Abstract
Aims and Objective:
In recent period, basal cell markers high molecular weight cytokeratin (HMWCK), P63 and prostate biomarker AMACR have been used as adjuvant to morphology in diagnostically challenging cases with a very high sensitivity and specificity.
Materials and Methods:
In this prospective study, total of 80 cases including 40 cases of malignant lesions and 40 cases of benign lesions of the prostate were taken. Tumor grade was determined according to Gleason's grading system. AMACR, HMWCK, P63 expressions were determined by immunohistochemical staining.
Results:
This study showed AMACR had a sensitivity of 90%, specificity of 100%. AMACR was not expressed in any of the 40 cases of benign lesions of the prostate while in malignant lesions of prostate it was expressed in 36 of 40 (90%) cases; 15 of 16 (93%) of well-differentiated carcinoma were positive for AMACR expression; 17 of 19 (89.47%) moderately differentiated and 4 of 5 (80%) cases of poorly differentiated tumors were positive for AMACR. There was statistically significant difference in expression of AMACR between benign and malignant lesions of the prostate (P = 0.001). In benign lesions, HMWCK and P63 were expressed in all the 40 (100%) cases, while in malignant lesions of prostate it was not expressed in any of the (0%) case. AMACR expression was not seen in any of the benign lesion. Out of 40 malignant cases, 4 cases were negative for AMACR, HMWCK and P63, 36 cases were positive only for AMACR, but no case was positive for HMWCK and P63.
Conclusions:
As an adjunct to biopsy, AMACR, HMWCK and P63 have potential for combating diagnostically challenging cases.
Keywords: AMACR, high molecular weight cytokeratin, P63, prostate carcinoma, prostate-specific antigen
Introduction
Prostate cancer is globally the second most frequently diagnosed cancer and the sixth leading cause of cancer death in males, accounting for 14% (903,500) of new cancer cases and 6% (258,400) of cancer deaths in male since 2008.[1,2] Tissue examination of a prostate is mandatory for the diagnosis of prostate cancer. However, tissue diagnosis can be difficult and inaccurate if the cancer focus is very small (<1 mm) because the establishment of a pathologic diagnosis requires the presence of combination of multiple histological features of tumor cells such as pattern of growth, nuclear atypia, absence of basal cells, and the presence of characteristic extracellular material in malignant glands.[3] There are many benign mimickers of prostate malignancy such as adenosis (ADEN), atrophy, partial atrophy.[4] In such cases, over diagnosis may cause unnecessary treatment of men without prostate cancer and lead to incontinence or impotency. Under diagnosis affects the prognosis of patient. Unfortunately, there is small but significant error rate in the pathologic diagnosis of prostate cancer in general practice, because of limited biopsy specimen. The accuracy of pathologic diagnosis of prostate cancer may be improved by the application of a more objective and reliable tumor-specific marker. PSA is not a cancer-specific marker, as it is present in benign and malignant prostatic epithelial cells.[5] Basal cells are absent in prostate adenocarcinoma, high-molecular-weight cytokeratin (34βE12)[6,7] and P63[8,9,10] immunostains specific for basal cells have been used for the diagnosis of prostate cancer. The identification of the basal cells of prostate glands indicates the presence of benign glands.[5] However, a limitation of using this negative marker for the diagnosis of carcinoma is that basal cells can have a patchy or discontinuous distribution in some benign lesions (i.e. adenosis). Consequently, negative staining for basal cell staining in a few glands suggestive of cancer is not proof of their malignancy.[5]
A positive immunohistochemical marker specific for prostate cancer along with negative basal cell marker with proper histopathological examination would therefore be of great value in increasing the level of confidence required to establish a definitive malignant diagnosis. Amethylacyl CoA racemase (AMACR), a new potential prostatic adenocarcinoma specific marker, has been reported to have sensitivity ranging from 82% to 100% respectively.[11,12,13,14,15,16] The present study is carried out with the aim to evaluate the expression of AMACR in prostate cancer and its correlation with Gleason grade.
Materials and Methods
Case selection
This study was conducted in the Department of Pathology during the period from 2016 to 2018. In this prospective study, total of 80 cases including 40 malignant lesions and 40 benign lesions of the prostate were taken. Two trained pathologist evaluated the prostate adenocarcinoma and benign prostatic hyperplasia cases and confirmed the diagnosis. In our study, Hematoxylin and Eosin is gold standard. Confirmed case of prostate carcinoma and benign prostatic hyperplasia subjected to immunohistochemistry evaluation. The tissue samples obtained during transurethral electro resection, needle biopsies were considered. Inadequate biopsies and cases with marked inflammation were excluded. Brief clinical data were noted from case records.
