Abstract
Background
Patients with esophageal cancer (EC) frequently have multiple primary cancers. We conducted the present study to assess the risk of multiple primary malignancies for patients with squamous cell carcinoma (SCC) and adenocarcinoma (AC) of the esophagus and to investigate the influence of multiple primary tumors on the prognosis of EC patients.
Methods
Using the data of 44,091 EC patients from the Surveillance Epidemiology and End Results (SEER) database, we calculated the standardized incidence ratios (SIRs) for overall multiple primary cancers and cancers at particular sites among EC survivors. The SIRs of esophageal SCC and AC patients were compared using Poisson regression. The Kaplan-Meier (KM) method was used for survival analysis.
Results
Multiple primary cancer risk was significantly increased among both esophageal SCC and AC survivors (SIR: 2.28 and 1.57, respectively; P<0.001). Among SCC patients, the highest SIRs were found in the oral cavity and pharynx (SIR: 16.54), esophagus (SIR: 10.02), and larynx (SIR: 10.34). Also, the highest SIRs following AC cases were observed in the esophagus (SIR: 8.81), stomach (SIR: 9.29), and small intestine (SIR: 4.95). SIRs for the oral cavity and pharynx, lung, and larynx were significantly higher among SCC survivors than AC survivors (all P<0.05). KM analysis revealed no significant difference of overall survival (OS) for multiple primary cancers, including those of the esophagus, stomach, oral cavity and pharynx, and lung among EC patients (log rank =2.04; P=0.564), except for prostate cancer (log rank =96.65; P<0.001).
Conclusions
Multiple primary malignancy risk differed by the histological type of esophageal SSC and AC survivor. However, no significant relationship between survival and the multiple primary cancer sites, except for prostate cancer, was observed.
Keywords: Esophageal cancer (EC), multiple primary cancer, standardized incidence ratios (SIRs)
Introduction
Esophageal cancer (EC) is an aggressive upper gastrointestinal malignancy and is estimated to be among the 10 most common malignancies worldwide (1). The two major distinct histological types of EC are squamous cell carcinoma (SCC) and adenocarcinoma (AC). There are marked differences between their pathological patterns in terms of incidence, natural history, and therapeutic scheme (1-3). More than one-half of the new cases in the United States and Western Europe were diagnosed as AC, while the predominate type in East Asian countries, such as China, was SCC (1,2). EC generally presents as locally advanced disease and requires multidisciplinary treatment (3,4). Prominent progress has been made in early detection techniques, surgical procedures, and perioperative adjuvant therapies (4,5). Despite improvements in its detection and management, the prognosis of patients with EC remains poor, with a 5-year survival rate of less than 20% (3). The relatively high occurrence rate of multiple primary malignancies has been suggested to be one of the major prognostic factors, and second cancer may contribute to the poor prognosis after curative surgical resection (6,7).
An earlier study consisting of 1,259 cases was conducted by Warren and Gates in 1932, and multiple primary carcinomas were considered rare (8). Multiple primary malignancies are histologically different from primary cancer and occur at a different site. In contrast to multiple primary carcinomas, second or double primary malignancies can affect the same organ but are anatomically distinct from the primary tumor and represent neither a metastatic nor recurrent tumor from the initial malignancy (9). In recent decades, the incidence and relative risk of developing second or multiple primary tumors have increased (10). Possible explanations are the improvement of follow-up care and progress in diagnostic techniques. A previous study reported that the number of second malignancies accounted for ~16% of all cases registered in 2003 based on the Surveillance Epidemiology and End Results (SEER) database (11). Also, Otowa et al. demonstrated that approximately 36.2% of ECs were observed with multiple primary cancers (12). However, the risk factors related to second cancer differ between SCC and AC and have not been discussed separately (13,14). Matsubara et al. reported that antecedent malignancy was a significant factor that affected the risk of developing other malignancies after esophagectomy, which was only performed on patients with SCC of the thoracic esophagus (6). Therefore, we conducted a study to assess the multiple primary cancer risks of EC patients separately for SCC and AC.
