Short abstract
Objective
This study was performed to evaluate the diagnostic value of the neutrophil CD64 index in patients with sepsis in the intensive care unit (ICU).
Methods
Patients with sepsis who were treated at the ICU of the authors’ institution from December 2016 to June 2018 were retrospectively reviewed. The controls comprised age- and sex-matched patients who underwent coronary bypass and had no evidence of infection. The neutrophil CD64 index, C-reactive protein (CRP) level, and procalcitonin level were compared between the two groups. The diagnostic performance of these measures, including the sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve, was examined.
Results
In total, 35 patients with sepsis and 27 control patients were included in the data analysis. The sensitivity of the neutrophil CD64 index, CRP level, and procalcitonin level was 83%, 74%, and 77%, respectively. The specificity was 88%, 86%, and 81%, respectively. The area under the ROC curve was 0.923 [95% confidence interval (CI), 0.856–0.989], 0.904 (95% CI, 0.832–0.976), and 0.863 (95% CI, 0.776–0.950), respectively.
Conclusion
The neutrophil CD64 index is a valuable biomarker for diagnosing sepsis in patients in the ICU.
Keywords: CD64, sepsis, neutrophil, C-reactive protein, procalcitonin, intensive care unit
Introduction
Sepsis is a multifactorial clinical syndrome involving life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Despite vigorous respiratory and cardiovascular support and antibiotic therapy, sepsis continues to be associated with unacceptably high mortality rates, especially among patients in the intensive care unit (ICU).2 The Sepsis Occurrence in Acutely Ill Patients (SOAP) study in Europe showed a 33% incidence rate and 27% overall mortality rate in ICUs.3 Advances in early diagnosis and treatment have led to a modest decline in the sepsis-associated mortality rate, but such advances have not led to a significant reduction in the absolute number of sepsis-associated deaths.4 Sepsis accounts for more than 210,000 deaths per year in the United States.5
Early diagnosis of sepsis is crucial for implementation of appropriate and timely management and ultimately for improvements in patient outcomes.6 Commonly used biomarkers for early diagnosis of sepsis include the leukocyte count, C-reactive protein (CRP) level, and procalcitonin level. However, the specificity and diagnostic value of these markers are not sufficiently reliable.7
A key contributor to the pathogenesis and progression of sepsis is dysregulation of innate and adaptive immunity. CD64 is a high-affinity receptor that binds to monomeric immunoglobulin G. Increased CD64 expression is a very early indicator of the host immune response to bacterial infection. CD64 expression in neutrophils is low in the resting state and sharply increases upon bacterial activation. Recent studies have indicated that the neutrophil CD64 index might be useful for differentiation of sepsis with fairly good sensitivity and specificity.8,9 In the current study, we compared the diagnostic value of the neutrophil CD64 index versus other established biomarkers, including the neutrophil count, CRP level, and procalcitonin level, in patients with sepsis in the ICU.
Patients and methods
Patients
Consecutive patients with sepsis treated from December 2016 to June 2018 were identified in the medical records at the ICU of Nanjing Jiangbei People’s Hospital Affiliated to Nantong University. The diagnosis of sepsis was based on evident signs of bacterial infection and a Sequential Organ Failure Assessment (SOFA) score of ≥2.1 A patient was excluded if he or she was aged <18 years, had malignancy, or had received treatment with interferon-γ, granulocyte colony-stimulating factor (G-CSF), or glucocorticoids. Patients who died within 4 hours of ICU admission were also excluded. Patients who underwent coronary bypass surgery during the same period and showed no signs of bacterial infection were included as the control group.
The study protocol was approved by the hospital’s ethics committee. Written informed consent was obtained from either the patients or their legal surrogates.
Patient evaluation
The following data were extracted on ICU admission: age, sex, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, SOFA score, and reason for ICU admission. Body temperature, leukocyte count, presence of shock, previous use of antimicrobial agents, length of ICU stay, and ICU mortality were also recorded.
