Abstract
Adipose tissue engineering using adipose-derived stem cells (ADSCs) has emerged as an opportunity to develop novel approaches to postmastectomy breast reconstruction with the potential for an autologous tissue source with a natural appearance and texture. As of yet, the role of ADSCs in breast cancer development and metastasis is not completely understood; therefore, we must consider the oncological safety of employing an autologous source of ADSCs for use in breast regeneration. This study investigated the regenerative properties of ADSCs isolated from breast cancer patients, including those who had received neoadjuvant chemotherapy, and noncancer controls. The ADSCs were characterised for several parameters central to tissue regeneration, including cell viability, proliferation, differentiation potential, and cytokine secretion. A stem cell population was isolated and confirmed by flow cytometry and multilineage differentiation. There was no difference in cell phenotype or surface antigen expression between ADSCs from different sources. Adipose-derived stem cells isolated from the breast of cancer patients exhibited reduced adipogenic differentiation potential compared with ADSCs from other sources. The greatest degree of adipogenic differentiation was observed in ADSCs isolated from the subcutaneous abdominal fat of noncancer controls. The proliferation rate of ADSCs isolated from the breast of cancer patients was increased compared with other sources; however, it was decreased in ADSCs isolated from breast cancer patients who had recently been treated with neoadjuvant chemotherapy. A number of cytokines were detected in the cell conditioned media of ADSCs from different sources, including matrix metalloproteinase-2 (MMP-2), which was detected at higher levels in the secretome of ADSCs from breast cancer patients compared with noncancer controls. This study provides important information relating to the suitability of ADSCs as an autologous cell source for adipose tissue engineering in postcancer reconstruction. Results indicate that while the surface phenotype does not differ, the differentiation capacity, proliferative rate, and secreted cytokine profile are affected by the presence or treatment of breast cancer. These findings support further investigation into the regenerative potential of these ADSCs, if they are to be considered in clinical reconstructive strategies.
Keywords: Breast reonstruction, tissue engineering, adipose derived stem cells, breast cancer
Introduction
Breast reconstruction improves psychosocial and quality-of-life outcomes for the 40% of breast cancer patients who will require mastectomy to achieve locoregional disease control.1,2 Rates of mastectomy are currently increasing secondary to increased diagnosis of BRCA and other high-risk genetic mutations and an increased demand for contralateral prophylactic mastectomy.3-5 There is also an increasing trend for patients who are eligible for breast-conserving surgery to opt for mastectomy.6 According to the 2009 National Institute for Health and Care Excellence (NICE) guidelines, immediate breast reconstruction should be offered to all suitable patients undergoing mastectomy.7
While satisfactory aesthetic outcomes are possible with existing breast-reconstruction methods, there are significant limitations associated with all currently used breast-reconstruction strategies. Implant reconstruction is currently the most commonly performed breast-reconstruction procedure,8 secondary to cited advantages such as shorter operation times, lack of donor-site morbidity, and more rapid returns to normal activities.9 However, this breast-reconstruction method is associated with limitations such as the potential need for a second operative procedure to replace tissue expanders with a permanent implant, implant rupture and extrusion, capsular contracture, the need for implant replacement every 5 to 10 years, and the more recently recognised breast-implant-associated anaplastic large-cell lymphoma.10-12 Autologous procedures are currently the gold standard for breast reconstruction.13 However, these procedures are associated with morbidity at both the donor and recipient sites. Tissue flap necrosis and loss may occur secondary to ischaemia of transferred tissue. Complications such as wound dehiscence and abdominal wall herniae may also occur at donor sites. These procedures also require a longer operative time and longer hospital stays and involve a prolonged recovery.14 Autologous reconstruction procedures incorporating microvascular anastomoses are also exquisitely complex, requiring a high level of surgical skill and training, thus making these reconstructive options only available in specialist centres.15
In recent decades, there has been an increasing research focus on stem cells and their use in regenerative surgery with the potential to repair, replace, and/or regenerate cells, tissues, and organs to restore those lost through a disease. Adipose-derived stem cells (ADSCs) and adipose tissue engineering have emerged as an opportunity to develop novel approaches to breast reconstruction with the potential for an autologous tissue source with a natural appearance and texture after mastectomy. Adipose-derived stem cells are rapidly becoming the gold standard cell source for tissue-engineering purposes, as they are abundant in adipose tissue, which is easily harvested via liposuction procedures, allowing for autologous stem cell donation.16 They are capable of being differentiated into multiple mature cell types and have an immunophenotype that is more than 90% identical to bone-marrow-derived stem cells.17 Adipose-derived stem cells secrete growth factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor 2 (FGF-2), and insulin-like growth factor 1 (IGF-1), all of which are involved in adipose tissue regeneration and vasculogenesis, adding to their suitability for adipose tissue engineering.18,19 Adipose-derived stem cells are central to the new and emerging field of regenerative surgery and are currently being trialled in several clinical applications such as in the treatment of osteoarthritis, ischaemic heart disease, chronic limb ischaemia and bladder bioengineering.20-24
The primary challenge of adipose tissue engineering in breast reconstruction is generating an adequate tissue volume to recreate the breast mound after mastectomy. However, an additional challenge is posed when using autologous stem cells in a population that has had a cancer diagnosis. As of yet, the role of stem cells in breast cancer development and metastasis is not completely understood; therefore, we must consider the oncological safety of an autologous source of ADSCs for use in breast regeneration. The concern regarding the use of autologous stem cells in tissue engineering is as a result of the same cell characteristics that make them attractive for tissue regeneration: immune-modulatory, prosurvival, proangiogenic, and antiapoptotic effects; immunosuppression; tissue growth; and cellular homing are also dysregulated in tumorigenesis.25 It is possible that ADSCs introduced to a site where a malignant tumour has been excised will contribute to stromal support for cancer cells and deliver local inflammatory cytokines and/or growth factors, facilitating residual cancer cell survival and growth. Adipose-derived stem cells have been used clinically in autologous fat-grafting and cell-assisted lipotransfer procedures. A retrospective clinical study by Petit et al26 demonstrated an increased rate of disease recurrence in patients with intraepithelial neoplasia who underwent fat-grafting procedures. However, larger studies of invasive breast cancer have demonstrated no evidence of increased rates of disease recurrence. However, there are few prospective clinical trials in this area and for those that have been published; the length of follow-up is short. The Regenerative Cells Transplanted to Reconstruct Breast Deformities After Lumpectomy (RESTORE-2) trial demonstrated encouraging results with no increased risk of disease recurrence after ADSC-enriched fat grafting; however, this study only has a 12-month follow-up; therefore, these results are not conclusive.27 A systematic review by Waked et al28 concluded that there is, as of yet, discrepancy between experimental and clinical study findings regarding adipokines within ADSCs that could potentially promote tumour initiation and growth and the actual rate of locoregional recurrence in patients being treated with autologous fat grafting.
This study investigated the regenerative properties of ADSCs isolated from breast cancer patients, including those who had received neoadjuvant chemotherapy (NACT), and noncancer controls. The ADSCs were characterised for several parameters central to tissue regeneration, including cell viability, proliferation, differentiation potential, and cytokine secretion specifically focusing on the effect of (a) adipose tissue harvest site, (b) the presence of a breast tumour, and (c) the treatment received.
Materials and Methods
ADSC donor/patient groups
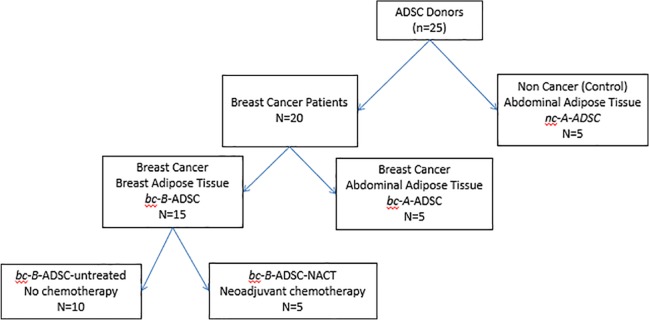
Adipose tissue samples were collected prospectively from breast cancer patients and healthy controls, with informed consent and ethical approval from the Galway University Hospital Clinical Research Ethics Committee (CREC) for the provision of a breast cancer biobank research resource for use in molecular and cellular studies and clinical trials (Ref 45/05). The samples collected were categorised according to site of adipose tissue harvest, diagnosis of breast cancer, and whether systemic cytotoxic chemotherapy had been received by the patient prior to ADSC harvest, as follows (Figure 1):
Figure 1.
ADSC patient groups.
- Breast adipose tissue from breast cancer patients this included:
- (a) Patients who had received no treatment to date (bc-B-ADSC untreated).
