Abstract
Aims
A growing body of evidence suggests that a higher maternal pre-pregnancy body mass index results in higher offspring’s blood pressure, but there is inconsistency about the impact of father’s body mass index. Furthermore, evidence is limited with regard to low and middle income countries. We aimed to determine the association between parental pre-pregnancy body mass index and offspring’s blood pressure during the first year of life.
Methods
In 587 infants of the BReastfeeding Attitude and Volume Optimization (BRAVO) trial systolic and diastolic blood pressure were measured twice at the right leg in a supine position, using an automatic oscillometric device at day 7, month 1, 2, 4, 6, 9 and 12. Parental pre-pregnancy body mass index was based on self-reported weight and height. Linear mixed models were performed to investigate the associations between parental pre-pregnancy body mass index and offspring blood pressure patterns.
Results
Each unit increase in maternal body mass index was associated with 0.24 mmHg (95% confidence interval 0.05; 0.44) and 0.13 mmHg (0.01; 0.25) higher offspring’s mean systolic and diastolic blood pressure, respectively, during the first year of life. A higher offspring blood pressure with increased maternal pre-pregnancy body mass index was seen at birth and remained higher during the first year of life. The association with systolic blood pressure remained similar after including birth size and offspring’s weight and height over time. The association with diastolic blood pressure attenuated slightly to a non-significant result after including these variables. Paternal body mass index was not associated with offspring’s blood pressure.
Conclusion
Higher maternal pre-pregnancy body mass index, but not paternal pre-pregnancy body mass index, is associated with higher offspring blood pressure already from birth onwards.
Keywords: Blood pressure, infant, parental pre-pregnancy body mass index, body mass index mother, body mass index father
Introduction
Many low and middle income countries are in an epidemiological transition, and will experience a strong increase in non-communicable diseases in the coming years. In Indonesia there is major concern about the rising cardiovascular disease (CVD) prevalence – currently 37% of all deaths – and the increasing numbers of overweight and obesity, which are important risk factors for CVD.1, 2 This is accompanied by concerns about an increasing number of future parents with elevated body mass index (BMI). A systematic review suggests that higher maternal pre-pregnancy BMI results in higher offspring’s blood pressure (BP),3 but evidence is still limited especially with regard to low and middle income countries. Moreover, no consensus has been reached concerning the effect of fathers’ pre-pregnancy BMI on offspring’s BP.4, 5 It is also unknown whether this potential maternal effect is already detectable very early in life, when infants are barely exposed to environmental factors that could influence their BP values. It is important to examine the effects of early life factors on BP, because BP tracks from childhood to adulthood.6 Although the immediate effects will in general be small, their relevance is that they can affect childhood to midlife BP trajectories, which in turn predict CVD risk.7 Moreover, in Indonesia smoking8 and alcohol consumption9 among women are extremely rare, in contrast to women originating from western countries. This enables us to investigate the effect of pre-pregnancy BMI on offspring BP without the likely confounding effects of maternal smoking and alcohol consumption. Finally, infancy and childhood are increasingly recognised as seminal periods with regard to cardiovascular and cardiometabolic risk. All over the world, including Indonesia, pregnancy is a relatively short period in which healthy young women receive frequent professional care, and at that young age this creates a window of opportunity for preventive measures, including those that could benefit both mothers and their children. For these reasons, it is important to know if pre-pregnancy risk in parents is transmitted specifically through pregnancy to infant offspring, or whether such transmission is a mere reflection of shared familial traits and lifestyles.10 The aim of our study was to investigate whether there is an association between parental pre-pregnancy BMI and offspring’s BP during the first year of life, and if so from which time period this association is detectable.
Methods
Study design and setting
The BReastfeeding Attitude and Volume Optimization (BRAVO) trial is a parallel groups randomised trial in Jakarta, Indonesia, which was designed to study breastfeeding effects on early life signs of later life cardiovascular and cardiometabolic outcomes. The design of the BRAVO trial is described in detail elsewhere.11 Briefly, women were recruited from Budi Kemuliaan Hospital, a private specialised referral centre for maternal and child care in the municipality of Jakarta. Although this is a private centre, it serves mostly the low middle socioeconomic status (SES) group, including those covered by government insurance in the period of recruitment. Women were also recruited from local primary care centres, including primary care centres in the Senen and Jatinegara districts. From July 2012 onwards, eligible pregnant women were randomly allocated either to receive primary care as usual or a programme aimed at add-on breastfeeding optimisation which extends from late pregnancy to 6 months after delivery. Women who, after random assignment, had babies that were stillborn, born prematurely (birthweight < 2500 g, gestational age < 37 weeks), had congenital heart disease or multiple congenital anomalies were excluded. Of the 1000 pregnant women who had been randomly assigned, 267 were already lost to follow up before giving birth. Of 693 out of 733 newborns at least one of the parental BMI measurements was available, of whom 587 had BP measured at least at one time point (Figure 1).