Blood samples were collected within 12 hours of ICU admission to determine the neutrophil CD64 index, CRP level, and procalcitonin level at the central hospital laboratory. The plasma CRP concentration was measured using an immunoturbidimetry-based test (QuikRead CRP test kit; Orion Diagnostica, Espoo, Finland). The intra-assay coefficient of variation of the CRP assay ranged from 2% at 140 mg/L to 15% at 9 mg/L. The serum procalcitonin concentration was measured using immunofluorescence chromatography with a sandwich technique and a chemiluminescent detection system (LumiTest; Brahms Diagnostica, Berlin, Germany). Samples were stored at −80°C for a maximum of 12 hours prior to assay.
Flow cytometry
CD64 expression in neutrophils was measured using quantitative flow cytometry as previously described.10 Key agents used in the assay included anti-CD64-FITC and CD45-PC5 antibodies (FC500; Beckman Coulter, Brea, CA, USA). The neutrophil CD64 index was calculated as the mean CD64 fluorescence intensity in neutrophils divided by the mean CD64 fluorescence intensity in lymphocytes.
Statistical analysis
Statistical analyses were performed with SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Categorical variables (e.g., sex) were analyzed using Fisher’s exact test. Continuous variables were first tested for normality using the Shapiro–Wilk test. Between-group comparisons were conducted using Student’s t-test for independent data if normally distributed and the Mann–Whitney U test otherwise. Variables with a normal distribution are reported as mean ± standard deviation and as median (25th–75th percentile) otherwise. Receiver operating characteristic (ROC) analysis was conducted to compare the performance of the CD64 index versus other measures (leukocyte count, CRP level, and procalcitonin level) in the identification of sepsis. The threshold was determined based on Youden’s index, which maximizes the sum of the sensitivity and specificity (J = max[sensitivity + specificity − 1]). Statistical significance was set at p < 0.05 (two-sided).
Results
Figure 1 shows the study flowchart. In total, 56 patients with sepsis were admitted to the ICU during the study period. Sixteen patients were excluded because they had malignant tumors, and 5 were excluded because of the use of interferon-γ, G-CSF, or glucocorticoids. The final data analysis included 35 patients with sepsis (22 men and 13 women). Their median age was 75 years (range, 27–90 years). The control group comprised 27 age- and sex-matched patients without signs of bacterial infection after coronary bypass surgery (16 men and 11 women; median age, 72 years; age range, 19–80 years).
Figure 1.
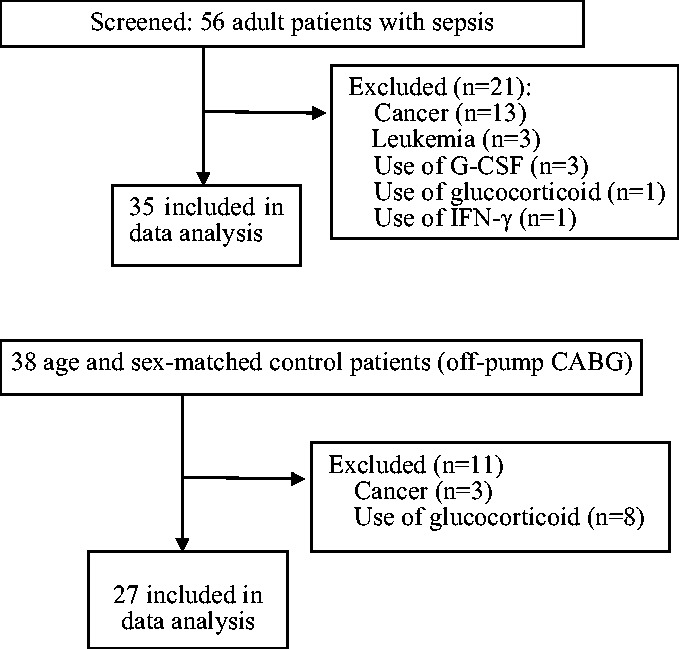
Study flowchart. (a) Sepsis group. (b) Control group. G-CSF, granulocyte colony-stimulating factor; IFN, interferon; CABG, coronary artery bypass grafting;
Patients in the sepsis group had significantly higher APACHE II scores (21.17 ± 8.34 vs. 12.00 ± 3.27; p < 0.01) and SOFA scores (11.26 ± 5.95 vs. 6.56 ± 2.74; p < 0.01) than patients in the control group (Table 1). Nine patients in the sepsis group died during the ICU stay, with a mortality rate of 26%.
Table 1.