- (b) Patients who had received NACT (bc-B-ADSC-nact).
Abdominal adipose tissue from breast cancer patients (bc-A-ADSC).
Abdominal adipose tissue from noncancer control donors (nc-A-ADSC).
Inclusion criteria for groups 1 and 2 in this study were a biopsy-proven breast cancer diagnosis without any previous cancer history. Samples were taken at time of primary tumour excision (mastectomy) in both the breast and abdominal tissue groups. In the neoadjuvant group (bc-B-ADSC-nact), samples were obtained at time of tumour excision after completion of NACT. For the noncancer control group 3 (nc-A-ADSC), samples were obtained during abdominoplasty procedures in patients with no previous cancer diagnosis. None of the patients in this study had been commenced on hormonal therapy or had radiotherapy prior to tissue samples being obtained.
ADSC harvest and culture
Adipose tissue was obtained via lipectomy from tumour-associated adipose tissue of breast cancer patients and the abdominal region of breast cancer patients and healthy controls. Resected fat was placed in complete media (Dulbecco’s Modified Eagle Medium [DMEM] + GlutaMax, heat-inactivated foetal bovine serum, penicillin/streptomycin) in a sterile pot and transferred immediately to the primary cell culture laboratory. The adipose tissue was rinsed using penicillin/streptomycin and minced. This was then digested overnight in Type III collagenase. The stromal vascular fraction (SVF) was subsequently isolated by centrifugation and resuspended in culture media (DMEM + GlutaMax + foetal bovine serum + penicillin/streptomycin). Cell media were changed every 2 to 3 days. Comparative analyses were performed with ADSCs derived from the abdominal adipose tissue of healthy controls (nc-A-ADSCs) and the breast and abdominal adipose tissue of breast cancer patients, including a subset who had received NACT prior to harvest of ADSCs (as mentioned previously and in Figure 1). Cells were used for experiments between passages 3 and 5.
Cell morphology
Cell morphology of ADSCs was examined using a light microscope in a T175 flask at 75% confluency. Images of the cells within the T175 flask were obtained using an Olympus c-7070 Wide Zoom digital camera.
Cell proliferation
Cell proliferation was assessed by an AlamarBlue® assay (ThermoFischer Scientific, Waltham Massachussets). Three test wells were set up in a 24-well plate and 10 000 cells seeded into each well. Cell proliferation was measured at days 1, 4, and 7. The number of viable cells correlated with the magnitude of dye reduction and was expressed as a percentage of AlamarBlue reduction.
Flow cytometric analysis
Flow cytometry was carried out for immunophenotypic characterisation of cell-surface markers and to confirm a stromal cell population at passage 3. Cells were incubated with monoclonal antibodies directed against CD105, CD73, CD90, CD45, CD34, and CD14 and against isotype controls PeQCy, PE, PeCy5.5 RPE, APC, and FITC. The cell suspension was transferred to a 96-well plate for analysis using the flow cytometer (Guava easyCyte 8HT, Merck Millipore, Darmstadt, Germany). Data analysis was carried out using Guava 2.2 InCyte software (Merck Millipore).
Multilineage differentiation
Osteogenic differentiation
Adipose-derived stem cells were trypsinised and seeded into 6-well plates and treated with osteogenic media (DMEM + GlutaMax + foetal bovine serum + penicillin/streptomycin + 1 mM dexamethasone + 10 mM ascorbic acid + β-glycerophosphate). Media were changed twice a week and the assay harvested after 10 days. Cells were then stained with Alizarin Red to identify calcium and osteogenic differentiation.
Adipogenic differentiation
Adipose-derived stem cells were trypsinised and seeded into 6-well plates (40 000 cells per well) and treated with adipogenic induction media (DMEM + GlutaMax + foetal bovine serum + penicillin/streptomycin + insulin 10 mg/mL + indomethacin 100 mM + 500 mM mix). Adipose-derived stem cells were treated with induction media for 3 days, which were then exchanged for maintenance media (DMEM + GlutaMax + foetal bovine serum + penicillin/streptomycin + insulin 10 mg/mL) for a day. The maintenance media were then replaced by induction media for a further 3 days. These media changes were repeated until three cycles in induction and maintenance media had been completed. After three cycles, ADCSs were left in maintenance media for 7 days. After this, cells were fixed and stained with Oil Red O. Adipogenic differentiation potential was then quantified using photospectometry. The Oil Red O contained within the cell cytoplasm was extracted using 99% isopropranolol. Absorbance of the extracted Oil Red O stain was measured on a plate reader at 540 nm to quantify the Oil Red O stain uptake by differentiated/mature adipocytes.
Quantitative real-time polymerase chain reaction
RNA was extracted from a cell pellet of ADSCs, either predifferentiation or postdifferentiation to mature adipocytes, using the MagNA Pure Compact system (Roche, Penzburg Germany). Once extracted, RNA concentration and integrity were measured using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies Inc, Wilmington, DE, USA). For cDNA synthesis, aliquots of total RNA equivalent to 1 µg were reverse transcribed using SuperScript III Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA, USA). Polymerase chain reaction (PCR) was the technique used to amplify DNA across several orders of magnitude, generating multiple copies of a specific DNA sequence. The real-time qualitative polymerase chain reaction (RQ-PCR) instrument used was the ABI Prism® 7000 Sequence Detection System (Applied Biosystems, Foster City California). The genes investigated were adipogenic genes—lipoprotein lipase (LPL), peroxisome proliferator-activated receptor gamma (PPAR-γ), and fatty-acid-binding protein 4 (FABP4).
Cytokine assessment
Cytokine analysis of cell conditioned media from ADSCs was performed to quantify the level of tumour-promoting cytokines secreted by undifferentiated ADSCs and ADSCs at early and late stages of adipogenesis. Adipose-derived stem cells were seeded at 40 000 cells per well in a 6-well plate and placed in an incubator at 37°C, 5% CO2 for 24 hours. Cell conditioned media were harvested at this time point for analysis (ie, undifferentiated ADSCs). Adipose-derived stem cells were then treated with adipogenic media as per the aforementioned protocol. Cell conditioned media were harvested at day 8 for cytokine analysis and again at day 19, when adipogenic differentiation was complete. Cell conditioned media were analysed using the Proteome Profiler Human XL Oncology Array Kit (R&D Systems, Minneapolis, Minnesota, USA), which facilitates quantification of the following proteins; (Protein estimation before carrying out the ChemiArray was carried out using the ThermoScientific™ Pierce™ BCA Protein Assay (Pierce Technology, Rockford, Illinois, USA). Protein concentrations were determined and reported with reference to standards of a common protein such as bovine serum albumin (BSA). ChemiArray analysis was performed for ADSC lysate and cell conditioned media from undifferentiated ADSCs from each source. ChemiArray membranes were analysed using a Bio-Rad ChemiDoc MP Imaging system, which measures chemiluminescence. Analysis of membrane images was carried out using ImageLab™ 5.2.1 software (Bio-Rad, Hercules California).
Enzyme-linked immunosorbent assay
An enzyme-linked immunosorbent assay (ELISA) was performed to validate the results of the ChemiArray and to further investigate cytokines of interest during adipogenic differentiation. Time points for analysis included undifferentiated ADSCs and ADSCs at days 8 and 19 of the adipogenic differentiation protocol. These time points were selected to investigate the ADSC secretome at early and late stages of adipogenesis. Cytokines selected for validation were matrix metalloproteinase (MMP)-2, MMP-3, MMP-11, and FGF-2. A Total ELISA Quantikine Kit was used for each of the cytokines being studied (R&D Systems).
Results
Clinicopathological details
Adipose-derived stem cells from a total of 25 donors were analysed, including 20 breast cancer patients and 5 noncancer controls (Figure 1). The clinicopathologic details are outlined in Table 1. All those treated with NACT received dose-dense adjuvant chemotherapy (ACT; doxorubicin + cyclophosphamide followed by paclitaxel). One patient who received NACT had a complete pathological response to treatment at the time of tumour excision. The remaining four patients were shown to have had a Miller-Payne Grade 3 (between an estimated 30%-90% reduction in tumour cells) response to treatment at the time of tumour excision.
Table 1.