Methods
Patients
Data from 44,091 patients initially diagnosed with EC and 2,256 patients diagnosed with multiple primary cancers based on the multiple primary standardized incidence ratio (MP-SIR) session were retrieved from the SEER program. The SEER program of the National Cancer Institute collects data for all cancer patients in 18 defined geographic regions across the United States. It collects and publishes approximately 28% of the American population’s cancer incidence and survival information. The database we selected was SEER 18 regs, excluding AK Custom Data (with additional treatment fields), which was submitted in November 2016 (2000 to 2014) (15). The inclusion criteria for data extraction in the current study were patients diagnosed with EC (site and morphology site recode B ICD-O-3/WHO 2008= “esophagus”). The exclusion criteria included (I) patients with a pathology type other than SCC or AC; (II) patients with missing or incomplete data, such as survival status, time, and pathological type. Finally, a total of 14,540 and 23,909 SCC and AC patients, respectively, who met all the inclusion criteria, were included in the baseline analysis. As the patients included in our study were retrieved from the SEER program, the ethics approval was waived.
Follow-up began at the time of primary EC diagnosis and ended at the earliest occurrence of multiple original cancer diagnoses, cause-specific death, or the end of the study period. The demographic and clinicopathological data for all eligible cases were collected and retrospectively analyzed.
Statistical analysis
For each multiple primary cancer site, we compared the SIR between esophageal SCC and AC cases using Poisson regression. The overall SIRs were calculated and stratified by time since EC diagnosis (1, 3, 5, and 10 y) (16). The survival curves were plotted by the Kaplan-Meier (KM) method to compare different cancer sites. Poisson regression was performed using STATA (College Station, TX, USA, version 15.1). SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for the classic survival analysis. SEER Stat 8.3.5 software was used to extract the study cohort from the SEER dataset (17). For all analyses, P<0.05 was considered statistically significant.
Results
Patient characteristics and overall survival (OS)
Patient demographics grouped by histological type are summarized in Table 1. Male patients accounted for most of the cases (78.2%) in our series, with 30,052 male patients and 8,397 female patients. The mean age was 69.0 and 70.3 years at SCC and AC EC diagnosis, respectively, and cases aged 60 to 80 years showed the maximum proportion. Roughly half of the patients underwent surgical treatment for esophageal carcinoma: 43.2% and 50.8% for SCC and AC patients, respectively. Most patients received adjuvant treatment, including radiation and chemotherapy, which accounted for approximately 51.5–61.2% of patients. SCC patients received radiation more frequently than AC patients (61.2% vs. 51.5%), while chemotherapy rates were similar for SCC and AC patients (57.8% and 59.5%, respectively).
Table 1. Characteristics of patients with esophageal carcinoma stratified by the histology.
