Key Points
Question
Is there an association between treatment with metformin vs sulfonylureas and major adverse cardiovascular events (MACE) among patients with diabetes and reduced kidney function?
Findings
In this retrospective cohort study of 49 478 patients with diabetes and reduced kidney function, the incidence of MACE for those treated with metformin vs sulfonylurea monotherapy was 23.0 per 1000 person-years vs 29.2 per 1000 person-years, a difference that was statistically significant.
Meaning
Monotherapy treatment with metformin, compared with a sulfonylurea, was associated with a lower risk of MACE among patients with diabetes and reduced kidney function.
Abstract
Importance
Before 2016, safety concerns limited metformin use in patients with kidney disease; however, the effectiveness of metformin on clinical outcomes in patients with reduced kidney function remains unknown.
Objective
To compare major adverse cardiovascular events (MACE) among patients with diabetes and reduced kidney function who continued treatment with metformin or a sulfonylurea.
Design, Setting, and Participants
Retrospective cohort study of US veterans receiving care within the national Veterans Health Administration, with data supplemented by linkage to Medicare, Medicaid, and National Death Index data from 2001 through 2016. There were 174 882 persistent new users of metformin and sulfonylureas who reached a reduced kidney function threshold (estimated glomerular filtration rate <60 mL/min/1.73 m2 or creatinine ≥1.4 mg/dL for women or ≥1.5 mg/dL for men). Patients were followed up from reduced kidney function threshold until MACE, treatment change, loss to follow-up, death, or study end (December 2016).
Exposures
New users of metformin or sulfonylurea monotherapy who continued treatment with their glucose-lowering medication after reaching reduced kidney function.
Main Outcomes and Measures
MACE included hospitalization for acute myocardial infarction, stroke, transient ischemic attack, or cardiovascular death. The analyses used propensity score weighting to compare the cause-specific hazard of MACE between treatments and estimate cumulative risk accounting for the competing risks of changing therapy or noncardiovascular death.
Results
There were 67 749 metformin and 28 976 sulfonylurea persistent monotherapy users; the weighted cohort included 24 679 metformin and 24 799 sulfonylurea users (median age, 70 years [interquartile range {IQR}, 62.8-77.8]; 48 497 men [98%]; and 40 476 white individuals [82%], with median estimated glomerular filtration rate of 55.8 mL/min/1.73 m2 [IQR, 51.6-58.2] and hemoglobin A1c level of 6.6% [IQR, 6.1%-7.2%] at cohort entry). During follow-up (median, 1.0 year for metformin vs 1.2 years for sulfonylurea), there were 1048 MACE outcomes (23.0 per 1000 person-years) among metformin users and 1394 events (29.2 per 1000 person-years) among sulfonylurea users. The cause-specific adjusted hazard ratio of MACE for metformin was 0.80 (95% CI, 0.75-0.86) compared with sulfonylureas, yielding an adjusted rate difference of 5.8 (95% CI, 4.1-7.3) fewer events per 1000 person-years of metformin use compared with sulfonylurea use.
Conclusions and Relevance
Among patients with diabetes and reduced kidney function persisting with monotherapy, treatment with metformin, compared with a sulfonylurea, was associated with a lower risk of MACE.
This cohort study uses data from the National Veterans Health Administration, Medicare, Medicaid, and National Death Index to compare major adverse cardiovascular events among patients with diabetes and reduced kidney function who continued treatment with metformin or sulfonylurea.
Introduction
In 2012, there were approximately 30 million US adults diagnosed as having type 2 diabetes, of whom 20% also had impaired kidney function.1 Metformin is the initial recommended diabetes treatment based on the beneficial results reported in 1998 from the UK Prospective Diabetes Study (UKPDS) 34.2,3 The UKPDS demonstrated that metformin reduced the incidence of macrovascular complications compared with sulfonylureas or insulin independent of glycemic control.2,4 Several large observational studies support the UKPDS findings.4,5,6,7
Metformin is eliminated by the kidneys and can accumulate as estimated glomerular filtration rate (eGFR) declines. Based on the negative clinical experience with phenformin and the potential for metformin-associated lactic acidosis, the US Food and Drug Administration (FDA) issued a safety warning restricting metformin for patients with serum creatinine levels of 1.5 mg/dL or greater for men or 1.4 mg/dL or greater for women.8 In 2016, the FDA changed its guidance based on evidence regarding metformin safety in patients with mild to moderate kidney disease; however, the effectiveness of metformin for clinical outcomes in those with reduced kidney function remains unknown. Large clinical trials that investigated diabetes treatment effects on cardiovascular outcomes excluded patients with reduced eGFR, rendering this population understudied.2,5,9,10,11,12
The aim of this study was to test the hypothesis that among patients with diabetes who develop reduced kidney function, continued metformin use is associated with lower risk of fatal or nonfatal major adverse cardiovascular events (MACE) than sulfonylureas.
Methods
Study Design and Data Sources
We assembled a retrospective cohort of Veterans Health Administration (VHA) patients.4 Pharmacy data included medication, date filled, days supplied, and number of pills dispensed. Demographic, diagnostic, and procedure information identified inpatient and outpatient VHA encounters. We collected laboratory results and vital signs data from clinical sources. For Medicare or Medicaid enrollees, we obtained enrollment, claims files, and prescription (Part D) data for Medicare enrollees.13,14 We obtained dates and cause of death from vital status and the National Death Index files.15,16 The institutional review board of VHA Tennessee Valley Healthcare System approved this study with a waiver of informed consent.
Study Population
The source population comprised veterans aged 18 years and older who were regular users of the VHA care, defined as an encounter or prescription fill at least once every 365 days for 2 or more years prior to cohort entry. We identified patients with new-onset type 2 diabetes by selecting those who were new users of metformin, glipizide, glyburide, or glimepiride. New users were patients who filled a first glucose-lowering prescription without any diabetic drug fill in the 180 days prior to that first fill. We followed up these patients with diabetes longitudinally and selected patients who experienced a decline in kidney function. Patients were required to persist with their initial monotherapy with no medication gaps for more than 180 days or medication switching prior to reaching the kidney threshold to be eligible for cohort entry.
The date of cohort entry and start of follow-up was the day of reaching a reduced kidney function threshold (eFigure 1 in the Supplement), defined as either an eGFR of less than 60 mL/min/1.73 m2 or serum creatinine level of 1.5 mg/dL for men or 1.4 mg/dL for women. Cohort entry was between January 1, 2002, and December 30, 2015, to allow sufficient collection of baseline data and follow-up. We excluded patients who added or switched glucose-lowering medications at or prior to the kidney threshold or had 2 or more episodes of dialysis, organ transplantation, or hospice care within the 2 years prior to reaching the kidney threshold.