Patient demographics and baseline characteristics
| Variable | Control (n = 27) | Sepsis (n = 35) | p |
|---|---|---|---|
| Age, years | 72 (25–85) | 75 (27–90) | 0.073 |
| Sex | |||
| Male | 16 (59) | 22 (63) | 0.773 |
| APACHE II score | 12.00 ± 3.27 | 21.17 ± 8.34 | <0.001 |
| SOFA score | 6.56 ± 2.74 | 11.26 ± 5.95 | <0.001 |
| In-hospital mortality | 0 (0) | 9 (26) | 0.004 |
| Cause of death | |||
| Shock | 0 (0) | 5 (14) | 0.041 |
| Respiratory failure | 0 (0) | 3 (9) | 0.119 |
| Heart failure | 0 (0) | 1 (3) | 0.376 |
| Site of infection (n = 35) | |||
| Respiratory | 0 (0) | 15 (43) | <0.001 |
| Abdominal | 0 (0) | 12 (34) | <0.001 |
| Skin/soft tissue | 0 (0) | 5 (14) | 0.041 |
| Urinary tract | 0 (0) | 2 (6) | 0.207 |
| Central nervous system | 0 (0) | 1 (3) | 0.376 |
| Comorbidities | |||
| Coronary artery disease | 27 (100) | 2 (6) | <0.001 |
| Valvular heart disease | 2 (7) | 0 (0) | 0.008 |
| Hypertension | 16 (59) | 10 (28) | 0.032 |
| Congestive heart failure | 4 (15) | 2 (6) | 0.005 |
| Chronic pulmonary disease | 12 (44) | 9 (26) | 0.122 |
| Diabetes mellitus | 15 (55) | 11 (31) | 0.006 |
| Renal failure | 4 (15) | 2 (6) | 0.715 |
| Liver disease | 1 (4) | 3 (9) | 0.439 |
Data are presented as median (range), n (%), or mean ± standard deviation.
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
Biomarkers of sepsis
The leukocyte count was not different between the two groups (Table 2). In contrast, the plasma CRP level was significantly higher in the sepsis than control group (146.9 ± 68.27 vs. 46.5 ± 35.8 mg/L; p < 0.01). The sepsis group also had a significantly higher serum procalcitonin level than the control group (31.82 ± 35.77 vs. 1.87 ± 2.75 ng/L; p < 0.01). The neutrophil CD64 index was significantly higher in the sepsis group than control group (9.03 ± 5.59 vs. 3.18 ± 1.50; p < 0.01). The CD64 index was not different in patients with pulmonary infection (9.15 ± 5.62; n = 15) or intra-abdominal infection (9.02 ± 6.51; n = 12).
Table 2.
Key laboratory measures in the sepsis and control groups
| Variable | Control (n = 27) | Sepsis (n = 35) | p |
|---|---|---|---|
| Neutrophil CD64 index | 3.18 ± 1.50 | 9.03 ± 5.59 | <0.001 |
| CRP, mg/L | 46.5 ± 35.8 | 146.9 ± 68.27 | <0.001 |
| Procalcitonin, ng/mL | 1.87 ± 2.75 | 31.82 ± 35.77 | 0.01 |
| WBC count, ×109/L | 11.16 ± 3.51 | 13.04 ± 9.26 | 0.28 |
Data are presented as mean ± standard deviation.
CRP, C-reactive protein; WBC, white blood cell.
