Short abstract
Objective
Smoking has been proven to increase systemic inflammation in previous studies using different biomarkers. The eosinophil-to-lymphocyte ratio (ELR), neutrophil-to-lymphocyte ratio (NLR), and lymphocyte-to-monocyte ratio (LMR) are new indicators of systemic inflammation that are used as predictors of systemic inflammation, morbidity, and mortality associated with many diseases. We investigated the effects of smoking on these inflammatory indicators.
Methods
In total, 616 consecutive smoking healthy subjects and 387 age-matched nonsmoking healthy subjects were enrolled. White blood cell counts (neutrophils, lymphocytes, basophils, eosinophils, and monocytes) were determined by electrical impedance with an automatic blood cell counting device. The ELR, LMR, and NLR were calculated based on these cell counts. Smoking habits of participants were calculated as pack/year.
Results
The NLR and ELR were significantly higher and the LMR was significantly lower in smokers than nonsmokers. The pack-years were positively correlated with the NLR and ELR and negatively correlated with the LMR.
Conclusion
A high NLR and ELR and low LMR are associated with cigarette smoking and may be useful indicators of systemic inflammation activity, even in healthy smokers. Smokers with a high NLR and ELR and low LMR can easily be identified during routine blood analysis and might benefit from preventive treatment.
Keywords: Cigarette smoking, systemic inflammation, complete blood cell count, biomarkers, eosinophil-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio
Introduction
Cigarette smoking is a leading cause of morbidity and mortality associated with cardiovascular disease and has been implicated in the pathogenesis of atherosclerosis and thrombotic events.1,2 Smoking has been proven to increase systemic inflammation in previous studies using different biomarkers.3–5 Smoking has also been shown to increase the leukocyte and eosinophil counts in peripheral blood, although the underlying mechanism remains unknown. Blood test parameters reportedly return to the reference range after 5 years of smoking cessation.6 Several studies have shown that various types of white blood cells (WBCs) are damaged by smoking.7,8 WBCs are the principal cells affected by inflammation and are considered responsible for undesirable situations in patients with cardiovascular disease. Various WBC types are known as indicators of the inflammatory status.9,10 Increased neutrophil, basophil, and eosinophil counts and low lymphocyte counts induced by stress signalize alterations in the immune system.11–13
Several studies have shown that smoking is associated with changes in various hematological parameters such as the neutrophil, lymphocyte, monocyte, and eosinophil counts, all of which are active in inflammation, as well as with the neutrophil-to-lymphocyte ratio (NLR).14–16 In addition to these known parameters, the eosinophil-to-lymphocyte ratio (ELR) and lymphocyte-to-monocyte ratio (LMR) have recently been used as new indicators of systemic inflammation, morbidity, and mortality.17–19
To the best of our knowledge, however, the connection among smoking, the LMR, and the ELR has not been studied. A complete blood cell (CBC) count can be easily and inexpensively performed in all laboratories. Thus, the NLR, LMR, ELR, and various whole blood cell counts can be evaluated by clinicians using a rapid, low-cost method. The present study was performed to analyze the effects of smoking on the following inflammatory indicators: the ELR, LMR, NLR, and several whole blood cell counts.
Methods
Study population
This cross-sectional observational study involved consecutive smoking healthy subjects and age-matched, nonsmoking healthy subjects with no smoking history who were treated at the cardiology outpatient clinics of Dr. Ersin Arslan Education and Research Hospital in Gaziantep, Turkey from June 2017 to August 2018. None of the participants had any systemic diseases or risk factors for atherosclerosis with the exception of hyperlipidemia. Cardiac ischemia and other cardiac pathologies were detected by an exercise stress test and transthoracic echocardiography. Smokers were defined as those who consumed at least one cigarette per day. The number of pack-years was calculated by multiplying the number of cigarettes smoked with the number of smoked years and then dividing this total by 20. Hypertension was defined as a systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg and treatment with antihypertensive drugs. Diabetes was defined as treatment with an oral antidiabetic drug and/or insulin or a fasting blood glucose level of ≥126 mg/dL. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee (Presidency of R. T. Gaziantep University Ethics Committee). All participants in the study provided written informed consent.
Exclusion criteria
The exclusion criteria in this study were diabetes mellitus, coronary artery disease, hypertension, heart failure, chronic kidney disease, chronic lung disease, connective tissue disease, metabolic syndrome, anemia, thyroid dysfunction, nonsteroidal anti-inflammatory drug use within the previous week, steroid use within the previous 6 months (including steroid creams), upper respiratory tract infection diagnosed within the previous 3 weeks, pregnancy, leukocytosis, leukopenia or any other hematological, biochemical, or serological abnormalities, routine alcohol intake, marijuana intake, consumption of other tobacco products, ex-smokers, and a body mass index of >30 kg/m2.