Exposure
The study exposures were persistent use of metformin or a sulfonylurea (glyburide, glipizide, and glimepiride) after reaching the kidney threshold. Follow-up began on the date the kidney threshold (eGFR <60 mL/min/1.73 m2 or serum creatinine level, 1.4/1.5 mg/dL) was fulfilled and continued through an outcome (below); nonpersistence, defined as 90 days without an antidiabetic drug or the addition of or switch to a different glucose-lowering drug; censoring, defined as the 181st day of no VHA contact (inpatient, outpatient, or pharmacy use); noncardiovascular death; or study end (December 31, 2016). Seventy percent of the population received 90-day prescriptions and, in this population, allowing 90 days to refill medications approximates 80% adherence.17
Outcomes
The composite outcome was MACE including hospitalization for acute myocardial infarction (AMI), ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or date of cardiovascular death. The outcome date was the date of hospital admission for AMI, stroke, or TIA or the date of cardiovascular death. The primary discharge diagnosis, either International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) or ICD-10 codes before or after October 1, 2015, respectively, was used to identify outcomes.
We defined AMI by codes 410.x or I21.x. Stroke hospitalizations encompassed those with an ischemic stroke (433.x1, 434 [excluding 434.x0], and 436 or I63.30, I63.40, I63.50, I66.09, I66.19, I66.29, I66.9, I67.848, and I67.89), intracerebral hemorrhage (431 or I61.x), and subarachnoid hemorrhage (430 or I60.x). TIA hospitalizations were defined by codes 435 or G45.0, G45.1, G45.8, or G45.9. Medical record review and validation of a sample of these codes have shown high specificity and positive predictive values of 90% for AMI and 81% for stroke when compared with VHA medical record review.18
Cardiovascular deaths were identified from death certificates with an ICD-10 underlying cause of death compatible with cardiac death, fatal myocardial infarction, stroke, or cardiomyopathy (I00-I78 excluding I30.X [diseases of the pericardium]) or unattended sudden cardiac death (R98, R99, R960, and R961). This definition included the Centers for Disease Control and Prevention’s broad definition of cardiac death and a validated strategy for identification of sudden cardiac deaths.19
The secondary outcome excluded TIA as part of the composite MACE event. Because not all patients who sustain a TIA are admitted to the hospital, we determined whether the addition of TIA emergency department visits that did not lead to hospital admission would influence the outcome event rates. TIA emergency department visits included the above codes for TIA in the primary (first listed) diagnosis position of outpatient emergency department visits.
Covariates
Study covariates were measured up to 720 days before the reduced kidney function threshold and included age, sex, race, fiscal year, number of months from initial antidiabetic medication to kidney threshold (diabetes duration), and Veterans Integrated Service Networks (VISN) of care. Each VISN of care is a geographic designation for VHA and allowed a more granular estimation of geographic variation of diabetes care. Physiologic variables were defined as the most recent measure prior to kidney threshold and included body mass index (calculated as weight in kilograms divided by height in meters squared), blood pressure, hemoglobin A1c (HbA1c), low-density lipoprotein, hemoglobin, proteinuria, and creatinine values (both historical and the creatinine at cohort entry).
Creatinine was used to calculate eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.20,21 The Isotope Dilution Mass Spectroscopy calibration date was collected and accounted for in estimation of eGFR for 70% of the VHA facilities. Facilities where no Isotope Dilution Mass Spectroscopy switch date was identified had all eGFRs downward adjusted by 5%.22 Health care utilization (hospitalization, nursing home, number of outpatient visits or medications, and Medicare or Medicaid insurance use) was measured in the year prior to the reduced kidney function threshold. We collected data on smoking and comorbidities as defined in eTable 1 in the Supplement. Selected medications filled within 180 days prior to the reduced kidney function threshold were also covariates. Because race is associated with cardiovascular outcomes, it was included in all models. We selected patient self-reported categorical race from VHA data and supplemented with Medicare patient self-reported categorical race data to minimize missing values.
Statistical Analyses
The primary analysis compared the cause-specific hazard of MACE between medication groups in a propensity score–weighted cohort. The propensity score modeled the probability of metformin or sulfonylurea continuation at reduced kidney function threshold given covariates, VISN, and an indicator for imputed covariates. Missing covariates were handled using 20 iterations of chained imputations and adjusting for canonical variates.23 We used matching weights to balance both exposure groups on observed covariates (detailed methods in eTable 2 and eFigures 2-4 in the Supplement).24,25 Standardized mean differences are the absolute value of the difference in means or proportions divided by a pooled standard deviation. Standardized mean differences were calculated as the difference between groups in number of standard deviations and is a more meaningful measure than P values from t tests for large samples.
Cox proportional hazards models estimated the cause-specific hazard ratios (HRs) for metformin vs sulfonylurea (referent) in the weighted cohort, adjusted for covariates. Statistical significance for the 2-sided P value was set at .05. The proportional hazards assumptions were verified through examination of Schoenfeld residuals over time.26 The cause-specific hazard allowed estimation of the medication association with MACE in those patients who were event free.27 Nonparametric estimates of the cumulative incidence of MACE accounted for 2 competing risks: medication nonpersistence and noncardiovascular death. Cumulative incidence curves were generated using the Aalen-Johansen estimator. The nonparametric Aalen-Johansen estimator was preferred over the semiparametric Fine and Gray model because it allowed more flexibility when modeling the cumulative incidence function.28,29
Sensitivity and Subgroup Analyses
The first sensitivity analysis evaluated a cohort with chronic kidney disease and required patients to have a second measured eGFR less than 60 mL/min/1.73 m2 between 30 and 180 days after the first eGFR less than 60 mL/min/1.73 m2 and began cohort entry at 180 days from the first eGFR less than 60 mL/min/1.73 m2. The second sensitivity analysis assumed patients remained in their initial exposure groups and did not censor follow-up based on regimen changes or the 90-day refill requirement (ie, persistent exposure was not required). This analysis is akin to an intention-to-treat analysis in clinical trials and increases follow-up time and events but allows exposure time misclassification (eFigure 1 in the Supplement). A third sensitivity analysis excluded patients who were enrolled in Medicare Advantage at baseline and censored patients at Medicare Advantage enrollment to determine whether results were influenced by Advantage status. Subgroup analyses tested for effect modification by including interaction terms (treatment by subgroup) in the model for the following groups: history of cardiovascular disease (yes, no), age (≥65 years, <65 years), race (black, nonblack), baseline eGFR (45-59, 30-45, or <30 mL/min/1.73 m2), and if the patient entered the cohort based on reaching the FDA creatinine threshold or a reduced eGFR with a serum creatinine less than the FDA threshold. Analyses were conducted using R (http://www.r-project.org).
