Abstract
Background
Ginkgo biloba extract (EGb761), a standard extract of the Chinese traditional medicine Ginkgo biloba, plays an anti-tumor role in various cancers. However, whether EGb761 is involved in the invasion and metastasis of gastric cancer remains unclear.
Material/Methods
In the current study, cell viability assay, Western blotting, wound-healing assay, Transwell invasion assay, and orthotopic transplantation model were performed to explore the effects of EGb761 on gastric cancer.
Results
In vitro, the results showed that EGb761 suppressed the proliferation of gastric cancer cells in a dose-dependent manner. Furthermore, the migration and invasiveness were weakened and the protein levels of p-ERK1/2, NF-κB P65, NF-κB p-P65, and MMP2 were decreased by EGb761 or U0126 (an inhibitor of ERK signaling pathway) exposure in gastric cancer cells. Moreover, the combined treatment with EGb761 and U0126 significantly inhibited ERK, NF-κB signaling pathway, and the expression of MMP2 than those of single drug. In vivo, EGb761 inhibited the tumor growth and hepatic metastasis of gastric cancer in the mouse model. Results of immunohistochemistry indicated that the expression of ERK1/2, NF-κB P65 and MMP2 were decreased by EGb761 in the tumor tissues.
Conclusions
EGb761 plays a vital role in the suppression of metastasis and ERK/NF-κB signaling pathway in gastric cancer.
MeSH Keywords: Extracellular Signal-Regulated MAP Kinases, Ginkgo Biloba, Neoplasm Invasiveness, Neoplasm Metastasis, NF-kappa B, Stomach Neoplasms
Background
Gastric cancer (GC) is a common malignancy, accounting for 10% of all cancer deaths among females and 7% among men worldwide [1]. Due to the absence of symptoms at early stages, most patients with GC are in the advanced stage when diagnosed. The mortality rate of advanced gastric cancer has not declined in the past decade due to lack of effective treatment methods [2,3]. At present, medication is still a major treatment for metastasis of advanced GC, but the efficacy is unsatisfied. Therefore, it is urgent to explore the potential mechanism involved in invasion and metastasis of GC and to find an effective GC therapy.
Ginkgo biloba extract (EGb761), a standard herbal extract, consists of 2 major ingredients with specific pharmacological profiles: 22–27% flavonoids (including quercetin, kaempferol and isorhamnetin) and 5.4–6.6% terpene trilactones (including ginkgolide and bilobalide) [4]. EGb761 has been used for the treatment of Alzheimer’s disease, cognitive disorders, and vascular diseases for many years [5]. Recent evidence suggests that EGb761 has potent anti-cancer effects by inducing apoptosis, reducing angiogenesis, and suppressing tumor growth [6,7]. Moreover, studies indicated that EGb761 inhibits invasiveness and metastasis of non-small cell lung cancer and colorectal cancer [8,9], but little is known about the influence of EGb761 on the invasion and migration of GC.
ERK signaling cascades have vital roles in regulating cell processes such as growth, differentiation, and apoptosis under physiological conditions as well as stress conditions [10]. Dysregulation of ERK participates in the progression of malignancy [11]. It is reported that aberrant activation of the ERK signaling pathway increases the synthesis and secretion of matrix metalloproteinases (MMPs) and sequentially degrades extracellular matrix (ECM), and promotes invasion and metastasis of carcinoma [12]. U0126, a specific inhibitor of the ERK signaling pathway, weakens the invasive ability of hepatocellular carcinoma cells via reducing the expressions of MMP2 and MMP9 [13]. The invasion and metastasis of breast cancer cells were inhibited after blockage of the ERK/NF-κB signaling axis by reversing epithelial-mesenchymal transition (EMT) and stem cell characteristics [14]. Our previous investigation also showed that ERK expression was associated with lymph node metastasis of GC, and EGb761 increased chemotherapy sensitivity of GC cells via inhibition of the KSRI/ERK signaling pathway [15]. Moreover, quercetin is an active ingredient of EGb761 that inhibits the invasion and metastasis of breast cancer through decreasing MMP9 expression mediated by the ERK/AP-1 signaling pathway [16]. Accordingly, we hypothesized that EGb761 restrains invasion and metastasis of GC cells via suppressing the ERK signaling pathway and regulating its downstream signals. To test this hypothesis, the present study assessed the effects of EGb761 on invasion and metastasis of GC and explored the underlying mechanism in vitro and in vivo.
