Abstract
Background
The secondary lymphoid tissues (LTs), lymph nodes (LNs) and gut-associated lymphoid tissue (GALT) are considered reservoirs for HIV. Antiretrovirals (ARVs) have lower penetration into LT. In vitro models predictive of ARV LT penetration have not been established.
Objectives
To develop an in vitro model of LT bioavailability using human lymphoid endothelial cells (HLECs) and investigate its predictability with in vivo pharmacokinetic (PK) studies in mice.
Methods
ARV bioavailability in HLECs was evaluated at the maximum plasma concentration (Cmax) observed in HIV-infected patients. ARVs were: abacavir, atazanavir, darunavir, dolutegravir, efavirenz, elvitegravir, emtricitabine, maraviroc, raltegravir, rilpivirine, ritonavir, tenofovir disoproxil fumarate and the PK booster cobicistat. The LT PK of representative drugs showing high (efavirenz), intermediate (dolutegravir) and low (emtricitabine) HLEC bioavailability was investigated in BALB/c mice given 50/10/30 mg/kg efavirenz/dolutegravir/emtricitabine orally, daily for 3 days. The concordance of in vitro and in vivo ARV bioavailability was examined.
Results
ARVs showed high (>67th percentile; rilpivirine, efavirenz, elvitegravir and cobicistat), intermediate (67th–33rd percentile; ritonavir, tenofovir disoproxil fumarate, dolutegravir and maraviroc) and low (<33rd percentile; atazanavir, darunavir, raltegravir, emtricitabine and abacavir) HLEC bioavailability. The hierarchy of efavirenz, dolutegravir and emtricitabine bioavailability in LN, gut and brain tissues of mice was: efavirenz>dolutegravir>emtricitabine.
Conclusions
ARVs displayed distinct HLEC penetration patterns. PK studies of representative ARVs in LT of mice were concordant with HLEC bioavailability. These findings support further development of this approach and its translational predictability in humans.
Introduction
HIV establishes persistent and stable reservoirs in lymphoid tissues (LTs).1,2 LT reservoirs persist even in patients suppressed to undetectable levels of plasma HIV RNA with ART and are a major obstacle for virus eradication.2 Antiretroviral (ARV) concentrations are lower in LTs than in PBMCs, and may allow persistent viral replication in simian immunodeficiency virus (SIV)-infected macaques and HIV-infected persons.3–6 This evidence suggests that LTs may be a sanctuary for HIV because of insufficient drug exposure. Our knowledge of key determinants of drug penetration into LTs is limited. An in vitro model to predict LT bioavailability of ARVs is not available and could be a useful tool for understanding ARV pharmacology.
Our objective was to develop such an in vitro model, using human lymphoid endothelial cells (HLECs), and investigate its agreement with ARV tissue penetration in mice.
Methods
Experimental procedures
ARVs
Abacavir, atazanavir, darunavir, efavirenz, emtricitabine, maraviroc, rilpivirine and ritonavir were obtained from the NIH AIDS Reagent Program (MD, USA). Elvitegravir and cobicistat were from Gilead (CA, USA), raltegravir was from Merck (NJ, USA), tenofovir disoproxil fumarate was from Matrix Scientific (Columbia, USA) and dolutegravir was from Advanced Chemblocks Inc. (CA, USA).
Cell culture
Lymph node (LN)- and skin-derived HLECs (ScienCell Laboratories, CA, USA) were cultured according to the manufacturer’s protocol and maintained in a 37°C incubator with 5% CO2.