Results
Study Cohort and Patient Characteristics
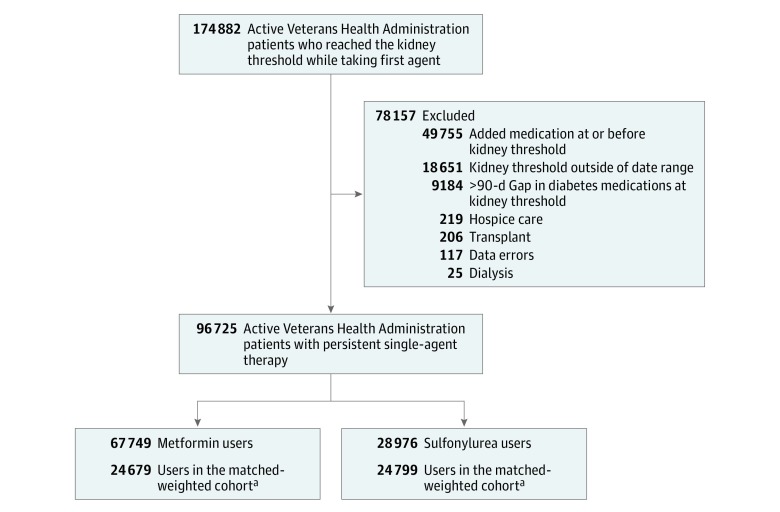
We identified 67 749 new metformin users and 28 976 new sulfonylurea users who persisted with treatment, met the reduced kidney function threshold, and satisfied cohort entry criteria (Figure 1). These cohort patients represent 55.3% of the 174 882 new persistent users who had a baseline creatinine level and reached the reduced kidney function threshold. We excluded 49 755 who added another diabetes medication on or before the kidney threshold, 18 651 who met the kidney threshold outside the study time frame, 9184 who had no supply of metformin or sulfonylurea in the 90 days before reaching the kidney threshold, and those with organ transplant (n = 206), hospice care (n = 219), dialysis use in the past 2 years (n = 25), or data error (n = 117). The weighted cohort included 24 679 metformin users and 24 799 sulfonylurea users (54% glipizide, 45% glyburide, and 1% glimepiride).
Figure 1. Eligible Patients in the Veterans Health Administration.
aMatched weighted cohort was formed using matching weights, derived using propensity scores, and up or downweighting patients to more closely resemble each other.
Cohort patients were 98% male and 81.8% white. Metformin users were younger than sulfonylurea users (median age, 67 vs 71 years; eFigure 3 in the Supplement) and a larger proportion of metformin users reached the kidney threshold in later study years.30,31 HbA1c (6.6% [interquartile range {IQR}, 6.1-7.2]; 49 mmol/mol [IQR, 43-55]), eGFR at cohort entry (55.8 mL/min/1.73 m2 [IQR, 51.6-58.2]), and historical eGFR before cohort entry (69.6 mL/min/1.73 m2 [IQR, 64.7-77.0]) were similar between exposures. Standardized mean differences were less than 0.10 after weighting (Table 1).
Table 1. Characteristics of Patients at the Time They Reached a Reduced Kidney Function Threshold.
| Characteristic | Full Unweighted Cohort | SMDa | Propensity Score–Weighted Cohort | SMDa | ||
|---|---|---|---|---|---|---|
| Metformin (n = 67 749) |
Sulfonylurea (n = 28 976) |
Metformin (n = 24 679) |
Sulfonylurea (n = 24 799) |
|||
| Patient Characteristics | ||||||
| Age, median (IQR), y | 67.3 (62.1-74.4) | 71.1 (63.2-78.8) | 0.28 | 70.1 (62.9-77.8) | 70.0 (62.8-77.8) | 0.001 |
| Sex, No. (%) | ||||||
| Male | 64921 (95.8) | 28459 (98.2) | 0.14 | 24189 (98.0) | 24308 (98.0) | <0.001 |
| Female | 2828 (4.2) | 517 (1.8) | 490 (2.0) | 491 (1.9) | ||
| Race, No. (%)b | ||||||
| White | 56392 (83.2) | 23524 (81.2) | 0.07 | 20186 (81.8) | 20290 (81.8) | 0.001 |
| Black | 9883 (14.6) | 4924 (17.0) | 4035 (16.4) | 4047 (16.3) | ||
| Other | 1474 (2.2) | 528 (1.8) | 458 (1.9) | 462 (1.9) | ||
| Medication start to kidney threshold (diabetes duration), median (IQR), mo | 16.2 (6.5-35.1) | 13.6 (5.9-29.0) | 0.15 | 14.0 (5.8-30.2) | 14.0 (6.0-30.3) | 0.01 |
| Year reduced kidney threshold was reached, No. (%) | ||||||
| 2002-2003 | 3167 (4.7) | 4904 (16.9) | 0.76 | 2924 (11.8) | 2918 (11.3) | 0.03 |
| 2004-2005 | 5786 (8.5) | 5737 (19.8) | 4479 (18.2) | 4442 (17.2) | ||
| 2006-2007 | 9075 (13.4) | 6101 (21.1) | 5207 (21.1) | 5437 (21.1) | ||
| 2008-2009 | 9952 (14.7) | 4051 (14.0) | 3876 (15.7) | 3895 (15.1) | ||
| 2010-2011 | 12237 (18.1) | 3341 (11.5) | 3367 (13.6) | 3289 (12.7) | ||
| 2012-2013 | 12854 (18.9) | 2619 (9.0) | 2651 (10.7) | 2600 (10.1) | ||
| 2014-2015 | 14678 (21.7) | 2223 (7.7) | 2175 (8.8) | 2218 (8.6) | ||
| Laboratory Variables | ||||||
| Hemoglobin A1c, median (IQR), % | 6.5 (6.1-7.0) | 6.6 (6.1-7.3) | 0.15 | 6.5 (6.1-7.1) | 6.6 (6.1-7.2) | 0.005 |
| No. with available measure | 64981 | 27838 | 23668 | 23805 | ||
| Creatinine at kidney threshold, median (IQR), mg/dL | 1.33 (1.24-1.43) | 1.33 (1.24-1.43) | 0.05 | 1.33 (1.24-1.43) | 1.33 (1.24-1.43) | 0.002 |
| Estimated glomerular filtration rate, median (IQR), mL/min/1.73 m2 | ||||||
| Before kidney threshold | 70.5 (65.1-78.6) | 69.3 (64.5-76.6) | 0.13 | 69.6 (64.6-77.0) | 69.7 (64.7-77.0) | 0.001 |
| At kidney threshold | 55.9 (51.6-58.3) | 55.8 (51.5-58.2) | 0.02 | 55.8 (51.6-58.2) | 55.8 (51.6-58.2) | 0.002 |
| Hemoglobin, median (IQR), g/L | 14.0 (12.9-150) | 14.1 (13.0-15.2) | 0.05 | 14.1 (13.0-15.1) | 14.1 (13.0-15.2) | 0.002 |
| No. with available measure | 64119 | 27264 | 23167 | 23292 | ||
| Low-density lipoprotein cholesterol, median (IQR), mg/dL | 85 (67-106) | 89 (72-111) | 0.13 | 88 (70-110) | 88 (71-110) | 0.002 |