Morphological evaluation
Prostate fragments were fixed in 10% formalin, paraffin-embedded, sectioned and standard H and E stained sections were studied under the light microscope and classified into benign and malignant lesions. Carcinoma cases were histologically graded according to Gleason's grading system, and Gleason's score was noted (well differentiated 6, moderately differentiated 7, poorly differentiated 8–10). Associated prostatic tissue changes, such as tumor invasion, prostatic intraepithelial neoplasia (PIN), prostatitis, and others if any, were also analyzed. Special stain used as per requirement.
Immunohistochemical analysis
Immunohistochemistry (IHC) profile of the tumor was assessed by subjecting one section each from a representative block to AMACR/P504S and HMWCK, P63 immunostain. Immunohistochemistry was performed on 4 μm thick sections from 10% formalin-fixed paraffin–embedded specimens, according to the streptavidin-biotinimmunoperoxidase technique (Dako-cytomation). Positive and negative controls were run simultaneously.
Positive staining for AMACR pertained to dark diffuse or granular, cytoplasmic or luminal, but circumferential. The percentage positivity was graded from 0 to 3+ as follows: 0% cells (0+, negative), 1%–10% cells (1+, mild), and 11%–50% cells (2+, moderate), >51% cells (3+, strong). The adjacent benign glands should not show more than weak, partial (no circumferential) staining if any. Negative staining pertained to no staining or focal, weak non-circumferential fine granular staining[6], whereas in case of HMWCK: Cytoplasmic positivity/negative and continuous/discontinuous.[8] Immunostaining for p63 was interpreted as positive/negative and continuous/discontinuous. Positive staining was defined as positive staining of nuclei of basal cells.[8]
Statistical analysis
The data were entered into excel sheet and statistical analysis of data was performed using Chi-square test and Fisher exact test whichever was appropriate. The correlation between Gleason's grade and IHC expression was analyzed using the Chi-square test with an accompanying P value.
Results
Total cases with age group
The age group of prostate adenocarcinoma cases ranged from 42 to 84 years with mean age of 65 years. Patient with benign lesion of the prostate were in the age range of 41-80 years with mean age of 65 years.
Gleason's grade
Among all the 40 prostatic adenocarcinoma case studied the most common Gleason score 7 in 19 cases constituting 47.5%, followed by score 6 in 16 cases constituting 40%, score 8 in 4 cases constituting 10%, score 9 in 1 case constituting 2.5%. The Gleason scores were 3 + 3(6) (N = 16), 4 + 3(7) (N = 19), and 4 + 4(8) (N = 4) 5 + 4(9) (n = 1). Out of 40 cases, 19 (47.5%) were moderately differentiated (Gleason score 7), 5 (12.5%) were poorly differentiated (Gleason score 8–10), and 16 (40%) were well differentiated (Gleason score 2–6).
Alpha-methyl acyl-coenzyme A racemase immunoreactivity
AMACR was not expressed in any of the 40 cases of benign lesions of the prostate while in malignant lesions of prostate it was expressed in 36 of 40 (90%) cases. AMACR expression was significantly up-regulated in malignant lesions of the prostate (P < 0.001) as compared to benign prostatic lesions. Out of 16 cases of well-differentiated tumors, 7 (43.75%) cases showed 2+ positivity, while other 8 (50%) cases showed 3+ positivity: One case negative (6.25%). Out of 19 cases of moderately differentiated tumor, 9 (47.36%) cases revealed 3+ positivity while 5 cases (26.31%) revealed 2+ positivity followed by 2 (10.52%) cases with negative staining, and 3 (15.78%) cases with 1 + positivity. Out of 5 cases of poorly differentiated tumors, 3 cases (60%) revealed 3+ positivity followed by 1 (20%) cases with 1+ positivity, and 1 (20%) case with negativity. AMACR positivity was shown in poorly differentiated carcinoma.
[Figure 1] as well as in moderately differentiated carcinoma [Figure 2]. AMACR stain negative and grade wise positive in [Figure 3]. Statistically significant correlation was not observed between AMACR expression and Gleason's grade of malignant lesions of the prostate (P = 0.88) [Table 1].
