Abstract
Cleaning and disinfecting tasks and product use are associated with elevated prevalence of asthma and respiratory symptoms among healthcare workers; however, the levels of exposure that pose a health risk remain unclear. The objective of this study was to estimate the peak, average, and determinants of real-time total volatile organic compound (TVOC) exposure associated with cleaning tasks and product-use. TVOC exposures were measured using monitors equipped with a photoionization detector (PID). A simple correction factor was applied to the real-time measurements, calculated as a ratio of the full-shift average TVOC concentrations from a time-integrated canister and the PID sample, for each sample pair. During sampling, auxiliary information, e.g. tasks, products used, engineering controls, was recorded on standardized data collection forms at 5-min intervals. Five-minute averaged air measurements (n = 10 276) from 129 time-series comprising 92 workers and four hospitals were used to model the determinants of exposures. The statistical model simultaneously accounted for censored data and non-stationary autocorrelation and was fit using Markov-Chain Monte Carlo within a Bayesian context. Log-transformed corrected concentrations (cTVOC) were modeled, with the fixed-effects of tasks and covariates, that were systematically gathered during sampling, and random effect of person-day. The model-predicted geometric mean (GM) cTVOC concentrations ranged from 387 parts per billion (ppb) for the task of using a product containing formaldehyde in laboratories to 2091 ppb for the task of using skin wipes containing quaternary ammonium compounds, with a GM of 925 ppb when no products were used. Peak exposures quantified as the 95th percentile of 15-min averages for these tasks ranged from 3172 to 17 360 ppb. Peak and GM task exposures varied by occupation and hospital unit. In the multiple regression model, use of sprays was associated with increasing exposures, while presence of local exhaust ventilation, large room volume, and automatic sterilizer use were associated with decreasing exposures. A detailed understanding of factors affecting TVOC exposure can inform targeted interventions to reduce exposures and can be used in epidemiologic studies as metrics of short-duration peak exposures.
Keywords: cleaning products, healthcare, modeling, peaks, real-time exposures, tasks
Introduction
In healthcare settings, cleaning and disinfecting is critical for maintaining infection control. The high cost of healthcare-associated infections (HAI) coupled with the risk posed to the safety of patients and healthcare workers, has increased demand for effective cleaning and disinfection products (Weber et al., 2012; Zimlichman et al., 2013). At the same time, epidemiologic studies have reported increased risk of asthma, rhinitis, and respiratory symptoms among workers performing cleaning and disinfecting tasks in multiple occupations (Zock et al., 2010). These tasks include: cleaning surfaces, sterilizing instruments, using products on patients, and using cleaning and disinfecting products such as general-purpose cleaners (e.g. bleach), high-level disinfectants (HLD, e.g. glutaraldehyde), and spray products (Zock et al., 2010; Siracusa et al., 2013; Folletti et al., 2017). Cleaning and disinfecting products are complex chemical mixtures that often contain multiple respiratory sensitizers and irritants (Wolkoff et al., 1998; Quirce and Barranco, 2010; Gerster et al., 2014). However, the specific chemical exposures and the levels at which they pose a health risk is not well understood. Quantitative exposure data are needed in epidemiological studies to better understand the levels at which health risks occur, and to begin the discussion on balancing exposure control measures with infection control needs.
Comprehensive exposure assessments for cleaning and disinfecting chemicals are rare in healthcare settings. The few studies conducted in hospitals have reported exposure to alcohols (ethanol, 2-propanol); ketones (acetone); terpenes (d-limonene, α-pinene); peroxygen compounds (hydrogen peroxide, peracetic acid); monoethanolamines; glycol ethers (e.g. 2-butoxyethanol, ethylene glycol mono-n-butyl ether); benzyl alcohol; aldehydes (formaldehyde, glutaraldehyde); quaternary ammonium compounds (e.g. benzyldimethyldodecyl ammonium chloride, benzyldimethyltetradecyl ammonium chloride); and aliphatic, aromatic, and halogenated hydrocarbons, using personal, mobile-area or stationary, time-integrated samplers (Teschke et al., 2002; Bessonneau et al., 2013; LeBouf et al., 2014; Melchior Gerster et al., 2014; Hawley et al., 2017; LeBouf et al., 2017). Modeling factors affecting exposure to cleaning and disinfecting chemicals is essential to explain sources of exposure variability, but is also rare in the healthcare setting. One study identified product type, task, room volume and ventilation, and product concentration as significant predictors of 2-butoxyethanol exposures in a quasi-experimental study (Bello et al., 2013). A recent study of several healthcare occupations evaluated the determinants of exposure to full-shift total volatile organic compounds (TVOC), ethanol, 2-propanol, acetone, d-limonene, α-pinene, and chloroform, focusing on tasks, product-application, amount of product, background activities, product ingredients, and local exhaust ventilation as predictors (Su et al., 2018). The study also provided estimates of geometric mean (GM) exposures to total and specific VOCs during product-application tasks.
TVOC exposure may be a useful surrogate of the complex mixture of chemicals present in cleaning and disinfecting products, and real-time measurements can provide additional exposure characteristics, such as peak exposures that may be relevant for asthma and irritation symptoms. Real-time measurements with observations or self-reported activity diaries can be particularly useful for identifying high-exposure tasks to target interventions. The objectives of this study were to (i) estimate TVOC exposure for cleaning product-application tasks based on real-time measurements, (ii) evaluate the influence of time-varying and time-independent covariates on TVOC exposures, and (iii) estimate metrics of peak exposure for product-application tasks based on exposure quantiles [e.g. 95th percentile (P95)]. A better understanding of factors affecting exposure to cleaning and disinfecting chemicals combined with estimates of means, variability, and quantiles will allow identification and prioritization of controls, and development of short-duration peak exposure metrics for use in epidemiologic studies (Heederik, 2014; Quinn et al., 2015).
Methods
Healthcare workers from 14 occupations were recruited from five hospitals comprising three U.S. Veterans Affairs (VA) and two teaching hospitals (Saito et al. 2015); only four hospitals are included in these analyses as explained below. Exposure assessment was conducted during spring and summer of 2009–2011. The occupations monitored included: clinical laboratory technician, certified nursing assistant, dental assistant, dental laboratory technician, endoscopy technician, floor stripper/waxer, housekeeper, licensed practical nurse, medical appliance technician, medical equipment preparer, pharmacy technician, registered nurse, respiratory therapist, and surgical technologist. Employees in these occupations worked in 12 areas of the hospital, denoted as hospital units. Real-time mobile-area and personal TVOC samples were collected using ppbRae 2000 and ToxiRae PGM-1800 monitors (RAE Systems, Inc.) respectively, and each instrument was paired with their respective time-integrated 6 liter or 0.4 liter Silonite™ evacuated canister (Entech Instruments, Inc.) (Supplementary Figure S1, available at Annals of Occupational Hygiene online) (Lebouf et al., 2014). Mobile-area samples were kept in a basket that was transported by a technician, and was maintained within ~1.5 m of the healthcare worker at all times. Real-time instruments were equipped with photoionization detectors (PID) with 10.6-eV lamps; their specifications are provided in Supplementary Table S1 (available at Annals of Occupational Hygiene online). Canister samples were analyzed using gas chromatography/mass spectrometry (GC/MS) for ethanol, acetone, 2-propanol, methylene chloride, hexane, chloroform, benzene, methyl methacrylate, toluene, ethylbenzene, m,p-xylene, o-xylene, α-pinene, d-limonene, and the quantified 14 VOCs were summed as TVOC (LeBouf et al., 2012). A real-time temperature and relative humidity meter (PRHTEMP 101, MadgeTech) that recorded measurements at 1-min intervals was placed in the mobile-area basket. During sampling, information on tasks, products used and their amounts, work location, engineering controls, and personal protective equipment use, as well as cleaning tasks and product-application by nearby workers, was recorded on standardized data collection forms at 5-min intervals (Saito et al. 2015; Su et al. 2018). Measurements from the PID samplers and temperature and humidity meter were averaged over 5-min intervals to match the observation data. Additional details on the sampling strategy, choice of sampling and analysis method, and results of specific VOC exposures and distributions of healthcare workers’ activities are reported elsewhere (LeBouf et al., 2012, LeBouf et al., 2014, Saito et al. 2015).
