Short abstract
Objective
This systematic review aimed to explore the potential association between dietary cholesterol intake and esophageal cancer risk.
Methods
A literature search was conducted using PubMed, Embase, and Web of Science databases from inception to March 2019 according to specific inclusion and exclusion criteria. Pooled estimates with odds ratio (ORs) and 95% confidence intervals (CIs) were obtained using random effects models.
Results
Nine articles of 12 independent studies were included in the final meta-analysis. Pooled analysis suggested that dietary cholesterol intake may increase the risk of esophageal cancer (summarized OR = 1.424, 95% CI = 1.191–1.704). Consistent results were found in American (summarized OR = 1.410, 95% CI = 1.130–1.758) and European populations (summarized OR = 1.556, 95% CI = 1.021–2.373). Subgroup analysis by disease type showed that dietary cholesterol intake had a significant association with the development of esophageal adenocarcinoma and esophageal squamous cell carcinoma.
Conclusion
Our findings indicated that dietary cholesterol intake could significantly increase the risk of developing esophageal cancer in both European and American populations. Further high-quality studies are necessary to confirm the effects of cholesterol intake.
Keywords: Diet, cholesterol, esophageal cancer, meta-analysis, risk factor, adenocarcinoma
Introduction
Cancer is the second leading cause of death globally, and GLOBOCAN estimates suggest that it was responsible for 9.6 million deaths in 2018.1 Esophageal cancer is the ninth most common cancer in the world, with over 300,000 new cases annually of which 80% occur in developing countries.1 Esophageal cancer mainly includes esophageal adenocarcinoma and esophageal squamous cell carcinoma.2,3 Genetic factors are thought to play an important role in its occurrence,4,5 while dietary factors and other major environmental risk factors may also potentially affect its development.3,6–12
Previous meta-analyses have investigated the association between cholesterol intake and some cancers. Chen et al.13 and Wang et al.14 showed that a high intake of cholesterol increases the risk of pancreatic cancer, while Lin et al.15 found that cholesterol intake increased the risk of lung cancer. However, no meta-analysis has studied the effect of cholesterol intake on esophageal cancer risk. The recent increase in new evidence has led to diverging opinions about the precise effects of cholesterol intake on the risk of esophageal cancer. Moreover, studies of small sample sizes have failed to demonstrate whether cholesterol intake increases esophageal cancer risk. Thus, this meta-analysis aimed to explore the effect of cholesterol intake on the risk of esophageal cancer.
Methods
Study selection and data extraction
An independent literature search was conducted by two reviewers (YYJ and TY) using PubMed, Embase, and Web of Science databases from inception to March 2019. The following MESH terms were used for the search strategy: ‘cholesterol’ AND ‘esophageal’ AND ‘cancer’ OR ‘tumor’. This study did not require approval by an ethics review committee because it is a meta-analysis.
Based on titles and abstracts, full texts of potentially relevant studies were retrieved and assessed for eligibility criteria. Additionally, the cited references in the included articles were manually assessed for eligibility.
The following inclusion criteria were employed: (1) studies about cholesterol intake and esophageal cancer risk; (2) studies about humans; (3) observational studies; (4) articles published in English; and (5) available data of odds ratios (ORs) and 95% confidence intervals (CIs). Exclusion criteria were: (1) overlapping studies or populations; (2) conference reports, editor comments, reviews, or case reports; and (3) animal studies.
The following data were extracted from the included studies by the two reviewers using a customized data extraction sheet: first author, year of publication, study design, country, age of patients, type of disease, number of cases and participants, dietary assessment, ORs and 95% CIs, and adjustment or matched for factors. Disagreements between the two reviewers were resolved by a third reviewer (DYL).
Statistical analysis
Pooled ORs and 95% CIs were calculated using a random effects model.16 I2 statistics enabled the evaluation of collected data statistical heterogeneity.17 I2 > 50% indicated high heterogeneity. Meta-regression was used to explore the potential reason of between-study heterogeneity.18 Sensitivity analysis was performed to assess whether a single study could affect the overall estimate. Egger’s test19 and a funnel plot20 were used to determine the presence of publication bias. Statistical analysis was performed using Review Manager software (version 5.3; Cochrane Collaboration, London, UK) with statistical significance set at P < 0.05.