| Demographics | Histology | |
|---|---|---|
| SCC | AC | |
| No. of patients | 14,540 | 23,909 |
| No. of person—years | 23,010.62 | 44,809.91 |
| Gender, n (%) | ||
| Male | 9,530 (65.5) | 20,522 (85.8) |
| Female | 5,010 (34.5) | 3,387 (14.2) |
| Age, n (%) | ||
| <40 | 75 (0.5) | 378 (1.6) |
| 40–60 | 4,065 (28.0) | 7,225 (30.2) |
| 60–80 | 7,673 (52.7) | 12,019 (50.3) |
| >80 | 2,727 (18.8) | 4,287 (17.9) |
| Race, n (%) | ||
| White | 8,988 (61.8) | 22,608 (94.6) |
| Black | 4,106 (28.2) | 653 (2.7) |
| Other/unknown | 1,446 (10.0) | 108 (0.5) |
| Calendar year of EC diagnosis, n (%) | ||
| 2000–2005 | 4,342 (29.9) | 4,803 (20.1) |
| 2005–2010 | 5,160 (35.5) | 8,469 (35.4) |
| 2010+ | 5,038 (34.6) | 10,861 (45.4) |
| Esophagectomy, n (%) | ||
| Yes | 6,278 (43.2) | 12,155 (50.8) |
| No | 2,692 (18.5) | 5,682 (23.8) |
| Unknown | 5,572 (38.3) | 6,072 (25.4) |
| Adjuvant therapy, n (%) | ||
| Any radiotherapy | 8,904 (61.2) | 12,320 (51.5) |
| No/unknown radiotherapy | 5,636 (38.8) | 11,589 (48.5) |
| Any chemotherapy | 8,398 (57.8) | 14,228 (59.5) |
| No/unknown chemotherapy | 6,142 (42.2) | 9,681 (40.5) |
| Stage of EC, n (%) | ||
| Local | 3,032 (20.9) | 5,339 (22.3) |
| Regional | 5,018 (34.5) | 7,179 (30.0) |
| Distant | 4,612 (31.7) | 9,239 (38.6) |
| Unknown | 1,878 (12.9) | 2,152 (9.1) |
| Prognosis, n (%) | ||
| Alive | 2,307 (15.9) | 5,311 (22.2) |
| Death | 12,233 (84.1) | 18,598 (77.8) |
SCC, squamous cell carcinoma; AC, adenocarcinoma; EC, esophageal cancer.
Additionally, the proportion of SCC cases remained stable during the study period, while the proportion of AC histology increased over time, constituting approximately 45.4% of ECs in the most recent calendar period (2010 to 2014). In the SEER registries, White patients made up the majority of AC patients (94.6%), and SCC patients were mainly composed of White and Black patients (61.8% and 28.2%, respectively). During the follow-up period, a total of 7,618 patients were alive, and 30,831 had patients died.
SIRs of different second cancer sites for SCC and AC patients
Table 2 presents the SIRs of multiple primary malignancies in different sites of SCC and AC of the esophagus. The most frequent multiple original cancers among SCC patients were the lungs (229 cases), oral cavity and pharynx (153 cases), and prostate (74 cases), while the most frequent multiple original cancers among AC cases were the lungs (221 cases), prostate (154 cases), esophagus (103 cases), and colon (124 cases). Overall, the SIRs for all multiple primary cancer sites of SCC and AC patients were significantly increased [SIR: 2.28, 95% confident interval (CI): 2.13 to 2.43; SIR: 1.57, 95% CI: 1.48 to 1.65, respectively; P<0.001]. Also, among SCC patients, the highest SIRs were found in the oral cavity and pharynx (SIR: 16.54), esophagus (SIR: 10.02), and larynx (SIR: 10.34). The highest SIRs following AC cases were observed in the esophagus (SIR: 8.81), stomach (SIR: 9.29), and small intestine (SIR: 4.95). Moreover, both SCC and AC patients suffered a significantly increased risk for cancers sites of the oral cavity and pharynx, esophagus, stomach, small intestine, lung, liver, and kidney. The increased risk was only observed in the multiple primary cancer of the larynx for SCC patients and the thyroid and non-Hodgkin lymphoma for AC survivors. However, AC patients were at a significantly decreased risk for cancers of the myeloma and prostate. Additionally, significant differences in SIRs were found between cancer sites, including the oral cavity and pharynx, larynx, lung, and breast (all P<0.05). The SIRs for the oral cavity and pharynx, larynx, and lung were significantly higher in SCC than in AC survivors (all P<0.05) (Table 2).
