Short abstract
Objective
Thyroid hormones affect airway contraction, but the specific effects of thyroid hormones on airways are controversial.
Methods
We divided 78 advanced-age men with asthma into 3 groups: type I respiratory failure (RF1), type II respiratory failure (RF2), and no respiratory failure (NRF). Pulmonary function was measured after asthma stabilization.
Results
The free triiodothyronine (FT3) level was significantly higher in the RF1 than RF2 group, but the free thyroxine (FT4), total thyroxine (TT4), and thyroid-stimulating hormone (TSH) levels were not significantly different. In the RF1, RF2, and NRF groups, the correlation coefficients between FT3 and the forced expiratory volume in1 s (FEV1) were 0.317, 0.627, and 0.213; those between FT3 and the FEV1/forced vital capacity (FVC) ratio were 0.287, 0.412, and 0.156; those between FT4 and FEV1 were 0.214, 0.231, and 0.167; and those between FT4 and the FEV1/FVC ratio were 0.211, 0.215, and 0.218, respectively. In the RF1 and RF2 groups, the correlation coefficients between the sum activity of peripheral deiodinases (SPINA-GD) and the FEV1/FVC ratio were 0.164 and 0.421, and those between SPINA-GD and FEV1 were 0.284 and 0.491, respectively.
Conclusion
The correlation between FT3 and pulmonary function is likely caused by changes in thyroid enzymology.
Keywords: Asthma, older adults, acute exacerbation, thyroid hormone, pulmonary function, respiratory failure
Background
Recent observations have shown that the prevalence of asthma in older adults is not different from that in younger populations.1 The management of asthma in patients of advanced age represents one of the greatest conundrums for experts in this field. The term “geriatric asthma” describes a key clinical phenotype of asthma.2
The effects of thyroid hormones on airway contractility are unclear. Rats rendered hypothyroid via thyroidectomy exhibited attenuated susceptibility to the development of experimental asthma, and this susceptibility was reestablished with thyroxine (T4) supplementation.3 Triiodothyronine (T3) supplementation in euthyroid children with asthma has been shown to relieve symptoms and allow children to taper the use of asthma medication.4 The protective effect of the absence of T4 in rats and the beneficial effects of the addition of T3 in children with asthma and normal levels of other thyroid hormones seem counterintuitive. Together, these observations suggest that the relationship between thyroid hormones and the risk of asthma is complex.
In the present study, we retrospectively analyzed changes in older adults with acute exacerbation of bronchial asthma to deepen our understanding of the preliminary effects of thyroid hormones on asthma. Our findings will help to clarify the relationship between serum thyroid hormone levels and the pathogenesis of asthma in patients of advanced age.
Methods
Participants and subgroups
We retrospectively analyzed advanced-age patients with acute exacerbation of asthma who were treated in the Emergency Department of Qingdao City Hospital from 2014 to 2017. All patients were male smokers with a smoking history of >400 pack-years. Similarly aged healthy men who also had a smoking history of >400 pack-years comprised the normal control group. The participants were divided into three groups according to the point during the treatment process at which the experimental measures were assessed: patients with acute exacerbation of asthma who underwent measurements prior to treatment, patients with acute exacerbation of asthma who underwent measurements after treatment, and normal controls. The patients were divided into three subgroups based on their arterial blood gas analysis results: type I respiratory failure (RF1), type II respiratory failure (RF2), and no respiratory failure (NRF). The study was approved by the Qingdao Municipal Hospital Research Ethics Committee. All patients were fully informed about the purpose and procedures of the study, and all provided written consent to participate.
Exclusion criteria
Patients were excluded from the study if they had a history of thyroid disease; had a history of chronic obstructive pulmonary disease, pulmonary embolism, lung lesions, interstitial lung disease, or other respiratory diseases; had received drugs that affect thyroid hormones, such as amiodarone hydrochloride, within the last 3 months; had a history of long-term use of glucocorticoids; or had severe heart failure requiring drug control.
Diagnostic criteria
Asthma exacerbation was diagnosed according to the 2017 edition of the Global Initiative for Asthma program5 and was based on a partial pressure of arterial oxygen (PaO2) of <60 mmHg with or without a partial pressure of arterial carbon dioxide (PaCO2) of >50 mmHg under sea-level conditions in a resting state, which excludes intracardiac anatomical shunts, primary cardiac discharge, and other factors that may be diagnosed as respiratory failure. The diagnostic criteria for RF1 were a PaO2 of <60 mmHg accompanied by a decreased or normal PaCO2. The diagnostic criteria for RF2 were a PaO2 of <60 mmHg accompanied by a PaCO2 of >50 mmHg.