Figure 1.
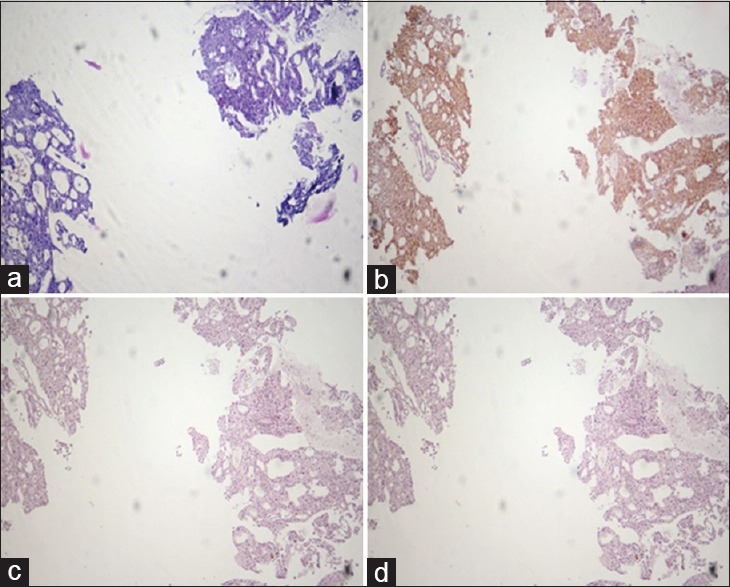
Carcinoma prostate: poorly differentiated. Comparison of hematoxylin and Eosin (a), AMACR (b), HMWCK (c), and P63 (d). Staining in serial sections of a small focus of prostatic Adenocarcinoma. (b) Diffuse intense cytoplasmic staining of the neoplastic glands with AMACR. The diagnosis of prostatic adenocarcinoma was confirmed by the negative basal cell staining with both HMWCK (c) and P63 (d). (×10)
Figure 2.
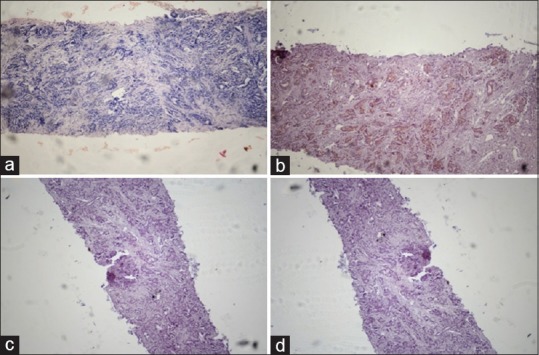
Carcinoma prostate: moderately differentiated. Comparison of hematoxylin and eosin (a), AMACR (b), HMWCK (c), and P63 (d). Staining in serial sections of a small focus of prostatic adenocarcinoma. (b) Diffuse intense cytoplasmic staining of the neoplastic glands with AMACR. The diagnosis of prostatic adenocarcinoma was confirmed by the negative basal cell staining with both HMWCK (c) and P63. (d) (×10)
Figure 3.
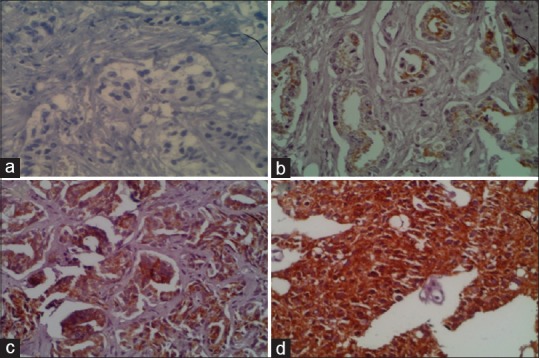
Section of prostatic adenocarcinoma: (a) Negative staining for AMACR. (b) Grade I positivity for AMACR. (c) Grade II positivity for AMACR. (d) Grade III positivity for AMACR. (×40)
Table 1.