Diagnostic performance of neutrophil CD64 index
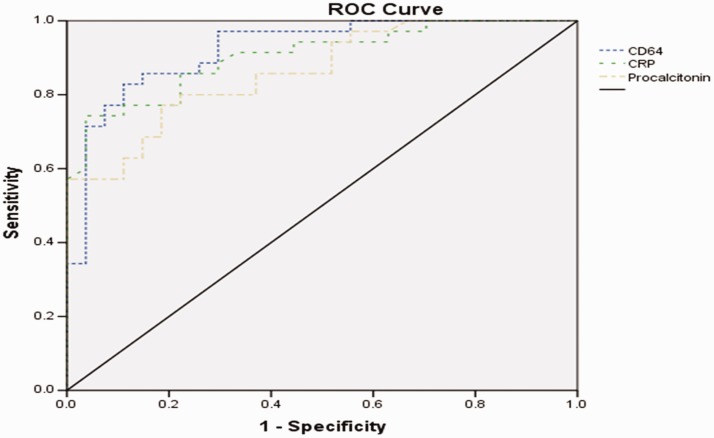
At a cut-off of 4.56, the neutrophil CD64 index had a sensitivity of 83% and specificity of 88% (Table 3). At a cut-off of 98 mg/L, the CRP level had a sensitivity of 74% and specificity of 86%. At a cut-off of 2.81 ng/mL, the procalcitonin level had a sensitivity of 77% and specificity of 81%. The area under the ROC curve was 0.923 [95% confidence interval (CI), 0.856–0.989] for the neutrophil CD64 index, 0.904 (95% CI, 0.832–0.976) for the CRP level, and 0.863 (95% CI, 0.776–0.950) for the procalcitonin level (p < 0.05) (Figure 2).
Table 3.
Diagnostic performance of biomarkers in diagnosing sepsis.
| Biomarker | Cut-off | Sensitivity, % | Specificity, % |
|---|---|---|---|
| CD64 index | 4.56 | 83 | 88 |
| CRP, mg/mL | 98 | 74 | 86 |
| Procalcitonin, ng/mL | 2.81 | 77 | 81 |
CRP, C-reactive protein.
Figure 2.
ROC curve for neutrophil CD64 index, CRP level, and procalcitonin level. ROC, receiver operating characteristic; CRP, C-reactive protein.
Discussion
The current study demonstrated a significantly higher neutrophil CD64 index in patients with sepsis than in control patients. The neutrophil CD64 index was more sensitive and specific than the CRP and procalcitonin levels for diagnosing sepsis.
CD64 is a receptor for the Fc fragment of immunoglobulin G and serves as a link between humoral and cellular immunity.11,12 CD64 is mainly distributed on the surface of monocytes, macrophages, and dendritic cells. In neutrophils, CD64 expression is low in the resting state but sharply increases upon activation by a variety of stimuli, including lipopolysaccharide, tumor necrosis factor-α, interferon-γ, and G-CSF. Increased CD64 expression in neutrophils initiates and amplifies the immune response to bacterial infection, including phagocytosis, antibody-dependent cytotoxicity, and release of cytokines, and thus plays a critical role in host defense against bacterial infection.13,14 CD64 overexpression in neutrophils occurs as early as 12 hours after bacterial infection and lasts for at least 36 hours; therefore, this parameter could be used as a biomarker for early diagnosis of infection and sepsis.15
A previous report16 using the 1992 version of the sepsis diagnostic criteria17 showed relatively good sensitivity and specificity of the neutrophil CD64 index in identifying sepsis. The current study extended such findings to include the most recently revised criteria for sepsis diagnosis.1
Commonly used biomarkers for early diagnosis of bacterial infection include the leukocyte count, CRP level, and procalcitonin level. However, the leukocyte count can be influenced by many other factors, including trauma, stress, and tumor invasion; therefore, it may not be a suitable biomarker for bacterial infection.18 Consistent with this notion, the leukocyte count did not differ significantly between the sepsis group and the control group in the present study.
CRP is an acute-phase protein that increases within 4 to 6 hours upon stimulation by pro-inflammatory cytokines and peaks at 36 to 50 hours.19 CRP is often used for early diagnosis of infection and as a biomarker for sepsis,18,20 but it has relatively low specificity.19
Procalcitonin is a glycoprotein that consists of 116 amino acids and has a half-life of 20 to 24 hours. Studies using animal models have shown that procalcitonin becomes elevated at 3 to 6 hours and peaks at 6 to 8 hours after bacterial infection.21 A meta-analysis suggested that procalcitonin cannot be used to distinguish between infectious and non-infectious diseases.22 However, a recent study suggested reasonable performance of procalcitonin for sepsis, with 77% sensitivity and 75% specificity.23 In the current study, the serum procalcitonin level had similar sensitivity and specificity (77% and 81%, respectively).