| No. with available measure | 66426 | 27837 | 23883 | 24002 | ||
| Microalbumin to creatinine ratio stage, No. (%) with available measure | 38 869 | 14632 | 12913 | 12969 | ||
| A1 (<30 mg/g: normal to mild increase albuminuria) | 29656 (43.8) | 10625 (36.7) | 0.16 | 9470 (38.4) | 9530 (38.4) | 0.003 |
| A2 (30-300 mg/g: moderate increase albuminuria) | 7398 (10.9) | 3076 (10.6) | 2674 (10.8) | 2676 (10.8) | ||
| A3 and positive unable to quantify (>300 mg/g: severely increased albuminuria) | 1815 (2.7) | 931 (3.2) | 769 (3.1) | 763 (3.1) | ||
| Proteinuria by urinalysis, No. (%) with available measure | 45852 | 19167 | 16372 | 16466 | ||
| Negative | 32963 (48.7) | 13516 (46.7) | 0.08 | 11648 (47.2) | 11704 (47.2) | 0.002 |
| Urine protein trace or 1+ | 10071 (14.9) | 4185 (14.4) | 3574 (14.5) | 3606 (14.5) | ||
| Proteinuria present at 2+ | 2186 (3.2) | 983 (3.4) | 803 (3.3) | 809 (3.3) | ||
| Proteinuria present at 3+ or 4+ | 632 (0.9) | 483 (1.7) | 347 (1.4) | 348 (1.4) | ||
| Clinical Variables | ||||||
| Blood pressure, median (IQR), mm Hg | ||||||
| Systolic | 129 (117-139) | 130 (119-142) | 0.12 | 131 (119-142) | 131 (119-142) | 0.002 |
| Diastolic | 73 (65-80) | 71 (64-80) | 0.10 | 72 (64-80) | 72 (64-80) | <0.001 |
| Body mass index, median (IQR)c | 31.1 (27.7-35.2) | 30.1 (26.9-34.1) | 0.16 | 30.4 (27.1-34.4) | 30.3 (27.1-34.3) | 0.005 |
| No. with available measure | 56235 | 23243 | 20070 | 20163 | ||
| Baseline Comorbidities, No.(%)d | ||||||
| Cardiovascular/diabetes complications | ||||||
| Cardiovascular disease | 17700 (26.1) | 9811 (33.9) | 0.17 | 7797 (31.6) | 7868 (31.7) | 0.003 |
| Arrhythmia | 9510 (14.0) | 5469 (18.9) | 0.13 | 4289 (17.4) | 4320 (17.4) | 0.001 |
| Congestive heart failure | 5526 (8.2) | 4218 (14.6) | 0.20 | 2987 (12.1) | 3009 (12.1) | 0.001 |
| Cardiac valve disease | 1894 (2.8) | 1196 (4.1) | 0.07 | 897 (3.6) | 907 (3.7) | 0.001 |
| Stroke | 1900 (2.8) | 1030 (3.6) | 0.04 | 832 (3.4) | 830 (3.3) | 0.001 |
| Transient ischemic attack | 710 (1.0) | 410 (1.4) | 0.03 | 321 (1.3) | 331 (1.3) | 0.003 |
| Retinopathy | 508 (0.7) | 399 (1.4) | 0.06 | 290 (1.2) | 291 (1.2) | <0.001 |
| Amputation | 230 (0.3) | 170 (0.6) | 0.04 | 116 (0.5) | 120 (0.5) | 0.002 |
| Pulmonary | ||||||
| Chronic obstructive pulmonary disease | 10303 (15.2) | 5266 (18.2) | 0.08 | 4196 (17.0) | 4234 (17.1) | 0.002 |
| Smoking | 8749 (12.9) | 3551 (12.3) | 0.02 | 3062 (12.4) | 3085 (12.4) | 0.001 |
| History of respiratory failure | 1967 (2.9) | 963 (3.3) | 0.02 | 792 (3.2) | 792 (3.2) | 0.001 |
| History of pneumonia | 2179 (3.2) | 1426 (4.9) | 0.09 | 1056 (4.3) | 1074 (4.3) | 0.003 |
| Neurologic/psychiatric | ||||||
| Serious mental illness | 16588 (24.5) | 5825 (20.1) | 0.11 | 5046 (20.4) | 5121 (20.6) | 0.005 |
| Parkinson disease | 496 (0.7) | 310 (1.1) | 0.04 | 228 (0.9) | 231 (0.9) | 0.001 |
| Infectious | ||||||
| Urinary tract infection | 2267 (3.3) | 1375 (4.7) | 0.07 | 1035 (4.2) | 1046 (4.2) | 0.001 |
| History of sepsis | 961 (1.4) | 511 (1.8) | 0.03 | 397 (1.6) | 403 (1.6) | 0.001 |
| Osteomyelitis | 309 (0.5) | 198 (0.7) | 0.03 | 155 (0.6) | 153 (0.6) | 0.002 |
| HIV | 235 (0.3) | 118 (0.4) | 0.01 | 95 (0.4) | 97 (0.4) | 0.001 |
| Oncologic/metabolic comorbidity | ||||||
| Malignancy | 7199 (10.6) | 3514 (12.1) | 0.05 | 2892 (11.7) | 2909 (11.7) | <0.001 |
| Liver disease | 1131 (1.7) | 820 (2.8) | 0.08 | 596 (2.4) | 593 (2.4) | 0.002 |
| History of kidney disease | 73 (0.1) | 52 (0.2) | 0.02 | 35 (0.1) | 38 (0.2) | 0.002 |
| Markers of frailty | ||||||
| Osteoporosis | 475 (0.7) | 239 (0.8) | 0.01 | 196 (0.8) | 202 (0.8) | 0.002 |
| Falls | 147 (0.2) | 73 (0.3) | 0.007 | 55 (0.2) | 57 (0.2) | 0.001 |
| Fractures | 1257 (1.9) | 679 (2.3) | 0.03 | 549 (2.2) | 549 (2.2) | 0.001 |
| Use of Medications, No. (%) | ||||||
| Angiotensin-converting enzyme inhibitors | 43222 (63.8) | 18809 (64.9) | 0.02 | 15963 (64.7) | 16087 (64.9) | 0.004 |
| β-Blockers | 33337 (49.2) | 14797 (51.1) | 0.04 | 12511 (50.7) | 12585 (50.7) | 0.001 |
| Thiazide and potassium-sparing diuretics | 29980 (44.3) | 11572 (39.9) | 0.09 | 10101 (40.9) | 10194 (41.1) | 0.004 |
| Calcium channel blockers | 19720 (29.1) | 8665 (29.9) | 0.02 | 7379 (29.9) | 7412 (29.9) | <0.001 |
| Loop diuretics | 10315 (15.2) | 6621 (22.8) | 0.20 | 4956 (20.1) | 4983 (20.1) | <0.001 |