Frequency of AMACR expression in relation to tumor differentiation and Gleason grade (n=40)
| AMACR expression (%) | Gleason grade (%) | Total | ||
|---|---|---|---|---|
| Well differentiated tumors | Moderately differentiated tumor | Poorly differentiated tumor | ||
| 0 (0) | 1 | 2 | 1 | 4 |
| 1+ (1-10) | 0 | 3 | 1 | 4 |
| 2+ (11-50) | 7 | 5 | 0 | 12 |
| 3+ (>51) | 8 | 9 | 3 | 20 |
| Total | 16 | 19 | 5 | 40 |
High molecular weight cytokeratin and P63 immunoreactivity
In benign lesions, HMWCK and p63 was expressed in all the 40 (100%) cases while in malignant lesions of prostate it was not expressed in any of the (0%) cases [Figure 1 and 2c, d]. There was statistically significant difference in staining of HMWCK and p63 between cases of benign and malignant lesions prostate, indicated by P = 0.001. Of 40 benign cases, BPH revealed continuous staining pattern in all cases.
PIN staining
Low-grade PIN (LPIN) associated with one case of BPH showed grade 2 + positivity with AMACR and revealed discontinuous staining pattern with HMWCK and P63. We did not get cases of high-grade PIN.
Discussion
The arrival of prostate-specific antigen screening has led to a significant increase both in the number of prostate needle biopsies performed and in the number of difficult biopsies with limited amount of cancer. The diagnosis of prostate cancer when only few malignant glands are present on small prostatic specimen is one of the major diagnostic challenges in surgical pathology. There are several histological benign mimics of cancer.[4] Diagnostic difficulty in indeterminate cases concerns 1.5%–9% of prostate biopsy with potential liability for pathologists.[8] AMACR/P504S is used as a positive marker for prostate cancer in conjunction with morphology and a basal cell-specific marker. Using AMACR as a positive marker alone might be misleading because the weak expression of AMACR might be seen in benign glands and expression of AMACR is also seen in HGPIN and AAH.[8] Therefore, using AMACR as a positive marker along with a basal cell-specific negative marker, HMWCK (34βE12) and P63 will enhance diagnostic accuracy in prostate cancer and reduce the chance of misdiagnosis.[5,6,7,8,9,10]
The results of our study of age distribution among benign and malignant lesions of prostate are almost similar to the results of previous studies, which showed that cases of malignant lesions of prostate progressively rise after the age of 50 years with a peak incidence at and above 70 years.[6,17] Patients with prostate cancer were in the higher age group as compared to patients with benign lesions.
Results of our study showed that Gleason score 6 and 7 as the most common pattern. The results of our study quite close to study done by Jain, et al. and Djavan et al. which states Gleason score 6 as the commonest pattern.[6,17] In our study, maximum number of cases, that is, 47.50% were moderately differentiated (Gleason score 7), followed by well differentiated, that is, 40% (Gleason score 3–6), and poorly differentiated that is, 12.5% (Gleason score 8–10). The results were in the similar line with the observations of Gleason who noted that majority of cases of malignant lesions of the prostate were of intermediate grade.[18]
In the present study, AMACR expression was significantly expressed in prostate cancer (P = 0.001) as compared with benign prostatic lesions. The sensitivity of AMACR is 90% and specificity is 100% in our study. This finding was in agreement with Jian et al., Luo et al., Rubin et al., Magi-Galluzi et al., and Moliniie et al., who also found that this marker was highly expressed in prostate cancer as compared with benign lesions of prostate (P < 0.001).[11,12,13,14,15,16] Similarly above study showed sensitivity of 82%-100% and specificity of 80%-100%. Our findings of sensitivity and specificity of AMACR are similar to above studies.[11,12,13,14,15,16] Jiang et al. and Rashed et al. also observed statistical significant (P < 0.001) difference in AMACR index between benign and malignant prostatic lesions.[10,11]