Material and Methods
Cell lines and reagents
Human GC SGC-7901 and MGC-803 cell lines were obtained from the Chinese Academy of Sciences (Shanghai, China). EGb761 was purchased from Dr. Willmar Schwabe GmbH&Co/Co. (Karlsruhe, Baden-Württemberg, Germany). U0126 was from SelleckChem (Radnor, PA, USA). RPMI-1640 medium, fetal bovine serum (FBS), and 0.25% EDTA trypsin were all bought from Gibco (New York, NY, USA). Six-well plates, 24-well plates, and Matrigel were acquired from Corning (New York, NY, USA). Rabbit antibodies against ERK and p-ERK were obtained from Cell Signaling Technology (Boston, MA, USA), rabbit antibodies against MMP2 were gained from Abcam (Cambridge, UK), rabbit antibodies against NF-κB p-P65 were from Bioss (Beijing, China), and rabbit antibodies against NF-κB P65 and GAPDH were from Proteintech Group (Wuhan, China). The secondary antibody, horseradish peroxidase (HRP)-linked goat anti-rabbit IgG, was purchased from EarthOX (San Francisco, CA, USA). RIPA buffer and phosphate-buffered saline (PBS) were bought from LEAGENE (Beijing, China). Proteinase inhibitor (PMSF) and TBST were from Solarbio Biotech (Beijing, China). Phosphatase inhibitor was from Beyotime (Shanghai, China) and OB glue was from Baiyunshan (Guangzhou, China). The polyvinylidene fluoride (PVDF) membranes were purchased from Millipore (Billerica, MA, USA).
Drug interventions and cell culture
Cells were cultured in RPMI-1640 medium supplemented with 10% FBS and penicillin-streptomycin mixture (100 U/ml of penicillin G and 100 mg/ml of streptomycin) at 37°C in a humidified atmosphere of 5% CO2. Cells were pretreated with or without U0126 (10 μM) for 2 h. After washing with PBS, cells were further treated with EGb761 (400 μg/ml) for 24 h. Cells treated with the same volume of RPMI-1640 culture medium containing 10% FBS served as the control group.
Cell viability assay
Cell Counting Kit-8 (CCK8) assay was used to detect the effects of EGb761 on SGC-7901 and MGC-803 cells. Briefly, the cells (4×103 cells/well) in 10% FBS culture medium (100 μl) were seeded into each well of a 96-well plate and allowed to attach overnight. Then, cells were treated with different concentrations (0, 200, 400, or 600 μg/ml) of EGb761 for 24 h. CCK8 solution (10 μl) was added to each well and the plate was further incubated at 37°C for 2 h. A microplate reader was used to measure the optical density (OD) at 450 nm. Data were from at least 3 independent experiments.
Western blot analysis
Cells were lysed in RIPA buffer supplemented with 1× proteinase inhibitor and 1×phosphatase inhibitor for 30 min on ice. Cell lysates were centrifuged at 12 000 rpm for 15 min at 4°C. Protein quantification was performed with the BCA assay. Subsequently, equal amounts of proteins (30 μg) were separated by 10–15% SDS-PAGE gel and then transferred onto the PVDF membranes. After blocking in 1×TBST containing 5% non-fat milk at room temperature (RT) for 1 h, the membranes were incubated with primary antibodies including ERK (1: 1000), p-ERK (1: 1000), NF-κB p-P65 (1: 500), NF-κB P65 (1: 1000), and MMP2 (1: 1000) overnight at 4°C. The membranes were washed with 1×TBST 3 times and then incubated with the secondary antibody for 1 h at RT in a lucifugal environment. GAPDH (1: 500) was used as the loading control. The protein band detection was performed using the Odyssey CLx Infrared Imaging system (LI-COR Biosciences, Lincoln, NE, USA) according to manufacturer’s instructions.
Transwell invasion assay
Matrigel glue was dissolved overnight at 4°C and diluted with cold serum-free RPMI-1640 at a concentration of 1: 8. Then, 100 μl of the diluted Matrigel was poured onto the upper chamber and incubated overnight at 37°C. The density of cells in different groups was adjusted to 1×105 cells/1000 μl with serum-free culture medium. The upper chamber of 24-well Transwell chambers were supplemented with 100 μl cell suspension, and 600 μl RPMI-1640 culture medium containing 20% FBS was added to the lower chamber. The 24-well plate was incubated in a 5% CO2 humidified atmosphere at 37°C for 24 h. After removing the cells in the upper chamber by swabs, the chambers were fixed in 4% paraformaldehyde for 30 min, then stained by crystal violet for 30 min. After washing with PBS and drying, the chambers were placed under the microscope to count the invading cells in 5 random fields. The number of cells was counted to calculate the average number of invading cells per region.
Wound-healing assay
GC cells (1×106) were seeded in 6-well plates. After reaching 80% confluency, a 100-μl pipette tip was used to make a scratch. The detached cells were removed by PBS. Then, according to the groups mentioned above, serum-free RPMI-1640 culture medium and drugs were added into different wells to treat cells. The wound areas were examined by microscope at 0 h and 24 h. Healing rate (%)=[(A1–At)/A1]×100%. At and A1 are the wound areas on the detection day (24 h) and wounding day (0 h), respectively.
