Short abstract
Objectives
To explore the efficacy of three-dimensional printing physical model-assisted laparoscopic partial nephrectomy (3D-LPN) in patients with renal tumors.
Methods
We retrospectively assessed all patients who underwent LPN with or without 3D-printed physical model assistance from January 2016 to February 2018 at our institution. The demographic characteristics, operative findings, and clinical outcomes from the procedure were collected and analyzed.
Results
Sixty-nine patients underwent 3D-LPN and 58 underwent traditional LPN. The groups showed no differences in demographics, RENAL score, surgical approach, operative time, estimated intra-/postoperative blood loss, increased creatinine level, or complications. In the 3D-LPN group, warm ischemia time was shorter, whereas surgery waiting time was longer, compared with those parameters in the LPN group. Subgroup analysis indicated that for patients with RENAL score ≥8, the 3D-LPN group had significantly shorter warm ischemic time and less intraoperative blood loss than the traditional LPN group. Intra- and postoperative hospital complication rates were similar for 3D-LPN and traditional LPN groups (8.7% vs. 13.7%).
Conclusions
3D printing provides an additional tool to assist with LPN. Use of a 3D model can assist in planning and performance of LPN in patients with RENAL score ≥8.
Keywords: Three-dimensional printing, laparoscopic partial nephrectomy, renal cancer, warm ischemia time, surgical blood loss, kidney neoplasms, surgical time
Introduction
Three-dimensional (3D) printing is widely used in clinical practice for the production of physical models based on patient imaging data.1–3 Thus far, 3D printing has been used primarily for patient education during practical planning and surgical training in management of renal cancer.1,4 In addition, urologic research has indicated that 3D printing is a reliable tool for planning of partial nephrectomy and intraoperative navigation because it can reveal the real size, depth, and location of both the kidney mass and arteriovenous systems, and may thus prevent damage to the surrounding structures.5,6 In the past 2 years, we have explored the feasibility of 3D printing-assisted laparoscopic partial nephrectomy (3D-LPN). To quantitatively assess the feasibility and utility of 3D-LPN, we retrospectively collected clinical data and outcomes to compare the clinical effectiveness of 3D- LPN with traditional LPN.
Methods
Population
We performed this retrospective analysis of patients who underwent either 3D-LPN or traditional LPN in our center between January 2016 and February 2018. Patient data were collected from the date of the surgery until the end of this study (28 February 2018). Based on their imaging results, all patients had been diagnosed with T1-2bN0M0 renal cancer, using the UICC TNM classification of malignant tumors.7 Patients were informed of the benefits and risks of surgery, as well as the benefits and risks of both surgical methods. The surgical method was determined by each patient, prior to treatment. We excluded patients who underwent LPN for renal tuberculosis or cystonephrosis, as well as those with solitary kidneys. We did not assess long-term prognosis due to the short follow-up period for some patients. This study was approved by the Ethics Committee Board of Hunan Cancer Hospital (No. 2016[05]). All methods were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from each participant prior to enrollment in the study.
Procedure
Prior to surgery, individualized 3D-printed physical anatomic kidney models were created as described previously.6,8 In brief, the patients’ enhanced computed tomography (CT) images were obtained (Figure 1). All imaging data were entered in the Mimics 18.0 system (Materialise, Leuven, Belgium) for model reconstruction. The tumors, arteries, veins, and pelvis were represented by different colors in the 3D-printed physical models (Figures 2 and 3a, b).
