Abstract
Introduction:
Clostridium difficile infection (CDI) and nontyphoidal Salmonella infection (NSI) have similar clinical manifestations and are seldom seen simultaneously. The decision-making in terms of antibiotic therapy becomes difficult when both the pathogens are isolated from the same patient.
Case Presentation:
We describe a case of Clostridium difficile (CD) enterocolitis in a healthcare provider who concomitantly tested positive for nontyphoidal Salmonella.
Discussion:
To the best of our knowledge after extensive literature review (English), this is only the fourth report highlighting this association.
Conclusion:
Although Salmonella is not a risk factor for CDI, it can cause intestinal inflammation and alteration in the intestinal flora. When two pathogens are isolated from the same patient, it is tempting to treat both with antibiotics as highlighted. When it involves healthcare workers, there is no difference in guidelines and should not be prescribed antibiotics with intent of reducing secondary transmission.
Keywords: Antibiotics, Clostridium difficile, diarrhea, healthcare worker, nontyphoidal Salmonella
Case Presentation
A 45-year-old emergency room nurse was admitted to the hospital with complaints of lower abdominal pain, three episodes of nonbloody diarrhea, and fever of 1 day duration. The abdominal pain was nonradiating and was colicky in nature. She denied any recent sick contacts except during her clinical work in the emergency room and she had no recent travel. One week prior to her current admission, she was treated with 1 week course of nitrofurantoin for cystitis as outpatient and experienced complete resolution of her urinary symptoms. Her medical history included general anxiety disorder for which she has been on paroxetine for many years. At the time of admission, she was noted to be febrile with a temperature of 38.7°C; she was tachycardic with heart rate of 110/min and blood pressure of 155/79 mmHg. She was in no respiratory distress and was saturating 100% on room air. The remainder of her physical examination was remarkable for hyperactive bowel sounds with generalized abdominal tenderness. There was no rigidity or rebound tenderness. Her respiratory and cardiovascular examination was unremarkable.
Laboratory data revealed leucopenia and no immature forms with total white cell count of 3.0 × 1000/μL (3.9–11.0 × 1000/μL) with normal hemoglobin, hematocrit, and platelet counts. She had normal serum chemistry with creatinine of 0.63 mg/dL and calculated glomerular filtration rate of 110 mL/min. Serum electrolytes and liver function tests were normal. Her erythrocyte sedimentation rate was 14 mm/h (2–15 mm/h), but C-reactive protein was significantly elevated to 124 mg/dL (0.0–4.9 mg/dL). A computed tomography scan of the abdomen and pelvis with contrast showed findings consistent with active colitis involving the caecum and ascending colon with adjacent inflammatory stranding [Figure 1]. The distal small bowel was also noted to be fluid-filled which was concerning for concurrent small bowel enteritis [Figure 2a and b].
Figure 1.
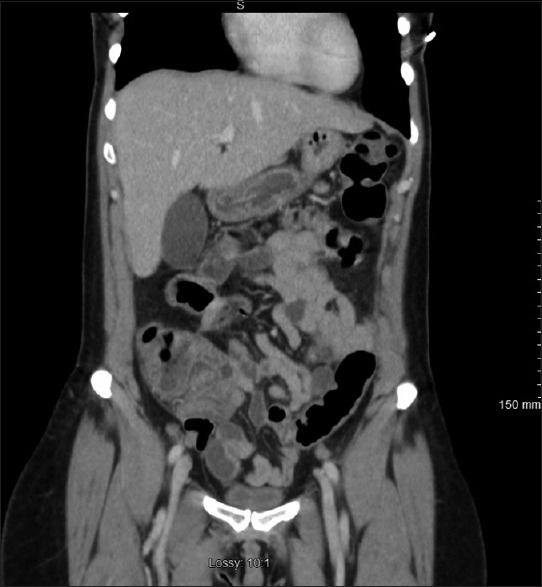
Axial section of CT scan of abdomen with intravenous contrast showing wall thickening of cecum with adjacent mild inflammatory stranding
Figure 2.