Study methods and observation indexes
Blood samples were collected from patients with acute exacerbation of asthma on an empty stomach at 7:00 am for the first 2 days. The serum levels of thyroid hormones [total T3 (TT3), total T4 (TT4), free T3 (FT3), free T4 (FT4), and thyroid-stimulating hormone (TSH)] were measured by chemiluminescence. The sum activity of peripheral deiodinases (SPINA-GD), Jostel’s TSH index (JTI), and the thyroid’s secretory capacity (SPINA-GT) were also evaluated. Hospitalized patients received standardized treatment. Blood was withdrawn from patients when their condition improved. Samples were also collected from patients on an empty stomach at 7:00 am 1 day before discharge to measure serum hormone and cytokine levels. Blood samples were collected from patients in the healthy control group on an empty stomach at 7:00 am to measure serum thyroid hormones. Pulmonary function was tested during the asthma stabilization period.
Statistical analysis
All data were analyzed using SPSS for Windows, Version 16.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data are expressed as mean ± standard deviation. The results from the two groups of samples were compared using the t test. Multiple samples were compared using the Kruskal–Wallis test when the data were not normally distributed. Correlation coefficients between the FT4 level and pulmonary function and between FT3 and pulmonary function were calculated in different stages of asthma were also calculated. Exact P values were considered statistically significant at <0.05.
Results
Patients’ baseline characteristics
The study comprised 78 older men with acute exacerbation of asthma (mean age, 70 ± 12 years; age range, 62–82 years) and 42 healthy male controls (mean age, 69 ± 11 years; age range, 61–80 years). The RF1, RF2, and NRF groups comprised 28, 20, and 30 patients, respectively. As shown in Table 1, there were no significant differences in body mass index, pack-years, blood pressure, or age among all study participants. There were also no significant differences in the C-reactive protein level among the RF1, RF2, and NRF groups. However, there was a significant difference in the C-reactive protein level between the control group and the patient groups (RF1, RF2, and NRF) (P < 0.05).
Table 1.
Baseline characteristics of all older adults with asthma.
| GroupProject | RF1 | RF2 | NRF | Control group |
|---|---|---|---|---|
| Age, years | 70.6 ± 8.6 | 72.1 ± 9.1 | 70.1 ± 7.1 | 69 ± 11 |
| C-reactive protein, mg/L | 35.7 ± 5.6 | 34.8 ± 4.9 | 34.9 ± 5.1 | 9.7 ± 0.56▲ |
| Blood pressure,mmHg | 110 ± 8.9/70 ± 12.1 | 115 ± 9.1/75 ± 10.2 | 113 ± 7.4/68 ± 8.9 | 111 ± 9.3/69 ± 8.8 |
| BMI, kg/m2 | 25.1 ± 2.1 | 24.8 ± 2.3 | 25.2 ± 2.2 | 25.3 ± 2.3 |
| Pack-years | 419.3 ± 17.8 | 418.7 ± 16.7 | 419.7 ± 14.8 | 418.3 ± 13.9 |
Data are presented as mean ± standard deviation.
RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; BMI, body mass index.
▲Compared with control group, P < 0.05
Comparison of thyroid hormone levels before and after treatment
Table 2 shows the serum thyroid hormone levels in patients with bronchial asthma before and after treatment for asthma exacerbation and participants in the normal control group. The TT4, TT3, and FT3 levels were significantly lower before than after treatment (P < 0.05). The TT4, TT3, and FT3 levels were significantly lower in the after-treatment group than in the control group (P < 0.05). There was a significant difference in the FT4 level between the before- and after-treatment groups (P <0.05). However, there was no significant difference in the FT4 level between the after-treatment group and the control group. The differences in the TSH level among the three groups were not statistically significant. The SPINA-GT, SPINA-GD, and JTI were significantly lower before than after treatment (P < 0.05). The SPINA-GT, SPINA-GD, and JTI were also significantly lower in the after-treatment group than in the control group (P < 0.05).
Table 2.
Comparison of serum thyroid hormone levels before and after treatment in older adults with acute exacerbation of asthma.
| Group | n | TT3nmol/L | TT4nmol/L | FT3pmol/L | FT4pmol/L | TSHmIU/L | SPINA-GTpmol/s | SPINA-GDnmol/s | JTI |
|---|---|---|---|---|---|---|---|---|---|
| Asthma exacerbation | |||||||||
| Before treatment | 78 | 0.85 ± 0.21*,# | 79 ± 17*,# | 2.6 ± 0.61*,# | 14.8 ± 2.3# | 1.30 ± 0.5 | 1.32 ± 0.35*,# | 15.6 ± 3.6*,# | 1.01 ± 0.21*,# |
| After treatment | 78 | 1.33 ± 0.29* | 90 ± 19* | 3.35 ± 0.57* | 17.1 ± 2.5 | 1.32 ± 0.64 | 2.21 ± 0.52* | 24.7 ± 2.8* | 2.32 ± 0.33* |
| Normal control group | 42 | 1.58 ± 0.31 | 112 ± 21 | 4.19 ± 0.64 | 17.2 ± 2.1 | 1.34 ± 0.75 | 5.2 ± 0.83 | 35.7 ± 3.8 | 3.25 ± 0.41 |
Data are presented as mean ± standard deviation.