Animals experiments
All procedures involving animals were performed following protocols approved by our local Animal Care and Use Committee, in compliance with Guangxi Medical University Application for Ethics Approval for Research Involving Animals. SGC-7901 cells (1×107) were injected into the flank subcutaneous tissues of nude mice. Once the tumors grew to approximately 1 cm3, they were cut into 1-mm3 pieces, then were orthotopically implanted into the gastric serosa layer of 10 mice with medical OB glue. After the glue coagulated, the peritoneum was closed with a no. 5 absorbable suture. During surgical procedures, mice were anesthetized intraperitoneally with 0.5% pentobarbital sodium. A heater was used to accelerate the revival of mice. Ten mice were randomly divided into 2 groups: 40 mg/kg of EGb761 was intraperitoneally injected into the experimental mice twice a week, while the other 5 mice were inoculated with 0.9% saline as controls. Two different groups were observed at least 5 weeks before they were sacrificed. The tumors and livers were measured and fixed in formalin for further experiments.
Immunohistochemistry and HE staining
The tumor and liver tissues were embedded in paraffin. After being sectioned at 4 μm, the slides were baked, dewaxed, hydrated, and rinsed. The liver sections were stained with H&E for routine histological examinations and morphometric analysis. The tumor sections were put in citrate buffer (0.01 M, pH 6.0) for antigen retrieval, and the endogenous peroxidase activity was inactivated by 3% H2O2, followed by washing with PBS. After blocking in 5% normal goat serum at RT for 1 h, the slides were successively incubated with primary antibodies: ERK1/2 (1: 250), NF-κB P65 (1: 250), and MMP2 (1: 500) at 4°C overnight, then were incubated with horseradish peroxidase-conjugated secondary antibody. The procedure was performed according to the instructions of the SP Immunohistochemistry kit (Beijing, China). A negative control was obtained by using PBS rather than primary antibodies. The intensity of staining and the percentage of positive cells were observed in random fields under a microscope.
Statistical analysis
Data were analyzed using SPSS16.0. The measurement data are presented as mean ± SD. The difference among groups was compared with the t test or one-way ANOVA. A difference was considered to be statistically significant at p<0.05.
Results
Effects of EGb761 on the proliferation of GC cells
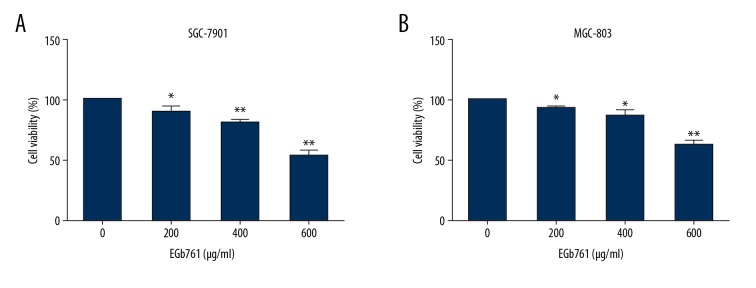
The SGC-7901 cell line was derived from moderately differentiated lymph node metastatic gastric adenocarcinoma, and the MGC-803 cell line was derived from poorly differentiated gastric mucinous adenocarcinoma [17]. To detect the effect of EGb761 on GC cells with various differentiation grades and origins, SGC-7901 and MGC-803 were selected as the target cell lines. To assess the effects of EGb761 on growth of GC cells, SGC-7901 and MGC-803 cells were treated with various concentrations (0, 200, 400, or 600 μg/ml) of EGb761 for 24 h. The results of CCK8 assay suggested that EGb761 treatment significantly decreased the viability of GC cells in a dose-dependent manner (Figure 1).
Figure 1.
EGb761 inhibited the proliferation of gastric cancer cells. SGC-7901 (A) and MGC-803 (B) were treated with different concentrations (0, 200, 400, or 600 μg/ml) of EGb761 for 24 h. Cell viability was detected by CCK8 assay. N=3, mean ±SD, * P<0.05 or ** P<0.01 vs. control group.