Jiang et al. also reported strongly positive staining in HGPIN, and noted that when HGPIN partially involved a gland, staining was confined to the HGPIN and did not extend into the normal epithelial cells within the same gland.[11] With respect to staining amount, Luo et al. reported that both invasive carcinoma and HGPIN had higher IHC staining scores than normal prostate epithelium; however, the score for carcinoma was significantly higher than that for HGPIN.[12] Similar results were also reported by Rubin et al.[13]
The present study revealed no statistically significant correlation between AMACR positivity and Gleason's grade (P = 0.88) which was in concordance with studies by Jain et al. and Rubin et al. (P > 0.05).[6,13] In our study, four cases were negative for AMACR. Negative staining in prostate adenocarcinoma can be explained by heterogeneous expression of AMACR in prostate cancer. Heterogeneousity refers to different AMACR staining intensities were associated with different Gleason patterns or grades within tumor. In 2007, Murphy et al. in their study showed a striking finding was that a significant proportion of tumors (38%) showed heterogeneous AMACR expression, with 28% of positive cases showing weak or absent staining in over half of the tumor.[19]
Molinié et al. showed AMACR reacted with only 2% of normal glands (4/260) with a focal weak staining, and 97% of prostatic cancer showed AMACR over expression with a heterogeneous staining pattern from weak (30%) or moderate (31%) to strong (36%) intensity, independently of the Gleason score (P = 0.29), and the fixative technique (P = 0.27).[15] Luo et al. gave AMACR mean IHC scores for stratified by Gleason score, and pathological stage and noted no relation between AMACR IHC score and Gleason grade, pathological stage, patient age, or preoperative serum PSA (all P = 0.05).[12] Beach et al. also found 82% AMACR/P504S expression in prostate cancer whatever the morphological aspect and Gleason score or additional treatment such as hormone therapy or radiotherapy.[20]
The most commonly used basal cell–specific markers in prostate cancer are HMWCK and P63. HMWCK and P63 show continuous, intact, circumferential staining of basal cells in benign lesion but discontinuous staining in malignant lesions. In our study of benign lesions, HMWCK and P63 was expressed in all the 40 (100%) cases while in malignant lesions of prostate it was not expressed in any of the (0%) case. There was statistically significant difference in expression of HMWCK and P63 between cases of benign and malignant lesions prostate, indicated by P = 0.001. Similar findings were seen in the study by Shah et al., Singh et al.[5,8,9,21,22] In our study, all cases were of benign prostatic hyperplasia.
In our study, all 40 benign lesions were positive for HMWCK and P63. No benign lesion was positive for only AMACR or AMACR, HMWCK and P63. Out of 40 malignant cases, 4 cases were negative for AMACR, HMWCK and P63, 36 cases were positive only for AMACR, but no case was positive for HMWCK and P63 [Table 2]. These data suggest that positive cancer specific marker AMACR along with negative basal cell marker can increase the level of confidence in establishing a definitive diagnosis of prostate adenocarcinoma on limited biopsy.
Table 2.
AMACR/HMWCK and P63 status
| AMACR/HMWCK and P63 status | No of cases (n=80) | Percentage | |
|---|---|---|---|
| Benign (40) | Malignant (40) | ||
| AMACR+/HMWCK and P63+ | 0 | 0 | 0 |
| AMACR+/HMWCK and P63- | 0 | 36 | 45 |
| AMACR-/HMWCK and P63+ | 40 | 0 | 50 |
| AMACR-/HMWCK and P63- | 0 | 4 | 5 |
| Total | 40 | 40 | 100 |
Nowadays, fruitful health policy of government resulted in more access of urological health services at primary care level. For early detection purpose, the use of prostate-specific antigen (PSA)-based screening and digital rectal examination have resulted in the increased number of prostate biopsy with minimal prostate adenocarcinoma and benign mimics of prostate adenocarcinoma.[4] Immunohistochemistry with positive AMACR marker with negative basal cell marker HMWCK and P63 help in combating diagnostically challenging cases of minimal prostate adenocarcinoma. There by affecting the prognosis of patient.
