Abstract
Objective:
Ameloblastoma is a rare odontogenic neoplasm with high recurrence rates if improperly treated. If left untreated (or is treated inadequately), it can cause substantial morbidity, disfigurement, and even death. Hence, there is a need to explore the stromal cells too, which might play an important role in assessing its aggressive behavior and may help to predict the recurrence of different clinical variants of ameloblastoma. Myofibroblasts (MFs) are such cells which have been studied in various lesions.
Materials and Methods:
This retrospective study involved archival tissues of ameloblastoma. Among a total of 40 cases, 12 cases of SMA (solid multicystic ameloblastoma), 10 cases of unicystic ameloblastoma (UA), 8 cases of desmoplastic ameloblastoma, and 10 cases of oral squamous cell carcinoma were selected as control. Immunohistochemical staining with anti-alpha-smooth muscle actin antibody was done. Interpretation of ten examined fields was counted by three observers.
Results:
Significant difference in the number of MFs in SMA and UA and desmoplastic ameloblastoma and UA (P < 0.05) was found. However, there was no statistically significant difference in MFs of SMA and desmoplastic ameloblastomas (P > 0.05). In addition, there was no statistically significant difference in the staining intensity between the three variants (P > 0.05).
Conclusion:
A significant correlation was obtained between the number of MF in all the three clinical variants, i.e., SMA, UA, and desmoplastic ameloblastoma (P = 0.02), which is the unique feature of the study.
Keywords: Alpha-smooth muscle actin, ameloblastoma, clinical variants, types
INTRODUCTION
Ameloblastoma is a rare, benign tumor of odontogenic epithelium affecting exclusively the jawbones. It shows various clinical types. It has been proved that unicystic and peripheral extraosseous ameloblastomas tend to grow slowly and show little evidence of invasion; however, solid multicystic ameloblastomas are destructively invasive with high recurrence rate.[1,2] In spite of being the most common odontogenic tumor, paucity still exists in understanding the molecular mechanisms associated with the pathogenesis and biological behavior of ameloblastomas.[3]
The aggressive clinical behavior of ameloblastoma and its histological deceptive benign features constitute a mystifying paradox. Some justifications concerning this bizarre clinical–histologic queries have been analyzed.[4]
As stated by Ruiter et al., “tumor microenvironment”implies the entire functional and structural assemblage of neoplastic and nonneoplastic cells and their extracellular components, including cytokines, chemokines, and growth factors, and their secretions in tumor tissue.[5,6,7]
The tumor microenvironment has now become the focus of intense research. Fibroblasts are among the most abundant cell type in the microenvironment of solid tumors, being particularly prominent in various neoplasms.[8] Attempts have been made to assess tumor behavior to explore nonepithelial factors by employing immunocytochemical markers. These include, among other phenomena, the existence of myofibroblasts (MFs) as discussed by Vered et al.[9,10] Gabbiani et al.[11,12] coined the term “myofibroblasts”for these contractile cells.
MFs share a phenotype between fibroblasts and smooth muscle cells and are characterized by the expression of a specific smooth muscle isoform of alpha-smooth muscle actin (α-SMA). MFs are variants of fibroblasts which express α-SMA, the activation of which results in the development of the fibrotic response. Active MFs cease to proliferate and start to secrete large amounts of extracellular proteins. The expression of α-SMA correlates with the activation of MFs.[13] Anti-α-SMA is an excellent immunostain for tumors of smooth muscle differentiation.[14] “Alpha-SMA-positive stromal cells belong to the myofibroblast group and are found in pathological conditions but not in normal tissue.”[15]
Sherlin et al.[3] suggested that the MFs which are present in the stroma could be a mature myofibroblast, and their presence may aggravate tumor progression, which can be attributed to the invasiveness of desmoplastic ameloblastoma as proposed by Hinz and Gabbiani.[16]
Several types of odontogenic lesions such as ameloblastoma have the potential for aggressive behavior.[17] Thus, it is thought-provoking to know whether the microenvironment of ameloblastomas has been modified by MFs, causing invasion and aggressiveness.[10] This research analyzed the frequency of stromal MFs in different clinical types of ameloblastomas in order to predict their aggressive biological behavior, as similar studies have not been reported in literature. This study aims at demonstrating and comparing the expression of alpha-smooth muscle actin (α-SMA) by MFs in clinical variants of ameloblastoma, i.e., SMA, unicystic ameloblastoma (UA), and desmoplastic ameloblastoma, which have not been explored so far.
