Abstract
Background
Lungs are one of the target organs of metastases of primary lung, breast, liver, colorectal, and esophageal cancer. While computed tomography (CT) is the most widely used modality for detecting lung metastases, it is still very challenging to detect them at the earlier stages. If lung metastases could be found on CT scans at the earliest time points, patients would benefit by beginning treatment earlier. The objective of this study was to demonstrate that CT can reveal lung metastases in many cases at even earlier stages than current radiological practice may find.
Methods
One hundred patients with lung metastases were randomly selected and their surveillance CT scans were analyzed retrospectively. The patients had primary cancer in the breasts, lungs, esophagus, colorectum, and liver. All patients had multiple CT examinations of the lungs and their metastases, if any, were confirmed by subsequent CT scans. The earliest CT scans were examined to determine whether lung metastases at the same locations had been diagnosed or missed. Missed lung metastases, categorized by type of the primary cancer and adjacency to nearby blood vessels, were statistically analyzed.
Results
There were 36/100 (36%) cases of missed lung metastases, including 15 cases of single metastasis and 21 cases of multiple metastases. There were a total of 174 missed loci of lung metastases. Where metastases were missed, there was a statistically significant difference (P<0.001) in their distribution within the sub-regions of the lungs. Adjacency to blood vessels appeared to be a significant factor in metastases being missed during diagnosis (P<0.001).
Conclusions
There was a considerable percentage of early lung metastases that were missed by radiologists but actually appeared on CT scans. The capability of CT to reveal such early metastases opens up an opportunity to move up the time points of detecting lung metastases through clinical and training improvement and technology development such as computer-aided detection.
Keywords: Computed tomography (CT), lung metastases, early detection
Introduction
Lungs are one of the target organs of metastases of primary lung, breast, liver, colorectal, and esophageal cancer. Patients with lung metastases have a 5-year survival rate of only 20% (1). Due to its greater sensitivity compared to other modalities such as magnetic resonance imaging (MRI) (2-4) and positron emission tomography (PET) (5-7), computed tomography (CT) is the most widely used modality for detecting lung metastases (8). Cancer patients routinely undergo CT examinations to monitor potential lung metastasis (9). Despite CT’s great sensitivity, detecting lung metastases at their earliest stages remains extremely challenging (10,11). Often, it is not until a later CT scan that lung metastases are found and treatment started. A critical question remains why lung metastases cannot be detected at the earliest CT scan. According to guidelines, such as those of the National Comprehensive Cancer Network (NCCN) or the Fleischner Society, high-risk small nodules in patients with a history of cancer should have follow-up in a short time, we know that lung metastases grow fast. If lung metastases could be found on CT scans at earlier time points, patients would benefit because they can begin treatment earlier and not have to wait until later CT scans when more lung metastases may have developed or existing ones may have become larger.
Our hypothesis was that many lung metastases could have been revealed at the earliest CT scans but radiologists missed them due to their small sizes and low density. Given the multiple scans performed months apart for cancer patients, we used later scans to confirm the presence of lung metastases on the early scans. The large metastases seen on the later scans were also corroborated through biopsy. For most cases in our study, we found that using the second scan to confirm the presence of lung metastases on the first scans was sufficient to find many missed metastases. We then mapped the metastatic loci on the second scans to the same locations on the first scans to examine whether metastases were detectable on the first scan but were missed by routine readings. We retrospectively analyzed CT scans of cancer patients to determine and categorize scenarios with one being whether lung metastases manifested as weak low density and were missed on the first CT scans but were confirmed by CT scans at later time points. We found that, in one-third of cases, the first CT scans could reveal the abnormal density corresponding to lung metastases that were missed by radiologists. To the best of our knowledge, this is the first study whose aim it was to determine and categorize the frequency with which lung metastases were missed on a CT scan at the first time point only to be detected on later CT scans. The findings of our study provided insight into the process of improving the detection of lung metastases on surveillance CT scans. More importantly, we identified an overlooked opportunity to move up the detection of lung metastases to the earliest time points so patients can receive treatment more promptly.
Methods
Institutional review board approval was obtained prior to initialization of this retrospective study. Data from January 2011 to April 2017 on 100 cancer patients at our hospital were retrospectively reviewed and analyzed. CT images were extracted from our Picture Archiving and Communication Systems and reviewed and re-reviewed by board-certified radiologists.