Clinicopathological details.
| Breast cancer |
Noncancer control |
|||
|---|---|---|---|---|
|
bc-B-ADSC-untreated
(n = 10) |
bc-B-ADSC-nact
(n = 5) |
bc-A-ADSC (n = 5) |
nc-A-ADSC (n = 5) |
|
| Age | 51 (21-66) | 59 (46-68) | 58 (38-71) | 52 (32-59) |
| T-size (T) | ||||
| T1 | 5 (14.5 [9-19]) mm | 1 (14) mm | 3 (18 [17-19]) mm | n/a |
| T2 | 4 (24.5 [21-30]) mm | 2 (25 [24-26]) mm | 2 (36 [32-40]) mm | |
| T3 | 1 (80) mm | 2 (79 [76-82]) mm | 0 | |
| Nodal status (n) | ||||
| N0 | 6 | 4 | 0 | n/a |
| N1 | 4 | 0 | 3 | |
| N2 | 0 | 1 | 2 | |
| Tumour biologic subtype | ||||
| Luminal A (ER+, PR+, and Her2–) | 6 | 2 | 5 | n/a |
| Luminal B (ER+, PR±, and Her2+) | 4 | 3 | 0 | |
| Neoadjuvant chemotherapy received | no | Dose-dense ACT | no | n/a |
| Pathological response | n/a | 1 × complete pathological response 4 × Grade 3 Miller-Payne Response |
n/a | n/a |
Abbreviation: ADSC, adipose-derived stem cell.
Cell morphology
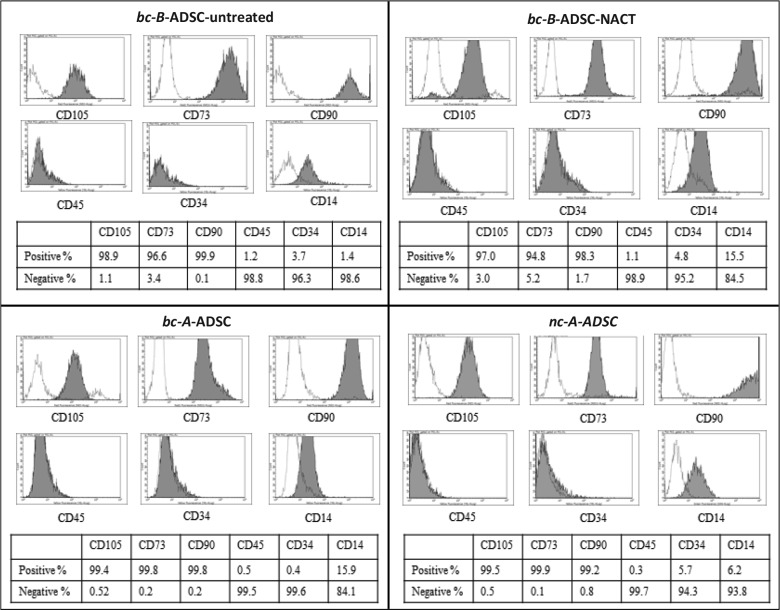
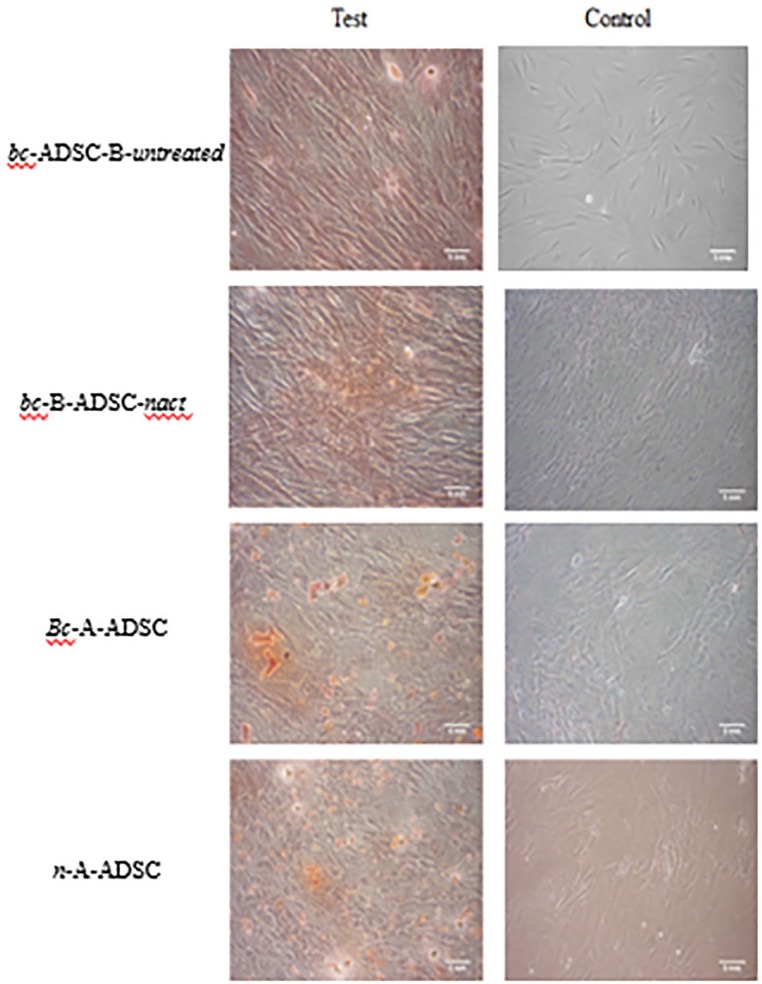
Adipose-derived stem cells were successfully isolated from the tissue of all donors. Cells that morphologically resembled mesenchymal stem cells (MSCs) could be early as 2 days after plating. Cells were imaged at passage 3 at 10× magnification (Figure 2). Adipose-derived stem cells isolated and harvested from all four patient groups demonstrated similar spindle shape fibroblastic cell morphology, and no spontaneous differentiation was observed in any of the groups. Cell phenotype, analysed by flow cytometric analysis of surface expression molecules, demonstrated that the ADSC populations originating from different sources exhibited a similar immunophenotype. The cells displayed a characteristic pattern of MSCs29; ADSCs from breast cancer patients were positive for CD105, CD90, and CD73 and negative for hematopoietic markers CD45 and CD34, and phenotypically similar to those of healthy controls (Figure 3). The haematopoietic marker CD14 was expressed on some of the cells, although at a low percentage, ranging from 1.4% in the bc-B-ADSC-untreated to 15.9% in the bc-A-ADSCs.
Figure 2.
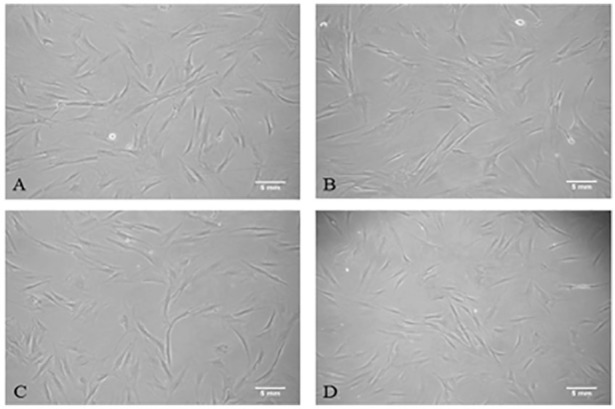
Fibroblastic cell morphology of adipose-derived stem cells at 10× magnification. (A) bc-B-ADSC-untreated (B) bc-B-ADSC-NACT (C) bc-A-ADSC (D) nc-A-ADSC
Figure 3.
Immunophenotype of cells isolated from various adipose tissue depots demonstrating a stem cell population.