Effects of EGb761 on the invasion capability of GC cells
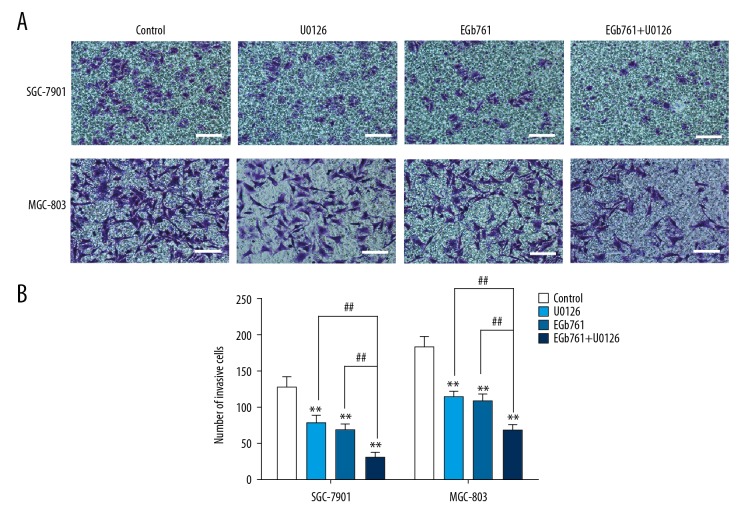
The number of cells penetrating the Matrigel in the EGb761 or U0126 group were decreased as compared with the control group. Moreover, the numbers of invading cells in the groups simultaneously exposed to 400 μg/ml EGb761 and 10 μM U0126 were obviously lower than in the group treated with EGb761 or U0126 separately (Figure 2).
Figure 2.
The inhibition of invasion with EGb761 and (or) U0126 treatments in GC SGC-7901 and MGC-803 cells. (A) Cells were pretreated with or without U0126 (10 μM) for 2 h and then treated with EGb761 (400 μg/ml) for 24 h. Representative images of Transwell invasion assay. (B) The number of invasive cells in different experimental groups. N=3, mean ±SD, ** P<0.01 vs. control group; ## P<0.01 vs. EGb761+U0126 group. Scale bar: 100 μm.
Effects of EGb761 on the migration capability of GC cells
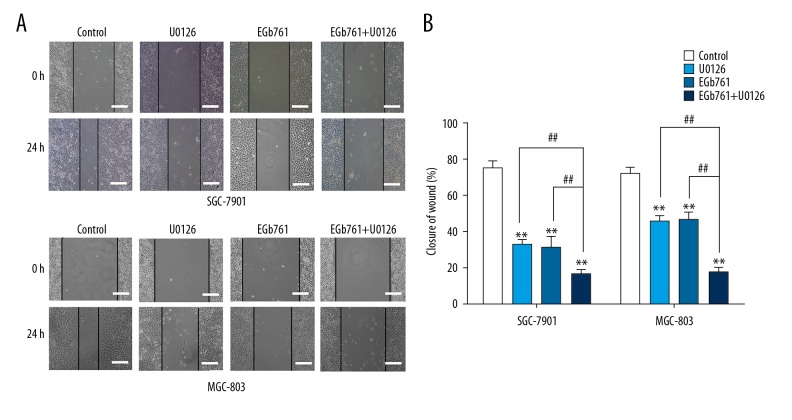
The wound-healing rates of the EGb761 or U0126 group were lower than in the control group, and the wound-healing rate was clearly lower when 400 μg/mL EGb761 and 10 μM U0126 were exposed together (Figure 3).
Figure 3.
The inhibition of invasion with EGb761 and (or) U0126 treatments in GC SGC-7901 and MGC-803 cells. (A) Cells were pretreated with or without U0126 (10 μM) for 2 h and then treated with EGb761 (400 μg/ml) for 24 h. Representative images of wound-healing assay. (B) The wound-healing rate in different experimental groups. N=3, mean ±SD, ** P<0.01 vs. control group; ## P<0.01 vs. EGb761+U0126 group. Scale bar: 200 μm.
Effects of EGb761 and (or) U0126 on the protein expression of ERK1/2, p-ERK1/2, NF-κB P65, NF-κB p-P65, and MMP2 in GC cells
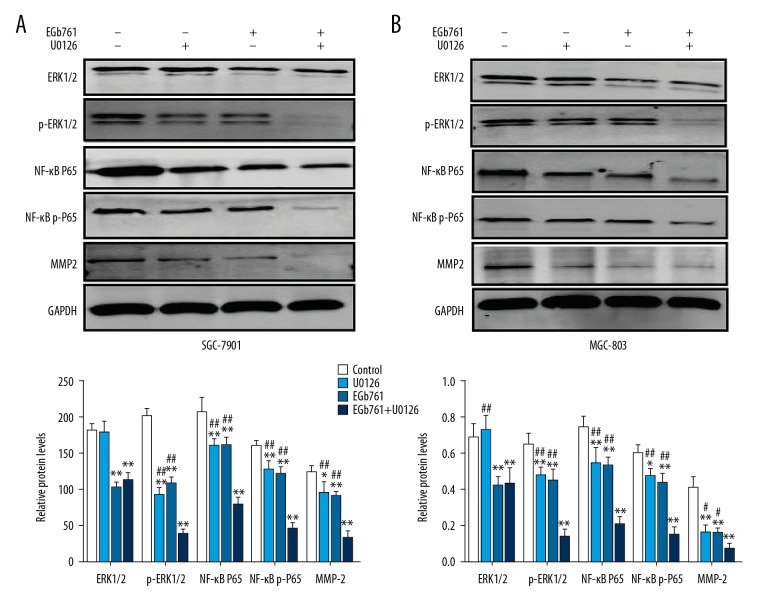
In comparison to the control group, the expression of ERK1/2, p-ERK1/2, NF-κB P65, NF-κB p-P65, and MMP2 were markedly decreased in the cells treated with 400 μg/ml EGb761 or 10 μM U0126. Moreover, combined treatment with 400 μg/ml EGb761 and 10 μM U0126 produced the most significant reduction in protein levels (Figure 4).