Calibration of PID instruments
Prior to each day of sampling, PIDs were calibrated according to manufacturer’s recommendations, which included zeroing the instrument with zero-grade air and span calibration with a single concentration of 10 parts per million (ppm) isobutylene. In addition, a simple correction factor was calculated as a ratio of the full-shift average TVOC concentration from the canister to the full-shift average TVOC concentration from the PID for each sample pair. This correction factor was applied to the 5-min average TVOC concentrations from the mobile-area and personal PIDs for each PID-canister sample pair, to adjust their respective readings to obtain canister-equivalent corrected TVOC concentrations (cTVOC). The manufacturer recommended mixed exposure response factor (MRF), which requires knowledge of the composition and proportions of the VOCs, was not used because the exposure mixture in the workplace likely varied over the full-shift with the use of different products. Moreover, a chamber study comparing responses of real-time PID samplers to time-integrated sorbent tubes for known VOC concentrations showed that the MRF-corrected TVOC concentration was less accurate than the uncorrected concentration, even when the VOC composition and proportion was known (LeBouf and Coffey, 2015). In the present analyses, correction factors could not be calculated for samples collected from the first of the five hospitals because the analytical method to measure the VOCs from the canister samples quantified only seven of the 14 VOCs, and thus the resulting TVOC concentrations could not be used to calibrate the PIDs; data from only four hospitals were used in the analysis.
Predictor variables
The 145 tasks and 222 products recorded in the observation sheets were combined and grouped into 27 product-application tasks, with a focus on cleaning product use for specific cleaning tasks, e.g. using alcohol wipes to clean patient skin, denoted as ‘alcohol skin wipe’ or using cleaning products containing quaternary ammonium compounds to clean surfaces, denoted as ‘quats surface cleaner’. Other non-cleaning tasks using products that can release VOCs were also coded, e.g. using formaldehyde in laboratory or using dental products. All other 5-min intervals of tasks where no products were used were combined into one task group, i.e. ‘no product use’. Henceforth, product-application tasks will be referred to as ‘tasks’. Other covariates included three time-invariant factors: hospital, occupation and hospital unit, and 12 time-varying factors: presence of local exhaust, presence of general exhaust, number of air changes per hour, room pressure negative/positive, distance of mobile-area sampler from worker, room volume, use of sprays and wipes, automatic sterilizer use, types of mops used, amount of product used, relative humidity and temperature. Room volume was categorized as large (>1232 ft3, the mode) or small (≤1232 ft3). Amount of product used was dichotomized into small (gram, milligram, milliliter, teaspoon of quantity used) or large (gallon, kilogram, liter of quantity used). The 27 tasks were also combined with occupation to create occupation-specific tasks e.g. ‘alcohol skin wipe-RN’, or hospital unit to create unit-specific tasks e.g. ‘quats floor cleaner-critical care’, because some tasks were unique to certain occupations or units, e.g. using dental products in dental occupations or in a dental clinic or laboratory.
Statistical analysis
All data management and descriptive analyses were performed in SAS software version 9.4 (SAS Institute Inc.). Summary statistics were calculated for the TVOC concentrations including scatter plots of full-shift average concentrations from the canister and PID samplers, scatter plots of mobile-area versus personal full-shift average and 5-min average measurements, and frequencies of predictor variables including tasks, three time-invariant factors and 12 time-varying factors.
Time-series of 5-min averages from the PID samplers were summarized using the method described by Houseman and Virji (2017). Briefly, this method provides an approach to model real-time exposure data that accounts for non-stationary autocorrelation via a spline basis, and censored data by integrating over the left tail of the distribution. The model was fit using Markov-Chain Monte Carlo (MCMC) within a Bayesian paradigm using non-informative priors; 10 000 MCMC samples thinned by 10, were drawn from the posterior distribution to characterize the model parameters. For the spline, the choice of knots was based on the expected ‘roughness’, or meaningful variation of the measurements over short durations, and was set at every 7.5 min. The model incorporates fixed-effects covariates, hierarchical random effects, and provides a range of summary measures such as the mean, task-specific contributions to standard deviations and various quantiles of interest. Instructions on downloading the complete R-code to run this model is provided in Supplementary Methods (available at Annals of Occupational Hygiene online). The model is of the form:
| (1) |
where Yir is the repeated log-transformed exposure measurement for subject i, observation r (at time tir), α(wir) is mean log-concentration for task wir, performed by subject i at time tir β is the vector of parameters corresponding to the matrix of fixed-effect covariates associated with subject i at time tir, is a series-specific vector of random spline coefficients, b(tir) a vector of B-splines, is a series-specific random intercept, and σe2 is the variance of the task-specific contribution to variation. The series-specific spline term represents a smooth, series-specific function of time capturing the autocorrelated error present within the series. Note that the overall variation of Yir is captured not by alone, but rather by var. Note also that the autocorrelation parameter is not finite, but rather entails an autocorrelation function which is approximated with a finite set of autocorrelation parameters. The covariation between two observations within the same series can be expressed by the covariation function , so that
The posterior distribution of quantiles of Yir (such as the P95) can be generated by calculating the corresponding quantiles of the right-hand side of equation (1) from the MCMC samples generated by the model. Similarly, posteriors of quantiles of time-aggregated values, such as 15-min averages, for possible comparison to short-term exposure limits (STEL) or averages of other time intervals (more generally , where R = number of 5-min average measurements) are easily produced by generating the corresponding distributions (over 10 000 simulation samples) for each MCMC sample and estimating the appropriate quantile for each task. In this case, we chose the P95 of 15-min averages for each tasks to represent peak exposure. Finally, we note that this Bayesian model generates posterior parameter distributions and credible intervals (CI), not parameter estimates and confidence intervals; however, within a practical context, posterior means (p.mn.) and posterior medians (p.md.) have similar interpretation as parameter estimates, and CI have similar interpretations as confidence intervals.
All models used log-transformed cTVOC concentrations as the outcome variable Yir and included one main fixed-effect term for task (wir) and a random-effect term for person-day (indexed by i). Crude posterior distributions of the GM, geometric standard deviation (GSD) and P95 of cTVOC concentrations for tasks were obtained from this single predictor model. In the text, for simplicity we only report the p.md. for the GM, GSD and P95, whereas in the tables, the p.mn. and CI are also reported. Occupation- and unit-specific task posterior distributions were also obtained by replacing the task variable wr with the occupation- or unit- specific task variables in the model noted above. Models for tasks were then fit with one additional fixed-effect covariate from the three time-invariant and 12 time-varying factors to evaluate their influence on the cTVOC exposures. Consistent with a growing awareness of the limitations of significance testing and the importance of considering effect size and sample size (Nuzzo, 2014; Wasserstein and Lazar, 2016), variables whose parameters were in the expected direction were included in the final multiple regression model regardless of whether their CI included 0. Finally, for ease of interpretation of the model parameters α and β from a log-transformed model, percent change in cTVOC associated with one unit change in continuous variable or going from 0 to1 for an indicator variable was calculated as (exp(α or β)−1)*100%. Statistical modeling was done using the R-code described above, and plots were generated using the ggplot2 package both run in R 3.3.1 (R Foundation for Statistical Computing) and SigmaPlot 14.0 (Systat Software Inc.).
