Ovarian cancer can originate in the fallopian tube. We show that ovarian colonization, exposure of the ovarian extra-cellular matrix as would occur during ovulation, and loss of PTEN in the fallopian tube are important for spread of this disease.
Abstract
High-grade serous ovarian cancer (HGSOC) can originate in the fallopian tube epithelium (FTE), but the role of the ovary in these tumors is unclear. Tumorigenic murine oviductal epithelial (MOE) cells allografted in the ovarian bursa resulted in aggressive tumors that spread throughout the peritoneum whereas intraperitoneal xenografting the same number of cells did not form tumors, indicating that colonization of the ovary may play a role in metastasis. Physical tearing of the ovarian surface to mimic rupture of the ovary during ovulation (independent of hormonal changes) resulted in more MOE and HGSOC cells adhering to the ovary compared with intact ovaries. More MOE cells also adhered to three-dimensional (3D) collagen and primary ovarian stromal cells than to ovarian surface epithelia, indicating that FTE cells adhered to the extracellular matrix exposed during ovulation. However, plating cells on 3D collagen reduced the viability of normal FTE but not cancer cells. Mutation of p53 (R273H or R248W) and activation of Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) (G12V) did not increase the viability of MOE cells on 3D collagen. In contrast, loss of phosphatase and tensin homolog (PTEN) allowed MOE cells to retain normal viability on 3D collagen. Loss of PTEN activated AKT and RAC1/c-jun N-terminal kinase signaling that each contributed to the increased viability, invasion and attachment in the collagen rich ovarian microenvironment. These results show that loss of PTEN activates multiple pathways that together enhance colonization of the ovary due to access to 3D collagen, which is a critical organ in the colonization of FTE-derived HGSOC.
Introduction
All epithelial ovarian cancers were thought to originate in the ovarian surface epithelium (OSE), but it is now clear that high-grade serous ovarian cancer (HGSOC) originates from the fallopian tube epithelium (FTE) ( 1–5). The current model of FTE-derived HGSOC indicates that FTE cells acquire mutations in Tp53, resulting in stabilization of the p53 protein (called a p53 signature). Additional mutations result in the formation of serous tubal intraepithelial carcinomas (STICs). STICs metastasize to the ovary, form tumors and then eventually spread throughout the peritoneum ( 2). However, the role of the ovary in the spread of FTE-derived tumors is unclear ( 6).
The number of ovulations is associated with a woman’s risk of developing HGSOC ( 7, 8). In mice, superovulation increases the formation of ovarian tumors following intrauterine xenograft of tumorigenic cells ( 9). In primates, OSE cells are lost during ovulation as a stigma forms at the future site of ovulation ( 10, 11), exposing the underlying extracellular matrix (ECM). The ovulatory wound may provide a site for tumorigenic FTE cells to attach to the ovary; however, the role of chemotactic factors produced by the follicle ( 9, 12) and physical disruption of the ovarian surface are difficult to separate in vivo.
The only gene ubiquitously mutated in HGSOC is Tp53 ( 13), though several different pathway alterations that result in increased PI3K/AKT and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling in serous ovarian cancer have been identified ( 14). Our laboratory has recently shown that knockdown or knockout of PTEN in the FTE of mice results in FTE-derived ovarian cancer whereas myristoylated AKT (AKTmyr) does not ( 15, 16), suggesting that loss of PTEN alters signaling in some aspects that differ from AKT activation. The objectives of the current research were to (i) evaluate the role of ovarian colonization in metastases of FTE-derived cells to the peritoneum; (ii) determine if exposure of the ovarian stroma and collagen during ovulation increases colonization by fallopian tube epithelial cells; (iii) identify signaling pathways that allow the FTE to remodel the ovarian ECM and (iv) elucidate the pathways activated by PTEN loss in FTE that are important in colonization of the ovary.
Materials and methods
Cell culture and transfections
Murine oviductal epithelial (MOE) and murine ovarian surface epithelial (MOSE) were originally generated from FVB mice and donated by Barbara Vanderhyden, PhD, University of Ottawa ( 15). MOE, MOSE, OVCAR3, OVCAR4, OVCAR5, OVCAR8 and OVSAHO cells were maintained in media as described previously ( 12, 17, 18). MOE cells stably expressing scrambled shRNA (SCRshRNA), empty vector (NEO), PTENshRNA, AKTmyr, KRASG12V and mutant p53 were previously generated ( 15, 19, 20) and were reconfirmed here by reduced PTEN levels, increased phospho-AKT levels, increased phospho-extracellular-signal-regulated kinase or stabilized p53 protein, respectively ( Supplementary Figure 1A–E, available at Carcinogenesis Online). Production of fluorescent cell lines, transient transfections and treatment with small-molecule inhibitors are described in the Supplementary Materials and Methods, available at Carcinogenesis Online. Efficacy of MK2206 (AKT inhibitor), NSC23766 (RAC1 inhibitor) and c-jun N-terminal kinase (JNK) inhibitor VIII (JNK inhibitor) was validated in MOE cells by treating the cells with a dose response of each inhibitor for 24 h and measuring phospho-AKT, phospho-JNK and phospho-cJun, respectively, via western blot ( Supplementary Figure 1F–H, available at Carcinogenesis Online).
Allograft experiment and immunohistochemistry
All animal procedures were approved by the Institution Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals. MOE KRASG12V + PTENshRNA cells (50 000 in phosphate-buffered saline) were injected into the peritoneum [intraperitoneal (IP)] or ovarian bursa [intrabursal (IB)] of 8-week-old female athymic nu/nu mice (Taconic, Germantown, NY). The health of mice was monitored daily, and mice were killed at humane end points. At necropsy, the peritoneal space was visually inspected for ascites and tumors, which were fixed in 4% paraformaldehyde overnight, dehydrated in ethanol and embedded in paraffin. Immunochemistry ( Supplementary Table 2, available at Carcinogenesis Online) was carried out as described previously ( 15).
Ex vivo adhesion assay
An assay to measure adhesion of fluorescently tagged cells to murine ovaries has been recently described ( 16). Ovaries were removed from CD-1 mice between days 16 and 18. Ovaries were left intact or the surface was disrupted with a scalpel blade to mimic ovulation. Each ovary was placed in a 1.8 ml microfuge tube with 1 ml of media and 30 000 cells labeled with green fluorescent protein (GFP) or red fluorescent protein (RFP). Tubes were incubated on an orbital shaker for 24 h at 37°C at 40 r.p.m. Ovaries were washed 2× in phosphate-buffered saline to remove non-adhered cells, and ovaries were inspected with a Nikon Eclipse TS100 inverted microscope. The number of fluorescent cells attached to the ovary was counted on both sides and averaged. A representative fluorescent and bright field image was taken, and the fluorescent pixels were pasted on top of the bright field image using ImageJ (imagej.nih.gov) to produce the overlay image.
