Abstract
Introduction:
Polycystic ovarian syndrome (PCOS) presents as an extremely heterogeneous condition that includes chronic anovulation, hyperandrogenism, hyperlipidemia, and hyperinsulinemia along with enlarged polycystic ovaries. Insulin resistance is thought to be a pathogenic factor in women having PCOS along with increased incidence of metabolic disturbances.
Materials and Methods:
After obtaining ethical clearance, the girls of various colleges in Patiala were verbally sensitized to the symptoms of PCOS, and then given semistructured proformas subsequent to written informed consent. The students suspected on self-evaluation, and on evaluation of the proformas were investigated in the Out Patient Department, and patients reporting to Obstetrics and Gynecology Department with similar symptoms were also included after written informed consent. The patients were divided with simple randomization into two groups of 25 each. Group 1 (treatment group) was put on metformin therapy for three months (500 mg thrice a day), and Group 2 (control group) were given placebo thrice a day for the same duration. The patients were followed up after three months of metformin therapy (500 mg thrice a day) in Group 1, and placebo therapy in Group 2. Body weight, fasting blood glucose, blood glucose after 2 h of 75 g of glucose, and fasting serum insulin levels were assessed initially, and then after three months.
Results:
The mean age of patients in the present study was 21.46 ± 4.45 years. About 54% patients in the present study had BMI ≥25 kg/m2. About 68% of the patients had menstrual irregularity and PCO on ultrasonography (USG). About 44% patients in Group 1 (metformin) had increased insulin levels initially (>20 μU/ml), which were decreased to 16% after three months of metformin therapy. About 52% patients in Group 2 had increased insulin levels that were decreased to 48% after the placebo therapy.
Conclusion:
It has been concluded from this study that metformin significantly lowers insulin levels in patients with PCOS; in both obese and nonobese; which points towards its potential usefulness in treatment of PCOS patients, though it had no significant effect on body mass index in 12 weeks.
Keywords: Insulin levels, metformin therapy, polycystic ovarian syndrome
Introduction
Polycystic ovarian syndrome (PCOS) is estimated to occur in 15%–20% of women of reproductive age.[1] It has been seen that about 50% of the women with PCOS are either overweight or obese.[2] Women diagnosed with PCOS have key clinical features of hirsutism, acne, obesity, acanthosis nigricans, anovulatory menses, and multiple cystic appearances in ovaries. None of these features are universal or uniform.[3,4] Therefore, it has an extremely heterogeneous picture including chronic anovulation, hyperandrogenism, hyperlipidemia, and hyperinsulinemia along with enlarged polycystic ovaries.[5,6] The etiology of PCOS remains unclear. Resistance toward insulin is known to be a major risk factor for the development of type-2 diabetes mellitus. Thus, PCOS women are seen to be at higher risk for the development of type-2 diabetes mellitus due to the same factors.[7] Insulin resistance is thought to be the pathogenic factor in women having PCOS with increased incidence of metabolic disturbances.[8] This well explains the various features associated with PCOS like menstrual irregularities and hyperandrogenism, and other metabolic manifestations like central obesity, cardiovascular diseases, hypertension, dyslipidemia, insulin resistance, diabetes, cancers, psychological problems, and obstructive sleep apnea.[9] Improving insulin sensitivity through both lifestyle and pharmacological interventions can ameliorate these abnormalities to an extent. Metformin is an oral antidiabetic agent that has been used for the treatment of diabetes mellitus since a long time. It works by sensitizing the peripheral tissues to insulin, and has been found to reduce insulin levels in PCOS patients.[10]
Metformin is one of the effective drugs for treatment of PCOS, which has been gaining intense interest of many researchers and doctors. Hence, the present study conducted at the Department of Obstetrics and Gynecology, Government Medical College, Patiala, India, was undertaken to study the effects of metformin on insulin levels, body mass index (BMI), and other parameters in PCOS patients.
Materials and Methods
The present study was done in the Department of Obstetrics and Gynecology, and Department of Biochemistry, Government Medical College, Patiala, Punjab, India.
After obtaining approval from Institutional Ethics Committee, the girls of various colleges in Patiala were verbally sensitized to the symptoms of PCOS, and then provided a designed pretested questionnaire proformas. The students suspected on self-evaluation, and on evaluation of the proformas were investigated in the Out Patient Department, and patients reporting to Obstetrics and Gynecology Department with similar symptoms were also included after written informed consent.
The presence of any two of the following criteria was used to diagnose PCOS as laid down by the Rotterdam ESHRE/ASRM PCOS consensus workshop group (2004).[11]
Oligoovulation or anovulation
Clinical and/biochemical hyperandrogenism[12]
Polycystic ovaries.
