Short abstract
Objective
We aimed to compare the survival and perioperative outcomes of patients with stage II endometrial cancer (EC) undergoing simple hysterectomy (SH) or radical hysterectomy (RH), to validate the various guidelines.
Methods
A total of 155 consecutive patients diagnosed with stage II EC from 2000 to 2014 were reviewed. We identified 40 pairs of patients (40 SH and 40 RH) who were matched in terms of age, pathological type, and lymphovascular space invasion status using matched-pair analysis. Patient data were collected from medical records and outcomes were determined by telephone follow-up.
Results
Among the 80 patients in the two groups, seven died from tumor recurrence. However, cancer-related survival rates were not significantly different between the SH and RH groups. The 3-year cancer-related survival rates in the SH and RH groups were 94.97% and 92.53%, and the 5-year survival rates were 92.40% and 90.03%, respectively. Regarding perioperative outcomes, the SH group had significantly less intraoperative bleeding and a significantly shorter catheter-indwelling time than the RH group.
Conclusions
SH provides similar survival outcomes and a superior perioperative quality of life compared with RH in patients with stage II EC.
Keywords: Endometrial cancer, simple hysterectomy, radical hysterectomy, survival, quality of life, postoperative complication
Introduction
Endometrial cancer (EC) is the second most prevalent gynecological malignancy in China according to the National Central Cancer Registry of China, with a 5-year prevalence rate of 31.6 per 100,000 women.1 Current treatment strategies for EC are based on the International Federation of Gynecologists and Obstetricians (FIGO) staging system. The 1988 FIGO staging system defined stage II EC as EC with cervical mucosal and stromal involvement; however, the 2009 revised staging criteria indicated that stage II EC should only include patients with cervical stromal invasion.2 Radical hysterectomy (RH) with bilateral salpingo-oophorectomy (BSO) is recommended for patients with stage II EC on the basis of the 2014 European Society for Medical Oncology (EMSO) and National Comprehensive Cancer Network (NCCN) guidelines.3–5 However, recently published NCCN guidelines for uterine neoplasms in 2018 recommend both simple hysterectomy (SH) and RH for operable patients with cervical involvement, together with BSO, cytology (peritoneal lavage), and lymph node dissection if indicated.6 In addition, the latest ESMO guidelines do not recommend RH for the management of stage II EC, and the recommendation is for EC up to grade B.7
RH refers to excision of the uterus with the parametrium (i.e., round, broad, cardinal, and uterosacral ligaments) and the upper one-third to one-half of the vagina. However, stage II EC does not invade the parametrium or vagina. Furthermore, RH is associated with a high postoperative incidence of sexual dysfunction, significant blood loss, bladder and bowel dysfunction, and fistula formation.8,9 Nevertheless, several clinical studies have demonstrated the benefits of RH compared with SH in patients with stage II EC, and showed that RH or modified RH might improve overall survival compared with SH.10–12 However, other studies have provided contradictory results, and showed that SH provided comparable survival outcomes to RH in patients with FIGO stage II EC.13–15 Therefore, despite changes in the guidelines, the need for more invasive surgical methods for stage II EC remains controversial.
We aimed to address this controversy and the validity of the changes in the guidelines by examining previous articles published over several decades, and by comparing the survival and perioperative outcomes of matched patients with stage II EC who underwent SH or RH at the Obstetrics and Gynecology Hospital of Fudan University.
Materials and methods
Study selection for literature review
We screened studies investigating survival outcomes of patients with stage II EC treated with SH or RH worldwide over several decades. We performed a MEDLINE search with the keywords simple hysterectomy, radical hysterectomy, stage II endometrial cancer, and survival. We also further searched the reference lists of relevant publications to identify missed studies.
Study participants
A total of 1171 patients were diagnosed with EC and operated on at the Obstetrics and Gynecology Hospital of Fudan University from 2000 to 2014. Patients with pathological stage II EC according to the FIGO 2009 guidelines were included in the study. Patients with only cervical glandular invasion and patients who were lost to follow-up were excluded. The remaining patients were divided into an RH and SH group, matched according to age, pathological type, and lymphovascular space invasion (LVSI) using a case-matching method. BSO, lymphadenectomy, omentum resection, and appendectomy were performed as appropriate. Patients were under observation or received subsequent therapy, including chemotherapy, radiotherapy, or endocrine therapy, if necessary. Demographic, surgical, pathological, and postoperative outcome data were extracted from the resident admission notes, discharge records, surgical records, pathology reports, progress notes, and nursing records in the medical archives of the Obstetrics and Gynecology Hospital of Fudan University. Information on patient outcomes was obtained by telephone follow-up at specified intervals. The study was approved by the ethics committee of the Obstetrics and Gynecology Hospital of Fudan University. All enrolled patients provided written informed consent.