Echocardiography
A Vivid 5 ultrasound system (GE Medical Systems, Milwaukee, WI, USA) was used to perform transthoracic echocardiography. A 2.5-MHz transducer and harmonic imaging were used in accordance with the recommendations of the American Society of Echocardiography. M-mode echocardiography was applied to obtain the diastolic and systolic diameters of the left ventricle. The Teichholz method was applied to assess the left ventricular ejection fraction.20
Exercise stress test
The Bruce or modified Bruce treadmill protocol was used to perform the stress test (Cardiosis TEPA Exercise Stress Test device; TEPA Medicaland Electronic Products Industry and Trade Company, Ankara, Turkey). Bruce treadmill protocols were performed as non-invasive measurements of functional capacity tolerance for exercise and for existence of cardiovascular disorders in participants.21
Laboratory measurements
Ante-cubital vein was used to gain 6 mL for full biochemistry and 5 mL for total blood count (CBC) samples following 12 hours of fasting. The blood samples were collected in vacuum tubes filled with 15% K3 ethylene diamine tetraacetic acid as an anticoagulant (Sarstedt, Essen, Belgium). CBC parameters were evaluated using a Sysmex XN-1000 hematology analyzer (Sysmex Europe GmbH, Sysmex Corporation, Hamburg, Germany) according to the manufacturer’s instructions. Venous blood samples were taken after a 12-hour fast to measure biochemical parameters using a cobas device (Roche Diagnostics, Basel, Switzerland).
Statistical evaluation
SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The Kolmogorov–Smirnov test was performed to check whether data were normally distributed. The Mann–Whitney U-test was then applied to analyze continuous data with non-normal distributions. Non-normally distributed variables are shown as median (25th–75th percentile). Pearson’s correlation test was used for correlation analyses. All p-values were two-tailed, and p < 0.05 was accepted as statistically significant.
Results
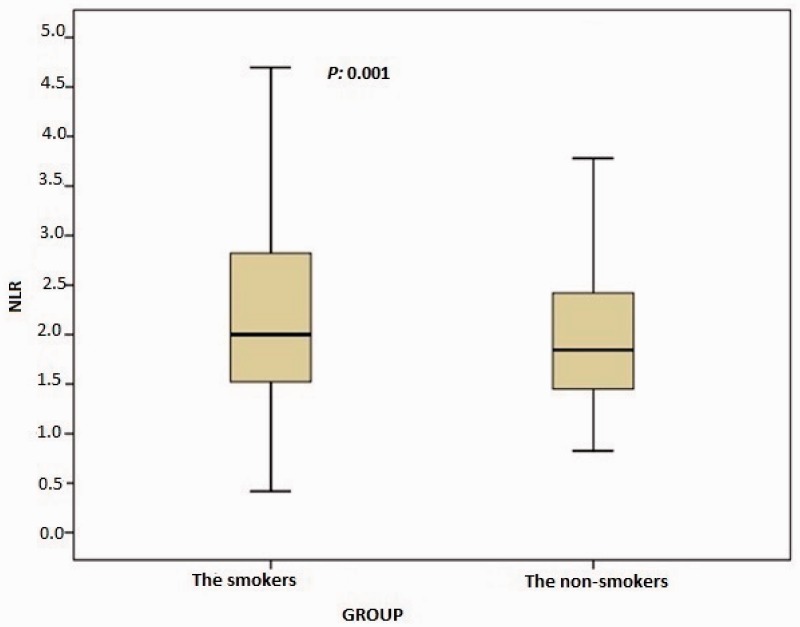
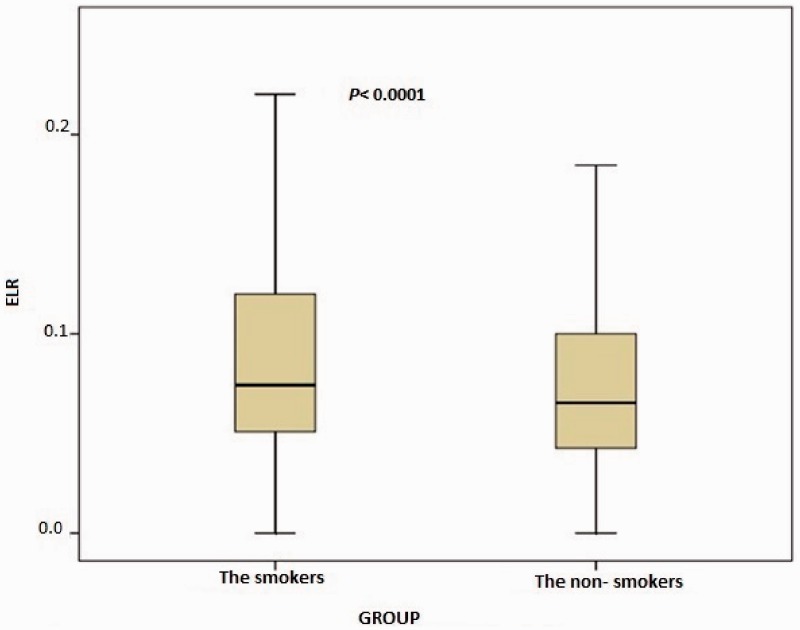
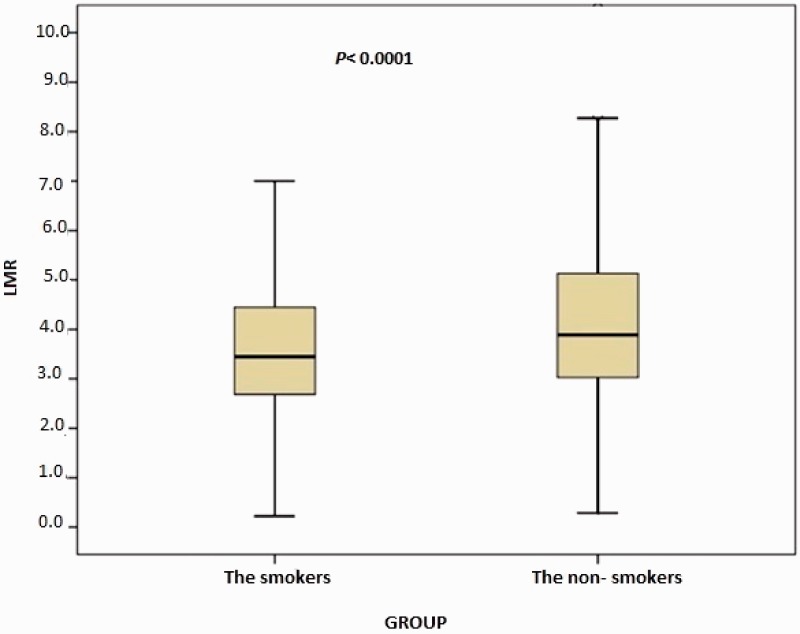
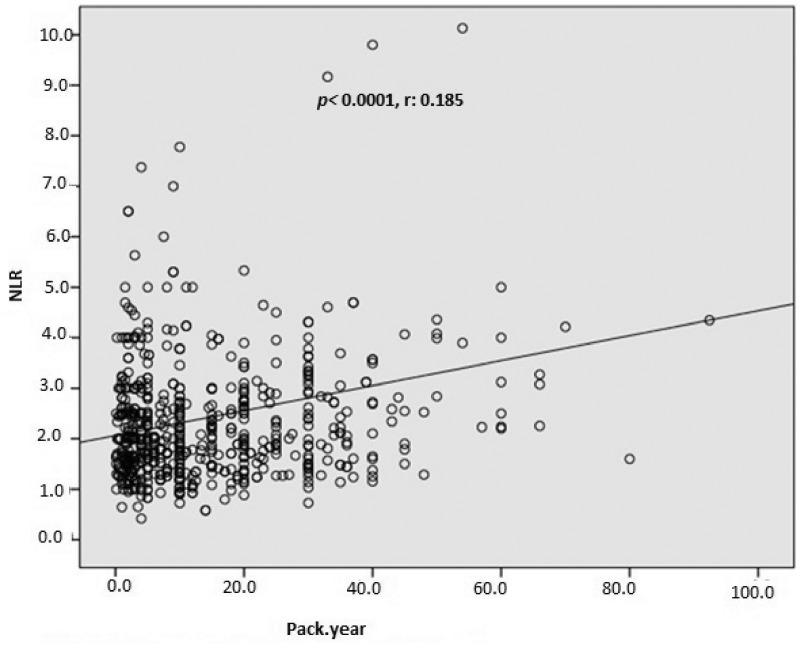
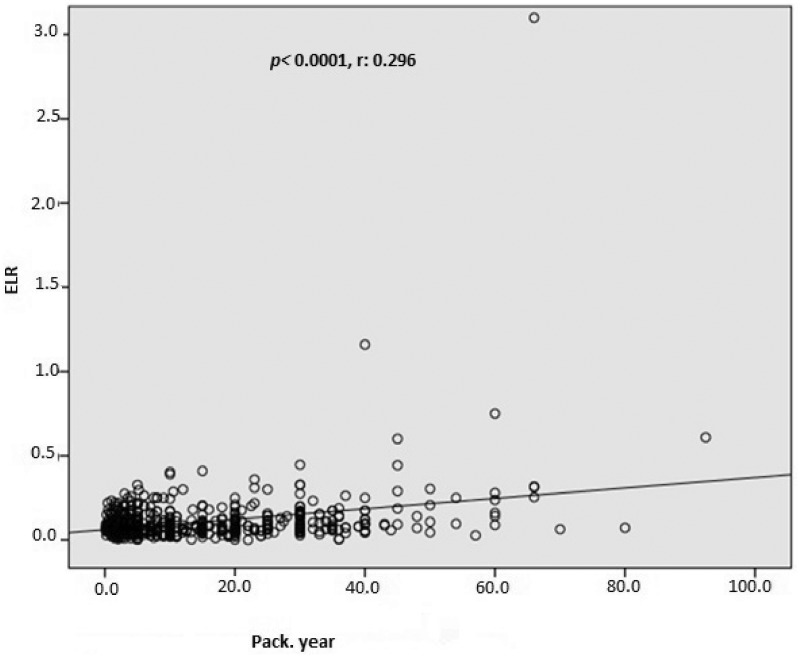
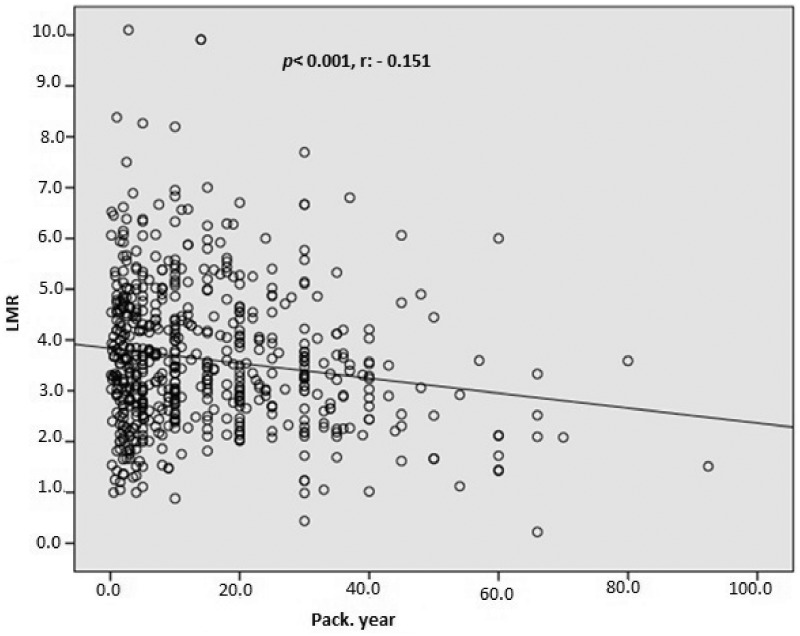
In total, 1003 consecutive subjects were initially included. After application of the exclusion criteria, the study population comprised 616 smokers (male, n = 489) and 387 nonsmokers (male, n = 289) ranging in age from 17 to 70 years, The nonsmoking group exhibited a significantly lower total WBC count (p < 0.0001), monocyte