Abstract
The Pacific region, also referred to as Oceania, is a geographically widespread region populated by people of diverse cultures and ethnicities. Indigenous people in the region (Melanesians, Polynesians, Micronesians, Papuans, and Indigenous Australians) are over-represented on national, regional, and global scales for the burden of infectious and non-communicable diseases. Although social and environmental factors such as poverty, education, and access to health-care are assumed to be major drivers of this disease burden, there is also developing evidence that genetic and microbiotic factors should also be considered. To date, studies investigating genetic and/or microbiotic links with vulnerabilities to infectious and non-communicable diseases have mostly focused on populations in Europe, Asia, and USA, with uncertain associations for other populations such as indigenous communities in Oceania. Recent developments in personalized medicine have shown that identifying ethnicity-linked genetic vulnerabilities can be important for medical management. Although our understanding of the impacts of the gut microbiome on health is still in the early stages, it is likely that equivalent vulnerabilities will also be identified through the interaction between gut microbiome composition and function with pathogens and the host immune system. As rapid economic, dietary, and cultural changes occur throughout Oceania it becomes increasingly important that further research is conducted within indigenous populations to address the double burden of high rates of infectious diseases and rapidly rising non-communicable diseases so that comprehensive development goals can be planned. In this article, we review the current knowledge on the impact of nutrition, genetics, and the gut microbiome on infectious diseases in indigenous people of the Pacific region.
Keywords: Pacific, Oceania, microbiome, nutrition, genetics, infectious disease, non-communicable disease
Introduction
The Pacific region is a loosely defined group of countries and territories that share a border with the Pacific Ocean. The region, sometimes referred to as Oceania, is diverse in its cultures, ethnicities, economic development, and living standards. Reflecting this diversity, health indicators vary considerably across the region, with life expectancy ~20 years higher and infant mortality up to 15 times lower in the best performing countries (Australia—life expectancy: 82.8; infant mortality: 4/1000 live births; and New Zealand—life expectancy: 81.6; infant mortality: 6/1000 live births) relative to the poorest performing countries (Papua New Guinea—life expectancy: 62.9; infant mortality: 57/1000 live births; and Solomon Islands—life expectancy: 69.2; infant mortality: 28/1000 live births) (1).
Although indigenous populations in the Pacific have developed complex stratagems and adapted highly effectively to their sometimes extreme environment, Australian Aboriginal & Torres Straits Islanders, Micronesian, Melanesian, and Polynesian populations are overrepresented among severe cases and deaths related to certain diseases, both communicable and non-communicable (2–5). A partial explanation of this over-representation is the challenges faced in many Pacific island countries in the delivery and uptake of: health services, improved water and sanitation, and education; problems faced in many low-income countries globally. However, factors specific to the region and its people, such as rapidly changing diets and the historic isolation of these ethnic groups, need also be considered.
Studies conducted on the relationships between host genetics, microbiome, and diseases are mainly carried out in Europe, Asia, or USA, and include very few or no indigenous participants from the Pacific despite the high burden of some diseases they face. Conclusions drawn from studies conducted in other geographical areas determine global trends, but have little specific application to Pacific populations (6–8), leading to a dearth of knowledge on how the microbiome may impact upon and/or interact with susceptibility to disease and immunomodulation.
Despite current limitations, numerous studies have investigated the ethnography and relatedness of the Pacific people. Data exists on burden of diseases in Pacific nations, and to a lesser extent in Pacific migrants living overseas. Using these data and drawing on primarily international literature on immunomodulation and the gut microbiome; we explored these key factors and their role on health and disease in Pacific people.
Historical Peopling of the Pacific
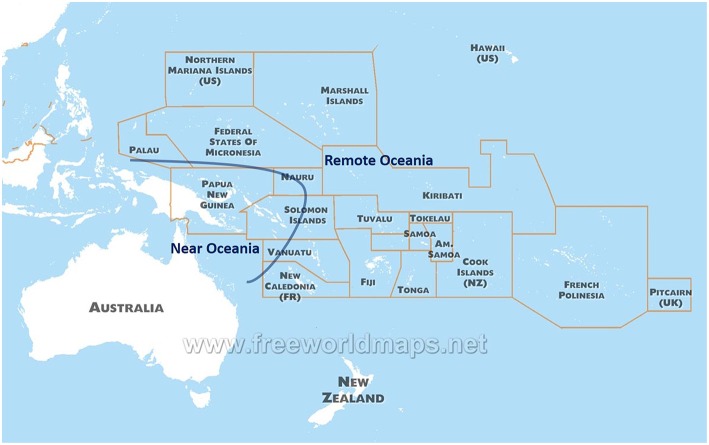
Indigenous populations in the Pacific arose from the last great human migration into an uninhabited continent, and differ genetically from other populations present across the Globe. The Pacific has two distinct regions in anthropology: Near Oceania (Australia, New Guinea Island, and Solomon Islands) and Remote Oceania (to the east of Solomon Islands, including up to Easter Island and Hawaii Islands) (9) (Figure 1). Archaeological evidence suggests human settlement in Near Oceania around 47,500–55,000 years before present (10). Recent sequence analysis of genomes from indigenous groups in the Pacific has revealed that ~3–6% of DNA from Melanesian and Indigenous Australian people derive from an ancient human lineage called the Denisovans (11, 12). This DNA was possibly introduced during the early migration to Near Oceania, and further illustrates the historic isolation and uniqueness of the Pacific people.
Figure 1.
A map of the countries and territories of Oceania.
Human settlement of Remote Oceania occurred only within the past 3,100 years: New Caledonia 1,200 BC, Tonga 900 BC, Samoa 800 BC, Hawaiian Islands 900 AD, and New Zealand 1,200 AD (13). The indigenous people of Remote Oceania probably originated from the East Asian region, in particular the Southern regions in China or Taiwan. This is supported by linguistic evidence as indigenous populations in Taiwan and Remote Oceania both speak Austronesian languages (14). Genomic studies also suggest that there was admixture between East Asian and Near Oceania ancestry. However, it is still debated where the admixture occurred: at the northern coastal region of the New Guinea Islands while East Asian ancestry moved toward Remote Oceania (9); or in Remote Oceania, where Near Oceania ancestry later arrived and mixed with East Asian ancestry (15).
Interestingly, in the context of this review and the impact of gut microbiome on health (below), supporting evidence of the two waves of migration in the region comes from phylogenetic study of Helicobacter pylori; a pathogen associated with stomach ulcers that was present in human populations during the human migration out of Africa and has been previously used to provide insights into human migrations (16, 17). Haplotyping of H. pylori in indigenous populations in the Pacific reflects the time since divergence, with the H. pylori hpSahul haplotype associated with indigenous people living in Near Oceania diverging from an Asian haplotype some 20,000 to 25,000 years before the H. pylori hspMaori haplotype isolated from indigenous people living in Remote Oceania diverged (18).