Patients
One hundred randomly-selected patients who were diagnosed with a primary cancer in the breasts, lungs, esophagus, colorectum, and liver, and had multiple surveillance CT scans comprised the study cohort. The 100 patients were selected randomly to avoid creating bias related to any pre-determination of how many of them had lung metastases correctly detected on their very first CT scans. There were 62 males and 38 females with an average age of 59.38±10.58 years. The detailed demographics and types of cancer of the patients are shown in Table 1. All except one patient had undergone treatment for the primary cancer. Sixty-nine (69/100, 69%) patients had surgical resections: 10 (10/100, 10%) had surgeries only, 48 (48/100, 48%) had adjuvant chemotherapy, and the remaining 11 (11/100, 11%) patients had local radiotherapy. Thirty-one (31/100, 31%) patients had non-operative treatment, including 20 (20/100, 20%) patients who underwent chemotherapy, 6 (6/100, 6%) patients had chemotherapy plus local radiotherapy, 3 (3/100, 3%) patients were treated by traditional Chinese medicine, 1 (1/100, 1%) patient had targeted therapy, and 1 (1/100, 1%) patient declined treatment. Following the identification of lung metastases, 61 (61/100, 61%) patients received chemotherapy, 14 (14/100, 14%) patients were surgically treated to remove nodules, 5 (5/100, 5%) patients underwent local radiotherapy for the metastases, 11 (11/100, 11%) patients received adjuvant traditional Chinese medicine, and the remaining 9 (9/100, 9%) patients chose not to receive any treatment.
Table 1. Patient characteristics.
| Primary cancer | Number (male/female) | Age (in years, mean ± SD) |
|---|---|---|
| Lung | 59 (40/19) | 60.98±10.27 |
| Colorectum | 15 (10/5) | 57.13±9.66 |
| Breast | 8 (0/8) | 60.00±15.45 |
| Liver | 5 (5/0) | 50.40±9.53 |
| Esophagus | 4 (4/0) | 54.75±10.75 |
| Other (<3 cases/cancer) | 9 (3/6) | 57.69±8.88 |
SD, standard deviation.
Study methods
Inclusion criteria
CT data on patients with primary cancer in the breasts, lungs, esophagus, colorectum, or liver and whose primary cancer had been confirmed by pathology were eligible for inclusion in the study. Additional inclusion criteria were that patients must have had at least two surveillance CT scans with lung metastases detected on the second CT scan and confirmed by either pathology or other clinical diagnostics. Lung metastases are diagnosed in clinics when, compared to a previous CT scan, nodules are newly found or existing nodules become larger and increase in number in the later scan(s). As the first step, we randomly selected 100 cancer patients from our database whose second CT scans had been interpreted to have lung metastases. As the second step, we then retrieved the first CT scans for analysis to determine whether the initial diagnosis involved the detection or missing of lung metastases.
CT parameters
CT examinations were performed using either a 16-detector row Toshiba Aquilion or a 128-detector row Siemens Somatom CT scanner. Both scanners were calibrated each day prior to scanning. Scanning parameters for the Aquilion machine were tube voltage 120 kV, tube current 30–230 mAs, helical pitch 0.837, rotation time 0.4 second, acquisition matrix 512×512 pixels, scanned slice thickness 1.0 mm, and interval 2.0 mm, with a reconstruction parameter Fc01. The Somatom machine was set with a tube voltage 120 kV, tube current 30–290 mAs, helical pitch 1.2, rotation time 0.5 second, acquisition matrix 512×512 pixels, scanned slice thickness 0.60 mm, and interval 2.0 mm, with a reconstruction parameter K51. The tube current was controlled by the CARE Dose 4D technique, which is an automatic exposure control that adjusted current during each rotation so as to reduce the magnitude for projection views with less attenuation and increase the magnitude for projection views with more attenuation. The advantage of auto mA is the improved performance in small lesion detection.
CT imaging
All the patients underwent multiple surveillance CT scans over a period of several months. The average interval between two CT scans was 173.4±161.6 days for a range of 13–1,068 days. For all the patients, we labeled the first scan as S1 and second scan as S2. For all the 100 cases, the mean interval between S1 and S2 was 156.6±140.3 days for a range of 19–728 days. Eleven of the S1 scans were pre-operative scans, 57 were post-operative scans, and 32 were non-operative scans. All the S2 scans had detectable lung metastases. There were 64 true negative cases among the S1 scans in which the metastases were located in the same areas as in the corresponding S2 scans, and, in 36 cases, the metastases had been missed. Among the missed metastases, three cases were missed in pre-operative scans (27%, 3/11), 22 cases were missed in post-operative scans (39%, 22/57), and 11 cases were missed in non-operative scans (34%, 11/32). The mean interval between S1 and S2 for the missed metastases was 97.2±61.5 days.