Results
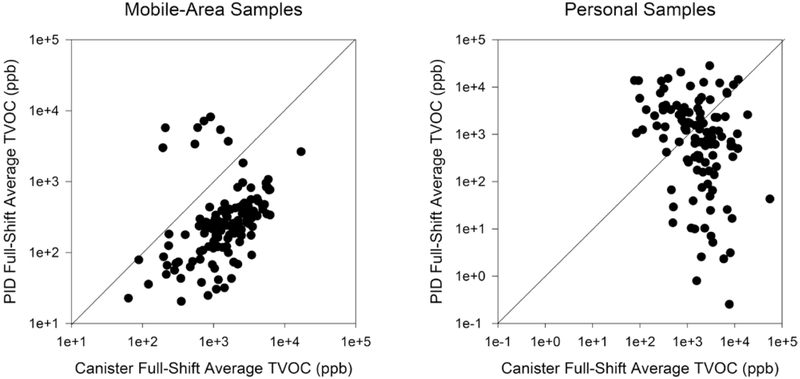
Altogether 129 mobile-area samples from 92 participants on 40 days, and 111 personal samples from 82 participants on 39 days were used for summarizing and modeling the determinants of exposures. 5.1% of the 10 276 five-minute average mobile-area and 29.2% of the 8846 five-minute average personal TVOC measurements were below their respective LOD of 1 and 100 ppb. Figure 1 displays the scatter plots of full-shift average TVOC concentrations from the canister compared to the PID, for the mobile-area samples (Fig. 1a) and personal samples (Fig. 1b). The full-shift average concentrations from the mobile-area PIDs were mostly lower than the corresponding measurements from the canister samples and showed a moderate correlation (rs = 0.52, P < 0.05). The full-shift averages from the personal PIDs were highly variable and showed a negative correlation with the personal canister samples (rs = −0.32, P < 0.05), suggesting that the random errors were too great for meaningful analyses using personal PID data. Although a relationship exists between mobile-area and personal measurements, as reflected by the moderate correlation (rs = 0.60, P < 0.05) for the full-shift averages from canister samples (Supplementary Figure S2a, available at Annals of Occupational Hygiene online), poor (rs = 0.36, P < 0.05) to no relationships (rs = −0.19, P = 0.20) were observed for 5-min average (Supplementary Figure S2b, available at Annals of Occupational Hygiene online) or full-shift average (Supplementary Figure S2c, available at Annals of Occupational Hygiene online) of the PID samples; the personal and mobile-area PIDs were different instrument models with different LODs and sampling modes (passive versus active). Thus, further analysis using the personal PID data was not conducted.
Figure 1.
Scatter plot of full-shift average TVOC concentrations from time-integrated canister and full-shift average PID samples (a) mobile-area samples and (b) personal samples.
The frequency of the task and other predictor variables is presented in Table 1. The most common tasks were: using quaternary ammonium compounds (quats) containing products to clean surfaces (4.4%), using quats products to clean floors (3.4%), using alcohol wipes on skin (2.3%), using products containing ethanolamines, benzyl alcohol or glycol ethers to strip floors (2.0%), and using enzymatic cleaners on instruments (1.5%). Housekeepers, registered nurses, and floor strippers/waxers were the most frequently measured occupations. Most measurements were collected in the operating room/gastroenterology, wards, and critical care areas. The 5-min average temperature ranged from 17 to 36°C with a mean of 21.8°C and median of 21.9°C; relative humidity ranged from 28 to 83% with a mean of 53.8% and median of 53%.
Table 1.
Frequencies of the main fixed effect, time-invariant and time-varying fixed effects covariates.
| Main fixed effect |
N (%) |
Time-invariant covariates |
N (%) |
Time-varying covariates |
N (%) |
|---|---|---|---|---|---|
| Task | Occupation | Air changes per hour | |||
| No product use (reference) | 7993 (77.8) | Clinical laboratory techs (Reference) | 576 (5.6) | ≤10 (Reference) | 1627 (15.8) |
| Alcohol skin wipe | 232 (2.3) | Nursing assistants | 560 (5.5) | >10 | 2760 (26.9) |
| Alcohol surface cleaning | 38 (0.4) | Dental assistants | 327 (3.2) | N/A | 5889 (57.3) |
| Bleach surface cleaning | 46 (0.5) | Dental laboratory techs | 331 (3.2) | General ventilation | |
| Chlorine skin wipe | 39 (0.4) | Endoscopy techs | 675 (6.6) | Absent (reference) | 8198 (79.8) |
| Dental products | 9 (0.1) | Floor strippers/waxers | 991 (9.6) | Present | 236 (2.3) |
| Detergent bathroom cleaning | 27 (0.3) | Housekeepers | 2430 (23.7) | N/A | 1842 (17.9) |
| Detergent instrument cleaning | 64 (0.6) | Licensed practical nurses | 312 (3.0) | Local exhaust hood | |
| Detergent skin cleaning | 3 (0.03) | Med equipment preparers | 602 (5.9) | Absent (reference) | 8262 (80.4) |
| Detergent surface cleaning | 17 (0.2) | Pharmacy techs | 455 (4.4) | Present | 171 (1.7) |
| EA/BA/GE floor stripping | 206 (2.0) | Registered nurses | 2334 (22.7) | N/A | 1843 (17.9) |
| EA/GE/ glass cleaning | 62 (0.6) | Respiratory therapists | 527 (5.1) | Sampler distance | |
| EA/GE surface cleaning | 51 (0.5) | Surgical technologists | 156 (1.5) | ≤3 feet (reference) | 5588 (54.4) |
| Enzymatic cleaning | 149 (1.5) | Unit group | >3 feet | 4688 (45.6) | |
| Formaldehyde in laboratory | 26 (0.3) | Critical care (reference) | 1167 (11.4) | Room volume | |
| HLD on instruments | 40 (0.4) | Clinical laboratory | 576 (5.6) | Small (reference) | 1560 (15.2) |
| Iodine skin wipe | 36 (0.4) | Dental clinic | 327 (3.2) | Large | 2971 (28.9) |
| Phenolics products | 109 (1.1) | Dental laboratory | 331 (3.2) | N/A | 5745 (55.9) |
| Quats bathroom cleaning | 14 (0.1) | Dialysis | 352 (3.4) | Tool mop use | |
| Quats floor cleaning | 346 (3.4) | Emergency room | 705 (6.9) | No mop use (reference) | 148 (1.4) |
| Quats skin wipe | 23 (0.2) | Floor | 991 (9.6) | Regular mop | 333 (3.2) |
| Quats surface cleaning | 450 (4.4) | OR/GI | 2580 (25.1) | Micro fiber mop | 91 (0.9) |
| Solvent products | 6 (0.1) | Pharmacy | 455 (4.4) | N/A | 9704 (94.4) |
| Non-cleaning-other product | 192 (1.9) | Central supply | 602 (5.9) | Automatic sterilizer | |
| Non-cleaning-dialysis buffer | 7 (0.1) | Ward | 2190 (21.3) | No (reference) | 101 (1.0) |
| Non-cleaning-lab reagent | 28 (0.3) | Hospital | Yes | 69 (0.7) | |
| Non-cleaning-medications | 63 (0.6) | VA Hospital 1 (reference) | 1994 (19.4) | N/A | 10 106 (98.3) |
| VA Hospital 2 | 3326 (32.4) | Product amount | |||
| University Hospital 1 | 1266 (12.3) | Small (Reference) | 1114 (10.8) | ||
| University Hospital 2 | 3690 (35.9) | Large | 820 (8.0) | ||
| Non-cleaning product | 349 (3.4) | ||||
| N/A | 7993 (77.8) | ||||
| Room pressure | |||||
| Positive (reference) | 2662 (25.9) | ||||
| Negative | 1869 (18.2) | ||||
| N/A | 5745 (55.9) | ||||
| Tool—Spray & sponge | |||||
| No tool used (reference) | 546 (5.3) | ||||
| Sponge only | 655 (6.4) | ||||
| Spray only | 33 (0.3) | ||||
| Sponge and spray | 150 (1.5) | ||||
| N/A | 8892 (86.5) |
N = number of 5-min average measurements; EA/BA/GE = ethanolamines/benzyl alcohols/glycol ethers; Techs = technicians; OR/GI = operating room/gastroenterology; N/A = variable not relevant.
