Abstract
Context:
Hypothyroidism is the most prevalent endocrine disorder worldwide, with a prevalence of 4%–5%. Thyroid hormone has a role in neurological development, and hormone deficiency can be manifested by many neurological signs and symptoms such as behavioral disturbances, anxiety, and depressive symptoms.
Aims:
To estimate the prevalence of depression among hypothyroid patients attending primary healthcare and endocrine clinic of King Fahad Hospital of the University (KFHU) and to assess the relationship between depression and different factors.
Settings and Design:
A cross-sectional study was conducted to estimate the prevalence of depression among hypothyroid patients attending the primary healthcare and endocrine clinics of KFHU in Al Khobar.
Materials and Methods:
Patients were screened for depression using Patient Health Questionnaire-9 screening tool, in addition to obtaining their sociodemographic data, details of their thyroid function status, and other risk factors for depression. Also, patient medical files were used to obtain the laboratory results.
Statistical Analysis Used:
Data were analyzed using the Statistical Package for the Social Science (SPSS) version 23. Continuous data were displayed using mean and standard deviation; categorical data were displayed in numbers and percentage. Chi-square test was used to assess the relationship between the variables. A P value of less than 0.05 was considered as statistically significant.
Results:
It was found that 33.9% of patients were depressed with varying degree of depression. Certain symptoms were found to be associated with higher risk of depression such as fatigue, memory problems, hair loss, and gland enlargement.
Conclusion:
Depression was concluded to be prevalent among hypothyroid patients. And screening for depression among hypothyroid patients is recommended.
Keywords: Depression, hypothyroidism, Patient Health Questionnaire-9
Introduction
Hypothyroidism is the most prevalent endocrine disorder worldwide.[1] It can be defined as a disorder of the endocrine system in which there is a deficiency in thyroid hormone production. Many factors affect the prevalence of thyroid disorders such as age, sex, ethnicity, and iodine intake.[2]
Numerous studies in developed countries suggested that hypothyroidism prevalence is estimated to be 4%–5% of the population worldwide.[3] It is estimated to be 5% among the population of the United States over the age of 12 years,[4] while in other counties such as the United Kingdom and India, it was estimated to be about 2% and 10.95%, respectively.[3,5] In Libya in 2008, it was estimated to be 6.18%,[6] whereas in Saudi Arabia in Makkah 2011, the estimated prevalence of hypothyroidism was 47.34% of the study's population.[6] Manifestations of hypothyroidism include tiredness, weight gain, cold intolerance, constipation, forgetfulness, slow speech, and depression.[7]
Thyroid hormone plays a role in normal neurological development function of the central nervous system by stimulating the development of neuronal processes, axons, and dendrites, and it increases the rate of neuronal proliferation.[8] Hypothyroidism can cause behavioral disturbances, depressive symptoms, and anxiety, and it can impact certain aspects of cognitive functioning, such as slowed information processing speed, reduced efficiency in executive functions, poor learning and memory, mood disturbance, and problems in verbal fluency. More severe hypothyroidism can mimic melancholic depression and dementia.[8] A study conducted among hypothyroid patients in King Fahad Hospital of the University (KFHU) during 1995 found the prevalence of depression as the first manifestation of hypothyroidism to be 18%.[9]
Depression can be diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria, which consist of depressed mood, anhedonia (loss of interest in pleasurable activity), change in appetite or weight, feeling of worthlessness or guilt, disturbance in sleep, decreased concentration, psychomotor agitation or retardation, loss of energy, and recurrent thoughts of death. Five out of these nine symptoms have to be experienced for at least 2 weeks for the diagnosis to be made.[10] Screening of depression can be made through various instruments; the most practically and commonly used is the Patient Health Questionnaire-9 (PHQ-9) screening tool.[11]
There are multiple factors that may lead to depression in hypothyroid patients, for instance, the presence of chronic disease, sociodemographic factors, stressful life events, level of thyroid-stimulating hormone (TSH), change in dose of levothyroxine, and some medications.[12] A study conducted in southern Brazil in 2009 estimated the prevalence of depression among chronic disease patients to be 16.2%. Depression is found to be higher among women, older individuals, widowed or divorced, hospitalized patients, and patients with low socioeconomic status.[12] Stressful life events have shown to increase the prevalence of depression.[13]