TT3, total triiodothyronine; TT4, total thyroxine; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; SPINA-GT, thyroid’s secretory capacity; SPINA-GD, sum activity of peripheral deiodinases; JTI, Jostel’s thyroid-stimulating hormone index.
*P < 0.05 compared with control group; #P < 0.05 compared with treatment group.
Comparison of thyroid hormone levels among the study groups
Table 3 shows the serum thyroid hormone levels in the RF1, RF2, and NRF groups. All thyroid hormone levels (FT3, FT4, TT3, and TT4) were much lower in the RF1 and RF2 groups than in the NRF group (P < 0.05). The TT3 and FT3 levels were markedly higher in the RF1 than RF2 group (P < 0.05). There was no significant difference in the FT4 or TT4 level between the RF1 and RF2 groups. There was also no significant difference in the TSH level among the RF1, RF2, and NRF groups.
Table 3.
Comparison of thyroid hormone levels between patients with and without respiratory failure among advanced-age patients with acute exacerbation of asthma.
| Group | n | TT3nmol/L | TT4nmol/L | FT3pmol/L | FT4pmol/L | TSHmIU/L |
|---|---|---|---|---|---|---|
| RF1 | 28 | 0.75 ± 0.14#,* | 76 ± 13* | 2.51 ± 0.64#,* | 14.1 ± 2.1* | 1.30 ± 0.56 |
| RF2 | 20 | 0.69 ± 0.12* | 71 ± 15* | 2.29 ± 0.59* | 14.2 ± 1.9* | 1.29 ± 0.31 |
| NRF | 30 | 1.05 ± 0.22 | 93 ± 16 | 3.06 ± 0.61 | 16.1 ± 2.3 | 1.29 ± 0.46 |
Data are presented as mean ± standard deviation.
RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; TT3, total triiodothyronine; TT4, total thyroxine; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
*P < 0.05 compared with NRF group; #P < 0.05 compared with RF2 group.
Comparison of thyroid hormone levels between patients with exacerbation and stabilization of asthma
Table 4 compares the serum thyroid hormone levels between patients with exacerbation and stabilization of asthma. Among the patients with acute exacerbation, the PaCO2 level was significantly lower in the RF1 than RF2 group (P < 0.05). Among the patients with stabilization of asthma, there were no significant differences in the PaCO2 level. The PaO2 level was significantly lower in the RF1 group than in the NRF and control groups (P < 0.05). There were no significant differences in the PaO2 level between the RF1 and RF2 groups. Analysis of pulmonary function in the patients with stabilization of asthma showed that the forced expiratory volume in 1 s (FEV1) and the FEV1/forced vital capacity (FVC) ratio were significantly better in the RF1 than RF2 group (P < 0.05), significantly better in the NRF group than in the RF1 and RF2 groups (P < 0.05), and significantly better in the control group than in the RF1 and RF2 groups (P < 0.05).
Table 4.
Serum thyroid hormone levels in advanced-age patients with exacerbation and stabilization of asthma.
| GroupFeatures |
Exacerbation |
Stabilization |
|||||
|---|---|---|---|---|---|---|---|
| RF1 | RF2 | NRF | RF1 | RF2 | NRF | Control | |
| Pulmonary function | |||||||
| FEV1/FVC% | – | – | – | 73.5 ± 7.1◇ | 70.1 ± 6.7 | 76.2 ± 6.7□,◇ | 87.3 ± 5.7□,◇ |
| FEV1% | – | – | – | 64.2 ± 5.3◇ | 55.2 ± 4.7 | 77.3 ± 6.3□,◇ | 88.1 ± 6.1□,◇ |
| Arterial blood gas | |||||||
| pH | 7.41 ± 0.05 | 7.42 ± 0.06 | 7.39 ± 0.03 | 7.39 ± 0.03 | 7.38 ± 0.02 | 7.41 ± 0.04 | 7.42 ± 0.05 |
| PaO2, mmHg | 55.1 ± 3.1 | 56.7 ± 2.7 | 69.1 ± 4.3 | 65.4 ± 3.2 | 64.8 ± 3.4 | 73.2 ± 4.6◇,□ | 75.3 ± 4.2◇,□ |
| PaCO2, mmHg | 37.7 ± 4.7◆ | 56.2 ± 4.6 | 37.6 ± 3.2 | 39.1 ± 4.2 | 40.3 ± 3.9 | 36.4 ± 3.6 | 38.2 ± 4.1 |
| BMI, kg/m2 | 25.1 ± 2.1 | 24.8 ± 2.3 | 25.2 ± 2.2 | 25.1 ± 2.1 | 24.8 ± 2.3 | 25.2 ± 2.2 | 25.3 ± 2.3 |
Data are presented as mean ± standard deviation.
RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; BMI, body mass index.
Exacerbation of arterial blood gas: ◆P < 0.05 compared with RF2 group.
Stabilization of pulmonary function and arterial blood gas: ◇P < 0.05 compared with RF2 group. □P < 0.05 compared with RF1 group.
Correlation analysis between FT3 and pulmonary function
As shown in Table 5, the correlation coefficients between serum FT3 and FEV1 were 0.317 (RF1 group), 0.627 (RF2 group), and 0.213 (NRF group). The correlation coefficients between serum FT3 and the FEV1/FVC ratio were 0.287 (RF1 group), 0.412 (RF2 group), and 0.156 (NRF group).
Table 5.
Correlation coefficients between serum FT3 level and pulmonary function among the study groups.
| GroupsFT3 | FEV1 | FEV1/FVC |
|---|---|---|
| RF1 | 0.317* | 0.287* |
| RF2 | 0.627* | 0.412* |
| NRF | 0.213* | 0.156* |
| Normal control group | – | – |
FT3, free triiodothyronine; RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
*P < 0.05, –P > 0.05.
Correlation analysis between FT4 and pulmonary function
As shown in Table 6, the correlation coefficients between FT4 and FEV1 were 0.214 (RF1 group), 0.231 (RF2 group), and 0.167 (NRF group). The correlation coefficients between FT4 and the FEV1/FVC ratio were 0.211 (RF1 group), 0.215 (RF2 group), and 0.218 (NRF group).
Table 6.
Correlation coefficients between serum FT4 level and pulmonary function among the study groups.
| GroupsFT4 | FEV1 | FEV1/FVC |
|---|---|---|
| RF1 | 0.214* | 0.211* |
| RF2 | 0.231* | 0.215* |
| NRF | 0.167* | 0.218* |
| Normal control group | – | – |
FT4, free thyroxine; RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
*P < 0.05, –P > 0.05.
Discussion
Asthma was historically considered mainly a childhood disease. However, asthma is currently an important cause of morbidity and mortality in patients of advanced age.1
Clinical studies of patients with many nonthyroid diseases (e.g., respiratory and heart failure) have shown that changes in thyroid hormones (e.g., a reduced T3 level) are key predictors of disease prognosis.6,7
The term “geriatric asthma” refers to patients who meet the diagnostic criteria for asthma and are ≥60 years of age.8 This type of asthma is an important clinical phenotype of bronchial asthma. However, aging itself also has significant effects on the methacholine response and airway hyperreactivity.9 Therefore, higher annual declines in FEV1 are observed in patients with late-onset asthma,10,11 especially people of advanced age and smokers with a history of >10 pack-years.12–14 Women are more likely than men to be affected by late-onset asthma.15 In the present study, we measured different levels of thyroid hormones in advanced-age patients with different stages of asthma. All participants were male smokers, which effectively controlled for experimental bias.
When the availability of energy or glutathione does not match their consumption, active thyroid hormones are expected to be selectively downregulated. By analogy to classic concepts, we refer to this phenomenon as type 1 thyroid allostasis.16
Bronchial asthma in association with various levels of TT3 and FT3 is a stress response, but it is also a reversible and protective mechanism. Lower oxygen consumption is conducive to tissue and organ function. Studies have shown that thyroid hormone levels are key factors in acute exacerbation of bronchial asthma.17
Euthyroid sick syndrome results from severe acute or chronic nonthyroid disease, trauma, or other causes of thyroid function abnormalities in the circulating blood, and it often manifests in serum as a decrease in FT3 and T3. In contrast, FT4 and T4 are normal or decreased, and TSH is generally in the normal range. Euthyroid sick syndrome is one of a group of syndromes that present clinically without significant thyroid dysfunction.18 Recent studies have shown that T3 is an independent predictor of survival.19
The results of the present study showed that the serum TT4, TT3, FT4, and FT3 levels were lower in advanced-age patients with acute exacerbation of asthma than in normal control patients. There was no significant difference in the TSH level between the two groups. FT3 and FT4 reflect the status of thyroid function when thyroid-binding globulin (TBG) remains normal, and these hormones are not affected by changes in TBG quantity, TBG function, or albumin content. FT3 and FT4 more accurately reflect the thyroid function status than do TT3 and TT4 when the TBG concentration is abnormal. In the present study, the FT3 level was lower in advanced-age patients with acute exacerbation of asthma than in those with stabilization. Stabilization of the thyroid hormone levels in older patients with acute exacerbation of asthma suggests that both the acute exacerbation and hypothyroidism are short and reversible. In patients of advanced age, the combination of asthma and RF2 is critical. Hypoxia and carbon dioxide retention decrease the levels of thyroid hormones, especially FT3, which more likely reflects the disease severity and may indicate the severity of acute exacerbations.