MATERIALS AND METHODS
This laboratory-based, retrospective study conducted from June 2014 to December 2014 involved the use of buffered formalin, paraffin-embedded tissues of previously histopathologically diagnosed cases of ameloblastoma and oral squamous cell carcinoma (OSCC) between 1992 and 2015, retrieved from the archives of the Department of Oral and Maxillofacial Pathology, Dr. D. Y. Patil Dental College, Nerul, Navi-Mumbai Maharashtra, India. Institutional ethical approval had been taken from the same institute (no. DYPUSOD/GS-OP-AT/887-A/2014).
Inclusion and exclusion criteria
Cases which fulfilled the Vickers and Gorlin (V&G) criteria and those devoid of considerable inflammation were selected as it has been suggested by Mashhadiabbas et al. that inflammatory infiltrate may interfere with MF differentiation.[18] Cases with insufficient tissues were excluded from the study.
Among a total of 40 cases, 12 cases of SMA, 10 cases of UA, 8 cases of desmoplastic ameloblastoma (study group), and 10 cases of OSCC were selected as positive control. Immunohistochemical staining of all the samples was done using anti-alpha-smooth muscle actin antibody (Biogenex life sciences Pvt. Ltd., CA, USA). The technique used was based on the labeled streptavidin biotin method. Endogenous peroxidase was blocked by first activating the section in 0.6% H2O2. The specimen was then incubated with primary antibody followed by sequential incubations with biotinylated link antibody and peroxidase-labeled streptavidin. Staining was completed after incubation with substrate chromogen solution. Polylysine-coated (Biogenex CA, USA) slides were used for the proper adherence of tissue section (other adhering agents which can be used are gelatin chrome, 3-Aminopropyltriethoxysilane (APES), and aminopropyltriethoxysilane). Unstained sections of 5 μm thicknesses were cut using a microtome and were transferred on polylysine-coated slides from the paraffin blocks. The sections were deparaffinized and rehydrated. The next step of antigen removal was done using freshly prepared tris-ethylenediaminetetraacetic acid buffer solution in which sodium citrate and citric acid were added with distilled water. Antigen retrieval is a process by which antigenic epitopes, made unavailable because of fixation-associated protein cross linking, are rendered accessible to antibodies for binding. The staining protocol included incubating the specimen with a peroxidase block (Biogenex CA, USA) for 15 min. After washing, the slides were covered with a power block (Biogenex CA, USA) for 10 min. The slides were then blot dried, and the sections were covered with optimally diluted primary antibody of anti-α-SMA (Biogenex CA, USA) and incubated for 1 h. The slide was then flooded with Super Enhancer (Biogenex CA, USA) and incubated for 20 min. This was followed by adding Poly HRP Reagent (horseradish peroxidase) (Biogenex life sciences Pvt. Ltd., CA, USA) and incubating for 30 min at room temperature. After the slides were wiped, freshly prepared substrate chromogen solution was applied to cover the section and incubated at room temperature for 10 min. The sections were then counterstained with Harris hematoxylin, and the stained sections were dehydrated, cleared, and mounted in Distrene dibutyl pthalate xylene.
Interpretation was done by evaluating the staining intensity and the number of alpha-SMA-positive cells based on methods followed by Vered et al. and Etemad-Moghadam et al.[10,19] The positive control, i.e., OSCC, was examined for the presence of a colored end product at the site of the target antigen (Dab chromogen brown end product). The presence of these colors was interpreted as a positive staining result, indicating proper performance of the kit reagents. Stromal spindle cells positive for α-SMA were regarded as MFs. Blood vessels with muscle walls were excluded.
Ten representative fields were randomly selected in each immunohistochemically stained section. The expression pattern of alpha-SMA was studied adjacent to the cystic epithelial lining for UA and for the solid tumors it was immediately adjacent to the periphery of the tumoral islands/nests/cords. The number of alpha-SMA-positive cell, excluding those surrounding the blood vessels, was counted, and the total number of positive cells for all the ten examined fields, per case, was counted by three observers who were blindfolded. This allowed calculation of the mean number of alpha-SMA-positive cells per field. The staining intensity was considered 0 when there was no staining; 1, in parts where positivity was observed only at a magnification of ×400; 2, in cases where the staining was obvious at ×100, but not at ×40; and 3, in fields where immunopositive cells were seen even at ×40.[18] Any disagreements within the three observers were resolved with a penta-head microscope.