| Angiotensin II receptor blockers | 8697 (12.8) | 3109 (10.7) | 0.07 | 2816 (11.4) | 2807 (11.3) | 0.003 |
| Other antihypertensive medications | 18458 (27.2) | 7831 (27.0) | 0.005 | 6715 (27.2) | 6726 (27.1) | 0.002 |
| Statin lipid-lowering drugs | 49906 (73.7) | 18670 (64.4) | 0.20 | 16545 (67.0) | 16695 (67.3) | 0.006 |
| Nonstatin lipid-lowering agents | 13166 (19.4) | 4665 (16.1) | 0.09 | 4244 (17.2) | 4272 (17.2) | 0.001 |
| Antiarrhythmics digoxin and inotropes | 4395 (6.5) | 3143 (10.8) | 0.16 | 2260 (9.2) | 2272 (9.2) | <0.001 |
| Anticoagulants | 6027 (8.9) | 3099 (10.7) | 0.06 | 2488 (10.1) | 2495 (10.1) | 0.001 |
| Nitrates | 7812 (11.5) | 4715 (16.3) | 0.14 | 3628 (14.7) | 3664 (14.8) | 0.002 |
| Aspirin | 14371 (21.2) | 6542 (22.6) | 0.03 | 5359 (21.7) | 5407 (21.8) | 0.002 |
| Platelet inhibitors, not aspirin | 6240 (9.2) | 3100 (10.7) | 0.05 | 2574 (10.4) | 2591 (10.4) | 0.001 |
| Antipsychotics | 5414 (8.0) | 1992 (6.9) | 0.04 | 1740 (7.0) | 1762 (7.1) | 0.002 |
| Oral glucocorticoids | 5050 (7.5) | 2139 (7.4) | 0.003 | 1795 (7.3) | 1812 (7.3) | 0.001 |
| Indicators of Health Care Utilization | ||||||
| Hospitalized within year, No. (%) | ||||||
| With claim in Veterans Health | 9076 (13.4) | 4516 (15.6) | 0.06 | 3574 (14.5) | 3630 (14.6) | 0.004 |
| With claim in Medicare/Medicaid | 5634 (8.3) | 3597 (12.4) | 0.16 | 2770 (11.2) | 2789 (11.2) | 0.001 |
| Hospitalized in 30 d, No. (%) | ||||||
| With claim in Veterans Health | 2510 (3.7) | 1197 (4.1) | 0.02 | 942 (3.8) | 962 (3.9) | 0.003 |
| With claim in Medicare/Medicaid | 987 (1.5) | 581 (2.0) | 0.04 | 440 (1.8) | 452 (1.8) | 0.003 |
| Nursing home encounter in last year, No. (%) | 201 (0.3) | 136 (0.5) | 0.03 | 96 (0.4) | 100 (0.4) | 0.002 |
| No. of medications, median (IQR) | 7 (5-11) | 7 (4-10) | 0.06 | 7 (4-10) | 7 (4-10) | 0.003 |
| Outpatient visits in past year, median (IQR) | 6 (3-11) | 7 (4-12) | 0.05 | 6 (4-11) | 6 (4-11) | 0.002 |
| Insurance use, No. (%) | ||||||
| Medicare in last year | 21435 (31.6) | 10538 (36.4) | 0.10 | 8807 (35.7) | 8812 (35.5) | 0.003 |
| Medicaid in last year | 663 (1.0) | 435 (1.5) | 0.05 | 323 (1.3) | 331 (1.3) | 0.002 |
| Medicare Advantage | 10251 (15.1) | 4339 (15.0) | 0.004 | 3770 (15.3) | 3784 (15.3) | 0.001 |
Abbreviations: IQR, interquartile range; SMD, standardized mean difference.
SI conversion factors: to convert creatinine to μmol/L, multiply by 88.4; low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259.
SMDs are the absolute difference in means or percentage divided by an evenly weighted pooled standard deviation, or the difference between groups in number of standard deviations. In the weighted cohort, all standardized differences were less than 0.01, suggesting there were no important imbalances (see eFigure 3 in the Supplement for the plot of the mean standardized differences of the preweighted and weighted cohort).
Other races include American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.
Calculated as weight in kilograms divided by height in meters squared.
Definitions of comorbidities are listed in eTable 1 in the Supplement.
Median follow-up in the weighted cohort was 1.0 year (IQR, 0.4-2.6) for patients taking metformin and 1.2 years (IQR, 0.5-2.7) for sulfonylurea users. At 3 years of follow-up, 84.7% vs 82.4% metformin and sulfonylurea users, respectively, had stopped or switched treatment; 3.0% vs 4.1% had experienced noncardiovascular death; 2.5% vs 3.3% were censored for leaving the VHA; and 5.6% vs 4.7% reached study end.
MACE Outcomes
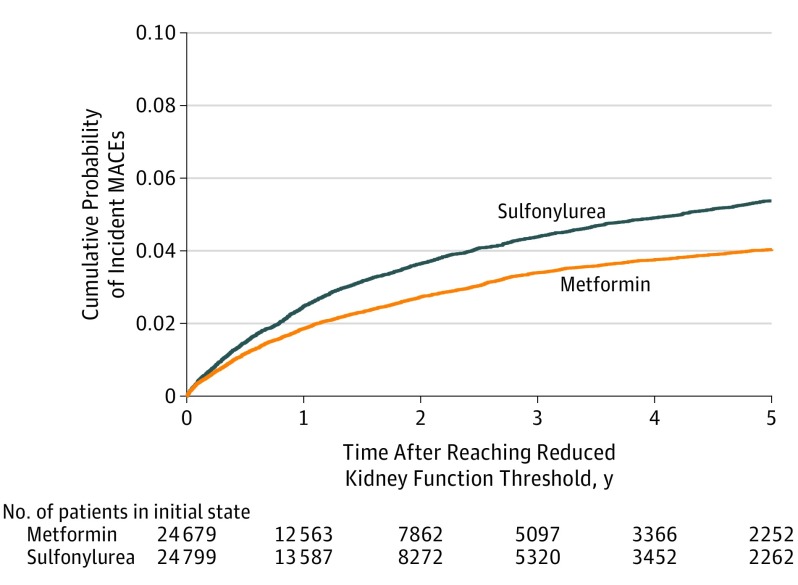
After propensity score weighting, there were 1048 composite events among metformin patients with reduced kidney function and 1394 events among sulfonylurea patients, yielding 23.0 (95% CI, 21.7-24.4) vs 29.2 (95% CI, 27.7-30.7) events per 1000 person-years of use, respectively. After covariate adjustment, the cause-specific adjusted HR (aHR) for MACE was 0.80 (95% CI, 0.75-0.86) among metformin users compared with sulfonylurea. The adjusted incident rate difference was 5.8 (95% CI, 4.1-7.3) fewer events per 1000-person years for metformin compared with sulfonylurea users. The cumulative probability of MACE for patients in the metformin group vs the sulfonylurea group was 1.9% vs 2.5% at 1 year, 3.4% vs 4.4% at 3 years, and 3.8% vs 4.9% at 4 years (Figure 2).