Figure 4.
Effects of EGb761 and (or) U0126 treatments on the protein levels of ERK1/2, p-ERK1/2, NF-κB p65, NF-κB p-p65, and MMP2 in GC SGC-7901 and MGC-803 cells. Cells were pretreated with or without U0126 (10 μM) for 2 h and then treated with EGb761 (400 μg/ml) for 24 h. (A) Representative images of Western blot assay and the quantification of the relative protein expression in SGC-7901 cells. (B) Representative images of Western blot assay and quantification of relative protein expression in MGC-803 cells. N=3, mean ±SD, * P<0.05 or ** P<0.01 vs. control group; ## P<0.01 vs. EGb761+U0126 group.
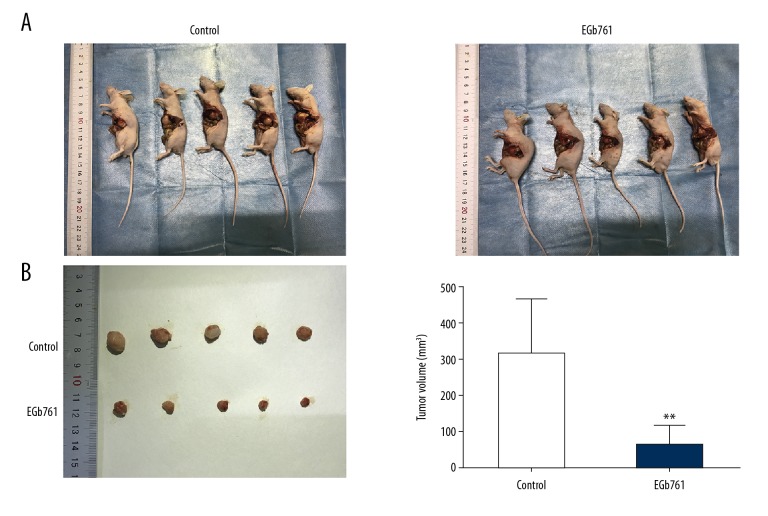
EGb761 restrained the growth and metastasis of orthotopic tumors in vivo
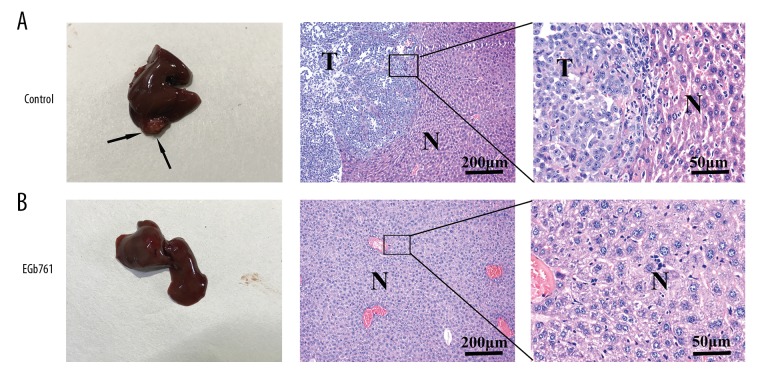
Orthotopic tumors in the EGb761 group were smaller than in the control group (Figure 5). The metastatic potential of GC SGC7901 cells was examined by HE staining of liver tissue, showing that 3 of 5 (60%) animals were confirmed to have hepatic metastases in the control group (not treated with EGb761), but no animals had hepatic metastases in the EGb761 group (Figure 6).
Figure 5.
EGb761 suppressed the growth of orthotopic tumors in an orthotopic transplantation model. Orthotopic transplantation mice were randomly divided into 2 groups for treatment – intraperitoneal injection of EGb761 (40 mg/kg) or saline (0.9%) – twice a week. (A) The orthotopic transplantation nude mice in EGb761 and control groups. (B) The size of orthotopic tumors in transplanted nude mice. ** P<0.01 vs. control group.
Figure 6.
EGb761 inhibited the hepatic metastases of orthotopic tumors in an orthotopic transplantation model. Orthotopic transplantation mice were randomly divided into 2 groups for treatment – intraperitoneal injection of EGb761 (40 mg/kg) or saline (0.9%) – twice a week. HE staining was used to evaluate liver histology in different groups. Hepatic metastases were found in the control group (A) but not in the EGb761 group (B). “T” means tumor. “N” means normal tissue. Representative images are presented.