All the findings were compiled and analyzed for various comparative analyses using all the parameters with the help of ANOVA test, Kolmogorov–Smirnov test, and least significant difference (LSD) test. Data were presented using frequency, percentage, and descriptive statistics such as mean, standard deviation, and standard error, followed by appropriate charts and graphs.
Further analysis was done using one-way ANOVA followed by LSD test. The level of significance was set at 5%. Statistical significance was set at P < 0.05. IBM SPSS 20.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). software was used for analysis.
The results were obtained from the analyzed data. Conclusions were drawn from the obtained results.
RESULTS
The MFs observed in SMA, UA, and desmoplastic ameloblastomas have been compared with those in control group [Figure 1]. All the findings have been tabulated.
Figure 1.
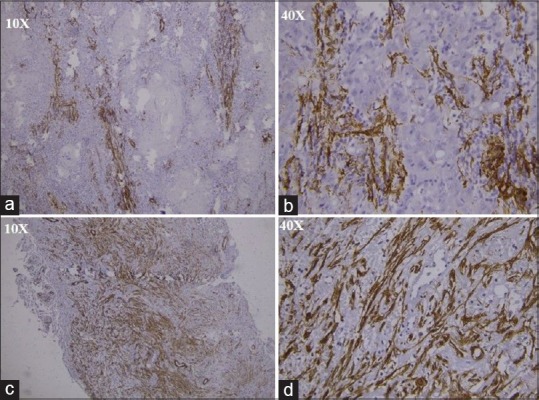
(a) Immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in oral squamous cell carcinoma under ×10, (b) immunohistochemistry stained section showing myofibroblasts in oral squamous cell carcinoma under ×40, (c) immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in solid multicystic ameloblastoma under ×10, (d) immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in solid multicystic ameloblastoma under ×40
Immunohistochemical expression for MFs in solid multicystic, desmoplastic, and unicystic according to observer 1, observer 2, and observer 3 Table 1 shows that solid multicystic ameloblastoma has comparatively more values for MFs.
Table 1.
Immunohistochemical expression of myofibroblasts in various clinical subtypes of ameloblastoma
| Desmoplastic (n=8) | Solid multicystic (n=12) | Unicystic (n=10) | One-way ANOVA (F, P) | ||||
|---|---|---|---|---|---|---|---|
| Mean±SE | 95% CI | Mean±SE | 95% CI | Mean±SE | 95% CI | ||
| Observer-1 | 43.75±15.29 | 7.60-79.90 | 56.92±18.45 | 16.32-97.51 | 19.87±9.24 | −1.04-40.78 | 1.555, 0.229 |
| Observer-2 | 44.71±15.52 | 8.01-81.41 | 56.28±18.36 | 15.86-96.70 | 19.30±9.02 | −1.12-39.72 | 1.574, 0.226 |
| Observer-3 | 45.08±15.96 | 7.34-82.82 | 57.39±18.70 | 16.22-98.56 | 20.63±9.01 | 0.24-41.02 | 1.491, 0.243 |
| Overall average | 44.51±15.57 | 7.70-81.33 | 56.86±18.50 | 16.15-97.57 | 19.93±9.08 | −0.62-40.48 | 1.541, 0.232 |
CI: Confidence interval, SE: Standard error
Results indicate that there was a statistically significant difference in the number of MFs in solid multicystic and UA and desmoplastic and UA (P < 0.05). However, there was no statistically significant difference in the number of MFs of solid multicystic and desmoplastic ameloblastomas (P > 0.05).
On multiple comparisons, the pair-wise significance of difference found in SMA, desmoplastic ameloblastoma, and UA according to different observers was tested using LSD test. The mean difference was significant at the 0.05 level. No significant difference was observed between the number of MFs in solid multicystic and desmoplastic ameloblastoma (P > 0.05) [Table 2].
Table 2.