Figure 2. Competing Risk Cumulative Incidence Match-Weighted Cohort.
Aalen-Johansen cumulative probability of incident major adverse cardiovascular events (MACE) among sulfonylurea vs metformin cohort with reduced kidney function. The median follow-up time in the weighted cohort was 1.0 year (interquartile range, 0.4-2.6) for patients taking metformin and 1.2 years (interquartile range, 0.5-2.7) for sulfonylurea users.
Results were consistent for each component of the primary outcome, including cardiovascular hospitalizations (aHR, 0.87 [95% CI, 0.80-0.95]) and cardiovascular deaths (aHR, 0.70 [95% CI, 0.63-0.78]) (Table 2). eFigure 5 in the Supplement demonstrates the cumulative incidence of MACE accounting for the competing risks of medication nonpersistence and noncardiovascular death. The secondary outcome, which included AMI, stroke, and cardiovascular death and excluded TIA, demonstrated consistent results (Table 2). Addition of TIA emergency department visits added 10 events in the weighted cohort (5 each for metformin and sulfonylurea users) and the estimates were unchanged.
Table 2. Rates and Adjusted Hazard Ratios for Major Adverse Cardiovascular Events (MACE) in Weighted Cohort.
| Metformin | Sulfonylurea | |
|---|---|---|
| Persistent Exposure Requireda | ||
| No. at risk in weighted cohort | 24 679 | 24 799 |
| Primary outcome: composite MACE | 1048 | 1394 |
| Person-years | 45542 | 47762 |
| Unadjusted rate/1000 person-years (95% CI) | 23.0 (21.7 to 24.4) | 29.2 (27.7 to 30.7) |
| Adjusted hazard ratio (95% CI)b | 0.80 (0.75 to 0.86) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −5.8 (−7.3 to −4.1) | |
| Component of primary outcome: cardiovascular hospitalization (AMI, stroke, or TIA) | 708 | 874 |
| Unadjusted rate/1000 person-years (95% CI) | 15.5 (14.4 to 16.7) | 18.3 (17.1 to 19.5) |
| Adjusted hazard ratio (95% CI)b | 0.87 (0.80 to 0.95) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −2.4 (−3.7 to −0.9) | |
| Component of primary outcome: cardiovascular death | 407 | 623 |
| Person-years | 46 484 | 49 066 |
| Unadjusted rate/1000 person-years (95% CI) | 8.8 (8.0 to 9.6) | 12.7 (11.7 to 13.7) |
| Adjusted hazard ratio (95% CI)b | 0.70 (0.63 to 0.78) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −3.8 (−4.7 to −2.8) | |
| Secondary outcome: AMI, stroke, or cardiovascular death | 953 | 1297 |
| Person-years | 45719 | 47987 |
| Unadjusted rate/1000 person-years (95% CI) | 20.8 (19.6 to 22.2) | 27.0 (25.6 to 28.5) |
| Adjusted hazard ratio (95% CI)b | 0.78 (0.72 to 0.84) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −5.9 (−7.6 to −4.3) | |
| Sensitivity Analysis: Population With 2 Reduced eGFRs Who Remain Persistent With Medication | ||
| No. at risk in weighted cohort | 3586 | 4287 |
| Primary outcome: composite MACE | 160 | 273 |
| Person-years | 6676 | 8797 |
| Unadjusted rate/1000 person-years (95% CI) | 24.0 (20.6 to 27.9) | 31.0 (27.6 to 34.9) |
| Adjusted hazard ratio (95% CI)b | 0.85 (0.70 to 1.02) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −4.7 (−9.3 to 0.6) | |
| Component of primary outcome: cardiovascular hospitalization (AMI, stroke, or TIA) | 103 | 162 |
| Person-years | 6676 | 8797 |
| Unadjusted rate/1000 person-years (95% CI) | 15.4 (12.7 to 18.7) | 18.4 (15.8 to 21.4) |
| Adjusted risk difference (95% CI) | −1.7 (−5.2 to 2.6) | |
| Adjusted hazard ratio (95% CI)b | 0.91 (0.72 to 1.15) | 1 [Reference] |
| Component of primary outcome: cardiovascular death | 67 | 134 |
| Person years | 6809 | 8980 |
| Unadjusted rate/1000 person-years (95% CI) | 9.8 (7.7 to 12.4) | 15.0 (12.6 to 17.7) |
| Adjusted risk difference (95% CI) | −4.4 (−7.1 to −1.1) | |
| Adjusted hazard ratio (95% CI)b | 0.71 (0.53 to 0.93) | 1 [Reference] |
| Sensitivity Analysis: Persistent Exposure Not Requiredd | ||
| No. at risk in weighted cohort | 24 679 | 24 799 |
| Primary outcome: composite MACE | 4479 | 4722 |
| Person-years | 153 840 | 148 115 |
| Unadjusted rate/1000 person-years (95% CI) | 29.1 (28.2 to 30.0) | 31.9 (31.0 to 32.8) |
| Adjusted hazard ratio (95% CI)b | 0.90 (0.87 to 0.93) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −3.2 (−4.1 to −2.2) | |
| Sensitivity Analysis: Excluding Medicare Advantage | ||
| No. at risk in weighted cohort | 20 909 | 21 015 |
| Primary outcome: composite MACE | 893 | 1195 |
| Person-years | 36 670 | 38 674 |
| Unadjusted rate/1000 person-years (95% CI) | 24.4 (22.8 to 26.0) | 30.9 (29.2 to 32.7) |
| Adjusted hazard ratio (95% CI)b | 0.80 (0.74 to 0.87) | 1 [Reference] |
| Adjusted incident rate difference (95% CI)c | −6.2 (−8.0 to −4.0) | |
Abbreviations: AMI, acute myocardial infarction; eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack.
Primary analysis considers patients persistent with regimen until they do not have oral antidiabetic medications for 90 days.
Cox proportional hazards model for MACE, adjusted for demographics, clinical information derived from the electronic health record, comorbidities, use of medications, and health care utilization (see eTable 1 in the Supplement). All continuous variables were modeled as restricted cubic splines.
The decrease in the number of events per 1000 person-years of metformin use compared with sulfonylurea use among patients with reduced kidney function. The adjusted rate difference is estimated by multiplying the unadjusted incident rate for sulfonylurea by the adjusted hazard ratio minus 1. Confidence bounds are calculated using the respective bounds from the hazard ratio.
Patients remain in their exposure group, regardless of persistence with drug therapy, until outcome or end of the study.
