Short abstract
Symptomatic arachnoid cysts are relatively rare, and no case reports have described recurrence of such cysts almost 30 years after surgery. We herein report a case in which a symptomatic intradural arachnoid cyst recurred 29 years after fenestration of the primary lesion. The patient was a 64-year-old woman who presented with paralysis of the left lower limb. She had undergone surgical treatment for an intradural arachnoid cyst at the T12 level 29 years previously. Magnetic resonance imaging (MRI) revealed an intradural mass at the T12–L1 level. The mass was compressing the spinal cord and cauda equina. Its localization and shape on MRI were similar to those of the primary cyst 29 years previously. Partial resection was performed under a diagnosis of a recurrent intradural arachnoid cyst. After surgery, the patient’s left lower limb paralysis improved. The pathological findings were suggestive of an intradural arachnoid cyst. The MRI findings 29 years previously provided useful information. The possibility of very late recurrence should be considered in patients who undergo surgical removal of intradural arachnoid cysts.
Keywords: Spinal arachnoid cyst, recurrence, paralysis, magnetic resonance imaging, computed tomography myelography, fenestration
Introduction
Symptomatic arachnoid cysts are relatively rare,1,2 but they sometimes cause paralysis of the lower limbs when they arise as intradural lesions.3,4 Recent advances in magnetic resonance imaging (MRI) have facilitated the diagnosis of symptomatic arachnoid cysts, and such cysts are being incidentally detected in an increasing number of patients.5 Surgery is routinely performed to treat symptomatic intradural arachnoid cysts, and resection or wide marsupialization is recommended.6 One study showed that incomplete fenestration of symptomatic intradural arachnoid cysts led to recurrence in 9.5% of patients during a 5-year maximum follow-up period.7 However, no case reports have described the recurrence of an arachnoid cyst almost 30 years after the initial surgery.
Case report
We de-identified the patient’s details such that her identity cannot be ascertained in any way. A 64-year-old woman presented with a 6-month history of muscle weakness of the left lower limb. She had undergone fenestration of an intradural arachnoid cyst at the T12 level 29 years previously. Because the primary lesion was located on the ventral side of the spinal cord, the intraoperative diagnosis was intramedullary edema. Therefore, no pathological examination was performed. After the initial surgery, her left lower limb paralysis resolved.
At the current presentation, the manual muscle test (MMT) scores of the left quadriceps muscle, hamstrings, tibialis anterior, peroneus longus, and extensor hallucis longus were 4, 4, 3, 4, and 2, respectively. All right lower limb muscles exhibited MMT scores of 5. Left-sided hypesthesia was observed from the thigh downward, but no sensory disturbance was detected in the right lower limb. The bilateral patellar tendon and Achilles tendon reflexes were enhanced, and Babinski’s sign was noted on both sides.