Image analysis
All scans were analyzed independently by two radiologists, each of whom had more than 5 years of experience. An axial lung window of a width 1,500 HU and level −650 HU were used. Discrepancies between the two radiologists were resolved by a third radiologist. There is good consistency between the two radiologists. The diagnostic identical rate was 95%. Only five cases required the involvement of a third radiologist, and all the radiologists concurred on the final lung metastasis diagnosis. We divided the image of each lung into nine regions from the top to the bottom with upper, center, and lower regions and with inner, middle, and outer regions from the inner side to the outer side. The boundary between the upper and center regions was at the aortic arch level, and the boundary between the center and lower regions was at the level of 3 cm above the diaphragm. For depiction on dividing the lungs into sub-regions, please refer to the supplementary materials. We manually measured the maximum diameter of each nodule and also measured the distance between nodules to the nearest blood vessels and the diameters of the blood vessels. For all these features, we measured each twice and used the average as the read-out.
Statistical analysis
The data were analyzed using SPSS Statistics 22.0. All the results are presented as mean ± SD (standard deviation). Independent samples t-test was used to compare the independence of two groups. Three groups of independent samples were compared by one-way analysis of variance (ANOVA). Data were compared by the chi-square or Fisher’s exact test. We considered P<0.05 as indicative of a statistically significant difference.
Results
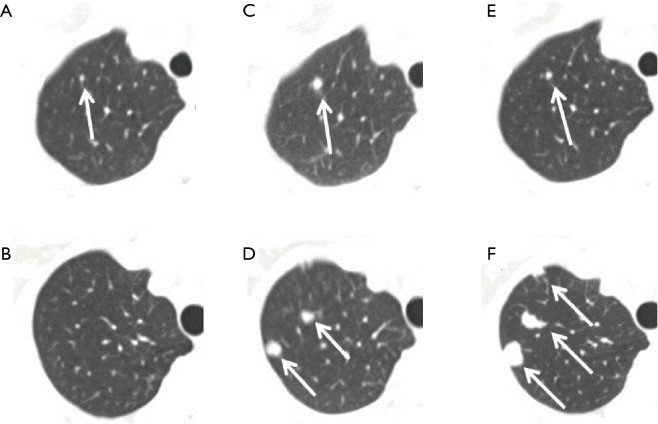
Using scanning results at S2 to re-examine lung metastases at S1, we found that there were 64 (64/100, 64%) cases of true negatives and 36 (36/100, 36%) false negatives at S1. Figure 1A,B shows examples of two female breast cancer patients of 63 and 53 years old, respectively, whose first CT scans already showed lung metastases, but they were deemed negative by a radiologist. The radiologist found the metastases on the second scans (Figure 1C,D), or 7 and 8 months later, respectively, after the corresponding first scans. On the third scan of the first patient at 5 months later, the metastasis of the first patient was reduced due to chemotherapy (Figure 1E). On the third scan for the second patient, at 1 year late, the metastasis increased in size (Figure 1F). Figure 2A shows another example of missed metastasis of a 46-year-old male liver cancer patient at the first CT scan, even though abnormal signal hyperintensity was present. The metastasis was found on the second scan at 4 months late (Figure 2B). Among the 36 false negatives, there were a total of 174 missed nodules. The mean size of the missed nodules was 2.66±1.27 mm (range, 0.67–7.60 mm). Among the 36 false negatives, there were 15 (15/36, 42%) cases of solitary nodules and 21 (21/36, 58%) cases of multiple nodules. The average diameter of solitary nodules was 4.04±1.73 mm, range 2.33–7.60 mm, and the average diameter of multiple nodules was 2.53±1.13 mm (range, 0.67–7.45 mm). There were statistically significant differences between the sizes of the solitary and multiple nodules (P=0.005). For each type of primary cancer, there were 22 cases in lung cancer (37%, 22/59), three cases in breast cancer (38%, 3/8), two cases in liver cancer (40%, 2/5), six cases in colorectal cancer (40%, 6/15), and two cases of esophageal cancer (50%, 2/4). There was one case of a missed nodule in an ovarian cancer patient.
Figure 1.
Two cases of missed nodules and their outcomes. (A,C,E) A case of a 63-year-old breast cancer patient with a missed nodule detection at the initial CT scan. (A) A missed nodule (arrow) on the first scan. The nodule was found only after mapping the position of the detected nodule (arrow) on the second scan 7 months later (C) to the first scan. (E) After chemotherapy, the nodule (arrow) was seen becoming smaller on the third scan performed another 5 months later. (B,D,F) A case of a 53-year-old breast cancer patient. The initial scan (B) was truly negative. Eight months later two nodules (arrows) were detected on the second scan (D). An additional 12 months later, the nodules (arrows) were significantly larger and a new nodule was found on the third scan (F).
Figure 2.