Surgical methods
SH involved complete removal of the uterus and cervix, with or without BSO, and RH involved additional resection of the lateral parametrium and ventral parametrium. RH was further classified into four types according to the range of parametrial resection, based on criteria published in 2011.16 All types of RH, except type 4, met the study inclusion criteria.
Performance of lymphadenectomy associated with SH or RH, as well as omentum resection, was decided based on pre- and perioperative findings.
Statistical analysis
Categorical and numerical demographic, surgical, pathological, and postoperative variables in the SH and RH groups were analyzed and compared using χ2 and t-tests. P < 0.10 or P < 0.05 was considered to indicate statistical significance.
The main outcome was cancer-related survival, with death as an event. Survival was compared using the log-rank test and Kaplan–Meier analysis. Multivariate analysis was carried out by Cox proportional hazards regression.
Results
Previous studies of survival following SH and RH for stage II EC
A search of the MEDLINE database for studies of patients with stage II EC treated with SH or RH identified 11 relevant articles published from 1993 to 2017. Three articles were based on data from the Surveillance, Epidemiology, and End Result (SEER) database, and the other eight were retrospective studies from America, Italy, Turkey, and Japan. There was thus a lack of data from China in relation to survival after SH and RH among patients with stage II EC. All four studies conducted before 2011 showed that survival after RH was superior to that after SH, while all five studies conducted after 2009 showed similar survival after RH and SH (Table 1). These results suggest that the survival advantage of RH in patients with stage II EC gradually disappeared, and the procedure may have resulted in more serious perioperative complications. In addition, studies from America and Italy found that survival after RH was superior to that after SH, while studies from Turkey and Japan found similar survival after RH and SH (Table 1). The conclusions regarding the relative outcomes of RH and SH may thus be affected by both geographic region and study period.
Table 1.
Summary of previous articles comparing survival of patients with stage II endometrial cancer following SH or RH.
| Author | Publication year | Study period | Region | Sample size | Survival (SH vs RH) |
|---|---|---|---|---|---|
| Boente et al.26 | 1993 | 1972–1988 | USA | 202 | RH superior to SH(77% vs 86%)5 year-related OS |
| Cornelison et al.12 | 1999 | 1988–1994 | SEER database | 932 | RH superior to SH(84% vs 92.96%)5 year-related OS |
| Mariani et al.27 | 2001 | 1984–1993 | Italy | 82 | RH superior to SH(68% vs 76%)5 year-related OS |
| Sartori et al.10 | 2001 | 1980–1995 | Italy | 203 | RH superior to SH(79% vs 94%)5 year-related OS |
| Ayhan et al.28 | 2004 | 1982–2000 | Turkey | 48 | RH equal to SH(83% vs 90%)5 year-related OS |
| Cohn et al.11 | 2007 | 1982–2004 | USA | 162 | RH superior to SH(81% vs 88%)5 year-related OS |
| Wright et al.29 | 2009 | 1988–2004 | SEER database | 1577 | RH equal to SH(79% vs 82%)5 year-related OS |
| Miyamoto et al.30 | 2016 | 1990–2009 | Japan | 247 | RH equal to SH |
| Phelippeau and Koskas15 | 2016 | 1998–2012 | SEER database | 819 | RH equal to SH(88.7% vs 94.1%)3 year-related OS |
| Takano et al.13 | 2013 | 1995–2009 | Japan | 300 | RH equal to SH(84% vs 83.6%)5 year-related OS |
| Ozgul et al.14 | 2018 | 2002–2015 | Turkey | 250 | RH equal to SH(83% vs 89%)5 year-related OS |
SH: simple hysterectomy, RH: radical hysterectomy, OS: overall survival.