Both populations in Near Oceania and Remote Oceania are assumed to be historically isolated from other populations; as highlighted by over 800 language groups in Papua New Guinea (19), and also supported by H. pylori phylogenetics (18). The populations in Near Oceania rarely interbred with neighboring populations in the past. For example, the endogamy rate in the 1940-50s in both the Kombio-speaking group (Sepik, Papua New Guinea) and the Gidra-speaking group (Oriomo Plateau, Papua New Guinea) were >95% (20, 21). Indigenous populations in Remote Oceania were geographically isolated on islands that were separated by vast distances. However, genetic, linguistic, and archaeological evidence suggests that the Polynesian people of Remote Oceania maintained a complex trade network that extended from Hawaii in the north, New Zealand in the south, and Easter Island in the east (22, 23)—the so-called “Polynesian triangle.”
Demographic and Health Indicators in the Pacific
The Oceanian territories (land mass) in the South Pacific total about 8.5 million km2 (adapted from Pocket Statistical Summary: https://prism.spc.int/) over a total area of about 80 million km2 (estimated using GoogleEarth Pro: https://www.google.com/earth/). It is populated by ~41 million inhabitants, although approximately two-thirds of those people live in Australia and New Zealand, where European settlement accounts for the majority of the population and has had major cultural and socio-economic impacts. There are ~12.3 million inhabitants of the Pacific that have whole or partial indigenous Oceanian origins [details of the demographics of indigenous people in the Pacific are reviewed in (24)]; including the Indigenous Australian (Aboriginal and Torres Strait Islanders) and New Zealand Maori populations. This distinction between indigenous Oceanians and descendants of immigrant ancestries (arrived within the past 230 years) is useful in a public health context, because health indicators differ greatly, reflecting both the differing challenges and vulnerabilities faced by population subgroups, and access to healthcare and education.
The Pacific consists of many countries and territories that are geographically small and isolated, with comparatively small populations. This has consequences in terms of health delivery, but also on the local impact on global health priorities and the ability to attract international awareness and subsequent funding. To this end, there is some value in considering the total population of indigenous Oceanians, which is comparable in number to that of South Sudan, Rwanda, Somalia, or Chad. These countries are located at or near the 67th percentile of populations in countries/territories (25) and have health indicators that are broadly comparable to Papua New Guinea (the most populated Pacific country of primarily indigenous people), but appear to be a higher priority in global health.
Importantly, while the collective population of the Pacific is comparable to a moderately populated country, it is undergoing rapid growth. According to United Nations projections, the total population of Oceania (including Australia) will reach about 47 million in 2030 and 57 million in 2050 (25). Much of this growth is predicted to come from countries such as Papua New Guinea and other countries of predominantly indigenous populations, and will put further pressure on already challenged health systems.
A high Burden of Disease Among Indigenous Populations in the Pacific
Indigenous Oceanians face many of the health challenges identified in similar low-income settings, though cultural and biological constructs associated with health and disease differ. There is relatively abundant and well-documented data to suggest that certain health issues disproportionately affect Maoris in New Zealand, Polynesians in Hawaii and the Aboriginal & Torres Straits Islanders in Australia compared to their non-indigenous counterparts in the same countries (2–4, 26). The incidence of infectious diseases is strongly conditioned by social determinants of health (food and nutrition, housing, sanitation, prenatal stress, etc.), the less favorable of which are often concentrated among the most marginalized populations (5, 27–33). The respective roles of the social determinants of health (education, food, poverty, etc.), genetics and microbiota in the incidence of these diseases among indigenous populations in the Pacific, however, remain to be determined.
In the age of personalized medicine, genetics becomes an important consideration. The majority of known gene variations that distinguish one human group from another are not associated with diseases but display comparable functionality at the clinical level and only illustrate human genetic diversity (34). Identifying genetic vulnerabilities among populations can be very important for medical management (6, 35). For example, the identification of a P2X7R polymorphism has been associated with an increased risk of tuberculosis in mice (36) and later a Tibetan Chinese population (37). Further studies have suggested new therapeutic avenues through the use of natural agonists to P2X7R that promote death of Mycobacterium tuberculosis and apoptosis of infected monocytes and macrophages (38).
In addition to pre-existing immunity, vulnerability to a particular disease is partly due to a population not having been exposed for a long time to the pathogen that causes it. Specific vulnerabilities can arise from genetic characteristics selected for the advantage they confer in the face of another, more prevalent or severe infectious risk (39, 40). Certain pathologies linked to genetic characteristics may also be more frequent in some subgroups, mainly because of the emergence of recessive traits due to human reproduction in isolated settler populations; whether these are isolated from a geographical or environmental standpoint, or for cultural, linguistic or religious reasons. In the global context an example of such a disease is Tay-Sachs disease among Ashkenazi Jews, with the recessive carriage rate (1 in 30) approximately 10 times higher in Ashkenazi Jews compared to the general population in United States (39, 41).
Indigenous populations in the Pacific, however large and geographically scattered, could derive from a very small number of surviving individuals at some point in history (genetic bottlenecks), because groups were small and/or possibly faced with extreme conditions, including those encountered in central Australia, Papua New Guinea or during long transoceanic migrations (18, 42–44). This “perilous travels theory” is disputed by some (45, 46); but is difficult to discount as being a factor in at least some populations in the Pacific.
Global extrapolations supported by limited geographically specific data suggest that genetic selection pressures could, in part, explain the significantly higher burden of some diseases in indigenous populations in the Pacific. The interactions between genetics and burden/susceptibility for infectious and immune mediated diseases such as influenza and rheumatic fever, and non-infectious diseases such as obesity and type 2 diabetes, likely contribute to the health of Pacific people. Selected infectious and immune-mediated diseases are discussed below based on their disproportionate burden in indigenous populations in the Pacific and the emerging lines of research that may help to explain their impact in these populations.
Influenza
Indigenous populations in the Pacific suffered a disproportionately high mortality during the influenza pandemic of 1918–1919 (47–52). While it is estimated that 3% of the world's population died of influenza during the 1918 pandemic, mortality reached 22% in some Pacific nations such as Western Samoa (50, 51), and 50% in some Australian Aboriginal communities (53). The mortality rate in indigenous Hawaiians was also four-times higher than their non-indigenous counterparts (54). Although the factors that underlie these excess mortalities remain debated (55–59), recent evidence suggests that modern indigenous populations in Pacific Island countries (49, 60–71) are overrepresented 2- to 5-fold among the severe forms of influenza virus infection, such as during the 2009 influenza pandemic in New Caledonia (62, 68). Furthermore, between 2002 and 2014 (excluding 2009) the rate of reported influenza was up to 6.4 times higher among Australian Aboriginals compared to non-Aboriginals; the hospitalization rate for influenza was up to 3.5 times higher, and death rate up to 5.5 times higher (72). This is not only true for pandemic influenza: in 2016, the incidence of seasonal influenza in New Zealand was lower among Maori compared to “Europeans or others” (40 vs. 64 per 100,000). However, the incidence of hospital admissions for severe influenza was 2.5 times higher in the Maori population during that period (73).
The clearly documented vulnerability to severe influenza among indigenous populations in the Pacific may be due to the higher prevalence of identified comorbidities (e.g., diabetes, obesity) (62). It may also be due to lower population immunity or immunization coverage, a more adverse socio-economic context, differential access to care, or other factors. However, genetic and microbiotic factors have also been theorized to play a major role (40, 47, 74, 75). For instance, following the 2009 H1N1 influenza pandemic, researchers identified multiple genetic polymorphisms in diverse populations linked with an increased risk to the H1N1 pandemic virus (76–79).