Non-thyroidal illness syndrome is a disease-independent risk factor for survival; therefore, it is important to understand the mechanisms underlying this condition.20 Patients with low FT3 show significantly higher mortality rates and a significantly longer duration of mandatory ventilation.20,21 Furthermore, low FT3 is a strong prognostic predictor in patients with B-cell lymphomas.22 The alterations during the acute phase of non-thyroidal illness syndrome in patients with critical illness occur within hours or days and are defined by increased release of anterior pituitary hormones, low levels of anabolic peripheral effector hormones, a reduced TBP concentration, reduced binding affinity, reduced expression of thyroid hormone transporters, decreased thyroid hormone uptake, and altered expression of D1 and D3 activity and thyroid hormone receptor alpha 1.
The expression of thyroid hormones is induced in advanced-age patients with asthma mainly for the following reasons: (1) Conversion of T4 to T3 in peripheral tissue may decline because of hypoxia, and conversion of T4 to reverse T3 may be enhanced. (2) Tissue hypoxia and carbon dioxide retention stimulate the thalamus–pituitary–adrenal system, which leads to catecholamine and cortisol synthesis. Both of these activities elevate blood glucose, fat mobilization, and protein decomposition to maintain normal energy metabolism and meet the basic physiological needs of organs. Metabolic changes in the thalamus–pituitary complex result in feedback inhibition of TSH release, which inhibits peripheral tissue T4 and T3 mutual transformation and reduces T3 synthesis. (3) Acute exacerbation of asthma in older patients leads to increases in a series of cytokines, such as interleukins and tumor necrosis factor, which inhibit the biological activity of TSH and suppress T4 conversion to T3.23 (4) Acute infection and hypoxia decrease the TBG level, which reduces the T3 and T4 levels. Acute exacerbation of asthma places advanced-age patients at increased risk of heart failure, reduced renal blood flow, and renal tubular reabsorption of iodine. Diuretics accelerate iodine excretion.
FT4 is also an important thyroid hormone. Some studies have shown that FT4 is an important determinant of refractory asthma.24 FT4 affects blood glucocorticoid levels in patients with asthma.25
In the present study, the correlation coefficients between FT3 and pulmonary function were higher than those between FT4 and pulmonary function, which suggests that the pathogenesis of asthma in patients of advanced age is different from that of asthma in younger patients. During the development of the disease, the correlation coefficient between FT3 and pulmonary function was higher in the RF2 than RF1 group. This result suggests that thyroid hormones may be involved in the development of asthma in patients of advanced age.
The JTI is measured to quantitatively estimate the thyrotropic (i.e., thyroid-stimulating) function of the anterior pituitary lobe. The equation was derived from the logarithmic standard model of thyroid homeostasis. Recent research has demonstrated that the JTI is inversely correlated with the SPINA-GT and thyroid volume.26 In the present study, the JTI improved significantly after treatment. This suggests that the SPINA-GT level in acute exacerbation of bronchial asthma is acceptable and that the JTI returns to normal27 as the condition improves.
SPINA-GD represents the maximum amount of T3 produced per time unit under conditions of substrate saturation.28 It is assumed to reflect the activity of deiodinases outside the central nervous system and other isolated compartments. Recent research has revealed that total deiodinase activity is higher in patients with hypothyroidism as long as thyroid tissue is still present. This effect may ensue from the existence of an effective TSH–deiodinase axis or TSH–T3 shunt. After total thyroidectomy or high-dose radioiodine therapy (e.g., in treated thyroid cancer) as well as after initiation of substitution therapy with levothyroxine, the activity of step-up deiodinases decreases and the correlation of SPINA-GD with the TSH concentration is lost. SPINA-GD is also reduced in patients with some chronic diseases, such as euthyroid sick syndrome. The present results showed that SPINA-GD improved significantly after treatment. After treatment, SPINA-GD was lower than that of normal patients. The index was still within the normal range.29 SPINA-GD is closely correlated with pulmonary function. (Figures 1–4)
Figure 1.