Figure 1.
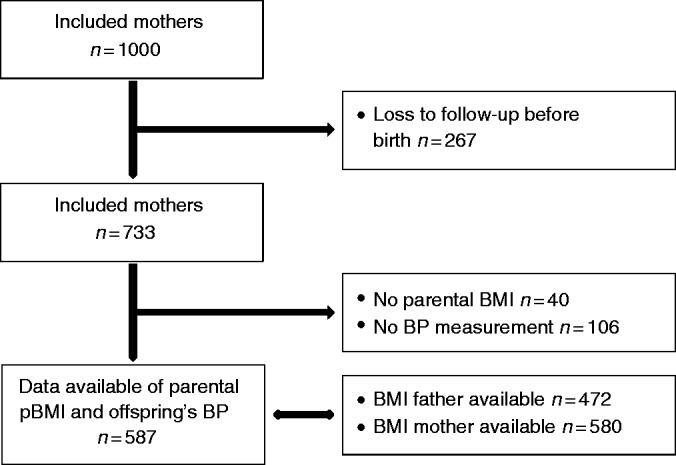
Flowchart of the study population.
Intervention
Care as usual means that there was no interference with breastfeeding propagation practice in current Indonesian care which, in general, follows the World Health Organization (WHO) recommendations for successful breastfeeding. The BRAVO programme consisted of antenatal, perinatal and postnatal interventions, and special support for working mothers. At private counselling in late pregnancy, women and families were enabled to visit a lactation counsellor for 30 minutes, mainly to build motivation for breastfeeding. At group counselling, women and closest family members were subjected to a commercially available video (http://injoyvideos.com/) including steps to perform better breastfeeding. Group counselling also allowed mothers to share opinions with peers for mutual encouragement. Postnatally, the intervention arm received one extra home visit from appointed midwives/breastfeeding counsellors to establish breastfeeding, address problems and check preparations at home. As part of the postnatal intervention, women received lactation support and mothers in the intervention group, who returned to work before 6 months after delivery, received special support.11
This study was ethically approved by the institutional review board of the Faculty of Medicine University of Indonesia/Cipto Mangunkusumo General Hospital (reference no.: 913/UN2.F1/ETIK/X/2015).
Pre-pregnancy BMI
Pregnant women of 24–36 weeks gestational age were asked to fill out an eligibility screening questionnaire, including their pre-pregnancy weight and their latest height. During an antenatal visit, pregnant women were asked to complete a questionnaire dealing with the health behaviour of her and her husband, including paternal weight and height. Maternal pre-pregnancy BMI and paternal BMI were calculated as weight in kilograms divided by height in meters squared (kg/m2).
Blood pressure
The offspring’s systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice at the right lower leg (popliteal artery) in a supine position, using an automatic oscillometric device (GE CRITIKON Dinamap) at day 7, month 1, 2, 4, 6, 9 and 12. Appropriate cuff sizes relative to the diameter of the leg were used. The first measurement was made by a paediatric research nurse after 5 minutes rest and 2 minutes quiet was observed between the measurements. The means of all available SBP and DBP recordings were used for analyses.
Confounding and intermediates
Parental characteristics
In the analyses parental characteristics – obtained by perinatal questionnaires – were considered as possible intermediates and confounders. Mother’s age at giving birth was considered as a confounder, as BMI increases with ageing12 and a higher maternal age might affect the intrauterine environment and programme the offspring to a higher BP.13 Although maternal (pre-)eclampsia (yes/no) during pregnancy, gestational diabetes (yes/no), gestational hypertension (yes/no), maternal smoking and alcohol use during pregnancy (yes/no) were considered as intermediates, we did not include these variables in separate models as percentages of (pre-)eclampsia (3%), gestational diabetes (0.6%), gestational hypertension (0.5%), maternal smoking (2%) and alcohol use (0%) during pregnancy were very low in our study population. We did provide two models in which we excluded the offspring of women who smoked during pregnancy and the offspring of women who had any gestational disease ((pre-)eclampsia, gestational diabetes or gestational hypertension). It has been suggested that second-hand smoke exposure during pregnancy also leads to higher offspring BP,14, 15 and smoking is related to obesity.16 Therefore, prenatal (second-hand) smoke exposure (yes/no) indicated by smoking during pregnancy by the mother, father or household members in the presence of the mother was included as a possible confounder. In our research question we considered SES a proxy for maternal BMI and adjusting for SES would lead to spurious results. A lower SES is associated with a higher BMI in women17 and with increased offspring’s BP, but higher (maternal) BMI partly explains this association,18 probably because it is an indicator for clustered health behaviours.19 Thus we did not consider SES a confounding bias of our association of interest.