Sensitivity and Subgroup Analyses
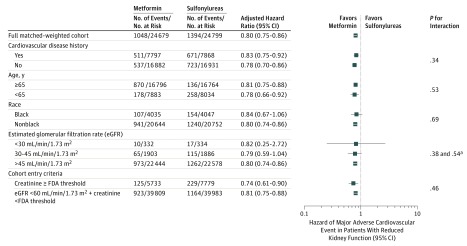
Among the 14 589 patients who had a second confirmatory eGFR less than 60 mL/min/1.73 m2 and remained persistent with their regimen, the median number of days to the second confirmatory eGFR was 112 days (IQR, 73-147). Among this chronic kidney disease cohort, there were 3586 weighted metformin users and 4287 weighted sulfonylurea users, and results were similar but no longer statistically significant for the primary MACE outcome. However, results reached statistical significance for the outcome of cardiovascular death. After removing the requirement for glucose-lowering medication persistence and excluding Medicare Advantage patients, all results were consistent (Table 2). Subgroup analyses stratified by history of cardiovascular disease, age, race, eGFR at kidney threshold, and if the patient entered via reaching the FDA-defined elevated creatinine or reduced eGFR threshold with creatinine below the FDA guidance were consistent with the main analysis, with no evidence of effect modification (all P values >.20). For smaller subgroups, HR confidence intervals were wide (Figure 3 and eTable 3 in the Supplement).
Figure 3. Adjusted Hazard Ratios for Major Adverse Cardiovascular Events by Subgroups.
FDA indicates Food and Drug Administration.
aP value for eGFR prime term (it was modeled as a spline so there are multiple terms).
Discussion
Among patients with diabetes who developed reduced kidney function, persistent use of metformin compared with sulfonylurea use was associated with a decreased hazard of MACE. This study and the results add to the limited observational evidence for the beneficial association of metformin compared with sulfonylurea and cardiovascular outcomes among those who develop reduced kidney function.32
Although there is consensus that metformin is first-line diabetes treatment, metformin is discontinued in many patients when kidney disease develops. Flory and Hennessy33 reported that nearly 1 million US patients with diabetes and eGFR between 31 and 89 mL/min/1.73 m2 could take metformin but do not. In April 2016, the FDA issued a safety announcement and revised label regarding metformin use in patients with reduced kidney function.34 The revised label states that metformin can be safely used in patients with mild kidney function impairment (45-60 mL/min/1.73 m2) and some patients with moderate kidney function impairment (eGFR, 30-45 mL/min/1.73 m2).
At the same time, the FDA also recommended that kidney function be evaluated with eGFR rather than creatinine.34 This US guidance is now more aligned with recommendations from the United Kingdom, Canada, and Australia, which emphasize metformin use based on eGFR criteria rather than creatinine because eGFR more accurately measures kidney function.35,36 Patients with reduced eGFR may use metformin with frequent monitoring and dose reduction, but metformin is contraindicated at an eGFR less than 30 mL/min/1.73 m2.
The FDA decision about metformin was based in part on data from 2 comprehensive reviews. The systematic review by Inzucchi et al37 included 65 studies (the largest had 10 000 patients) and found no increased risk of metformin-associated lactic acidosis in patients with mild to moderate kidney disease. The meta-analysis by Crowley and colleagues32 describes the existing evidence on metformin effectiveness in kidney disease. This meta-analysis included 6 studies (5 cohort studies and 1 nested case-control study) of patients with diabetes and chronic kidney disease. All the studies compared clinical outcomes between patients using metformin and non-metformin regimens, including multiple drugs. Five of the 6 studies (n = 33 442) examined all-cause mortality. The relative risk of all-cause mortality was lower in patients taking metformin than for patients not taking metformin (HR, 0.78 [95% CI, 0.63-0.96]; I2 = 79.8%).
Only 2 of the 6 studies (n = 14 408) examined the association between diabetes treatments and MACE in patients with reduced kidney function. Both compared metformin vs non-metformin regimens. The first, by Ekström et al,38 used a Swedish registry to define MACE as diagnosis of myocardial infarction, angina, hemorrhagic or ischemic stroke, peripheral vascular disease, or coronary disease procedure. They found no significant difference in MACE between metformin patients with eGFR of 45 to less than 60 mL/min/1.73 m2 (n = 6655; HR, 0.94 [95% CI, 0.84-1.05]) and 30 to less than 45 mL/min/1.73 m2 (n = 1894; HR, 1.00 [95% CI, 0.83-1.19]) compared with other regimens (including but not restricted to sulfonylureas). The second study was conducted in the United States by Masoudi et al39 and examined heart failure readmission in patients with reduced kidney function. They demonstrated lower readmission risk (n = 5859; HR, 0.91 [95% CI, 0.84-0.99]) for metformin compared with sulfonylurea or insulin use. The current study adds to the body of evidence from these 2 prior studies by examining important cardiovascular outcomes (MACE) in a large population who persisted with their initial diabetes treatment once they reached reduced kidney function threshold.
Limitations
This study has several limitations. First, incident therapy persistence with either metformin or sulfonylureas at the kidney threshold was required and excluded many patients who discontinued, added, or switched to newer medications at or before reaching the kidney threshold. The study design also excluded those who began diabetes treatment after the onset of reduced kidney function. While reducing sample size, this design choice allowed the evaluation of those patients who continued taking their initial glucose-lowering monotherapy despite changing kidney function. Furthermore, a competing risk model was used to address concerns that nonpersistence with glucose-lowering medications or noncardiovascular death would preclude assessment of MACE outcomes. Findings from this study cannot be generalized to patients who already have a reduced eGFR at the time of metformin initiation.
Second, veterans may not receive all their care at VHA facilities, and some MACE outcomes may have been missed despite the linkage to Medicare and Medicaid data.
Third, cohort entry and the start of follow-up was either an elevated serum creatinine or reduced eGFR less than 60 mL/min/1.73 m2. It is possible that for some patients this kidney threshold may represent an acute kidney injury event rather than progression to chronic kidney disease. There was inadequate statistical power to evaluate differences in MACE events in patients with persistently reduced kidney function. The sensitivity analysis, which required a confirmatory reduced eGFR, found results consistent with the main findings but without statistical significance; therefore, overall results cannot be extrapolated to this group of patients.
Fourth, although propensity score weighting and direct covariate adjustment were used to address confounding, there is likely residual confounding.
Fifth, the study did not include a dose analysis or compare those who continued metformin use with those who switched to a newer agent to determine whether the findings were associated with specific doses of metformin or sulfonylurea or whether results were consistent when compared with a newer drug class.
Sixth, the study population was mostly elderly white men, and may not be representative of the larger population of patients with diabetes and reduced kidney function. This should be considered when generalizing the study results to other populations.
Seventh, it cannot be determined from these analyses whether metformin is associated with a reduced risk or sulfonylureas are associated with an increased risk of MACE outcomes.
