added year of admission to the models as a covariate.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statements.25 All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). A 2-sided P value of < 0.05 was considered to indicate statistical significance.
Ethics statement
The collection of clinical information for the CRCS-5 registry was approved by the local Institutional Review Boards (IRBs) in all participating centers with a waiver of patient consent because of the anonymity of the collected data and the minimal risk to participants. We obtained further approval for the use of the registry database for the purpose of the present study (Seoul National University Bundang Hospital IRB No. 2017-09-002-001).
RESULTS
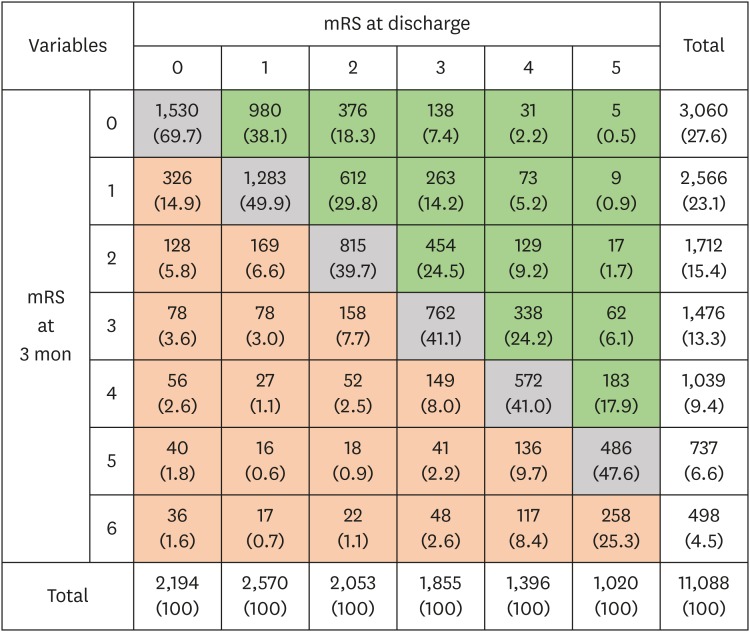
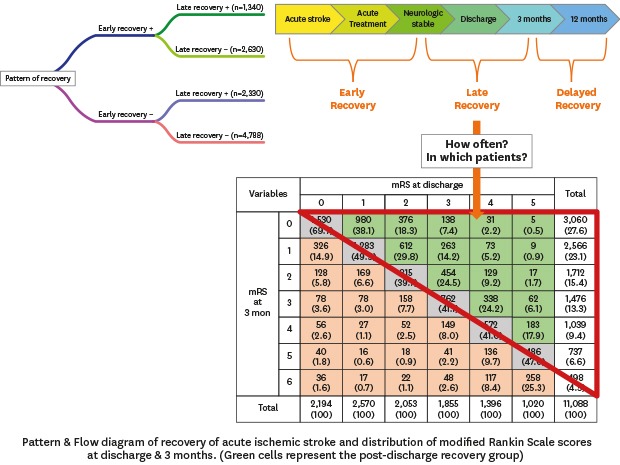
Among 14,943 patients hospitalized at the 12 participating centers during the study period, 1,803 patients were excluded due to duration of hospitalization more than 30 days (n = 723), in-hospital death (n = 370), and non-availability of functional outcome (n = 710). Finally, 11,088 (men, 55%; age, 67 ± 13 years) met the eligibility criteria (Supplementary Fig. 2). Missing imputation was performed in 4 subjects for NIHSS and in 504 subjects for blood glucose at admission. The median NIHSS score was 3 (IQR, 1–7) at admission and 2 (IQR, 1–5) at discharge. The mean value of onset-to-admission time was 10.0 ± 5.6 hours, and 40.8% of patients were hospitalized within 6 hours of onset. The mean duration of hospitalization was 9.5 ± 5.6 days, which differed significantly by center and year of admission (P's < 0.001 by analysis of variance). Favorable functional outcomes (mRS score 0–2) were noted among 61% of patients at discharge and among 66% of patients at 3 months from stroke onset (Fig. 1).