The posterior distributions of the mobile-area GM, GSD, and P95 cTVOC concentrations for tasks (exponentiated values), based on the MCMC chains and additional simulations for P95, from the model with a single variable are displayed in Fig. 2 and Supplementary Table S2 (available at Annals of Occupational Hygiene online). There was a high overall average cTVOC exposure reflected by the GM of 925 ppb for the reference task of ‘no product use’, which we denote as average ‘background’ exposures across hospitals, units and occupations. The recurrent use of hand sanitizers and cleaning of floors and surfaces is likely responsible for the high background level of TVOC exposure in hospitals. The GM concentrations of cTVOC were similar across tasks and ranged from 2,091 ppb for ‘quats skin wipes’ to 387 ppb for ‘formaldehyde in laboratory’ (Fig. 2a). Other high-exposure cleaning tasks included: ‘enzymatic cleaner’, ‘chlorine skin wipes’, ‘EA/GE based glass cleaner’, ‘HLD instrument cleaner’. Note, the exposures associated with tasks are for TVOC, and not for specific chemicals in the products such as chlorine or formaldehyde. The GSDs were similar across tasks (Fig. 2c), and were among the highest for ‘detergent bathroom cleaner’, ‘HLD instrument cleaner’, ‘alcohol skin wipe’, and ‘alcohol surface cleaner’. The posterior distributions of both the GM and GSD were quite variable with a wide range of CI. The P95 representing peak exposures (Fig. 2b) showed discernible differences among tasks, and ranged from a median of 17,360 ppb for ‘quats skin wipes’ to 3,172 ppb for ‘formaldehyde in laboratory’. The GM and P95 were highly correlated (rp = 0.91, P < 0.05), but there were many differences in the rank order of adjacent values between the two metrics across the tasks.
Figure 2.
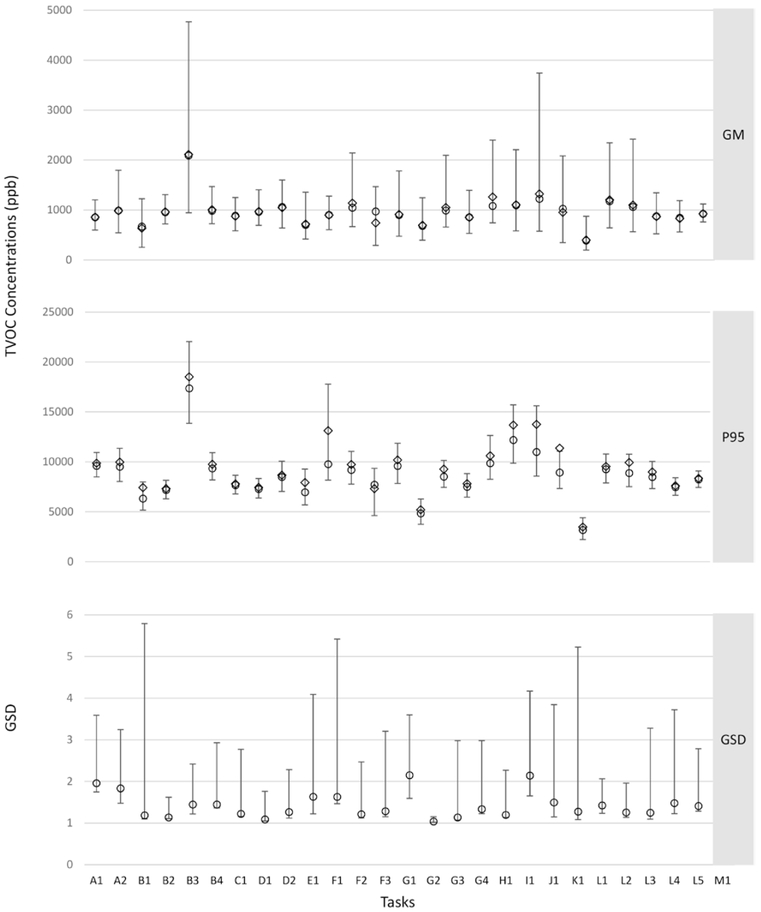
Posterior distributions of the cTVOC summary measures for tasks showing: (a) GM and 95% CI, (b) P95 and IQR, and (c) GSD and 95%CI. Footnotes: Open diamond = mean; open circle = median; intervals around the GM and GSD are 95% CI; intervals around the P95 are inter quartile range (25–75%); model parameters are exponentiated to get GM, GSD, P95. A1: Alcohol Skin Wipe; A2: Alcohol Surface Cleaner; B1: Quats Bathroom Cleaner; B2: Quats Floor Cleaner; B3: Quats Skin Wipe; B4: Quats Surface Cleaner; C1: Phenolics Cleaner; D1: Chlorine Surface Cleaner-Bleach; D2: Chlorine Skin Wipe; E1: Iodine Skin Wipe; F1: Ethanolamine/Glycol Ether Floor Stripper; F2: Ethanolamine/Glycol Ether Glass Cleaner; F3: Ethanolamine/Glycol Ether Surface Cleaner; G1: Detergent Bathroom Cleaner; G2: Detergent Skin Cleaner; G3: Detergent Surface Cleaner; G4: Detergent Instrument Cleaner; H1: Enzymatic Instrument Cleaner; I1: High-Level Instrument Cleaner; J1: Dental Product; K1: Solvent Cleaner; L1: Formaldehyde in Laboratory; L2: Non-Cleaner in Laboratory; L3: Non-Cleaners in Dialysis; L4: Non-Cleaner Medication; L5: Other Non-Cleaner; M1: No Product Used.
The predicted GM task exposures from models with the main fixed effect of task and a single time-invariant covariate are presented in Supplementary Figure S3 (available at Annals of Occupational Hygiene online), which shows variable task exposures across occupation and unit. Posterior distribution statistics from the time-invariant and time-varying covariates are presented in Supplementary Table S3 (available at Annals of Occupational Hygiene online). Seven of the 12 time-varying covariates had p.mn. in the expected direction, and included use of sprays only (17.5%) which was associated with increasing exposures, while presence of local exhaust ventilation (−14.1%), large room volume (−10.4%), automatic sterilizer use (−32.7%), sampler distance >3 ft. from worker (−0.6%), negative room pressure (−1.5%), and increasing humidity (−1.1%) were all associated with decreasing exposures. These seven covariates were included in the final multiple regression model. Air changes per hour >10, presence of general ventilation, microfiber mop use, and increasing temperature were associated with increasing exposure, while large product amount was associated with decreasing exposure; these patterns were in the opposite direction than expected.
The posterior distributions of the GM and P95 cTVOC exposures for selected tasks-occupation combinations (with n > 5) related to (i) patient cleaning, (ii) surface cleaning, and (iii) instrument cleaning are displayed in Fig. 3 and Supplementary Table S4 (available at Annals of Occupational Hygiene online). It is note-worthy that using products containing alcohol, chlorine or quats used on patient skin or for surface cleaning had among the highest GM cTVOC exposures for several occupations. High GM cTVOC exposures were also associated with the use of products containing ethanolamine/glycol ether-containing products for glass and surface cleaning among housekeepers. Lowest GM cTVOC exposures were predicted for instrument cleaning tasks. Similar trends were observed for the P95, though the rank order across occupation-tasks was occasionally different and a high correlation (rp = 0.86, P < 0.05) was observed between the two metrics. Differences among task-occupation exposures were more discernible with the P95 metric than with the GM exposure metric. Complementary results for the unit-specific tasks are presented in Supplementary Table S5 and Figure S4 (available at Annals of Occupational Hygiene online). Patient cleaning and surface cleaning tasks using quats products in dental clinics, wards, central supply, and operating room/gastroenterology unit had among the highest GM cTVOC exposures. Cleaning surfaces and instruments using several products such as quats, enzymatic cleaners, phenolics, and HLD were also associated with high GM cTVOC exposures in the operating room/gastroenterology unit. Similar trends were observed for the P95 with high correlation between the metrics (rp = 0.91, P < 0.05), though the rank order across unit-tasks was occasionally different between the two metrics.