The serum TSH level was associated with the severity of depressive symptoms in hypothyroid patients, and levothyroxine hormone replacement therapy was found to improve depression in hypothyroidism.[14] Symptoms of hypothyroidism such as weight gain and hair loss have shown to increase the risk of depression, where those who are overweight and obese tend to have a higher risk of depressive symptoms than those with normal weight.[15]
Complaints of hair loss, especially among adult female patients, were associated with a greater prevalence of depression.[16] Some medications have shown to increase the risk of developing depression such as β-blockers, corticosteroids, and isotretinoin, yet the nature of those effects is yet to be understood.[17,18,19] Around 10% of patients on corticosteroids will experience severe psychiatric adverse effects, ranging from depression to psychosis and delirium.[17] Isotretinoin has shown to increase the risk of developing depression among patients.[18] B-blocker medications that are able to cross blood–brain barrier increase the risk of developing neuropsychological symptoms, such as drowsiness, fatigue, and lethargy.[19]
A case–control study conducted in Eastern Mediterranean region in Iran 2016 on 30 patients showed that depression score using (depression Anxiety Stress Scale-21) was higher among hypothyroid women compared with the control group.[20] An Indian case–control study done in Mumbai 2017 studied the relationship between depression and hypothyroidism and concluded that inflammatory cytokines are associated with depression that can be relieved by treating autoimmune hypothyroidism, so they recommended that all patients with depression should be screened for hypothyroidism.[21] Two cross-sectional studies conducted in India (2016–2017) to estimate the prevalence of depression among hypothyroid patients on 100 and 144 patients showed that about 60% and 12.5% of hypothyroid patients had depression, respectively.[8,22] Another cross-sectional study was done in Brazil (2009) on 1298 women, and it showed that patients who have high levels of TSH (high TSH and low T3, T4) were at increased risk of developing depression than the general population.[23]
There are limited studies conducted to estimate the prevalence of depression among hypothyroid patients worldwide and especially in Saudi Arabia, all studies were focused on studying the hypothyroid status rather than screening them for depression.
Materials and Methods
Study design and setting
A descriptive, cross-sectional study was conducted during December 2018 to March 2019, to estimate the prevalence of depression among hypothyroid patients attending the primary healthcare and endocrine clinics of KFHU.
Study population
A convenient sample size of 56 patients was calculated according to Epi Info, and it included patients of either sex above the age of 18 years and diagnosed with hypothyroidism either due to high TSH level (more than 4.94 uIU/mL) and low T3 or T4 level (T3 less than 1.71 pg/mL, T4 less than 0.7 ng/dL) or due to low TSH (less than 0.35 uIU/mL) and low T3 or T4.
Patients younger than 18 years, patients with personal or family history of psychiatric disorder, serious medical illnesses, for example, cancer and chronic kidney disease, and patients within 6 weeks postpartum period were excluded.
Materials
-
A questionnaire was collected by interviewing the patients or asking them to fill the following data:
- Sociodemographic data (age, sex, nationality, marital status, educational level, and occupation)
- Details of thyroid function state (time of diagnosis, symptoms, TSH level, and dose of medication)
- Risk factors for depression (stressful life events, medical diseases, family history of psychiatric illness, and medications)
- PHQ-9 for depression assessment.
PHQ-9 is a valid screening instrument used for screening and monitoring of mood disorders. It has 61% sensitivity and 94% specificity in adults. PHQ-9 consists of nine questions that the patient has to rate it from 0 (not experienced at all) to 3 (experienced nearly every day), and then he or she will be given a final score out of 27 which classifies the depression severity into none, mild, moderate, moderately severe, and severe.[11] A validated Arabic version of PHQ-9 was used.[24] A score of 10 or higher on the PHQ-9 is considered positive for depression.[25]
-
2.
Patient medical files to obtain the laboratory results.