Fig. 1. Distribution of mRS scores at discharge and 3 months after stroke onset. Green cells represent the post-discharge recovery group. Orange cells represent the aggravated group. Gray represent the non-changing group. Data are number of patients (%).
mRS = modified Rankin scale.
The median value of change in NIHSS scores from admission to discharge (NIHSS at admission – at discharge) was 0 (IQR, 0–2). In-hospital recovery (an improvement of 4 points or > 40% in a NIHSS score) was observed in 36% of patients. Worsening of the NIHSS score by 1 point or more from admission to discharge occurred in 12% of patients (Fig. 1). Patients who showed in-hospital recovery were more likely to be younger, men, current smokers, previously healthy (pre-stroke mRS score = 0); they were also more likely to have a history of atrial fibrillation, more severe neurological deficits at admission, lower levels of blood glucose at admission, and shorter onset-to-admission time, as well as to be treated with recanalization therapy; but they were less likely to have a history of stroke, diabetes mellitus, or hyperlipidemia (Table 1). Cardioembolism was more frequently noted among patients who showed in-hospital recovery, while LAA and other stroke subtype were more frequently noted among those who did not show in-hospital recovery; the prevalence of SVO was similar between patients with and without in-hospital recovery.
Table 1. Effect of 3-month outcome determinants in in-hospital and post-discharge recovery after acute ischemic stroke.
| Variables | No in-hospital recovery (n = 7,118) | In-hospital recovery (n = 3,970) | P value | No post-discharge recovery (n = 7,418) | Post-discharge recovery (n = 3,670) | P value | |
|---|---|---|---|---|---|---|---|
| Age, yr | 67.75 ± 12.6 | 66.8 ± 13.1 | < 0.01 | 70.4 ± 12.0 | 65.3 ± 12.7 | < 0.001 | |
| Men | 4,119 (57.9) | 2,406 (60.6) | < 0.01 | 4,228 (57.0) | 2,297 (62.6) | < 0.001 | |
| BMI, kg/m2 | 24.1 ± 13.1 | 23.9 ± 11.1 | 0.64 | 24.0 ± 14.0 | 24.0 ± 8.2 | 0.03 | |
| Onset-to-admission time, hr | 29.0 ± 37.0 | 21.8 ± 32.1 | < 0.001 | 26.9 ± 35.5 | 26.8 ± 35.3 | 0.46 | |
| Blood glucose at admission, mg/dL | 120.5 ± 53.4 | 115.8 ± 47.0 | < 0.001 | 118.7 ± 50.0 | 119.1 ± 53.8 | 0.12 | |
| SBP at admission, mmHg | 148.6 ± 26.8 | 147.7 ± 27.1 | 0.09 | 147.8 ± 27.1 | 149.2 ± 26.6 | 0.02 | |
| Previous stroke | 1,538 (21.6) | 764 (19.2) | < 0.01 | 1,676 (21.9) | 676 (18.4) | < 0.001 | |
| Hypertension | 4,961 (69.7) | 2,727 (68.7) | 0.27 | 5,197 (70.1) | 2,491 (67.9) | 0.02 | |
| Diabetes mellitus | 2,461 (34.6) | 1,227 (30.9) | < 0.001 | 2,454 (33.1) | 1,234 (33.6) | 0.57 | |
| Hyperlipidemia | 2,397 (33.7) | 1,258 (31.7) | 0.03 | 2,390 (32.2) | 1,265 (34.5) | 0.02 | |
| Smokera | < 0.001 | < 0.001 | |||||
| Current | 1,803 (25.3) | 1,121 (28.2) | 1,862 (25.1) | 1,062 (28.9) | |||
| Ex-smoker | 657 (9.5) | 426 (10.7) | 729 (9.8) | 373 (10.2) | |||
| Non-smoker | 4,639 (65.7) | 2,423 (61.0) | 4,827 (65.1) | 2,235 (60.9) | |||
| CHF | 121 (1.7) | 60 (1.5) | 0.45 | 141 (1.9) | 40 (1.1) | 0.002 | |