Patient characteristics
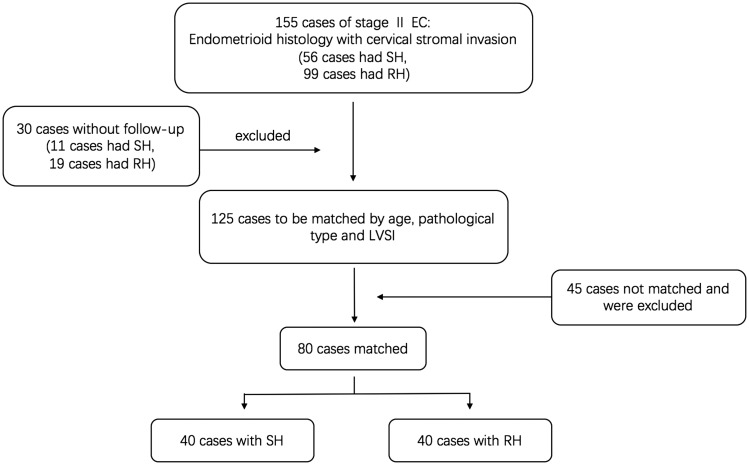
Among 1171 patients diagnosed with EC and operated on from 2000 to 2014, 155 women aged 23 to 77 years were diagnosed with stage II EC according to final pathology reports during the same period. Of these 155 patients, 56 had undergone SH and 99 had undergone RH. Sixteen of these 155 patients died of tumor recurrence but no patients died of other causes during the follow-up period. We excluded 30 patients who were lost to follow-up (11 SH and 19 RH). Following case-matching, 80 patients (40 SH and 40 RH) were therefore included in our final study (Figure 1). The demographic, surgical, and pathological characteristics of the patients are presented in Table 2.
Figure 1.
Flowchart of case selection in matched-pair analysis. A case-matched method was used to obtain two homogeneous groups according to age, pathological type, and LVSI. EC: endometrial cancer, SH: simple hysterectomy, RH: radical hysterectomy, LVSI: lymphovascular space invasion.
Table 2.
Patient demographic, surgical, and pathological characteristics.
| Simplehysterectomy | Radical hysterectomy | χ2P value | |
|---|---|---|---|
| Age (years) | 52.75 ± 9.32 | 52.80 ± 9.44 | 0.981 |
| Pathological type | |||
| Endometrioid adenocarcinoma | 30 | 29 | 0.800 |
| Other type* | 10 | 11 | |
| Grade (among endometrioid adenocarcinomas) | |||
| 1 | 18 | 9 | 0.072 |
| 2 | 10 | 15 | |
| 3 | 2 | 5 | |
| Tumor size according to final pathologic record | |||
| <2 cm | 16 | 8 | 0.137 |
| 2–5 cm | 17 | 21 | |
| >5 cm | 7 | 11 | |
| Myometrial invasion depth | |||
| <1/2 | 27 | 26 | 0.813 |
| ≥1/2 | 13 | 14 | |
| Cervical stromal invasion depth | |||
| <1/2 | 33 | 28 | 0.293 |
| ≥1/2 | 7 | 12 | |
| LVSI | |||
| No | 32 | 28 | 0.302 |
| Yes | 8 | 12 | |
| Hypertension | |||
| No | 31 | 33 | 0.576 |
| Yes | 9 | 7 | |
| Diabetes | |||
| No | 33 | 34 | 0.762 |
| Yes | 7 | 6 | |
| Surgical pathway | |||
| Laparotomy | 21 | 23 | 0.186 |
| Laparoscopic surgery | 19 | 17 | |
| Lymphadenectomy | |||
| No | 6 | 2 | 0.136 |
| Yes | 34 | 38 | |
| Omentum resection | |||
| No | 36 | 38 | 0.396 |
| Yes | 4 | 2 | |
| Postsurgical treatment | |||
| Observation | 16 | 16 | 0.506 |
| Chemotherapy | 17 | 17 | |
| Chemotherapy+radiotherapy | 7 | 5 | |
| Endocrine therapy | 0 | 2 |
*Serous carcinoma, mucinous adenocarcinoma, clear cell adenocarcinoma, and mixed cell adenocarcinoma. LVSI: lymphovascular space invasion.
The groups were comparable with regard to age, pathological type, pathological grade, tumor size, depth of myometrial invasion, depth of cervical stromal invasion, LVSI, hypertension, diabetes, surgical pathway, postoperative therapy, lymphadenectomy, and omentum resection.