Collagen treatment, migration assay and invasion assays
Production of conditioned media, scratch assays to measure migration and Boyden chamber invasion assays were done according to well-established protocols. Collagen was added to wells as a two-dimensional (2D) or three-dimensional (3D) layer as described previously ( 21). A complete description is available in the Supplementary Materials and Methods, available at Carcinogenesis Online.
Adhesion assays
Isolation and growth of the murine ovarian stroma (MOST) cells is described in the Supplementary Materials and Methods, available at Carcinogenesis Online. MOE GFP cells (100 000) were added to wells with a confluent monolayer of MOST, a monolayer of MOSE or a 3D collagen gel and incubated for 2 h. Washed monolayers were imaged with an inverted fluorescent microscope, and fluorescence was measured with a BioTek Synergy Plate Reader. Adhesion to fibronectin, vitronectin, laminin, collagen and collagen IV was measured with the Millicoat ECM Screening Kit (ECM205; Millipore, Billerica, MA) per the manufacturer’s instructions.
To determine if MOE cells adhere on top of or in between MOSE cells, MOSE cells were seeded at indicated concentrations on 96-well plates pretreated with 2D type I collagen. The next day, media were removed, and the cells were stained with CellTracker Red DMPTX dye (C34552; Life Technologies, Waltham, MA). Next, 100 000 MOE GFP cells were seeded into each well. After 2 h, images were captured with a 4× objective. Subsequently, the media were removed from each well, the cells were rinsed with phosphate-buffered saline and 100 μl lysis buffer was added to each well. The plates were frozen at −80°C, thawed and 80 µl lysis buffer was transferred to white 96-well plates (3789; Corning, Corning, NY). The relative number of MOE cells in each well was determined by measuring fluorescence intensity of GFP with the BioTek Synergy Plate Reader.
Viability assay
Cells (20 000–50 000 dependent on cell line) were plated on 2D collagen or 3D collagen in a 96-well plate in 100 μl media and cultured for 24 h. Viability was measured with CellTiter-Glo Luminescent Cell Viability Kit (G7570; Promega) per the manufacturer’s instructions. Results are expressed as the ratio of viability of the same cell line or treatment on 3D collagen to viability on 2D collagen (3D:2D viability ratio).
RNA isolation, qPCR, RNAseq and western blot
RNA isolation, quantitative PCR, RNA sequencing (RNAseq) and western blots were carried out as described previously ( 12, 15, 18). A detailed description of these methods is available in the Supplementary Materials and Methods available at Carcinogenesis Online.
Statistical analysis
All data are presented as mean ± SEM and n = 3–5. Data were analyzed by a one-sample t-test compared to 1, a two-sample t-test or an analysis of variance (ANOVA) followed by a Dunnett’s or Tukey’s post hoc test, depending on the hypothesis and number of treatments. Data were analyzed with Prism, version 7.0a (www.graphpad.com). Significance was defined at P < 0.05.
Results
Colonization of the ovary is important for peritoneal metastasis of FTE-derived tumors
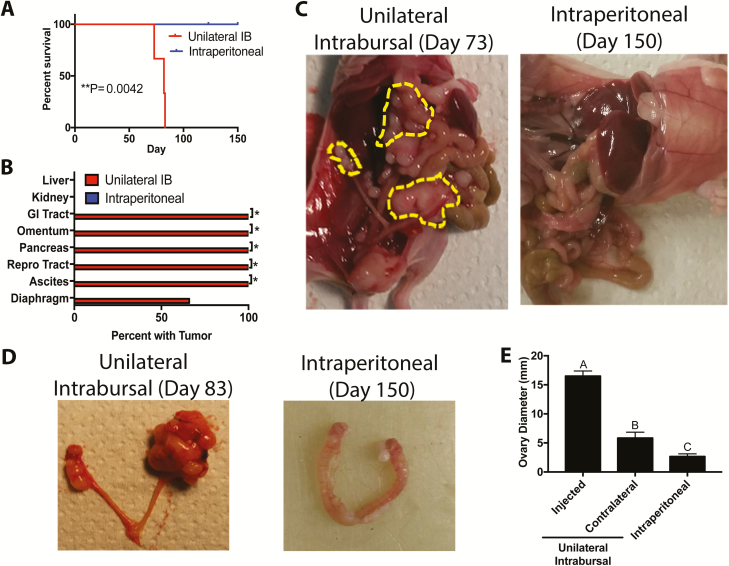
Previous evidence suggested that ovarian cancer cells preferentially colonized the ovary, an event facilitated by ovulation ( 9, 22). We demonstrated that MOE KRASG12V + PTENshRNA cells were tumorigenic after intraperitoneal (IP) xenograft of 10 million cells ( 15). To determine if MOE KRASG12V + PTENshRNA could form tumors after intrabursal (IB) xenograft, which limits the xenograft to a small volume, 50 000 MOE KRASG12V + PTENshRNA cells were allografted into the right and left bursa of two mice. Mice developed aggressive tumors, requiring killing on days 64 and 97. Tumors were observed in every peritoneal organ examined ( Supplementary Figure 2A, available at Carcinogenesis Online). Next, to evaluate the role of ovarian colonization in metastasis of FTE-derived cancer cells, 50 000 MOE KRASG12V + PTENshRNA cells were allografted into the left ovarian bursa (unilateral IB) in three mice. To mimic what would happen if an equal number of cells exfoliated from the fallopian tube, but were not directly interacting with the ovary, 50 000 cells were xenografted intraperitoneally into five mice. No tumors were observed in any of the IP-injected mice for the duration of the experiments (150 days; Figure 1A). One IP mouse was killed on day 123 for unrelated health reasons, but no tumors were observed ( Supplementary Figure 2B, available at Carcinogenesis Online). All unilateral IB allografted mice developed tumors, necessitating killing due to tumor burden by day 90 (P < 0.05). All unilateral IB mice (3/3) developed tumors in the gastrointestinal tract, omentum, pancreas, reproductive tract and ascites, and two-thirds of mice had tumors in the diaphragm ( Figure 1B and C). In unilateral IB-injected mice, the left ovary was on average 16.5 mm in diameter compared with 5.84 mm for the right ovary (P < 0.0001) due to tumor burden, and both ovaries in unilateral IB-injected mice were larger than ovaries of IP mice (2.65 mm; P < 0.05; Figure 1D–E).
Figure 1.
Colonization of the ovary is an important step in metastasis of FTE-derived cancer cells. (A) Kaplan–Meier analysis of mice following xenograft of MOE KRASG12V + PTENshRNA cells into the left bursa (unilateral IB) or IP. (B) Distribution of visible tumors in the peritoneum after unilateral IB and IP xenografts. (C and D) Representative images of mice and reproductive tracts from unilateral IB and IP xenografts. (E) Diameter of ovaries in unilateral IB and IP xenografts mice (n = 3–5). Bars without common letter differ, A–CP < 0.05. Significantly different from unilateral IB, *P < 0.05.