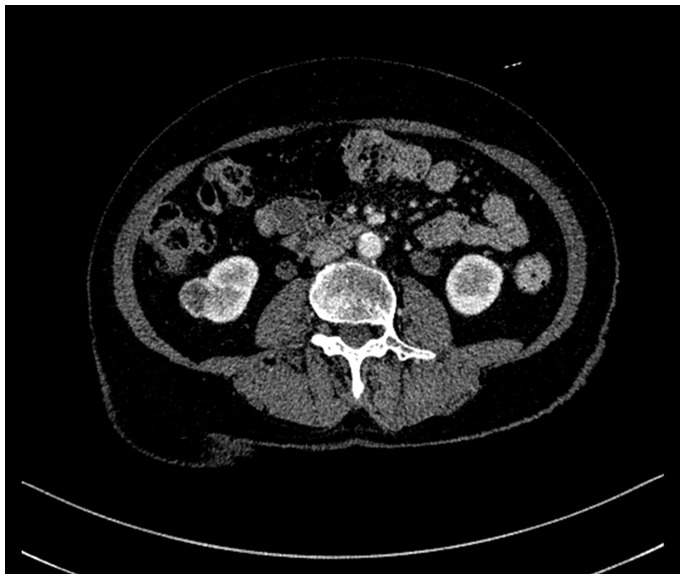
Figure 1.
Traditional two-dimensional computed tomography image of the kidney, used in lateral partial nephrectomy.
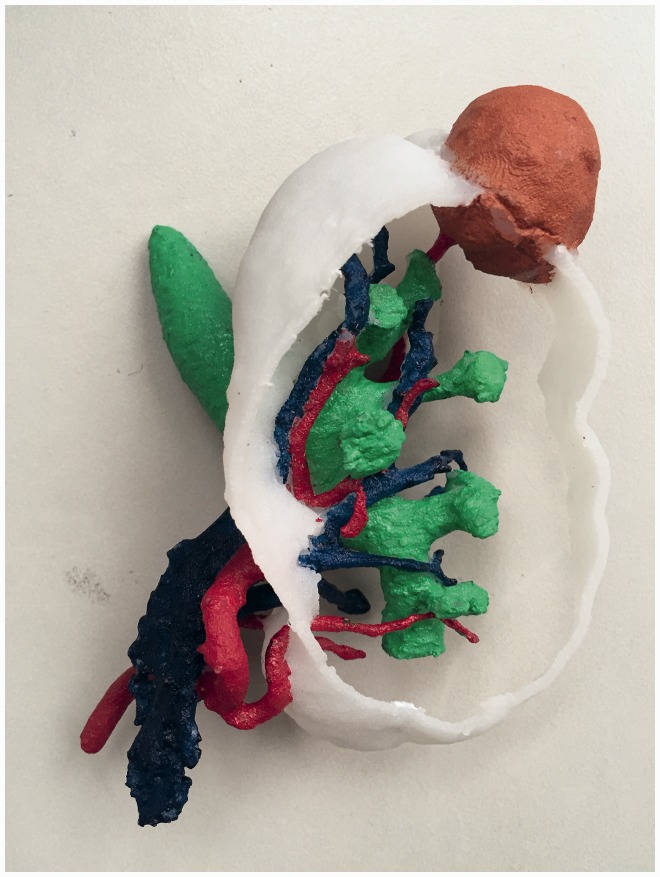
Figure 2.
Three-dimensional (3D)-printed physical anatomic kidney model. Compared with two-dimensional computed tomography images, the 3D-printed physical anatomic model clearly demonstrates the locations and relationships among the kidney mass, segmental arteries of the kidney, and renal pelvis.