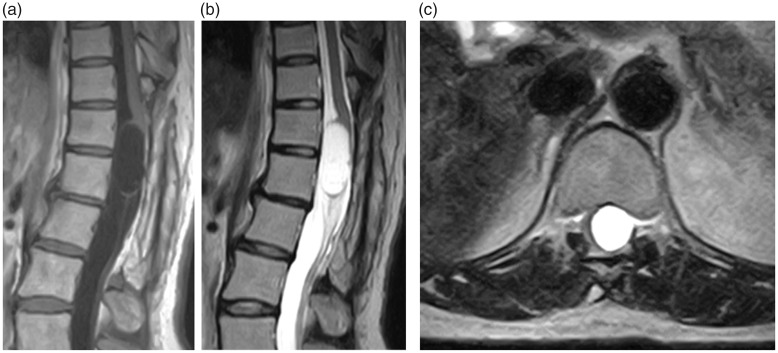
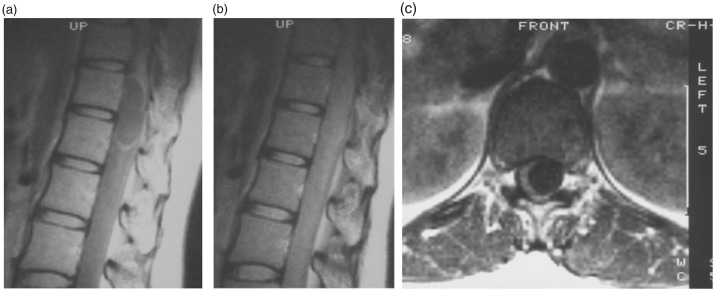
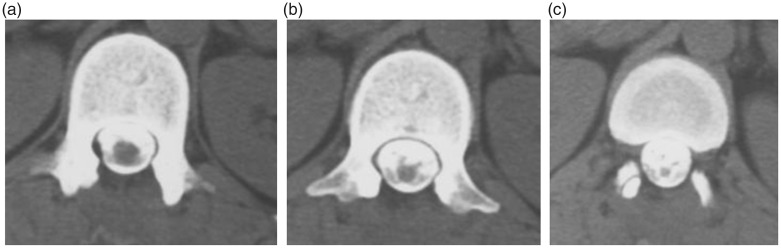
MRI revealed an intradural mass at the T12–L1 level (Figure 1). The mass was detected as a region of low signal intensity on T1-weighted images and an area of uniform high signal intensity on T2-weighted images. The mass was compressing the spinal cord and cauda equina, pushing them to the right (Figure 1(c)). In the MRI scan performed 29 years previously, proton-density-weighted images were obtained, which is not the current standard imaging method. In addition, the resolution was low (0.5 tesla). However, proton-density-weighted sagittal images (repetition time [TR]: 1.60 ms, echo time [TE]: 30 ms) showed an intradural mass involving the T12–L1 level. At the periphery of the lesion, a region of high signal intensity was detected. At the center of the mass, an area of uniform isointensity was detected (Figure 2(a)). However, the isointense borders of the mass were unclear on T2-like-weighted sagittal images (TR: 1.60 ms, TE: 68 ms) (Figure 2(b)). On proton-density-weighted transverse images (TR: 0.50 ms, TE: 30 ms), the spinal cord was pushed to the right by a mass displaying uniform low signal intensity (Figure 2(c)). The localization and shape of the primary lesion were similar to those of the recurrent lesion (Figure 2). Furthermore, computed tomography myelography performed 1 month after the initial surgery confirmed that the spinal cord and cauda equina had returned to their normal positions and that the mass had disappeared (Figure 3).
Figure 1.
(a) A T1-weighted sagittal thoracic magnetic resonance imaging section showing an intradural, extramedullary low-signal-intensity mass involving the T12–L1 level. (b) A T2-weighted sagittal section showing a mass exhibiting uniform high signal intensity. (c) A T2-weighted transverse section showing that the spinal cord had been pushed to the right by a high-signal-intensity mass at the T12 level.
Figure 2.
Proton-density-weighted sagittal thoracic magnetic resonance images [repetition time (TR): 1.60 ms, echo time (TE): 30 ms] obtained 29 years previously showing an intradural mass involving the T12–L1 level. (a) At the periphery of the mass, a region of high signal intensity was detected. At the center of the mass, an area of uniform isointensity was detected. (b) On a T2-like-weighted sagittal section (TR: 1.60 ms, TE: 68 ms), the borders of the mass were unclear and displayed isointensity. (c) On a proton-density-weighted transverse section (TR: 0.50 ms, TE: 30 ms), the spinal cord had been pushed to the right by a mass exhibiting uniform low signal intensity.
Figure 3.
Computed tomography myelogram of the spinal cord obtained 1 month after the initial surgery (29 years before the current presentation) showing that the spinal cord and cauda equina had returned to their normal positions. (a) Cephalic side at the T12 level. (b) Caudal side at the T12 level. (c) T12/L1 level.