Cell viability and cell-associated drug quantitation assays
HLECs were cultured at 2.5 × 104 density in 200 μL of medium in a 96-well plate until a monolayer was formed and then separately treated with ARVs for 72 h. ARVs (concentrations in ng/mL) were: abacavir (4000), atazanavir (4500), darunavir (7500), dolutegravir (3500), efavirenz (2500), elvitegravir (1500), emtricitabine (2000), maraviroc (600), raltegravir (1500), rilpivirine (150), ritonavir (1000), tenofovir disoproxil fumarate (450) and cobicistat (1000). These concentrations are equivalent to the maximum plasma concentration (Cmax) observed in HIV-infected persons. After drug treatment, we measured HLEC viability using an MTT cell proliferation kit (Sigma–Aldrich, IL, USA) according to the manufacturer’s protocol. Additional details are provided in the Supplementary Methods (available as Supplementary data at JAC Online). For drug quantitation, 5 × 105 HLECs were cultured in each well of a 6-well plate until a monolayer was formed and treated with ARV Cmax for 72 h. After drug treatment, cells were washed once with 1× PBS and resuspended in 0.5 mL of 70% methanol in ultrapure water (v/v). Cell extracts were stored at −80°C until drug analysis. ARV quantification was performed by LC/MS/MS as described previously.3,6–9 These methods were adapted for cobicistat, elvitegravir, maraviroc and rilpivirine with detection by their specific mass transitions 776.3/606.2, 448.1/344.1, 514.3/280.2 and 367.2/195.1, respectively, with optimized voltages and other MS parameters. Mobile phase was substituted for rilpivirine with MeOH/25 mM ammonium acetate/HCOOH (65/35/0.1, v/v/v) and for maraviroc with MeOH/H2O/HCOOH (40/60/0.1, v/v/v).
Experimental animals
Adult, healthy male BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) that were 8–10 weeks old were used for pharmacokinetic (PK) studies.
Housing and husbandry
Mice were housed at the University of Nebraska Medical Center (UNMC) vivarium, acclimatized for 14 days, provided access to food and water and followed light/dark cycles.
Ethics
UNMC follows American Association for Laboratory Animal Care guidelines. All procedures were approved by the Institutional Animal Care and Use Committee according to NIH guidelines.
Study design
Drug formulation containing efavirenz/dolutegravir/emtricitabine at 12.5/2.5/7.5 mg/mL was prepared in a vehicle [DMSO/PEG400/PG/ethanol/kolliphor EL/1× PBS (8/25/15/10/7/35% v/v)] according to a previously described protocol.10 Mice were administered the efavirenz/dolutegravir/emtricitabine (EFV/DTG/FTC) formulation, at a human-equivalent dose of 50/10/30 mg/kg of body weight (<250 μL) by oral gavage,10 daily for three consecutive days, and sacrificed on day 3 (4 h after administration of the third dose).
Sample size
Four independent biological replicates for in vitro HLEC experiments and six for in vivo mice experiments were used based on investigator judgement of optimal scientific rigour.
Tissue collection and processing
Mice were anaesthetized with isoflurane. Blood was collected from the submandibular vein and then brain, gut and LN samples were collected, washed with HPLC-grade water, air dried and stored at −80°C until homogenization. Plasma samples were prepared as described previously.10 Brain and gut tissues were homogenized in 70% methanol at a 1:5 ratio (w/v) and LN tissues at a 1:20 ratio (w/v) with a Precellys Evolution cryolys homogenizer (Bertin Technologies, USA) in a temperature-controlled chamber (<10°C) according to the manufacturer’s protocol. Homogenates were centrifuged at 10000 rpm and supernatant containing drugs was stored at −80°C until drug quantitation.
Data analyses
ARV bioavailability in HLECs was calculated as the ratio of measured ARV intracellular concentration (ICC; fM per million cells) to the concentration used in cell culture medium (Cmax; fM). In mice, ARV bioavailability was calculated as the ratio of ARV concentration in tissues to that in plasma. The pharmacologically active moiety of emtricitabine is emtricitabine-triphosphate that cannot be given orally, does not exist in plasma and is only formed intracellularly; therefore, we used the ratio of emtricitabine-triphosphate in HLECs and mouse tissues to emtricitabine in cell culture medium or plasma to represent emtricitabine bioavailability.
Results
Cytotoxicity of ARVs in HLECs
Viability studies in LN-derived HLECs showed that all tested ARVs except dolutegravir were non-toxic (>85% viability) at their Cmax; dolutegravir showed modest cytotoxicity (64.7% viability) (see the Supplementary Results and Figure S1).