Results
Study characteristics
Our search yielded 631 potentially relevant studies, of which 35 were reserved for full text reading and further assessment after the initial review. According to the inclusion and exclusion criteria, nine articles21–29 were included and analyzed in our meta-analysis. Three articles25,26,29 simultaneously and independently reported esophageal adenocarcinoma and esophageal squamous cell carcinoma cases. Therefore, 12 independent studies involving 1555 cases and 6497 participants were included in the analysis. The flow diagram of study selection is shown in Figure 1, and the main characteristics of the included studies are shown in Table 1.
Figure 1.
Flow chart of the meta-analysis.
Table 1.
Characteristics of studies about cholesterol intake and esophageal cancer risk.
| Study, year | Design | Age (years) | Participants (cases) | Country | Outcome | Assessment of intake | Categories | OR (95% CI) | Adjusted for or matched for |
|---|---|---|---|---|---|---|---|---|---|
| Tzonou et al., 1996 | HBCC | NA | 256 (56) | Greece | Esophageal adenocarcinoma | FFQ | Q5 vs. Q1 | 1.06 (0.75–1.51) | Age, sex, birth place, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake. |
| Tzonou et al., 1996 | HBCC | NA | 243 (43) | Greece | Esophageal squamous cell carcinoma | FFQ | Q5 vs. Q1 | 1.21 (0.85–1.72) | Age, sex, birth place, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake. |
| Zhang et al., 1997 | HBCC | NA | 214 (90) | United States | Esophageal adenocarcinoma | HHHQ | Q4 vs. Q1 | 1.0 (0.7–1.4) | Age, sex, race, education, smoking, alcohol intake, BMI, and total dietary intake in calories. |
| De Stefani et al., 1999 | HBCC | 40–89 | 330 (82) | Uruguay | Esophageal cancer | FFQ | T3 vs. T1 | 1.59 (0.79–3.20) | Age, sex, residence, urban/rural status, education, BMI, tobacco smoking, total alcohol intake and total energy intake. |
| Mayne et al., 2001 | PBCC | 30–80 | 969 (282) | United States | Esophageal adenocarcinoma | FFQ | Q4 vs. Q1 | 1.74 (1.36–2.23) | Age, site, sex, race, proxy status, BMI, income, education, smoking, and alcohol consumption. |
| Mayne et al., 2001 | PBCC | 30–80 | 893 (206) | United States | Esophageal squamous cell carcinoma | FFQ | Q4 vs. Q1 | 1.63 (1.22–2.18) | Age, site, sex, race, proxy status, BMI, income, education, smoking, and alcohol consumption. |
| Wolfgarten et al., 2001 | PBCC | 62.2 ± 1.9 | 140 (40) | Germany | Esophageal adenocarcinoma | FFQ | >0.42 g/d vs. <0.25 g/d | 2.3 (0.7–7.4) | Age, gender, height, weight, BMI and socioeconomic data such as marital status and earning capacity. |
| Wolfgarten et al., 2001 | PBCC | 58.1 ± 1.2 | 145 (45) | Germany | Esophageal squamous cell carcinoma | FFQ | >0.42 g/d vs. <0.25 g/d | 1.7 (0.6–5.0) | Age, gender, height, weight, BMI and socioeconomic data such as marital status and earning capacity. |
| De Stefani et al., 2006 | HBCC | 40–89 | 1170 (234) | Uruguay | Esophageal squamous cell carcinoma | FFQ | Q4 vs. Q1 | 1.06 (0.66–1.71) | Age, sex, residence, urban/rural status, birthplace, education, BMI, smoking status, years since quitting smoking, number of cigarettes smoked per day, alcohol drinking, meat consumption, and total energy intake. |
| Wu et al., 2007 | PBCC | 30–74 | 1514 (206) | United States | Esophageal adenocarcinoma | FFQ | Q4 vs. Q1 | 1.63 (0.7–3.7) | Age, sex, race, birthplace, education, smoking, BMI, reflux, use of vitamins, total calories, and fat intake. |
| Jessri et al., 2011 | HBCC | 40–75 | 143 (47) | Iran | Esophageal squamous cell carcinoma | SFFQ | T3 vs. T1 | 1.53 (1.41–4.13) | Age, sex, reflux, BMI, smoking, physical activity, and education. |
| O’Doherty et al., 2011 | PBCC | <80 | 480 (224) | Ireland | Esophageal adenocarcinoma | FFQ | 484.7 mg/d vs. 260.6 mg/d | 3.59 (1.71–7.54) | Age at interview, sex, smoking status, BMI 5 years prior to interview date, job type, education, energy intake, fruit intake, vegetable intake, alcohol intake, Helicobacter pylori infection, nonsteroidal antiinflammatory drug use 5 years prior to interview date, gastroesophageal reflux symptoms, and location. |
OR: odds ratio; CI: confidence interval; PBCC: population-based case–control study; HBCC: hospital-based case–control study; NA: not available; HHHQ: health habits and history questionnaire; FFQ: food frequency questionnaire; SFFQ: Semi-quantitative food frequency questionnaire; BMI: body mass index.