The pair-wise significance of difference in various clinical subtypes of ameloblastoma for overall average using the least significant difference test
| Lesion type | Mean±SD | Mean rank | χ2, P* |
|---|---|---|---|
| Desmoplastic | 2.38±0.74 | 15.38 | 1.762, 0.414 |
| Solid multicystic | 2.58±0.67 | 17.58 | |
| Unicystic | 1.80±1.39 | 13.10 |
*Kruskal-Wallis test. SD: Standard deviation
When multiple comparisons were assessed for the intensity of staining using LSD, it was found that that there was no significant difference in the staining index according to histopathological subtypes (P > 0.05). The results indicate that there was a significant difference in the number of MFs in solid multicystic ameloblastoma and UA and desmoplastic ameloblastoma and UA (P < 0.05). However, there was no significant difference in MFs of solid multicystic and desmoplastic ameloblastomas. (P > 0.05). Furthermore, there was no significant difference in the staining intensity between the three variants (P > 0.05) [Table 3 and Figure 2].
Table 3.
The pair-wise significance of difference for expression of myofibroblasts (intensity of staining) for overall average using the least significant difference test
| Mean difference | SE | P* | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Desmoplastic versus unicystic | 24.58 | 23.48 | 0.304 | −23.60 | 72.76 |
| Solid multicystic versus desmoplastic | 12.35 | 22.60 | 0.589 | −34.02 | 58.71 |
| Solid multicystic versus unicystic | 36.93 | 21.20 | 0.093 | −6.56 | 80.42 |
*Pair-wise comparisons using LSD method. LSD: Least significant difference, SE: Standard error, CI: Confidence interval
Figure 2.
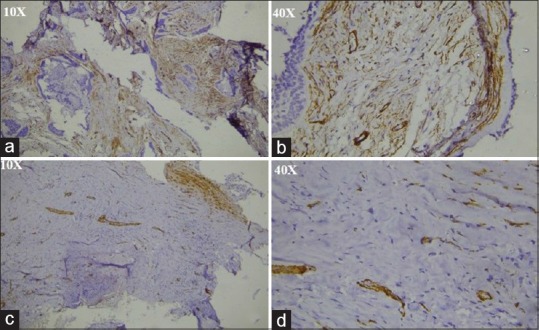
(a) Immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in desmoplastic ameloblastoma under ×10, (b) immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in desmoplastic ameloblastoma under ×40, (c) immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in unicystic ameloblastoma under ×10, (d) immunohistochemistry stained section showing alpha-smooth muscle actin-positive myofibroblasts in unicystic ameloblastoma under ×40
DISCUSSION
Ameloblastomas, though benign, behave aggressively and may be lethal in recurrent cases.[20,21]
Researchers have been trying to explore hidden factors for such aggressive behavior within the epithelial component, but, now, the research has been shifted on stromal component due to its high recurrence. MFs are stromal cells exhibiting the behavior of both fibroblasts and smooth muscle cells and are responsible for the biological behavior of various lesions.[22,23] MFs exist as a minor fibroblast subpopulation which is widely distributed and secrete cytokines, growth factors, chemokines, hormones, inflammatory mediators, adhesion proteins, and most abundantly extracellular matrix proteins.[24,25]
Several studies have demonstrated an increase in the density of myofibroblasts in different lesions, including malignant neoplasms.[26]
Hence, this study was undertaken to determine the immunohistochemical expression of MFs present in stroma in the three clinical variants of ameloblastoma, i.e., SMA, UA, and desmoplastic ameloblastoma.
The results from the present study indicate that there was a significant difference in the number of MFs between SMA and UA and desmoplastic ameloblastoma and UA. On pair-wise comparisons among the three variants, there was a significant difference between the number of MFs in SM and UA and desmoplastic ameloblastoma and UA. However, there was no significant difference in the number of MF of solid multicystic and desmoplastic ameloblastomas.
Thus, from the results of the present study, a positive link can be established between the number of MFs and the aggressive nature of the lesion. Our findings confirm to Smith et al. who reported a recurrent infiltrative ameloblastoma whose stroma contained abundant MFs and concluded that these cells accompany the invasive behavior of this tumor. A similar study was carried out by Shruthi et al.[27] to assess the frequency of stromal MFs in odontogenic keratocyst (OKC) and ameloblastomas and to correlate the same with the behavior of these lesions, where they found that the mean number of MFs in ameloblastoma was more than that of OKC. Even they found a positive link between the presence and number of MFs to the aggressive behavior of the odontogenic cyst/tumor. Another study confirmed the invasive behavior of a recurrent aggressive ameloblastoma due to a high number of MFs in the stroma.[28] A study done on odontogenic myxoma which is considered to be a slow-growing invasive tumor, by Effiom et al.,[29] showed that abundant stromal MFs are associated with its invasive behavior and further hypothesized that stromal MFs modify extracellular matrix by secreting various cytokines, causing epithelial invasion.