Selection criteria
Oligomenorrhea (<6 cycles per year), secondary amenorrhea, or hypomenorrhea (bleeding <1 day) with obesity
Oligomenorrhea (<6 cycles per year), secondary amenorrhea, or hypomenorrhea (bleeding <1 day) with hirsutism
Oligomenorrhea (<6 cycles per year), secondary amenorrhea, or hypomenorrhea (bleeding <1 day) with acne
Oligomenorrhea (<6 cycles per year), secondary amenorrhea, or hypomenorrhea (bleeding <1 day) with infertility.
Exclusion criteria
Infertility due to male factors
Infertility due to tubal factors
Infertility due to cervical factors
Hyperprolactinemia
Thyroid disorders
Adrenal hyperplasia
Cushing's disease
Androgen producing tumors.
The patients fulfilling the above criteria were included in the study. A detailed history was recorded with particular reference to menstrual irregularity, obesity, hirsutism, acne, and family history of diabetes and PCOS.[13]
Patients were divided into two groups of 25 each with simple randomization. Group 1 (treatment group) was put on metformin therapy for three months (500 mg thrice a day) and Group 2 (control group) were given a placebo thrice a day for the same duration. This study was single blinded. The menstrual cycle was described as oligomenorrheic (cycle longer than 35 days but less than 6 months), and amenorrheic (no mensuration for more than six months) or hypomenorrheic (bleeding lasting less than one day).
Height, weight, BMI, and a complete general examination was done. Evidence for hirsutism, acne, and acanthosis nigricans was looked for. Routine investigations that were done for all participants are as follows:
BMI–Quetlet's index;
Hemoglobin;
Fasting blood sugar;
Ultrasonography; and
Serum insulin levels (fasting).
Assay used
Fasting blood sugar—Asatoor and King method was used
Ultrasonography—A transabdominal ultrasonographic examination of pelvic organs was done on the patients using GE, LOGIQ-alpha-200 machines, with a 3.5-MHz sector probe
Insulin estimation—DRG insulin ELISA kit, a solid phase enzyme linked immunosorbent assay based on sandwich principle was used.
The patients were followed up after three months of metformin therapy (500 mg thrice a day) in Group 1, and placebo therapy in Group 2. Weight, fasting blood glucose, blood glucose after 2 h of 75 g of glucose, and fasting serum insulin levels were repeated. All the results were evaluated using Paired t-test, and Chi-square test at significance level of P < 0.05.
Results
The present study was conducted in OPD of Department of Obstetrics and Gynecology of the Tertiary Care Hospital. A total of 50 patients of PCOS after detection and investigations were included in the study after their written informed consent. The various parameters studied in both the groups are given in Table 1.
Table 1.
Distribution of participants in both groups on basis of various variables
| Variable | Observation | Group 1 Metformin | Group 2 Placebo | Relation |
|---|---|---|---|---|
| 1. Age | 16-30 | 16-28 | Nonsignificant | |
| Mean=23.4 | Mean=22.5 | “t”= 0.834 | ||
| S.D. = +/- 4.25 | S.D. = +/- 3.51 | |||
| 2. BMI | Normal (46%) | 11 | 12 | χ2=0.09 |
| Overweight (42%) | 11 | 10 | df=2 | |
| Obese (12%) | 3 | 3 |
P>0.05 Nonsignificant |
|
| 3. Ultrasonography | Not done (10%) | 3 | 2 | χ2=6.54 |
| PCO on USG (68%) | 13 (52%) | 21 (84%) | df=2 | |
| PCO on USG (22%) | 9 (36%) | 2 (8%) |
P<0.05 Significant |
|
| 4. Menstrual irregularity | Irregularities present (68%) normal mensuration (22%) |
16 (64%) | 18 (72%) | χ2=0.36 |
| 9 (36%) | 7 (28%) | df=1 | ||
|
P>0.05 Nonsignificant |
||||
| 5. Marital status | Married (48%) | 13 (52%) | 11 (44%) | χ2=0.32 |
| Unmarried (52%) | 12 (48%) | 14 (56%) | df=1 | |
| P>0.05 | ||||
| Nonsignificant | ||||
| 6. Family history | Positive (62%) | 15 (60%) | 16 (64%) | χ2=0.08 |
| Negative (38%) | 10 (40%) | 9 (36%) | df=1 | |
| P>0.05 | ||||
| Nonsignificant |
Note: The age range was 16-30 years for the study group with 46% patients in the normal BMI range. It was observed that both the groups were comparable
The results of biochemical parameters and BMI after administration of Metformin and Placebo for 3 months for Group 1 and Group 2 are depicted in Tables 2, 3 and Figure 1.
Table 2.