All included studies in our analysis had a case–control design; six studies were population-based case–control studies (PBCCs) and six were hospital-based case–control studies (HBCCs). Positive results were only found in PBCCs (summarized OR = 1.773, 95% CI = 1.490–2.110), not in HBCCs. Significant associations were found in both American populations (summarized OR = 1.410, 95% CI = 1.130–1.758) and European populations (summarized OR = 1.556, 95% CI = 1.021–2.373) compared with other populations. Detailed results are shown in Table 2.
Table 2.
Summarized overall and subgroup results.
| Subgroups | Number of studies | Number of cases | OR (95% CI) | Z test | P for trend | Heterogeneity test |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Total | 12 | 1555 | 1.424 (1.191–1.704) | 3.87 | <0.001 | 43.2 | 0.055 |
| Disease type | |||||||
| Esophageal adenocarcinoma | 6 | 898 | 1.525 (1.075–2.163) | 2.36 | 0.018 | 68.7 | 0.007 |
| Esophageal squamous cell carcinoma | 5 | 575 | 1.394 (1.157–1.681) | 3.49 | <0.001 | 0.0 | 0.516 |
| Study design | |||||||
| PBCC | 6 | 1003 | 1.773 (1.490–2.110) | 6.46 | <0.001 | 0.0 | 0.542 |
| HBCC | 6 | 552 | 1.146 (0.966–1.358) | 1.57 | 0.118 | 0.0 | 0.710 |
| Geographic location | |||||||
| America | 6 | 1100 | 1.410 (1.130–1.758) | 3.04 | 0.002 | 44.0 | 0.112 |
| Europe | 5 | 408 | 1.556 (1.021–2.373) | 2.06 | 0.040 | 59.2 | 0.044 |
| Asia | 1 | 47 | – | – | – | – | – |
OR: odds ratio; CI: confidence interval; PBCC: population-based case–control studies; HBCC: hospital-based case–control studies.
Meta-analysis results
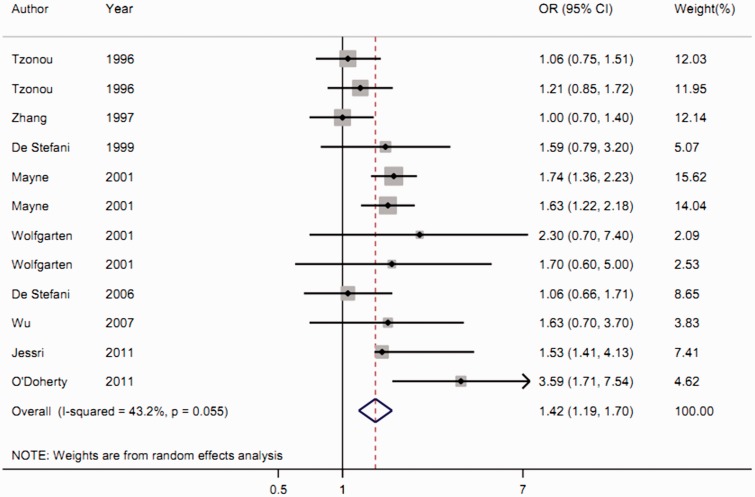
The highest category of cholesterol intake was shown to significantly increase the risk of esophageal cancer compared with the lowest category (summarized OR = 1.424, 95% CI = 1.191–1.704, Z test = 3.87, Pfor trend < 0.001), with moderate heterogeneity (I2 = 43.2%, Pfor heterogeneity = 0.055) (Figure 2).
Figure 2.
Forest plot of the association between cholesterol intake and esophageal cancer risk.