Rheumatic Heart Disease
Acute Rheumatic Fever (ARF) is an immune syndrome that can occur following an infection with group A Streptococcus (GAS). The related aberrant inflammatory response can lead to permanent heart damage in about 60% of cases, resulting in the development of a chronic Rheumatic Heart Disease (RHD) (80). The occurrence of ARF and consequent RHD remain a major public health problem in vulnerable populations (81, 82). In 2015, it was estimated that RHD affected more than 30 million people worldwide each year with more than 300,000 related deaths (81).
RHD is mainly diagnosed in children and adolescents. It is seldom found in developed countries but remains common in emerging countries, particularly in Asia, Africa, and Oceania (80, 83–86), where nearly 84% of the global RHD cases are documented (87–91).
Indigenous populations in the Pacific present some of the highest rates of RHD in the world. In 1985, one study estimated RHD prevalence at 800 per 100,000 in Polynesia, while in New Zealand this risk was 650 per 100,000 among Maori (compared to 90 among non-Maori) (92). In Australian Aborigines in remote rural parts of Northern Territory the incidence was 651 per 100,000: at the time described as the highest incidence of RHD in the world (93). Rheumatic heart disease is also frequently described among Melanesian and Polynesian populations in New Caledonia (94–98).
Epidemiological studies have shown that socio-economic and environmental conditions, including access to healthcare systems, remain among the major determinants of ARF/RHD prevention (98–100). Moreover, several GAS strains identified as “rheumatogenic” were associated with ARF and differentially influence host immune response (101, 102). Genetic susceptibilities of the host should also be taken into account when the immune response to an infectious agent is being characterized. Recently, a meta-analysis of several published case-control studies showed a correlation between polymorphisms on several genes encoding for cytokines and predisposition for RHD (103–106). Studies in Europe have associated RHD to particular HLA (107–109), however, HLA results were inconsistent, and two recent genome-wide association studies (GWAS) conducted in Oceanian populations and European ancestry individuals showed no significance with the risk of developing RHD or ARF, respectively (110, 111). Interestingly, a novel susceptibility signal was identified in the immunoglobulin heavy chain (IGH) locus (110).
Dengue
Dengue is the most emergent vector-borne viral disease worldwide (112). The behavior of mosquitoes and the circulation of dengue viruses may be profoundly affected by global climate change, to which territories in the Pacific are particularly vulnerable.
Between 2001 and 2008, dengue epidemics in four Asian countries (Cambodia, Malaysia, Philippines, and Vietnam) caused >1 million reported dengue cases, of which nearly 5,000 (0.5%) died. During this same period, dengue epidemics described in six Pacific territories (Polynesia, New Caledonia, Cook Islands, American Samoa, Palau, and Federated States of Micronesia) caused ~50,000 cases, of which 34 (0.07%) died (113). Other available data also suggest a comparatively lower reported case-fatality rate for dengue in the Pacific (114–116), despite genetically similar viruses circulating in Asia and the Pacific (114).
The relatively lower frequency of deaths due to severe dengue in the Pacific with a high proportion of indigenous populations compared to Asian countries could be due to many biases, including: (i) undefined differences in the virulence of strains circulating in Asia and in the Pacific; (ii) underreporting of dengue cases in Asia and the overrepresentation of severe cases admitted to hospital; (iii) better health in general and better hospital care in particular in the Pacific countries in which these studies were conducted. Conversely, studies have correlated dengue severity with obesity and diabetes (117), both of which are highly prevalent in the Pacific and in indigenous populations especially (118–120). Whether the associations are causative or the result of confusion bias in lower socio-economic groups vulnerable to both dengue and obesity/diabetes remains to be determined.
Recent genome wide association and admixture mapping studies have investigated the natural protection and susceptibility for severe forms of dengue infection. Research teams have identified at least seven single nucleotide polymorphisms that are associated with the risk of severe outcomes during dengue infection (121–123). Although, more detailed understanding of the genetic mechanisms related to susceptibility of dengue infection is needed, associations based on ethnicity have been identified that reveal that people with African ancestry are best protected against severe dengue, while Asian and European populations are more susceptible (124, 125). To date, this has not been investigated in the Pacific to our knowledge.
Leptospirosis
Leptospirosis is a zoonotic disease that most frequently occurs following an environmental infection with pathogenic Spirochaetes from the genus Leptospira. The infection can range from asymptomatic in most cases (126) to severe life-threatening disease with a high case-fatality rate (127).
Despite the large spectrum of clinical forms, very little research has focused on the host genetic susceptibility to Leptospira infection. Following an outbreak in triathletes in Illinois, the first study on genetic susceptibility associated with leptospirosis evidenced the HLA-class II HLA-DQ6 as a significant risk factor (128). However, further studies in independent populations did not confirm this finding but rather identified HLA-class I and the ancestral HLA haplotype (A1, B8, DR3) as well as several SNPs in innate immune genes as conferring susceptibility to leptospirosis (129, 130). Globally, there is still conjecture, reflecting a lack of studies with a significant number of well-defined confirmed leptospirosis cases. Notably, none of the few studies reported to date have included people of Oceanian ancestry. Whether factors from skin or mucosal microbiota contribute to the initial phase of Leptospira infection remains to be determined but would deserve further studies (131).
Leptospirosis mostly impacts tropical rural or suburban young active males from vulnerable populations worldwide, imposing a global burden similar to that of schistosomiasis, leishmaniasis, or lymphatic filariasis (132). Of note, Pacific islanders pay the highest toll to this neglected disease by far, with a morbidity rate 10 times higher than global figures (132, 133). Although social and environmental determinants might contribute to this high burden, more research attention should be paid to population-specific genetic and microbiotic factors contributing to this high burden.
Indigenous Diets in the Pacific and the Transition to a Western Diet
Indigenous diets in Near Oceania are contrasted between the Papua New Guinea highlands and other regions. The Papua New Guinea highlands is believed to be one of the global origins of agriculture, with taro (Colocasia esculenta) and banana (Musa spp.) domesticated in the area 10,000 and 7,000 years before present, respectively (134). Banana and taro were the staple foods in the Papua New Guinea highlands before the introduction of sweet potato from Central America ~300 years ago. The intensified agricultural system has enabled continuous cultivation of sweet potato production, which has supported high population density in the region. Pigs pigs which are used as bride price or for compensation of deaths during tribal fighting (135), are reared by feeding sweet potatoes, but the nutritional contribution of pig meat is scarce. Studies in Papua New Guinea have reported that sweet potatoes accounted for over 70% of the total energy intake and that only a limited amount of animal protein was consumed (136).
In the non-Highlands regions of Papua New Guinea and Solomon Islands, shifting cultivation of tubers (taro, yam), banana, sugarcane, and leafy vegetables has been conducted. This type of agricultural system has been formulated on the basis of the crops and agricultural technologies introduced by the Austronesia people a few thousand years before, probably mixed with crops domesticated in the Papua New Guinea Highlands. In some parts of these regions, starch extracted from the stem of the sago palm, which is naturally fermented using traditional practices, is consumed as a staple food (137, 138). Since the sago starch contains only small amounts of nutrients other than carbohydrate, hunted animals, and gathered wild plants are also consumed. In coastal areas, aquatic animals such as fish and crustaceans are also commonly consumed.