EGb761 inhibited the expression of ERK1/2, NF-κB P65 and MMP2 in orthotopic tumors
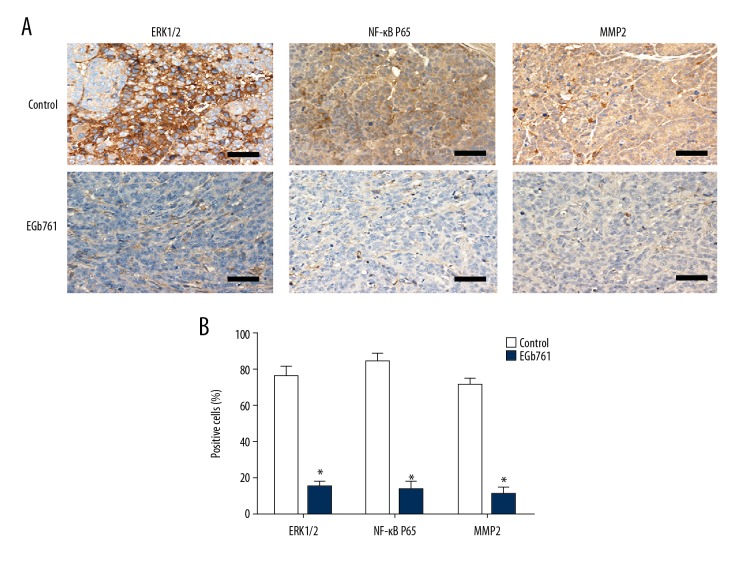
Immunohistochemistry staining results showed that in the orthotopic tumor tissues, the positive cell staining rates of ERK1/2, NF-κB P65, and MMP2 in the EGb761 group were significantly lower than in the control group (Figure 7).
Figure 7.
The expression levels of ERK1/2, NF-κB p65, and MMP2 in orthotopic tumors tissues. Orthotopic transplantation mice were randomly divided into 2 groups for treatment: intraperitoneal injection of EGb761 (40 mg/kg) or saline (0.9%) twice a week. Orthotopic tumors tissues were subjected to immunohistochemistry for ERK1/2, NF-κB p65, and MMP2. (A) The positive expression of ERK1/2, NF-κB p65, and MMP2 in the orthotopic tumors of control group and EGb761 group. Representative images are presented. (B) Positivity for ERK1/2, NF-κB p65, and MMP2 in cells in the EGb761 group and control group. N=5, mean ±SD, ** P<0.01 vs. control group. Scale bar: 50 μm.
Discussion
With the development of endoscopic technique, the diagnostic efficiency of early-stage GC has improved distinctly in recent years; however, the metastasis of advanced GC is still the main cause of cancer-related deaths [1]. Therefore, finding a way to inhibit tumor metastasis is key to treatment of advanced GC.
EGb761 has several bioeffects, including anti-inflammation, cardioprotection, neuroprotection, free radical scavenging, and anti-tumor effects, and the anti-tumor effect of EGb761 consisted of the suppression of cancer cells proliferation, induction of cell apoptosis, and inhibition of malignancy metastasis [5]. The present study confirmed that EGb761 not only suppressed the invasion and migration of GC cells in vitro, but also inhibited the growth of orthotopic tumors and hepatic metastases in an orthotopic transplantation model. Moderately differentiated and poorly differentiated GC cell lines were both inhibited by EGb761, which indicates that EGb761 plays effective anti-cancer roles in GC, and we explored the mechanism in subsequent experiments.
The ERK-related intracellular signal transduction pathway has abnormal overexpression in about half of all malignancies and participates in tumor progression [18]. Our investigation demonstrated that EGb761 inhibited activation of the ERK signaling pathway in a dose-dependent manner in GC (data not shown). A report indicated that the growth capability of capillaries and the migration of endothelial cells were suppressed by EGb761 via inhibiting the ERK signaling pathway [19]. Similarly, our previous study also demonstrated that EGb761 improved chemotherapy sensitivity of GC to cisplatin by inhibition of the ERK signaling pathway, and the ERK expression was associated with lymph node metastasis of GC [15]. Hence, EGb761 may suppress the invasion and migration of GC cells by inhibiting activation of the ERK signaling pathway.