According to the results of the present study, in known aggressive lesions such as SM and desmoplastic ameloblastoma, the mean number of MFs was more as compared to that of UA, which is not aggressive in nature. This was in accordance with the study conducted by Sherlin et al., Vered et al., and Smith and Bartov.[3,10,26,30,31] We found no significant difference between the number of MFs in SMA and desmoplastic ameloblastoma, and the reason could be because both are known aggressive lesions with a tendency to recur. In addition, in the present study, there was no significant difference in the staining intensity of the three lesions.
Similarly, Vered et al.[10] quantitatively compared odontogenic cysts and tumors and reported that stroma of these lesions harbors MF as reflected by α-SMApositive cells. Furthermore, they showed that the mean number of MF, in aggressive odontogenic lesions, for example, SMA and parakeratinized OKC-P, was more similar to that in OSCC. In contrast, known nonaggressive lesions, for example, UA, orthokeratinized OKC-O, and dentigerous cyst (DC), significantly showed less MF compared to SMA and/or OKC-P. Thus, they proved a strong link between the number of MF and the aggressive nature of the neoplasm. Odontogenic epithelium, mainly in SMA and OKC-P, can stimulate stromal MF in a manner similar to OSCC. Thus, more MFs in the stroma directly related to a more aggressive behavior of the odontogenic cysts.[18] The results indicated that lesser MFs were present in the cyst stroma of keratocystic odontogenic tumors than DC and radicular cysts.
Mashhadiabbas et al.[18] analyzed the distribution and proliferation of MFs in DC, OKC, and ameloblastoma and concluded that the high frequency of stromal myofibroblast in the OKC implies that MF can contribute to the aggressive nature of this cyst, but between odontogenic cysts and ameloblastoma, the presence of stromal MF has no correlation with invasiveness.
As MFs are probably the best indicators or markers for the aggressive potential of any neoplasm, they should be evaluated in the surgical/resected margins too, of the neoplasm. However, in the present study, it was randomly studied in the biopsy tissue.
Limitations of the study
Further studies with more number of cases could further throw light on the biology of ameloblastomas as the sample size was small. Patients’ detailed clinical findings should also be considered to correlate with histopathological correlation.
CONCLUSION
The present study has provided us with valuable information regarding the role and quantification of MFs in the three clinical variants of ameloblastoma, i.e., SMA, UA, and desmoplastic ameloblastoma, especially their aggressive behavior. There was a significant difference in the number of MFs, between SMA and UA and desmoplastic and UA. However, there was no significant difference in the number of MFs of solid multicystic and desmoplastic ameloblastomas. This showed that odontogenic lesions with a high aggressive biological behavior contain more MFs in their stroma as compared to the nonaggressive UA. Thus, increased expression of smooth muscle actin in ameloblastomas marks its aggressiveness, thereby predicting their biological behavior which should be assessed before therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brazis PW, Miller NR, Lee AG, Holliday MJ. Neuro-ophthalmologic aspects of ameloblastoma. Skull Base Surg. 1995;5:233–44. doi: 10.1055/s-2008-1058921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iordanidis S, Makos C, Dimitrakopoulos J, Kariki H. Ameloblastoma of the maxilla. Case report. Aust Dent J. 1999;44:51–5. doi: 10.1111/j.1834-7819.1999.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 3.Sherlin HJ, Natesan A, Ram P, Ramani P, Thiruvenkadam C. Immunohistochemical profiling of ameloblastomas using cytokeratin, vimentin, smooth muscle actin, CD34 and S100. Ann Maxillofac Surg. 2013;3:51–7. doi: 10.4103/2231-0746.110084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal M, Weihsin H, Garg B, Sood R, Singh M. Atypical proliferative ameloblastoma with a rare histopathological feature and review of literature. Oral Maxillofac Pathol J. 2015;6:582–5. [Google Scholar]