ARV bioavailability in HLECs
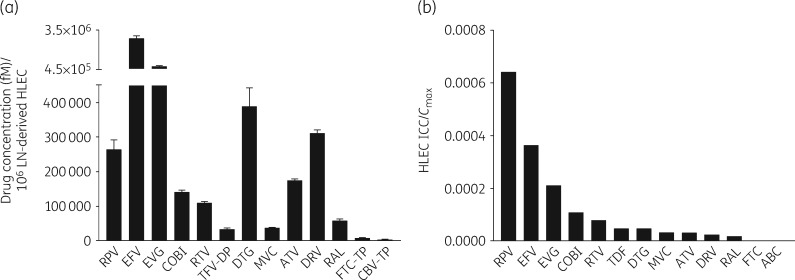
ARV concentrations in HLECs demonstrated distinct penetration patterns (Figure 1a and the Supplementary Results) as did the HLEC ICC/Cmax ratio as a measure of bioavailability (Figure 1b). Based on the HLEC ICC/Cmax ratio, ARVs were divided into tertiles (the log-transformed HLEC/Cmax ratio had a normal distribution and there was no suggestion of significant non-linearity). Rilpivirine, efavirenz, elvitegravir and cobicistat were categorized as high-bioavailability drugs (>67th percentile); ritonavir, tenofovir disoproxil fumarate, dolutegravir and maraviroc were categorized as intermediate-bioavailability drugs (67th–33rd percentile) and atazanavir, darunavir, raltegravir, emtricitabine and abacavir were categorized as low-bioavailability drugs (<33rd percentile). ARVs achieved similar concentrations in human skin-derived dermal HLECs (Figure S2).
Figure 1.
ARV concentrations and bioavailability in HLECs. (a) ARV concentrations per million LN-derived HLECs. A monolayer of LN-derived HLECs was treated with each of the 13 different ARVs: abacavir (ABC; 4000 ng/mL), atazanavir (ATV; 4500 ng/mL), cobicistat (COBI; 1000 ng/mL), darunavir (DRV; 7500 ng/mL), dolutegravir (DTG; 3500 ng/mL), efavirenz (EFV; 2500 ng/mL), elvitegravir (EVG; 1500 ng/mL), emtricitabine (FTC; 2000 ng/mL), maraviroc (MVC; 600 ng/mL), raltegravir (RAL; 1500 ng/mL), rilpivirine (RPV; 150 ng/mL), ritonavir (RTV; 1000 ng/mL) and tenofovir disoproxil fumarate (TDF; 450 ng/mL) at the Cmax usually observed in the plasma of ART-treated HIV-infected patients; the cells were washed once with 1× PBS and harvested 72 h post-treatment for drug analysis. Drug concentrations were quantified using LC/MS/MS. For ABC, FTC and TDF, the antivirally active compounds carbovir-triphosphate (CBV-TP), FTC-triphosphate (FTC-TP) and tenofovir-diphosphate (TFV-DP), respectively, were quantified. (b) Bioavailability of ARVs in HLECs. ARV concentration in HLECs was normalized to the concentration of that drug used in the HLEC culture.
ARV bioavailability in mouse LNs, gut and brain tissues
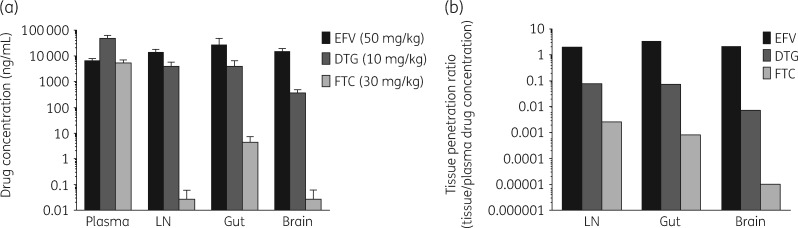
Efavirenz, dolutegravir and emtricitabine, showing high, intermediate and low HLEC bioavailability, respectively, were tested for LT penetration in six healthy mice. Animals maintained good health, and no adverse conditions were reported. Efavirenz showed the highest concentrations in LNs, gut and brain tissues followed by dolutegravir and emtricitabine-triphosphate (Figure 2a). Tissue/plasma ratios also showed that efavirenz had the highest tissue bioavailability: LN, efavirenz (2.07) >dolutegravir (0.082) >emtricitabine-triphosphate (0.0026); gut, efavirenz (3.49) >dolutegravir (0.076) >emtricitabine-triphosphate (0.00082); and brain, efavirenz (2.23) >dolutegravir (0.0077) >emtricitabine-triphosphate (0.00001) (Figure 2a and b).