Figure 3.
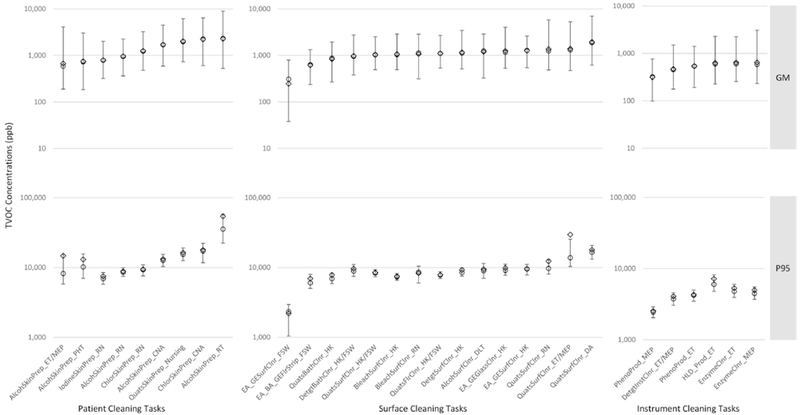
Posterior distributions of the GM (top panels), and P95 (bottom panels) of cTVOC summary statistics for selected (a) patient cleaning, (b) surface cleaning, and (c) instrument cleaning task-occupation combinations. Footnotes: Open diamond = mean; open circle = median; intervals around the GM are 95% CI; intervals around the P95 are inter quartile range (25–75%); model parameters exponentiated to get GM and P95; CLT = clinical laboratory technician; CAN = certified nursing assistant; DA = dental assistant; DLT = dental laboratory technician; ET = endoscopy technician; FSW = floor stripper/waxer; HK = housekeeper; LPN = licensed practical nurse; MAT = medical appliance technician; MEP = medical equipment preparer; PHT = pharmacy technician; RN = registered nurse; RT = respiratory therapist; and ST = surgical technologist.
The final multiple regression model for the (log-transformed) cTVOC concentrations provides the mean and median parameters for tasks and their 95% CI, the task-specific standard deviation (SD) and their 95% CI, the % change from the reference category, and parameters for the spline and random effect variances (Table 2). The full multiple regression model included the following covariates and their influence on cTVOC exposure: use of automatic sterilizer (−36.8%), presence of local exhaust (−13.4%), large room size (−11.2%), sampler distance >3ft from worker (−1.6%), increasing humidity (−1.3%) and use of spray products only (20.6%). The parameter for room pressure switched signs in the final model with the effect in the opposite direction than expected, and was thus not included in the final model.
Table 2.
Final multiple regression model of log-transformed cTVOC concentrations summarizing the posterior distributions from the MCMC chains of model parameters for covariates and random effects.
| Variable | N | Mean (α or β) (% change) |
Median (α or β) (% change) |
α or β (95% CI) |
Median SD (95% CI) |
|
|---|---|---|---|---|---|---|
| Main fixed effects | No product (intercept) | 7993 | 8.05 | 7.96 | 7.63, 8.86 | 0.29 (0.28, 0.94) |
| Alcohol skin wipe | 232 | −0.08 (−8.1) | −0.08 (−7.7) | −0.24, 0.05 | 0.66 (0.55, 1.26) | |
| Alcohol surface cleaning | 38 | 0.02 (1.9) | 0.02 (2.1) | −0.4, 0.46 | 0.55 (0.37, 1.14) | |
| Bleach surface cleaning | 46 | −0.16 (−14.4) | −0.14 (−13.4) | −0.37, 0.02 | 0.12 (0.07, 0.57) | |
| Chlorine skin wipe | 39 | 0.14 (15.2) | 0.16 (17.2) | −0.16, 0.35 | 0.19 (0.12, 0.84) | |
| Dental products | 9 | 0.3 (35.3) | 0.27 (30.8) | −0.27, 1.07 | 0.34 (0.16, 1.17) | |
| Detergent bathroom cleaning | 27 | −0.17 (−15.4) | −0.18 (−16.6) | −0.56, 0.27 | 0.7 (0.44, 1.26) | |
| Detergent instrument cleaning | 64 | −0.15 (−14.2) | −0.14 (−13.5) | −0.5, 0.13 | 0.25 (0.13, 1.07) | |
| Detergent skin cleaning | 3 | −0.27 (−23.3) | −0.29 (−25.0) | −0.61, 0.1 | 0.04 (0.02, 0.15) | |
| Detergent surface cleaning | 17 | 0.04 (4.4) | 0.01 (1.1) | −0.19, 0.46 | 0.1 (0.08, 1.1) | |
| EA/BA/GE floor stripping | 206 | −0.04 (−4) | −0.03 (−3.3) | −0.23, 0.11 | 0.47 (0.38, 1.67) | |
| EA/GE/ glass cleaning | 62 | 0.04 (4.2) | 0.01 (1.3) | −0.24, 0.41 | 0.18 (0.13, 0.84) | |
| EA/GE surface cleaning | 51 | −0.17 (−15.7) | 0.04 (4.2) | −1.06, 0.27 | 0.27 (0.17, 1.1) | |
| Enzymatic cleaning | 149 | 0.19 (21.4) | 0.13 (13.4) | −0.03, 0.58 | 0.16 (0.13, 0.73) | |
| Formaldehyde in laboratory | 26 | −0.78 (−54) | −0.75 (−52.8) | −1.36, −0.22 | 0.32 (0.21, 0.7) | |
| HLD on instruments | 40 | 0.29 (33.3) | 0.28 (31.9) | −0.22, 0.81 | 0.71 (0.49, 1.4) | |
| Iodine skin wipe | 36 | −0.27 (−23.9) | −0.3 (−25.8) | −0.58, 0.15 | 0.39 (0.19, 1.38) | |
| Non-cleaning-other product | 192 | −0.1 (−9.3) | −0.08 (−7.6) | −0.32, 0.05 | 0.32 (0.26, 1.03) | |
| Non-cleaning-dialysis buffer | 7 | 0.22 (25.2) | 0.2 (22.4) | −0.12, 0.74 | 0.14 (0.08, 1.15) | |
| Non-cleaning-lab reagent | 28 | 0.24 (27.6) | 0.22 (24.7) | −0.14, 0.7 | 0.2 (0.12, 0.64) | |
| Non-cleaning-medications | 63 | −0.03 (−3.3) | −0.02 (−2.1) | −0.33, 0.19 | 0.36 (0.25, 1.29) | |
| Phenolics products | 109 | −0.09 (−8.4) | −0.06 (−6.2) | −0.38, 0.09 | 0.2 (0.13, 0.99) | |
| Quats bathroom cleaning | 14 | −0.39 (−32.5) | −0.33 (−28.0) | −1.12, 0.03 | 0.16 (0.09, 1.5) | |
| Quats floor cleaning | 346 | −0.06 (−5.9) | −0.05 (−5.0) | −0.24, 0.06 | 0.12 (0.1, 0.48) | |
| Quats skin wipe | 23 | 0.82 (126.2) | 0.78 (119.0) | 0.25, 1.45 | 0.34 (0.2, 0.86) | |
| Quats surface cleaning | 450 | −0.02 (−1.8) | −0.02 (−1.7) | −0.16, 0.11 | 0.36 (0.31, 1.07) | |
| Solvent products | 6 | −0.01 (−1.2) | 0.06 (6.1) | −0.96, 0.44 | 0.15 (0.09, 1.57) | |
| Covariates | Local exhaust hood: present | 171 | −0.11 (−10.7) | −0.14 (−13.4) | −0.33, 0.26 | |
| Local exhaust hood: N/A | 1843 | 0 (0.2) | 0 (0.2) | −0.06, 0.06 | ||
| Sampler distance >3 ft | 4688 | −0.02 (−1.7) | −0.02 (−1.6) | −0.07, 0.03 | ||
| Room volume: large | 2971 | −0.13 (−11.8) | −0.12 (−11.2) | −0.25, −0.02 | ||
| Room volume: N/A | 5745 | −0.12 (−11.1) | −0.11 (−10.8) | −0.21, −0.03 | ||
| Tool—spray & sponge: sponge only | 655 | 0.02 (1.8) | 0.02 (2.3) | −0.09, 0.12 | ||
| Tool—spray & sponge: spray only | 33 | 0.2 (22.3) | 0.19 (20.6) | −0.08, 0.57 | ||
| Tool—spray & sponge: sponge & spray | 150 | 0.08 (8.4) | 0.08 (8.3) | −0.04, 0.21 | ||
| Tool—spray & sponge: N/A | 8892 | −0.08 (−7.5) | −0.05 (−4.85) | −0.29, 0.07 | ||
| Automatic sterilizer | 69 | −0.46 (−37.1) | −0.46 (−36.8) | −0.9, −0.1 | ||
| Automatic sterilizer: N/A | 10 106 | −0.33 (−28.4) | −0.28 (−24.2) | −0.67, −0.14 | ||
| Humidity | −0.01 (−1.3) | −0.01 (−1.3) | −0.02, −0.01 | |||
| SD | Spline (σζ): median (95% CI) | 1.2 (0.02, 1.24) | ||||
| Random (σα): median (95% CI) | 1.0 (0.88, 1.13) |
N = number of 5-min average measurements; α = posterior parameter for task; β = posterior parameter for covariates; %change = (exp(α) − 1)*100% for tasks or (exp(β) − 1)*100% for covariates; EA/BA/GE = ethanolamines/benzyl alcohols/glycol ethers; EA/GE = ethanolamines/glycol ethers; N/A = variable not relevant.