Mean values of various parameters in Group 1 (treatment group) results with metformin
| Parameter | Before treatment | After treatment | Percentage change | “P” | Significance |
|---|---|---|---|---|---|
| BMI | 25.34±3.67 | 25.26±3.57 | 0.32 | >0.05 | Nonsignificant |
| Fasting blood sugar | 80.16±7.62 | 80.44±7.46 | 0.35 | >0.05 | Nonsignificant |
| 2-h blood glucose | 106.0±14.1 | 107.0±11.8 | 0.94 | >0.05 | Nonsignificant |
| Fasting serum insulin | 19.15±6.83 | 14.67±4.75 | 23.4 | <0.05 | Significant |
Table 3.
Mean values of various parameters in Group 2 (treatment group) results with placebo
| Parameter | Before treatment | After treatment | Percentage change | “P” | Significance |
|---|---|---|---|---|---|
| BMI | 26.44±3.25 | 26.31±3.16 | 0.49 | >0.05 | Nonsignificant |
| Fasting blood sugar | 78.60±7.97 | 80.12±7.24 | 1.93 | >0.05 | Nonsignificant |
| 2-h blood glucose | 104.4±17.34 | 103.6±14.97 | 0.77 | >0.05 | Nonsignificant |
| Fasting serum insulin | 21.60±6.16 | 21.56±6.15 | 0.19 | >0.05 | Nonsignificant |
Figure 1.
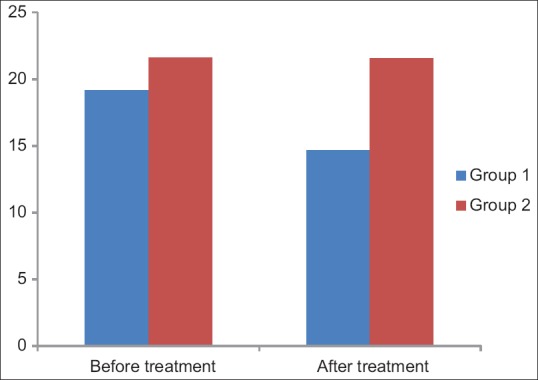
Bar diagram showing mean fasting serum insulin levels before and after treatment in Group 1 and Group 2
Discussion
PCOS is considered a problem of persistent anovulation with a spectrum of etiologies, and clinical manifestations that include hyperinsulinemia as well as hyperandrogenism. Although this metabolic syndrome has high prevalence worldwide, yet it is a neglected entity. Since it is an oft encountered gynecological disorder among adolescents demanding constant care by a family physician for both pharmacotherapy, and lifestyle modifications. Hence, the present study was designed to sensitize and detect PCOS cases among the college students. Another objective of this study was to observe the effect of metformin on insulin levels, blood sugar levels (2 h after 75 g of glucose), and BMI in these cases.
In previous studies, the patients with age at 26 ± 4 years[14] or at 24.6 ± 1.8 years[15] showed high prevalence of this disease in comparison to less mean age of 21.46 ± 4.45 years observed in the current study. It might be due to the younger age group of the participants selected for this study as the present study included a number of college students. Patients presenting with features of PCOS in this study with BMI > 25 were 54%, whereas it was 38.4%,[16] 66.9%,[17] 62%,[18] and 52%[19] in other studies. Menstrual irregularity was reported as 66.2%,[16] 73.0%,[17] and 100%[20] in various studies in comparison to 68% patients reported in the present study. About 62% of the patients reported a positive family history of diabetes mellitus, obesity, and PCOS in the present study. In a previous study conducted by Battalgia et al.[21] to determine the presence of PCO in daughters of patients with PCOS, a high prevalence of 93% was achieved, which might be due to specific selection of subjects. Similarly, Dunaif and Thomas[22] reported that PCO, and hyperandrogenemia are present in approximately 50% of sisters of affected women. Moran et al.[23] reported 57% patients with PCOS on USG, Rodin et al.[24] reported 52%, Taponen et al.[25] 82.3% in comparison to 68% reported in the present study.
Obesity is another factor that may favor menstrual and ovulatory disorders during adolescence. In the present study, both obese/overweight and normal patients were included and were randomly assigned to the metformin or the control group. The change in BMI after three months of metformin therapy was 0.08, which was nonsignificant. A significant weight loss was observed in previous studies with metformin doses higher than 2000 mg/day.[23,26] However, in a study done by Aggarwal and Kumar, the change in BMI was found to be -5.7% after three months, which was also nonsignificant. In another study by Bridger et al.[27] metformin therapy had no effect on body weight after 12 weeks. Zafar[28] also reported no significant weight reduction with metformin even after six months therapy.
Fasting insulin levels in metformin group significantly decreased from 19.15 to 14.67 μU/mol. Similar results were obtained in a study done by Zafar[28], where insulin level was decreased to 23.6–20.2 μU/mol. In another study by Agarwal et al., a significant reduction in insulin level was also detected from 20.45 to 12.59 μU/mol.[18] In Group 2, no significant reduction in BMI was seen after 12 weeks therapy (P > 0.05). No significant effects were seen on the fasting blood glucose, 2 h post prandial blood glucose, and fasting serum insulin during the study in the placebo group.