| Atrial fibrillation | 1,176 (16.5) | 877 (22.1) | < 0.001 | 1,481 (20.0) | 572 (15.6) | < 0.001 | |
| NIHSS score at admission. | 3 (1–6) | 4 (2–9) | < 0.001 | 3 (1–7) | 3 (1–6) | < 0.001 | |
| Pre-stroke mRS score | < 0.001 | < 0.001 | |||||
| 2–5 | 938 (13.2) | 428 (10.8) | 1,056 (14.2) | 310 (8.4) | |||
| 0–1 | 6,180 (86.8) | 3,542 (89.2) | 6,362 (85.8) | 3,360 (91.6) | |||
| TOAST classification | < 0.001 | < 0.001 | |||||
| LAA | 2,803 (39.4) | 1,404 (35.4) | 2,748 (37.0) | 1,459 (39.8) | |||
| SVO | 1,379 (19.4) | 754 (19.0) | 1,333 (18.0) | 800 (21.8) | |||
| Cardioembolism | 1,264 (17.8) | 956 (24.1) | 1,581 (21.3) | 639 (17.4) | |||
| Other | 1,672 (23.5) | 856 (21.6) | 1,756 (23.7) | 772 (21.0) | |||
| Recanalization therapy | 608 (8.5) | 838 (21.1) | < 0.001 | 975 (13.1) | 471 (12.8) | 0.65 | |
| Prior statin use | 1,138 (16.0) | 686 (17.3) | 0.08 | 1,201 (16.2) | 623 (17.0) | 0.29 | |
| Statin use at discharge | 5,710 (80.2) | 3,193 (80.4) | 0.79 | 5,869 (79.1) | 3,034 (82.7) | < 0.001 | |
Data are presented as mean ± standard deviation or median (interquartile range) or number (%).
BMI = body mass index, SBP = systolic blood pressure, CHF = congestive heart failure, NIHSS = National Institute of Health Stroke Scale, mRS = modified Rankin scale, TOAST = Trial of Org 10172 in Acute Stroke Treatment, LAA = large artery atherosclerosis, SVO = small vessel occlusion.
aSmoking status was divided by current, ex-smoker (quit within 5 years), and non-smoker (never smoker or quit > 5 years).
Post-discharge recovery was noted in 33% of patients, whereas mRS scores worsened in 18% of patients (Fig. 1). Patients who showed post-discharge recovery were more likely to be younger, men, and current smokers, as well as to have diabetes mellitus or hyperlipidemia (Table 1). Patients who showed post-discharge recovery had better premorbid status and lower NIHSS scores at admission and discharge; such patients were also more likely to receive statins at discharge. LAA and SVO were more frequent among patients who showed post-discharge recovery, while cardioembolism was more frequent among those who did not show post-discharge recovery.
Multivariate analysis revealed that age, pre-stroke mRS, NIHSS at admission, and stroke subtype were associated with both in-hospital and post-discharge recovery. However, onset-to-admission time, blood glucose levels at admission, SBP at admission, history of stroke, CHF, recanalization therapy, and prior statin use were associated only with in-hospital recovery, whereas gender and statin use at discharge were associated only with post-discharge recovery (Table 2). Tests for OR equality between in-hospital and post-discharge recovery showed that age, onset-to-admission time, blood glucose levels at admission, SBP at admission, smoking status, NIHSS score at admission, and recanalization therapy differentially affected in-hospital and post-discharge recovery (all P values for OR equality < 0.05).
Table 2. Multivariate analysis for determining factors associated with in-hospital and post-discharge recovery after acute ischemic stroke.