Offspring’s characteristics
Offspring’s birth size – as proxy for fetal growth – and weight and height during the first year of life were also considered as intermediates. Birthweight, birth length and gestational age were used as indicators for birth size. Weight and height measurements were performed by a paediatric research nurse at birth and during immunisation visits at day 7, month 1, 2, 4, 6, 9 and 12.
Data analysis
The means and variances of parent and child characteristics were calculated based on the maternal pre-pregnancy BMI tertiles and frequencies were tested using Pearson’s chi-squared tests or – in case of too low expected values – Fisher’s exact tests. Differences in continuous variables were tested using analysis of variance (ANOVA) or Kruskal–Wallis test in the case of skewed data.
As the number of BP measurements over time differed per child, the crude associations between parental pre-pregnancy BMI and BP were assessed using linear mixed models (crude model), by using the variance components structure, and based on complete case analysis. Model fits for linear and quadratic models were compared, showing the best fit for the quadratic model. Model 1 includes additionally mother’s age at giving birth and second-hand smoke exposure. Although we investigated the association independent of the status of the intervention, we intended to make sure that there was no trial effect in this association. Therefore, model 2 additionally included the status of the intervention to model 1. Models 3 and 4 are explanatory models and consist of model 1 and, respectively, birth size and weight and height measured at the same visits as when BP was measured. Furthermore, an interaction term between birthweight and parental BMI was tested to investigate whether the association between parental BMI and BP is modified by fetal growth. Similarly, interaction terms between maternal BMI and intervention status (intervention vs. control), care centre (primary care vs. private specialised centre), breastfeeding (any breastfeeding during the first year of life), parity and gender were tested. Furthermore, to investigate whether the effect over time is different for different parental BMI values, interaction terms between time and quadratic time with parental BMI were tested by the likelihood ratio test. The offspring BP pattern over time was plotted for parental BMI, by using the estimated marginal means of BP of model 1, which were calculated at each month during the first year of life and for four random chosen BMI values: 20, 25, 30 and 35 kg/m2.
The results are expressed as mean differences with 95% confidence intervals (CIs) and P values. CIs not including zero, corresponding to P values less than 0.05 were considered as statistically significant. All analyses were performed using SPSS version 21.0 for Windows.
Results
The mean (SD) maternal and paternal pre-pregnancy BMI were 22.4 (4.5) and 22.9 (3.6) kg/m2, respectively. Mean offspring’s SBP and DBP ranged, respectively, from 93 to 109 mmHg and 50 to 64 mmHg during the first year of life. Table 1 demonstrates the general characteristics by maternal pre-pregnancy BMI tertiles. Compared to the lowest tertile, mothers in the highest tertile had 22 kg higher maternal pre-pregnancy weight, fathers had 2 kg/m2 higher BMI and offspring had 179 g higher birthweight. The baseline characteristics of participants and women who were not in the data analysis due to loss to follow-up or lacking data of the determinant of outcome were comparable (see Supplementary Appendix Table 1). SES, education, occupational status and type of job were comparable between these groups in both parents, but the family income of participants was higher compared to the loss to follow-up group.