The islands in remote Oceania can be mostly categorized into two groups depending on how they were formed (139). One group of islands were formed by volcanic activity (“volcano islands”), while the other group of islands were formed when a coral reef rose (“reef islands”). The volcano islands have relatively larger space, higher mountains, and richer fauna/flora than reef islands. This impacts on the abundance and availability of many crop foods.
Protein deficiency has been confirmed by a number of studies in the Pacific region (140). A recent study found that over 80% of the population in a remote region of the Papua New Guinea highlands had protein intakes below the estimated required level (141). Individuals whose protein intakes are below the required level are expected to show clinical signs of protein deficiency, such as decreased muscle mass (142). Yet despite their apparently deficient protein intake people in the Papua New Guinea highlands rarely exhibit such clinical signs (143). Some researchers have speculated that the Papua New Guinea highlands people have biologically adapted to a low-protein diet (144). Indeed, a recent study found evidence of nitrogen fixation in the gut microbiota of Papua New Guinean people (145). However, no evidence was found that nitrogen fixation substantially contributed to the host nitrogen balance.
During the period before colonization, the people who resided in Oceania were almost completely free from obesity (146). The people consumed a low-energy-density diet and engaged in relatively high physical activity for their subsistence, which resulted in a balance between energy intake and energy expenditure (147). Signs of a “nutritional transition” were first observed in Remote Oceania, then in the coastal and islands region of Near Oceania and finally in the Highlands region of Near Oceania (148). During the period of nutritional transition, the people came to consume energy-dense foods imported from Australia and New Zealand (e.g., rice, canned fish, canned meat, lamb/mutton flaps, vegetable oil, beef tallow, and instant noodles). The nutritional transition occurred more rapidly in the urban areas in each region.
According to the theory of epidemiological transition (149), people in subsistence societies before the initial stage of industrialization are faced with high burdens of communicable diseases. As food security, public health, and medicine improve due to industrialization, the burdens of communicable diseases are replaced with those of non-communicable diseases. During the period of epidemiological transition, mortality rates decrease drastically followed by fertility decline. The South Pacific countries have not shown typical patterns of epidemiological transition. Figure 2 shows a scatter plot between infant mortality rates in 2010 and age-adjusted prevalence of overweight adult females (>18 years of age) in 2016 for 13 countries in the South Pacific. A high infant mortality rate reflects the burdens of communicable diseases, while the prevalence of overweight individuals reflects the burdens of non-communicable diseases. As shown in Figure 2, several South Pacific countries such as Papua New Guinea, Kiribati, Micronesia, Nauru, and Tuvalu have high burdens of communicable and non-communicable diseases simultaneously. Such double burdens will provide considerable challenges to the health sectors of these countries.
Figure 2.

The double burden of infectious and non-communicable diseases in Oceania: a scatter plot between infant mortality rates in 2010 and age-adjusted prevalence of overweight adult females (>18 years of age) in 2016.
Oceanians are the most exposed, after the populations of sub-Saharan Africa, to the loss of disability-adjusted life years (DALYs) because of metabolic diseases. Indeed, Oceania residents (all genders and all populations) had the largest increase in BMI in the world for the period 1980–2008, the average BMI in Nauru being the highest in the world in 2008 (118). The link between obesity/inflammatory status (150, 151) and obesity/excess mortality (152, 153) is well-documented, both among Pacific populations and on other continents. After adjustment for age, the prevalence of type 2 diabetes mellitus (T2DM) in the South Pacific was the highest in the world (15–16%). This prevalence increased most strongly in the world between 1980 and 2008 (154), and it is expected that this prevalence will continue to increase in some areas of the Pacific (155). The prevalence of age-adjusted or unadjusted T2DM is highly variable among indigenous Oceanian populations, and can reach 20–30% of the population in some studies (156). By way of comparison, it was estimated in 2012 at about 5.5% of the population in France, and at least 9.4% in the US in 2017 (157, 158). In a New Zealand study, the prevalence of T2DM in Polynesian participants was 2–2.5 times that of participants of European origin (159). The incidence of T2DM was 4–8 times higher among Australian Aborigines than in the general population or compared to the non-indigenous population (160, 161). A review of T2DM in global aboriginal populations found varying prevalence of T2DM between different indigenous groups, which was hypothesized as at least partially due to genetic susceptibility (162). Indeed, a genetic polymorphism in the HNF1A gene (G319S mutation) has been identified in the native Canadian Oji-Cree population that was strongly associated with an increased risk of T2DM (163).
The human gut microbiome
Pacific Microbiome Relative to a Global Microbiome
Great advances in our understanding of the human microbiome have been made over the past two decades, though the field is arguably still in its infancy. Much of the focus of the human microbiome has been on intestinal microflora and its associated genes; with the current and broadly accepted theories stating that the gut microbiome has the potential to impact greatly on human health and disease. Indeed, most of the microbiome data reported to date are solely focused on bacterial species and their interactions with the human host. Further insights will be gained through elucidating the impact of commensal viruses, archaea, fungi, and protozoans on human health.
The composition of the gut microbiota evolves from birth, with the greatest change occurring at around the age of weaning. By the age of 2 years the gut microbiota of a child resembles that of an adult (164–166), though with some ongoing compositional differences to adult microbiota throughout adolescence (167). Once established, the adult gut microbiota is considered stable, yet subject to perturbation.
Limited studies exist on the gut microbiota/microbiome in the Pacific. The first culture independent study to characterize the gut microbiota in the region used targeted reverse transcription quantitative PCR (168). This approach is likely to be adequately accurate in determining relative numbers (169), but is considerably limited in scope of microbes detected. Nonetheless, findings were indicative of broad similarities between the microbial composition of the gut of PNG study participants and those in other low-income countries, particularly those with some traditional aspects of diet intact as found in a study into the microbiome of people from a rural African village in Burkina Faso (170, 171).
A subsequent study by Martinez et al. (172) used 16S RNA sequencing to investigate the gut microbiota of people in PNG (rural, traditional setting), and compared the composition to study participants in USA (urban Nebraska). This study supported the observation made previously by Greenhill et al. (168) that the microbial composition of people in Papua New Guinea had similarities to other subsistence dwellers (166, 170, 172). Specifically, the presence of a high ratio of Prevotella relative to Bacteriodes, which is common in people consuming traditional diets where there is a high dependence on fibrous, plant-derived foods (170) found in the Pacific (above).
Microbiome studies conducted in the Pacific, albeit only a small number to date, have helped inform theories of global diversity and distribution of bacteria and mobile genetic elements of the human digestive tract. Global microbiota studies consistently support the notion of greater diversity, or at the very least additional lineages, in populations living a traditional (non-western) lifestyle relative to people in industrialized countries (172, 173). Based on comparison of gut microbial composition of people in Papua New Guinea to people living in USA, Martinez and co-workers proposed that diversity could be explained by the impact of lifestyle on ecological assembly processes. In particular, aspects of traditional life such as diet and lack of sanitation and hygiene likely impact microbial composition.