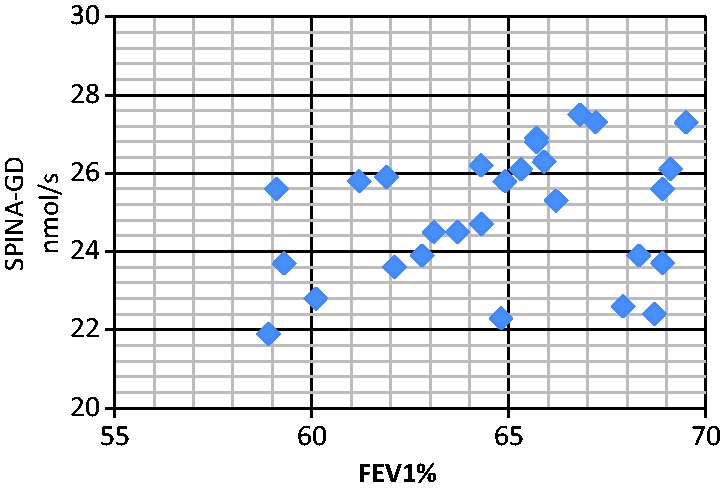
Correlation analyses between serum SPINA-GD level and FEV1 (RF1). The correlation between SPINA-GD and FEV1 was r = 0.284 (RF1 group), P < 0.05. SPINA-GD, sum activity of peripheral deiodinases; FEV1, forced expiratory volume in 1 s; RF1, type I respiratory failure.
Figure 2.
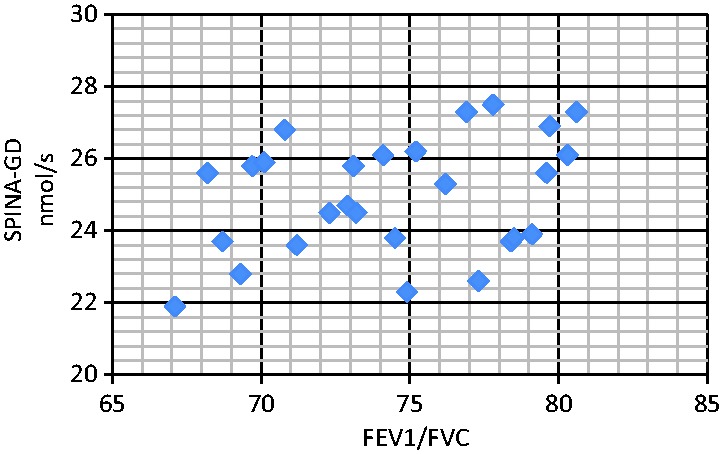
Correlation analyses between serum SPINA-GD level and FEV1/FVC (RF1). The correlation between SPINA-GD and FEV1/FVC was r = 0.164 (RF1 group), P < 0.05. SPINA-GD, sum activity of peripheral deiodinases; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RF1, type I respiratory failure.
Figure 3.
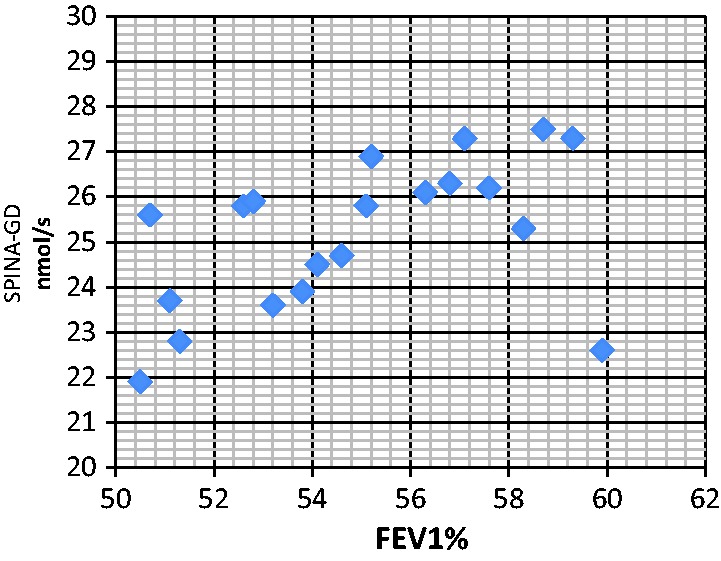
Correlation analyses between serum SPINA-GD level and FEV1 (RF2). The correlation between SPINA-GD and FEV1 was r = 0.491 (RF2 group), P < 0.05. SPINA-GD, sum activity of peripheral deiodinases; FEV1, forced expiratory volume in 1 s; RF2, type II respiratory failure.
Figure 4.
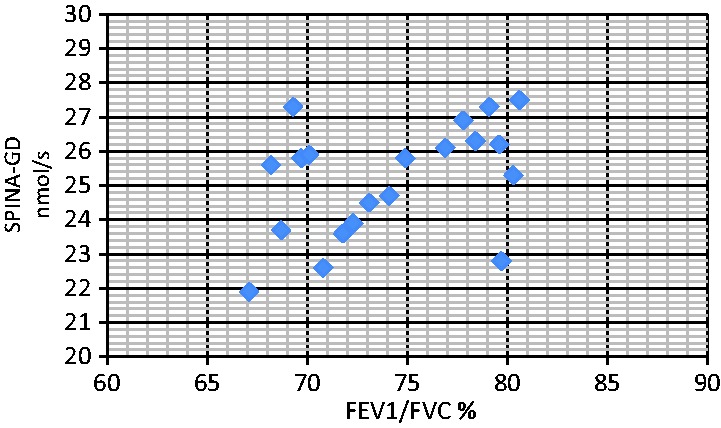
Correlation analyses between serum SPINA-GD level and FEV1/FVC (RF2). The correlation between SPINA-GD and FEV1/FVC was r = 0.421 (RF2 group), P < 0.05. There was no correlation between SPINA-GD and FEV1 in the NRF group or in the normal control group. There was also no correlation between SPINA-GD and FEV1/FVC in the NRF group or in the normal control group. SPINA-GD, sum activity of peripheral deiodinases; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RF2, type II respiratory failure; NRF, no respiratory failure.