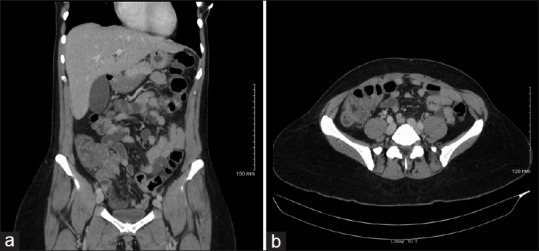
(a) Coronal section of CT scan of abdomen with intravenous contrast showing wall thickening of distal small bowel with fluid-filled bowel indicating concurrent enteritis. (b) Axial section of CT scan of abdomen with intravenous contrast showing similar findings as Figure 2(a) in a different plane
There was no evidence of intraabdominal collection or perforation. With the concern for active colitis on the imaging study, the patient was given a single dose of intravenous cefoxitin in the emergency room. Due to the recent use of antimicrobial for cystitis, we entertained the possibility of CDI and oral vancomycin was introduced and cefoxitin was discontinued while the stool samples were sent for CD toxin and other stool studies including Shigella, Salmonella, Campylobacter, and Escherichia coli.
The stool studies returned positive for toxigenic Clostridium difficile (CD) using real-time polymerase chain reaction (Cepheid Xpert C. difficile/Epi, Cepheid, Sunnyvale CA, USA) and was negative for epidemic strain BI/NAP1/027. The patient showed remarkable improved within 48 h of treatment with oral vancomycin with resolution of diarrhea, fever, and abdominal pain. On the third day of hospital admission, stool cultures returned positive for Salmonella sp. (Cary-Blair stool culture transport medium; Quest Diagnostics NY/NJ, USA) and was negative for Shigella, Campylobacter, and enterohemorrhagic E. coli. Her blood remained sterile. Since the patient's symptoms were resolving with the current antibiotic regime and absence of any other systemic complaints, decision was made to treat CDI with 10 days of oral vancomycin and no additional treatment for Salmonella in stools. At her 2 weeks of follow-up, the patient was asymptomatic with no further episodes of abdominal pain, diarrhea, or fever. She had no untoward effects from the oral antibiotics therapy.
Discussion
Clostridium difficile infection (CDI) causes close to 500,000 infections annually worldwide, although the exact magnitude depends on the type of diagnostic tests used.[1] Nontyphoidal Salmonella infection (NSI) is a common foodborne illness causing 93 million enteric infections worldwide annually and 1.4 million cases in the United States.[2,3,4,5] The majority of Salmonella cases in the United States are of nontyphoidal type.[3] Both infections, CDI and NSI, have similar clinical manifestations and are seldom seen simultaneously in a patient and can be difficult to distinguish if presented at the same time. The decision-making in terms of antibiotic therapy becomes difficult when both the pathogens are isolated from the same patient. To increase the complexity, if the patient is involved in a profession where disease transmissibility is a concern, the temptation to treat the carrier state is tangible. We have been able to find three reports in the literature (English) with simultaneous CDI and NSI, and none of the patients was a healthcare worker.[6,7,8]
Epidemiologically, it has been shown that up to 3% on healthy adults may be colonized asymptomatically with CD and this number increases significantly in the healthcare setting.[9,10] Healthcare workers are clearly at increased risk of developing CDI owing to their direct contact with patients, and this population includes physicians, nurses, and laboratory personnel.[10,11] CD spores are present ubiquitously on inanimate objects and are resistant to commonly used decontaminants; they can persist for long periods of time without loss of viability and are transmitted via feco-oral pathway.[10,12]
Concomitant CDI and Salmonella infection in immunocompetent host is not commonly seen. To the best of our knowledge after extensive literature review (English), this is only the fourth report highlighting this association. Whether it was a coinfection versus colonization and detection of coexisting nontyphoidal Salmonella is debatable. In either case, the detection of these two pathogens in clinical setting raises concerns for antibiotics of choice and duration. The differentiation between colonization versus active infection with nontyphoidal Salmonella may not always be possible in a clinical setting; when it presents in a healthcare worker, the decision becomes even more challenging considering concerns of infection transmission. Literature review from the previous reports is described in Table 1.
Table 1.