Table 1.
Baseline characteristics of BRAVO participants by maternal pre-pregnancy BMI tertiles.
| Maternal pre-pregnancy BMI
(kg/m2) |
|||||
|---|---|---|---|---|---|
| N | 14–20 (n = 194) | 20–24 (n = 192) | 24–47 (n = 194) | P value | |
| Status trial (intervention) | 580 | 50.0 | 55.7 | 49.0 | 0.36 |
| Maternal characteristics | |||||
| Mean (SD) age at giving birth (years) | 484 | 27 (6) | 28 (5) | 30 (6) | <0.001 |
| Mean (SD) pre-pregnancy weight (kg) | 580 | 44 (5) | 52 (5) | 66 (9) | <0.001 |
| Mean (SD) height (cm) | 580 | 156 (6) | 155 (6) | 156 (6) | 0.11 |
| Education (%) | 0.51 | ||||
| Uneducated, elementary/junior school | 155 | 24.2 | 31.1 | 25.4 | |
| Senior high school | 381 | 69.6 | 61.6 | 66.8 | |
| University | 41 | 6.2 | 7.4 | 7.8 | |
| Occupational status (employed) (%) | 577 | 27.6 | 23.4 | 24.4 | 0.61 |
| Type of job (white collar) (%) | 141 | 56.9 | 59.1 | 60.0 | 0.95 |
| Family income (per month) (%) | 0.69 | ||||
| ≤ IDR 750,000 | 76 | 12.2 | 11.8 | 16.3 | |
| IDR 750,000– ≤ IDR 3,000,000 | 318 | 56.1 | 56.1 | 56.3 | |
| IDR 3,000,000– > IDR 9,000,000 | 172 | 31.7 | 32.1 | 27.3 | |
| (Pre-)eclampsia (yes) (%) | 462 | 2.6 | 2.5 | 4.1 | 0.75 |
| Prenatal smoking (yes) (%) | 450 | 2.7 | 0.7 | 2.7 | 0.34 |
| Prenatal alcohol use (yes) (%) | 476 | 0.0 | 0.0 | 0.0 | >0.99 |
| Median (IQR) parity (n) | 578 | 2 (1) | 2 (2) | 2 (2) | <0.001 |
| Paternal characteristics | |||||
| Mean (SD) BMI (kg/m2) | 465 | 22 (3) | 23 (4) | 24 (4) | <0.001 |
| Mean (SD) weight (kg) | 466 | 61 (10) | 64 (12) | 66 (11) | <0.001 |
| Mean (SD) height (cm) | 466 | 166 (7) | 167 (6) | 166 (6) | 0.39 |
| Education (%) | 0.57 | ||||
| Uneducated, elementary/junior school | 105 | 21.9 | 24.4 | 19.9 | |
| Senior high school | 320 | 71.0 | 64.6 | 66.7 | |
| University – undergraduate | 50 | 7.1 | 10.9 | 13.5 | |
| Occupational status (employed) (%) | 475 | 95.5 | 98.2 | 97.5 | 0.36 |
| Type of job (white collar) (%) | 454 | 31.3 | 23.9 | 27.0 | 0.36 |
| Smoking (yes) (%) | 451 | 68.2 | 66.0 | 66.0 | 0.89 |
| Child characteristics | |||||
| Gender (men) (%) | 575 | 57.3 | 55.5 | 57.3 | 0.92 |
| Mean (SD) gestational age (weeks) | 446 | 39 (2) | 39 (1) | 39 (1) | 0.12 |
| Mean (SD) birthweight (g) | 501 | 3017 (383) | 3137 (416) | 3196 (445) | <0.001 |
| Mean (SD) birth length (cm) | 496 | 48 (2) | 48 (2) | 49 (2) | 0.10 |
| Prenatal second hand smoke exposure (%) (yes) | 452 | 23.0 | 17.5 | 20.7 | 0.50 |
| Breastfeeding (%) (any, during first year) | 524 | 92.6 | 97.7 | 93.2 | 0.08 |
BMI: body mass index; IQR: interquartile range.
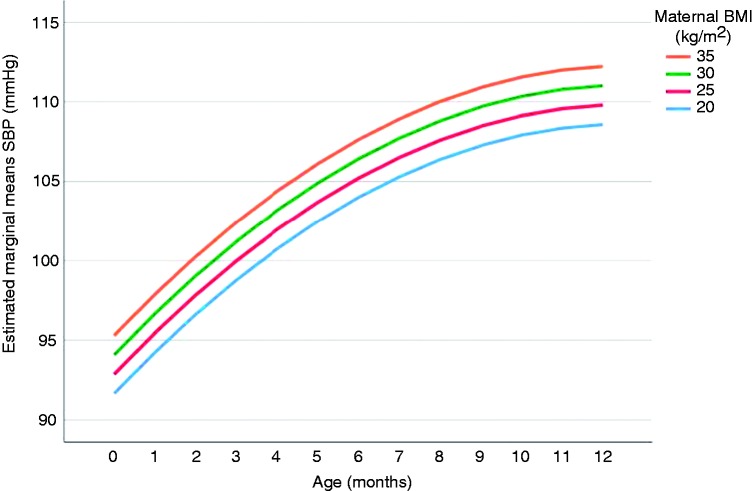
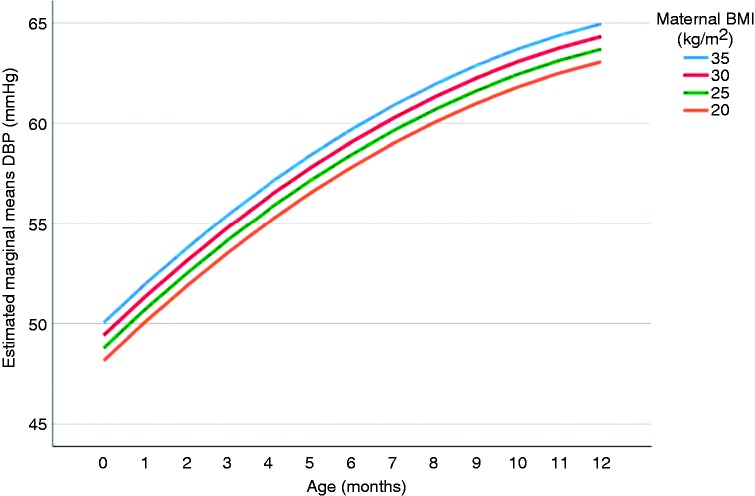
Table 2 provides the associations between parental BMI and offspring’s BP. A one unit increase in maternal BMI (kg/m2) was associated with 0.24 mmHg (95% CI 0.05; 0.44) and 0.13 mmHg (95% CI 0.01; 0.25) higher offspring’s mean SBP and DBP, respectively, over the first year of life (model 1). This higher offspring BP with increased maternal pre-pregnancy BMI was already set from birth onwards. There was no effect of the trial status (model 2) on the association of interest. Additional adjustment for birth size (0.27, 95% CI 0.05; 0.49) or offspring’s weight and length gain (0.20, 95% CI 0.01; 0.40) did not explain the association with SBP. The associations attenuated slightly to a non-significant result in the model with DBP including birth size (0.12, 95% CI –0.01; 0.25) and weight and length measurements over time (0.11, 95% CI –0.01; 0.23). There was no effect modification by birthweight, intervention status, care