Subgroup analysis by disease type revealed an increased risk of esophageal adenocarcinoma (summarized OR = 1.525, 95% CI = 1.075–2.163) and esophageal squamous cell carcinoma (summarized OR = 1.394, 95% CI = 1.157–1.681) with high cholesterol intake.
Publication bias sensitivity analysis
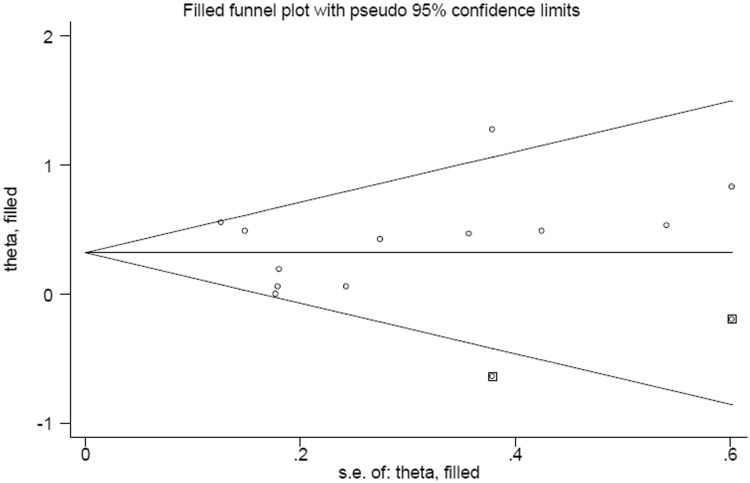
Funnel plots (Figure 3) and Egger’s test revealed no publication bias in this meta-analysis. Sensitivity analysis suggested that no single study affected the overall estimate.
Figure 3.
Funnel plot of publication bias regarding cholesterol intake and esophageal cancer risk.
Discussion
Findings from the current study suggested that cholesterol intake significantly increases the risk of developing esophageal cancer in both American and European populations. Positive associations were found between esophageal adenocarcinoma risk and esophageal squamous cell carcinoma risk with high cholesterol intake.
Because a high cholesterol diet may indicate that lifestyles are prone to health-related problems such as cardiovascular disease and cancer, the relationship between dietary cholesterol and cancer risk has recently attracted widespread attention.14,30 Some mechanisms have been suggested to explain the possible role of cholesterol in the development of cancer. For example, changes in lipid and apolipoprotein levels may result in cellular inflammation.31 Moreover, decreased high-density lipoprotein cholesterol levels and elevated levels of low-density lipoprotein cholesterol and total cholesterol are associated with increased pro-inflammatory cytokines, including tumor necrosis factor-α and interleukin-6.32
To the best of our knowledge, this is the first meta-analysis of the relationship between cholesterol intake and esophageal cancer risk. Its inclusion of more cases and participants than a single study means that a more precise conclusion can be obtained. However, despite this, there were a number of limitations. First, we did not perform a dose-response analysis about cholesterol intake and esophageal cancer risk because no detailed information about cholesterol intake was provided in the individual studies. Second, all included studies had a case–control design which may have resulted in selection bias and recall bias. That the association was non-significant in HBCCs may reflect the additional number of confounding factors in hospital-based populations. Third, we only found a positive association in European and American populations, not in other populations. Therefore, our results may only be applicable to these populations, probably because of their dietary habits. Additionally, only one study derived from Asia so we could not conclude about the effect of cholesterol intake on esophageal cancer risk in Asians. Therefore, more studies in Asia and other countries are warranted to further explore these associations. Fourth, subgroup analysis by sex was not conducted because few studies contained sufficient data, which limited conclusions. Finally, moderate between-study heterogeneity was found in the overall analysis. Analysis by meta-regression revealed that study design could increase between-study heterogeneity. Indeed, when we performed subgroup analysis by study design, I2 was reduced to 0.0% both in PBCCs and HBCCs.