In unilateral IB-injected mice, the ipsilateral ovary was completely degraded by tumor cells as evidenced by the absence of observable follicles or corpus lutea , and tumors had pockets of necrotic tissue with a lack of nuclear staining. Immunostaining for PAX8, a marker of the FTE, was widespread in the left ovary, confirming that tumor cells were derived from the FTE. Tumors from MOE KRASG12V + PTENshRNA tumors were highly proliferative and had widespread Ki67 staining. Tumor tissue and some follicles and corpus lutea in the contralateral ovaries had high Ki67 immunostaining. No evidence of ovarian colonization was found in the IP-injected mice, with defined follicles and corpus luteum in the hematoxylin and eosin sections. PAX8 immunostaining was negative and Ki67 immunostaining was low ( Supplementary Figure 3, available at Carcinogenesis Online).
Exposure of ovarian collagen increases attachment to the ovary
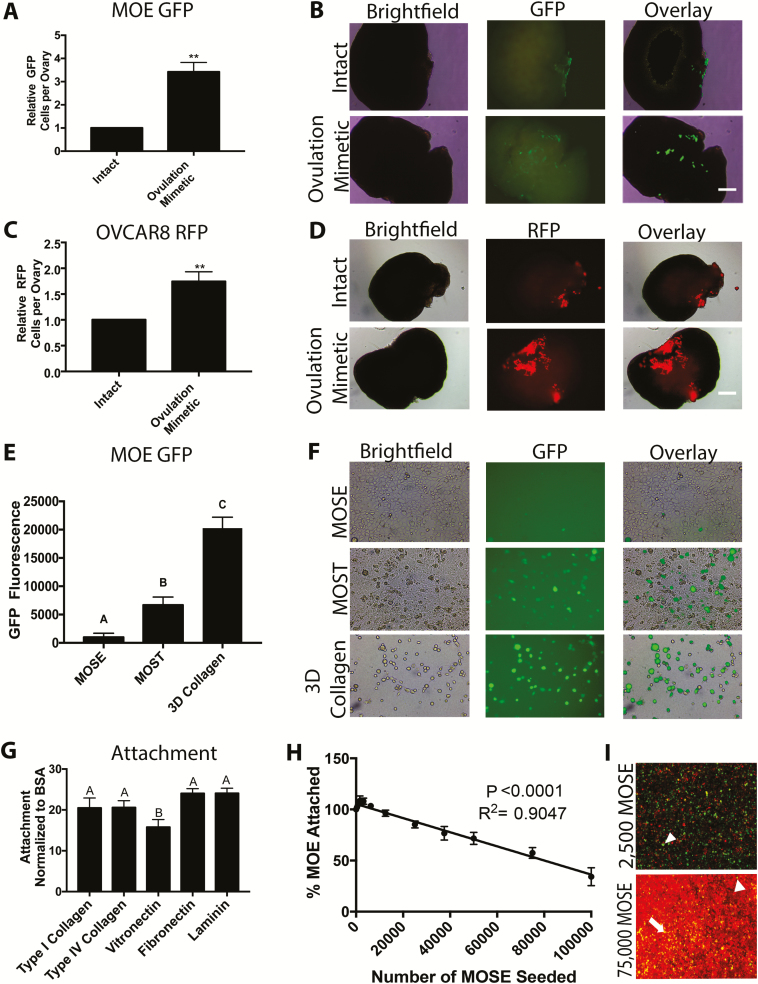
The individual contributions of hormones and physical tearing of the ovary during ovulation on tumor cell colonization are difficult to separate in vivo. Therefore, an ex vivo adhesion assay was used to isolate and test the role of physical rupture of the ovary, which would expose underlying 3D collagen, on attachment of FTE cells ( 16). Murine ovaries were either left intact or the surface was ruptured with a scalpel to mimic ovulation independent of hormonal changes. The wound resulted in loss of the OSE and disruption of the ovarian surface (hematoxylin and eosin images in Supplementary Figure 4A–B, available at Carcinogenesis Online) ( 11). Intact and ovulation mimetic treated ovaries were incubated with MOE GFP cells for 24 h on an orbital shaker. Ovulation mimetic increased the number of MOE GFP cells adhering to the surface of each ovary by 3.2-fold (P < 0.01; Figure 2A and B). Similarly, the ovulation mimetic increased attachment of HGSOC cell lines, OVCAR8 RFP and OVCAR4 RFP cells (2.5- and 1.7-fold increase, respectively; P < 0.05; Figure 2C and D and Supplementary Figure 4C, available at Carcinogenesis Online). Next, MOE PTENshRNA cells were incubated with ovulation mimetic ovaries followed by immunohistochemistry to determine where MOE cells were adhering. PAX8 immunostaining was clearly observed at the wounded surface ( Supplementary Figure 4D, available at Carcinogenesis Online) with sporadic PAX8 staining on the intact OSE-lined surfaces of the ovary. Numerous MOE PTENshRNA cells were also observed attached at the hilus region ( Supplementary Figure 4E, available at Carcinogenesis Online), suggesting that the MOE cells are preferentially adhered to any area where the ovarian surface is disrupted. To confirm that the effect of ovulation mimic was not due to the increased secretion of chemotactic factors leaking out after wounding, conditioned media were produced using intact and ovulation mimetic ovaries. Conditioned media from intact and wounded ovaries equally increased migration of MOE cells (P− = 0.96) by approximately 3-fold (P < 0.01) ( Supplementary Figure 4F, available at Carcinogenesis Online). These data confirmed that the ovulation mimetic model did not increase the release of chemotactic factors by the ovary.
Figure 2.
Mimicking ovulation by wounding the ovary increases attachment of FTE cells. (A–D) Rupture of the ovarian surface (ovulation mimetic) increases attachment of MOE GFP (equivalent to fallopian tube) (A, B) and OVCAR8 RFP (C, D) to the ex vivo cultured murine ovaries. (E and F) Attachment of MOE GFP cells to a monolayer of MOSE , monolayer of MOST or 3D type I collagen gels. (G) Attachment of MOE cells to five ECM proteins present in the basement membrane and stroma. (H and I) Attachment of MOE GFP to 2D type I collagen in wells with increasing number of MOSE cells labeled with CellTracker Red dye (arrowhead = MOE GFP attached to plastic; arrow = MOE GFP cell attached on top of MOSE). n = 3–5. Scale bar = 100 μm. Significantly different from intact, **P < 0.01. Bars without common letter differ, A–CP < 0.05. BSA, bovine serum albumin.