Results
Univariate analysis
I. Sociodemographic characteristics
The sample size was 56 hypothyroid patients attended primary healthcare and KFHU endocrine clinic affiliated to the university. The mean age was 42.05 ± 11.49 years, ranging from a minimum of 23 years to a maximum of 72 years.
The majority of patients were females and were Saudis (94.6% and 83.9%, respectively). Two-thirds of patients were married (66.1%) and 55.4% carried bachelor or diploma degree, and only 7.1% were illiterate. More than three quarters (76.9%) were non-working [Table 1].
Table 1.
Distribution of the hypothyroid patient according to sociodemographic data
| Characteristics | n=56 | Percentage=100% |
|---|---|---|
| Gender | ||
| Male | 3 | 5.4 |
| Female | 53 | 94.6 |
| Nationality | ||
| Saudi | 47 | 83.9 |
| Non-Saudi | 9 | 16.1 |
| Marital status | ||
| Married | 37 | 66.1 |
| Single | 13 | 23.2 |
| Divorced | 5 | 8.9 |
| Widow | 1 | 1.8 |
| Education | ||
| Illiterate | 4 | 7.1 |
| Elementary | 5 | 8.9 |
| Intermediate | 5 | 8.9 |
| High school | 8 | 14.3 |
| Bachelor/diploma | 31 | 55.4 |
| Retired | 2 | 3.6 |
| Postgraduate | 3 | 5.4 |
| Occupation | ||
| Nonworking | 38 | 76.9 |
| Working | 16 | 28.6 |
II. Hypothyroidism-related characteristics
Regarding the time of diagnosis; more than one-third of the patients (35.7%) were diagnosed since more than 10 years, while nearly one-third (32.1%) were diagnosed since more than 2 to less than 10 years. Normal TSH levels were encountered among 57.1% of the patients, while elevated TSH levels were reported in 35.7% of them. More than three quarters reported measuring TSH level every 3–6 months (78.6%).
Almost all the study samples were on thyroxine replacement therapy except for two cases, of whom more than two-thirds (68.5%) were on levothyroxine dose between 25 and 100 μg daily and 25.9% were on a dose between 125 and 175 μg daily and only 5.6% were on high dose between 200 and 350 μg daily. Nearly three quarters needed to change the dose, either increase (40.7%) or decrease (31.5%) [Table 2].
Table 2.
Distribution of the hypothyroid patient according to timing of diagnosis and TSH level and treatment
| Characteristics | n=56 | Percentage=100% |
|---|---|---|
| Time of diagnosis | ||
| <6 months | 5 | 8.9 |
| 6 months-2 years | 13 | 23.2 |
| >2 years-10 years | 18 | 32.1 |
| >10 years | 20 | 35.7 |
| TSH level | ||
| Normal | 32 | 57.1 |
| High | 20 | 35.7 |
| Low | 4 | 7.1 |
| Frequency of measuring TSH | ||
| <3 months | 4 | 7.1 |
| Every 3-6 months | 44 | 78.6 |
| >6 months | 8 | 14.3 |
| Dose (µg)* | ||
| 25-100 | 37 | 68.5 |
| 125-175 | 14 | 25.9 |
| 200-350 | 3 | 5.6 |
| Change in dose* | ||
| No change | 15 | 27.8 |
| Increase | 22 | 40.7 |
| Decrease | 17 | 31.5 |
TSH: thyroid-stimulating hormone. *n=54
Regarding symptoms of hypothyroidism, the highest percentage was encountered among fatigue, hair loss, and weight gain (85.7%, 78.6% and 73.2%, respectively) [Figure 1].
Figure 1.
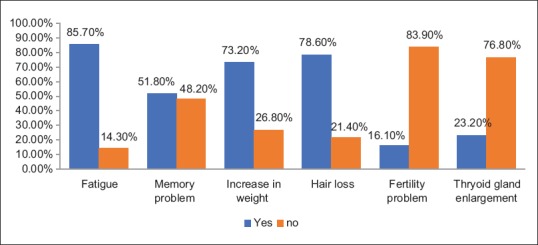
Symptoms of hypothyroidism
III. Depression-related characteristics
According to PHQ-9 as shown in Figure 2, it is found that only 33.9% of the hypothyroid patients were depressed ranging from moderately depression (10.7%), moderately severely depressed (19.6%), to severely depressed (3.6%).