| Variables | In-hospital recovery | Post-discharge recovery | P value for OR equality | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Age, 5-year increments | 0.96a | 0.95–0.98 | 0.93a | 0.91–0.94 | < 0.01 | |
| Gender, men | 1.05 | 0.95–1.16 | 1.20a | 1.08–1.32 | 0.08 | |
| BMI, kg/m2 | 1.00 | 1.00–1.00 | 1.00 | 0.99–1.00 | 0.59 | |
| Onset-to-admission time, hr | 0.93a | 0.90–0.96 | 1.00 | 0.97–1.03 | < 0.001 | |
| Blood glucose at admission, 10 mg/dL | 0.98a | 0.97–0.99 | 1.00 | 0.99–1.01 | < 0.001 | |
| SBP at admission, 10 mmHg | 0.98a | 0.96–0.99 | 1.01 | 0.99–1.02 | 0.01 | |
| Previous stroke | 0.85a | 0.76–0.95 | 0.92 | 0.82–1.03 | 0.32 | |
| Hypertension | 1.04 | 0.95–1.14 | 0.98 | 0.89–1.08 | 0.39 | |
| Diabetes mellitus | 0.97 | 0.88–1.06 | 1.01 | 0.92–1.12 | 0.48 | |
| Smokerb | 0.02 | |||||
| Current | 1.10 | 0.99–1.23 | 0.89 | 0.80–1.00 | ||
| Ex-smoker | 1.06 | 0.93–1.21 | 0.88 | 0.77–1.00 | ||
| Non-smoker | Reference | Reference | ||||
| CHF | 0.71a | 0.51–1.00 | 0.76 | 0.53–1.10 | 0.78 | |
| Atrial fibrillation | 1.13 | 0.96–1.32 | 1.07 | 0.91–1.26 | 0.64 | |
| NIHSS score at admission | 1.05a | 1.04–1.06 | 0.99a | 0.98–0.999 | < 0.001 | |
| Pre-stroke mRS score 2–5 | 0.79a | 0.69–0.91 | 0.68a | 0.59–0.79 | 0.16 | |
| TOAST classification | 0.05 | |||||
| LAA | 0.85a | 0.76–0.95 | 0.98 | 0.87–1.09 | ||
| SVO | Reference | Reference | ||||
| Cardioembolism | 0.90 | 0.76–1.07 | 0.77a | 0.65–0.92 | ||
| Other | 0.80a | 0.70–0.91 | 0.79a | 0.69–0.90 | ||
| Recanalization therapy | 1.99a | 1.75–2.26 | 1.12 | 0.97–1.28 | < 0.001 | |
| Prior statin use | 1.13a | 1.01–1.27 | 1.08 | 0.96–1.21 | 0.55 | |
| Statin use at discharge | 1.11 | 1.00–1.24 | 1.18a | 1.06–1.32 | 0.40 | |
The relationship between in-hospital and post-discharge recovery had a correlation coefficient of 0.004.
OR = odds ratio, CI = confidence interval, BMI = body mass index, SBP = systolic blood pressure, CHF = congestive heart failure, NIHSS = National Institute of Health Stroke Scale, mRS = modified Rankin scale, TOAST = Trial of Org 10172 in Acute Stroke Treatment, LAA = large artery atherosclerosis, SVO = small vessel occlusion.
aStatistically significant with P < 0.05; bSmoking status was divided by current, ex-smoker (quit within 5 years), and non-smoker (never smoker or quit > 5 years).
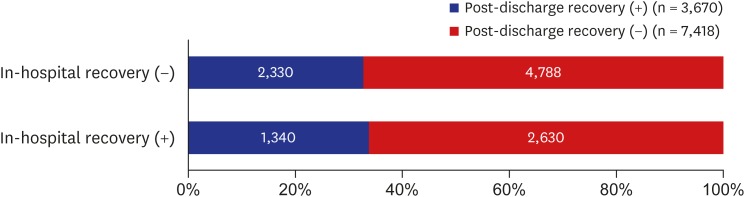
Both in-hospital and post-discharge recovery were observed in 12% of the study population. On the other hand, 43% of patients showed neither in-hospital nor post-discharge recovery. The incidence of post-discharge recovery in patients who experienced in-hospital recovery was similar to that in those who did not (33.8% vs. 32.7%, respectively; P = 0.27 by Pearson's χ2 test) (Fig. 2). The multivariable logistic regression analysis showed no significant association between in-hospital and post-discharge recovery (P = 0.37), suggesting that post-discharge recovery occurred independent of in-hospital recovery (Supplementary Table 1).
Fig. 2. Incidence of in-hospital and post-discharge recovery after acute ischemic stroke.