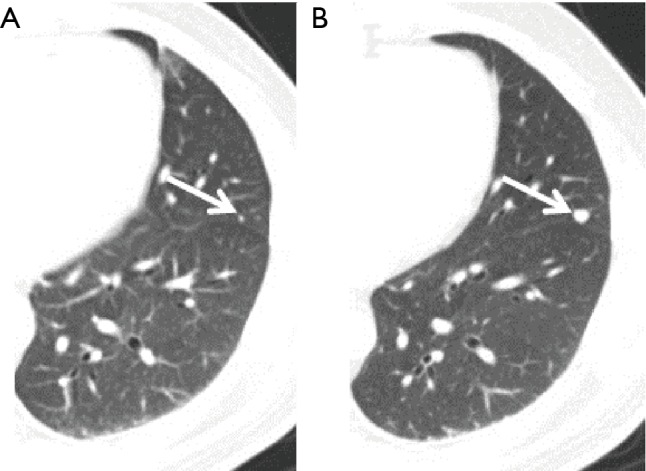
A case of a 46-year-old male liver cancer patient. (A) The metastasis was missed by a radiologist (arrow) on the first scan; (B) the metastasis, with an increased size, was found by a radiologist on the second scan at 4 months late (arrow).
In terms of the location of the missed nodules, there were a total of 53 (53/174, 30%) missed nodules in the left lung and 121 (121/174, 70%) missed nodules in the right lung. Considering both lungs together, there were 33 (33/174, 19%) missed nodules in the upper region, 113 (113/174, 65%) missed nodules in the center region, and 28 (28/174, 16%) missed nodules in the lower region (Table 2). The delineation of the upper, center, and lower regions is shown in supplement Figure S1. There were 32 (32/174, 18%) missed nodules in the inner region, 47 (47/174, 27%) missed nodules in the middle region, and 97 (97/174, 56%) missed nodules in the outer region (Table 2). The delineation of the inner, middle, and outer regions is shown in supplement Figure S2. No statistically significant difference was found in the sizes of missed nodules in the left and right lungs, either in scans S1 or S2. However, the sizes of the missed nodules in the upper, center, and lower regions of the lungs showed statistically significant differences in both scans S1 and S2 (Table 3). There were no statistically significant differences in the sizes of the nodules in the inner, middle, and outer regions (Table 3). There were statistically significant differences between the sizes of missed nodules in scan S1 and the correctly detected nodules in scan S2 (Table 4). In S1, there were statistically significant differences between the distance from missed nodules to the nearest blood vessels and the distance from correctly detected nodules to the nearest blood vessels (Table 4). In addition, there were statistically significant differences in the size of blood vessels that were near a missed nodule or near a correctly detected nodule (Table 4).
Table 2. Distribution of missed nodules in different sub-regions.
| Region | Inner zone, n (%) | Middle zone, n (%) | Outer zone, n (%) | Total, n (%) |
|---|---|---|---|---|
| Left lung | ||||
| Upper region | 1 (1.89) | 4 (7.55) | 0 | 5 (9.43) |
| Center region | 5 (9.43) | 8 (15.09) | 23 (43.40) | 36 (67.92) |
| Lower region | 2 (3.77) | 5 (9.43) | 5 (9.43) | 12 (22.64) |
| Total | 8 (15.09) | 17 (32.08) | 28 (52.83) | 53 |
| Right lung | ||||
| Upper region | 4 (3.31) | 9 (7.44) | 15 (12.40) | 28 (23.14) |
| Center region | 15 (12.40) | 19 (15.70) | 43 (35.54) | 77 (63.64) |
| Lower region | 5 (4.13) | 2 (1.65) | 9 (7.44) | 16 (13.22) |
| Total | 24 (19.83) | 30 (24.79) | 67 (55.37) | 121 |
Figure S1.
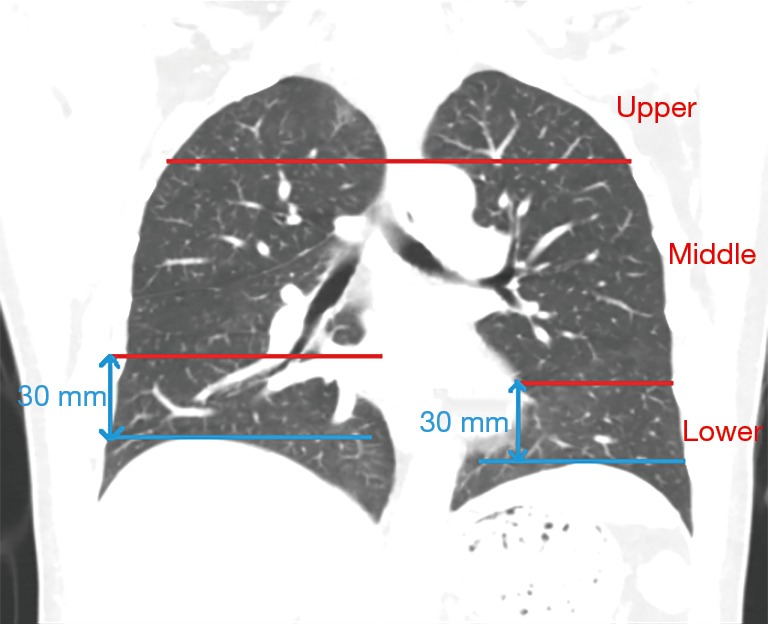
Division of the lungs into the upper, center, and lower regions.