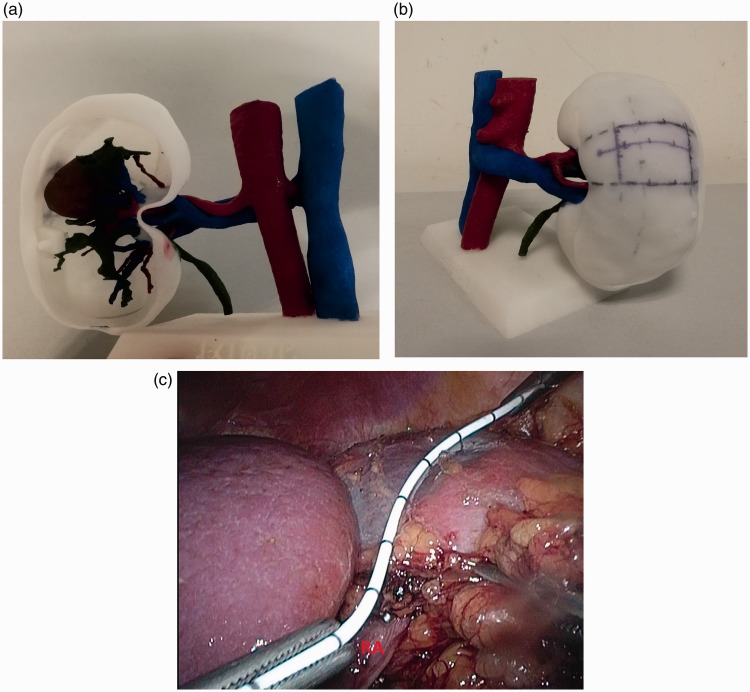
Figure 3.
Three-dimensional (3D)-printed physical anatomic models used for surgical planning and navigation. a, b: 3D-printed physical anatomic models used to identify the location of the kidney tumor during surgical planning. c: 3D-printed physical anatomic model used during intraoperative navigation to facilitate rapid and accurate establishment of tumor position and assist with exposure of the tumor area.
All patients underwent LPN. All operations were performed by the same surgeon, who had carried out >200 LPN procedures. Traditional LPN was carried out with two-dimensional (2D) CT images, as described previously.9,10 The individualized 3D-printed physical anatomic kidney models were used in preoperative surgical planning and intraoperative navigation, as described previously.6 Briefly, the preoperative surgical planning and intraoperative navigation procedures were performed as follows:
The individualized 3D-printed physical anatomic kidney models were used in preoperative surgical planning. Before the surgery, a urologist surgeon and multiple radiologists checked the tumor-specific kidney model reliability through CT images to reduce deviation between the model and the patient’s actual anatomy. Subsequently, the same surgeon and same group of radiologists discussed the complexity and feasibility of laparoscopic partial nephrectomy and potential complications in relation to the arteriovenous distribution, collecting system, and tumor characteristics. The surgeon and the surgical assistants determined the design of the operations based on the individualized 3D kidney models.
The individualized 3D-printed physical anatomic kidney models were used for intraoperative navigation by mapping the location to guide target dissociation (Figure 3a, b). The surgical assistant aligned the models with the laparoscopic screen image (Figure 3c) to aid the surgeon in rapid and accurate location of the tumor.
Data
Based on each patient’s 3D-printed physical anatomic kidney model, all kidney tumors were evaluated and classified in accordance with the RENAL nephrometry scoring system, as described by Kutikov.11 We stratified patients based on RENAL score indicative of tumor complexity (<8 and ≥8) because of sample limitations.12,13 Postoperative complications were categorized using the Clavien–Dindo Classification of Surgical Complications.14 We clinically assessed several parameters,9,15 including operative time (OT), warm ischemia time (WIT), estimated intraoperative blood loss (EIBL), estimated postoperative blood loss (EPBL), increased creatinine level (ICL), intra- and postoperative hospital complications (Clavien–Dindo classification ≥Grade II), and surgery waiting times. We compared 3D tumor model sizes with resected tumors to assess the reliabilities of the 3D-printed physical anatomic kidney models.