Literature review for previous reports describing the association of CDI and Salmonella
| Study | Age/sex | Recent history of antibiotic use | Steroid use or other immunosuppression | Treatment | Outcome |
|---|---|---|---|---|---|
| Brettle et al.[6] | 24/F | Erythromycin | None | Vancomycin | Survived |
| Brettle et al.[6] | 65/F | Co-trimoxazole | Prednisone | Vancomycin Chloramphenicol | Survived |
| Brettle et al.[6] | 79/M | Ampicillin | None | Not mentioned | Survived |
| Flucloxacillin | |||||
| Brettle et al.[6] | 75/M | Chloramphenicol | Chronic obstructive pulmonary disease (COPD) (possible steroid use) | Not mentioned | Died |
| Amoxicillin | |||||
| Erythromycin | |||||
| Grinblat et al.[7] | 86/M | None | None | Metronidazole | Survived |
| Grinblat et al.[7] | 80/F | None | None | Metronidazole | Survived |
| Ciprofloxacin | |||||
| Halvorson et al.[8] | 20/F | None | None | Imipenem | Died |
| Cilastatin | |||||
| Vancomycin | |||||
| Metronidazole |
Our patient was exposed to nitrofurantoin which is a lower risk of inducing CDI compared to antibiotics such as clindamycin, carbapenems, and flouroquinolones.[13]
Coinfection with Salmonella and CD has been reported in the past. Monkemuller et al. described a case where nontyphoidal Salmonella infection mimicked pseudomembranous colitis as a severe presentation of the disease.[14] Although Salmonella is not a risk factor for CDI, it can cause intestinal inflammation and alteration in the intestinal flora.[15,16] Some researchers have postulated that CD toxin had a protective effect by decreasing the transepithelial and paracellular migration of enteropathic bacteria, thereby reducing chances of bacterial colitis caused by pathogens like Salmonella.[17] Salmonella can both colonize and cause active infection; however, certain Salmonella species are more adapted for causing human infections when compared to others. Salmonella typhi is known to be an obligate human pathogen, whereas nontyphoidal Salmonella can be seen in cattle as well.[18]
Clinical presentation of Salmonella gastroenteritis and CDI can be very similar. When both pathogens are isolated from the same patient, it is tempting to treat both with antibiotics as highlighted in our literature review [Table 1]. Despite clear guidelines, often times patients are prescribed antibiotics with the intent of reducing the transmission of Salmonella. Hand hygiene appears to be the cornerstone to reduce transmission of bacteria.[19,20] When it comes to healthcare workers, there is no difference in guidelines and should not be prescribed antibiotics with intent of reducing secondary transmission.[20] Normally, patients shed bacteria for up to 4 weeks after acute infection and antibiotics given during the acute phase with prolong the carrier state by altering the normal gut flora.[21,22] As far as infection control measures and fitness for work are concerned, healthcare workers can return to work after cessation of diarrhea.[23]
Treatment guidelines for CDI are being revised regularly. Current recommendations are in favor for treatment of CDI with oral vancomycin 125 mg four times a day resulting in quicker response than metronidazole.[24,25] In critically ill patients with severe disease, early surgery is found to reduce mortality in the group of patients with intact immune system, age 65 years and more, mounting good leucocytotic response and with moderate rise in serum lactate values.[24,26]
CDI is also associated with older age, recent hospitalization, multiple comorbidities, use of gastric acid blockers, inflammatory bowel disease, and immunosuppression. It has become more common in younger and healthier patients in community settings. However, in cases where a patient is tested for nontyphoidal Salmonella as in the case that we present; the decision-making becomes more difficult. Treatment includes discontinuing the contributing antibiotic, if possible. Appropriate diagnostic testing and restricted use of antibiotics (Antimicrobial stewardship programs) by an astute internist and family physician in the community can be of significant impact in this matter.[27]
In conclusion, awareness of cases where two pathogens are concurrently isolated in a patient is of prime importance, especially in this era of rising prevalence of C difficile-associated disease (CDAD) and drug resistance. The importance of decision-making based on the clinical response of patient after antibiotic therapy cannot be overemphasized. Also, the guidelines state clear directives with regard to no additional antibiotic use for healthcare professionals suffering such infections and no additional need for quarantine after resolution of gastrointestinal symptoms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66:987–94. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 2.Trong TA, Nicholas AF, Melita AG, Karen HK, Frederick JA, John AC. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis J. 2015;21:941. doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38(Suppl 3):S127–34. doi: 10.1086/381578. [DOI] [PubMed] [Google Scholar]