count (p < 0.0001), eosinophil count (p < 0.0001), basophil count (p < 0.0001), neutrophil count (p < 0.0001), NLR (p = 0.001), ELR (p < 0.0001), triglyceride concentration (p < 0.0001), low-density lipoprotein cholesterol (LDL-C) concentration (p = 0.001), hematocrit (p < 0.0001), and hemoglobin (p < 0.0001) than the smoking group (Table 1, Figure 1, and Figure 2). However, the nonsmoking group exhibited a significantly higher LMR (p < 0.0001) and high-density lipoprotein cholesterol (HDL-C) concentration (p < 0.0001) than the smoking group (Table 1 and Figure 3). Positive correlations were found between the pack-years and the total cholesterol concentration (p = 0.005), triglyceride concentration (p < 0.0001), LDL-C concentration (p = 0.003), basophil count (p < 0.0001), neutrophil count (p = 0.030), eosinophil count (p < 0.0001), monocyte count (p < 0.0001), NLR (p < 0.0001), and ELR (p < 0.0001) (Table 2, Figures 3–5). Negative correlations were found between the pack-years and the HDL-C concentration (p = 0.009) and LMR (p < 0.0001) (Table 2, Figure 6). No statistically significant differences were found in hyperlipidemia, age, or other investigated laboratory parameters between the smokers and nonsmokers (Table 1).
Table 1.
Intergroup comparison of demographic data.