To determine if cells were adhering to ovulation mimetic ovaries due to exposure of the ECM, MOE GFP cells were plated on monolayers of MOSE cells to model the intact ovarian surface, which is covered with a single layer of ovarian surface cells. MOE GFP cells were also plated onto monolayers of MOST cells to mimic rupture sites within the ovary ( Supplementary Figure 5A, available at Carcinogenesis Online). Lastly, MOE GFP were plated onto 3D type I collagen gels, because this is the primary ECM in the ovarian stroma ( Supplementary Figure 5B, available at Carcinogenesis Online). As predicted, 6.7× more MOE GFP cells bound to MOST relative to MOSE monolayers (P < 0.05). Supporting a role for collagen as a binding surface for metastatic FTE-derived HGSOCs, 3-fold more cells bound to 3D collagen gel relative to MOST cells (P < 0.0001; Figure 2E and F). In a 2D adhesion assay, MOE cells bound equally well to type I collagen (primarily a stromal ECM protein), type IV collagen and laminin, which are all primarily basement membrane ECM proteins. About 25% less MOE cells bound to vitronectin, a glycoprotein found in high concentrations in serum ( Figure 2G; P < 0.05). Finally, the ability of OSE cells to hinder binding to underlying type I collagen was tested by plating an increasing number of MOSE cells on top of 2D type I collagen in a 96-well plate and then performing adhesion assays with MOE GFP cells. Increasing the number of MOSE cells in each well led to a linear decrease in MOE GFP attachment (P < 0.0001, R2 = 0.9047; Figure 2H). Staining the MOSE monolayer with CellTracker Red dye showed that in subconfluent wells, MOE GFP adhered between MOSE cells. In confluent wells, MOE GFP adhered between MOSE cells when possible, but some MOE GFP cells did adhere on top of the MOSE monolayer ( Figure 2I). These results suggest that FTE preferentially adhere to ECM proteins (including type I collagen) that are under the ovarian surface and are exposed during ovulation ( 10, 11).
Loss of PTEN increases the ability of FTE cells to grow on 3D type I collagen
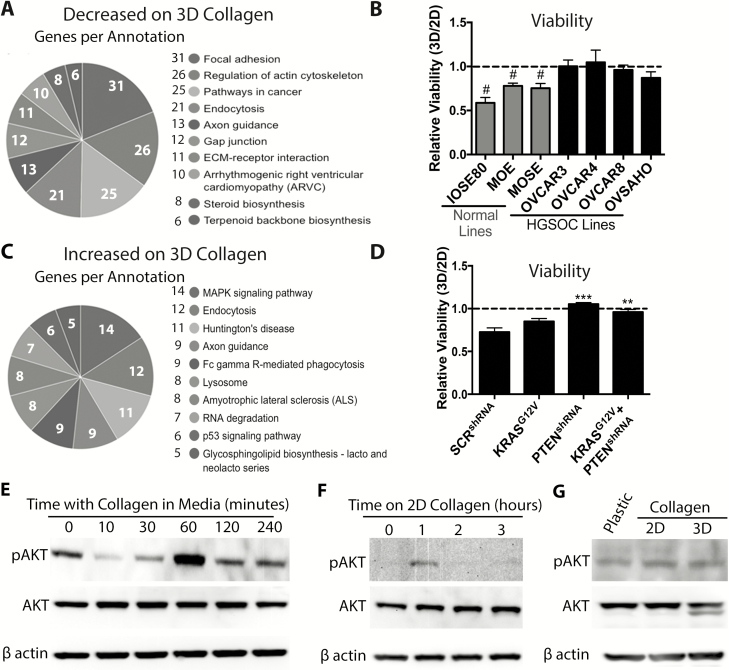
RNAseq was used to compare the transcriptome of MOE cells grown on 2D or 3D collagen. The time period of adhesion to 3D collagen was optimized by monitoring connective tissue growth factor (CTGF) expression, which decreased after 6 h (P < 0.05; Supplementary Figure 5C, available at Carcinogenesis Online) ( 21). RNAseq-identified 847 transcripts decreased (including CTGF) and 692 transcripts increased by 3D collagen ( Supplementary Figure 5D, available at Carcinogenesis Online; GSE112397). Interestingly, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of transcripts that were downregulated by 3D collagen identified pathways regulating focal adhesion, actin cytoskeleton and ECM–receptor interaction ( Figure 3A), suggesting that untransformed MOE cells may respond poorly to a microenvironment rich in 3D collagen. Next, we measured the viability of a panel of normal and HGSOC cell lines 24 h after plating on 2D and 3D collagen. To account for intrinsic differences in the viability of different cell lines, viability of each cell line on 3D collagen was normalized to the viability of the same cell line on 2D collagen (3D:2D viability ratio). The 3D:2D viability ratio of three normal cell lines (MOE, MOSE and IOSE80) was reduced on 3D collagen relative to their viability on 2D collagen (3D:2D viability ratio <1; P < 0.05). In contrast, the viability of all four HGSOC cell lines (OVCAR3, OVCAR4, OVCAR8 and OVSAHO) was unaffected by 3D collagen (3D:2D viability ratio = 1; Figure 3B, morphology on plastic, 2D collagen and 3D collagen shown in Supplementary Figures 6 and 7, available at Carcinogenesis Online). These data indicate that even though normal FTE attached to the collagen exposed by ovulation, HGSOC lines are better at surviving in a microenvironment rich in 3D type I collagen than normal epithelial cells.
Figure 3.
Loss of PTEN allows FTE cells to survive in a collagen-rich microenvironment. (A) KEGG analysis of genes decreased by culture on 3D collagen for 6 h. (B) 3D:2D viability ratio of normal (IOSE80, MOE and MOSE) and HGSOC cells (OVCAR3, OVCAR4, OVCAR8 and OVSAHO). (C) KEGG analysis of genes increased by culture on 3D collagen. (D) 3D:2D viability ratio of MOE cells stably expressing a control vector, KRASG12V, PTENshRNA or KRASG12V + PTENshRNA. (E) Representative western blots for phospho-AKT, AKT and β actin of MOE cells treated with 100 ng/ml of collagen for indicated time points. (F) Representative western blot for MOE cells plated on 2D collagen for indicated times. (G) Representative western blots for phospho-AKT, AKT and β actin of MOE cells cultured on plastic, 2D collagen and 3D collagen for 24 h. Data are plotted as mean ± SEM (n = 3–5). Significantly different from 1, #P < 0.05. Significantly different from control, **P < 0.01, ***P < 0.001.
KEGG analysis of transcripts increased in response to growth on 3D collagen found significant differences in the p53 pathway ( Figure 3C). Analysis of the data found that the KEGG pathway identified only six transcripts associated with p53 signaling that were significantly altered ( Supplementary Figure 8A, available at Carcinogenesis Online). Therefore, the list of genes altered by 3D collagen was compared with a list of canonical p53 targets ( 23). Of the 41 well-established canonical p53 targets identified by Nikulenkov et al., only 17% were significantly different in the RNAseq dataset ( Supplementary Figure 8B, available at Carcinogenesis Online). In addition, plating MOE cells on 2D or 3D collagen for 24 h did not result in stabilization of the p53 protein based on western blots ( Supplementary Figure 8C, available at Carcinogenesis Online). Functionally, the viability of MOE cells expressing the two most common mutations in p53 (R273 and R248) was reduced on 3D collagen to a similar extent (20–30%) as the vector control cells ( Supplementary Figure 8D, available at Carcinogenesis Online). These data indicate that mutation in p53 was not sufficient to allow cells to thrive in a microenvironment rich in 3D collagen.