centre, breastfeeding, parity, gender or time. The P value for interaction between maternal BMI and parity was 0.08 in the adjusted model with offspring DBP as the outcome. Linear regression coefficients (95% CI) were –0.01 (–0.22; 0.21) and 0.20 (0.05; 0.35) for the first pregnancy and the second or more than two pregnancies, respectively. The BMI of mothers with a first pregnancy was significantly lower than the BMI of mothers with a parity of two or more than two pregnancies. The offspring SBP and DBP pattern over time plotted for maternal BMI, by using the estimated marginal means of the BP of model 1, are provided in Figures 2 and 3, respectively. Effect estimates were similar in models which excluded the offspring of women who had any gestational disease or women who smoked during pregnancy (Supplementary Appendix Table 2).
Table 2.
Associations between maternal pre-pregnancy BMI and offspring mean BP over the first year of life.
| Maternal pre-pregnancy BMI
(kg/m2) |
||
|---|---|---|
| Linear regression coefficients (95% confidence
intervals) |
||
| SBP (mmHg) | DBP (mmHg) | |
| Crude | 0.25 (0.07; 0.43) | 0.14 (0.03; 0.25) |
| Model 1a | 0.24 (0.05; 0.44) | 0.13 (0.01; 0.25) |
| Model 2b | 0.24 (0.05; 0.44) | 0.12 (0.004; 0.24) |
Crude model including additionally mother’s age at giving birth and second-hand prenatal smoke exposure.
Model 1 and status trial.
BMI: body mass index; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Figure 2.
The offspring systolic blood pressure (SBP) pattern over the first year of life (in months). Estimated marginal means of SBP are estimated based on model 1 (including SBP, maternal body mass index (BMI), age, age2, maternal age and prenatal (second-hand) smoke exposure) for maternal BMI values of 20, 25, 30 and 35 for each month during the first year of life.
Figure 3.
The offspring diastolic blood pressure (DBP) pattern over the first year of life (in months). Estimated marginal means of DBP are estimated based on model 1 (including DBP, maternal body mass index (BMI), age, age2, maternal age and prenatal (second-hand) smoke exposure) for maternal BMI values of 20, 25, 30 and 35 at each month during the first year of life.
Paternal BMI was not associated with offspring’s BP (SBP –0.02 mmHg/kg/m2 (95% CI –0.27; 0.23); DBP −0.05 mmHg/kg/m2 (95% CI –0.21; 0.10)).
Discussion
A higher maternal pre-pregnancy BMI, but not paternal BMI, was associated with a higher offspring’s BP during the first year of life. This was for DBP partly explained by offspring’s anthropometry. The offspring of mothers with a higher pre-pregnancy BMI had higher BP values around birth and remained higher during the first year of life, but their change in BP was similar compared to the offspring of mothers with a lower pre-pregnancy BMI.
To appreciate these findings some aspects of the study need to be addressed. To our knowledge, ours is the first study that investigated the association between parental pre-pregnancy BMI and offspring’s BP in a lower middle income country. The relevance of this is that Indonesia is in an epidemiological transition and will deal with the increasing prevalence of CVD (risk factors) in the coming years. A strength of the current study is the very low number of women who smoked or used alcohol during pregnancy, which enabled us to analyse the data without these confounding effects. As BP varies between and across visits,20 we intended to reduce the effects of individual variation over time – regression to the mean – by including multiple BP measurements at different visits. Moreover, this approach enabled us to estimate the BP patterns based on parental BMI during the first year of life.