Statistical analysis
The demographic data are presented as numbers and percentages or as means, ranges, and standard deviations for each characteristic. Clinical data and outcomes were compared between groups with Fisher’s exact test or Student’s t-test. Two-sided P values were used, and differences were considered to be statistically significant when P < 0.05. All statistical analyses were conducted with SPSS software (version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
A total of 127 patients were eligible for enrollment in this study. Of these, 69 underwent 3D-LPN, and 58 underwent traditional LPN. The patients’ clinical characteristics are described in Table 1. The two groups did not differ in terms of age, sex, body mass index, American Society of Anesthesiologists status, tumor size, RENAL score, or surgical approach. The perioperative results showed that the mean WITs in the 3D-LPN group and LPN group were 24.1 ± 5.1 and 26.6 ± 4.2 minutes, respectively; WIT was significantly shorter in the 3D-LPN group (P < 0.05). Neither of the surgical methods was significantly associated with OT, EIBL, EPBL, or ICL (Table 2). The mean surgery waiting time in the 3D-LPN group was 13.6 ± 3.4 days, whereas it was 7.0 ± 0.6 days in the LPN group (P < 0.05; Table 2); patients in the 3D-LPN group had a significantly longer waiting time than patients in the LPN group, due to the added time for completion of 3D-printed physical anatomic kidney model printing prior to surgery. In terms of reliability, the mean size deviation of 3D-printed physical anatomic kidney models from resected tumors was 0.28 ± 0.30 cm (range: 0–0.60 cm). Postoperative pathology examinations showed that all resected mass margins were tumor-free in both groups.
Table 1.
Baseline demographic and clinical characteristics.
| Patient demographics | 3D-LPN group (n = 69) | LPN group (n = 58) | P value |
|---|---|---|---|
| Age (years), mean (±SD, range) | 48.0 (±11.9, 24–75) | 50.0 (±11.4, 26–70) | >0.05 |
| Sex (male/female), n | 38/31 | 26/32 | >0.05 |
| BMI (kg/m2) mean (±SD, range) | 23.7 (±3.0, 17.7–32.0) | 23.2 (±4.4, 17.8–34.9) | >0.05 |
| ASA status (Grade I/II/III), n | 44/20/5 | 34/21/3 | >0.05 |
| Tumor size (cm), mean (±SD, range) | 4.0 (±2.6, 1.5–11.0) | 3.9 (±1.4, 1.2–6.7) | >0.05 |
| Left/Right, n | 35/34 | 25/33 | >0.05 |
| RENAL score (<8/≥8), n | 33/36 | 39/19 | >0.05 |
| Surgical approach (retro/trans-peritoneal), n | 41/28 | 37/21 | >0.05 |
LPN, laparoscopic partial nephrectomy; SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists.
Table 2.
Operative parameters and clinical outcomes.
| Patient Demographics | 3D-LPN Group (n = 69) | LPN Group (n = 58) | P value |
|---|---|---|---|
| OT (minutes), mean ± SD) | 169.7 (±56.4) | 159.2 (±39.6) | >0.05 |
| WIT (minutes), mean (±SD) | 24.1 (±5.1) | 26.6 (±4.2) | <0.05* |
| EIBL (mL), mean (±SD) | 118.7 (±83.6) | 131.1 (±80.2) | >0.05 |
| EPBL (mL), mean (±SD) | 162.6 (±155.6) | 187.1 (±143.9) | >0.05 |
| ICL (mg/dL), mean (±SD) | 13.9 (±3.0) | 23.2 (±5.7) | >0.05 |
| Positive margins, n (%) | 0 (0) | 0 (0) | |
| Histology of primary lesion, n | |||
| Clear cell | 48 | 45 | |
| Papillary | 15 | 8 | |
| Chromophobe cell | 4 | 2 | |
| Others | 2 | 3 | |
| Complications, n (%) | 6 (8.7) | 8 (13.8) | >0.05 |
| Surgery waiting times (days), mean (±SD) | 13.6 (±3.4) | 7.0 (±0.6) | <0.05* |
*Statistically significant difference.
LPN, laparoscopic partial nephrectomy; OT, operative time; SD, standard deviation; WIT, warm ischemia time; EIBL, estimated intraoperative blood loss; EPBL, estimated postoperative blood loss; ICL, increased creatinine level.
The combined intra- and postoperative hospital complication rate in the 3D-LPN group was 8.7%, including 4.3% (3 cases) of postoperative complications and 4.3% (3 cases) of change in surgical modality. In the LPN group, the combined complication rate was 13.8%, including 8.6% (5 cases) of postoperative complications and 5.2% (3 cases) of change in surgical modality. The symptoms involving these complications were mild, with a Clavien–Dindo Grade of II, and were treated via pharmacotherapy (Table 2).