Figure S2.
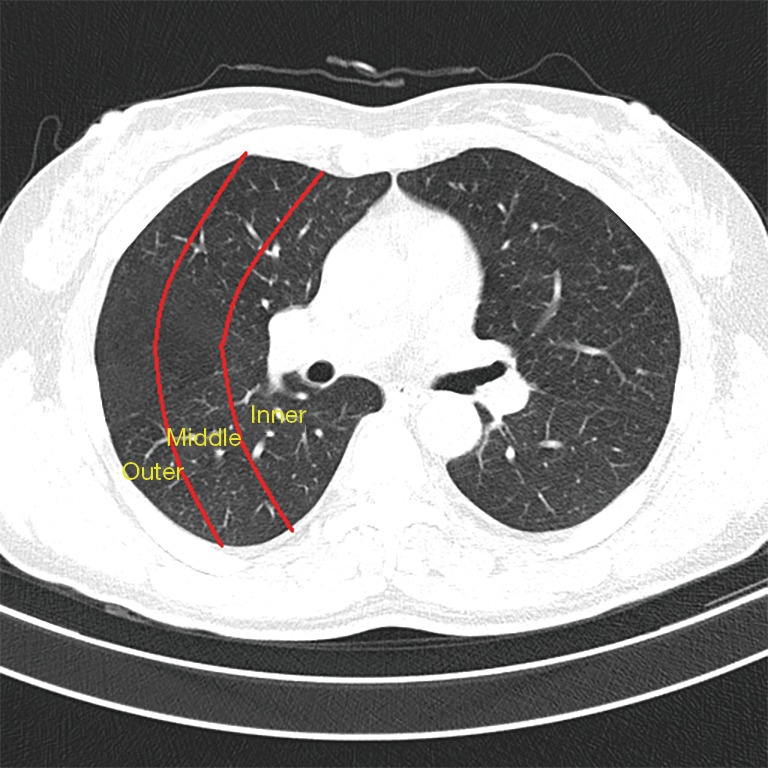
Division of the lungs into the inner, middle, and outer regions.
Table 3. Characteristics of missed nodules on scans S1 and S2.
| Location | S1 | S2 |
|---|---|---|
| Region | ||
| Upper region (n=33) | 2.23±0.72 (0.92–3.94) | 3.60±1.58 (1.81–9.78) |
| Center region (n=133) | 2.65±1.26 (0.67–7.60) | 3.85±1.67 (1.52–8.88) |
| Lower region (n=28) | 3.18±1.59 (1.25–7.48) | 5.53±3.03 (1.89–15.63) |
| P value | 0.013 | 0.001 |
| Zone | ||
| Inner zone (n=32) | 2.89±1.48 (1.14–7.48) | 4.49±2.76 (1.69–15.63) |
| Middle zone (n=47) | 2.43±0.94 (1.06–5.59) | 3.77±1.81 (1.52–11.90) |
| Outside zone (n=95) | 2.69±1.32 (0.67–7.60) | 4.08±1.83 (1.81–9.78) |
| P value | 0.263 | 0.299 |
Results are presented as mean ± SD (range). SD, standard deviation.
Table 4. The distance of adjacent blood vessels to the nodules and the sizes of the blood vessels.
| Variables | Miss nodules (n=174) | Non-missed nodules (n=64) | P value |
|---|---|---|---|
| Distance (mm) | 4.34±1.68 (1.48–11.15) | 7.66±3.79 (2.86–23.46) | 0.001 |
| Size of vessel (mm) | 1.39±0.49 (0.51–3.20) | 1.64±0.66 (0.64–4.53) | 0.001 |
Results are presented as mean ± SD (range). Distances were measured from the center of a nodule to the center of its nearest blood vessel. SD, standard deviation.
Discussion
Metastases account for more than 90% of tumor-related deaths that can be largely attributable to the significant challenges in tumor detection at an early stage (12). Lungs are a primary location to which many types of cancer metastasize. For example, 60–70% of breast cancer patients eventually die of lung metastases (1). For patients with liver cancer, the 5-year survival rate is 96.1% if there are no lung metastases, but, if there are lung metastases, the survival rate drops to 50.1% (13). To detect lung metastases, CT remains the most widely used technique due to its higher resolution and sensitivity than MRI and PET (14). However, despite CT’s resolution and sensitivity, its use still results, in our analysis, in a considerable rate of missed (36%, 36/100) detections of lung metastases on the earliest scans.