MMPs, a type of endogenous proteolytic enzyme, participated in oncogenesis and metastasis by degradation of ECM [20,21]. Drugs that inhibit the expression of MMPs, such as MMP2 and MMP9, significantly suppress metastasis and have become an effective treatment of invasion and metastasis [22]. Investigations suggested that overexpression of TRIB1 promotes invasion and metastasis of colorectal cancer SW480 cells via increasing MMP2 expression mediated by the ERK signaling pathway, and inhibition of the ERK signaling pathway suppressed the expression of MMP2/9 and attenuated invasion and metastasis in colorectal cancer [23]. Similarly, the invasion and metastasis ability of lung cancer was inhibited via inhibition of the ERK/MMP2 signaling pathway [24]. In GC, the expression of MMP2 is an important prognostic indicator [25]. Invasion of GC AGS and MKN-45 cells was promoted by abnormally activating the ERK signaling pathway and increasing MMP2 expression, and U0126 interfered with the activation of ERK/MMPs mediated by cytarabine [26]. The present study further confirms that EGb761 suppresses the expressions of p-ERK1/2 and MMP2 in GC, which has an effect like U0126. Hence, inhibition of ERK/MMP2 is a possible mechanism of EGb761 activity in prevention of invasiveness.
Abnormal activation of the NF-κB signaling pathway plays a crucial role in the progression of malignancies [27]. Reports show that silencing the DEC2 gene by shRNA induced phosphorylation of ERK1/2 and NF-κB P65, enhancing the ability of invasion and metastasis in GC, while U0126 blocked stimulation of the ERK/NF-κB signaling pathway mediated by knockdown of DEC2 [28]. In the present study, we showed that U0126 suppressed the invasion and migration and decreased the expressions of p-ERK, NF-κB P65, and NF-κB p-P65 in GC cells. These results show that the ERK signaling pathway, upstream of the NF-κB signaling pathway, participates in the regulation of invasion and metastasis in malignancy, and U0126 may play an anti-cancer role via inhibiting ERK and its downstream signaling pathway. Furthermore, a report suggested that kaempferol, an active ingredient of EGb761, suppresses angiogenesis of tumors, regulated by the ERK/NF-κB signaling pathway [29]. Similarly, the present study shows that blockage of the ERK/NF-κB signaling pathway suppresses MMP2 expression by EGb761 in GC. There is evidence that the invasion and metastasis of GC are promoted by overexpression of MMP2, mediated by the NF-κB signaling pathway [30], and the MMP2 expression and the invasion ability were both suppressed by inhibiting of the NF-κB P65 signaling pathway by PDTC in tumor cells [31]. Therefore, MMP2 may be regulated via the ERK/NF-κB signaling pathway, which is involved in the metastasis of GC. Research has demonstrated that EGb761 could be used as an ancillary drug and could enhance the efficacy of cisplatin and 5-fluorouracil in anti-tumor therapy [15,32]. In the present study, we also found that EGb761 elevated the effect of U0126 in suppressing invasion and metastasis of GC. Combination treatment with EGb761 and U0126 blocked the ERK/NF-κB/MMP2 signaling pathway significantly more than use of drug alone. The results suggest that combined therapy with EGb761 and U0126, at least in part, exerts a synergistic anti-tumor effects via regulating the ERK/NF-κB/MMP2 signaling pathway.
Conclusions
Collectively, these data indicate that EGb761 inhibits invasion and metastasis of GC cells, at least partly by suppressing the ERK/NF-κB/MMP2 signaling cascade in vitro and in vivo. Therefore, EGb761 may serve as an effective anti-tumor treatment.
Abbreviations
- EGb761
Ginkgo biloba extract
- GC
gastric cancer
- RT
room temperature
- MMPs
matrix metalloproteinases
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (no. 81460380), the Natural Science Foundation of Guangxi, China (no. 2017GXNSFAA198019), the Natural Science Foundation of Guangxi, China (no. 2011GXNSFA018182) and the Project Foundation from the Health Department of Guangxi, China (no. GZKZ 10-107)
Conflict of interests
None.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka T, Yashiro M. Biomarkers of gastric cancer: Current topics and future perspective. World J Gastroenterol. 2018;24:2818–32. doi: 10.3748/wjg.v24.i26.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hua K, Li Y, Zhao Q, et al. Downregulation of Annexin A11 (ANXA11) inhibits cell proliferation, invasion, and migration via the AKT/GSK-3beta pathway in gastric cancer. Med Sci Monit. 2018;24:149–60. doi: 10.12659/MSM.905372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: Areview of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52:727–49. doi: 10.1007/s40262-013-0074-5. [DOI] [PubMed] [Google Scholar]
- 5.Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: Biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(3):211–44. doi: 10.1080/10590500701569414. [DOI] [PubMed] [Google Scholar]
- 6.Chen XH, Miao YX, Wang XJ, et al. Effects of Ginkgo biloba extract EGb761 on human colon adenocarcinoma cells. Cell Physiol Biochem. 2011;27:227–32. doi: 10.1159/000327948. [DOI] [PubMed] [Google Scholar]