In this study, we used the RENAL scoring system to evaluate the complexity of the surgery. Thirty-three patients in the 3D-LPN group and 39 patients in the LPN group had RENAL score < 8, while 36 patients in the 3D-LPN group and 19 patients in the LPN group had RENAL score ≥8. Among patients with RENAL score ≥8, WIT and EIBL significantly differed between the 3D-LPN and LPN groups (P < 0.05); however, there were no significant differences between the 3D-LPN and LPN groups in OT, EIBL, EPNL, or ICL. Among patients with RENAL score < 8, the 3D-LPN and LPN groups showed no significant differences in any of the assessed clinical parameters (Table 3).
Table 3.
Operative parameters and clinical outcomes according to RENAL scores.
| Variable |
RENAL score |
|||||
|---|---|---|---|---|---|---|
|
<8 |
≥ 8 |
|||||
| 3D-LPN group (n = 33) | LPN Group (n = 39) | P value | 3D-LPN group (n = 36) | LPN group (n = 19) | P value | |
| OT (minutes), mean (±SD) | 147.0 (±39.6) | 154.6 (±33.2) | >0.05 | 190.5 (±61.7) | 168.4 (±49.9) | >0.05 |
| WIT (minutes), mean (±SD) | 23.4 (±5.0) | 25.4 (±4.4) | >0.05 | 24.6 (±4.3) | 29.2 (±2.3) | <0.05* |
| EIBL (mL), mean (±SD) | 102.7 (±87.5) | 107.7 (±72.1) | >0.05 | 131.9 (±78.5) | 179.2 (±76.1) | <0.05* |
| EPBL (mL), mean (±SD) | 142.0 (±88.6) | 153.9 (±150.9) | >0.05 | 191.4 (±193.6) | 214.0 (±122.1) | >0.05 |
| ICL (mg/dL), mean (±SD) | 18.9 (±13.6) | 28.8 (±21.0) | >0.05 | 22.2 (±20.2) | 19.4 (±21.1) | >0.05 |
| Complications, n (%) | 3 (9.1) | 3 (7.7) | >0.05 | 3 (8.3) | 5 (26.3) | >0.05 |
*Statistically significant difference.
LPN, laparoscopic partial nephrectomy; OT, operative time; SD, standard deviation; WIT, warm ischemia time; EIBL, estimated intraoperative blood loss; EPBL, estimated postoperative blood loss; ICL, increased creatinine level.
Discussion
We recently showed the feasibility of a 3D-printed physical anatomic kidney model for surgical planning and navigation in laparoscopic partial nephrectomy.6 A 3D-printed physical anatomic model, which reveals the spatial characteristics of kidney masses, can facilitate the understanding of kidney characteristics and assist in preoperative preparation and intraoperative navigation. To evaluate the effectiveness of using a 3D-printed physical anatomic model in LPN, we compared operative parameters and complication rates between 3D-LPN and traditional LPN (assisted by CT alone). We included 69 patients who underwent 3D-LPN and 58 patients who underwent traditional LPN. We demonstrated that the use of a 3D-printed physical anatomic kidney model for surgical planning and navigation could shorten surgical WIT and reduce EIBL for patients with RENAL score ≥8. Compared with traditional 2D radiographic imaging, a 3D-printed physical anatomic model is intuitive, capable of 3D representation, and easy to understand; therefore, it can aid in preoperative planning and intraoperative navigation.