DISCUSSION
The present study may be the first investigation based on a multicenter, nationally representative, stroke register to assess the effects of known determinants of 3-month functional outcome while distinguishing recovery during hospitalization and the subsequent period (i.e., from hospital discharge to 3 months from stroke onset). A study from the German Stroke Data Bank reported functional outcomes using a nationally representative dataset, but it provided only descriptive information on 3-month functional outcome.15 Another study from the Swedish Stroke Register analyzed functional outcomes at 3 months and one year, but it did not distinguish improvement from hospitalization to discharge.26
The present study showed that a number of determinants of 3-month functional outcome had a differential effect on in-hospital and post-discharge recovery and could be divided into 3 groups depending on their associations with each recovery phase. Specifically, there were seven variables associated with in-hospital recovery only, which included onset-to-admission time, blood glucose levels at admission, SBP at admission, history of stroke, CHF, recanalization therapy and prior statin use (Table 2). These variables are known as determinants of early neurologic improvement in patients with acute ischemic stroke.9,10,11,27 As might be expected, the associations were negative regarding onset-to-admission time, glucose levels at admission, SBP at admission, history of stroke, and CHF, and positive regarding recanalization therapy and prior statin use. These findings are clinically relevant and concordant with results reported in previous studies.9,10
The second group of variables, which showed association with post-discharge recovery only, included gender and statin use at discharge. Compared to men, women have been reported to have poorer functional outcomes after stroke.28,29,30 This difference can be explained by the fact that women have higher life expectancy (and thus chance to experience stroke at an older age), higher prevalence of living alone or in social isolation, poorer pre-stroke function, and higher incidence of post-stroke mood disorder.28 It is quite notable that prior statin administration was beneficial for both in-hospital recovery and statin use at discharge for post-discharge recovery. The pleiotropic effects of statins, which affect angiogenesis, neurogenesis, and synaptogenesis, have been proposed as possible biological mechanisms accounting for the relevance of statin use in recovery after stroke.31
The third group of variables, which showed associations with both in-hospital and post-discharge recovery, included age, NIHSS score at admission, pre-stroke disability, and stroke subtype. Conceptually, the contribution of age does not seem to be confined to either the in-hospital or post-discharge recovery phase, but age did show a different effect in in-hospital and post-discharge recovery (P for OR equality < 0.01) (Table 2). This differential effect can be explained, at least in part, by social aspects of aging such as an increased possibility of being widowed, which seems to bear more influence on post-discharge recovery. Interestingly, higher NIHSS scores at admission increased the probability of in-hospital recovery but decreased the probability of post-discharge recovery, which is probably related to definition of each recovery (i.e., in-hospital recovery was defined in terms of NIHSS score improvement, while post-discharge recovery was defined in terms of mRS score improvement). The effects of stroke subtype on recovery were complex, and further study is warranted to clarify the mechanism of each effect. Our findings regarding the effects of age, NIHSS score at admission and pre-stroke disability were in agreement with our expectations as well as with results reported previously.32,33,34 Furthermore, the tests for OR equality indicated that 7 factors had a differential effect on in-hospital and post-discharge recovery, suggesting that in-hospital and post-discharge recovery may proceed via different mechanisms.
Finally, we found that in-hospital and post-discharge recovery occur independently (Supplementary Table 1), which implies that patients discharged from acute stroke care hospitals have the same chance of further recovery, regardless of the incidence of in-hospital recovery. Future research is warranted to clarify the mechanisms underlying the independence of in-hospital and post-discharge recovery, as well as to explore mechanisms of post-discharge recovery.
In our study, in-hospital recovery was noted in 36% of the study population. In-hospital recovery is a useful surrogate marker for recanalization of occluded arteries and good functional outcomes,27 and its incidence was reported to range from 18% to 37%.11,12,27 We found that recanalization therapy, prior statin use, and higher NIHSS score at admission independently increased the probability of in-hospital recovery, while older age, longer onset-to-admission time, higher levels of blood glucose at admission, higher SBP at admission, history of stroke, CHF, and pre-stroke disability decreased the probability of in-hospital recovery; such observations are in agreement with results of previous studies.12,27,35
We defined post-discharge recovery as recovery from the time of discharge from acute stroke care services to 3 months after stroke onset, when it is expected that recovery may slow down and plateau.36 Studies on post-discharge recovery are scarce,16 and therefore information about the incidence of post-discharge recovery is limited. We found that post-discharge recovery occurred in approximately one third of the study population. Men and statin use at discharge increased the probability of post-discharge recovery, while older age, higher NIHSS score at admission, and pre-stroke disability decreased the probability of post-discharge recovery.