Figure 2.
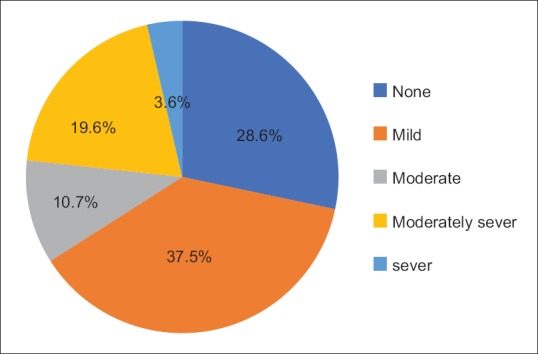
Hypothyriod patient according to depression level
The most stressful problems reported among hypothyroid patients were family (28.6%), social (21.4%), and emotional problems (17.9%), and 63.3% of the patients have had any stressful life conditions and 35.7% had no such conditions [Figure 3].
Figure 3.
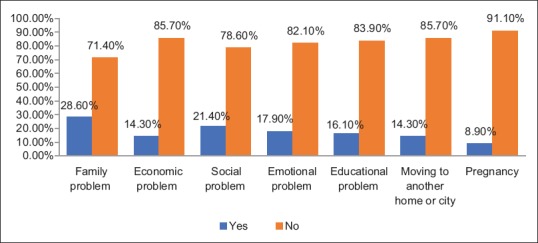
Stressful events among hypothyroid patients
Nearly one-fifth (17.9%) of the patients had diabetes mellitus [Figure 4], and regarding the use of some medications that might cause depressive symptoms, it was reported that a majority or nearly all patients did not use such medications [Figure 5].
Figure 4.
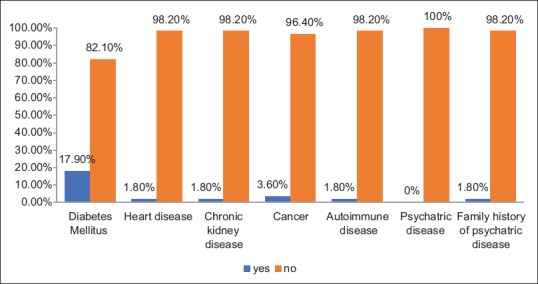
Medical diseases among hypothyroid patients
Figure 5.
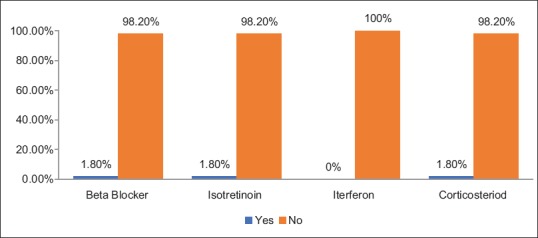
Medications used causing depression among hypothyroid patients
Bivariate analysis
I. Relationship between depression and sociodemographic
The highest percentages of depressed hypothyroid patients were encountered among working patients (43.8%) and single patients (61.5%) with no statistically significant association between depression and occupation or marital status [Table 3].
Table 3.
Association between depression in hypothyroid patients and occupation, marital status
| Sociodemographic characteristics | Nondepressed | Depressed | Test of significance P | ||
|---|---|---|---|---|---|
| n=37 | % | n=19 | % | ||
| Occupation | χ22=1.809 P=0.405 | ||||
| Not working | 26 | 68.4 | 12 | 31.6 | |
| Working | 9 | 56.3 | 7 | 43.8 | |
| Retired | 2 | 100 | 0 | 0 | |
| Marital status | |||||
| Single | 5 | 38.5 | 8 | 61.5 | χ23=6.539 P=0.088 |
| Married | 28 | 75.7 | 9 | 24.3 | |
| Divorced | 3 | 60 | 2 | 40 | |
| Widowed | 1 | 100 | 0 | 0 | |
II. Relationship between depression- and hypothyroidism-related characteristics
The highest percentages of depressive patients were mostly encountered among those with high TSH level (40%), those with the dose of levothyroxine between 125 and 175 μg daily (42.9%), and those who needed to increase their dose (40.9%) [Table 4].