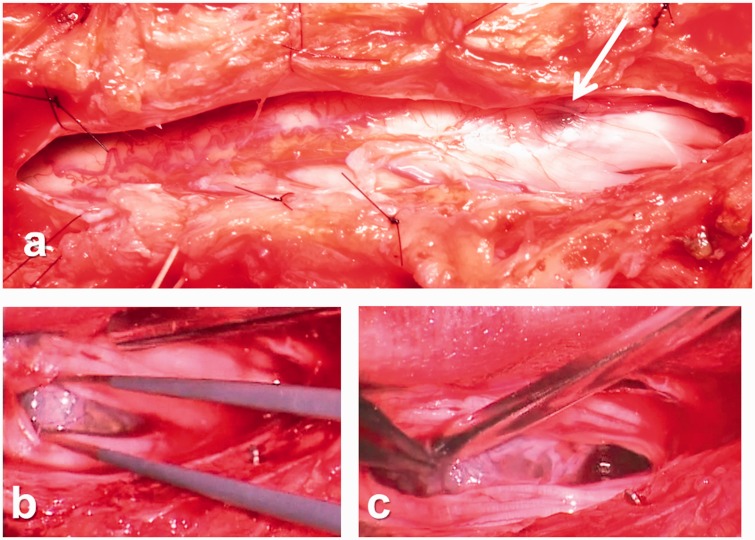
Partial resection was carried out under a diagnosis of a recurrent intradural arachnoid cyst (based on the MRI findings of the primary and recurrent lesions and the patient’s postoperative course after the initial surgery). Intraoperative ultrasonography showed a mass on the ventral side compressing the spinal cord and cauda equina toward the right side. The lesion had developed on the conus medullaris at the L1 level (Figure 4(a)), and the cyst wall was partially resected. The mass was white and had partially adhered to the spinal cord and cauda equina (Figure 4(b), (c)). We resected almost all of the cyst without leaving a partial lesion adhered to the spinal cord. After using intraoperative ultrasonography to confirm that the cyst had decreased in size and that the spinal cord was decompressed, the surgery was completed.
Figure 4.
Intraoperative findings. (a) Intraoperatively, a cyst-like mass was seen on the left conus medullaris at the L1 level after the opening of the dura (arrow). (b, c) The cyst wall was white and had partially adhered to the spinal cord and cauda equina on the ventral side of the spinal cord.
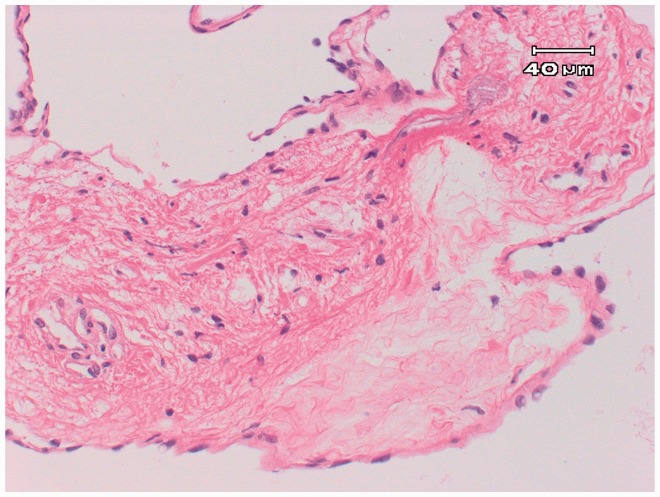
After surgery, the paralysis affecting the patient’s left lower limb improved. Six months after surgery, she could walk without crutches. The pathological findings suggested that the lesion was an intradural arachnoid cyst (Figure 5).
Figure 5.
Photomicrograph revealing a cystic lesion with a fibrous wall. The lesion was suspected to be an arachnoid cyst. The inner area was covered with squamous cells, and partial aggregation of meningeal cells was observed. Hematoxylin and eosin staining, original magnification: ×200.
This study was approved by the Nihon University Institutional Review Board and was conducted in conformity with ethical and human principles of research. Written informed consent was obtained from the patient before treatment.