In HGSOC, alterations that activate PI3K/AKT and mitogen-activated protein kinase/extracelluar regulated kinase are common, and these pathways are well known to regulate cell viability and survival in many other contexts ( 24, 25). Therefore, we tested the viability of MOE cells stably expressing KRASG12V, PTENshRNA or KRASG12V + PTENshRNA. MOE KRASG12V cells showed a slightly better 3D:2D viability ratio as compared with normal cells, but this did not reach statistical significance (P = 0.088; Figure 3D). In contrast, stable expression of PTENshRNA completely rescued the 3D:2D viability ratio of MOE cells. The 3D:2D viability ratio of MOE KRASG12V+ PTENshRNA was similar to that of MOE PTENshRNA alone ( Figure 3D), indicating that PTENshRNA, by itself, is sufficient to rescue the relative viability of MOE cells on 3D collagen.
Given that collagen is well known to activate PI3K/AKT signaling in other cell types ( 26, 27), we tested the ability of collagen to increase phospho-AKT in MOE cells by western blot. Because of the transient nature of AKT phosphorylation in response to extracellular stimulation and the time required for cells to attach to collagen after plating (~1–2 h), we added 100 ng/ml collagen to the media of MOE cells that were grown on plastic and serum-starved for 24 h. Treatment with collagen resulted in a dramatic increase in phospho-AKT levels at 60 min, which returned to baseline at 120 and 240 min ( Figure 3E). Plating MOE cells on 2D collagen led to an increase in phospho-AKT at 1 h that was absent by 2 and 3 h ( Figure 3F), confirming a transient increase in AKT activation. To investigate prolonged phospho-AKT activation due to collagen, MOE cells were plated on plastic, 2D collagen and 3D collagen for 24 h, and no differences in phospho-AKT levels were detected ( Figure 3G). These results suggest that sustained activation of PI3K signaling enhances MOE cellular survival in a microenvironment rich in 3D collagen.
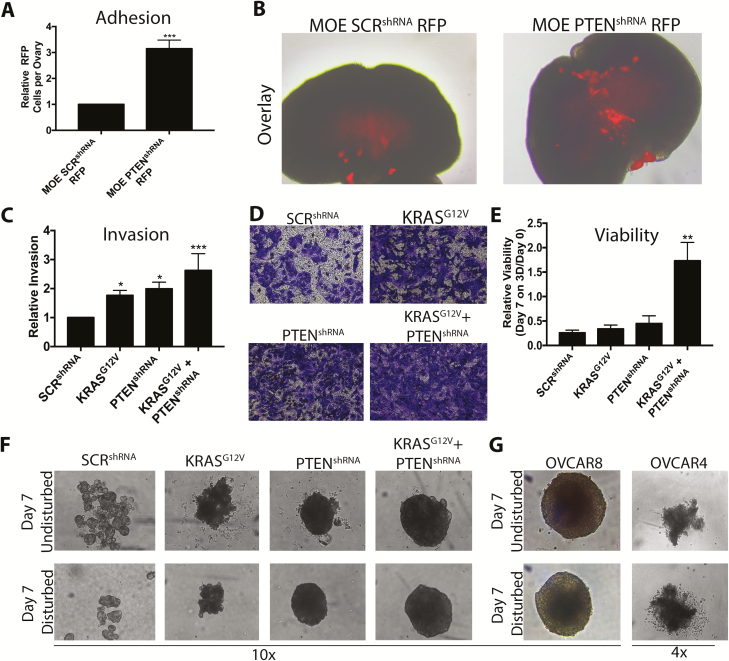
Colonization of the ovary is a complex process. In addition to just surviving in a 3D collagen-rich microenvironment, cells must first attach to the ovary and then invade. Therefore, the ability of MOE cells stably expressing KRASG12V or PTENshRNA to attach to type I collagen was further characterized. The ability of MOE cells stably expressing a scrambled shRNA (SCRshRNA) with RFP and MOE PTENshRNA RFP cells to adhere to murine ovaries was measured. MOE PTENshRNA RFP cells attached 3-fold more to ovulation mimetic ovaries than MOE SCRshRNA RFP (P < 0.001; Figure 4A and B). Expression of KRASG12V, PTENshRNA and KRASG12V+PTENshRNA significantly increased invasion of MOE cells thorough collagen (1.7- to 2.6-fold over MOE SCRshRNA; P < 0.05; Figure 4C and D). After 7 days on 3D collagen, the relative viability of KRASG12V + PTENshRNA was higher than that of control cells, KRASG12V cells and PTENshRNA cells ( Figure 4E), suggesting that the combination was necessary for long-term viability on 3D collagen. Surprisingly, MOE PTENshRNA and KRASG12V + PTENshRNA cells formed spheroids in 3D collagen ( Figure 4F), similar to the spheroids formed by OVCAR8 cells ( Figure 4G). MOE KRASG12V cells formed spheroid-like structures, but they did not exhibit the typical smooth, spherical morphology. These results indicate that silencing PTEN increases the ability of FTE cells to attach to ovaries and invade, survive and form spheroids in a type I collagen-rich microenvironment.
Figure 4.
Loss of PTEN increases the ability of FTE cells to adhere, invade and form spheroids in 3D collagen. (A and B) Attachment of MOE SCRshRNA RFP and MOE PTENshRNA RFP cells to ovulation mimetic ovaries. (C and D) Boyden chamber invasion through 3D collagen of MOE cells stably expressing SCRshRNA (control), PTENshRNA, KRASG12V or KRASG12V + PTENshRNA. (E) Viability of MOE cell lines plated on 3D collagen for 7 days. (F and G) Representative images of structures formed by MOE cell lines (F) or HGSOC lines (G) after 7 days. (F) Data are plotted as mean ± SEM (n = 3–5). Significantly different from MOE SCRshRNA, *P < 0.05, **P < 0.01, ***P < 0.001.