Conclusion
Hence, we conclude that though histopathological examination is the gold standard, AMACR with positive marker and HMWCK, P63 negative marker in combination will enhance the diagnostic accuracy in prostate cancer and reduce the chance of misdiagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures 2003. Atlanta, GA: American Cancer Society; 2003. [Google Scholar]
- 2.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI. Diagnostic criteria of limited adenocarcinoma of the prostate on needle biopsy. Hum Pathol. 1995;26:223–9. doi: 10.1016/0046-8177(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 4.Srigley JR. Benign mimickers of prostatic adenocarcinoma. Mod Pathol. 2004;17:328–48. doi: 10.1038/modpathol.3800055. [DOI] [PubMed] [Google Scholar]
- 5.Shah RB, Zhou M, LeBlanc M, Snyder M, Rubin MA. Comparison ofthe basal cell-specific markers, 34betaE12 and p63, in the diagnosisof prostate cancer. Am J Surg Pathol. 2002;26:1161–8. doi: 10.1097/00000478-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Gupta S, Marwah N, Kalra R, Gupta V, Gill M, et al. Evaluation of role ofalpha-methyl acyl-coenzyme A racemase/P504S and high molecular weight cytokeratin in diagnosing prostatic lesions. J Can Res Ther. 2017;13:21–5. doi: 10.4103/0973-1482.206239. [DOI] [PubMed] [Google Scholar]
- 7.Kumaresan K, Kakkar N, Verma A, Mandal AK, Singh SK, Joshi K. Diagnostic utility of α-methylacyl CoA racemase (P504S) and HMWCK in morphologically difficult prostate cancer. Diagn Pathol. 2010;5:83. doi: 10.1186/1746-1596-5-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh V, Manu V, Malik A, Dutta V, Mani NS, Patrikar S. Diagnostic utility of p63 and α-methyl acyl CoA racemasein resolving suspicious foci in prostatic needle biopsy and transurethral resection of prostate specimens. J Can Res Ther. 2014;10:686–92. doi: 10.4103/0973-1482.138194. [DOI] [PubMed] [Google Scholar]
- 9.Boran C, Kandirali E, Yilmaz F, Serin E, Akyol M. Reliability of the 34βE12, keratin 5/6, p63, bcl-2, and AMACR in the diagnosis of prostate carcinoma. Urol Oncol. 2011;29:614–23. doi: 10.1016/j.urolonc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Rashed HE, Kateb MI, Ragab AA, Shaker SS. Evaluation of minimal prostate cancer in needle biopsy specimens using AMACR (p504s), p63 and ki67. Life Sci J. 2012;9:12–21. [Google Scholar]
- 11.Jiang Z, Woda BA, Rock KL, Xu Y, Savas L, Khan A, et al. P504S: A new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol. 2001;25:1397–404. doi: 10.1097/00000478-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, et al. Alpha-methylacyl-CoA racemase: A new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–6. [PubMed] [Google Scholar]
- 13.Rubin MA, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, et al. Alpha-methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287:1662–70. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Wu CL, Woda BA, Dresser K, Xu J, Fanger GR, et al. P504S/alpha-methylacyl-CoA racemase: A useful market for diagnosis of small foci of prostatic carcinoma on needle biopsy. Am J Surg Pathol. 2002;25:1169–74. doi: 10.1097/00000478-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Molinie V, Fromont G, Sibony M, Vieillefond A, Vassiliu V, Cochand-Priollet B, et al. Diagnostic utility of a p63/alphamethyl-CoA-racemase (p504s) cocktail in atypical foci in the prostate. Mod Pathol. 2004;17:1180–90. doi: 10.1038/modpathol.3800197. [DOI] [PubMed] [Google Scholar]
- 16.Magi-Galluzzi C, Luo J, Isaacs WB, Hicks JL, de Marzo AM, Epstein JI. Alpha-methylacyl-CoA racemase: A variably sensitive immunohistochemical marker for the diagnosis of small prostate cancer foci on needle biopsy. Am J Surg Pathol. 2003;27:1128–33. doi: 10.1097/00000478-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: When should we stop? J Urol. 2001;166:1679–83. [PubMed] [Google Scholar]
- 18.Gleason DF. The veterans administration cooperative urologic Research group histologic grading system. Histologic grading system of prostate cancer. Hum Pathol. 1992;23:273–9. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 19.Murphy AJ, Hughes CA, Lannigan G, Sheils O, O’Leary J, Loftus B. Hetrogeneousexpression of alpha-methylacyl-CoA Racemase in prostatic cancer correlates with Gleason score. Histopathology. 2007;50:243–51. doi: 10.1111/j.1365-2559.2007.02572.x. [DOI] [PubMed] [Google Scholar]
- 20.Beach R, Gown AM, De Peralta-Venturina MN, Folpe AL, Yaziji H, Salles PG, et al. P504S immunohistochemical detection in 405 prostatic specimens including 376 18-gauge needle biopsies. Am J Surg Pathol. 2002;26:1588–96. doi: 10.1097/00000478-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lakhtakia R, Bharadwaj R, Kumar VK, Mandal P, Nema SK. Immunophenotypic characterization of benign and malignant prostatic lesions. Med J Armed Force India. 2007;63:243–8. doi: 10.1016/S0377-1237(07)80145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotakidou R, Lakis S, Papamitsou T, Mavridou M, Varvaresou D, Koutsou A, et al. Diagnostic utility of immunohistochemical marker 34βe12 (keratin 903) in prostate pathology. Aristotle Univ Med J. 2007;34:31–6. [Google Scholar]