Our study has several limitations. First, we dichotomized the recovery period into an in-hospital phase and a post-discharge phase based on the discharge time. According to some previous studies on patterns of recovery after stroke,2,37,38 greater recovery is achieved during the first month after stroke, followed by a decrease in the rate of recovery. While the time from hospital admission to neurological stabilization differs in each case, the time to discharge tends to be similar across the population of acute stroke patients. Thus, dividing the recovery period into a phase before and after discharge from acute stroke care services is expected to be a reasonable approach. Second, although we assumed the study population have representativeness of stroke patients in Korea, participating centers of CRCS-5 were regional stroke centers or university hospitals and could have introduced selection bias. However, our national healthcare system provides a public insurance program which allow easy accessibility to any hospitals in Korea. In addition, the baseline characteristics of age and gender of this study was almost identical to that of a statistical report from the National Health Insurance Review Board in Korea.39 Third, this study is a retrospective analysis of the prospectively collected multicenter registry database, therefore other biases related to the retrospective nature of this study are inevitable. Lack of information on post-discharge care, and no flexibility in defining outcome measures including in-hospital and post-discharge recovery could be listed. Forth, information on the rehabilitation services, an aspect likely to affect recovery, was not scrutinized because such information was not included in the CRCS-5 registry.40,41 Fifth, the events including stroke recurrence after discharge may affect the post-discharge recovery and biased the study results. However, the results of the post-hoc sensitivity analysis excluding patients who had vascular events after discharge were not different from those of the original analysis (Supplementary Table 2).
Despite its limitations, our study showed that various factors known as determinants of 3-month functional outcome had a differential effect on in-hospital and post-discharge recovery, and that post-discharge recovery occurred independent of in-hospital recovery. Characterizing the determinants of in-hospital and post-discharge recovery after stroke represents an important step in the development of interventional programs, appropriate allocation of medical resources, and, ultimately, improvement of outcomes in both phases of recovery after stroke.
Footnotes
Funding: This research was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020, HI16C1078), and Hallym University Specialization Fund (HRF-S51)
Disclosures: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Bae HJ, Jang MU, Kang J, Kim BJ, Hong JH, Yeo MJ, Han MK, Lee BC, Yu KH, Oh MS, Choi KC, Lee SH, Hong KS, Cho YJ, Park JM, Cha JK, Kim DH, Park TH, Lee KB, Lee SJ, Lee J,1 Kim JT, Kim DE, Choi JC.
- Formal analysis: Lee JS, Jang MU, Bae HJ.
- Methodology: Jang MU, Bae HJ, Lee JS, Lee J.2
- Software: Lee JS.
- Validation: Lee J,2 Jang MU, Bae HJ.
- Writing - original draft: Jang MU, Bae HJ.
- Writing - review & editing: Bae HJ, Kang J, Kim BJ, Hong JH, Han MK, Lee BC, Yu KH, Oh MS, Choi KC, Lee SH, Hong KS, Cho YJ, Park JM, Cha JK, Kim DH, Park TH, Lee KB, Lee SJ, Lee J,1 Kim JT, Kim DE, Choi JC, Gorelick PB.
Lee J,1 Jun Lee; Lee J,2 Juneyoung Lee.
SUPPLEMENTARY MATERIALS
Multivariable logistic regression analysis for determining factors associated with post-discharge recovery after acute ischemic stroke
Sensitivity analysis of Table 1 (Effect of 3-month outcome determinants in in-hospital and post-discharge recovery after acute ischemic stroke) using different population which exclude patients with vascular events after discharge
Participating centers.
Flow diagram of enrollment of study subjects from the registry database.