- 5.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: A critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–48. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 6.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 7.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: Structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 8.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 9.Li TJ, Kitano M. Reviewing the unicystic ameloblastoma: A clinicopathologically distinct entity. Oral Med Pathol. 1997;2:61–8. [Google Scholar]
- 10.Vered M, Allon I, Buchner A, Dayan D. Stromal myofibroblasts and malignant transformation in a 4NQO rat tongue carcinogenesis model. Oral Oncol. 2007;43:999–1006. doi: 10.1016/j.oraloncology.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 12.Gabianni G. The evolution of the myofibroblast concept: A key cell for wound healing and fibrotic diseases. G Gerontol. 2004;52:280–2. [Google Scholar]
- 13.Chemg S, Young J, Ma H. Alpha-smooth muscle actin. J Am Sci. 2008;4:7–9. [Google Scholar]
- 14.Gaffney EF, Dervan PA, Fletcher CD. Pleomorphic rhabdomyosarcoma in adulthood. Analysis of 11 cases with definition of diagnostic criteria. Am J Surg Pathol. 1993;17:601–9. [PubMed] [Google Scholar]
- 15.Nakayama H, Enzan H, Miyazaki E, Toi M. Alpha smooth muscle actin positive stromal cells in gastric carcinoma. J Clin Pathol. 2002;55:741–4. doi: 10.1136/jcp.55.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Reichart PA, Philipsen HP. London: Quintessence Publishing Co., Ltd; 2004. Odontogenic Tumours and Allied Lesions; p. 82. [Google Scholar]
- 18.Mashhadiabbas F, Atarbashi S, Moshref M, Elahi M. Immunohistochemical detection and ultrastructure of myofibroblasts in the stroma of odontogenic cysts and ameloblastoma. Iran Red Crescent Med J. 2010;12:453–7. [Google Scholar]
- 19.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38:639–43. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 20.Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J Cell Physiol. 2010;222:703–12. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- 21.Bello IO, Alanen K, Slootweg PJ, Salo T. Alpha-smooth muscle actin within epithelial Islands is predictive of ameloblastic carcinoma. Oral Oncol. 2009;45:760–5. doi: 10.1016/j.oraloncology.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Fregnani ER, Sobral LM, Alves FA, Soares FA, Kowalski LP, Coletta RD. Presence of myofibroblasts and expression of matrix metalloproteinase-2 (MMP-2) in ameloblastomas correlate with rupture of the osseous cortical. Pathol Oncol Res. 2009;15:231–40. doi: 10.1007/s12253-008-9110-4. [DOI] [PubMed] [Google Scholar]
- 23.Bagul N, Ganjre A, Goryawala SN, Kathariya R, Dusane S. Dynamic role of myofibroblasts in oral lesions. World J Clin Oncol. 2015;6:264–71. doi: 10.5306/wjco.v6.i6.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 25.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 26.Santos PP, Nonaka CF, Freitas RA, Pereira Pinto L, Souza LB. Immunohistochemical analysis of myofibroblasts, TGF-β1, and IFN-γ in epithelial odontogenic lesions. J Oral Pathol Med. 2017;46:365–70. doi: 10.1111/jop.12494. [DOI] [PubMed] [Google Scholar]
- 27.Shruthi DK, Tegginamani AS, Karthik B. A possible role of myofibroblast in biological behavior of odontogenic keratocyst and ameloblastoma: A comparative study. Univ Res J Dent. 2014;4:115–7. [Google Scholar]
- 28.Rothouse LS, Majack RA, Fay JT. An ameloblastoma with myofibroblasts and intracellular septate junctions. Cancer. 1980;45:2858–63. doi: 10.1002/1097-0142(19800601)45:11<2858::aid-cncr2820451123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Effiom OA, Adewole RA, Odukoya O. Clinicopathological characteristics of odontogenic myxoma in Nigerians. West Afr J Med. 2011;30:255–61. [PubMed] [Google Scholar]
- 30.Smith SM, Bartov SA. Ameloblastoma with myofibroblasts: First report. J Oral Pathol. 1986;15:284–6. doi: 10.1111/j.1600-0714.1986.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 31.Smitha GP, Shenoy S, Narayan TV, Jayaram R. Comparison of myofibroblasts between solid/Multicystic ameloblastoma and unicystic ameloblastoma: An immunohistochemical analysis. J Clin Diagn Res. 2016;10:ZC52–7. doi: 10.7860/JCDR/2016/14380.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]