Conclusions
Among patients with diabetes and reduced kidney function persisting with monotherapy, treatment with metformin, compared with a sulfonylurea, was associated with a lower risk of MACE.
eTable 1. Definitions of Comorbid Conditions and Medications, on the Basis Of Codes in 720 Days Before Reaching the Reduced Kidney Function Threshold and Prescriptions in the 180 Days Before Kidney Threshold
eTable 2. Description of Propensity Score Model and Treatment Weights
eTable 3. Risk of Major Adverse Cardiovascular Events in Subgroups Stratified by History of Cardiovascular Disease, Age, Race, Estimated Glomerular Filtration Rate at Time of Reaching Kidney Threshold, and Cohort Entry Criteria Reduced Estimated Glomerular Filtration Rate or Elevated Creatinine
eFigure 1. Study Design Schematic
eFigure 2. Distribution of Propensity Scores by Drug
eFigure 3. Mean Standardized Differences Comparing Metformin versus Sulfonylurea Before and After Weighting the Cohort
eFigure 4. Deviance for the Propensity Score Model
eFigure 5. Complete Aalen-Johansen Cumulative Probability of Major Adverse Cardiovascular Events With the Competing Risks of Nonpersistence and Death From Noncardiovascular Cause in Cohort Using Matching Weights
References
- 1.Geiss LS, Kirtland K, Lin J, et al. Changes in diagnosed diabetes, obesity, and physical inactivity prevalence in US counties, 2004-2012. PLoS One. 2017;12(3):e0173428. doi: 10.1371/journal.pone.0173428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 3.Standards of medical care in diabetes 2017: summary of revisions. Diabetes Care. 2017;40(suppl 1):S4-S5. doi: 10.2337/dc17-S003 [DOI] [PubMed] [Google Scholar]
- 4.Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157(9):601-610. doi: 10.7326/0003-4819-157-9-201211060-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565-1576. doi: 10.1056/NEJMoa0806359 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. doi: 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 7.Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40(5):706-714. doi: 10.2337/dc16-1943 [DOI] [PubMed] [Google Scholar]
- 8.Food Drug Administration Center for Drug Evaluation and Research application number: 20-357/s019: final printed label: Glucophage. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20357S019_Glucophage_prntlbl.pdf. Accessed January 5, 2015.
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Genuth S, et al. ; ACCORD Study Group . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828. doi: 10.1056/NEJMoa1006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi: 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 12.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 13.Humensky J, Carretta H, de Groot K, Brown MM, Tarlov E, Hynes DM. Service utilization of veterans dually eligible for VA and Medicare fee-for-service: 1999-2004. Medicare Medicaid Res Rev. 2012;2(3):mmrr.002.03.a06. doi: 10.5600/mmrr.002.03.A06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hynes DM, Koelling K, Stroupe K, et al. Veterans’ access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45(3):214-223. doi: 10.1097/01.mlr.0000244657.90074.b7 [DOI] [PubMed] [Google Scholar]
- 15.McCarthy JF, Valenstein M, Kim HM, Ilgen M, Zivin K, Blow FC. Suicide mortality among patients receiving care in the Veterans Health Administration health system. Am J Epidemiol. 2009;169(8):1033-1038. doi: 10.1093/aje/kwp010 [DOI] [PubMed] [Google Scholar]
- 16.Center of Excellence for Suicide Prevention Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository: National Death Index (NDI). http://vaww.virec.research.va.gov/Mortality/Overview.htm. Accessed December 2018.
- 17.Greevy RA Jr, Huizinga MM, Roumie CL, et al. Comparisons of persistence and durability among three oral antidiabetic therapies using electronic prescription-fill data: the impact of adherence requirements and stockpiling. Clin Pharmacol Ther. 2011;90(6):813-819. doi: 10.1038/clpt.2011.228 [DOI] [PubMed] [Google Scholar]
- 18.Niesner K, Murff HJ, Griffin MR, et al. Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology. 2013;24(2):334-335. doi: 10.1097/EDE.0b013e3182821e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167. doi: 10.1001/archpsyc.58.12.1161 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518-2531. doi: 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011;162(3):548-554. doi: 10.1016/j.ahj.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 23.vanBuuren S. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press/Taylor and Francis Group; 2012. [Google Scholar]
- 24.D’Agostino R, Rubin D. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95(451):749-759. doi: 10.1080/01621459.2000.10474263 [DOI] [Google Scholar]
- 25.Franklin JM, Eddings W, Austin PC, Stuart EA, Schneeweiss S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat Med. 2017;36(12):1946-1963. [DOI] [PubMed] [Google Scholar]
- 26.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 27.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Scheike TH, Zhang MJ. Checking Fine and Gray subdistribution hazards model with cumulative sums of residuals. Lifetime Data Anal. 2015;21(2):197-217. doi: 10.1007/s10985-014-9313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(466):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 30.Huizinga MM, Roumie CL, Elasy TA, et al. Changing incident diabetes regimens: a Veterans Administration cohort study from 2000 to 2005. Diabetes Care. 2007;30(8):e85. doi: 10.2337/dc07-0650 [DOI] [PubMed] [Google Scholar]
- 31.Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, Griffin MR. Diabetes treatment intensification and associated changes in HbA1c and body mass index: a cohort study. BMC Endocr Disord. 2016;16(1):32. doi: 10.1186/s12902-016-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166(3):191-200. doi: 10.7326/M16-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flory JH, Hennessy S. Metformin use reduction in mild to moderate renal impairment: possible inappropriate curbing of use based on food and drug administration contraindications. JAMA Intern Med. 2015;175(3):458-459. doi: 10.1001/jamainternmed.2014.6936 [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/Drugs/ood DrugSafety/ucm493244.htm. Published 2016. Accessed April 8, 2016.
- 35.European Medications Agency Use of metformin to treat diabetes now expanded to patients with moderately reduced kidney function. https://www.ema.europa.eu/en/medicines/human/referrals/metformin-metformin-containing-medicines. Accessed June 13, 2019.
- 36.Diabetes Canada Canadian Journal of Diabetes https://guidelines.diabetes.ca/docs/CPG-2018-full-EN.pdf. Published April 2018. Accessed June 13, 2019.
- 37.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668-2675. doi: 10.1001/jama.2014.15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2(4):e001076. doi: 10.1136/bmjopen-2012-001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111(5):583-590. doi: 10.1161/01.CIR.0000154542.13412.B1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Comorbid Conditions and Medications, on the Basis Of Codes in 720 Days Before Reaching the Reduced Kidney Function Threshold and Prescriptions in the 180 Days Before Kidney Threshold
eTable 2. Description of Propensity Score Model and Treatment Weights
eTable 3. Risk of Major Adverse Cardiovascular Events in Subgroups Stratified by History of Cardiovascular Disease, Age, Race, Estimated Glomerular Filtration Rate at Time of Reaching Kidney Threshold, and Cohort Entry Criteria Reduced Estimated Glomerular Filtration Rate or Elevated Creatinine
eFigure 1. Study Design Schematic
eFigure 2. Distribution of Propensity Scores by Drug
eFigure 3. Mean Standardized Differences Comparing Metformin versus Sulfonylurea Before and After Weighting the Cohort
eFigure 4. Deviance for the Propensity Score Model
eFigure 5. Complete Aalen-Johansen Cumulative Probability of Major Adverse Cardiovascular Events With the Competing Risks of Nonpersistence and Death From Noncardiovascular Cause in Cohort Using Matching Weights