Limitations
The evaluation of HOMA (hemostasis model assessment) insulin resistance rather than a single insulin value, which was done in the present study, may be a more sensitive indicator.
Conclusion
It has been concluded from this study that metformin significantly lowers insulin levels in patients with PCOS, both obese and nonobese, which points toward its potential usefulness in treatment of PCOS patients; though it had no significant effect on BMI in 12 weeks.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://academic.oup.com/jcem/article/88/10/4682/2845743 .
- 3. https://www.fertstert.org/article/S0015-0282 (00) 00525-2/fulltext .
- 4. https://www.fertstert.org/article/S0015-0282 (99) 00184-3/fulltext .
- 5.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, editors. Polycystic Ovary Syndrome. Cambridge: Blackwell Science; 1992. pp. 377–84. [Google Scholar]
- 6.Schneider D, Gonzalez JR, Yamamoto M, Yang J, Lo JC. The association of polycystic ovary syndrome and gestational hypertensive disorders in a diverse community-based cohort. J Pregnancy. 2019;2019:1–6. doi: 10.1155/2019/9847057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekaran S, Sagili H. Metabolic syndrome in women. Obstet Gynaecol. 2018;20:245–52. [Google Scholar]
- 8.Siklar Z, Berberoglu M, Camtosun E, Koccay P. Diagnostic characteristics and metabolic risk factors of cases with polycystic ovary syndrome during adolescence. J Pediatr Adolesc Gynecol. 2015;28:78–83. doi: 10.1016/j.jpag.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. https://academic.oup.com/humrep/article/21/6/1416/724392 .
- 11. https://obgyn.onlinelibrary.wiley.com/doi/full/10.1111/j.1471-0528.2006.01008 .
- 12. https://academic.oup.com/humrep/article-abstract/19/1/41/690226 .
- 13. https://www.fertstert.org/article/S0015-0282 (00) 01662-9/abstract?code=fns-site .
- 14.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: Is there a difference? Clin Endocronol. 2002;57:343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 15.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 16.Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–11. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez AC, Marin IH, Mendoza R, Tovar Rodriguez JM, Ayala AR. Polycystic ovarian disease: Clinical and biochemical expression. Ginecol Obstet Mex. 2003;71:253–8. [PubMed] [Google Scholar]
- 18.Agarwal R, Kumar P. PCOS- The role of obesity, hyperinsulinemia and metformin therapy. J Obstet Gynecol Ind. 2003;53:264–7. [Google Scholar]
- 19.Hahn S, Tan S, Elsenbruch S, Quadbeck B, Herrmann BL, Mann K, et al. Clinical and biochemical characterization of women with polycystic ovarysyndromein North Rhine-Westphalia. Horm Metab Res. 2005;37:438–44. doi: 10.1055/s-2005-870236. [DOI] [PubMed] [Google Scholar]
- 20.Orio F, Jr, Palomba S, Spinelli L, Cascella T, Tauchmanova L, Zullo F, et al. The cardiovascular risk of young women with polycystic ovary syndrome: An observational, analytical, prospective case-control study. J Clin Endocrinol Metab. 2004;89:3694–5. doi: 10.1210/jc.2003-032049. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia C, Regnani G, Mancini F, Lughetti L, Flamigni C, Venturoli S. PCO in childhood: A common finding in daughters of PCOS patients. A pilot study. Hum Reprod. 2002;17:771–6. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- 22.Dunaif A, Thomas A. Current concepts in the polycystic ovary syndrome. Annu Rev Med. 2001;52:401–19. doi: 10.1146/annurev.med.52.1.401. [DOI] [PubMed] [Google Scholar]
- 23.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: A position statement of the androgen excess and polycystic ovary syndrome society. Fertil Steril. 2009;92:1967–82. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol. 1998;49:91–9. doi: 10.1046/j.1365-2265.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 25.Taponen S, Martikainen H, Jarvelin MR, Sovio U, Laitinen J, Pouta A, et al. Metabolic cardiovascular disease risk factors in women with self reported symptoms of oligomenorrhoea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2004;89:2114–8. doi: 10.1210/jc.2003-031720. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson F, Rolland C, Broom J, Love J. Effectiveness of long-term (twelve months) nonsurgical weight loss interventions for obese women with polycystic ovary syndrome: A systematic review. Int J Women Health. 2010;2:393–9. doi: 10.2147/IJWH.S13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–6. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 28.Zafar S. Role of metformin in correcting hyperinsulinemia, menstrual irregularity and anovulation in polycystic ovary syndrome. J Ayub Med Coll Abbottabad. 2006;17:54–6. [PubMed] [Google Scholar]