Based on the analysis of the spatial distribution of the missed nodules, we found that there were 228% more missed nodules in the right lung than the left lung. The reason behind this observation could be that the right lung is larger and has more blood vessels than the left lung, with the latter situation allowing for more cancer cells to leave the circulation system and settle into the lung (15,16). The difference in the density of blood vessel in a local area may also explain why we found more missed nodules in the center region as this region is near the hilum and rich in blood vessels. In the outer region, the size of the blood vessels may be very similar to the size of nodules, causing more nodules to be missed by a radiologist. The sizes of the missed nodules located in the lower region were statistically larger than those in the upper and center region. We posited that, because the blood vessels in the lower region are large and comparable to the sizes of the nodules, detection of the nodules is difficult.
It is interesting to note that the distance from a nodule to the nearest blood vessel may have some effects on the likelihood of detection of the nodule. There was a statistically significant shorter distance between the missed nodules and nearby blood vessels than between the accurately detected nodules and nearby blood vessels. The close proximity of a nodule and a neighboring blood vessel may be a primary reason that some nodules are missed in diagnosis, particularly in scans S1 on which the nodules are small. Our results showed that the diameters of missed nodules ranged from 0.67 to 7.60 mm, and that nodules with diameters less than 2.66 mm were more likely to be missed; 66% (114/174) were smaller than 2.66 mm and 34% (60/174) were larger than 2.66 mm, as shown by the broken line in Figure 3. It has been reported that metastatic nodules less than 10 mm were likely to be missed in PET/CT examinations (17-19) whereas MRI examinations tended to miss nodules smaller than 4.0 mm (20,21). Due to the resolution of CT, our broken line is lower than that of the MRI.
Figure 3.
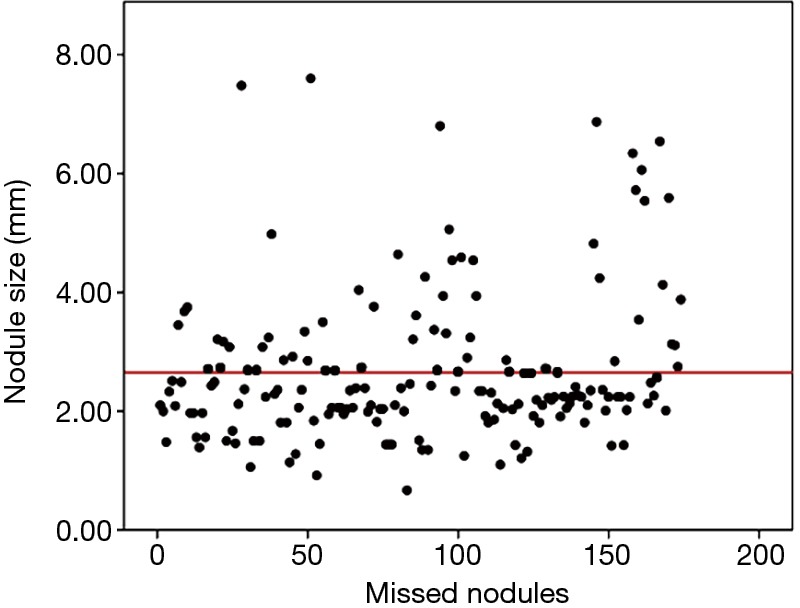
Sizes (in diameter) of the 174 missed pulmonary nodules measured on HRCT. Mean diameter of the nodules was 2.66 mm in diameter (broken line). Minimum and maximum diameters were 0.67 and 7.60 mm, respectively. HRCT, high-resolution computed tomography.
Typically, in clinics, solitary nodules are more likely to be missed than multiple nodules. However, we found more cases of missed multiple nodules in S1 scans. We posit two explanations for this finding: first, there are more cases of multiple nodules in lung metastases, constituting a large baseline; and, second, solitary nodules are generally larger than multiple nodules, as evidenced by the statistically significant differences in their sizes and, thus, solitary nodules are relatively easier to find.
Our study provided new insights into the process of detecting lung metastases by CT by showing that, with careful examination, many lung metastases can be detected on the initial CT scans, particularly small nodules that may be indicative of malignancy (22,23). Among the 174 missed nodules, 3% (5/174) remained approximately the same sizes on S2, 3% (5/174) became smaller on S2, and 94% (164/174) of the nodules became larger on S2. Schematically, several options are available if a metastasis is found: (I) fine-needle aspiration (FNA) biopsy may be performed to provide pathological confirmation (14); (II) an additional CT scan may be ordered sooner than the current guideline suggests; or (III) a PET/CT scan may be performed to provide additional information about the suspected malignancies (24,25). We found that close adjacency to blood vessels adversely affects the sensitivity of detecting nodules and, thus, our findings may suggest the need to facilitate the development of computer aided detection (CAD) techniques for distinguishing blood vessels from suspected lesions to assist clinicians in making better decisions (26-28). Through CAD techniques, the radiologic appearance of the nodules such as size, shape, and margin characteristics can be combined with clinical information to produce an integrated input for clinicians.