During the past decade, 3D-printed physical anatomic models have been extensively used in anatomy education, surgical training, preoperative planning, and intraoperative navigation.16–19 Regarding renal cancer, the most detailed studies thus far have explored the roles of 3D-printed physical anatomic models in surgical training and education. Research regarding 3D-printed physical anatomic models has shown that they improve understanding and characterization of kidney anatomy in both patients and medical students. Importantly, 3D printing techniques bridge the gap between imaging and actual anatomy, providing a more tangible method for evaluation and comprehension of pathology.1,20,21 Because of their high precision, 3D-printed physical anatomic models have also been used in the planning, simulation, and navigation of LPN. Shin et al.5 used a 3D-printed physical anatomic model to aid in partial nephrectomy in 10 patients with RENAL score ≥8, indicative of a complex renal tumor; all patients had acceptable clinical outcomes and showed good comprehension of the operation. Using a 3D-printed physical anatomic model in pre-surgical simulation of robotic-assisted LPN, von Rundstedt et al.22 demonstrated that the simulation platform could assist in surgical planning and improve surgical education. Our current study on 3D-printed physical anatomic models was focused on the effectiveness for planning and navigation in LPN. We found that 3D-printed physical anatomic models could help the surgeon fully understand the spatial structure of the individual kidney, assist in rapid and accurate location of the tumor site, reveal structures surrounding the kidney tumor, and assist in tumor area exposure. We evaluated the operations by using the RENAL system, which comprises risk stratification for partial nephrectomy based on tumor characteristics. The resection of highly complex tumors is considered a great challenge, as it often results in increased OT, WIT, and postoperative complication rates.23,24 Our results showed that 3D-LPN shortened surgical WIT and reduced EIBL in kidney tumors.
In this study, we evaluated mean model size deviation by comparing the diameters of tumor models and resected tumors. Previous studies noted that the CT scans must have 3- to 5-mm step intervals to minimize the discrepancies between the generated physical model and the patient’s actual anatomy.2,25 In our study, the slice thickness in the CT scans was fixed at 0.75 mm, and the mean size deviation of all 3D models was 0.28 ± 0.30 cm. Moreover, compared with conventional 2D radiographic images, 3D-printed physical anatomic models are intuitive, capable of 3D representation, and easy to understand; therefore, they can aid surgeons in preoperative planning and intraoperative navigation. 3D-printed physical anatomic models serve as additional tools for assisted partial nephrectomy, achieving high concordance with patient anatomy at a comparably low cost. In this study, the fabrication cost was 3200 RMB (approximately 500 USD) per patient. This cost will likely decrease with improvements in technology and materials.
The main limitation of the current study was its small sample size. In addition, we only evaluated intra- and postoperative hospital complications for a short follow-up period. Finally, this was a retrospective and unmatched study, and thus the results may have a degree of bias; however, to the best of our knowledge, this comprises the largest study of the use of a 3D-printed physical anatomic model in urologic research, as well as the first study to compare 3D-LPN and traditional LPN.
Conclusions
Despite its limitations, our study showed that the use of a 3D-printed physical anatomic model aided surgeons in understanding kidney and tumor anatomy. The use of a 3D-printed physical anatomic model may support assisted planning and performance of LPN in patients with RENAL scores ≥8, indicative of a complex renal tumor. Furthermore, 3D-printed physical anatomic models have a comparably low cost and are easy to disseminate among the surgical community.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the Health and Family Planning Commission of Hunan Province (grant number: A2016002), and the Sciences Research Council of Changsha (grant number: K91701043), and the Sciences Research Council of Hunan (grant number: 2018SK2125).