References
- 1.Horgan NF, O'Regan M, Cunningham CJ, Finn AM. Recovery after stroke: a 1-year profile. Disabil Rehabil. 2009;31(10):831–839. doi: 10.1080/09638280802355072. [DOI] [PubMed] [Google Scholar]
- 2.Wade DT, Wood VA, Hewer RL. Recovery after stroke--the first 3 months. J Neurol Neurosurg Psychiatry. 1985;48(1):7–13. doi: 10.1136/jnnp.48.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62(3):393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- 4.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62(4):569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, et al. Prognostic modeling for an efficacy and a safety of thrombolysis in acute ischemic stroke. J Korean Neurol Assoc. 2012;30:100–109. [Google Scholar]
- 6.Awadh M, MacDougall N, Santosh C, Teasdale E, Baird T, Muir KW. Early recurrent ischemic stroke complicating intravenous thrombolysis for stroke: incidence and association with atrial fibrillation. Stroke. 2010;41(9):1990–1995. doi: 10.1161/STROKEAHA.109.569459. [DOI] [PubMed] [Google Scholar]
- 7.von Kummer R, Mori E, Truelsen T, Jensen JS, Grønning BA, Fiebach JB, et al. Desmoteplase 3 to 9 hours after major artery occlusion stroke: the DIAS-4 trial (efficacy and safety study of desmoteplase to treat acute ischemic stroke) Stroke. 2016;47(12):2880–2887. doi: 10.1161/STROKEAHA.116.013715. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, et al. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47(9):2373–2379. doi: 10.1161/STROKEAHA.116.013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DL, Johnston KC, Wagner DP, Haley EC., Jr Predicting major neurological improvement with intravenous recombinant tissue plasminogen activator treatment of stroke. Stroke. 2004;35(1):147–150. doi: 10.1161/01.STR.0000105396.93273.72. [DOI] [PubMed] [Google Scholar]
- 10.Saposnik G, Di Legge S, Webster F, Hachinski V. Predictors of major neurologic improvement after thrombolysis in acute stroke. Neurology. 2005;65(8):1169–1174. doi: 10.1212/01.wnl.0000180687.75907.4b. [DOI] [PubMed] [Google Scholar]
- 11.Muresan IP, Favrole P, Levy P, Andreux F, Marro B, Alamowitch S. Very early neurologic improvement after intravenous thrombolysis. Arch Neurol. 2010;67(11):1323–1328. doi: 10.1001/archneurol.2010.265. [DOI] [PubMed] [Google Scholar]
- 12.Takagi T, Kato T, Sakai H, Nishimura Y. Early neurologic improvement based on the National Institutes of Health Stroke Scale score predicts favorable outcome within 30 minutes after undergoing intravenous recombinant tissue plasminogen activator therapy. J Stroke Cerebrovasc Dis. 2014;23(1):69–74. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Huybrechts KF, Caro JJ, Xenakis JJ, Vemmos KN. The prognostic value of the modified Rankin Scale score for long-term survival after first-ever stroke. Results from the Athens Stroke Registry. Cerebrovasc Dis. 2008;26(4):381–387. doi: 10.1159/000151678. [DOI] [PubMed] [Google Scholar]
- 14.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 15.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32(11):2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 16.Berges IM, Kuo YF, Ottenbacher KJ, Seale GS, Ostir GV. Recovery of functional status after stroke in a tri-ethnic population. PM R. 2012;4(4):290–295. doi: 10.1016/j.pmrj.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke. 2014;9(4):514–518. doi: 10.1111/ijs.12199. [DOI] [PubMed] [Google Scholar]
- 18.Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. 2015;17(1):38–53. doi: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veerbeek JM, Kwakkel G, van Wegen EE, Ket JC, Heymans MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. 2011;42(5):1482–1488. doi: 10.1161/STROKEAHA.110.604090. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Ko Y, Lee S, Chung JW, Han MK, Park JM, Kang K, et al. MRI-based algorithm for acute ischemic stroke subtype classification. J Stroke. 2014;16(3):161–172. doi: 10.5853/jos.2014.16.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin assessment. Neuroepidemiology. 2001;20(1):26–30. doi: 10.1159/000054754. [DOI] [PubMed] [Google Scholar]