Three patients (3/40, 7.5%) in the SH group had recurrence, including one in the pelvis, one in the liver, and one in the liver, pelvis, and intestines. Four patients (4/40, 10%) in the RH group had recurrence, including one in the vaginal cuff, two in the pelvis, and one with distant metastasis in the liver.
Survival of patients with stage II EC
The relationships between demographic, surgical, and pathological factors and overall survival were assessed by Kaplan–Meier analysis, which identified primary tumor pathological type (P = 0.069), LVSI (P = 0.063), surgical pathway (P = 0.059), and lymphadenectomy (P = 0.041) as significant predictors of overall survival (Table 3). These significant prognostic factors (P < 0.100) in the Kaplan–Meier analysis were then analyzed using a Cox proportional hazards model. However, after removing confounding factors, no factor had any significant effect on survival of patients with stage II EC (Table 4).
Table 3.
Prognostic indicators in patients with stage II endometrial cancer.
| Risk factor | Cases | Deaths | Cancer-related survival rate | χ2 | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| <60 | 58 | 5 | 91.4% | 0.005 | 0.945 |
| ≥60 | 22 | 2 | 90.9% | ||
| Grade (among endometrioid adenocarcinomas) | |||||
| 1 | 27 | 1 | 96.3% | 1.333 | 0.513 |
| 2 | 25 | 1 | 96.0% | ||
| 3 | 7 | 1 | 85.7% | ||
| Pathological type | |||||
| Endometrioid adenocarcinoma | 59 | 3 | 94.9% | 3.306 | 0.069 |
| Other* | 21 | 4 | 81.0% | ||
| Tumor size | |||||
| <2 cm | 24 | 2 | 91.7% | 2.436 | 0.296 |
| 2–5 cm | 38 | 5 | 86.8% | ||
| >5 cm | 18 | 0 | 100.0% | ||
| Myometrial invasion deep | |||||
| <1/2 | 53 | 3 | 94.3% | 1.715 | 0.190 |
| ≥1/2 | 27 | 4 | 85.2% | ||
| Cervical invasion deep | |||||
| <1/2 | 61 | 6 | 90.2% | 0.350 | 0.554 |
| ≥1/2 | 19 | 1 | 94.7% | ||
| LVSI | |||||
| Yes | 20 | 4 | 80.0% | 3.454 | 0.063 |
| No | 60 | 3 | 95.0% | ||
| Surgical method | |||||
| Simple hysterectomy | 40 | 3 | 92.5% | 0.090 | 0.764 |
| Radical hysterectomy | 40 | 4 | 90.0% | ||
| Surgical pathway | |||||
| Laparotomy | 44 | 6 | 86.4% | 3.564 | 0.059 |
| Laparoscopic | 36 | 1 | 97.2% | ||
| Lymphadenectomy | |||||
| Yes | 72 | 5 | 93.1% | 4.186 | 0.041 |
| No | 8 | 2 | 75.0% | ||
| Omentum resection | |||||
| Yes | 6 | 1 | 83.3% | 0.567 | 0.451 |
| No | 74 | 6 | 91.9% | ||
| Postsurgical treatment | |||||
| Observation | 32 | 1 | 96.9% | 3.464 | 0.325 |
| Chemotherapy | 34 | 5 | 85.3% | ||
| Chemotherapy + radiotherapy | 12 | 1 | 91.7% | ||
| Endocrine therapy | 2 | 0 | 100.0% |
*Serous carcinoma, mucinous adenocarcinoma, clear cell adenocarcinoma, and mixed cell adenocarcinoma. LVSI: lymphovascular space invasion.
Table 4.
Multivariate analysis of demographic, surgical, and pathological factors.
| Risk factor | Hazard ratio (95% CI) | P value |
|---|---|---|
| Pathological type (endometrioid adenocarcinoma compared withother types*) | 0.521 (0.102–2.669) | 0.434 |
| LVSI(no compared with yes) | 0.337 (0.068–1.672) | 0.183 |
| Surgical pathway(laparotomy compared with laparoscopic surgery) | 4.122 (0.418–40.669) | 0.225 |
| Lymphadenectomy(no compared with yes) | 1.700 (0.289–9.997) | 0.557 |
*Serous carcinoma, mucinous adenocarcinoma, clear cell adenocarcinoma, and mixed cell adenocarcinoma. CI, confidence interval, LVSI: lymphovascular space invasion.