Discussion
Arachnoid cysts are also termed “pouches” or “diverticula.” The history of their classification is complex. Nabors et al.4 classified spinal meningeal cysts into three major categories: type I, extradural cysts without nerve root involvement; type II, extradural cysts with nerve root involvement; and type III, intradural cysts. The present case corresponded to type III. Spinal meningeal cysts have been reported to occur more frequently in the thoracic vertebrae and in male patients, and symptomatic cysts most commonly occur in people aged 20 to 29 years. Furthermore, monoparesis is seen in 10% of patients who develop paralysis. In the present case, monoparesis was also observed before the initial surgery and after the lesion’s recurrence. The development of arachnoid cysts on the ventral side of the spinal cord is relatively rare, but such lesions can result in severe paralysis.8–14 In the current case, the lesion was located on the ventral side of the spinal cord, and the intraoperative diagnosis made during the initial surgery was intramedullary edema. In addition, several reports have described cases of arachnoid cysts related to minor trauma.8,15
The dominant theory regarding the mechanism responsible for cyst expansion is the passive fluid-transport theory, which involves pulsatile cerebrospinal fluid dynamics and an osmotic gradient with or without a valve-like mechanism.3,16–18
The postoperative recurrence of arachnoid cysts has been previously reported, but no case reports have described the recurrence of cysts almost 30 years after the initial surgery. Marsupialization is effective for the treatment of intradural arachnoid cysts. Kumar et al.6 investigated 31 patients with intradural arachnoid cysts and reported that none developed recurrence during the 4-year follow-up. Evangelou et al.7 indicated that recovery was achieved without neurological abnormalities in 94% of 21 patients and that recurrence was detected in 9.5% of these patients. In the latter cases, the maximum interval until recurrence was 5 years. However, Evangelou et al.7 emphasized the necessity of postoperative follow-up MRI and reported that the recurrence rate according to MRI was 5.3%. In another study, Takahashi et al.19 performed minimally invasive treatment involving percutaneous MRI-guided fenestration. Furthermore, shunt creation is reportedly a useful procedure for recurrent intradural arachnoid cysts.20 In the current case, however, partial resection was selected. The most appropriate surgical procedure for symptomatic spinal intradural arachnoid cysts remains unresolved.
MRI is the most useful technique for diagnosing arachnoid cysts.18 The MRI procedure used to diagnose our patient’s primary lesion 29 years previously differed from the current imaging method, and the resolution (0.5 tesla) was low; however, it was possible to evaluate the localization and shape of the lesion. The localization and shape of the primary lesion were similar to those of the recurrent lesion. Furthermore, computed tomography myelography of the spinal cord performed 1 month after the initial surgery confirmed that the spinal cord and cauda equina had returned to their normal positions. As a result, the present lesion was diagnosed as a recurrence of the primary lesion. Although long-term storage of images is often difficult because of problems with storage methods or costs, images of primary lesions can provide information that facilitates future surgery. Therefore, the long-term storage of images should be considered in cases involving diseases that might recur, such as tumors.
Conclusion
We have herein presented a case in which a symptomatic intradural arachnoid cyst recurred 29 years after fenestration of the primary lesion. After surgery, the patient’s left lower limb paralysis improved. The images obtained during the initial surgery 29 years previously provided useful information that aided the diagnosis and treatment of the recurrent lesion. Very late recurrence should be considered in patients who undergo surgical treatment of spinal intradural arachnoid cysts.
Acknowledgement
We would like to thank all the staff members at Nihon University Itabashi Hospital.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Note
We state that no portion of the contents of the paper have been presented or published previously.