Confirmation of stemness and comparison of ADSC differentiation capacity
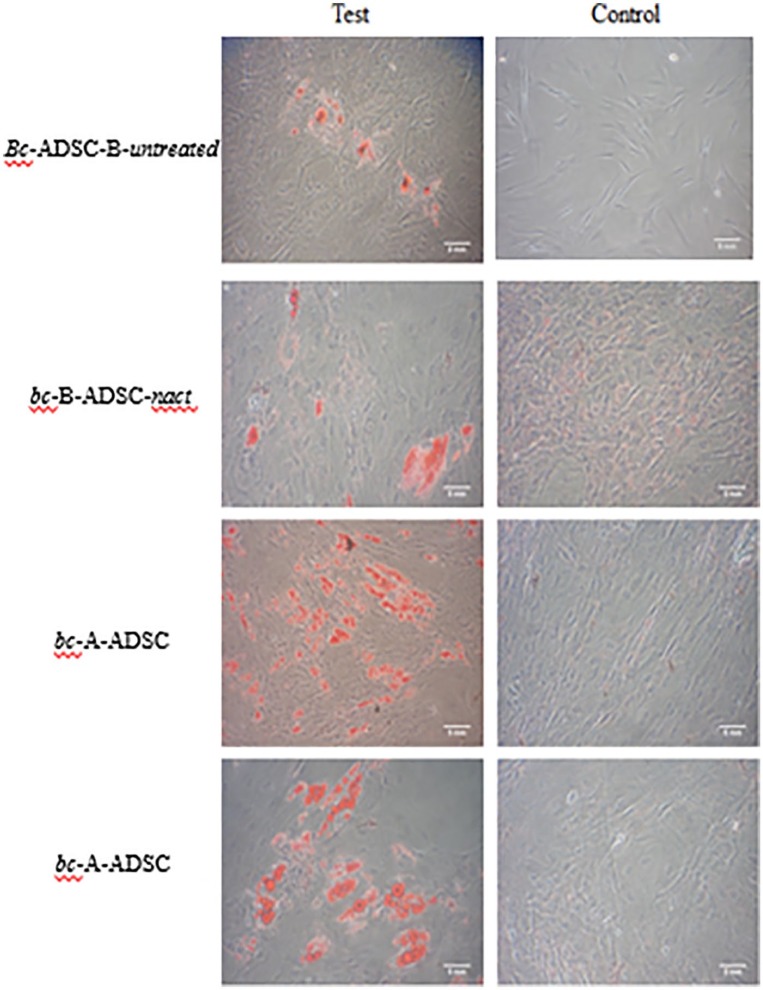
The differentiation potential of ADSCs from all sources was determined as a functional assay to confirm their identity as stem cells. Adipose-derived stem cells from all four groups had osteogenic differentiation potential as confirmed by positive Alizarin Red staining, demonstrating calcium deposits and evidence of differentiation to mature osteocytes (Figure 4). Adipose-derived stem cells from all sources also demonstrated adipogenic differentiation potential. This was confirmed by positive staining with Oil Red O to identify intracellular lipid deposits and evidence of transformation to a mature adipocyte cell type (Figure 5). As our primary interest is in the potential of these ADSCs for adipose tissue regeneration, the adipogenic differentiation potential of ADSCs from each source was quantified using photospectometry (Figure 6). Absorbance of light at 540 nm by the Oil Red O extracted from differentiated ADSCs was measured, reflecting the amount of Oil Red O contained within the cell group and, by extension, the amount of intracellular lipid present. Adipose-derived stem cells harvested from the breast of patients with a tumour in situ bc-B-ADSC-untreated demonstrated the lowest absorbance of light by photospectometry (0.18 ± 0.05), that is, the lowest yield of mature adipocytes by demonstrating. A higher yield of mature adipocytes was observed in ADSCs harvested from the breast adipose tissue of patients who had been treated with NACT (bc-B-ADSC-nact) (0.35 ± 0.13). There was a higher yield of mature adipocytes from ADSCs that had been harvested from the abdomen of patients with breast cancer (bc-A-ADSC) and the abdomen of noncancer controls (nc-A-ADSC) (0.47 ± 0.15) (P = .022)
Figure 4.
Alizarin Red S staining of ADSCs after osteogenic differentiation.
Figure 5.
Oil Red O staining of ADSCs after adipogenic differentiation.
Figure 6.
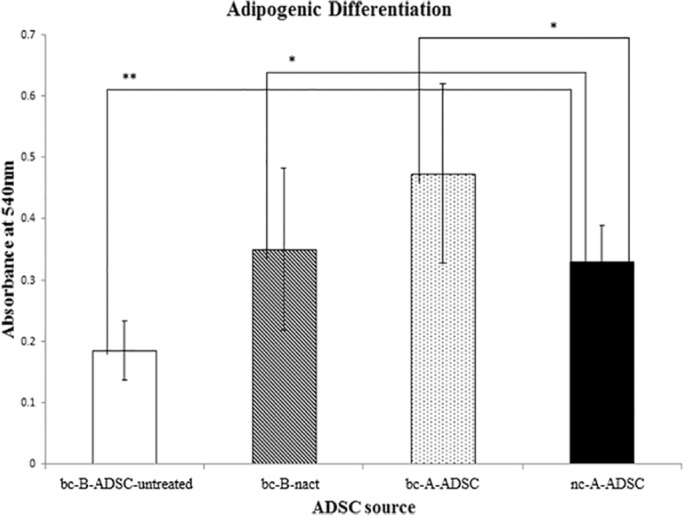
Absorbance of Oil Red O from mature adipocytes as a marker of adipogenic potential.
Gene expression analysis
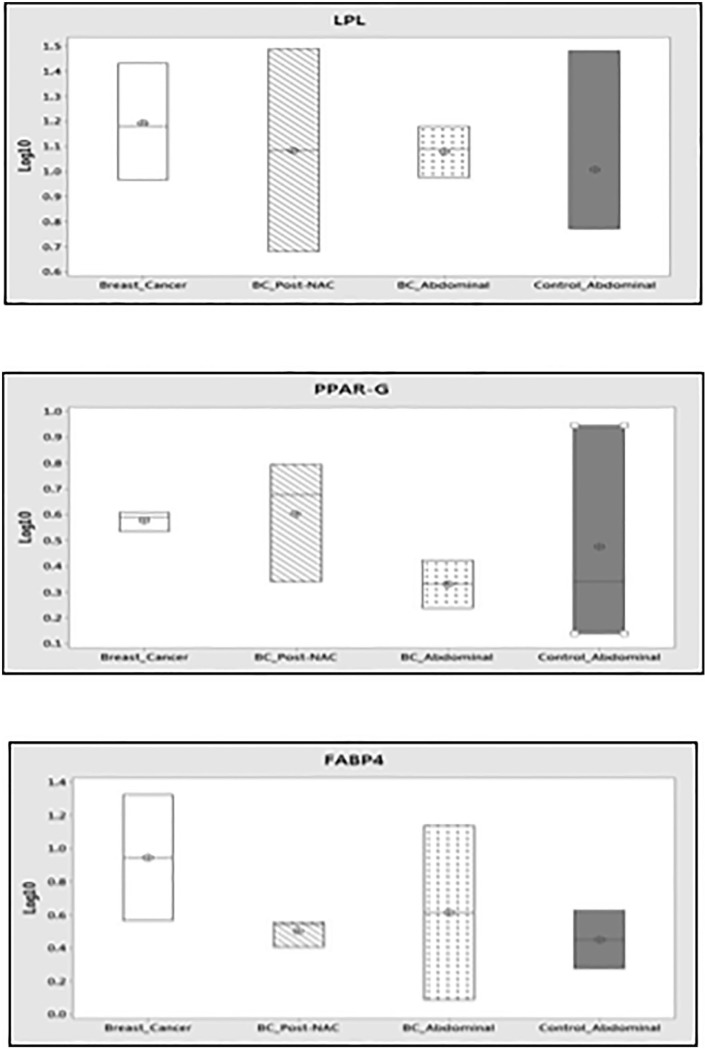
The capacity of ADSCs from different sources to differentiate into mature adipocytes was further investigated by gene expression analysis. Gene expression analysis was carried out on undifferentiated ADSCs and ADSCs following culture in adipogenic media over a 19-day protocol to induce adipogenic differentiation. Gene expression of adipogenic genes, such as LPL, PPAR-γ, and FABP4, was quantified using RQ-PCR. There was no significant difference in the expression of these genes between undifferentiated and differentiated ADSCs in any of the patient groups.
Expression of these genes at each time point (ie, undifferentiated or differentiated ADSCs) was then compared across the groups. In undifferentiated ADSCs, the only statistically significant difference observed was a higher expression of PPAR-γ in the ADSCs harvested from the breast (bc-B-ADSC-untreated) than from the abdomen (bc-A-ADSC) of patients with breast cancer, that is, tumour adjacent ADSCs compared to ADSCs harvested from a site distant from the tumour (P = .046). Lipoprotein lipase, PPAR-γ, and FABP4 were expressed in mature adipocytes (ADSCs following adipogenesis) from all sources; however, there was no statistically significant difference in expression between the groups.