Discussion
Healthcare workers are exposed to complex mixtures of chemicals that can include simultaneous exposure to multiple asthmagens (Quirce and Barranco, 2010). This complex exposure scenario varies with task, location, and across days depending on the combination of products used, application methods, control measures, and other factors (Su et al., 2018). Furthermore, both acute and chronic respiratory health outcomes are associated with short-term exposures (Siracusa et al., 2013; Hawley et al., 2017). However, comprehensive quantitative measures of exposures are lacking and challenging to collect, because multiple sampling and analytical methods are required, compounded by the difficulties in conducting personal monitoring of healthcare professionals. Additionally, traditional single-sample full-shift exposure measurements cannot capture within work-shift variability, and will thus not yield relevant measures of short-term exposures for use in epidemiologic studies, or for devising effective interventions. TVOC may serve as a useful surrogate of the complex mixture of chemicals present in cleaning and disinfecting products, especially as they may contain multiple or unknown asthmagens. Moreover, real-time TVOC measurements can provide additional exposure characteristics such as short-duration mean or peak exposures that may be particularly relevant for asthma and irritation symptoms, and essential for identifying high-exposure tasks for targeted interventions. In the absence of real-time instruments for measuring multiple chemicals simultaneously, decisions have to made whether to measuring a single chemical among several etiologically relevant chemicals or a non-specific surrogate of mixture, or whether to collect time-integrated versus real-time or personal versus mobile-area measurements; each decision entails a balance of the benefits and limitations of the selected approach, and balance between the potential bias from not considering some etiologically relevant exposures versus exposure misclassification arising from using a non-specific surrogate exposure for the mixture. Whereas portable GC/MS instruments are available that can accurately quantify multiple VOCs in near real-time, their regular use in the field is hindered by several factors including their cost, their requirement for gases and for sample injection at time intervals, need for a specially trained technician to collect the samples, and while these units are labeled portable, it would be very difficult to collect short-duration personal task samples from a mobile worker such as a nurse.
In this study, we used a newly developed statistical method to quantify short-duration mean and peak exposures to cTVOC associated with specific tasks. In addition to addressing the statistical challenges of modeling non-stationary time-series with censored data and hierarchical structure, the use of MCMC within a Bayesian context provides information on the distributions of the parameters of interest across credible values. These estimates may be used in probabilistic risk assessment, which incorporates exposure variability into the risk assessment process to provide a more complete characterization of risks. In this study, the overall cleaning-related mobile-area GM cTVOC exposures ranged from 670 ppb for ‘quats bathroom cleaning’ to 2091 ppb for ‘quats skin wipe’. Some of the highest mobile-area GM exposures were associated with the use of quats-, alcohol- and chorine-based skin wipes, quats- and ethanolamine/glycol ether-based surface cleaning tasks, as well as use of HLD for cleaning instruments among specific occupations and locations. Although no previous studies report task-specific TVOC concentrations in hospitals, similar results were reported in a study of simulated cleaning in which the authors found average personal TVOC concentration in the range of 560 ppb (cleaning toilet bowls) to 6490 ppb (sink cleaning) in a small bathroom, and 20 ppb (mirror cleaning) to 1360 ppb (sink cleaning)in a large bathroom (Bello et al., 2010). Likewise, in a multiple regression model of full-shift mobile-area TVOC exposures from canister samples collected on the same population as the present study, Su et al. (2018) included several product-application tasks that were significant in univariate analysis. Including all product-application tasks (regardless of their significance) in the multiple regression model yielded the following mobile-area TVOC task estimates (GM-ppb): alcohol skin prep (1560), alcohol surface cleaner (906), bleach surface cleaner (1022), chlorine skin prep (980), detergent bathroom cleaner (1121), detergent instrument cleaner (1888), detergent surface cleaner (1074), EA/BA/GE floor stripper (998), EA/GE glass cleaner (1303), EA/GE surface cleaner (869), enzymatic cleaner (640), iodine skin prep (243), phenolic surface cleaner (2597), quats bathroom cleaner (244), quats floor cleaner (2283), quats skin prep (1270), quats surface cleaner (1226) (data not reported by Su et al, 2018). For a majority of the tasks, the PID GM from the present study were lower than those calculated from the model of the canister samples. We are presently investigating the causes of differences in GM tasks predicted from a model using real-time measurements versus one using time-integrated measurements from the same population.
Metrics of peak exposure may be particularly relevant and important for investigating asthma and respiratory symptoms, but such metrics have not been used in studies of cleaning and disinfecting chemical exposure (Siracusa et al., 2013). Peak exposures are of concern because they can potentially overwhelm the capacity of normal defense mechanisms and induce adverse health effects, and may be relevant for disease processes related to inflammation, irritation, or immune sensitization (Smith, 2001; Kriebel et al., 2007; Smith and Kriebel, 2008). Numerous studies have reported associations between peak exposure, inadvertent exposure or spills and asthma symptoms in a variety of workplace settings, and in the general population (Goldstein and Weinstein, 1986; Andersson et al., 2003; Andersson et al., 2006; Amster et al., 2014). Furthermore, high exposure to irritants is associated with reactive airways disease syndrome (Heederik, 2003). Different metrics have been used to characterize peak exposures based on intensity, duration, time interval between peaks, and frequency and aggregation of peaks from real-time data (Preller et al., 2004). In this study of spray painting operations, principal components analysis of these peak metrics revealed three independent factors related to intensity, variability, and duration that sufficiently characterized peak exposures. In a commentary, Kumagai (2004) expressed these findings in terms of real-time exposure distribution parameters and autocorrelation, i.e. the GM, GSD, and autocorrelation coefficient, all of which are parameters obtained from our model, but the single autocorrelation coefficient is replaced by an autocorrelation function. Real-time exposure monitoring provides flexibility in post hoc definitions of peaks, and in examining the correlations among the various peak metrics and their utility in predicting risk of health effects. In this study, we used 15-min averages and quantified peaks as the median P95 of the posterior distributions for short-duration tasks. This metric, representing exposure intensity, is easy to interpret when used in epidemiologic studies, to make decisions on interventions, or to evaluate the efficacy of control measures. The overall cleaning-related peak mobile-area cTVOC exposures ranged from 4843 ppb for ‘detergent skin cleaning’ to 17 360 ppb for ‘quats skin wipe’. While the P95 were well correlated with the GM (rp = 0.86, P < 0.05), the rank order for the tasks varied between the two metrics. The range of mobile-area concentrations observed in our study are similar to the personal exposures from a study of simulated cleaning, in which the authors reported peak (highest) TVOC concentrations of 710 ppb for cleaning toilet bowls to 11 360/11 110 ppb for mirror cleaning/sink cleaning tasks in a small bathroom, and 140 ppb for mirror cleaning to 2130 ppb for sink cleaning in a large bathroom (Bello et al., 2010).