| Variable | Smokers (n = 616) | Nonsmokers (n = 387) | p value |
|---|---|---|---|
| Age, years | 35.9 (24.0–45.0) | 36.0 (23.0–46.0) | 0.594 |
| Platelets, ×103/mm3 | 261.8 (223.0–295.0) | 259.0 (218.0–286.0) | 0.185 |
| Mean platelet volume, fL | 10.4 (9.7–11.0) | 10.4 (9.8–11.0) | 0.465 |
| Glucose, mg/dL | 94.5 (86.4–100.0) | 95.4 (89.0–99.0) | 0.29 |
| Triglycerides, mg/dL | 149.4 (91.0–168.0) | 134.1 (75.0–154.0) | <0.0001 |
| LDL cholesterol, mg/dL | 108.0 (87.0–126.0) | 104.1 (82.0–120.0) | 0.001 |
| Total cholesterol, mg/dL | 181.0 (154.0–200.0) | 178.1 (151.2–198.0) | 0.95 |
| HDL cholesterol, mg/dL | 43.4 (42.0–49.0) | 48.6 (40.0–57.0) | <0.0001 |
| Hemoglobin, g/dL | 15.1 (13.9–14.7) | 14.5 (13.3–15.3) | <0.0001 |
| Hematocrit, % | 45.4 (41.6–47.1) | 43.6 (39.9–45.9) | <0.0001 |
| White blood cells, ×103/mm3 | 7.94 (6.40–8.99) | 7.35 (6.05–8.51) | <0.0001 |
| Neutrophils, ×103/mm3 | 4.80 (3.57–5.54) | 4.28 (3.25–5.19) | <0.0001 |
| Lymphocytes, ×103/mm3 | 2.24 (1.83–2.65) | 2.27 (1.86–2.64) | 0.696 |
| Eosinophils, ×103/mm3 | 0.20 (0.12–0.24) | 0.172 (0.100–0.220) | <0.0001 |
| Basophils, ×103/mm3 | 0.040 (0.030–0.046) | 0.343 (0.200–0.400) | <0.0001 |
| Monocytes, ×103/mm3 | 0.65 (0.53–0.77) | 0.599 (0.437–0.660) | <0.0001 |
| NLR, % | 2.42 (1.52–2.82) | 2.07 (1.43–2.41) | 0.001 |
| LMR, % | 3.62 (2.68–4.45) | 4.31 (3.02–5.13) | <0.0001 |
| ELR, % | 0.10 (0.50–1.12) | 0.78 (0.42–0.100) | <0.0001 |
| Urea, mg/dL | 27.9 (22.3–33.0) | 28.0 (22.0–33.0) | 0.750 |
| Creatinine, mg/dL | 0.69 (0.60–0.87) | 0.70 (0.61–0.86) | 0.674 |
| Sodium, mmol/L | 139.4 (138.0–142.0) | 139.8 (138.0–142.0) | 0.157 |
| Potassium, meq/L | 4.31 (4.00–4.58) | 4.29 (4.00–4.56) | 0.738 |
| Calcium, mg/dL | 9.64 (9.10–9.77) | 9.48 (9.14–9.80) | 0.269 |
Data are presented as median (25th–75th percentile). LDL, low-density lipoprotein; HDL, high-density lipoprotein; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; ELR, eosinophil-to-lymphocyte ratio.
Figure 1.
Comparison of neutrophil-to-lymphocyte ratio (NLR) between the smokers and nonsmokers. The box-whisker plots show the NLR for the smokers and nonsmokers. The heavy black horizontal lines for each group represent the means, the extremities of the box are the 25th and 75th percentiles, and the error bars are the minimum and maximum outliers.
Figure 2.
Comparison of eosinophil-to-lymphocyte ratio (ELR) between the smokers and nonsmokers. The box-whisker plots show the ELR for the smokers and nonsmokers. The heavy black horizontal lines for each group represent the means, the extremities of the box are the 25th and 75th percentiles, and the error bars are the minimum and maximum outliers.
Figure 3.
Comparison of lymphocyte-to-monocyte ratio (LMR) between the smokers and nonsmokers. The box-whisker plots show the LMR for the smokers and non-smokers. The heavy black horizontal lines for each group represent the means, the extremities of the box are the 25th and 75th percentiles, and the error bars are the minimum and maximum outliers.
Table 2.
Pearson correlation analysis between smoking (pack-years) and the NLR, LMR, ELR, and various laboratory parameters.
|
Pack-years |
||
|---|---|---|
| r | p | |
| Triglycerides, mg/dL | 0.149 | <0.0001 |
| Total cholesterol, mg/dL | 0.118 | 0.005 |
| LDL-C, mg/dL | 0.126 | 0.003 |
| HDL-C, mg/dL | −0.111 | 0.009 |
| WBC count, ×103/mm3 | 0.096 | 0.018 |
| Neutrophils, ×103/mm3 | 0.087 | 0.030 |
| Lymphocytes, ×103/mm3 | −0.049 | 0.226 |
| Monocytes, ×103/mm3 | 0.178 | <0.0001 |
| Eosinophils, ×103/mm3 | 0.311 | <0.0001 |
| Basophils, ×103/mm3 | 0.158 | <0.0001 |
| NLR, % | 0.185 | <0.0001 |
| LMR, % | −0.151 | <0.0001 |
| ELR, % | 0.296 | <0.0001 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; ELR, eosinophil-to-lymphocyte ratio.
Figure 4.
Pearson’s correlation analysis between the neutrophil-to-lymphocyte ratio (NLR) and pack-years.