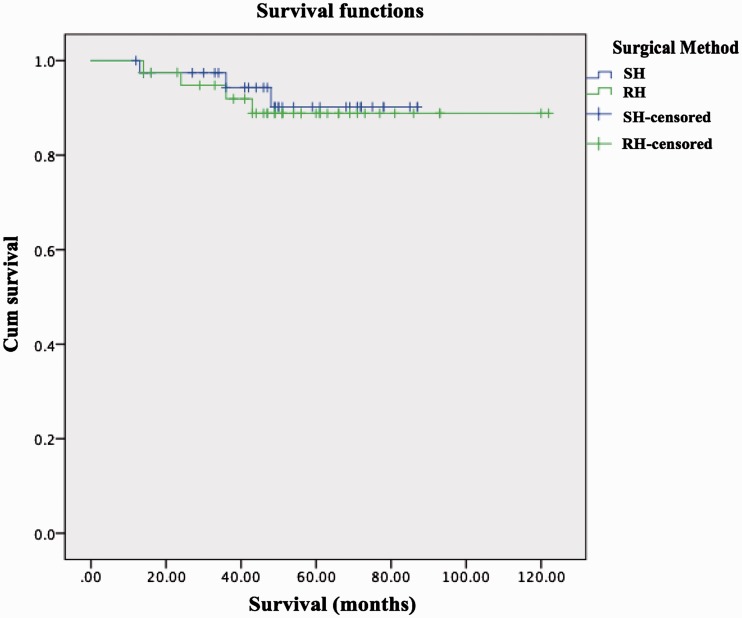
Furthermore, there was no significant difference in cancer-related survival rates between the SH and RH groups (Table 3). The 3-year cancer-related survival rates in the SH and RH groups were 94.97% and 92.53%, and the 5-year cancer-related survival rates were 92.40% and 90.03%, respectively. There were no significant differences in cancer-related survival rates between the two groups (Figure 2).
Figure 2.
Cancer-related survival curve according to hysterectomy type. There were no significant differences in survival between the SH and RH groups. The 3-year cancer-related survival rates in the SH and RH groups were 94.97% and 92.53% and the 5-year cancer-related survival rates were 92.40% and 90.03%, respectively. SH: simple hysterectomy, RH: radical hysterectomy, Cum survival: cumulative survival, censored: patients lost to follow-up and patients who survived until the end of the study.
Perioperative outcomes of SH and RH
In terms of choosing the optimal surgical method for stage II EC, we examined perioperative outcomes and quality of life, in addition to cancer-related survival rates (Table 5). SH was associated with significantly less intraoperative bleeding (mean ±standard deviation: 325.0 ± 307.7 mL vs 625.0 ± 581.4 mL, respectively, P = 0.010) and a shorter catheter-indwelling time compared with RH (4.8 ± 3.5 days vs 11.5 ± 3.9 days, P < 0.001). However, there was no significant difference in the length of hospital stay after surgery, hospitalization cost, therapeutic time with antibiotics, or total number of postoperative complications between the two groups.
Table 5.
Comparison of perioperative situations in patients undergoing simple hysterectomy and radical hysterectomy.
| Simple hysterectomy | Radical hysterectomy | P value | χ2 | |
|---|---|---|---|---|
| Length of postoperative hospital stay | 13.5 ± 4.6 | 15.4 ± 4.8 | 0.060 | |
| Hospitalization costs | 22428.1 ± 7654.9 | 22428.1 ± 12562.0 | 0.516 | |
| Hospitalization costs (after 2008) | 23115.2 ± 9134.8 | 27151.1 ± 11432.3 | 0.283 | |
| Intraoperative bleeding | 325.0 ± 307.7 | 625.0 ± 581.4 | 0.010 | |
| Therapeutic time of antibiotics | 3.4 ± 1.5 | 3.9 ± 2.0 | 0.259 | |
| Time of indwelling catheter | 4.8 ± 3.5 | 11.5 ± 3.9 | <0.001 | |
| Postoperative complications | 5 | 4 | 0.724 | 0.125 |
Moreover, 12.5% (5/40) of patients in the SH group had postoperative complications (postoperative morbidity, n = 3; incision infection, n = 1; pelvic abscess, n = 15), compared with 10.0% (4/40) in the RH group (intestinal obstruction, n = 1; postoperative morbidity, n = 1; Escherichia coli infection, n = 1; uroschesis and urinary tract infection, n = 1). There was no significant difference in complication rates between the two groups, but there were more types of complications after RH compared with SH.