AKT and RAC1/JNK mediates the effects of PTENshRNA in FTE cells
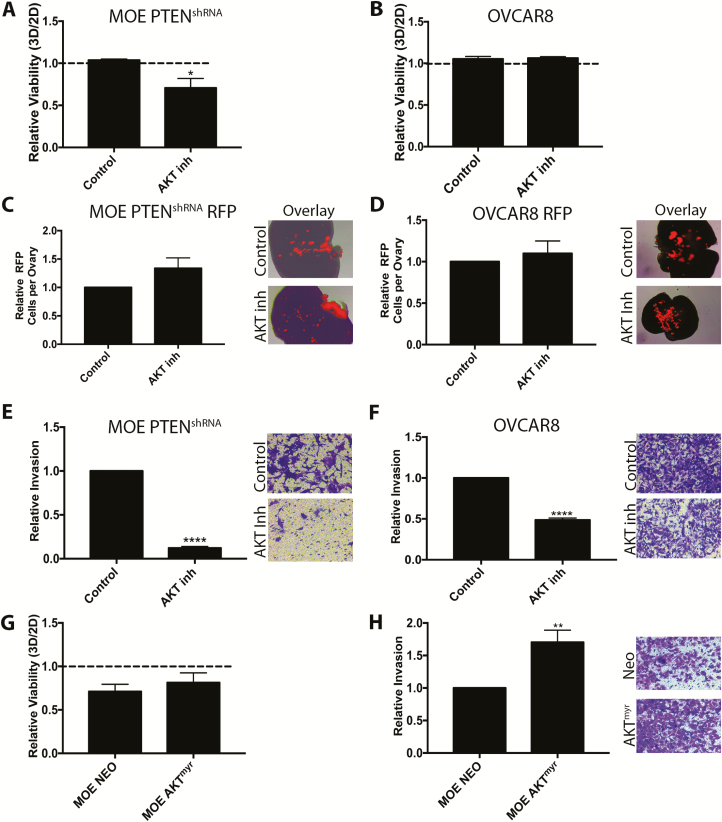
AKT activation occurs after PTEN loss ( Supplementary Figure 9A, available at Carcinogenesis Online); therefore, the role of AKT in mediating the phenotypes seen in MOE PTENshRNA cells was assessed using the AKT inhibitor MK2206. MK2206 (10 μM) reduced the 3D:2D viability ratio of MOE PTENshRNA from 1.05 to 0.71 (P < 0.05; Figure 5A). However, MK2206 had no effect on the 3D:2D viability ratio of OVCAR8 cells ( Figure 5B). Interestingly, MK2206 had no effect on the attachment of either MOE PTENshRNA RFP or OVCAR8 RFP cells to ovulation mimetic ovaries ( Figure 5C and D), suggesting this effect of PTENshRNA is not entirely dependent on AKT alone. MK2206 dramatically reduced invasion of MOE PTENshRNA cells through collagen by 87% (P < 0.0001; Figure 5E) and invasion of OVCAR8 cells through collagen by 52% (P < 0.0001; Figure 5F). To determine if activation of AKT could similarly increase viability and invasion observed with MOE PTENshRNA cells, the MOE AKTmyr cells were investigated. Stable expression of AKTmyr in MOE cells increased phospho-AKT to a similar extent as PTENshRNA ( Supplementary Figures 1C and 8A, available at Carcinogenesis Online). However, AKTmyr did not rescue the viability ratio of MOE cells ( Figure 5G). AKTmyr increased invasion of MOE cells (1.7-fold, P < 0.01; Figure 5H), though the effect was 30% less than seen in the MOE PTENshRNA cells. These data indicate that phospho-AKT only partially mediates the effects seen in MOE PTENshRNA cells and support recent reports from other tumor types that transformation following PTEN loss or PI3K activation increases signaling via multiple pathways ( 25, 26, 28).
Figure 5.
AKT is sufficient to increase invasion, required but not sufficient to increase viability and does not mediate increased ovarian attachment of MOE cells. (A and B) Viability ratio of MOE PTENshRNA (A) and OVCAR8 (B) cells cultured on 2D or 3D collagen with the AKT inhibitor (MK2206) or DMSO (control). (C and D) Attachment of MOE PTENshRNA RFP (C) and OVCAR8 RFP (D) cells to ovulation mimetic ovaries in the presence of dimethyl sulfoxide (DMSO) (control) or MK2206. (E and F) Boyden invasion assays through 3D collagen in the presence of DMSO (control) or MK2206. (G) 3D:2D viability ratio of control (MOE NEO) and MOE AKTmyr cells. (H) Invasion of MOE NEO and MOE AKTmyr through 3D collagen in Boyden assays. Data are plotted as mean ± SEM (n = 3–5). Significantly different from control or MOE NEO, *P < 0.05, **P < 0.01, ****P < 0.0001.
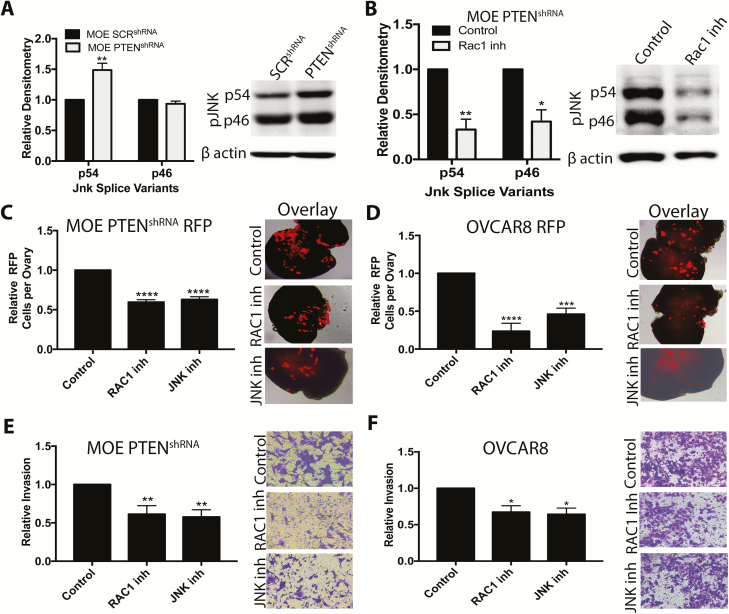
In other cell types, loss of PTEN activates RAC1 and JNK signaling ( 29–31), and recently we demonstrated that RAC1 stimulated activin A-induced migration of MOE cells ( 12). Western blots indicated higher phosphorylation levels of the p54 splice variant of JNK in MOE PTENshRNA relative to MOE SCRshRNA (P < 0.01), but no difference in phosphorylation level of the p46 splice variant ( Figure 6A). To interrogate the role of RAC1 and JNK, we used the small molecule inhibitors NSC23766 and JNK inhibitor V, respectively. Confirming that RAC1 mediates the increase in phospho-JNK, NSC2766 significantly reduced phospho-JNK levels in MOE PTENshRNA cells (P < 0.05; Figure 6B). Phospho-JNK levels were not altered in MOE AKTmyr relative to MOE NEO ( Supplementary Figure 9B, available at Carcinogenesis Online), confirming that PTENshRNA-induced activation of RAC1/JNK signaling is not dependent on phospho-AKT. Neither the RAC1 nor the JNK inhibitor reduced the 3D:2D viability ratio of MOE PTENshRNA or OVCAR8 cells ( Supplementary Figure 9C–D, available at Carcinogenesis Online). Both inhibitors, however, reduced attachment of MOE PTENshRNA RFP cells to ovulation mimetic ovaries by 40% (P < 0.0001; Figure 6C). These inhibitors were more effective in OVCAR8 RFP cells, reducing ex vivo adhesion by ~75% (P < 0.01; Figure 6D). In confirmation, a RAC1siRNA (previously validated ( 12)) reduced adhesion of MOE PTENshRNA RFP by approximately 40% (P < 0.05; Supplementary Figure 9E, available at Carcinogenesis Online). Inhibition of RAC1 and JNK also reduced invasion of MOE PTENshRNA through 3D collagen gels by 40% (P < 0.01; Figure 6E) and invasion of OVCAR8 cells by >30% (P < 0.05; Figure 6F). The guanine exchange factors PREX1 and PREX2 activate RAC1 after loss of PTEN in breast cancer ( 31). As PREX1 was undetectable in our RNAseq dataset, we tested the ability of a PREX2siRNA to reduce ovarian adhesion of MOE PTENshRNA RFP cells to murine ovaries. The PREX2siRNA dramatically reduced PREX2 protein levels over 3 days ( Supplementary Figure 9F–G, available at Carcinogenesis Online) but had no effect on ovarian adhesion of MOE PTENshRNA RFP cells ( Supplementary Figure 9H, available at Carcinogenesis Online). Collectively, these results indicate that RAC1/JNK contributes to the effects of PTENshRNA in MOE cells.