Figure 5.
Pearson’s correlation analysis between the eosinophil-to-lymphocyte ratio (ELR) and pack-years.
Figure 6.
Pearson’s correlation analysis between the lymphocyte-to-monocyte ratio (LMR) and pack-years.
Discussion
This study was performed to calculate the LMR, NLR, and ELR (recently established inflammatory, morbidity, and mortality markers) in healthy smokers and determine whether a correlation exists between smoking and these values. This study revealed that the ELR and NLR were higher in smokers than nonsmokers with a weak and positive correlation with pack-years and that the LMR was lower in smokers than nonsmokers with a weak and negative correlation with pack-years (Tables 1 and 2; Figures 1–6). These data indicate a possible association between a high WBC count, monocyte count, eosinophil count, basophil count, neutrophil count, NLR, and ELR and a low LMR with existing systemic inflammation in the pathophysiology of cigarette smoking. Our results also suggest a relationship between high hemoglobin and hematocrit concentrations and dyslipidemia in cigarette smokers.
Smoking causes chronic systemic inflammation even in individuals without additional health problems, and various biomarkers have been used to detect systemic inflammation in these individuals.3–5,22,23 Several recent studies have focused on the NLR as a systemic inflammatory marker in smokers.11,14 Although many studies have been performed to assess the correlations of CBC parameters with smoking, we are unaware of any studies evaluating the effect of smoking on the LMR and ELR.15,24,25
Lymphocytes, neutrophils, eosinophils, and monocytes are associated with the immune response and inflammation. Leukopenia is associated with cardiovascular adverse events.26,27 Additionally, elevated monocyte, neutrophil, and eosinophil counts are associated with cardiovascular disease. Both high monocyte, eosinophil, and neutrophil counts and a low lymphocyte count reflect systemic inflammation and physiologic stress and contribute to the development of cardiovascular disease.28–33
The relationships between the LMR, ELR, and NLR and adverse events in patients with oncological and cardiac diseases have been revealed in several recent studies. The NLR, ELR, and LMR, which are easily calculated from the CBC count, have been shown to be potential diagnostic and prognostic indicators of some malignant subtypes and cardiac diseases.17,19,30,31,34–38
Previous studies have also shown that the total WBC, neutrophil, monocyte, lymphocyte, basophil, and eosinophil counts, which are hematologic parameters, play important roles in the inflammatory process; these parameters are also used as indicators of inflammation and are closely related to smoking.11,39 Although many studies have been performed to explore the effects of smoking on hemogram parameters, few have focused on how smoking affects the NLR. Moreover, to the best of our knowledge, the relationship between smoking and the LMR and ELR has not been previously studied.
Similar to previously conducted studies, we found that while the WBC, neutrophil, eosinophil, and basophil counts were significantly higher in the smokers than nonsmokers, the lymphocyte count was not significantly different between the two groups.11,39 Additionally, we determined that the NLR and ELR were significantly higher in the smokers than nonsmokers (Table 1). However, the LMR was lower in the smokers than nonsmokers (Table 1). Although a positive and significant correlation was detected between the pack-years and the NLR and ELR (Table 2; Figures 3–5) and a negative and significant correlation was detected between the pack-years and the LMR, we found that these correlations were weak (Table 2, Figure 6). This result might be explained by the subjects included in the study being selected from among healthy individuals and being relatively young. In addition, three parameters (triglyceride, LDL-C, and HDL-C concentrations) were remarkably different between the two groups (Table 1). These data are in agreement with previous studies showing higher serum triglyceride and LDL-C concentrations along with a lower plasma HDL-C concentration in smokers than nonsmokers.40
Recent researches have clearly established that smoking causes an increase in cardiovascular events, morbidity, and mortality.41–43 Many studies have shown that the LMR, ELR, and NLR are closely related to systemic inflammation, morbidity, and mortality.17,19,34,35 The higher ELR and NLR and lower LMR in the smokers of the present study, even smokers with no health problems, may serve as indicators of systemic inflammation and many cardiovascular and other diseases caused by systemic inflammation.
Limitations
Second-hand smoking and exposure to environmental pollutants may increase systemic inflammation and affect the blood levels of biomarkers of systemic inflammation.44–47 In addition, studies have shown that decreasing the energy intake and increasing the physical activity level may be effective therapies for reducing overall inflammation; therefore, physical inactivity contributes to an enhanced proinflammatory burden.48,49 In the present study, second-hand smoking and environmental pollution exposure could not be controlled, and the participants were not questioned regarding their physical activities. The obtained data should thus be interpreted with caution.