SPINA-GT is elevated in primary hyperthyroidism and reduced in both primary hypothyroidism30–32 and untreated autoimmune thyroiditis.23 It has been observed to correlate with resting energy expenditure33 and thyroid volume.29 In a longitudinal evaluation, SPINA-GT showed lower intra-individual variation (i.e., higher reliability) than TSH, FT4, or FT3.34 Elevated SPINA-GT in patients with Graves’ disease is reversible with antithyroid treatment. In the present study, SPINA-GT improved significantly after treatment. After treatment, SPINA-GT was lower than that of normal patients. The index was still within the normal range.29 These results indicate that hypoxia during an acute attack of bronchial asthma leads to a decrease in enzymatic activity.
The use of thyroid hormones in the treatment of bronchial asthma is controversial.35 Changes in thyroid hormone levels only reflect the course of bronchial asthma in the process of systemic pathophysiological changes in parts of the body in an attempt to provide physical protection from the disease. The body additionally maintains a low metabolism level to reduce oxygen consumption and energy metabolism. The process described above can adapt in response to the environment, which is conducive to mitigation and recovery. Therefore, supplementing thyroid hormones does not improve the overall disease process but may instead damage the gradual formation of a relatively stable state.
In summary, thyroid hormone levels should be monitored during acute exacerbations of asthma in patients of advanced age to provide information related to disease progression and improvement. Monitoring the levels of thyroid hormones and performing timely assessments of the patient’s condition are helpful for assessing disease severity and forecasting the prognosis. Relatively few participants were included in the present study, all participants were male, and both the patients and controls were smokers. Future research should focus on female patients and nonsmokers.
Conclusion
A close relationship exists between thyroid hormone levels and the severity of asthma in older adults. Hypoxia may trigger mechanisms for saving oxygen, which downregulates step-up deiodinase activity, and the retention of carbon dioxide markedly reduces thyroid hormone levels. Therefore, it is more probable that hypoxia leads to type 1 thyroid allostasis rather than that hypothyroidism impairs pulmonary function. The FT3 level plays an important role in assessing the severity of asthma in patients of advanced age. The correlation between FT3 and pulmonary function is likely to be caused by changes in thyroid enzymology.
Abbreviations
T4, thyroxine; T3, triiodothyronine; FT4, free thyroxine; FT3, free triiodothyronine; TT4, total thyroxine; TT3, total triiodothyronine; TSH, thyroid-simulating hormone; RF1, type I respiratory failure; RF2, type II respiratory failure; NRF, no respiratory failure; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; SPINA-GD, sum activity of peripheral deiodinases; SPINA-GT, thyroid’s secretory capacity; JTI, Jostel’s thyroid-stimulating hormone index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TBG, thyroid-binding globulin.
Acknowledgements
The authors kindly thank all patients who participated in the study.
Authors’ contributions
Zhan Bingyan generated the hypothesis and acquired and analyzed the data. Wei Dong provided statistical support and assistance in the interpretation of the results. Both authors drafted, reviewed, and gave final approval of the manuscript as submitted.
Availability of data and materials
Anonymized data from the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by the president of the Municipal Hospital.
References
- 1.Yáñez A, Cho SH, Soriano JB, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J 2014; 7: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benfante A, Scichilone N. The geriatric asthma: pitfalls and challenges. Asthma Res Pract 2016; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manzolli S, Macedo-Soares MF, Vianna EO, et al. Allergic airway inflammation in hypothyroid rats. J Allergy Clin Immunol 1999; 104: 595–600. [DOI] [PubMed] [Google Scholar]
- 4.Duan QL, Du R, Lasky-Su J, et al. A polymorphism in the thyroid hormone receptor gene is associated with bronchodilator response in asthmatics. Pharmacogenomics J 2013; 13: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2017. http://www.ginasthma.org/.