Table 2. Risk of multiple primary cancers among EC survivors stratified by histology.
| Multiple primary cancer site | SCC | AC | P | ||||
|---|---|---|---|---|---|---|---|
| No. of observed | SIR | 95% CI | No. of observed | SIR | 95% CI | ||
| No. of patients | 14,540 | – | – | 23,909 | – | – | – |
| No. of person—years | 23,101.62 | – | – | 44,809.91 | – | – | – |
| All site | 913 | 2.28 | 2.13–2.43 | 1,272 | 1.57 | 1.48–1.65 | <0.001 |
| Oral cavity and pharynx | 153 | 16.54 | 14.03–19.38 | 31 | 1.38 | 0.94–1.96 | <0.001 |
| Esophagus | 49 | 10.02 | 7.41–13.25 | 103 | 8.81 | 7.19–10.68 | 0.660 |
| Stomach | 61 | 7.58 | 5.80–9.74 | 131 | 9.29 | 7.76–11.02 | 0.528 |
| Small intestine | 7 | 3.58 | 1.44–7.37 | 18 | 4.95 | 2.94–7.83 | 0.533 |
| Colon and rectum | 52 | 1.25 | 0.93–1.64 | 124 | 1.61 | 1.34–1.92 | 0.220 |
| Liver | 23 | 3.24 | 2.05–4.86 | 30 | 2.14 | 1.44–3.05 | 0.148 |
| Pancreas | 16 | 1.32 | 0.75–2.14 | 43 | 1.89 | 1.37–2.55 | 0.271 |
| Larynx | 40 | 10.34 | 7.39–14.08 | 11 | 1.28 | 0.64–2.30 | <0.001 |
| Lung and bronchus | 229 | 3.63 | 3.17–4.13 | 221 | 1.81 | 1.58–2.06 | <0.001 |
| Breast | 35 | 1.06 | 0.74–1.48 | 26 | 1.08 | 0.71–1.59 | <0.001 |
| Brain | 0 | – | – | 3 | 0.36 | 0.07–1.05 | 0.997 |
| Kidney | 31 | 2.52 | 1.71–3.58 | 90 | 3.31 | 2.66–4.07 | 0.055 |
| Myeloma | 5 | 0.73 | 0.24–1.70 | 4 | 0.34 | 0.09–0.86 | 0.185 |
| Non-hodgkin lymphoma | 21 | 1.35 | 0.83–2.06 | 49 | 1.41 | 1.04–1.86 | 0.488 |
| Hodgkin lymphoma | 0 | – | – | 4 | 2.11 | 0.58–5.41 | 0.315 |
| Thyroid | 7 | 1.77 | 0.71–3.64 | 27 | 3.69 | 2.43–5.37 | 0.107 |
| Leukemia | 14 | 1.33 | 0.73–2.23 | 34 | 1.41 | 0.98–1.98 | 0.487 |
| Prostate | 74 | 0.83 | 0.65–1.04 | 154 | 0.72 | 0.61–0.84 | 0.639 |
| Ovary | 3 | 0.90 | 0.18–2.62 | 2 | 0.84 | 0.10–3.05 | 0.240 |
P values comparing SIR for esophageal SCC versus esophageal AC survivors were calculated using Poisson regression. SCC, squamous cell carcinoma; AC, adenocarcinoma; EC, esophageal cancer; SIR, standardized incidence ratio; CI, confident interval.