As in all observational prospective cohort studies, we did have to deal with loss to follow-up. The reasons for loss to follow-up before giving birth is explained by the low commitment and often the husband disagreed with participation. Furthermore, most women were only temporary residents who moved to their mother’s house to give birth, which is a custom for a lot of Indonesian women. However, we consider selection bias implausible as the baseline characteristics were comparable and it is unlikely that the reported association between pre-pregnancy BMI and offspring BP is different in people who were lost to follow-up. Parental weight and height were self-reported by the mother during the third trimester of pregnancy. Due to the fact that these measures were self-reported and retrospectively asked, it could be that these values are imprecise. If so, we would expect an underestimated rather than an overestimated BMI for most people, which in turn would result in a dilution of the demonstrated effect estimates. Notably, we consider information bias improbable because parental weight and height were reported before the outcome was measured and offspring’s BP was measured automatically by a research nurse who was unaware of the research question. Parental BMI was analysed as a continuous variable rather than using the WHO classifications of body weight, as there is debate about the appropriate BMI cut-off for overweight or obesity in Asians,21 and we did expect that any association between BMI and offspring BP would be graded. BMI is an indicator for overall adiposity, but provides no information about fat distribution, which is important as an increase in visceral adipose tissue leads to a higher cardiometabolic risk compared to subcutaneous adipose tissue.22 Indonesians with the same BMI as Caucasians have, overall, a higher percentage of body fat.21
Despite these ethnicity-specific differences, our results with regard to increased offspring BP with increased maternal pre-pregnancy BMI are in line with the majority of the previous studies,4,5,23–31 but conflict with two studies which used maternal BMI categories.32,33 Similar to our study, this association between maternal pre-pregnancy BMI and SBP remained similar in all studies which included birthweight in the model,4,5,25,31 while it attenuated (slightly) after including offspring’s BMI.4,25,27,28,31 For DBP, in two out of three studies4,25,31 which investigated birth characteristics the association remained similar;25,31 previous studies support our findings that the association attenuated (slightly) when offspring’s anthropometry was additionally included in the model.4,25,28,31 In contrast to our study, two previous studies found increased offspring SBP with higher paternal pre-pregnancy BMI,4,5 but effect estimates were – in line with our results – higher for maternal than paternal pre-pregnancy BMI. Gaillard et al. reported, in accordance with our findings, no association between paternal pre-pregnancy BMI and offspring DBP.4
As we investigated the association of both maternal and paternal pre-pregnancy BMI, we could elucidate the potential underlying mechanism of this association of interest. Firstly, this could be a reflection of shared familial traits and lifestyles.10 However, the presence of the association with maternal pre-pregnancy BMI, but absence with paternal pre-pregnancy BMI, suggests that familial aggregation of body size and shared lifestyles does not completely explain this association of interest. As we lack information on parental BP, we cannot exclude familial aggregation of BP as an explanation. However, we consider an explanation for this association of interest by familial aggregation of height unlikely as the higher BMI values are caused by increased weight rather than being taller.
Secondly, parental overweight could already programme the offspring to an adverse cardiovascular and metabolic profile during conception and pregnancy by metabolic, mitochondrial and epigenetic changes. During this period, the gametes and early embryo are fully exposed to environmental conditions, e.g. parental obesity, which could alter development. Little evidence is available with regard to the impact of paternal pre-pregnancy BMI on offspring’s health. In animals, it is known that paternal obesity affects sperm quality, epigenetic status, DNA integrity and seminal fluid composition, which in turn could affect embryonal development. For instance, seminal fluid in mice influences blastocyst development, placental size, offspring glucose tolerance, adiposity and BP. However, offspring BP was not affected by paternal obesity in a meta-analysis including both maternal and paternal mouse models, while this was affected by maternal obesity.34 This supports our findings, and indicates an intrauterine mechanism influenced by the mother.
The follicles of obese mothers have elevated concentrations of follicular fluid insulin, lactate, triglycerides, leptin and other metabolic regulators. Moreover, the oocytes of obese mothers are smaller; and blastocysts have higher triglycerides, but lower glucose consumption. Mitochondrial defects in oocytes and chromosomal changes could exist and programme the offspring towards an adverse cardiometabolic profile.34 From the third trimester of pregnancy onwards, the elevated leptin concentrations in obese mothers affect the embryonal hypothalamus, which is responsible for the control of appetite and activation of efferent sympathetic pathways. This may lead to higher offspring’s leptin-mediated renal sympathetic activity and adiposity, which is related to an increased risk of developing obesity-related hypertension.35
As we found stronger estimates for maternal than paternal pre-pregnancy BMI, although associations attenuated slightly in the models including offspring’s anthropometry – we suppose that, at least partly, the association of interest is explained by an intrauterine mechanism that possibly affects fat metabolism. Different associations for maternal and paternal pre-pregnancy BMI indicates an intrauterine effect rather than familial clustering of body size or lifestyle. This is supported by the fact that higher offspring BP is already visible from birth onwards and does not affect the change in offspring BP over time.
In conclusion, higher maternal pre-pregnancy BMI, but not paternal pre-pregnancy BMI, is associated with higher offspring BP, which is partly explained by offspring’s anthropometry. This higher offspring BP with increased maternal pre-pregnancy BMI is already set from birth onwards and does not affect the change in BP over time.