In addition to the extant lineages associated with people living a traditional lifestyle (including those in the Pacific) and the resulting diversity of the microbiota, one study has investigated the distribution of mobile genes in a Pacific population and compared it to a Western population. In some aspects, the findings were similar to those for bacteria. Most mobile genes were present in some (62%) samples, akin to a core microbiota. Mobile genes for starch hydrolysis were higher in samples from agrarian Fijians than from USA participants, akin to a high Prevotella to Bacteriodes ratio. The presence of mobile genetic elements was said to be largely independent of species community composition (174).
To date, it has not been possible to elucidate the full composite mechanism that determines gut microbial composition. Ethnicity has some association with the gut microbiota, with Brooks et al. (175) suggesting that 12 taxa of bacteria reproducibly vary across four ethnicities. While conceivably a factor, heritability (thus ethnicity) appears to be a relatively minor influence on microbial composition (176). Indeed, immigration from a non-Westernized country to a Westernized country results in loss of diversity and associated function in the gut microbiome (177). Diet is undeniably an important factor, with people consuming a traditional diet consisting of starch-and fiber-rich foods having bacterial species and mobile genetic elements that reflect those dietary trends. Non-Westernized populations, including Pacific people, are likely to be a niche for extant species that are no longer detected in Western people (178).
Gut Microbiome, Genetics and Non-communicable Diseases
The prevalence of non-communicable diseases affecting affluent countries has prompted research efforts to focus on better understanding the immunomodulatory role of the microbiome (179, 180). Various studies have sought correlations between lost lineages and “Western diseases” such as obesity, autoimmune, and allergic diseases. However, this is likely an over-simplification of both the etiology of non-communicable diseases and the global burden of disease. Non-communicable diseases are an increasing burden in low-income countries, with heart disease, diabetes, and asthma among the leading 10 causes of all-age disability adjusted life years in the Pacific region (181).
One of the greatest health problems facing Pacific people is obesity, and interventions are urgently needed. The genetic selection of “thrifty genes” (182) has been theorized for indigenous people of the Pacific as a mechanism to cope with food shortages. Now, in the affluent sections of Pacific communities where food shortages are rare, this adaptation could contribute to obesity and type 2 diabetes. The thrifty gene mechanism has been explored in Pima Indians (from the Gila River Indian Community in south central Arizona), through a community-wide longitudinal study running since 1965, and found that the prevalence of T2DM decreases in those who report mixed ethnic ancestry (183–185). The thrifty gene theory remains disputed and seems to insufficiently explain the few data available on indigenous populations in the Pacific (45).
It may be that host genes play less of a role than the collective genes of the microbiome. The notion of an obese microbiome is well-established through both observational studies and transplant studies in animal models (186–189). Similarly, a role of microbiota in diabetes has been investigated, and characterization of the digestive metagenome predicts the presence of type 2 diabetes more robustly than body-mass index (BMI) in women in Sweden and China (190). Further research could help develop new and tailored approaches for post-prandial glycemic control (35, 191).
Immunomodulation of the Microbiome and Influence on Immune Health
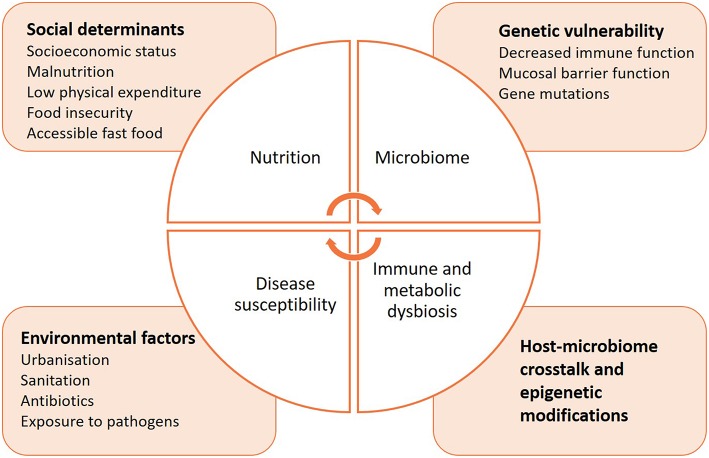
In comparison to non-communicable diseases, much less is known on how the microbiome may influence the course of communicable diseases, or increase susceptibility to infection (Figure 3). The delicate microbial ecosystem colonizing the human host fosters cooperative interactions with the immune system that is likely to influence how it responds to pathogenic assaults (192). Indeed, microbes synthesize short-chain fatty acids (SCFAs) and other metabolites, 95% of which are readily absorbed by the host (193–195). They can directly alter immunity, metabolism and reproductive functions, and even induce epigenetic modifications (196–201). Animal studies have facilitated the characterization of SCFAs, but the identification of specific bacterial species composing the human host responsible for similar activities has proven difficult (202). A possible reason is that the microbiome composition differs by geographic location due to differences in diet, lifestyle, and environmental exposures. Interestingly, a recent study highlighted the importance of considering the complex dynamics of the microbiota to understand how it affects immunity (166, 171, 203). Commensal viruses and fungi have a critical role in modulating bacterial metabolic pathways and therefore influence their release of specific SCFAs, which may provide a key element in profiling the microbiome in health and disease (204–207). In addition to categorizing the taxonomic diversity of the bacterial communities by sequencing to correlate a specific immune phenotype, the metabolomic signature of the microbiome should be determined, as it best determines the functional role of the microbial ecosystem and its influence on immunity.
Figure 3.
Intersections between nutrition, microbiome, immunity, and susceptibility to infectious diseases.
It is thought that the gut microbiota can have a negative impact on immune function, thus leading to increases in immune disorders. This led to the development of the hygiene hypothesis (208), which has evolved into more nuanced theories such as the old friends hypothesis and the microbiome depletion hypothesis (209, 210). From an ecological perspective it seems plausible that the loss of diversity, and in particular the loss of certain linages of microbes, could have negative implications for human health. This notion has the broad support of the World Allergy Organization, who point to the “mounting evidence” suggesting alterations in microbiota correlate with inflammatory disease (211).
It should also be noted that the original hygiene hypothesis was developed when we had a lesser appreciation of the importance of non-communicable diseases in low-income (“less-hygienic”) settings. Diseases such as asthma have likely been overlooked in low-income settings for decades, in part because the burden of asthma has been less than, for example, the burden of acute respiratory infections. Illustrating this point is that asthma was one of the top 10 contributors to death (for both children and all-ages) in Papua New Guinea in both 2007 and 2017 (http://www.healthdata.org/papua-new-guinea). While there was an apparent increase in the burden of asthma between 2007 and 2017, this probably reflects the decrease in burden of diarrhoeal disease during the same period. While not discounting the role of exposure and carriage of either commensal or pathogenic microbes in the risk of non-communicable diseases in the Pacific or elsewhere, further work is required to elucidate the merit and mechanisms of such hypotheses. As raised previously, such work should go beyond investigating the role of bacteria to include other microorganisms as well, a point also raised by World Allergy Organization (211). In brief, there is much to learn from furthering our understanding of interactions between the microbiota of Pacific people and immune diseases. Such studies should derive benefit for Pacific people, for which asthma and other immune diseases are a significant health problem; but may also inform our global understanding of these diseases.