Discussion
Although most women diagnosed with EC present with early-stage disease confined to the uterus, metastatic disease is identified in a significant percentage after comprehensive staging surgery,17 suggesting the need for more radical and thorough surgery in patients with stage II EC. Previous editions of the NCCN and EMSO guidelines recommended RH for clinical stage II EC with suspected gross cervical involvement. However, given the high incidence of sexual dysfunction and poor quality of life following RH, we investigated the possibility of replacing RH with SH in patients with stage II EC.
Several studies found that RH was associated with increased disease-free and overall survival. For example, Sartori et al.10 conducted a retrospective trial of 135 SH and 68 RH procedures for stage II EC and showed that the 5-year (94% vs 79%) and 10-year (94% vs 74%) survival rates were higher in the RH compared with the SH group. Likewise, Cohn et al.11 studied 162 patients with surgical stage II EC and found a significantly better 5-year disease-free survival rate in patients undergoing RH compared with extrafascial hysterectomy (94% vs 76%). In a larger sample size, Cornelison et al.12 based their results on the SEER database, which included 932 patients with stage II EC, and showed that RH was associated with better survival than SH (92.96% vs 84.36%), but there was no significant survival difference between patients with and without radiation in either surgical group.
However, the above-mentioned studies were performed almost 10 years ago, prior to the development of the FIGO 2009 staging system. They therefore included patients with both endocervical glandular involvement (1988 FIGO stage IIA) and cervical stromal invasion (1988 FIGO stage IIB). In addition, the prevalence of laparoscopy and improvements in surgical equipment, environment, and technology may also have increased survival after hysterectomy. Although several studies found no significant improvement in overall survival among patients with EC after laparoscopy compared with laparotomy, 18,19 some reports indicated that the morbidity of hysterectomy has decreased over recent decades.20–22 Averette et al.21 demonstrated a continuing trend towards decreasing morbidity and mortality rates after RH in the published literature. Likewise, Benjamin et al.22 reported significant reductions in blood loss, blood transfusions, and hospital stay after hysterectomy between the periods 1980 to 1993 and 1991 to 1993. In addition, advancements in pathological diagnosis and radiological technologies enable the discovery of small parametrial infiltrations in more patients, resulting in more extensive surgeries and thorough treatments and thus decreasing the incidence of misdiagnosed stage II EC and allowing patients to undergo non-radical surgeries.
Several recent studies have shown conflicting results. For example, Takano et al.13 reported on 300 patients with stage II EC who underwent RH, modified hysterectomy, or SH, and indicated that the type of hysterectomy was not a prognostic factor in patients with EC and gross cervical involvement, though perioperative and late adverse events were more frequent in patients treated with RH. Similarly, Ozgul et al.14 conducted a multicenter, retrospective trial in Turkey including 250 patients with stage II EC, of whom 199 underwent SH and the remaining 51 underwent RH. They found that age was the only independent risk factor for overall survival, and the type of hysterectomy had no effect on either disease-free or overall survival. Phelippeau et al.15 analyzed the data for 2886 patients with type 1 FIGO stage II EC from the SEER database. After one-to-two matching, 273 patients who underwent RH and 546 who received SH were included in the statistical analysis, which found no significant difference in 3-year cancer-related survival rates between the two groups (88.7% and 94.1%, respectively).
In the current study, we aimed to determine the survival and perioperative outcomes in patients with stage II EC according to the 2009 FIGO staging system, treated with different types of hysterectomy. We used a case-matched method to avoid any effects of population heterogeneity between the SH and RH groups on the final results. Our results showed that hysterectomy type had no impact on overall survival, although RH could lead to more intraoperative bleeding, a longer catheter-indwelling time, and more complex postoperative complications.