In this study we identified several factors that influenced exposure including automatic sterilizer use, presence of local exhaust, large room size, and use of spray products only. The few studies that have investigated determinants of exposures in healthcare settings have also identified some common exposure determinants. A study of radiographers found ventilation, workload, and time in certain work areas as important determinants of exposure to glutaraldehyde, acetic acid, and sulfur dioxide (Teschke et al., 2002). Bello et al. (2013) identified product type, task, room volume and ventilation, and product concentration as significant predictors of 2-butoxyethanol exposures, in a quasi-experimental study. In our previous work, modeling the determinants of exposure to full-shift mobile-area TVOC, ethanol, isopropanol, acetone, d-limonene, α-pinene and chloroform among several healthcare occupations identified tasks, product-application, product ingredients, and local exhaust ventilation as significant predictors (Su et al., 2018). Among cleaners in a variety of work settings, spraying and amount of product used were important determinants of monoethanolamine exposure, while spraying and cross ventilation were important predictors of glycol ether exposure (Melchior Gerster et al., 2014).
Reviews of epidemiologic studies have called for quantitative exposure estimates to better characterize the health risk of asthma outcomes (Siracusa et al., 2013). The GM and P95 cTVOC exposure estimates, along with the VOC-specific GM exposure estimates reported in Su et al. (2018) for occupation- or unit-specific tasks can be considered as a job-task or unit-task exposure matrix (JTEM or UTEM), and can be assigned to participants in epidemiologic studies who report tasks in their questionnaire, e.g. in the detailed cleaning and disinfecting modules used by different investigators (Kurth et al., 2017; Caridi et al., 2019). However, within-job/unit/ task differences stemming from work practices or workplace conditions are not accounted for, as everyone in the same J/U-TEM cell is assigned the same exposure. These assigned exposures can be refined by combining them with worker-specific information on the frequency and duration of tasks, to capture the between-subject variation in exposure and thus rendering the resulting estimates more representative of individual experience. In addition, the J/U-TEM exposure estimates can be further refined by incorporating other exposure characteristics reported in questionnaires and collected during exposure assessment, such as use of automatic sterilizers or sprays or other exposure determinants. These hybrid approaches are a significant improvement over simple application of a JTEM because they account for the often large between-worker variation within the same JTEM cell (Kromhout and Vermeulen, 2001; Semple et al., 2004). The exposure estimates generated in this study can also be combined with, or used to calibrate other JTEMs not based on quantitative exposure such as the JTEM of nurses described by Quinot et al. (2017) for exposure to various cleaning and disinfecting chemicals in different settings such as the emergency room, or the JTEM of 12 occupational categories described by Delclos et al. (2007), for different types of exposures including cleaning and disinfecting agents in different work settings. However, both these JTEMs include some occupations in healthcare settings outside of hospitals which will limit the extent of integration of the two approaches. The hybrid methods of combining multiple sources of information have been previously described (Friesen et al., 2012; Koh et al., 2014), and can be implemented within a Bayesian approach to improve exposure assessment for epidemiologic studies.
There are several limitations with the use of direct-reading instruments related to their performance characteristics, such as lack of specificity, issues of validity, precision, calibration, etc. Moreover, we could not account for differences in instrument responses for different chemical mixtures likely associated with different tasks, which would lead to increased uncertainty in task-specific exposure estimates. The PID manufacturer supplies response factors for specific VOCs to obtain a correction factor for a mixture using their mixture equation, or to estimate exposure to a specific VOC in a mixture. However, these response factors may not take into account the potential effects on the detector response of unpredictable mixture components, leading to uncertainty in the response factors provided. The proportion of the VOCs quantified in full-shift samples and the manufacturer-provided instrument response correction factors are presented in Supplementary Table S6 (available at Annals of Occupational Hygiene online) (Rae Systems Inc., 2013). Since the VOC mixture in healthcare is predominated by ethanol and 2-propanol which have response factors much greater than 1, we expected the PID to underestimate TVOC exposures. For situations when the proportion of alcohol was low and the composition was predominated by benzene, toluene, ethylbenzene and xylenes (BTEX) whose response factors are much less than 1, the PID likely overestimated TVOC exposures. Our findings reported in Fig. 1 are consistent with these observations. Without accurate knowledge of the VOC mixture composition over the short-term, the best approach was to use the simple correction factor to account for the underestimation of the TVOC by the PID. Although the TVOC may capture the total VOC load, a small signal from the combination of health-relevant VOCs may be masked by the very large background alcohol signal which is present at the highest concentrations of all VOCs. Thus the advantages of using real-time TVOC measurements that provide metrics of peak mixture exposure may be lost due to the high background level of TVOC that can obscure small differences in etiologically relevant exposures. A compromise in using TVOC as a surrogate of mixed exposure is the missed opportunity to identify specific etiologically relevant chemicals.
Conclusion
In this study, we used a newly developed statistical approach to quantify short-duration mean and peak exposures to TVOCs associated with specific cleaning product-application tasks that may be particularly relevant for epidemiologic studies of asthma and irritation symptoms. We also identified several factors such as automatic sterilizer use, presence of local exhaust, large room size, and use of spray products only that influenced TVOC exposures. Detailed understanding of factors affecting TVOC exposure can inform targeted interventions to reduce exposures. This study shows that real-time data can be appropriately analyzed to yield important information on short-term exposure variability and exposure determinants if contextual information is gathered systematically, and attention is paid to instrument calibration.
Supplementary Material
Acknowledgements
The authors thank Dr. Brie Hawley and Cynthia Hines from NIOSH for their review of this manuscript. The authors would like to acknowledge Douglas Dulaney MSEH, MHA, Director, Office of Occupational Safety, Health, and GEMS Programs Veterans Health Administration, Washington, DC who coordinated access to the VA Medical Centers. Funding for this project was provided by the National Institute for Occupational Safety and Health.
Footnotes
Supplementary Data
Supplementary data are available at Annals of Work Exposures and Health online.
Declaration for Publication
This work was funded through the National Institute for Occupational Safety and Health intramural research grant. All of the authors declare no conflict of interest relating to the material presented in this article.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Mention of a specific product or company does not constitute endorsement by the Centers for Disease Control and Prevention. In addition, citations to Web sites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these Web sites. Its contents, including any opinions and/or conclusions are solely those of the authors.