Probiotics have long been recognized as potentially beneficial to human health, with a focus in recent decades on their role as immunomodulatiors (212). This has also lead to a focus on dietary components that support the growth of probiotics and related, perceived beneficial taxa. The role of indigenous fermented foods, generally a rich source of probiotics, varies among the different Pacific cultures; but in summary their traditional role appears less than in Asia and Europe. In lowland and coastal PNG sago starch undergoes traditional fermentation (137), and breadfruit fermentation has been documented in the South Pacific (213); however, other fermentations in the region are seldom documented. It is likely that the traditional diet (section Indigenous Diets in the Pacific and the Transition to a Western diet), which is rich in what are now referred to as prebiotics, may impact on “gut health” and immunomodulation; though no correlative or experimental studies have been conducted specifically relating to Pacific people.
Gut Microbiome Interaction With Gastrointestinal Pathogens
Much of the global focus of gut microbiome studies has been on their interactions with non-communicable diseases. However, given the ongoing high burden of infectious diseases in low-income settings the interactions between the gut microbiome and pathogens should not be overlooked. Soil-transmitted helminths (STH) infect 2 billion people, and remain endemic in the majority of developing countries (214, 215). The most predominant organisms are the roundworms Ascaris lumbricoides, hookworms such as Necator americanus and Ancylostoma duodenale, and whipworms Trichuris trichiura, which heavily impact human health, and children in particular, via impairing nutrition and suppressing host immunity (215–217). Where data are available it appears that the burden of STHs is high in Pacific countries (218–221). It is estimated that 5.5 million people are infected with hookworm and 1.2 million people are infected with Ascaris sp. in Oceania, comprising ~1% of the global burdens for both of these infections (222–224).
Gastrointestinal protozoan infections are likely prevalent in the Pacific, although data are scarce. A large-scale surveillance of gastrointestinal protozoa in school children in Fiji sought to detect gastrointestinal protozoa. When detection was by PCR, the prevalence of Giardia was 22% and Entamoeba histolytica was 2.3% (225). A small study in pregnant women in the highlands of Papua New Guinea revealed high burdens of E. histolytica (43%) and Giardia (39%) (221). E. histolytica has also been detected in patients with hepatic abscesses in New Caledonia (226). The lack of data may well misrepresent the true burden of protozoan diseases in the Pacific. These parasites are likely to have important direct health impacts on people of the region, and may interact with the gut microbiome and immunoregulation.
Parasites are largely reported for their immunomodulatory properties to evade the host's immune system, which may as a consequence impact on the host's ability to respond to other pathogens (227). Chronic STH infection have been linked to decreased vaccination efficacy and increased susceptibility to co-infection (228, 229), suggesting that helminths—microbiome interactions need to be taken into consideration when envisaging immune-related intervention strategies in endemic areas.
Although the causality between parasitic infection, the microbiome and immune fitness has yet to be fully determined, it may be that such infections have a positive impact on human health. Iatrogenic infection with N. americanus was shown to promote species richness and evenness, and increase the production of fecal SCFAs (191, 224, 230). Similarly, it has been hypothesized that gastrointestinal protozoa may have a positive impact on the gut microbiome (231).
A potential role of the gut microbiome in susceptibility and/or resistance to other gastrointestinal pathogens such as bacterial and viral organisms has been raised, but is yet to be fully elucidated. Basic ecological principles suggest that a healthy and well-established gut microbiota will impede colonization by transient gastrointestinal pathogens (232, 233). However, the impacts are not limited to just the microbiota, and the microbiome and associated metabolomics characteristics are likely important factors in the probability of colonization, as is the case with gastrointestinal parasites (above). Bacterial gastrointestinal pathogens appear to be a major contributor to diarrhea in PNG, with shigellosis being an ongoing problem (234–236), and cholera being a sporadic problem (237). The high asymptomatic carriage of bacterial and viral diarrhoeal pathogens (238) in Papua New Guinea may be indicative of low levels of sanitation and hygiene, but nonetheless warrants further investigation in relation to immunity and gut microbiome function.
Conclusions
The indigenous people of Oceania currently face a variety of health challenges including a high burden of infectious and non-communicable diseases. Both of these are likely to be exacerbated by the ongoing climate change crisis in the region. The Pacific islands are among the most vulnerable to the impacts of climate change and resulting sea-level rise and changing weather patterns (239). Due to the limited health and basic service capacities in many of these countries they will be faced with considerable challenges to rapidly adapt and manage these risks. Alongside social and environmental determinants of health—genetic and microbiotic factors need to be considered and further investigated to ensure improved health outcomes for people in the region. Indeed, the inclusion of indigenous people from that Pacific in studies investigating conditions where indigenous populations may exhibit increased vulnerability (e.g., influenza, obesity, T2DM), or conversely increased resistance (e.g., severe dengue), may be important for global populations.
Author Contributions
PH, AT, CG, MM, EK, MU, SN, and AG contributed to drafting the manuscript. The final manuscript was reviewed and approved by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SN is supported by a fellowship from the Woolworth Centre for Childhood Nutrition Research and the Children's Hospital Foundation #50197 and #WIS0092018. EK is supported by the Government of New Caledonia.