- 23.Mikulik R, Ribo M, Hill MD, Grotta JC, Malkoff M, Molina C, et al. Accuracy of serial National Institutes of Health Stroke Scale scores to identify artery status in acute ischemic stroke. Circulation. 2007;115(20):2660–2665. doi: 10.1161/CIRCULATIONAHA.106.651026. [DOI] [PubMed] [Google Scholar]
- 24.Gauvreau K, Pagano M. The analysis of correlated binary outcomes using multivariate logistic regression. Biom J. 1997;39(3):309–325. [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Ullberg T, Zia E, Petersson J, Norrving B. Changes in functional outcome over the first year after stroke: an observational study from the Swedish stroke register. Stroke. 2015;46(2):389–394. doi: 10.1161/STROKEAHA.114.006538. [DOI] [PubMed] [Google Scholar]
- 27.Kharitonova T, Mikulik R, Roine RO, Soinne L, Ahmed N, Wahlgren N, et al. Association of early National Institutes of Health Stroke Scale improvement with vessel recanalization and functional outcome after intravenous thrombolysis in ischemic stroke. Stroke. 2011;42(6):1638–1643. doi: 10.1161/STROKEAHA.110.606194. [DOI] [PubMed] [Google Scholar]
- 28.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paolucci S, Bragoni M, Coiro P, De Angelis D, Fusco FR, Morelli D, et al. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006;37(12):2989–2994. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]
- 30.Kim JS, Lee KB, Roh H, Ahn MY, Hwang HW. Gender differences in the functional recovery after acute stroke. J Clin Neurol. 2010;6(4):183–188. doi: 10.3988/jcn.2010.6.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond lipid lowering. Am J Cardiol. 2003;91(4A):23B–29B. doi: 10.1016/s0002-9149(02)03270-8. [DOI] [PubMed] [Google Scholar]
- 32.Bagg S, Pombo AP, Hopman W. Effect of age on functional outcomes after stroke rehabilitation. Stroke. 2002;33(1):179–185. doi: 10.1161/hs0102.101224. [DOI] [PubMed] [Google Scholar]
- 33.Choi JC, Jang MU, Kang K, Park JM, Ko Y, Lee SJ, et al. Comparative effectiveness of standard care with IV thrombolysis versus without IV thrombolysis for mild ischemic stroke. J Am Heart Assoc. 2015;4(1):e001306. doi: 10.1161/JAHA.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dallas MI, Rone-Adams S, Echternach JL, Brass LM, Bravata DM. Dependence in prestroke mobility predicts adverse outcomes among patients with acute ischemic stroke. Stroke. 2008;39(8):2298–2303. doi: 10.1161/STROKEAHA.107.506329. [DOI] [PubMed] [Google Scholar]
- 35.Arboix A, García-Eroles L, Oliveres M, Targa C, Balcells M, Massons J. Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol. 2010;10(1):47. doi: 10.1186/1471-2377-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Formisano R, Pantano P, Buzzi MG, Vinicola V, Penta F, Barbanti P, et al. Late motor recovery is influenced by muscle tone changes after stroke. Arch Phys Med Rehabil. 2005;86(2):308–311. doi: 10.1016/j.apmr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Sharma N, Cohen LG. Recovery of motor function after stroke. Dev Psychobiol. 2012;54(3):254–262. doi: 10.1002/dev.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25(6):1181–1188. doi: 10.1161/01.str.25.6.1181. [DOI] [PubMed] [Google Scholar]
- 39.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, et al. Gender differences in the age-stratified prevalence of risk factors in Korean ischemic stroke patients: a nationwide stroke registry-based cross-sectional study. Int J Stroke. 2014;9(6):759–765. doi: 10.1111/ijs.12146. [DOI] [PubMed] [Google Scholar]
- 40.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 41.Lynch E, Hillier S, Cadilhac D. When should physical rehabilitation commence after stroke: a systematic review. Int J Stroke. 2014;9(4):468–478. doi: 10.1111/ijs.12262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariable logistic regression analysis for determining factors associated with post-discharge recovery after acute ischemic stroke
Sensitivity analysis of Table 1 (Effect of 3-month outcome determinants in in-hospital and post-discharge recovery after acute ischemic stroke) using different population which exclude patients with vascular events after discharge
Participating centers.
Flow diagram of enrollment of study subjects from the registry database.