ADSC proliferation
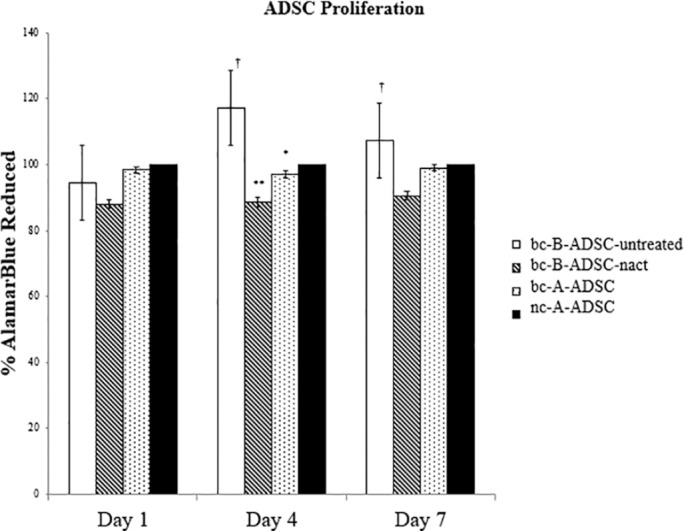
Proliferation of ADSCs from each source was measured at days 1, 4, and 7 after cell seeding (Figure 7). The proliferation rate of ADSCs isolated from the abdomen of noncancer subjects (nc-A-ADSC) were used as the control. Adipose-derived stem cells isolated from the breast adipose tissue of breast cancer patients (bc-B-ADSC-untreated) had a higher proliferation rate than that of abdominal ADSCs from normal healthy controls at day 4 (17% higher, P < .001) and day 7 (7% higher). Conversely, ADSCs from the breast adipose tissue of patients who had been treated with systemic chemotherapy (bc-B-ADSC-nact) had a lower proliferation rate (day 1 = 88%; day 4 = 88.6%; day 7 = 90.6%) than ADSCs from the other sources at all time points. The proliferation rate of abdominal ADSCs from both breast cancer patients and normal healthy controls were comparable across all time points (Figure 7).
Figure 7.
ADSC proliferation at day 1, 4, and 7.
ADSC secretome analysis
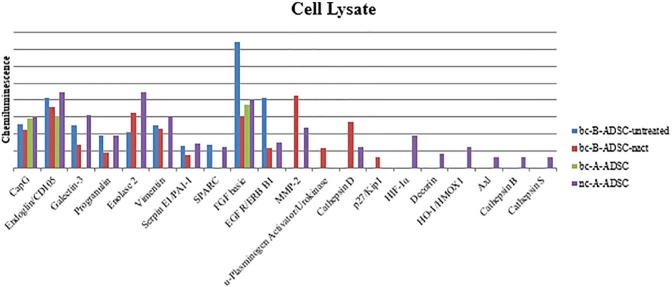
Cytokine analysis of cell-secreted factors released by ADSCs in culture was carried out using a commercially available ChemiArray (Proteome Profiler Array Human XL Oncology Array Kit, R&D Systems) which facilitates detection of 84 cancer-related proteins. Secreted factors were simultaneously measured in ADSC cell lysate and cell conditioned media from each ADSC source. Cytokines of interest were further validated by ELISA in additional samples and at additional time points in early (day 8) and late (day 19) adipogenesis.
Several cytokines were identified in the cell lysate (Figure 8). Fibroblast growth factor 2 was present in higher concentrations in the bc-B-ADSC-untreated group compared to all other groups.
Figure 8.
ChemiArray analysis results of ADSC lysate.
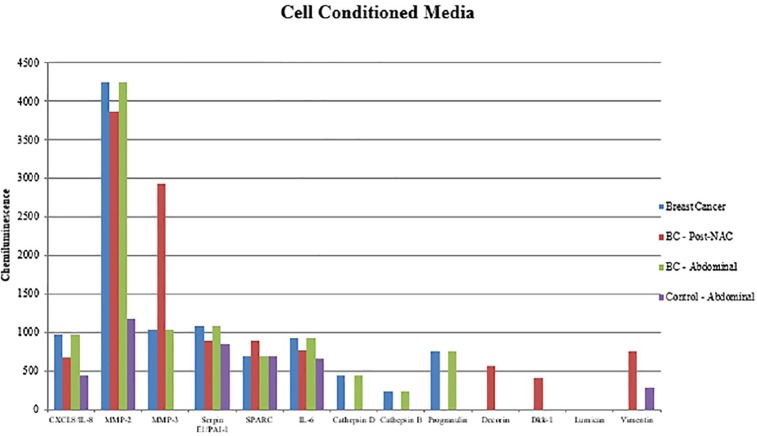
In cell conditioned media (Figure 9), several cytokines were secreted at different levels by ADSCs from different sources; MMP-2 was detected at higher levels in the cell conditioned media of breast cancer patient groups than in noncancer controls. Matrix metalloproteinase-3 was increased in cell conditioned media from the bc-B-ADSC-nact group compared with other breast cancer specimens and was not detected in cell conditioned media of ADSCs from noncancer controls.
Figure 9.
ChemiArray analysis results of undifferentiated ADSC cell conditioned media.
Enzyme-linked immunosorbent assay
For further investigation and validation of specific cytokines, the cell conditioned media of ADSCs from each source was assessed using ELISA for the presence of FGF-2, MMP-2, MMP-3, and MMP-11 in undifferentiated ADSCs and at early (day 8) and late (day 19) stages of adipogenesis.
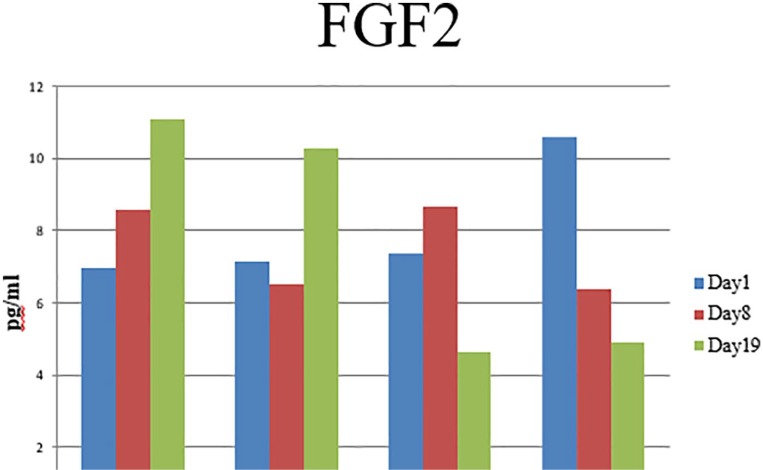
Fibroblast growth factor 2
Fibroblast growth factor 2 was selected for further analysis due to the detection of increased protein concentration in the cell lysate of ADSCs harvested from the breast adipose tissue of breast cancer patients (bc-B-ADSC-untreated) compared with other sources. On analysis of cell conditioned media, FGF-2 was detected in media from all sources at each time point (Figure 10). In ADSCs harvested from the breast of cancer patients (bc-B-ADSC-untreated and bc-B-ADSC-nact), the concentration of FGF-2 in the cell conditioned media increased as adipogenesis progressed and was highest on day 19. Conversely, for abdominal ADSCs harvested from breast cancer patients (bc-A-ADSC) and normal controls (nc-A-ADSC) the concentration of FGF-2 was higher in the cell conditioned media from undifferentiated ADSCs compared to those at day 19. Comparing across all ADSC sources and time points, the highest concentration of FGF-2 was detected in the cell conditioned media of breast cancer patients (bc-B-ADSC-untreated) (P < .001).
Figure 10.
ELISA analysis of FGF-2 secretion in cell conditioned media.
A panel of MMPs (MMP-2, MMP-3, and MMP-11) was also selected for validation.
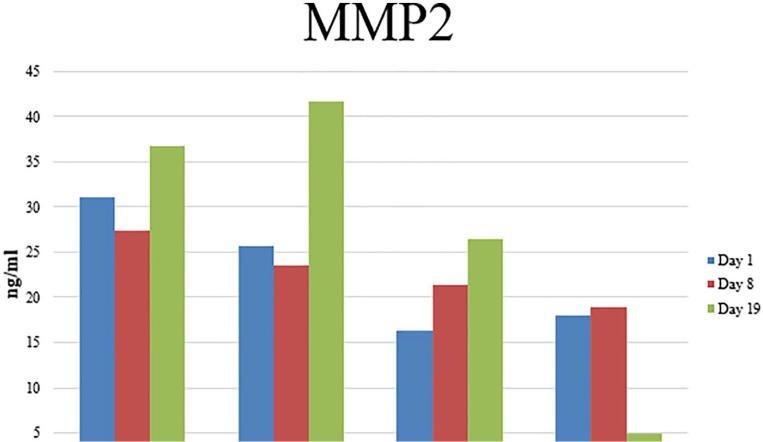
Matrix metalloproteinase-2 was detected in the cell conditioned media of ADSCs from all sources at all time points (Figure 11). There was no significant change in the quantity of MMP-2 secreted from cells upon adipogenic differentiation. However, higher levels of MMP-2 were detected in the cell conditioned media from ADSCs harvested from the breast adipose tissue of breast cancer patients (31.17 ng/mL ± 11.11) compared to ADSCs harvested from the abdomen of breast cancer patients (16.28 ng/mL ± 14.54, P = .045) and noncancer controls (17.93 ng/mL ± 8.26, P = .036).
Figure 11.
ELISA analysis of MMP-2 secretion in cell conditioned media.