References
- 1.OECD/WHO Health at a Glance: Asia/Pacific 2016: Measuring Progress Towards Universal Health Coverage. Paris: OECD Publishing; (2016). [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1545–602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Mortality and Causes of Death Collaborators 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2015 Risk Factors Collaborators 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1659–724. 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. (2009) 374:76–85. 10.1016/S0140-6736(09)60827-8 [DOI] [PubMed] [Google Scholar]
- 6.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. (2003) 348:1170–75. 10.1056/NEJMsb025007 [DOI] [PubMed] [Google Scholar]
- 7.Langenberg C, Lotta LA. Genomic Insights into the Causes of Type 2 Diabetes. Lancet. (2018) 391:2463–74. 10.1016/S0140-6736(18)31132-2 [DOI] [PubMed] [Google Scholar]
- 8.DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium, Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium South Asian Type 2 Diabetes (SAT2D) Consortium, Mexican American Type 2 Diabetes (MAT2D) Consortium Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples (T2D-GENES) Consortium et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. (2014) 46:234–44. 10.1038/ng.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellwood P. First Farmers: the Origin Of Agricultural Societies. Oxford: Blackwell Publishing; (2005). [Google Scholar]
- 10.Nielsen R, Akey JM, Jakobsson M, Pritchard JK, Tishkoff S, Willerslev E. Tracing the peopling of the world through genomics. Nature. (2017) 541:302–10. 10.1038/nature21347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reich D, Green RE, Kircher M, Krause J, Patterson N, Durand EY, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. (2010) 468:1053–60. 10.1038/nature09710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malaspinas AS, Westaway MC, Muller C, Sousa VC, Lao O, Alves I, et al. A genomic history of aboriginal Australia. Nature. (2016) 538:207–14. 10.1038/nature18299 [DOI] [PubMed] [Google Scholar]
- 13.Bellwood P. Holocene population history in the Pacific region as a model for worldwide food producer dispersals. Curr Anthropol. (2011): 52(S4):S363–78. 10.1086/658181 [DOI] [Google Scholar]
- 14.Gray RD, Drummond AJ, Greenhill SJ. Language phylogenies reveal expansion pulses and pauses in pacific settlement. Science. (2009) 323:479–83. 10.1126/science.1166858 [DOI] [PubMed] [Google Scholar]
- 15.Spriggs M, Valentin F, Bedford S, Pinhasi R, Skoglund P, Reich D, et al. Revisiting ancient DNA insights into the human history of the Pacific Islands. Archaeol Ocean. (2019) 54:53–6. 10.1002/arco.5180 [DOI] [Google Scholar]
- 16.Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science. (2003) 299:1582–5. 10.1126/science.1080857 [DOI] [PubMed] [Google Scholar]
- 17.Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. (2007) 445:915–8. 10.1038/nature05562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moodley Y, Linz B, Yamaoka Y, Windsor HM, Breurec S, Wu JY, et al. The peopling of the Pacific from a bacterial perspective. Science. (2009) 323:527–30. 10.1126/science.1166083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landweer ML, Unseth P. An introduction to language use in Melanesia. Intl J Soc Lang. (2012) 214:1–3. 10.1515/ijsl-2012-0017 [DOI] [Google Scholar]
- 20.Umezaki M, Ohtsuka R. Microdemographic analysis for population structure from a closed to open system: a study in the Kombio, Papua New Guinea. Man Cult Oceania. (1996) 12:19–30. [Google Scholar]
- 21.Ohtsuka R. Low rate of population increase of the Gidra papuans in the past: a genealogical-demographic analysis. Am J Phys Anthropol. (1986) 71:13–23. 10.1002/ajpa.1330710103 [DOI] [PubMed] [Google Scholar]
- 22.Weisler MI, Bolhar R, Ma J, St. Pierre E, Sheppard P, Walter RK, et al. Cook Island artifact geochemistry demonstrates spatial and temporal extent of pre-European interarchipelago voyaging in East Polynesia. Proc Natl Acad Sci USA. (2016) 113:8150–5. 10.1073/pnas.1608130113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudjashov G, Endicott P, Post H, Nagle N, Ho SYW, Lawson DJ. Investigating the origins of eastern Polynesians using genome-wide data from the Leeward Society Isles. Sci Rep. (2018) 8:1823. 10.1038/s41598-018-20026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantola A, Horwood PF, Goarant C, Bertrand S, Merilles OEA, Pedron T, et al. Counting oceanians of Non-European, Non-Asian Descent (ONENA) in the South Pacific to make them count in global health. Trop Med Infect Dis. (2019) 4:114. 10.3390/tropicalmed4030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations . Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.241 (2015). [Google Scholar]
- 26.Anderson I, Crengle S, Leialoha Kamaka M, Chen T-H, Palafox N, Jackson-Pulver L. Indigenous health in Australia, New Zealand, and the Pacific. Lancet. (2006) 367:1775–85. 10.1016/S0140-6736(06)68773-4 [DOI] [PubMed] [Google Scholar]
- 27.Daniel M, Lekkas P, Cargo M. Environments and cardiometabolic diseases in aboriginal populations. Heart Lung Circ. (2010) 19:306–15. 10.1016/j.hlc.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 28.Foster HME, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JMR, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank Cohort. Lancet Public Health. (2018) 3:e576–85. 10.1016/S2468-2667(18)30200-7 [DOI] [PubMed] [Google Scholar]
- 29.Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. (2009) 374:65–75. 10.1016/S0140-6736(09)60914-4 [DOI] [PubMed] [Google Scholar]
- 30.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. (2011) 59:279–89. 10.1016/j.yhbeh.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 31.Stringhini S, Bovet P. Socioeconomic status and risk factors for non-communicable diseases in low-income and lower-middle-income countries. Lancet Glob Health. (2017) 5:e230–31. 10.1016/S2214-109X(17)30054-2 [DOI] [PubMed] [Google Scholar]
- 32.Bann D, Johnson W, Li L, Kuh D, Hardy R. Socioeconomic inequalities in body mass index across adulthood: coordinated analyses of individual participant data from three british birth cohort studies initiated in 1946, 1958 and 1970. PLoS Med. (2017) 14:e1002214. 10.1371/journal.pmed.1002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kivimäki M, Vahtera J, Tabák AG, Halonen JI, Vineis P, Pentti J, et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes from childhood to middle age in the young finns study: a cohort study. Lancet Public Health. (2018) 3:e365–73 10.1016/S2468-2667(18)30111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marian AJ. Causality in Genetics. Circ Res. (2014) 114:e18–21. 10.1161/CIRCRESAHA.114.302904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. (2015) 163:1079–94. 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 36.Santos AA, Rodrigues-Junior V, Zanin RF, Borges TJ, Bonorino C, Coutinho-Silva R, et al. Implication of Purinergic P2X7 receptor in M. Tuberculosis infection and host interaction mechanisms: a mouse model study. Immunobiology. (2013) 218:1104–12. 10.1016/j.imbio.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Guo W, Ren G, He X, Hu Q, Zhang Y. P2X7R Gene Polymorphisms are associated with increased risk of pulmonary tuberculosis in the tibetan chinese population. Am J Trop Med Hyg. (2016) 95:1016–20. 10.4269/ajtmh.16-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soares-Bezerra RJ, Pinho RT, Bisaggio Rda C, Benévolo-de-Andrade TC, Alves LA. The search for new agonists to P2X7R for clinical use: tuberculosis as a possible target. Cell Physiol Biochem. (2015) 37:409–18. 10.1159/000430364 [DOI] [PubMed] [Google Scholar]