References
- Amster ED, Haim M, Dubnov J, Broday DM. (2014) Contribution of nitrogen oxide and sulfur dioxide exposure from power plant emissions on respiratory symptom and disease prevalence. Environmental Pollution; 186: 20–8. [DOI] [PubMed] [Google Scholar]
- Andersson E, Knutsson A, Hagberg S et al. (2006) Incidence of asthma among workers exposed to sulphur dioxide and other irritant gases. Eur Respir J; 27: 720–5. [DOI] [PubMed] [Google Scholar]
- Andersson E, Olin AC, Hagberg S et al. (2003) Adult-onset asthma and wheeze among irritant-exposed bleachery workers. Am J Ind Med; 43: 532–8. [DOI] [PubMed] [Google Scholar]
- Bello A, Quinn MM, Milton DK et al. (2013) Determinants of exposure to 2-butoxyethanol from cleaning tasks: a quasi-experimental study. Ann Occup Hyg; 57: 125–35. [DOI] [PubMed] [Google Scholar]
- Bello A, Quinn MM, Perry MJ et al. (2010) Quantitative assessment of airborne exposures generated during common cleaning tasks: a pilot study. Environ Health; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessonneau V, Mosqueron L, Berrubé A et al. (2013) VOC contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PLoS One; 8: e55535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi MN, Humann MJ, Liang X et al. (2019) Occupation and task as risk factors for asthma-related outcomes among healthcare workers in New York City. Int J Hyg Environ Health; 222: 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos GL, Gimeno D, Arif AA et al. (2007) Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med; 175: 667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folletti I, Siracusa A, Paolocci G. (2017) Update on asthma and cleaning agents. Curr Opin Allergy Clin Immunol; 17: 90–5. [DOI] [PubMed] [Google Scholar]
- Friesen MC, Coble JB, Lu W et al. (2012) Combining a job-exposure matrix with exposure measurements to assess occupational exposure to benzene in a population cohort in Shanghai, China. Ann Occup Hyg; 56: 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster FM, Vernez D, Wild PP et al. (2014) Hazardous substances in frequently used professional cleaning products. Int J Occup Environ Health; 20: 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IF, Weinstein AL. (1986) Air pollution and asthma: effects of exposures to short-term sulfur dioxide peaks. Environ Res; 40: 332–45. [DOI] [PubMed] [Google Scholar]
- Hawley B, Casey M, Virji MA et al. (2017) Respiratory symptoms in hospital cleaning staff exposed to a product containing hydrogen peroxide, peracetic acid, and acetic acid. Ann Work Expo Health; 62: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heederik D (2003) Allergen exposure and occupational respiratory allergy and asthma In Nieuwenhuijsen BW, editor. Exposure assessment in occupational and environmental epidemiology. New York, NY: Oxford University Press. [Google Scholar]
- Heederik D (2014) Cleaning agents and disinfectants: moving from recognition to action and prevention. Clin Exp Allergy; 44: 472–4. [DOI] [PubMed] [Google Scholar]
- Houseman EA, Virji MA. (2017) A Bayesian approach for summarizing and modeling time-series exposure data with left censoring. Ann Work Expo Health; 61: 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DH, Bhatti P, Coble JB et al. (2014) Calibrating a population-based job-exposure matrix using inspection measurements to estimate historical occupational exposure to lead for a population-based cohort in Shanghai, China. J Expo Sci Environ Epidemiol; 24: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel D, Checkoway H, Pearce N. (2007) Exposure and dose modelling in occupational epidemiology. Occup Environ Med; 64: 492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout H, Vermeulen R. (2001) Application of job-exposure matrices in studies of the general population-some clues to their performance. European Respiratory Review; 11: 80–90. [Google Scholar]
- Kumagai S (2004) Peaks of inhalation exposure. Ann Occup Hyg; 48: 653–4 [DOI] [PubMed] [Google Scholar]
- Kurth L, Virji MA, Storey E et al. (2017) Current asthma and asthma-like symptoms among workers at a Veterans Administration Medical Center. Int J Hyg Environ Health; 220: 1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBouf RF, Coffey CC. (2015) Effect of interferents on the performance of direct-reading organic vapor monitors. J Air Waste Manag Assoc; 65: 261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBouf RF, Stefaniak AB, Virji MA. (2012) Validation of evacuated canisters for sampling volatile organic compounds in healthcare settings. J Environ Monit; 14: 977–83. [DOI] [PubMed] [Google Scholar]
- LeBouf RF, Virji MA, Ranpara A et al. (2017) Air and surface sampling method for assessing exposures to quaternary ammonium compounds using liquid chromatography tandem mass spectrometry. Ann Work Expo Health; 61: 724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBouf RF, Virji MA, Saito R et al. (2014) Exposure to volatile organic compounds in healthcare settings. Occup Environ Med; 71: 642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior Gerster F, Brenna Hopf N, Pierre Wild P et al. (2014) Airborne exposures to monoethanolamine, glycol ethers, and benzyl alcohol during professional cleaning: a pilot study. Ann Occup Hyg; 58: 846–59. [DOI] [PubMed] [Google Scholar]
- Nuzzo R. (2014) Scientific method: statistical errors. Nature; 506: 150–2. [DOI] [PubMed] [Google Scholar]
- Preller L, Burstyn I, De Pater N et al. (2004) Characteristics of peaks of inhalation exposure to organic solvents. Ann Occup Hyg; 48: 643–52. [DOI] [PubMed] [Google Scholar]
- Quinn MM, Henneberger PK, Braun B et al. ; National Institute for Occupational Safety and Health (NIOSH), National Occupational Research Agenda (NORA) Cleaning and Disinfecting in Healthcare Working Group. (2015) Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control; 43: 424–34. [DOI] [PubMed] [Google Scholar]
- Quinot C, Dumas O, Henneberger PK et al. (2017) Development of a job-task-exposure matrix to assess occupational exposure to disinfectants among US nurses. Occup Environ Med; 74: 130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirce S, Barranco P. (2010) Cleaning agents and asthma. J Investig Allergol Clin Immunol; 20: 542–50; quiz 2p following 550. [PubMed] [Google Scholar]
- Rae Systems Inc. (2013). The PID Handbook: Theory and Application of Direct-Reading Photoionization Detectors (PIDs). 3rd edn. San Jose, CA: Rae Systems. [Google Scholar]
- Saito R, Virji MA, Henneberger PK et al. (2015) Characterization of cleaning and disinfecting tasks and product use among hospital occupations. Am J Ind Med; 58: 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SE, Dick F, Cherrie JW; Geoparkinson Study Group. (2004) Exposure assessment for a population-based case-control study combining a job-exposure matrix with interview data. Scand J Work Environ Health; 30: 241–8. [DOI] [PubMed] [Google Scholar]
- Siracusa A, De Blay F, Folletti I et al. (2013) Asthma and exposure to cleaning products - a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy; 68: 1532–45. [DOI] [PubMed] [Google Scholar]
- Smith T. (2001) Studying peak exposure: toxicology and exposure statistics In X2001–Exposure assessment in epidemiology and practice. Stockholm, Sweden: National Institute for Working Life; pp. 207–9. [Google Scholar]
- Smith TJ, Kriebel D. (2008) Why peaks matter: linking asthma attacks to exposure distributions. Epidemiology; 19: S357–S57. [Google Scholar]
- Su FC, Friesen MC, Stefaniak AB et al. (2018) Exposures to volatile organic compounds among healthcare workers: modeling the effects of cleaning tasks and product use. Ann Work Expo Health; 62: 852–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke K, Chow Y, Brauer M et al. (2002) Exposures and their determinants in radiographic film processing. AIHA J (Fairfax, Va); 63: 11–21. [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA. (2016) The ASA’s statement on p-values: context, process, and purpose. American Statistician; 70: 129–33. [Google Scholar]
- Weber DJ, Sickbert-Bennett EE, Brown V et al. (2012) Completeness of surveillance data reported by the National Healthcare Safety Network: an analysis of healthcare-associated infections ascertained in a tertiary care hospital, 2010. Infect Control Hosp Epidemiol; 33: 94–6. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Schneider T, Kildesø J et al. (1998) Risk in cleaning: chemical and physical exposure. Sci Total Environ; 215: 135–56. [DOI] [PubMed] [Google Scholar]
- Zock JP, Vizcaya D, Le Moual N. (2010) Update on asthma and cleaners. Curr Opin Allergy Clin Immunol; 10: 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimlichman E, Henderson D, Tamir O et al. (2013) Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med; 173: 2039–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.