Different second cancer sites and patient survival
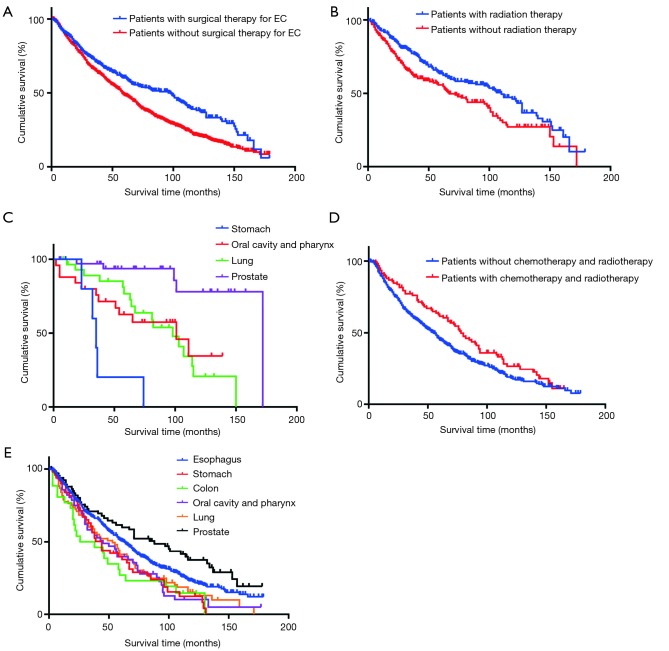
A total of 1,321 EC patients with multiple primary cancer were included in the survival analysis. During the follow-up period, there were 887 overall deaths. At the time of this analysis, the median OS is 44.00 months (95% CI: 40.35 to 47.65 months). The overall 1-, 3-, and 5-year, OS rates are 73.7%, 46.4%, and 29.4%, respectively, for all patients. We performed a KM analysis to evaluate whether the second cancer site was associated with prognosis. According to the KM survival curves, a significant difference in OS, with a median survival time of 59.00 months (95% CI: 53.02 to 64.98 months), was not observed for multiple primary cancers, such as the oral cavity and pharynx, esophagus, stomach, and lung among EC patients (log rank =2.04; P=0.564; Figure 1A). In contrast, survival rates with subsequent prostate cancer, with a median survival period of 139.00 months (95% CI: 119.64 to 158.36 months) and a 5-year survival rate of 82.7%, were significantly associated with better OS (log rank =96.65; P<0.001; Figure 1B). Similarly, we evaluated the survival and disease progression among different cancer sites. Low second-malignancy-specific mortality was only associated with prostate cancer (log rank =97.36; P<0.001; Figure 1C), which suggested a median disease progression period of 70.00 months (95% CI: 49.83 to 90.17 months). We further conducted a survival analysis in patients with and without surgical treatment for primary malignancy. Generally, better outcomes were observed for patients with surgery in esophageal lesion than those without surgical therapy (log rank= 22.45; P<0.001; Figure 2A). Considering that adjuvant treatment may be a confounding factor, stratified analysis was further performed. A better outcome was observed in patients who received radiotherapy for multiple cancer after they underwent esophagectomy for primary carcinoma (log rank =7.82; P=0.005; Figure 2B), especially for patients with multiple primary cancer of the prostate (log rank =30.80; P<0.001; Figure 2C). Chemotherapy was not evaluated because of the small sample size in the subgroup that had received the treatment. In patients without surgical treatment for EC, a favorable prognosis was observed in patients with chemotherapy and radiotherapy at the same time (log rank =5.09; P=0.024; Figure 2D). Also, patients with multiple primary cancers of the esophagus and prostate had the best response to the chemoradiotherapy, and the mean survival times were 75.50 and 92.20 months, respectively (log rank =25.80; P<0.001; Figure 2E).
Figure 1.
OS in the cohort grouped by different multiple primary cancers. (A) No significant difference was observed for multiple primary cancers, such as oral cavity and pharynx, esophagus, stomach, and lung among EC patients (log rank =2.04; P=0.564); (B) subsequent prostate cancer was significantly associated with better OS (log rank =96.65; P<0.001); (C) low second-malignancy-specific mortality was only associated with prostate cancer (log rank =97.36; P<0.001). OS, overall survival; EC, esophageal cancer.
Figure 2.
Survival analyses in patients with different treatment strategies. (A) Better outcomes were observed for patients with surgical treatment for original malignancy than those without surgical therapy (log rank =22.45; P<0.001); (B) a better outcome was found in patients received radiotherapy for the multiple cancer after underwent esophagectomy (log rank =7.82; P=0.005); (C) especially for patients with multiple primary cancer of prostate (log rank =30.80; P<0.001); (D) a favorable prognosis was observed in patients received chemotherapy and radiotherapy at the same time, but no operation for multiple cancers (log rank =5.09; P=0.024); (E) patients with multiple primary cancers of esophagus and prostate were the best response to the chemoradiotherapy (log rank =25.80; P<0.001). EC, esophageal cancer.