Parametrectomy is the most challenging part of an RH. It is responsible for postoperative urological dysfunction associated with autonomic nerve injury, and the interval before recovery of spontaneous voiding depends on the amount of parametrium removed. This accounts for why patients undergoing RH need a longer catheter-indwelling time than patients who undergo SH.23,24 As shown in the current and previous studies, RH can also lead to significant blood loss and more complex postoperative complications, which in turn prolong hospital stay. 9,13 Furthermore, RH is associated with a high incidence of sexual dysfunction, which may severely reduce the patient’s quality of life.8 Indeed, some patients in the current study complained of an unsatisfactory sex life during their telephone follow-up calls.
The above results suggest that reducing the universality of surgery in patients with stage II EC may not only decrease perioperative complications, but may also improve patient quality of life.
The current study had some limitations. We included patients diagnosed with stage II EC from 2000 to 2014, and this long time span may thus have introduced differences in relation to the operative methods, given that perioperative effects were related to the operation environment and technique of the surgeon. In addition, 30 patients were lost to follow-up, which would unavoidably cause some bias. Furthermore, the case-matched method meant that the sample size was relatively small, and investigations in a larger sample could enhance the credibility of these results. Finally, it should be noted that a P-value >0.05 does not necessarily indicate a lack of any difference, and we should learn to embrace uncertainty.25 Thus although our results were most compatible with a lack of any important difference between SH and RH in terms of survival outcomes in patients with stage II EC, it is not possible to make definitive decisions based solely on the P-value.
Conclusions
The results of the current retrospective study suggest that SH has similar survival outcomes to RH in patients with FIGO stage II EC. Furthermore, RH may be associated with more intraoperative bleeding, a longer catheter-indwelling time, and more complex postoperative complications than SH. Further prospective studies with large sample sizes are needed to confirm the outcomes of these two surgical procedures in patients with stage II EC.
Acknowledgements
We would like to thank all the patients and their families involved in our retrospective study and during the follow-up period. We wish them good health.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Science and Technology Commission of Shanghai Municipality (grant number: 16411953500), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20172003), and the National Natural Science Foundation of China (grant number: 81572836). The funding bodies supported our research team in collecting the data and guiding graduate students to analyze and summarize the data.
References
- 1.Zheng R, Zeng H, Zhang S, et al. National estimates of cancer prevalence in China, 2011. Cancer Lett 2016; 370: 33–38. DOI: 10.1016/j.canlet.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Burke WM, Orr J, Leitao M, et al. Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 2014; 134: 385–392. DOI: 10.1016/j.ygyno.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 3.Board PDQATE. Endometrial Cancer Treatment (PDQ(R)): Patient Version. PDQ cancer information summaries. Bethesda (MD): National Cancer Institute (US), 2002. [Google Scholar]
- 4.Koh WJ, Greer BE, Abu-Rustum NR, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw 2014; 12: 248–280. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: vi33–vi38. DOI: 10.1093/annonc/mdt353 [DOI] [PubMed] [Google Scholar]
- 6.Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018; 16: 170–199. DOI: 10.6004/jnccn.2018.0006 [DOI] [PubMed] [Google Scholar]
- 7.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol 2016; 27: 16–41. DOI: 10.1093/annonc/mdv484 [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Chen C, Liu P, et al. The morbidity of sexual dysfunction of 125 Chinese women following different types of radical hysterectomy for gynaecological malignancies. Arch Gynecol Obstet 2018; 297: 459–466. DOI: 10.1007/s00404-017-4625-0 [DOI] [PubMed] [Google Scholar]
- 9.Bouchard-Fortier G, Reade CJ, Covens A. Non-radical surgery for small early-stage cervical cancer. Is it time? Gynecol Oncol 2014; 132: 624–627. DOI: 10.1016/j.ygyno.2014.01.037 [DOI] [PubMed] [Google Scholar]