This study, to the best of our knowledge, is the first work to analyze CT examinations performed at different time points to show that more than one-third cases of metastases are already visible on the initial scans and, thus, it is feasible to use CT to detect lung nodules at the earliest stages. The findings provide the basis for our developing criteria for diagnosing lung metastases, an area that, so far, has had no specific guidelines. Although there are American College of Radiology (ACR) appropriateness criteria for detecting primary pulmonary nodules, there is currently no established role for the use of CT in monitoring lung metastasis. This work is significant in that it evidences the need and method for improving the sensitivity of diagnosing lung metastasis through the use of CT months earlier in the timeline of care of lung cancer patients.
In clinics, for primary lung cancer diagnosis, nodules less than 5 mm may require follow-up. However, for lung metastases, because they may develop very quickly, it is important to be able to identify them as early as possible. In addition, the detection of lung metastasis may change the staging of the cancer and this information may influence clinical decision-making; thus, missing early-stage lung metastases has a negative impact for clinics and finding them promptly will clinically benefit patients.
Our study has some limitations. First, due to the nature of this retrospective study, there is insufficient information on the follow-up with patients, whether their lung metastases were detected in S1 or S2. For some patients, after checking out from an institution and continuing their treatment at different institutions, it is difficult to maintain a close follow-up on their health status. Second, as the patients received different types of treatment on their primary cancer, this difference may have had some effect on the likelihood that they develop lung metastasis in the future. For example, it is unclear whether patients, who received systematic chemotherapy, experienced any effect in the delay or reduction of risk of lung metastases as compared to the patients who had surgery or other radiotherapy. From this perspective, it is beneficial to consider a patient’s clinical diagnosis and types of treatment to predict the probability of lung metastases and to integrate that information with radiology examinations for accurate and robust detection of lung metastasis.
In this work, the slice thickness was 2 mm, which was sufficient for finding nodules larger than 2 mm. For nodules smaller than 2 mm, unless they fall exactly between two slices, they can also be found. This work is confined to the finding of lung metastases with loci that were usually clearer than diffuse lung metastasis. In clinics, diffuse lung metastases are not difficult to detect. Therefore, this work has the benefit of assisting clinicians to find non-diffuse metastatic lesions.
Conclusions
There were a considerable percentage of early lung metastases that were missed by radiologists but appeared on CT scans. The capability of CT to reveal such early metastases opens up an opportunity to move up the time points of detecting lung metastases through clinical and training improvements and technology development, such as computer-aided detection and machine learning.
Acknowledgments
Funding: The work of H Chen was supported by the Postgraduate Education Innovation Planning Project of Guangdong Province, China (2018JGXM83) and the Science and Technology Planning Project of Guangzhou, Guangdong Province, China (201904010130) and the work of S Huang was supported by the Characteristic Innovation Projects of Colleges and Universities in Guangdong Province, China (2018KTSCX188).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board (medical research ethical review 2019, No. K47).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Eckhardt BL, Francis PA, Parker BS, et al. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov 2012;11:479-97. 10.1038/nrd2372 [DOI] [PubMed] [Google Scholar]
- 2.Schroeder T, Ruehm SG, Debatin JF, et al. Detection of pulmonary nodules using a 2D HASTE MR sequence: comparison with MDCT. AJR Am J Roentgenol 2005;185:979-84. 10.2214/AJR.04.0814 [DOI] [PubMed] [Google Scholar]
- 3.Rauscher I, Eiber M, Furst S, et al. PET/MR imaging in the detection and characterization of pulmonary lesions: technical and diagnostic evaluation in comparison to PET/CT. J Nucl Med 2014;55:724-9. 10.2967/jnumed.113.129247 [DOI] [PubMed] [Google Scholar]