Figure 2.
Tissue concentrations and bioavailability of efavirenz (EFV), dolutegravir (DTG) and emtricitabine (FTC) in mice. Mice (n = 6) were administered EFV/DTG/FTC at 50/10/30 mg/kg once daily by oral gavage for three consecutive days. EFV, DTG (in plasma and tissues) and FTC (in plasma) and FTC-TP ( in tissues) were determined by LC/MS/MS analysis. (a) EFV, DTG and FTC/FTC-TP concentrations in plasma, LNs, gut and brain. (b) EFV, DTG and FTC tissue bioavailability. Tissue bioavailability was calculated as the ratio of EFV, DTG and FTC-TP concentrations in LNs, gut and brain tissues to EFV, DTG and FTC concentrations in plasma. FTC-TP, FTC-triphosphate.
Discussion
We observed a hierarchy of ARV penetration in the HLEC model system (Figure 1a). In PK studies conducted in mice, ARV bioavailability in LNs was as predicted by the HLEC model: efavirenz >dolutegravir >emtricitabine (Figure 2b).
Penetration of drugs into LTs depends on several physiochemical characteristics including ionization, dissociation constant (pKa), lipophilicity (logP) and protein binding.11–13 The relevance of these factors can be observed, for example, by examination of the pKa, logP and water solubility values for efavirenz, dolutegravir and emtricitabine, showing high, intermediate and low HLEC bioavailability, respectively: pKa, efavirenz (10.2) >dolutegravir (8.2) >emtricitabine (2.65); logP, efavirenz (4.6) >dolutegravir (2.2) >emtricitabine (−1.4); and hydrophobicity based on water solubility, efavirenz (0.093 mg/mL) >dolutegravir (0.095 mg/mL) >emtricitabine (112 mg/mL). Thus, efavirenz, a highly bioavailable drug in HLECs, had the highest penetration into the LNs and accordingly higher pKa, logP and hydrophobicity.
Orally administered drugs can enter the lymphatic system by passing through an endothelial cell (EC) monolayer barrier present in lymphatic vessels. ECs also form a barrier while draining fluid from the interstitial space.14,15 While not a primary objective of this work, we found that the penetration of efavirenz, dolutegravir and emtricitabine into brain tissue of mice was completely consistent with their HLEC bioavailability (Figure 2a). The blood–brain barrier limits entry of ARVs into the brain.16 These data suggest that commonalities exist among determinants of ARV bioavailability in HLECs and brain ECs. Future studies may help to delineate the relative contributions of drug physicochemical properties, cell membrane architecture, drug-metabolizing enzymes, transporters and chylomicron-mediated transport on ARV penetration into LTs and the brain.17
We observed concordance between ARV bioavailability predicted by the HLEC model and the concentrations of efavirenz, dolutegravir and emtricitabine in mouse LNs, gut and brain tissues. We chose to use ARV-specific cell medium concentrations representative of the Cmax in patients in the HLEC model versus, for example, equal concentrations for all ARVs. While efavirenz, dolutegravir and emtricitabine PK in vivo followed the in vitro HLEC bioavailability pattern, our approach could limit direct molecule to molecule comparisons of penetration. We did, perhaps, observe discrepancies between some HLEC model predictions and other animal data. For example, maraviroc had intermediate bioavailability in the HLEC model. While an imaging study in rats showed that maraviroc in LNs exceeded that in plasma,18 a PK study in monkeys found a lower maraviroc LN/plasma ratio.19
Collectively, the data support further development of the HLEC model and evaluation of the tissue bioavailability of a broader array of ARVs in non-human primates because of their relevance to HIV infection in humans. Preliminary data have recently been presented on LN penetration of three integrase strand transfer inhibitors in 34 HIV-infected persons. The hierarchy of LN penetration was elvitegravir >dolutegravir >raltegravir, which is as predicted by the HLEC model.20 These data provide motivation for investigations into the ability of the HLEC model to predict LT drug penetration in humans, which could be a useful tool for drug development. Additionally, the model could provide support for innovative therapeutic strategies, such as whether an ART regimen with enhanced LT penetration improves suppression of HIV replication in viral reservoirs and sanctuaries mitigating limitations of current ART regimens.