- 6.Frączek MM, Gackowski A, Przybylik-Mazurek E, et al. The relation between the low T3 syndrome in the clinical course of myocardial infarction and heart failure. Polski Merkuriusz Lekarski Jourl 2016; 40: 380–383. [PubMed] [Google Scholar]
- 7.Hifumi T, Okada I, Kiriu N, et al. Thyroid hormone alterations in trauma patients requiring massive transfusion: an observational study. World J Emerg Med 2014; 5: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Stretton TB. Asthma in the elderly. Curr Geriatr Rep 2015; 1: 110. [Google Scholar]
- 9.Hopp RJ, Bewtra A, Nair NM, et al. The effect of age on methacholine response. J Allergy Clin Immunol 1985; 76: 609–613. [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004; 113: 101–108. [DOI] [PubMed] [Google Scholar]
- 11.Sorino C, Battaglia S, Scichilone N, et al. Diagnosis of airway obstruction in the elderly: contribution of the SARA study. Int J Chron Obstruct Pulmon Dis 2012; 7: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porsbjerg C, Lange P, Ulrik CS. Lung function impairment increases with age of diagnosis in adult onset asthma. Respir Med 2015; 109: 821–827. [DOI] [PubMed] [Google Scholar]
- 13.Tommola M, Ilmarinen P, Tuomisto LE, et al. The effect of smoking on lung function: a clinical study of adult-onset asthma. Eur Respir J 2016; 48: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 14.Park HW, Song WJ, Kim SH, et al. Classifcation and implementation of asthma phenotypes in elderly patients. Ann Allergy Asthma Immunol 2015; 114: 18–22. [DOI] [PubMed] [Google Scholar]
- 15.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax 2012; 67: 625–631. [DOI] [PubMed] [Google Scholar]
- 16.Chatzitomaris A, Hoermann R, Midgley JE, et al. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol (Lausanne) 2017; 8: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przybyłowski J, Piestrak J, Kowalski D. Triiodothyronine (T3) and thyroxine (T4) levels in patients with bronchial asthma. Wiadomości Lekarskie 1989; 42: 20–24. [PubMed] [Google Scholar]
- 18.Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am 2007; 36: 657. [DOI] [PubMed] [Google Scholar]
- 19.Bertoli A, Valentini A, Cianfarani MA, et al. Low FT3: a possible marker of frailty in the elderly. Clin Interv Aging 2017; 12: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism 2007; 56: 239–244. [DOI] [PubMed] [Google Scholar]
- 21.Bello G, Pennisi MA, Montini L, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest 2009; 135: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 22.De Groot LJ. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit Care Clin 2006; 22: 57–86. [DOI] [PubMed] [Google Scholar]
- 23.El Shabrawy RM, Atta AH, Rashad NM, et al. Serum anti-TPO and TPO gene polymorphism as a predictive factor for hidden autoimmune thyroiditis in patient with bronchial asthma and allergic rhinitis. Egypt J Immunol 2016; 23: 77–86. [PubMed] [Google Scholar]
- 24.Jerez FR, Plaza V, Tarrasa J, et al. Thyroid function and difficult to manage asthma. Arch Bronconeumol 1998; 34: 429–432. [DOI] [PubMed] [Google Scholar]
- 25.Arai H, Goto R, Matsuda T, et al. Relationship between free T4 levels and postnatal steroid therapy in preterm infants. Pediatr Int 2009; 51: 800–803. [DOI] [PubMed] [Google Scholar]
- 26.Hoermann R, Midgley JEM, Larisch R, et al. The role of functional thyroid capacity in pituitary thyroid feedback regulation. Eur J Clin Invest 2018; 48: e13003. [DOI] [PubMed] [Google Scholar]
- 27.Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition und evaluation of the TSH Index. Clin Endocrinol (Oxf) 2009; 71: 529–534. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich JW, Landgrafe-Mende G, Wiora E, et al. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol (Lausanne) 2016; 7: 57. doi:10.3389/fendo.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich JW. Der hypophysen-schilddrüsen-regelkreis. Berlin, Germany: Logos-Verlag Berlin, 2002. [Google Scholar]
- 30.Dietrich J, Fischer M, Jauch J, et al. SPINA-THYR: a novel systems theoretic approach to determine the secretion capacity of the thyroid gland. Eur J Intern Med 1999; 10: S34. [Google Scholar]
- 31.Dietrich JW. Thyroid storm. Med Klin Intensivmed Notfmed 2012; 107: 448–453. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Liu H, Chen J, et al. Metabolic characteristics in obese patients complicated by mild thyroid hormone deficiency. Horm Metab Res 2016; 48: 331–337. [DOI] [PubMed] [Google Scholar]
- 33.Kim MJ, Cho SW, Choi S, et al. Changes in body compositions and basal metabolic rates during treatment of Graves' disease. Int J Endocrinol 2018; 2018: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyroid Res 2012; 2012: 351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartalena L. The dilemma of non-thyroidal illness syndrome: to treat or not to treat? J Endocrinol Invest 2003; 26: 1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the current study are available from the corresponding author on reasonable request.