Conclusions
The ELR, NLR, and LMR are indicators of systemic inflammation and are used as predictors of morbidity and mortality associated with many diseases. Both healthy and clinically asymptomatic smokers may either develop inflammatory conditions or undergo worsening of their existing inflammatory conditions by smoking. The NLR, LMR, and ELR are simple, cost-effective parameters that can be used to predict systemic inflammatory responses in smokers. The detection of a high NLR and ELR and a low LMR by a simple CBC count during routine laboratory testing might help to intercept the development of inflammation. For this reason, these three indices should be given more attention when examining smokers to enhance both prevention and intervention of inflammatory conditions.
Contributorship statement
Study conception: Çekici Y, Yılmaz M, Seçen Ö. Study design: Çekici Y, Yılmaz M, Seçen Ö. Data acquisition: Çekici Y.Data analysis: Yılmaz M, Seçen Ö. Data interpretation: Çekici Y, Yılmaz M, Seçen Ö. Writing of the manuscript: Çekici Y, Yılmaz M, Seçen Ö.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Togna AR, Latina V, Orlando R, et al. Cigarette smoke inhibits adenine nucleotide hydrolysis by human platelets. Platelets 2008; 19: 537–542. [DOI] [PubMed] [Google Scholar]
- 2.Mobarrez F, Antoniewicz L, Bosson JA, et al. The effects of smoking on levels of endothelial progenitor cells and microparticles in the blood of healthy volunteers. PLoS One 2014; 9: e90314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007; 131: 1557–1566. [DOI] [PubMed] [Google Scholar]
- 4.Levitzky YS, Guo CY, Rong J, et al. Relation of smoking status to a panel of inflammatory markers: the Framingham offspring. Atherosclerosis 2008; 201: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CC, Piper ME, Gepner AD, et al. Longitudinal impact of smoking and smoking cessation on inflammatory markers of cardiovascular disease risk. Arterioscler Thromb Vasc Biol 2017; 37: 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bain B, Rothwell M, Feher M, et al. Acute changes in haematological parameters on cessation of smoking. J R Soc Med 1992; 85: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Hizoh I, Kovacs A, et al. Predictors of high on-clopidogrel platelet reactivity in patients with acute coronary syndrome. Platelets 2016; 27: 159–167. [DOI] [PubMed] [Google Scholar]
- 8.Sharma KH, Shah KH, Patel I, et al. Do circulating blood cell types correlate with modifiable risk factors and outcomes in patients with acute coronary syndrome (ACS)? Indian Heart J 2015; 67: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm RH, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA 1985; 254: 1932–1937. [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–2. [DOI] [PubMed] [Google Scholar]
- 11.Tulgar Y, Cakar S, Tulgar S, et al. The effect of smoking on neutrophil/lymphocyte and platelet/lymphocyte ratio and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016; 20: 3112–3118. [PubMed] [Google Scholar]
- 12.Meisel SR, Shapiro H, Radnay J, et al. Increased expression of neutrophil and monocyte adhesion molecules LFA-1 and Mac-1 and their ligand ICAM-1 and VLA-4 throughout the acute phase of myocardial infarction: possible implications for leukocyte aggregation and microvascular plugging. J Am Coll Cardiol 1998; 31: 120–125. [DOI] [PubMed] [Google Scholar]
- 13.Blum A, Sclarovsky S, Rehavia E, et al. Levels of T-lymphocyte subpopulations, interleukin-1β, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J 1994; 127: 1226–1230. [DOI] [PubMed] [Google Scholar]
- 14.Gumus F, Solak I, Eryilmaz M. The effects of smoking on neutrophil/lymphocyte, platelet//lymphocyte ratios. Bratisl Lek Listy 2018; 119: 116–119. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmi A, Anandhi Lakshmanan GKP, Saravanan A. Effect of intensity of cigarette smoking on haematological and lipid parameters. J Clin Diagn Res 2014; 8: BC11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreoli C, Bassi A, Gregg EO, et al. Effects of cigarette smoking on circulating leukocytes and plasma cytokines in monozygotic twins. Clin Chem Lab Med 2015; 53: 57–64. [DOI] [PubMed] [Google Scholar]
- 17.Han LH, Jia YB, Song QX, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev 2015; 16: 2245–2250. [DOI] [PubMed] [Google Scholar]
- 18.Ferro M, Musi G, Serino A, et al. Neutrophil, platelets, and eosinophil to lymphocyte ratios predict gleason score upgrading in low-risk prostate cancer patients. Urol Int 2019; 102: 43–50. [DOI] [PubMed] [Google Scholar]
- 19.Murat SN, Yarlioglues M, Celik IE, et al. The relationship between lymphocyte-to-monocyte ratio and bare-metal stent in-stent restenosis in patients with stable coronary artery disease. Clin Appl Thromb Hemost 2017; 23: 235–240. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. [DOI] [PubMed] [Google Scholar]
- 21.Bruce R, Blackmon J, Jones J, et al. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 1963; 32: 742–756. [PubMed] [Google Scholar]