Perspectives
Alongside the increased risks of maternal obesity on maternal and embryonal health during pregnancy,36 it seems that this also has an impact on offspring’s cardiometabolic profile after delivery. Although effect sizes are small, these could have long-term consequences as BP not only tracks from childhood to adulthood and BP trajectories are associated with CVD risk, BP differences are also reported to amplify over life. Small, favourable shifts in mean BP levels in the total population may have a large effect on the prevalence of hypertension in the total population.37 Therefore, for population prevention of hypertension and CVD, factors that affect BP early in life should be considered. This is of relevance for all pregnancies, but particularly in lower middle income countries, as those countries are in epidemiological transition and will experience an increased prevalence of CVD (risk factors). For the early prevention of hypertension at a population level it is important to reduce excessive body weight in future parents, especially in women given the potential intrauterine effect on offspring BP. In a systematic review and meta-analysis by Wewege et al. it is reported that overweight and obese people benefit from an exercising programme with regard to body composition.38 In addition to exercise training, caloric restriction resulted in a better body composition as well, when a hypocaloric diet led to greater weight loss compared to exercise training.39 In middle-aged overweight or obese adults with hypertension a combination of a hypocaloric diet with supervised aerobic exercise 2 days a week for a period of 16 weeks led to a reduction in weight and BP levels.40 Both exercise training and a hypocaloric diet influence components of the individual’s body composition and thereby improve the cardiometabolic profile.
Supplemental Material
Supplemental Material for Pre-pregnancy parental BMI and offspring blood pressure in infancy by Maria Adriana Cornelia Jansen, Geertje W Dalmeijer, Siti RF Saldi, Diederick E Grobbee, Mohammad Baharuddin, Cuno SPM Uiterwaal and Nikmah S Idris in European Journal of Preventive Cardiology
Acknowledgements
The authors gratefully acknowledge the children and their guardians for their contribution this study; Dr Irma Sapriani and the Budi Kemuliaan team for their support for setting up and conducting this study; and the BRAVO research staff for their dedicated work. They also wish to thank Dr Dina and Dr Kasturi (Kenari primary care centre) for their continuous support as well as all staff members of Senen and Jatinegara primary care centres.
Author contribution
MB, CSPMU and NSI contributed to the conception or design of the work. MACJ, GWD, SRFS, CSPMU and NSI contributed to the acquisition, analysis, or interpretation of data for the work. MACJ, GWD and CSPMU drafted the manuscript. All authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Clinical trials registration
ClinicalTrials.gov Identifier: NCT01566812 (https://clinicaltrials.gov/ct2/show/NCT01566812). Registered on 27 March 2012.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: BRAVO was made possible by an unrestricted grant from Nutricia Indonesia Fund, by several UMC Utrecht Global Health Support Program grants for PhDs, and by in-kind provisions by the centre for Clinical Epidemiology and Evidence-Based Medicine and Department of Child Health at Faculty of Medicine University of Indonesia/Cipto Mangunkusumo General Hospital, Jakarta, Indonesia, by Budi Kemuliaan Hospital, Jakarta, Indonesia, and by the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands. MAC Jansen has been financially supported by the Ter Meulen grant funded by the Royal Netherlands Academy of Arts and Sciences (KNAW), to perform this research in Jakarta, Indonesia. The funding sources had no role in the design of the study, the data collection, analysis and interpretation, and the writing of the manuscript.
References
- 1.World Health Organization. Indonesia: WHO statistical profile, 2015. www.who.int/gho/countries/idn.pdf?ua=1 (accessed 18 October 2017).
- 2.World Health Organization. Fact sheets. Obesity and overweight. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 18 October 2017).
- 3.Ludwig-Walz H, Schmidt M, Gunther ALB, et al. Maternal prepregnancy BMI or weight and offspring’s blood pressure: systematic review. Matern Child Nutr 2018; 14: e12561–e12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaillard R, Steegers EA, Duijts L, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 2014; 63: 683–691. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, Najman JM, Sterne J, et al. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation 2004; 110: 2417–2423. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 2008; 117: 3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015; 66: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palipudi K, Mbulo L, Kosen S, et al. A cross sectional study of kretek smoking in Indonesia as a major risk to public health. Asian Pac J Cancer Prev 2015; 16: 6883–6888. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Management of substance abuse. Global status report on alcohol and health 2014. Indonesia. www.who.int/substance_abuse/publications/global_alcohol_report/profiles/idn.pdf?ua=1 (accessed 18 October 2017).