Discussion
In the present study, we examined the multiple primary cancer risk separately for esophagus SCC and AC patients using the SEER database. We found that the risk of second cancers was significantly higher for esophageal SCC survivors than for AC survivors. Particularly, esophageal SCC patients had higher risks of multiple primary cancers of the oral cavity and pharynx, larynx, and lung.
We found that the incidence rates of multiple primary cancer for the oral cavity and pharynx, esophagus, stomach, and lung in SCC and AC patients were higher than those in the general population. It has been widely reported that the most common site for synchronous and metachronous second primary malignancy of EC is the upper aerodigestive organs, which include the oral cavity and pharynx, the stomach, and the lung (6,18-20), which is consistent with the findings of our study. However, most previous studies included only a small sample size of survivors with primary cancer after EC and did not analyze the histology separately. The current study was performed with a relatively large population and found that SCC patients had a significantly increased risk of the oral cavity and pharynx cancer than AC patients (SIRs: 16.54 and 1.38, respectively). The stomach was found to be the next most common site of multiple primary cancer (SIRs: 7.58 and 9.29 for SCC and AC, respectively). Although the cardiac and lesser gastric curvatures of the stomach were routinely resected to reconstruct the alimentary tract (21), a significantly high incidence of stomach cancer was still observed in patients who had undergone curative resection. Patients with oral and oropharyngeal SCC are often diagnosed with widespread multiple premalignant lesions in the upper aerodigestive tract (22). A potential mechanism for the development of multiple malignancies in organs close to the index tumor in EC survivors may be explained by the concept of ‘‘field cancerization’’, which theorizes that carcinogenic exposure could cause simultaneous genetic defects in the epithelium of the upper aerodigestive tract, putting the epithelium at high risk for the development of multiple lesions. Carcinogenic exposure may weaken DNA repair capabilities and ultimately results in genetic abnormalities in these patients (23).
Additionally, a relatively high incidence of second primary malignancies located in the esophagus was also observed (SIRs: 10.02 and 8.81 for SCC and AC, respectively). Therefore, an accurate evaluation of the esophageal lesion preoperatively is extremely important to guarantee the curative resection for esophageal carcinoma and remove the potential tumor cells. Moreover, we note the possibility that esophageal carcinoma recurrence may be misclassified as primary cancer, which may contribute to the increased incidence of subsequent esophageal malignancy.
An increased risk of lung cancer among EC survivors has been discussed (6,7). However, previous studies did not histologically distinguish esophagus cancers (6). In the current study, we found that EC patients were also at an increased risk of smoking-related multiple original cancers, particularly lung cancer. It is well-known that cigarette smoking is a risk factor for lung SCC and that smokers are mostly male (1,24,25). Cigarette smoke may lead to the exposure of the respiratory tract, from the mouth to the lungs, to environmental carcinogens. Therefore, carcinogenesis, such as tar and nicotine, may result in smoking-related cancers and multiple malignancies. Our study cohort was mainly composed of male patients (78.2%), which may account for the high incidence of multiple lung cancer after esophagectomy. Because information on the quantity of cigarette consumption in the SEER database was not sufficiently detailed for more precise analysis, we did not discuss the dose-response relationship. Previous studies have investigated the association between cumulative exposure to tobacco and the occurrence of aerodigestive tract cancers at length (26,27).