Supplementary Material
Acknowledgements
We acknowledge Dr Sushil Kumar for his assistance.
Funding
This study was funded by R01-AI124965 awarded to C. V. F. by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH, Bethesda, MD, USA.
Transparency declarations
None to declare.
Author contributions
C. V. F. and S. R. D. conceived the idea. S. R. D. developed the HLEC model, performed drug cytotoxicity and drug treatment experiments in HLEC and PK studies in mice and wrote the paper. N. G. prepared formulations for ARV PK studies in mice and, along with S. R. D., performed mice studies. Y. A. helped to design and supervise PK studies in mice. C. V. F. provided suggestions throughout the study and monitored the study findings. C. V. F. and A. T. P. were involved in scientific discussions. C. V. F., A. T. P. and Y. A. reviewed the manuscript. L. C. W. performed cobicistat, dolutegravir, elvitegravir, rilpivirine and carbovir-triphosphate quantitation in HLECs and plasma, LNs, brain and gut tissues of mice. J. A. W. performed emtricitabine-triphosphate, tenofovir-diphosphate and raltegravir quantitation in HLECs and plasma, LNs, brain and gut tissues of mice. T. M. M. performed atazanavir, ritonavir, efavirenz and darunavir quantitation in HLECs and plasma, LNs, brain and gut tissues of mice. K. M. C. performed quality control checks on LC/MS/MS raw data.
References
- 1. van Marle G, Church DL, van der Meer F. et al. Combating the HIV reservoirs. Biotechnol Genet Eng Rev 2018; 34: 76–89. [DOI] [PubMed] [Google Scholar]
- 2. Siliciano JM, Siliciano RF.. The remarkable stability of the latent reservoir for HIV-1 in resting memory CD4+ T cells. J Infect Dis 2015; 212: 1345–7. [DOI] [PubMed] [Google Scholar]
- 3. Fletcher CV, Staskus K, Wietgrefe SW. et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA 2014; 111: 2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourry O, Mannioui A, Sellier P. et al. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology 2010; 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solas C, Lafeuillade A, Halfon P. et al. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2003; 47: 238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dyavar SR, Ye Z, Byrareddy SN. et al. Normalization of cell associated antiretroviral drug concentrations with a novel RPP30 droplet digital PCR assay. Sci Rep 2018; 8: 3626.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Podany AT, Winchester LC, Robbins BL. et al. Quantification of cell-associated atazanavir, darunavir, lopinavir, ritonavir, and efavirenz concentrations in human mononuclear cell extracts. Antimicrob Agents Chemother 2014; 58: 2866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Podany AT, Bares SH, Havens J. et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32: 761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorenzo-Redondo R, Fryer HR, Bedford T. et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530: 51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gautam N, Roy U, Balkundi S. et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob Agents Chemother 2013; 57: 3110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ford J, Khoo SH, Back DJ.. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother 2004; 54: 982–90. [DOI] [PubMed] [Google Scholar]
- 12. Thompson CG, Cohen MS, Kashuba AD.. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr 2013; 63 Suppl 2: S240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashuba AD, Dyer JR, Kramer LM. et al. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother 1999; 43: 1817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trevaskis NL, Kaminskas LM, Porter CJ.. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov 2015; 14: 781–803. [DOI] [PubMed] [Google Scholar]
- 15. Onder L, Morbe U, Pikor N. et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity 2017; 47: 80–92.e4. [DOI] [PubMed] [Google Scholar]
- 16. Abbott NJ. Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anatomy 2002; 200: 629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson PL, Kiser JJ, Gardner EM. et al. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother 2011; 66: 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker DK, Bowers SJ, Mitchell RJ. et al. Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT). Xenobiotica 2008; 38: 1330–9. [DOI] [PubMed] [Google Scholar]
- 19. Massud I, Aung W, Martin A. et al. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol 2013; 87: 8952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fletcher CV. Comparative lymphoid tissue pharmacokinetics (PK) of integrase inhibitors (INSTI) In: Abstracts of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2018. Abstract-27. Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.