Table 4.
Association between depression in hypothyroid patients and hypothyroidism-related characteristics
| Hypothyroidism-related characteristics | Nondepressed | Depressed | Test of significance P | ||
|---|---|---|---|---|---|
| n=37 | % | n=19 | % | ||
| TSH level | χ22=0.574 P=0.751 | ||||
| Normal | 22 | 68.7 | 10 | 31.3 | |
| High | 12 | 60 | 8 | 40 | |
| Low | 3 | 75 | 1 | 25 | |
| Dose (µg)* | |||||
| 25-100 | 25 | 67.6 | 12 | 32.4 | FET=0.78 P=0.783 |
| 125-175 | 8 | 57.1 | 6 | 42.9 | |
| 200-350 | 2 | 66.7 | 1 | 33.3 | |
| Change in dose* | |||||
| No change | 11 | 73.3 | 4 | 26.7 | χ22=0.793 P=0.672 |
| Increase | 13 | 59.1 | 9 | 40.9 | |
| Decrease | 11 | 64.7 | 6 | 35.3 | |
TSH: Thyroid-stimulating hormone, FET: Fisher’s exact test. *n=54
Regarding the relationship of depression and hypothyroid symptoms, Table 5 shows that depression is mostly encountered with patients complaining of fatigue, memory problems, hair loss, and gland enlargement. The association is statistically significant where P < 0.05.
Table 5.
Association between depression in hypothyroid patients and hypothyroidism-related symptoms
| Symptoms of hypothyroidism | Nondepressed | Depressed | Test of significance P | ||
|---|---|---|---|---|---|
| n=37 | % | n=19 | % | ||
| Fatigue | χ21=4.793** | ||||
| No | 8 | 100 | 0 | 0 | |
| Yes | 29 | 60.4 | 19 | 39.6 | |
| Memory problem | |||||
| No | 25 | 92.6 | 2 | 7.4 | χ21=16.359** |
| Yes | 12 | 41.4 | 17 | 58.6 | |
| Increase weight | |||||
| No | 9 | 60 | 6 | 40 | χ21=0.337 P=0.562 |
| Yes | 28 | 68.3 | 13 | 31.7 | |
| Hair loss | |||||
| No | 11 | 91.7 | 1 | 8.3 | χ21=4.463a** |
| Yes | 26 | 59.1 | 18 | 40.9 | |
| Fertility problems | |||||
| No | 31 | 66 | 16 | 34 | χ21=002 P=0.967 |
| Yes | 6 | 66.7 | 3 | 33.3 | |
| Gland enlargement | |||||
| No | 32 | 74.4% | 11 | 25.6 | χ21=5.757** |
| Yes | 5 | 38.5% | 8 | 61.5 | |
**Significant P<0.05
III. Relationship between depression and stressful life conditions
Higher percentage of depressed patient was met among those who experienced any stressful life events, with no statistically significant association [Table 6].
Table 6.
Association between depression in hypothyroid patients and stressful life conditions
| Stressful life conditions | Nondepressed | Depressed | Test of significance P | ||
|---|---|---|---|---|---|
| n=37 | % | n=19 | % | ||
| Stress | χ21=0.214, P=0.644 | ||||
| No | 14 | 70 | 6 | 30 | |
| Yes | 23 | 63.9 | 13 | 36.1 | |
Discussion
Depression is a common problem encountered among hypothyroid patients worldwide. This study was conducted in primary healthcare and endocrine clinic of KFHU to address the prevalence of depression among hypothyroid patients and to correlate it with different factors such as certain sociodemographic factors, TSH level, symptoms of hypothyroidism, and stressful life events.