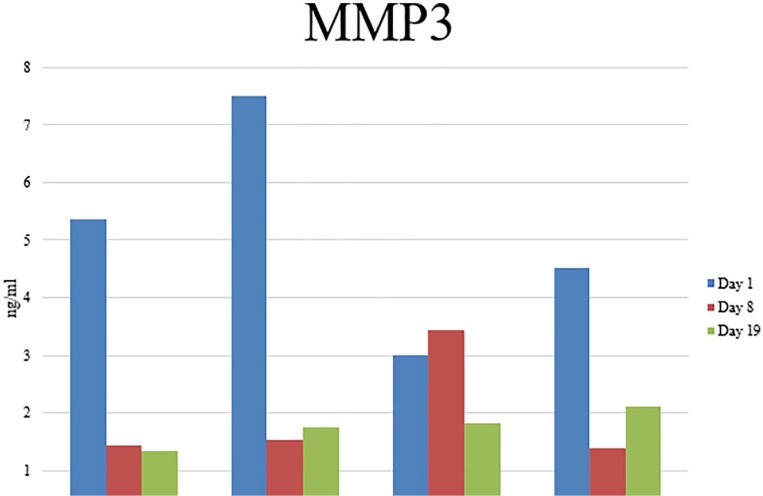
There was no significant difference in the secretion of MMP-3 from ADSCs of different sources and no significant change in MMP-3 secretion at different time points during adipogenesis (figure 13).
Figure 13.
ELISA analysis of MMP3 secretion in cell conditioned media.
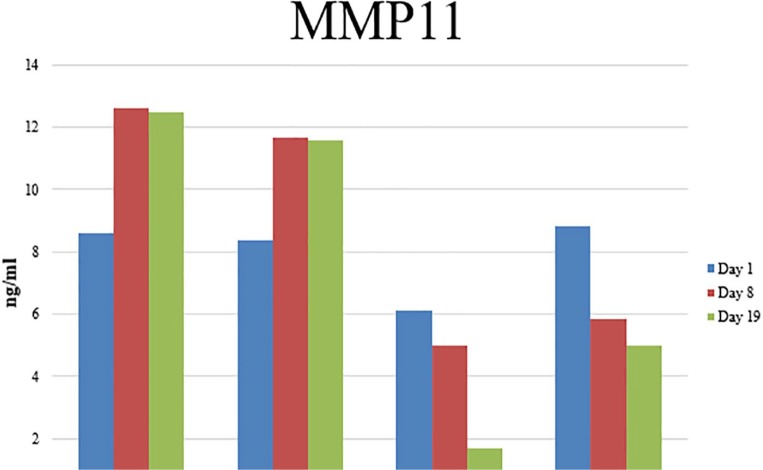
Matrix metalloproteinase-11 secretion was compared at all time points for ADSCs from each source (figure 14). There was no difference between the groups at day 1. Matrix metalloproteinase-11 secretion increased in all groups during adipogenesis (Figure 12). Increased levels of MMP-11 were detected in the cell conditioned media of ADSCs harvested from the breast adipose tissue of breast cancer patients (bc-B-ADSC-untreated: 12.61 ng/mL ± 1.32 and bc-B-ADSC-nact: 11.65 ng/mL ± 3.32) compared to ADSCs harvested from the abdomen of breast cancer patients (bc-A-ADCS: 4.99 ng/mL ± 4.66, P < .001) or noncancer controls (nc-A-ADSC: 5.84 ng/mL ± 3.54, P < .001).
Figure 14.
ELISA analysis of MMP11 secretion in cell conditioned media.
Figure 12.
ELISA analysis of MMP-11 secretion in cell conditioned media.
Discussion
Although there has been significant advancement in the field of adipose tissue engineering and in the use of ADSCs as a cell source in this regard, there are several outstanding issues that must be addressed before adipose tissue engineering can be considered for translation to the clinical setting in postmastectomy breast reconstruction for cancer patients. These include identification of the most appropriate adipose tissue depot from which to isolate ADSCs; the assessment of the effect systemic breast cancer treatment may have on ADSC behaviour and regenerative potential, and the identification of potential oncological risk in the use of autologous ADSCs from breast cancer patients in breast reconstruction. A number of studies have reported on ADSCs isolated from the abdominal wall subcutaneous fat.30-32 There are fewer studies in which ADSCs have been isolated from breast adipose tissue.33 There has been very limited work carried out in ADSCs isolated from the breast adipose tissue when there has been a breast tumour in situ.16,34,35 In addition, there is very limited data on ADSCs isolated from patients with breast cancer who have been treated with NACT. In reports to date, ADSCs in this scenario have been isolated from the tumour itself, as opposed to the surrounding adipose tissue.36
It is critical to investigate whether autologous ADSCs maintain their cellular function, phenotype, and regenerative potential. Initial experiments carried out in this study were undertaken to identify whether the cells isolated from the adipose tissue excised were indeed a stem cell population, and cells isolated from each of the four patient groups were shown to have a similar morphology, regardless of adipose tissue depot, cancer diagnosis, or NACT received. All isolated ADSCs demonstrated a fibroblastic, adherent morphology typical of stem cells. The immunophenotype of the isolated cells was then examined by flow cytometry. According to the International Society for Cellular Therapy, the minimal criteria for identification of a stem cell population includes a plastic adherent cell when maintained in standard cell culture conditions, a cell population that expresses CD105, CD73, and CD90 and shows no expression for CD45, CD34, and CD14. In addition, stem cells must also demonstrate multidifferential potential, as was shown in this study, by differentiation of the isolated cells into mature adipocytes and osteoblasts, as demonstrated by Oil Red O and Alizarin Red S staining.29 Thus, these experiments confirmed that ADSCs can be successfully isolated from the adipose tissue of breast and abdomen of breast cancer patients, including those that have been treated with NACT at the time of therapeutic surgery. The phenotype and surface antigen expression of breast-cancer-patient-derived ADSCs, including patients who had received cytotoxic chemotherapy, was not altered compared to noncancer controls. As such, there is potential for breast cancer patients to be autologous stem cell donors for breast reconstruction using ADSCs and tissue-engineering strategies.
The adipogenic differentiation capacity of ADSCs from each source was then assessed to determine which adipose tissue depot provided ADSCs that would be most effective for adipose tissue generation in regenerative surgery/tissue-engineering strategies. Overall, ADSCs isolated from abdominal adipose tissue generated more mature adipocytes than ADSCs isolated from breast adipose tissue, this difference may reflect the origin of the cells, indicating that abdominal adipose tissue is a superior cell source. However, there were several confounding factors in this study, so definitive conclusions cannot be drawn from this. These confounding factors include the body mass index (BMI) of patients which was not part of our inclusion or exclusion criteria. Patient BMI may have a significant influence on the adipogenic differentiation capacity of ADSCs isolated from patients. Further analysis of breast-derived ADSCs indicated that ADSCs isolated from adipose tissue near a breast tumour that were treated with adipogenic media generated the least amount of mature adipocytes and as such, had the poorest adipogenic potential. Conversely, the adipogenic potential of ADSCs isolated from the breast adipose tissue of patients treated with NACT had superior adipogenic differentiation capacity confirmed by photospectometric quantification of Oil Red O staining of intracellular lipid in mature adipocytes following the differentiation process. This is in contrast to the results published by Choron et al who found decreased adipogenic potential after chemotherapy39. However, Choron et al isolated ADSCs from the abdomen of healthy noncancer patients and subsequently treated these cells with paclitaxel in vitro, which differs from our approach of investigating the effects of clinical treatment of the cancer patient with systemic NACT on the subsequent functionality of their ADSCs. Adipose-derived stem cells treated with paclitaxel in vitro may have been subjected to much higher concentrations of paclitaxel than those exposed to therapeutic chemotherapy in vivo as in this study. Harris et al have reported on ADSCs’ ability to recover differentiation and proliferation function after exposure to paclitaxel38. The enhanced adipogenic differentiation observed in this study may reflect recovery of function of ADSCs during the period between cessation of NACT delivery and surgical resection of the tumour. It must be acknowledged, however, that as the samples in this study were obtained from breast cancer patients undergoing NACT, there is a certain degree of heterogeneity in terms of tumour size and receptor status, as is typical in any clinical cohort. It is conceivable that factors such as tumour size and biologic subtype/receptor phenotype may influence the ADSCs in the adjacent breast tissue, confounding the results; however, the sample size is too small to undertake any meaningful subgroup analysis.
Adipose-derived stem cells isolated from the breast adipose tissue of breast cancer patients demonstrated increased expression of PPAR-γ. This gene has been shown to be overexpressed in many tumour types, including breast cancer, and this may explain the increased expression observed in tumour adjacent ADSCs in our samples.37 There was no difference in expression of other adipogenic genes observed in ADSCs from different sources.