- 4.Raad RA, Friedman KP, Heacock L, et al. Outcome of small lung nodules missed on hybrid PET/MRI in patients with primary malignancy. J Magn Reson Imaging 2016;43:504-11. 10.1002/jmri.25005 [DOI] [PubMed] [Google Scholar]
- 5.Christensen JA, Nathan MA, Mullan BP, et al. Characterization of the solitary pulmonary nodule: 18F-FDG PET versus nodule-enhancement CT. AJR Am J Roentgenol 2006;187:1361-7. 10.2214/AJR.05.1166 [DOI] [PubMed] [Google Scholar]
- 6.Goo JM, Im JG, Do KH, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 2000;216:117-21. 10.1148/radiology.216.1.r00jl19117 [DOI] [PubMed] [Google Scholar]
- 7.Bakheet SM, Saleem M, Powe J, et al. F-18 fluorodeoxyglucose chest uptake in lung inflammation and infection. Clin Nucl Med 2000;25:273-8. 10.1097/00003072-200004000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Dabrowska M, Krenke R, Korczynski P, et al. Diagnostic accuracy of contrast-enhanced computed tomography and positron emission tomography with 18-FDG in identifying malignant solitary pulmonary nodules. Medicine 2015;94:e666. 10.1097/MD.0000000000000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swensen SJ, Brown LR, Colby TV, et al. Lung nodule enhancement at CT: prospective findings. Radiology 1996;201:447-55. 10.1148/radiology.201.2.8888239 [DOI] [PubMed] [Google Scholar]
- 11.Swensen SJ, Brown LR, Colby TV, et al. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology 1995;194:393-8. 10.1148/radiology.194.2.7824716 [DOI] [PubMed] [Google Scholar]
- 12.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006;127:679-95. 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Albanus DR, Apitzsch J, Erdem Z, et al. Clinical value of 68Ga-DOTATATE-PET/CT compared to stand-alone contrast enhanced CT for the detection of extra-hepatic metastases in patients with neuroendocrine tumours (NET). Eur J Radiol 2015;84:1866-72. 10.1016/j.ejrad.2015.06.024 [DOI] [PubMed] [Google Scholar]
- 14.Cardinale L, Ardissone F, Novello S, et al. The pulmonary nodule: clinical and radiological characteristics affecting a diagnosis of malignancy. Radiol Med 2009;114:871-89. 10.1007/s11547-009-0399-1 [DOI] [PubMed] [Google Scholar]
- 15.Sharara RS, Hattab Y, Patel K, et al. Introduction to the Anatomy and Physiology of Pulmonary Circulation. Crit Care Nurs Q 2017;40:181-90. 10.1097/CNQ.0000000000000157 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. 10.1038/nrc2622 [DOI] [PubMed] [Google Scholar]
- 17.Kanne JP, Jensen LE, Mohammed TL, et al. ACR appropriateness Criteria(R) radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1-3. 10.1097/RTI.0b013e31827657c8 [DOI] [PubMed] [Google Scholar]
- 18.Iwano S, Ito S, Tsuchiya K, et al. What causes false-negative PET findings for solid-type lung cancer? Lung Cancer 2013;79:132-6. 10.1016/j.lungcan.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 19.Erasmus JJ, Macapinlac HA. Low-sensitivity FDG-PET studies: less common lung neoplasms. Semin Nucl Med 2012;42:255-60. 10.1053/j.semnuclmed.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 20.Sawicki LM, Grueneisen J, Buchbender C, et al. Evaluation of the Outcome of Lung Nodules Missed on 18F-FDG PET/MRI Compared with 18F-FDG PET/CT in Patients with Known Malignancies. J Nucl Med 2016;57:15-20. 10.2967/jnumed.115.162966 [DOI] [PubMed] [Google Scholar]
- 21.Wang YX, Lo GG, Yuan J, et al. Magnetic resonance imaging for lung cancer screen. J Thorac Dis 2014;6:1340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 2001;21:403-17. 10.1148/radiographics.21.2.g01mr17403 [DOI] [PubMed] [Google Scholar]
- 23.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. 10.1164/ajrccm.165.4.2107006 [DOI] [PubMed] [Google Scholar]
- 24.Lowe VJ, Fletcher JW, Gobar L, et al. Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 1998;16:1075-84. 10.1200/JCO.1998.16.3.1075 [DOI] [PubMed] [Google Scholar]
- 25.Herder GJ, Golding RP, Hoekstra OS, et al. The performance of (18)F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging 2004;31:1231-6. 10.1007/s00259-004-1552-7 [DOI] [PubMed] [Google Scholar]
- 26.Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application. Radiology 1993;186:415-22. 10.1148/radiology.186.2.8421744 [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Yoshida H, Engelmann R, et al. Computerized analysis of the likelihood of malignancy in solitary pulmonary nodules with use of artificial neural networks. Radiology 2000;214:823-30. 10.1148/radiology.214.3.r00mr22823 [DOI] [PubMed] [Google Scholar]
- 28.Fraioli F, Catalano C, Almberger M, et al. Evaluation of effectiveness of a computer system (CAD) in the identification of lung nodules with low-dose MSCT: scanning technique and preliminary results. Radiol Med 2005;109:40-8. [PubMed] [Google Scholar]