- 4.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalker RB, Blaser MJ. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–24. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Brettle RP, Poxton IR, Murdoch JM, Brown R, Byrne MD, Collee JG. Clostridium difficile in association with sporadic diarrhoea. Br Med J (Clin Res Ed) 1982;284:230–3. doi: 10.1136/bmj.284.6311.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinblat J, Weiss A, Grosman B, Dicker D, Beloosesky Y. Diarrhea in elderly patients due to Clostridium difficile associated with Salmonella and Shigella infection. Arch Gerontol Geriatr. 2004;39:277–82. doi: 10.1016/j.archger.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 8.Halvorson SAC, Cedfeldt AS, Hunter AJ. Fulminant, non-antibiotic associated Clostridium difficile colitis following Salmonella gastroenteritis. J Gen Int Med. 2011;26:95–7. doi: 10.1007/s11606-010-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazier JS, Fitzgerald TC, Hosein I, Cefai C, Looker N, Walker M, et al. Screening for carriage and nosocomial acquisition of Clostridium difficile by culture: A study of 284 admissions of elderly patients to six general hospitals in Wales. J Hosp Infect. 1999;43:317–9. doi: 10.1016/s0195-6701(99)90431-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghose C. Clostridium difficile infection in the twenty- first century. Emerg Microbes Infect. 2013;2:e62. doi: 10.1038/emi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouza E, Martin A, Van den Berg RJ, Kuijper EJ. Laboratory-acquired clostridium difficile polymerase chain reaction ribotype 027: A new risk for laboratory workers? Clin Infect Dis. 2008;47:1493–4. doi: 10.1086/593109. [DOI] [PubMed] [Google Scholar]
- 12.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J, Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459–77. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 13.Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57:2326–32. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monkemuller K, Patasiute I, Walther F, Peitz U, Fry LC, Malfertheiner P. Pseudomembranous colitis due to Salmonella enterica serotype infantis. Endoscopy. 2006;38:546. doi: 10.1055/s-2006-925343. [DOI] [PubMed] [Google Scholar]
- 15.Santos RL, Raffatellu M, Bevins CL, Adams LG, Tükel C, Tsolis RM, et al. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 2009;17:498–506. doi: 10.1016/j.tim.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feltis BA, Kim AS, Kinneberg KM, Lyerly DL, Wilkins TD, Erlandsen SL, et al. Clostridium difficile toxins may augment bacterial penetration of intestinal epithelium. Arch Surg. 1999;134:1235–41. doi: 10.1001/archsurg.134.11.1235. discussion 1241-32. [DOI] [PubMed] [Google Scholar]
- 17.Feltis BA, Garni RM, Wells CL. Clostridium difficile toxins and enterococcal translocation in vivo and in vitro . J Surg Res. 2001;97:97–102. doi: 10.1006/jsre.2001.6130. [DOI] [PubMed] [Google Scholar]
- 18.Cianflone NFC. Salmonellosis and the GI tract: More than just peanut butter. Curr Gastroenterol Rep. 2008;10:424–31. doi: 10.1007/s11894-008-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65:1963–73. doi: 10.1093/cid/cix959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–51. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 21.Sirinavin S, Garner P. Antibiotics for treating Salmonella gut infections. Cochrane Database Syst Rev. 2000:Cd001167. doi: 10.1002/14651858.CD001167. [DOI] [PubMed] [Google Scholar]
- 22.Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012;11:CD001167. doi: 10.1002/14651858.CD001167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–9. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 24.Cózar-Llistó A, Ramos-Martinez A, Cobo J. Clostridium difficile infection in special high-risk populations. Infect Dis Ther. 2016;5:253–69. doi: 10.1007/s40121-016-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345–54. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 26.Lamontagne F, Labbe AC, Haeck O, Lesur O, Lalancette M, Patino C, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winslow BT, Onysko M, Thompson KA, Caldwell K, Ehlers GH. Common questions about Clostridium difficile infection. Am Fam Physician. 2014;89:437–42. [PubMed] [Google Scholar]