While our findings report that adipogenic differentiation capacity of ADSCs from the breast adipose tissue of breast cancer patients appeared to be reduced, we found that the proliferation rate and viability of ADSCs from this source was increased compared to ADSCs from abdominal adipose tissue, and ADSCs harvested from the breast of cancer patients who had been treated with NACT. The increased activity of ADSCs isolated from breast cancer patients with a tumour in situ, and therefore adjacent to the harvested ADSCs, raises concerns regarding the oncological safety of these ADSCs in adipose tissue engineering and also questions regarding the effect the tumour microenvironment have on ADSCs present in the adjoining breast tissues.
The ADSC secretome refers to the bioactive factors produced by these cells, which play an important role in the regulation of key biologic processes. Adipose-derived stem-cell-secreted factors, such as growth factors and cytokines, are known to exert paracrine signals responsible for chemoattractant, angiogenic, and prosurvival effects required for tissue regeneration. These same secreted factors, which stimulate tissue regeneration, also have the potential to promote cancer growth and metastasis.40 The secretome changes in response to physiological stressors or pathologic conditions and may be affected by a breast cancer diagnosis or a response to systemic chemotherapy. We analysed this effect by comparing the secretome from different ADSC sources and at different time points during adipogenesis. This was undertaken to gain some insight into factors that may affect the functionality, regenerative capacity, or the potential oncologic safety of these ADSCs.
Cytokine array analysis of both the cell lysate and cell conditioned media of undifferentiated ADSCs identified a range of factors including interleukin (IL)-8, IL-6, plasminogen activator inhibitor-1 (PAI-1), FGF-2, SPARC, vimentin, cathepsin B, and cathepsin D and MMPs that have previously been reported to be secreted by MSCs.41 A basic fibroblast growth factor (FGF-2) and a group of MMPs (MMP-2, MMP-3, and MMP-11) differed in their secretion profile between the ADSC groups and were selected for further validation and quantification at early and late adipogenesis.
Fibroblast growth factor-2 signalling is essential for both normal and cancer cell biology.42 Higher concentrations of FGF-2 were detected in the cell lysate of ADSCs from the breast adipose tissue of cancer patients compared to controls. Analysis of FGF-2 in the cell conditioned media at early and late stages of adipogenesis detected higher FGF-2 in cell conditioned media of ADSCs isolated from breast adipose tissue than in controls at the later stages of adipogenesis. Fibroblast growth factor-2 has been shown to suppress differentiation of MSCs by inducing Twist-2 and Sprouty4.43,44 In vivo evidence for the negative adipogenic function of FGF-2 also exists with the observation that MSCs from mice deficient in FGF-2 displayed enhanced adipogenic potential.44 The finding of increased FGF-2 in the cell lysate of ADSCs from breast cancer patients correlates with the reduced adipogenic capacity observed in these ADSCs in our study. Dysregulated FGF/fibroblast growth factor receptor (FGFR) signalling has also been shown to be associated with aggressive cancer phenotypes, enhanced chemotherapy resistance, and poor clinical outcomes, and FGF-2 is postulated be a tumour-promoting factor in the tumour microenvironment.42 Fibroblast growth factor-2 present within the tumour stroma and secreted by cancer-associated fibroblasts is known to promote tumour cell proliferation.45,46 Our observation of increased proliferation and FGF secretion in ADSCs harvested from breast cancer patients may thus raise concerns regarding oncological safety of the use of autologous stem cells from breast cancer patients in adipose tissue engineering.
Matrix metalloproteinases are responsible for the degradation of the extracellular matrix (ECM) within tissues, which is central to the processes of morphogenesis, wound healing, tissue repair, and remodelling in response to injury and in the progression of diseases, such as breast cancer.47 Matrix metalloproteinases are usually paracrine factors secreted by stromal cells such as ADSCs.48 They are typically synthesised and secreted by stromal cells near a tumour, and not by the genetically altered tumour cells themselves.49
Matrix metalloproteinase-11 was identified at significantly higher levels in the cell conditioned media of ADSCs from the breast of cancer patients during early differentiation of ADSCs towards an adipogenic lineage. This MMP is a known negative regulator of adipogenesis and may be responsible in part for the “dedifferentiation” of adipocytes and has also been shown to decrease preadipocyte differentiation.48,50 There is in vitro evidence of induction of mature adipocyte “dedifferentiation” in response to recombinant MMP-11.51 Therefore, MMP-11 may have contributed to the reduced adipogenic potential observed in the bc-B-ADSC-untreated group in this study. Matrix metalloproteinase-11 is known to be expressed by cancer-associated adipocytes (CAAs) found near invading cancer cells at the breast-cancer-invasive tumour front.50 This subset of adipocytes are mature adipocytes that have dedifferentiated into preadipocytes through loss of their lipid droplet and adopted a fibroblastic morphology and have been shown to play an active role in tumour progression and metastasis. This cell type increases tumour growth, tumour invasion via greater epithelial-mesenchymal transition (EMT) and results in a radio-resistant breast cancer phenotype.50 Matrix metalloproteinase-11 allows breast cancer cells to partake in bidirectional signalling with adipocytes and ADSCs near the tumour.52 In this study, the increased secretion of MMP-11 by ADSCs isolated from breast adipose tissue of breast cancer patients may be as a result of their close proximity to the breast tumour prior to excision, which is concerning from an oncological standpoint. In addition, the decreased secretion of MMP-11 in the ADSCs from patients who had been treated with NACT group may be as a result of the partial or complete pathological response to NACT observed in this patient group. The increase in MMP-11 secretion was only observed in ADSCs at an early stage of adipogenic differentiation (day 8 of the differentiation protocol) and was not observed in differentiated mature adipocytes. Thus, any functional effect this may exert would be expected to be observed during early adipogenic differentiation.
We observed a trend towards increased secretion of MMP-2 in the cell conditioned media of bc-B-ADSC-untreated and bc-B-ADSC-nact groups at all time points throughout the adipogenic process. Secretion of this protease is the lowest in the bc-A-ADSC and nc-A-ADSC groups. Adipose-derived stem cells from the latter two groups were harvested from a site distant from the breast tumour, that is, the abdomen, and are therefore not tumour-associated ADSCs. Matrix metalloproteinase-2 degrades the components of the ECM and has a role in cell migration and invasion of the basement membrane.53,54 It has been hypothesised that tumours are capable of enhancing MMP-2 expression in their surrounding tissues, accumulating it from the circulation and storing the molecules at the cell surface.54 Malignant breast cancer cells may stimulate MMP-2 secretion by stromal cells through paracrine mechanisms. Matrix metalloproteinases that are secreted by stromal cells can still be recruited to the cancer cell membrane. Matrix metalloproteinase-2 mRNA is found in stromal cells within human breast tumours and MMP-2 protein is identified on both stromal and cancer cell membranes.55 This proteinases production in stromal cells in the vicinity of the tumour in the breast helps to explain why its expression is decreased in abdominal adipose ADSCs in this study. The significantly increased expression of this cytokine in undifferentiated ADSCs isolated from the breast adipose tissue of breast cancer patients raises concerns regarding the oncological safety of these groups as a cell source for breast regeneration.
Secretion of MMP-3 was also investigated in the cell conditioned media of undifferentiated ADSCs and at early and late stages of adipogenic differentiation. Although differences in secretion did not reach statistical significance, MMP-3 secretion was initially increased in the ADSCs isolated from the breast adipose tissue of cancer patients, that is, tumour-associated ADSCs. Matrix metalloproteinase-3 has been observed in highly invasive breast cancer cell lines and shown to be involved in the metastatic process of breast cancer.