Figure 6.
RAC1/JNK signaling mediates the effects of PTEN loss on ovarian adhesion and migration through 3D collagen. (A and B) Representative western blots for phospho-JNK and β actin in MOE SCRshRNA and MOE PTENshRNA cells (A) and MOE PTENshRNA cells treated with a RAC1 inhibitor (NSC23766). (C and D) Adhesion of MOE PTENshRNA RFP cells (C) and OVCAR8 RFP (D) to ovulation mimetic ovaries in the presence of vehicle (control), a RAC1 inhibitor or a JNK inhibitor (JNK inhibitor VIII). (E and F) Invasion through type I collagen of MOE PTENshRNA (E) and OVCAR8 cells (F). Data are plotted as mean ± SEM (n = 3–5). Significantly different from control or MOE SCRshRNA, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
The current results suggest that colonization of the ovary may represent an important step in peritoneal metastasis of FTE-derived cancer. A mere 50 000 MOE KRASG12V + PTENshRNA cells injected into the ovarian bursa developed into aggressive tumors that spread throughout the peritoneum and resulted in death of the animals, whereas peritoneal grafting with this number of cells did not result in a single tumor forming. In a previous study, IP xenograft of 1 × 107 of the same cells resulted in tumor formation ( 15), with mice succumbing to the disease in a similar time frame as in this study. IB allografting results in a higher density of tumorigenic cells due to the confined nature of this membrane-encapsulated space. One theory could be that tumorigenic cells would be in higher concentration near the ovary as they are exfoliated from STIC lesions in the fallopian tube and dispersed into the peritoneal space. We hypothesize that the ovary represents a unique organ for primary metastasis in part due to its proximity to the fallopian tube. Previous studies confirmed that xenografting HGSOC cancer cells, but not other types of cancer cells, intravenously resulted in tumor formation in the ovary ( 22). In a transgenic mouse model (Brca, Tp53, Pten), ovariectomy reduced peritoneal metastasis, further demonstrating the importance of the ovary in peritoneal spread ( 32). Collectively, these data suggest that colonization of the ovary is a key step in metastasis of FTE-derived tumors and that understanding the mechanisms that drive tumorigenic FTE cells to colonize the ovary may improve strategies to disrupt this step and increase survival.
Physically cutting the ovarian surface was used in this study to mimic physical rupture of the ovary during ovulation, which might change the ovarian microenvironment in ways other than simply exposing the underlying ECM. However, conditioned media from intact and ovulation mimetic ovaries equally stimulated migration of MOE cells, indicating that alterations in adhesion were likely not due to chemotactic factors leaking out of the ovary after cutting in our experiments using ovulation mimetic ovaries. MOE cells attached to type I collagen and MOST cells better than to MOSE and increasing the number of MOSE cells hindered adhesion of MOE GFP cells to the underlying collagen. Yang-Hartwich et al. ( 9) showed that ovarian tumor initiating cells adhered to type IV collagen, but it was not clear if these were derived from FTE. The cell of origin may be important when considering factors that enhance ovarian colonization, such as increased WNT4 expression detected after loss of PTEN in fallopian-tube-derived tumor cell lines ( 16). All together, these investigations suggest a model where in women the ovary produces chemoattractant factors that draw FTE cells to the ovary, which then allows FTE cells to bind to the exposed ECM during and after ovulation.
We have shown previously that PTENshRNA, but not AKTmyr, confers tumorigenic properties to MOE cells ( 15). Here, multiple pathway alterations that are associated with HGSOC (p53 mutation, activation of KRAS, loss of PTEN) were tested; however, only loss of PTEN reversed the low 3D:2D viability ratio seen in normal MOE cells on 3D collagen. PTEN loss also increased attachment, invasion and spheroid formation, suggesting that loss of PTEN is sufficient to drive colonization of the ovary, which was supported by a transgenic mouse model ( 16). In both The Cancer Genome Atlas datasets for ovarian cancer, PTEN is genetically deleted in less than 7% of HGSOC cases ( 13). However, a recent study using whole-exome sequencing of laser-capture microdissections found that five of nine STICs had a loss of PTEN or a somatic mutation in the gene ( 2). Immunohistochemistry of STICs found 33% to be negative for PTEN ( 33), and Martins et al. ( 34) found that PTEN protein was low or absent in the tumor cells in over 50% of HGSOC cases. Multiple microRNAs that target PTEN have been shown to be overexpressed in ovarian cancer ( 35, 36), suggesting posttranscriptional mechanisms also contribute to loss of PTEN. Collectively, these results suggest that loss of PTEN may be more frequent than appreciated in HGSOC derived from FTE due to mechanisms that control PTEN expression beyond genetic loss.
Loss of PTEN or activation of PI3K is common across multiple types of cancer ( 29, 37, 38). Historically, the importance of these signaling alterations was attributed to activation of AKT. However, it is becoming increasingly clear that PTEN loss activates multiple pathways that are required for transformation. Transcriptomic analysis in A431 (epidermoid carcinoma), HCC827 (non-small-cell lung carcinoma) and SKBR-3 (mammary adenocarcinoma) cells with and without PTEN deletion identified increased JNK signaling ( 29). Importantly, this increase was mediated by RAC1 and was AKT independent. In agreement, restoration of PTEN in glioblastoma reduced phospho-JNK levels ( 38). In this study, AKT and RAC1/JNK signaling contributed to the increased invasion seen in MOE PTENshRNA cells, whereas RAC1/JNK increased adhesion. Interestingly, results from experiments using MOE PTENshRNA, MOE AKTmyr and the AKT inhibitor MK2206 suggest that AKT activation contributes to, but is not sufficient for, increased 3D:2D viability. Inhibition of the RAC1/JNK pathway did not reverse the phenotype seen in MOE PTENshRNA cells.
In this study, we used MOE cells due to their known tissue of origin and our collection of stable clones that mimic changes common in HGSOC ( 15, 20). We also used OVCAR8 cells due to their mutation in p53 ( 39), their ability to form aggressive tumors in vivo ( 40) and their classification as possibly HGSOC based on genetic analysis ( 41). However, it is possible that cancer cells propagated on plastic have acquired behavior that affects their response. Patient-derived xenografts were originally proposed to model in situ cancers from patients, but even those have recently been shown to evolve away from the original tumors ( 42). Therefore, primary cells would be a better model system, but many of the assays required GFP or RFP expression, necessitating at least short-term growth on plastic and clonal selection. Finally, primary normal FTE cells would not allow us to investigate genetic changes common in HGSOC whereas primary cells from HGSOC tumors would contain many mutations, making it challenging to investigate the effect of specific mutations.