The study included 56 hypothyroid patients; the majority were female, with a mean age of 42.05 ± 11.49 years. Depression was found in 33.9% of the sample, ranging from moderate 10.7%, moderately severe 19.6%, to severe depression 3.6%. The worldwide prevalence of depression among hypothyroid patients was estimated to be around 12.5% in India during 2016;[8] in the United States, it was 5% during 2018,[4] and it was 2% in the United Kingdom during 2013.[5] Differences in prevalence can be attributed to different sample size, gender, and other socioeconomic characteristics between different studied populations.
The highest percentages of depressed hypothyroid patients were encountered among working and single patients with no statistically significant association between depression and occupation or marital status. Similarly, to our study results, an Indian study conducted in 2017 found that 50% of depressed patients were unmarried.[22]
In this study, a positive association between hypothyroid symptoms and depression was shown. Our findings suggested that a high prevalence of depression is found in patients with fatigue, followed by hair loss and then weight gain. However, only statistically significant association was found between depression and fatigue, memory problems, hair loss, and gland enlargement. Whereas memory loss was the only statistically significant feature seen in depressed patients in the Indian studied population.[22]
Despite the majority of depressed patients mostly encountered among those with high TSH level, those with the dose of thyroxin between 125 and 175 μg daily, and those who needed to increase their dose, this study showed no statistically significant association between depression and TSH level, thyroxin dose, or change in thyroxin dose. Differently from our study, a study by Naseem et al. showed a significant association between higher TSH levels and prevalence of depression.[22]
Another study by Guimarães et al. showed a statistically significant association between TSH level of >10 mUI/mL and the presence of depressive symptoms.[23] These studies’ results have limited generalization ability due to the differences in the sample characteristics.
In our current study, although most of depressed patients were met among those who experienced stressful life events such as family problems (e.g. death of relative or marital separation), economic, social and emotional problems, educational obstacles, and pregnancy, it showed no statistical significant association between stressful life events and depression. This indicates that patients who were found to have depressive symptoms are more likely to be due to hypothyroid-related causes rather than other confounders such as stressful life events.
Conclusion
The study results estimated the prevalence of depression among hypothyroid patients and highlighted the most common symptoms that are associated with depression. A prevalence of 33.9% was found accounting for one-third of the sample which considered prevalent and consistent with the stated hypothesis.
In conclusion, hypothyroid patients are predisposed to depression independently from their TSH level or other risk factors such as socioeconomic issues. A population-based study is needed for more precise estimation of the prevalence of this disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chakera AJ, Pearce SH, Vaidya B. Treatment for primary hypothyroidism: Current approaches and future possibilities. Drug Des Devel Ther. 2012;6:1–11. doi: 10.2147/DDDT.S12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rana H, Mirah J, Al-Shahrani N, Nouf A, Afrah A, Basma O, et al. Incidence of thyroid diseases in female Saudi adults visiting a tertiary care hospital in Riyadh. Epidemiology. 2017;7:286. [Google Scholar]
- 3.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J EndocrinolMetab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennessey JV, Espaillat R. Current evidence for the treatment of hypothyroidism with levothyroxine/levotriiodothyronine combination therapy versus levothyroxine monotherapy. IntJClinPract. 2018;72:e13062. doi: 10.1111/ijcp.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werhun A, Hamilton W. Are we overusing thyroid function tests? Br J Gen Pract. 2013;63:404. doi: 10.3399/bjgp13X670589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad SAS, Ashraf EM, Khaled AS, Salih BS, Yousef S, Abeer AS, et al. The epidemiology of thyroid diseases in the Arab world: A systematic review. J Public Health Epidemiol. 2016;8:17–26. [Google Scholar]
- 7.Hueston W J. Treatment of hypothyroidism. AmFamily Physician. 2001;64:1717–24. [PubMed] [Google Scholar]
- 8.Bathla M, Singh M, Relan P. Prevalence of anxiety and depressive symptoms among patients with hypothyroidism. Indian J Endocrinol Metab. 2016;20:468–74. doi: 10.4103/2230-8210.183476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Sultan AI, Larbi EB, Magbool G, Karima T, Bagshi M. Clinical presentation of spontaneous primary hypothyroidism in adults. Ann Saudi Med. 1995;15:143–7. doi: 10.5144/0256-4947.1995.143. [DOI] [PubMed] [Google Scholar]
- 10.Ganti L, Kaufman M, Blitzstein SM. 4th ed. New York: McGraw-Hill Education; 2016. First Aid for the Psychiatry Clerkship; p. 230. [Google Scholar]
- 11.Maurer DM. Screening for depression. AmFamily Physician. 2012;85:139–44. [PubMed] [Google Scholar]
- 12.Boing AF, Melo GR, Boing AC, Moretti-Pires RO, Peres KG, Peres MA. [Association between depression and chronic diseases: Results from a population-based study] RevSaude Publica. 2012;46:617–23. doi: 10.1590/s0034-89102012005000044. [DOI] [PubMed] [Google Scholar]
- 13.Stroud K. Stressful life events and major depression 2010. Available from: https://www.family-institute.org/sites/ default/files/pdfs/csi_stroud_stress_depression.pdf .