- 22.Kurtoğlu E, Aktürk E, Korkmaz H, et al. Elevated red blood cell distribution width in healthy smokers. Arch Turk Soc Cardiol. 2013; 43: 199–206. [DOI] [PubMed] [Google Scholar]
- 23.Yılmaz M, Kayançiçek H. A new inflammatory marker: elevated monocyte to HDL cholesterol ratio associated with smoking. J Clin Med 2018; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malenica M, Prnjavorac B, Bego T, et al. Effect of cigarette smoking on haematological parameters in healthy population. Med Arch 2017; 71: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vadapalli K, Devarakonda BV, Sherke BA, et al. Evaluation of the sequential effect of quantity of cigarettes smoked per day on differential leukocyte count among North Eastern Indian adult males. Natl J Physiol Pharm Pharmacol 2016; 6: 572. [Google Scholar]
- 26.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005; 45: 1638–1643. [DOI] [PubMed] [Google Scholar]
- 27.Nunez J, Minana G, Bodi V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem 2011; 18: 3226–3233. [DOI] [PubMed] [Google Scholar]
- 28.van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J 2012; 163: 57–65.e2. [DOI] [PubMed] [Google Scholar]
- 29.Maekawa Y, Anzai T, Yoshikawa T, et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol 2002; 39: 241–246. [DOI] [PubMed] [Google Scholar]
- 30.Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2010; 105: 186–191. [DOI] [PubMed] [Google Scholar]
- 31.Ayhan SS, Öztürk S, Erdem A, et al. Relation of neutrophil/lymphocyte ratio with the presence and severity of coronary artery ectasia. Turk Kardiyol Dern Ars 2013; 41: 185–190. [DOI] [PubMed] [Google Scholar]
- 32.Demir M, Keceoglu S, Melek M. The relationship between plasma eosinophil count and coronary artery ectasia. Cardiol Res 2013; 4: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altas Y, Kurtoglu E, Yaylak B, et al. The relationship between eosinophilia and slow coronary flow. Ther Clin Risk Manag 2015; 11: 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutterer GC, Sobolev N, Ehrlich GC, et al. Pretreatment lymphocyte–monocyte ratio as a potential prognostic factor in a cohort of patients with upper tract urothelial carcinoma. J Clin Pathol 2015; 68: 351–355. [DOI] [PubMed] [Google Scholar]
- 35.Hu P, Shen H, Wang G, et al. Prognostic significance of systemic inflammation-based lymphocyte-monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One 2014; 9: e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holub K, Biete A. New pre-treatment eosinophil-related ratios as prognostic biomarkers for survival outcomes in endometrial cancer. BMC Cancer 2018; 18: 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Zhu L, Yang Y, et al. Prognostic role of neutrophil to lymphocyte ratio in ovarian cancer: a meta-analysis. Technol Cancer Res Treat 2018; 17: 1533033818791500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vano YA, Oudard S, By MA, et al. Optimal cut-off for neutrophil-to-lymphocyte ratio: fact or Fantasy? A prospective cohort study in metastatic cancer patients. PLoS One 2018; 13: e0195042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higuchi T, Omata F, Tsuchihashi K, et al. Current cigarette smoking is a reversible cause of elevated white blood cell count: cross-sectional and longitudinal studies. Prev Med Rep 2016; 4: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuzuya M, Ando F, Iguchi A, et al. Effect of smoking habit on age-related changes in serum lipids: a cross-sectional and longitudinal analysis in a large Japanese cohort. Atherosclerosis 2006; 185: 183–190. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder SA. New evidence that cigarette smoking remains the most important health hazard. Mass Medical Soc 2013; 368:389–390. [DOI] [PubMed] [Google Scholar]
- 42.Health Udo and Services H. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease, 2014. [Google Scholar]
- 43.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34: 509–515. [DOI] [PubMed] [Google Scholar]
- 44.Radwan LR. Impact of passive smoke on tongue blood vessels, blood cell count and blood glutathione level (experimental study) EC Dental Science 2015; 2: 423–434. [Google Scholar]
- 45.Dadvand P, Nieuwenhuijsen MJ, Agustí À, et al. Air pollution and biomarkers of systemic inflammation and tissue repair in COPD patients. Eur Respir J 2014; 44: 603–613. [DOI] [PubMed] [Google Scholar]
- 46.Lanki T, Hampel R, Tiittanen P, et al. Air pollution from road traffic and systemic inflammation in adults: a cross-sectional analysis in the European ESCAPE Project. Environ Health Perspect 2015; 123: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu YHM, Spiegelman D, Dockery DW, et al. Secondhand smoke exposure and inflammatory markers in nonsmokers in the trucking industry. Environ Health Perspect 2011; 119: 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruunsgaard H. Physical activity and modulation of systemic low‐level inflammation. J Leukoc Biol 2005; 78: 819–835. [DOI] [PubMed] [Google Scholar]
- 49.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 2005; 172: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]