References
- 1.Bernhard JC, Isotani S, Matsugasumi T, et al. Personalized 3D printed model of kidney and tumor anatomy: a useful tool for patient education. World J Urol 2016; 34: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010; 5: 335–341. [DOI] [PubMed] [Google Scholar]
- 3.Wake N, Rude T, Kang SK, et al. 3D printed renal cancer models derived from MRI data: application in pre-surgical planning. Abdom Radiol (NY) 2017; 42: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddox MM, Feibus A, Liu J, et al. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robot Surg 2018; 12: 27–33. [DOI] [PubMed] [Google Scholar]
- 5.Komai Y, Sugimoto M, Gotohda N, et al. Patient-specific 3-dimensional printed kidney designed for “4D” surgical navigation: a novel aid to facilitate minimally invasive off-clamp partial nephrectomy in complex tumor cases. Urology 2016; 91: 226–233. [DOI] [PubMed] [Google Scholar]
- 6.Fan G, Li J, Li M, et al. Three-dimensional physical model-assisted planning and navigation for laparoscopic partial nephrectomy in patients with endophytic renal tumors. Sci Rep 2018; 8: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak J, Fabian P. [Comments on the TNM classification of malignant tumours–7th edition]. Klin Onkol 2011; 24: 149–150. [PubMed] [Google Scholar]
- 8.Sodian R, Weber S, Markert M, et al. Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg 2008; 136: 1098–1099. [DOI] [PubMed] [Google Scholar]
- 9.Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol 2009; 182: 866–872. [DOI] [PubMed] [Google Scholar]
- 10.Gerber GS, Stockton BR. Laparoscopic partial nephrectomy. J Endourol 2005; 19: 21–24. [DOI] [PubMed] [Google Scholar]
- 11.Kutikov A, Uzzo RG. The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009; 182: 844–853. [DOI] [PubMed] [Google Scholar]
- 12.Stroup SP, Palazzi K, Kopp RP, et al. RENAL nephrometry score is associated with operative approach for partial nephrectomy and urine leak. Urology 2012; 80: 151–156. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Qi L, Yuan P, et al. Application of three-dimensional visualization technology in laparoscopic partial nephrectomy of renal tumor: a comparative study. J Laparoendosc Adv S 2017; 27: 516–523. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kural AR, Atug F, Tufek I, et al. Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy: comparison of outcomes. J Endourol 2009; 23: 1491–1497. [DOI] [PubMed] [Google Scholar]
- 16.Zheng JP, Li CZ, Chen GQ, et al. Three-dimensional printed skull base simulation for transnasal endoscopic surgical training. World Neurosurg 2018; 111: e773–e782. [DOI] [PubMed] [Google Scholar]
- 17.Hochman JB, Rhodes C, Wong D, et al. Comparison of cadaveric and isomorphic three-dimensional printed models in temporal bone education. Laryngoscope 2015; 125: 2353–2357. [DOI] [PubMed] [Google Scholar]
- 18.Mogali SR, Yeong WY, Tan HKJ, et al. Evaluation by medical students of the educational value of multi-material and multi-colored three-dimensional printed models of the upper limb for anatomical education. Anat Sci Educ 2018; 11: 54–64. [DOI] [PubMed] [Google Scholar]
- 19.Valverde I, Gomez-Ciriza G, Hussain T, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg 2017; 52: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 20.Silberstein JL, Thomas R. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study reply. Urology 2014; 84: 273. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Ge HW, Li NC, et al. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol 2016; 34: 533–537. [DOI] [PubMed] [Google Scholar]
- 22.von Rundstedt FC, Scovell JM, Agrawal S, et al. Utility of patient-specific silicone renal models for planning and rehearsal of complex tumour resections prior to robot-assisted laparoscopic partial nephrectomy. BJU Int 2017; 119: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmit GD, Thompson RH, Kurup AN, et al. Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol 2013; 189: 30–35. [DOI] [PubMed] [Google Scholar]
- 24.Minervini A, Siena G, Antonelli A, et al. Open versus laparoscopic partial nephrectomy for clinical T1a renal masses: a matched-pair comparison of 280 patients with TRIFECTA outcomes (RECORd Project). World J Urol 2014; 32: 257–263. [DOI] [PubMed] [Google Scholar]
- 25.Atalay HA, Ulker V, Alkan I, et al. Impact of three-dimensional printed pelvicaliceal system models on residents' understanding of pelvicaliceal system anatomy before percutaneous nephrolithotripsy surgery: a pilot study. J Endourol 2016; 30: 1132–1137. [DOI] [PubMed] [Google Scholar]