In conclusion, tumorigenic FTE cells grafted in the bursa colonized the ovary and then metastasized throughout the peritoneum. FTE cells preferentially bound to ECM, particularly collagen, found under the OSE; however, the viability of normal FTE cells was reduced by 3D type I collagen. Loss of PTEN activated phospho-AKT and RAC1/JNK, which led to increased cell viability on 3D collagen, increased attachment of FTE cells to ovaries and increased invasion through 3D collagen. These results suggest that loss of PTEN may be an important step in metastasis from the fallopian tube to the ovary and the colonization of the ovary is a critical step in progression of HGSOC.
Funding
Department of Defense (Grant OC130046); the Ovarian Cancer Research Fund Alliance Ann and Sol Schreiber Mentored Investigator Award (No. 543296); UG3 (ES029073).
Conflict of Interest Statement: The authors have no conflict of interest to declare.
Supplementary Material
Glossary
Abbreviations
- AKTmyr
myristoylated AKT
- 3D
three dimensional
- ECM
extracellular matrix
- FTE
fallopian tube epithelium
- GFP
green fluorescent protein
- HGSOC
high-grade serous ovarian cancer
- IB
intrabursal
- IP
intraperitoneal
- JNK
c-jun N-terminal kinase
- MOE
murine oviductal epithelial
- MOSE
murine ovarian surface epithelial
- MOST
murine ovarian stroma
- OSE
ovarian surface epithelium
- RFP
red fluorescent protein
- RNAseq
RNA sequencing
- SCRshRNA
scrambled shRNA
- STIC
serous tubal intraepithelial carcinoma
References
- 1. Marquez R.T., et al. (2005) Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin. Cancer Res., 11, 6116–6126. [DOI] [PubMed] [Google Scholar]
- 2. Labidi-Galy S.I., et al. (2017) High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun., 8, 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coscia F., et al. (2016) Integrative proteomic profiling of ovarian cancer cell lines reveals precursor cell associated proteins and functional status. Nat. Commun., 7, 12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falconer H., et al. (2015) Ovarian cancer risk after salpingectomy: a nationwide population-based study. J. Natl. Cancer Inst., 107, dju410. [DOI] [PubMed] [Google Scholar]
- 5. Hankinson S.E., et al. (1993) Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. JAMA, 270, 2813–2818. [PubMed] [Google Scholar]
- 6. Samimi G., et al. (2017) Opportunistic salpingectomy: what about the role of the ovary in ovarian cancer? Cancer, 123, 1699–1702. [DOI] [PubMed] [Google Scholar]
- 7. Merritt M.A., et al. (2013) Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum. Reprod., 28, 1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Titus-Ernstoff L., et al. (2001) Menstrual and reproductive factors in relation to ovarian cancer risk. Br. J. Cancer, 84, 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang-Hartwich Y., et al. (2014) Ovulation and extra-ovarian origin of ovarian cancer. Sci. Rep., 4, 6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright J.W., et al. (2010) Ovulation in the absence of the ovarian surface epithelium in the primate. Biol. Reprod., 82, 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wright J.W., et al. (2011) Dynamics of the primate ovarian surface epithelium during the ovulatory menstrual cycle. Hum. Reprod., 26, 1408–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean M., et al. (2017) Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett., 391, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancer Genome Atlas Research Network. (2011) Integrated genomic analyses of ovarian carcinoma. Nature, 474, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanrahan A.J., et al. (2012) Genomic complexity and AKT dependence in serous ovarian cancer. Cancer Discov., 2, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eddie S.L., et al. (2015) Tumorigenesis and peritoneal colonization from fallopian tube epithelium. Oncotarget, 6, 20500–20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russo A., et al. (2018) PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene, 37, 1976–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodgers L.H., et al. (2016) Loss of PAX8 in high-grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget, 7, 32785–32795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moyle-Heyrman G., et al. (2016) Genome-wide transcriptional regulation of estrogen receptor targets in fallopian tube cells and the role of selective estrogen receptor modulators. J. Ovarian Res., 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karthikeyan S., et al. (2016) Cadherin-6 type 2, K-cadherin (CDH6) is regulated by mutant p53 in the fallopian tube but is not expressed in the ovarian surface. Oncotarget, 7, 69871–69882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quartuccio S.M., et al. (2015) Mutant p53 expression in fallopian tube epithelium drives cell migration. Int. J. Cancer, 137, 1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbolina M.V., et al. (2009) Downregulation of connective tissue growth factor by three-dimensional matrix enhances ovarian carcinoma cell invasion. Int. J. Cancer, 125, 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coffman L.G., et al. (2016) New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl. Res., 175, 92–102.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikulenkov F., et al. (2012) Insights into p53 transcriptional function via genome-wide chromatin occupancy and gene expression analysis. Cell Death Differ., 19, 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia H., et al. (2004) Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem., 279, 33024–33034. [DOI] [PubMed] [Google Scholar]
- 25. Levental K.R., et al. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vasudevan K.M., et al. (2009) AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell, 16, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nho R.S., et al. (2005) Role of integrin-linked kinase in regulating phosphorylation of Akt and fibroblast survival in type I collagen matrices through a beta1 integrin viability signaling pathway. J. Biol. Chem., 280, 26630–26639. [DOI] [PubMed] [Google Scholar]
- 28. Orlacchio A., et al. (2017) SGK1 is a critical component of an AKT-independent pathway essential for PI3K-mediated tumor development and maintenance. Cancer Res., 77, 6914–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vivanco I., et al. (2007) Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell, 11, 555–569. [DOI] [PubMed] [Google Scholar]
- 30. Liliental J., et al. (2000) Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr. Biol., 10, 401–404. [DOI] [PubMed] [Google Scholar]
- 31. Mense S.M., et al. (2015) PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Sci. Signal., 8, ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perets R., et al. (2013) Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell, 24, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roh M.H., et al. (2010) High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod. Pathol., 23, 1316–1324. [DOI] [PubMed] [Google Scholar]
- 34. Martins F.C., et al. (2014) Combined image and genomic analysis of high-grade serous ovarian cancer reveals PTEN loss as a common driver event and prognostic classifier. Genome Biol., 15, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang H., et al. (2008) MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res., 68, 425–433. [DOI] [PubMed] [Google Scholar]
- 36. Lou Y., et al. (2010) MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med., 26, 819–827. [DOI] [PubMed] [Google Scholar]
- 37. Thorpe L.M., et al. (2015) PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer, 15, 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rong Y., et al. (2009) Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res., 69, 2540–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leroy B., et al. (2014) Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum. Mutat., 35, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitra A.K., et al. (2015) In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol., 138, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Domcke S., et al. (2013) Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun., 4, 2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ben-David U., et al. (2017) Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet., 49, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.