References
- 1.Cloward RB. Congenital spinal extradural cysts: case report with review of literature. Ann Surg 1968; 168: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gortvai P. Extradural cysts of the spinal canal. J Neurol Neurosurg Psychiatry 1963; 26: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gortvai P, el-Gindi S. Spinal extradural cyst. Case report. J Neurosurg 1967; 26: 432–435. DOI: 10.3171/jns.1967.26.4.0432. [DOI] [PubMed] [Google Scholar]
- 4.Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988; 68: 366–377. DOI: 10.3171/jns.1988.68.3.0366. [DOI] [PubMed] [Google Scholar]
- 5.Novegno F, Umana G, Di Muro L, et al. Spinal intramedullary arachnoid cyst: case report and literature review. Spine J 2014; 14: e9–e15. DOI: 10.1016/j.spinee.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Sakia R, Singh K, et al. Spinal arachnoid cyst. J Clin Neurosci 2011; 18: 1189–1192. DOI: 10.1016/j.jocn.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Evangelou P, Meixensberger J, Bernhard M, et al. Operative management of idiopathic spinal intradural arachnoid cysts in children: a systematic review. Childs Nerv Syst 2013; 29: 657–664. DOI: 10.1007/s00381-012-1990-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Cho DY. Symptomatic spinal intradural arachnoid cysts in the pediatric age group: description of three new cases and review of the literature. Pediatr Neurosurg 2001; 35: 181–187. DOI: 50419. [DOI] [PubMed] [Google Scholar]
- 9.Palmer JJ. Cervical intradural arachnoid cyst in a 3-year-old child. Report of a case. Arch Neurol 1974; 31: 214–215. [DOI] [PubMed] [Google Scholar]
- 10.Rabb CH, McComb JG, Raffel C, et al. Spinal arachnoid cysts in the pediatric age group: an association with neural tube defects. J Neurosurg 1992; 77: 369–372. DOI: 10.3171/jns.1992.77.3.0369. [DOI] [PubMed] [Google Scholar]
- 11.Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol 2003; 60: 49–55; discussion 55-46. [DOI] [PubMed] [Google Scholar]
- 12.Kumar K, Malik S, Schulte PA. Symptomatic spinal arachnoid cysts: report of two cases with review of the literature. Spine (Phila Pa 1976) 2003; 28: E25–E29. DOI: 10.1097/01.brs.0000041591.62378.9d. [DOI] [PubMed] [Google Scholar]
- 13.Aarabi B, Pasternak G, Hurko O, et al. Familial intradural arachnoid cysts. Report of two cases. J Neurosurg 1979; 50: 826–829. DOI: 10.3171/jns.1979.50.6.0826. [DOI] [PubMed] [Google Scholar]
- 14.Agnoli AL, Schonmayr R, Laun A. Intraspinal arachnoid cysts. Acta Neurochir (Wien) 1982; 61: 291–302. [DOI] [PubMed] [Google Scholar]
- 15.Muthukumar N. Anterior cervical arachnoid cyst presenting with traumatic quadriplegia. Childs Nerv Syst 2004; 20: 757–760. DOI: 10.1007/s00381-003-0903-1. [DOI] [PubMed] [Google Scholar]
- 16.McCrum C, Williams B. Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg 1982; 57: 849–852. DOI: 10.3171/jns.1982.57.6.0849. [DOI] [PubMed] [Google Scholar]
- 17.Lake PA, Minckler J, Scanlan RL. Spinal epidural cyst: theories of pathogenesis. Case report. J Neurosurg 1974; 40: 774–778. DOI: 10.3171/jns.1974.40.6.0774. [DOI] [PubMed] [Google Scholar]
- 18.Rohrer DC, Burchiel KJ, Gruber DP. Intraspinal extradural meningeal cyst demonstrating ball-valve mechanism of formation. Case report. J Neurosurg 1993; 78: 122–125. DOI: 10.3171/jns.1993.78.1.0122. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Morikawa S, Egawa M, et al. Magnetic resonance imaging-guided percutaneous fenestration of a cervical intradural cyst. Case report. J Neurosurg 2003; 99: 313–315. [DOI] [PubMed] [Google Scholar]
- 20.Jensen F, Knudsen V, Troelsen S. Recurrent intraspinal arachnoid cyst treated with a shunt procedure. Acta Neurochir (Wien) 1977; 39: 127–129. [DOI] [PubMed] [Google Scholar]