Additionally, we evaluated the survival time and different cancer sites for both OS and disease progression. No significant difference in OS was observed for cancer sites, including the esophagus, stomach, oral cavity and pharynx, and lung, among EC patients (Figure 1A). Only prostate cancer was significantly associated with better OS and lower specific mortality according to the KM survival analysis (Figure 1B,C, respectively). A previous epidemiological study of prostate cancer conducted in the United States demonstrated that the 5-year relative survival rates increased with a greater proportion of men diagnosed with localized early disease (11). Brawley suggested an impressively favorable prognosis for prostate cancer patients, in whom, for men with local and regional disease, the 5-year survival rate was 100%, the 10-year survival rate was 95%, and the 15-year survival rate was 82% (28). Considering the favorable outcomes for male prostate cancer patients, multiple primary cancer for prostate may not have a significant effect on the prognosis of EC patients. Also, we found that a significantly decreased risk of prostate cancer was observed in AC patients compared with the general population (SIR: 0.72). A prospective study conducted by Calle et al. with a population of more than 900,000 U.S. adults and a follow-up time of 16 years demonstrated that a higher body-mass-index value in men was a significant risk factor for being diagnosed and dying from prostate cancer (29). Malnutrition is a common condition for EC patients after esophagectomy (30,31). A previous study has reported that weight loss after EC surgery continues for at least 3 years after operation (31). Hynes found that about 86.9% of EC patients lost weight after surgical treatment (30). Therefore, we hypothesize that EC survivors lose weight, which may contribute to the lower risk of prostate cancer compared with the general population, especially those that are overweight.
Therapy differs between the lesions of multiple primary cancers. When the second original cancers are observed in distant organs, surgery removal is the primary treatment. However, when second cancer occurs in adjacent organs, the treatment modalities are limited because the post-operative adhesions and the reconstructed organs alter its structure. Most patients received chemotherapy and radiotherapy when reoperation was not feasible. The current study also showed that after undergoing esophagectomy for primary carcinoma, a better outcome was observed in patients who received radiotherapy for multiple cancer.
The strength of the current study was the assessment of a sufficiently large sample size based on the SEER database, guaranteeing the reliability of the findings. We found that the incidence rate of multiple original tumors was different for EC patients, and we analyzed it separately based on histology. As second malignancies of the EC are being observed more frequently, the relatively high incidence of cancer sites should be periodically and comprehensively inspected after surgery. For instance, an examination of the oral cavity and pharynx, esophagus, and stomach regions is an important follow-up procedure that enables the early detection of subsequent developing cancers. Our findings may help to guide the precise inspection area and the appropriate diagnostic approach. However, our study had several potential limitations, including insufficient clinical and treatment information, such as a history of smoking and surgical procedures. Additionally, some of the results we observed may have arisen from the misclassification of the EC histology and recurrence.
Conclusions
In conclusion, EC is highly associated with multiple primary cancers. We characterized multiple primary malignancy risks among esophageal SSC and AC survivors and analyzed the patterns of second cancers. We found that the SIRs for second cancers differed in the SCC and AC histological types. However, a significant relationship between survival and multiple primary cancer sites, except for in prostate cancer, was not observed. Further study is needed to define high-risk individuals who are prone to developing second primary cancers and clarify the mechanisms underlying EC and subsequent malignancies.
Acknowledgments
We want to extend our deepest gratitude to our mentor Dr. Zhesheng Wen, a respected and responsible scholar who has provided us with valuable guidance at every stage of the writing of this paper.