- 10.Sartori E, Gadducci A, Landoni F, et al. Clinical behavior of 203 stage II endometrial cancer cases: the impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer 2001; 11: 430–437. [DOI] [PubMed] [Google Scholar]
- 11.Cohn DE, Woeste EM, Cacchio S, et al. Clinical and pathologic correlates in surgical stage II endometrial carcinoma. Obstet Gynecol 2007; 109: 1062–1067. DOI: 10.1097/01.aog.0000260871.87607.25 [DOI] [PubMed] [Google Scholar]
- 12.Cornelison TL, Trimble EL, Kosary CL. SEER data, corpus uteri cancer: treatment trends versus survival for FIGO stage II, 1988-1994. Gynecol Oncol 1999; 74: 350–355. DOI: 10.1006/gyno.1999.5501 [DOI] [PubMed] [Google Scholar]
- 13.Takano M, Ochi H, Takei Y, et al. Surgery for endometrial cancers with suspected cervical involvement: is radical hysterectomy needed (a GOTIC study)? Br J Cancer 2013; 109: 1760–1765. DOI: 10.1038/bjc.2013.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozgul N, Boyraz G, Salman MC, et al. Oncological outcomes of stage II endometrial cancer: a retrospective analysis of 250 cases. Int J Gynecol Cancer 2018; 28: 161–167. DOI: 10.1097/igc.0000000000001133 [DOI] [PubMed] [Google Scholar]
- 15.Phelippeau J, Koskas M. Impact of radical hysterectomy on survival in patients with stage 2 Type1 endometrial carcinoma: a matched cohort study. Ann Surg Oncol 2016; 23: 4361–4367. DOI: 10.1245/s10434-016-5372-3 [DOI] [PubMed] [Google Scholar]
- 16.Cibula D, Abu-Rustum NR, Benedetti-Panici P, et al. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol 2011; 122: 264–268. DOI: 10.1016/j.ygyno.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 17.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987; 60: 2035–2041. [DOI] [PubMed] [Google Scholar]
- 18.Favero G, Anton C, Le X, et al. Oncologic safety of laparoscopy in the surgical treatment of Type II endometrial cancer. Int J Gynecol Cancer 2016; 26: 1673–1678. DOI: 10.1097/igc.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 19.Galaal K, Bryant A, Fisher AD, et al. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev 2012: 9: CD006655. DOI: 10.1002/14651858.CD006655.pub2 [DOI] [PubMed] [Google Scholar]
- 20.Underwood PB, Jr, Wilson WC, Kreutner A, et al. Radical hysterectomy: a critical review of twenty-two years' experience. Am J Obstet Gynecol 1979; 134: 889–898. [DOI] [PubMed] [Google Scholar]
- 21.Averette HE, Nguyen HN, Donato DM, et al. Radical hysterectomy for invasive cervical cancer. A 25-year prospective experience with the Miami technique. Cancer 1993; 71: 1422–1437. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin I, Barakat RR, Curtin JP, et al. Blood transfusion for radical hysterectomy before and after the discovery of transfusion-related human immunodeficiency virus infection. Obstet Gynecol 1994; 84: 974–978. [PubMed] [Google Scholar]
- 23.Covens A, Rosen B, Murphy J, et al. Changes in the demographics and perioperative care of stage IA(2)/IB(1) cervical cancer over the past 16 years. Gynecol Oncol 2001; 81: 133–137. DOI: 10.1006/gyno.2001.6158 [DOI] [PubMed] [Google Scholar]
- 24.Cibula D, Slama J, Velechovska P, et al. Factors affecting spontaneous voiding recovery after radical hysterectomy. Int J Gynecol Cancer 2010; 20: 685–690. DOI: 10.1111/IGC.0b013e3181d80ae3 [DOI] [PubMed] [Google Scholar]
- 25.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019; 567: 305–307. DOI: 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- 26.Boente MP, Yordan EL, Jr., McIntosh DG, et al. Prognostic factors and long-term survival in endometrial adenocarcinoma with cervical involvement. Gynecol Oncol 1993; 51: 316–322. [DOI] [PubMed] [Google Scholar]
- 27.Sartori E, Gadducci A, Landoni F, et al. Clinical behavior of 203 stage II endometrial cancer cases: the impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer 2001; 11: 430–437. [DOI] [PubMed] [Google Scholar]
- 28.Ayhan A, Taskiran C, Celik C, et al. The long-term survival of women with surgical stage II endometrioid type endometrial cancer. Gynecol Oncol 2004; 93: 9–13. [DOI] [PubMed] [Google Scholar]
- 29.Wright JD, Fiorelli J, Kansler AL, et al. Optimizing the management of stage II endometrial cancer: the role of radical hysterectomy and radiation. Am J Obstet Gynecol 2009; 200: 419.e1–7. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto M, Takano M, Aoyama T, et al. Is modified radical hysterectomy needed for patients with clinical stage I/II endometrial cancers? A historical control study. Oncology 2016; 90: 179–185. [DOI] [PubMed] [Google Scholar]