- 7.Qian Y, Xia L, Shi W, et al. The effect of EGB on proliferation of gastric carcinoma SGC7901 cells. Clin Transl Oncol. 2016;18:521–26. doi: 10.1007/s12094-015-1399-3. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JR, Liu PL, Chen YH, et al. Ginkgo biloba extract decreases non-small cell lung cancer cell migration by downregulating metastasis-associated factor heat-shock protein 27. PLoS One. 2014;9:e91331. doi: 10.1371/journal.pone.0091331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Zhang J, Chai Z, et al. Ginkgo biloba extract EGb 761-induced upregulation of LincRNA-p21 inhibits colorectal cancer metastasis by associating with EZH2. Oncotarget. 2017;8:91614–27. doi: 10.18632/oncotarget.21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roskoski R., Jr ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res. 2012;66:105–43. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:11673–79. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akter H, Park M, Kwon OS, et al. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumour Biol. 2015;36:6053–62. doi: 10.1007/s13277-015-3282-9. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Zhao L, He W, et al. Benzo[a]pyrene promotes gastric cancer cell proliferation and metastasis likely through the Aryl hydrocarbon receptor and ERK-dependent induction of MMP9 and c-myc. Int J Oncol. 2016;49:2055–63. doi: 10.3892/ijo.2016.3674. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Zhang S, Ji Y, et al. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SQ, Xu CY, Qin MB, et al. Ginkgo biloba extract enhances chemotherapy sensitivity and reverses chemoresistance through suppression of the KSR1-mediated ERK1/2 pathway in gastric cancer cells. Oncol Rep. 2015;33:2871–82. doi: 10.3892/or.2015.3923. [DOI] [PubMed] [Google Scholar]
- 16.Han D, Wu G, Chang C, et al. Disulfiram inhibits TGF-beta-induced epithelial-mesenchymal transition and stem-like features in breast cancer via ERK/NF-kappaB/Snail pathway. Oncotarget. 2015;6:40907–19. doi: 10.18632/oncotarget.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma PF, Chen JQ, Liu JL, et al. Comparison of the cell cycle and the invasive ability between human gastric cancer cell lines SGC-7901 and MGC-803 in vitro. Journal of Minimally Invasive Medicine. 2012;7:218–20. [Google Scholar]
- 18.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 19.Koltermann A, Liebl J, Furst R, et al. Ginkgo biloba extract EGb 761 exerts anti-angiogenic effects via activation of tyrosine phosphatases. J Cell Mol Med. 2009;13:2122–30. doi: 10.1111/j.1582-4934.2008.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shay G, Lynch CC Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44–46:200–6. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wu N, Pang B, et al. TRIB1 promotes colorectal cancer cell migration and invasion through activation MMP-2 via FAK/Src and ERK pathways. Oncotarget. 2017;8:47931–42. doi: 10.18632/oncotarget.18201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu HC, Horng CT, Lee YL, et al. Cinnamomum cassia extracts suppress human lung cancer cells invasion by reducing u-PA/MMP expression through the FAK to ERK pathways. Int J Med Sci. 2018;15:115–23. doi: 10.7150/ijms.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo L, Chang HC, Leu TH, et al. Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J Cell Physiol. 2006;207:729–34. doi: 10.1002/jcp.20616. [DOI] [PubMed] [Google Scholar]
- 26.Yang GL, Tao HR, Wang HW, et al. Ara-C increases gastric cancer cell invasion by upregulating CD-147-MMP-2/MMP9 via the ERK signaling pathway. Oncol Rep. 2015;33:2045–51. doi: 10.3892/or.2015.3748. [DOI] [PubMed] [Google Scholar]
- 27.Huang S, Pettaway CA, Uehara H, et al. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Jia YF, Ma XL, et al. DEC2 suppresses tumor proliferation and metastasis by regulating ERK/NF-kappaB pathway in gastric cancer. Am J Cancer Res. 2016;6:1741–57. [PMC free article] [PubMed] [Google Scholar]
- 29.Luo H, Rankin GO, Juliano N, et al. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFkappaB-cMyc-p21 pathway. Food Chem. 2012;130:321–28. doi: 10.1016/j.foodchem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wu H, Wu X, et al. Interleukin 17A promotes gastric cancer invasiveness via NF-kappaB mediated matrix metalloproteinases 2 and 9 expression. PLoS One. 2014;9:e96678. doi: 10.1371/journal.pone.0096678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin M, Liu S, Li A, et al. NIK- and IKK beta-binding protein promotes colon cancer metastasis by activating the classical NF-kappaB pathway and MMPs. Tumour Biol. 2016;37:5979–90. doi: 10.1007/s13277-015-4433-8. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Zeng L. Ginkgo biloba extract 761 enhances 5-fluorouracil chemosensitivity in colorectal cancer cells through regulation of high mobility group-box 3 expression. Am J Transl Res. 2018;10:1773–83. [PMC free article] [PubMed] [Google Scholar]