- 14.Talaei A, Rafee N, Rafei F, Chehrei A. TSH cut off point based on depression in hypothyroid patients. BMC Psychiatry. 2017;17:327. doi: 10.1186/s12888-017-1478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler-Brown AG, Ngo LH, Wee CC. The relationship between symptoms of depression and body weight in younger adults. Obesity (Silver Spring) 2012;20:1922–8. doi: 10.1038/oby.2011.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt JV, Ribeiro CF, Souza FH, Siqueira EB, Bebber FR. Hair loss perception and symptoms of depression in female outpatients attending a general dermatology clinic. AnBrasDermatol. 2012;87:412–7. doi: 10.1590/s0365-05962012000300010. [DOI] [PubMed] [Google Scholar]
- 17.Vidal E, Stewart JT, Catalano G. A case of corticosteroid-responsive depression. Psychosomatics. 2013;54:395–7. doi: 10.1016/j.psym.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Chen J, Wang W, Ai M, Zhang Q, Kuang L. Use of isotretinoin and risk of depression in patients with acne: A systematic review and meta-analysis. BMJ Open. 2019;9:e021549–e. doi: 10.1136/bmjopen-2018-021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drayer DE. Lipophilicity, hydrophilicity, and the central nervous system side effects of beta blockers. Pharmacotherapy. 1987;7:87–91. doi: 10.1002/j.1875-9114.1987.tb04029.x. [DOI] [PubMed] [Google Scholar]
- 20.Zavareh AT, Jomhouri R, Bejestani HS, Arshad M, Daneshmand M, Ziaei H, et al. Depression and hypothyroidism in a population-based study of iranian women. Rom J Intern Med. 2016;54:217–21. doi: 10.1515/rjim-2016-0033. [DOI] [PubMed] [Google Scholar]
- 21.Tayde PS, Bhagwat NM, Sharma P, Sharma B, Dalwadi PP, Sonawane A, et al. Hypothyroidism and depression: Are cytokines the link? Indian J Endocrinol Metab. 2017;21:886–92. doi: 10.4103/ijem.IJEM_265_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naseem YM, Nair A, Kumar SA, Beegum MS. Depression in Hypothyroidism and Risk Factors. Journal of Medical Science And clinical Research [Internet] 2017;05(03):219478–19484. Available from: http://jmscr.igmpublication.org/ v5-i3/182%20jmscr.pdf . [Google Scholar]
- 23.Guimarães JM, de Souza Lopes C, Baima J, Sichieri R. Depression symptoms and hypothyroidism in a population-based study of middle-aged Brazilian women. J Affect Disord. 2009;117:120–3. doi: 10.1016/j.jad.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 24.AlHadi AN, AlAteeq DA, Al-Sharif E, Bawazeer HM, Alanazi H, AlShomrani AT, et al. An arabic translation, reliability, and validation of patient health questionnaire in a Saudi sample. Ann Gen Psychiatry. 2017;16:32. doi: 10.1186/s12991-017-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams J, Nieuwsma J. Screening for depression in adults [Internet]. Up to date 2018. Available from: https://www. uptodate.com/home.