The results of the current study must be considered preliminary because of several limitations. First, this was a retrospective analysis and is therefore prone to selection bias. Another limitation is the small sample size and single-institution design. Additionally, the neutrophil CD64 index in the current study was higher than that reported in most previous studies. For example, a meta-analysis of the cut-off CD64 values for early identification of bacterial infection24 showed a relatively wide range (1.19–4.59). Many factors may have contributed to the relatively higher CD64 index in the current study. In our opinion, the higher CD64 index may reflect the unique ethnic background in the current study because a similarly high CD64 index was also reported in a previous study of a Chinese population.25
The use of patients undergoing coronary bypass without signs of infection as the control group also represents a source of potential bias. However, we believe that this was necessary because using patients with possible infection creates another set of confounding factors. Nevertheless, the results must be interpreted with caution to avoid overestimation of the diagnostic performance of the neutrophil CD64 index.
In summary, the current study has demonstrated that the neutrophil CD64 index has reasonably good sensitivity and specificity for early diagnosis of sepsis in patients in the ICU based on the 2016 criteria. If used in the context of other measures (e.g., clinical signs, CRP level, and procalcitonin level), the neutrophil CD64 index could be helpful in early identification of sepsis in the ICU setting.
Acknowledgements
The authors would like to acknowledge the contributions of Li Yang and Zhiming Liu from the hospital center laboratory.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312: 90–92. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 4.Martin G. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 2012; 10: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs. claims data, 2009–2014. JAMA 2017; 318: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 7.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci 2013; 50: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmeyer F, Witte K, Schmidt R. The high-affinity FcγRI on PMN: regulation of expression and signal transduction. Immunology 1997; 92: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gros A, Roussel M, Sauvadet E, et al. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intens Care Med 2012; 38: 445–452. [DOI] [PubMed] [Google Scholar]
- 10.Jamsa J, Huotari V, Savolainen ER, et al. Kinetics of leukocyte CD11b and CD64 expression in severe sepsis and non-infectious critical care patients. Acta Anaesthesiol Scand 2015; 59: 881–891. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Shi J, Fei A, et al. Neutrophil CD64 expression is a predictor of mortality for patients in the intensive care unit. Int J Clin Exp Pathol 2014; 7: 7806–7813. [PMC free article] [PubMed] [Google Scholar]
- 12.Elawady S, Botros SK, Sorour AE, et al. Neutrophil CD64 as a diagnostic marker of sepsis in neonates. J Investig Med 2014; 62: 644–649. [DOI] [PubMed] [Google Scholar]
- 13.Fossati G, Bucknall RC, Edwards SW. Fc gamma receptors in autoimmune diseases. Eur J Clin Invest 2001; 31: 821–831. [DOI] [PubMed] [Google Scholar]
- 14.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, Fc gamma RI, on human mast cells: up-regulation by IFN-gamma. J Immunol 2000; 164: 4332–4339. [DOI] [PubMed] [Google Scholar]
- 15.Sellge G, Barkowsky M, Kramer S, et al. Interferon–γ regulates growth and controls Fcγ receptor expression and activation in human intestinal mast cells. BMC Immunol 2014; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen FF, Petersen JA. Novel biomarkers for sepsis: a narrative review. Eur J Intern Med 2017; 45: 46–50. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Balk RA, Cerra FB, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–874. [PubMed] [Google Scholar]
- 18.Zarkesh M, Sedaghat F, Heidarzadeh A, et al. Diagnostic value of IL-6, CRP, WBC, and absolute neutrophil count to predict serious bacterial infection in febrile infants. Acta Med Iran 2015; 53: 408–411. [PubMed] [Google Scholar]
- 19.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther 2011; 9: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urban & Vogel. CRP infection marker may soon have company. MMW Fortschr Med 2015; 157: 16. [DOI] [PubMed] [Google Scholar]
- 21.Maruna P, Nedělníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res 2000; 49: 57–61. [PubMed] [Google Scholar]
- 22.Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis 2007; 7: 210–217. [DOI] [PubMed] [Google Scholar]
- 23.Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 426–435. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Huang X, Chen Z, et al. Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis. Int J Infect Dis 2013; 17: e12–e23. [DOI] [PubMed] [Google Scholar]
- 25.Xiong S, Pu L, Wang H, et al. Neutrophil CD64 index as a superior biomarker for early diagnosis of infection in febrile patients in the hematology department. Clin Chem Lab Med 2016; 55: 82–90. [DOI] [PubMed] [Google Scholar]