Funding: This work was supported by the National Natural Science Foundation (81871986).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As the patients included in our study were retrieved from the SEER program, the ethics approval was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Li H, Fang W, Yu Z, et al. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis 2018;10:2481-9. 10.21037/jtd.2018.03.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 2018;36:2796-803. 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. 10.1097/00000658-200208000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:4336-41. 10.1200/JCO.2003.12.074 [DOI] [PubMed] [Google Scholar]
- 7.Baba Y, Yoshida N, Kinoshita K, et al. Clinical and prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg 2018;267:478-83. 10.1097/SLA.0000000000002118 [DOI] [PubMed] [Google Scholar]
- 8.Warren S. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358-414. [Google Scholar]
- 9.Hildebrand P, Humke J, Oevermann E, et al. Influence of second or multiple tumours on the prognosis of patients with colorectal cancer. Acta Chir Iugosl 2012;59:31-8. 10.2298/ACI1201031H [DOI] [PubMed] [Google Scholar]
- 10.Das A, Chak A, Cooper GS. Temporal trend in relative risk of second primary colorectal cancer. Am J Gastroenterol 2006;101:1342-7. 10.1111/j.1572-0241.2006.00580.x [DOI] [PubMed] [Google Scholar]
- 11.Ries LAG, Harkins D, Krapcho M, et al. SEER cancer statistics review, 1975-2003, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2003/
- 12.Otowa Y, Nakamura T, Takiguchi G, et al. Successful treatment of quintuple primary cancer, including esophageal cancer: a case report. Oncol Lett 2015;9:2583-5. 10.3892/ol.2015.3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi AK, Kleinerman RA, Hildesheim A, et al. Second cancers after squamous cell carcinoma and adenocarcinoma of the cervix. J Clin Oncol 2009;27:967-73. 10.1200/JCO.2008.18.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst 1994;86:131-7. 10.1093/jnci/86.2.131 [DOI] [PubMed] [Google Scholar]
- 15.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev 1999;8:1117-21. [PubMed] [Google Scholar]
- 16.Liddell FD. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health 1984;38:85-8. 10.1136/jech.38.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology, and End Results (SEER) Program (available online: www.seer.cancer.gov) SEER*Stat Database: incidence—SEER 18 regs custom data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying)—linked to county attributes—total U.S., 1969–2016 counties, National Cancer Institute, DCCPS, Surveillance Research Program.
- 18.Poon RT, Law SY, Chu KM, et al. Multiple primary cancers in esophageal squamous cell carcinoma: incidence and implications. Ann Thorac Surg 1998;65:1529-34. 10.1016/S0003-4975(98)00177-5 [DOI] [PubMed] [Google Scholar]
- 19.Shibuya H, Wakita T, Nakagawa T, et al. The relation between an esophageal cancer and associated cancers in adjacent organs. Cancer 1995;76:101-5. [DOI] [PubMed] [Google Scholar]
- 20.Fogel TD, Harrison LB, Son YH. Subsequent upper aerodigestive malignancies following treatment of esophageal cancer. Cancer 1985;55:1882-5. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara T, Ueda M, Uchida C, et al. Modified stomach roll for safer reconstruction after subtotal esophagectomy. J Surg Oncol 2000;75:214-6. [DOI] [PubMed] [Google Scholar]
- 22.Chung KY, Mukhopadhyay T, Kim J, et al. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res 1993;53:1676-83. [PubMed] [Google Scholar]
- 23.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 1996;56:2488-92. [PubMed] [Google Scholar]
- 24.Lepage C, Drouillard A, Jouve JL, et al. Epidemiology and risk factors for oesophageal adenocarcinoma. Dig Liver Dis 2013;45:625-9. 10.1016/j.dld.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. 10.3748/wjg.v19.i34.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siemiatycki J, Krewski D, Franco E, et al. Associations between cigarette smoking and each of 21 types of cancer: a multi-site case-control study. Int J Epidemiol 1995;24:504-14. 10.1093/ije/24.3.504 [DOI] [PubMed] [Google Scholar]
- 27.Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer 1995;60:616-21. 10.1002/ijc.2910600508 [DOI] [PubMed] [Google Scholar]
- 28.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol 2012;30:195-200. 10.1007/s00345-012-0824-2 [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 30.Hynes O, Anandavadivelan P, Gossage J, et al. The impact of pre- and post-operative weight loss and body mass index on prognosis in patients with oesophageal cancer. Eur J Surg Oncol 2017;43:1559-65. 10.1016/j.ejso.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 31.Martin L, Lagergren P. Long-term weight change after oesophageal cancer surgery. Br J Surg 2009;96:1308-14. 10.1002/bjs.6723 [DOI] [PubMed] [Google Scholar]