- 10.Wang X, Xu X, Su S, et al. Familial aggregation and childhood blood pressure. Curr Hypertens Rep 2015; 17: 509–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savitri AI, Idris NS, Indawati W, et al. BReastfeeding Attitude and Volume Optimization (BRAVO) trial: study protocol for a randomized controlled trial. Trials 2016; 17: 271–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nooyens AC, Visscher TL, Verschuren WM, et al. Age, period and cohort effects on body weight and body mass index in adults: the Doetinchem Cohort Study. Public Health Nutr 2009; 12: 862–870. [DOI] [PubMed] [Google Scholar]
- 13.Brion MJ, Leary SD, Lawlor DA, et al. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet, and smoking. Pediatr Res 2008; 63: 593–598. [DOI] [PubMed] [Google Scholar]
- 14.Brion MJ, Leary SD, Smith GD, et al. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension 2007; 49: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge LL, Harris HR, Rich-Edwards JW, et al. Parental smoking in pregnancy and the risks of adult-onset hypertension. Hypertension 2013; 61: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One 2015; 10: e0123579–e0123579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton S, Braithwaite D, Akinyemiju TF. Socio-economic status over the life course and obesity: systematic review and meta-analysis. PLoS One 2017; 12: e0177151–e0177151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Berg G, van Eijsden M, Galindo-Garre F, et al. Explaining socioeconomic inequalities in childhood blood pressure and prehypertension: the ABCD study. Hypertension 2013; 61: 35–41. [DOI] [PubMed] [Google Scholar]
- 19.Petrovic D, de Mestral C, Bochud M, et al. The contribution of health behaviors to socioeconomic inequalities in health: a systematic review. Prev Med 2018; 113: 15–31. [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140: e20171904. [DOI] [PubMed] [Google Scholar]
- 21.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116: 39–48. [DOI] [PubMed] [Google Scholar]
- 23.Derraik JG, Ayyavoo A, Hofman PL, et al. Increasing maternal prepregnancy body mass index is associated with reduced insulin sensitivity and increased blood pressure in their children. Clin Endocrinol (Oxf) 2015; 83: 352–356. [DOI] [PubMed] [Google Scholar]
- 24.Filler G, Yasin A, Kesarwani P, et al. Big mother or small baby: which predicts hypertension? J Clin Hypertens (Greenwich) 2011; 13: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gademan MG, van Eijsden M, Roseboom TJ, et al. Maternal prepregnancy body mass index and their children’s blood pressure and resting cardiac autonomic balance at age 5 to 6 years. Hypertension 2013; 62: 641–647. [DOI] [PubMed] [Google Scholar]
- 26.Perng W, Gillman MW, Mantzoros CS, et al. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 2014; 24: 793–800.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen X, Triche EW, Hogan JW, et al. Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics 2011; 127: e713–e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West NA, Crume TL, Maligie MA, et al. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011; 54: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan HC, Roberts J, Catov J, et al. Mother’s pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr Diabetes 2015; 16: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oostvogels AJ, Stronks K, Roseboom TJ, et al. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5–6 years: the ABCD Study. J Clin Endocrinol Metab 2014; 99: 3845–3854. [DOI] [PubMed] [Google Scholar]
- 31.Laura HC, Menezes AB, Noal RB, et al. Maternal anthropometric characteristics in pregnancy and blood pressure among adolescents: 1993 live birth cohort, Pelotas, southern Brazil. BMC Public Health 2010; 10: 434–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daraki V, Georgiou V, Papavasiliou S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS One 2015; 10: e0126327–e0126327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenman JC, Sarzynski MA, Tucker J, et al. Maternal prepregnancy overweight and offspring fatness and blood pressure: role of physical activity. Pediatr Exerc Sci 2010; 22: 369–378. [DOI] [PubMed] [Google Scholar]
- 34.Fleming TP, Watkins AJ, Velazquez MA, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018; 391: 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf) 2014; 210: 508–523. [DOI] [PubMed] [Google Scholar]
- 36.Lawlor DA, Relton C, Sattar N, et al. Maternal adiposity – a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol 2012; 8: 679–688. [DOI] [PubMed] [Google Scholar]
- 37.Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on Hypertension. Lancet 2016; 388: 2665–2712. [DOI] [PubMed] [Google Scholar]
- 38.Wewege M, van den Berg R, Ward RE, et al. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev 2017; 18: 635–646. [DOI] [PubMed] [Google Scholar]
- 39.Verheggen RJ, Maessen MF, Green DJ, et al. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev 2016; 17: 664–690. [DOI] [PubMed] [Google Scholar]
- 40.Gorostegi-Anduaga I, Corres P, MartinezAguirre-Betolaza A, et al. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur J Prev Cardiol 2018; 25: 343–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Pre-pregnancy parental BMI and offspring blood pressure in infancy by Maria Adriana Cornelia Jansen, Geertje W Dalmeijer, Siti RF Saldi, Diederick E Grobbee, Mohammad Baharuddin, Cuno SPM Uiterwaal and Nikmah S Idris in European Journal of Preventive Cardiology