Conclusions
Our findings indicated that dietary cholesterol intake significantly increased the risk of esophageal cancer in European and American populations. Further high-quality studies are necessary to confirm the effects of cholesterol intake.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Globocan. Estimated cancer incidence, mortality and prevalence worldwide in 2018. International Agency for research on cancer WHO, 2018. Available online: http://gco.iarc.fr/ [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 3.Sardana RK, Chhikara N, Tanwar B, et al. Dietary impact on esophageal cancer in humans: a review. Food Funct 2018; 9: 1967–1977. [DOI] [PubMed] [Google Scholar]
- 4.Mao N, Nie S, Hong B, et al. Association between alcohol dehydrogenase-2 gene polymorphism and esophageal cancer risk: a meta-analysis. World J Surg Oncol 2016; 14: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Yu X, Li J, et al. Prognostic significance of p53 expression in patients with esophageal cancer: a meta-analysis. BMC Cancer 2016; 16; 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JL, Zhao Y, Guo CY, et al. Dietary vitamin B intake and the risk of esophageal cancer: a meta-analysis. Cancer Manag Res 2018; 10: 5395–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui L, Li L, Tian Y, et al. Association between dietary vitamin e intake and esophageal cancer risk: an updated meta-analysis. Nutrients 2018; 10: pii: E801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McRae MP. The benefits of dietary fiber intake on reducing the risk of cancer: an umbrella review of meta-analyses. J Chiropr Med 2018; 17: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Zhou H, Zhu Y, et al. Associations between dietary folate intake and risks of esophageal, gastric and pancreatic cancers: an overall and dose-response meta-analysis. Oncotarget 2017; 8: 86828–86842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhikara N, Kaur R, Jaglan S, et al. Bioactive compounds and pharmacological and food applications of Syzygium cumini - a review. Food Funct 2018; 9: 6096–6115. [DOI] [PubMed] [Google Scholar]
- 11.Chhikara N, Kushwaha K, Sharma P, et al. Bioactive compounds of beetroot and utilization in food processing industry: a critical review. Food Chem 2019; 272: 192–200. [DOI] [PubMed] [Google Scholar]
- 12.Chhikara N, Kour R, Jaglan S, et al. Citrus medica: nutritional, phytochemical composition and health benefits - a review. Food Funct 2018; 9: 1978–1992. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Qin S, Wang M, et al. Association between cholesterol intake and pancreatic cancer risk: evidence from a meta-analysis. Sci Rep 2015; 5: 8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Wang WJ, Zhai L, et al. Association of cholesterol with risk of pancreatic cancer: a meta-analysis. World J Gastroenterol 2015; 21: 3711–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Liu L, Fu Y, et al. Dietary cholesterol intake and risk of lung cancer: a meta-analysis. Nutrients 2018; 10: pii: E185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004; 23: 1663–1682. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 21.De Stefani E, Deneo-Pellegrini H, Boffetta P, et al. Meat intake and risk of squamous cell esophageal cancer: a case-control study in Uruguay. Int J Cancer 1999; 82: 33–37. [DOI] [PubMed] [Google Scholar]
- 22.De Stefani E, Ronco AL, Boffetta P, et al. Nutrient intake and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer 2006; 56: 149–157. [DOI] [PubMed] [Google Scholar]
- 23.Jessri M, Rashidkhani B, Hajizadeh B, et al. Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: a case-control study in Iran. Nutr J 2011; 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Doherty MG, Cantwell MM, Murray LJ, et al. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett's esophagus and esophageal adenocarcinoma. Int J Cancer 2011; 129: 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzonou A, Lipworth L, Garidou A, et al. Diet and risk of esophageal cancer by histologic type in a low-risk population. Int J Cancer 1996; 68: 300–304. [DOI] [PubMed] [Google Scholar]
- 26.Wolfgarten E, Rosendahl U, Nowroth T, et al. Coincidence of nutritional habits and esophageal cancer in Germany. Onkologie 2001; 24: 546–551. [DOI] [PubMed] [Google Scholar]
- 27.Wu AH, Tseng CC, Hankin J, et al. Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control 2007; 18: 713–722. [DOI] [PubMed] [Google Scholar]
- 28.Zhang ZF, Kurtz RC, Yu GP, et al. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer 1997; 27: 298–309. [DOI] [PubMed] [Google Scholar]
- 29.Mayne ST, Risch HA, Dubrow R, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 2001; 10: 1055–1062. [PubMed] [Google Scholar]
- 30.Li C, Yang L, Zhang D, et al. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr Res 2016; 36: 627–635. [DOI] [PubMed] [Google Scholar]
- 31.Ferretti G, Bacchetti T, Negre-Salvayre A, et al. Structural modifications of HDL and functional consequences. Atherosclerosis 2006; 184: 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Haddy N, Sass C, Droesch S, et al. IL-6, TNF-alpha and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis 2003; 170: 277–283. [DOI] [PubMed] [Google Scholar]