Overall, differences in regenerative potential, cell proliferation, and cytokine secretion were observed in ADSCs isolated from various adipose tissue depots of breast cancer patients undergoing distinct treatment protocols, and noncancer controls. Although the study is somewhat limited by small sample size and potential confounding effects of patient and tumour heterogeneity, these data from patient-derived ADSCs in treated and untreated breast cancer provide novel insight into the potential effects of both cancer and systemic cancer treatment on ADSC behaviour. ADSCs isolated from the breast adipose tissue of those with an untreated carcinoma appear to bear more hallmarks of CAAs than any other group. The delivery of systemic NACT prior to ADSC isolation appears to modify some of these genotypic and phenotypic effects, possibly making these stem cells safer for use in adipose-tissue-engineering purposes. ADSCs isolated from abdominal wall subcutaneous adipose tissue appear to be less influenced by the presence of a tumour within the breast and may be considered as more oncologically safe. This group also demonstrated the greatest adipogenic potential, suggesting these cells may produce the greatest adipose tissue volume when used for adipose-tissue-engineering strategies. Further in vivo investigation is warranted to assess which cell source allows for the greatest adipose tissue volume production to recreate the breast mound and also assess if there is a difference in the rate of breast cancer recurrence when various ADSC sources are used.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Niamh O’ Halloran - Experimental design, completion of experiments, article writing
Katie Gilligan, Sonja Khan – completion of experiments
Roisin Dwyer, Aoife Lowery, Michael Kerin – Experimental design, editing of manuscript
ORCID iD: Niamh O’Halloran  https://orcid.org/0000-0002-5153-3566
https://orcid.org/0000-0002-5153-3566
References
- 1. O’Halloran N, Lowery A, Kalinina O, et al. Trends in breast reconstruction practices in a specialized breast tertiary referral centre. BJS Open. 2017;1:148-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heneghan H, Prichard RS, Lyons R, et al. Quality of life after immediate breast reconstruction and skin-sparing mastectomy: a comparison with patients undergoing breast conserving surgery. Eur J Surg Oncol. 2011;37:937-943. [DOI] [PubMed] [Google Scholar]
- 3. Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437-3441. [DOI] [PubMed] [Google Scholar]
- 4. Dragun AE, Huang B, Tucker TC, Spanos WJ. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J. 2012;18:318-325. [DOI] [PubMed] [Google Scholar]
- 5. Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25:5203-5209. [DOI] [PubMed] [Google Scholar]
- 6. Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20:1436-1443. [DOI] [PubMed] [Google Scholar]
- 7. Harnett A, Smallwood J, Titshall V, Champion A. Diagnosis and treatment of early breast cancer, including locally advanced disease: summary of NICE guidance. BMJ. 2009;338:b438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15-23. [DOI] [PubMed] [Google Scholar]
- 9. O’Halloran N, Potter S, Kerin M, Lowery A. Recent advances and future directions in postmastectomy breast reconstruction. Clin Breast Cancer. 2018;18:e571-e585. [DOI] [PubMed] [Google Scholar]
- 10. Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I: a prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825-831. [DOI] [PubMed] [Google Scholar]
- 11. Headon H, Kasem AA, Mokbel K. Capsular contracture after breast augmentation: an update for clinical practice. Arch Plast Surg. 2015;42:532-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645-654. [DOI] [PubMed] [Google Scholar]
- 13. Wise MW, Hilaire HS, Sadeghi A, Dupin C. Autologous breast reconstruction. In: Riker A, ed. Breast Disease. New York, NY: Springer; 2015:279-304. [Google Scholar]
- 14. Platt J, Baxter N, Zhong T. Breast reconstruction after mastectomy for breast cancer. CMAJ. 2011;183:2109-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nahabedian MY, Momen B, Galdino G, Manson PN. Breast reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466-475. [DOI] [PubMed] [Google Scholar]
- 16. Chatterjee S, Laliberte M, Blelloch S, et al. Adipose-derived stromal vascular fraction differentially expands breast progenitors in tissue adjacent to tumors compared to healthy breast tissue. Plast Reconstr Surg. 2015;136:414e-425e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bielli A, Scioli MG, Gentile P, et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Combellack EJ, Jessop ZM, Naderi N, et al. Adipose regeneration and implications for breast reconstruction: update and the future. Gland Surg. 2016;5:227-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolonin MG, Evans KW, Mani SA, Gomer RH. Alternative origins of stroma in normal organs and disease. Stem Cell Res. 2012;8:312-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bansal H, Comella K, Leon J, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med. 2017;15:141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Salemi S, Tremp M, Plock JA, et al. Differentiated adipose-derived stem cells for bladder bioengineering. Scand J Urol. 2015;49:407-414. [DOI] [PubMed] [Google Scholar]
- 22. Chan YY, Sandlin SK, Kurzrock EA, Osborn SL. The current use of stem cells in bladder tissue regeneration and bioengineering [published online ahead of print January 6, 2017]. Biomedicines. doi: 10.3390/biomedicines5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Badimon L, Oñate BB, Vilahur G. Adipose-derived mesenchymal stem cells and their reparative potential in ischemic heart disease. Rev Esp Cardiol (Engl Ed). 2015;68:599-611. [DOI] [PubMed] [Google Scholar]
- 24. Zhi K, Gao Z, Bai J, et al. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci (Landmark Ed). 2014;19:768-776. [DOI] [PubMed] [Google Scholar]
- 25. Krastev T, Jonasse YY, Kon M. Oncological safety of autologous lipoaspirate grafting in breast cancer patients: a systematic review. Ann Surg Oncol. 2013;20:111-119. [DOI] [PubMed] [Google Scholar]
- 26. Petit J, Rietjens M, Botteri E, et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;24:1479-1484. [DOI] [PubMed] [Google Scholar]
- 27. Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: the RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382-389. [DOI] [PubMed] [Google Scholar]
- 28. Waked K, Colle J, Doornaert M, Cocquyt V, Blondeel P. Systematic review: the oncological safety of adipose fat transfer after breast cancer surgery. Breast. 2017;31:128-136. [DOI] [PubMed] [Google Scholar]
- 29. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [DOI] [PubMed] [Google Scholar]
- 30. Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834-3842. [DOI] [PubMed] [Google Scholar]
- 31. Flynn L. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715-4724. [DOI] [PubMed] [Google Scholar]
- 32. Hong L, Peptan IA, Colpan A, Daw JL. Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs. 2006;183:133-140. [DOI] [PubMed] [Google Scholar]
- 33. Yang J, Xiong L, Wang R, Sun J, Hirche C. Adipose-derived stem cells from the breast. J Res Med Sci. 2014;19:112-116. [PMC free article] [PubMed] [Google Scholar]
- 34. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116-122. [DOI] [PubMed] [Google Scholar]
- 35. Trivanovic D, Nikolić S, Krstić J, et al. Characteristics of human adipose mesenchymal stem cells isolated from healthy and cancer affected people and their interactions with human breast cancer cell line MCF-7 in vitro. Cell Biol Int. 2014;38:254-265. [DOI] [PubMed] [Google Scholar]
- 36. Zhang C, Zhai W, Xie Y, Chen Q, Zhu W, Sun X. Mesenchymal stem cells derived from breast cancer tissue promote the proliferation and migration of the MCF-7 cell line in vitro. Oncol Lett. 2013;6:1577-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kotta-Loizou I, Giagi nis C, Theocharis S. The role of peroxisome proliferator-activated receptor-γ in breast cancer. Anticancer Agents Med Chem. 2012;12:1025-1044. [DOI] [PubMed] [Google Scholar]
- 38. Harris WM, Zhang P, Plastini M, et al. Evaluation of function and recovery of adipose-derived stem cells after exposure to paclitaxel. Cytotherapy. 2017;19:211-221. [DOI] [PubMed] [Google Scholar]
- 39. Choron RL, Chang S, Khan S, et al. Paclitaxel impairs adipose stem cell proliferation and differentiation. J Surg Res. 2015;196:404-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hwang JH, Shim SS, Seok OS, et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akl MR, Nagpal P, Ayoub NM, et al. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget. 2016;7:44735-44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kakudo N, Shimotsuma AA, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359:239-244. [DOI] [PubMed] [Google Scholar]
- 44. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299-309. [DOI] [PubMed] [Google Scholar]
- 45. Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753-3761. [PubMed] [Google Scholar]
- 46. Clayton N, Wilson AS, Laurent EP, Grose RP, Carter EP. Fibroblast growth factor-mediated crosstalk in cancer etiology and treatment. Dev Dyn. 2017;246:493-501. [DOI] [PubMed] [Google Scholar]
- 47. Nagase H, Visse RR, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562-573. [DOI] [PubMed] [Google Scholar]
- 48. Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55:851-859. [DOI] [PubMed] [Google Scholar]
- 49. Sternlicht MD, Lochter A, Sympson CJ, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. O’Halloran N, Courtney D, Kerin MJ, Lowery AJ. Adipose-derived stem cells in novel approaches to breast reconstruction: their suitability for tissue engineering and oncological safety [published online ahead of print August 16, 2013]. Breast Cancer (Auckl). doi: 10.1177/1178223417726777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andarawewa KL, Motrescu ER, Chenard MP, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862-10871. [DOI] [PubMed] [Google Scholar]
- 52. Schweizer R, Tsuji W, Gorantla VS, Marra KG, Rubin JP, Plock JA. The role of adipose-derived stem cells in breast cancer progression and metastasis [published online ahead of print April 27, 2013]. Stem Cells Int. doi: 10.1155/2015/120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jezierska A, Motyl TT. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit. 2009;15:RA32-RA40. [PubMed] [Google Scholar]
- 55. Egeblad M, Werb ZZ. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. [DOI] [PubMed] [Google Scholar]