- 39.Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. (2014) 15:379–93. 10.1038/nrg3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quach H, Rotival M, Pothlichet J, Loh YE, Dannemann M, Zidane N, et al. Genetic adaptation and neandertal admixture shaped the immune system of human populations. Cell. (2016) 167:643–56. 10.1016/j.cell.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott SA, Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for sixteen ashkenazi jewish genetic diseases. Hum Mutat. (2010) 31:1240–50. 10.1002/humu.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykes B, Leiboff A, Low-Beer J, Tetzner S, Richards M. The origins of the Polynesians: an interpretation from mitochondrial lineage analysis. Am J Hum Genet. (1995) 57:1463–75. [PMC free article] [PubMed] [Google Scholar]
- 43.Oppenheimer S. Out-of-Africa, the peopling of continents and islands: tracing uniparental gene trees across the map. Philos Trans R Soc Lond B Biol Sci. (2012) 367:770–84. 10.1098/rstb.2011.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum JK, Jorde LB, Schiefenhovel W. Affinities among melanesians, micronesians, and polynesians: a neutral biparental genetic perspective. Hum Biol. (2002) 74:413–30. 10.1353/hub.2002.0031 [DOI] [PubMed] [Google Scholar]
- 45.Gosling AL, Buckley HR, Matisoo-Smith E, Merriman TR. Pacific populations, metabolic disease and 'just-so stories': a critique of the 'thrifty genotype' hypothesis in Oceania. Ann Hum Genet. (2015) 79:470–80. 10.1111/ahg.12132 [DOI] [PubMed] [Google Scholar]
- 46.Irwin G. Pacific seascapes, canoe performance, and a review of lapita voyaging with regard to theories of migration. Asian Perspect. (2008) 47:12–27. 10.1353/asi.2008.0002 [DOI] [Google Scholar]
- 47.Tarantola A, Horwood P, Richard V, Quintana-Murci L. Host and viral genetic diversity can contribute to explain 1918 influenza mortality in the Pacific. Lancet Infect Dis. (2018) 18:833–4. 10.1016/S1473-3099(18)30408-0 [DOI] [PubMed] [Google Scholar]
- 48.Mamelund SE. Geography may explain adult mortality from the 1918–20 influenza pandemic. Epidemics. (2011) 3:46–60. 10.1016/j.epidem.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 49.James SP. The General Statistics of Influenza in Australasia and Parts of Africa and Asia. Report on the pandemic of influenza, 1918–19. Reports on public health and medical subjects 4. London: Ministry of Health; (1920). p. 349–86. Available online at: http://influenza.sph.unimelb.edu.au/MOH_TOC.php [Google Scholar]
- 50.Shanks GD, Wilson N, Kippen R, Brundage JF. The unusually diverse mortality patterns in the Pacific region during the 1918–21 influenza pandemic: reflections at the pandemic's centenary. Lancet Infect Dis. (2018) 3099:30178–6. [DOI] [PubMed] [Google Scholar]
- 51.Shanks GD, Hussell T, Brundage JF. Epidemiological isolation causing variable mortality in Island populations during the 1918–1920 influenza pandemic. Influenza Other Respir Viruses. (2012) 6:417–23. 10.1111/j.1750-2659.2011.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. (2002) 76:105–15. 10.1353/bhm.2002.0022 [DOI] [PubMed] [Google Scholar]
- 53.Cleland Burton J. Disease among the Australian Aborigines. J Trop Med Hyg. (1928) 6:65. [Google Scholar]
- 54.Schmitt RC, Nordyke EC. Influenza deaths in Hawai'i, 1918–1920. Hawaii J Hist. (1999) 33:101–17. [Google Scholar]
- 55.Mayoral JM, Alonso J, Garín O, Herrador Z, Astray J, Baricot M, et al. Social factors related to the clinical severity of influenza cases in Spain during the A (H1N1) 2009 virus pandemic. BMC Public Health. (2013) 13:118. 10.1186/1471-2458-13-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nyland GA, McKenzie BC, Myles PR, Semple MG, Lim WS, Openshaw PJ, et al. Effect of ethnicity on care pathway and outcomes in patients hospitalized with influenza A(H1N1)pdm09 in the UK. Epidemiol Infect. (2015) 143:1129–38. 10.1017/S0950268814001873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tricco AC, Lillie E, Soobiah C, Perrier L, Straus SE. Impact of H1N1 on socially disadvantaged populations: systematic review. PLoS ONE. (2012) 7:e39437 10.1371/journal.pone.0039437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hadler JL, Yousey-Hindes K, Pérez A, Anderson EJ, Bargsten M, Bohm SR, et al. Influenza-related hospitalizations and poverty levels - United States, 2010–2012. MMWR Morb Mortal Wkly Rep. (2016) 65:101–5. 10.15585/mmwr.mm6505a1 [DOI] [PubMed] [Google Scholar]
- 59.Chandrasekhar R, Sloan C, Mitchel E, Ndi D, Alden N, Thomas A, et al. Social determinants of influenza hospitalization in the United States. Influenza Other Respir Viruses. (2017) 11:479–88. 10.1111/irv.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clemens EB, Grant EJ, Wang Z, Gras S, Tipping P, Rossjohn J, et al. Towards identification of immune and genetic correlates of severe influenza disease in indigenous Australians. Immunol Cell Biol. (2017) 95:648. 10.1038/icb.2017.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bandaranayake D, Huang QS, Bissielo A, Wood T, Mackereth G, Baker MG, et al. Risk factors and immunity in a nationally representative population following the 2009 influenza A(H1N1) pandemic. PLoS ONE. (2010) 5:e13211. 10.1371/journal.pone.0013211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Ruche G, Tarantola A, Barboza P, Vaillant L, Gueguen J, Gastellu-Etchegorry M, et al. The 2009 pandemic H1N1 influenza and indigenous populations of the Americas and the Pacific. Euro Surveill. (2009) 14:19366. 10.2807/ese.14.42.19366-en [DOI] [PubMed] [Google Scholar]
- 63.Trauer JM, Laurie KL, McDonnell J, Kelso A, Markey PG. Differential effects of pandemic (H1N1) 2009 on remote and indigenous groups, Northern Territory, Australia, 2009. Emerg Infect Dis. (2011) 17:1615–23. 10.3201/eid1709.101196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson N, Barnard LT, Summers JA, Shanks GD, Baker MG. Differential mortality rates by ethnicity in 3 influenza pandemics over a century, New Zealand. Emerg Infect Dis. (2012) 18:71–7. 10.3201/eid1801.110035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khieu TQ, Pierse N, Telfar-Barnard LF, Huang QS, Baker MG. Estimating the contribution of influenza to hospitalisations in New Zealand from 1994 to 2008. Vaccine. (2015) 33:4087–92. 10.1016/j.vaccine.2015.06.080 [DOI] [PubMed] [Google Scholar]
- 66.Chang MH, Moonesinghe R, Athar HM, Truman BI. Trends in disparity by sex and race/ethnicity for the leading causes of death in the United States-1999–2010. J Public Health Manag Pract. (2016) 22(Suppl. 1):S13–24. 10.1097/PHH.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 67.Wenger JD, Castrodale LJ, Bruden DL, Keck JW, Zulz T, Bruce MG, et al. Pandemic influenza A H1N1 in Alaska: temporal and geographic characteristics of spread and increased risk of hospitalization among Alaska Native and Asian/Pacific Islander people. Clin Infect Dis. (2011) 52(Suppl. 1):S189–97. 10.1093/cid/ciq037 [DOI] [PubMed] [Google Scholar]
- 68.Epidemiological Task Group for Overseas French Territories of the Pacific Influenza A(H1N1)2009 in the French Pacific territories: assessment of the epidemic wave during the austral winter. Clin Microbiol Infect. (2010) 16:304–8. 10.1111/j.1469-0691.2010.03172.x [DOI] [PubMed] [Google Scholar]
- 69.Verrall A, Norton K, Rooker S, Dee S, Olsen L, Tan CE, et al. Hospitalizations for pandemic (H1N1) 2009 among Maori and Pacific Islanders, New Zealand. Emerg Infect Dis. (2010) 16:100–2. 10.3201/eid1601.090994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker MG, Wilson N, Huang QS, Paine S, Lopez L, Bandaranayake D, et al. Pandemic influenza A(H1N1)v in New Zealand: the experience from April to August 2009. Euro Surveill. (2009) 14:19319. 10.2807/ese.14.34.19319-en [DOI] [PubMed] [Google Scholar]
- 71.Naidu L, Chiu C, Habig A, Lowbridge C, Jayasinghe S, Wang H, et al. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia 2006–2010. Commun Dis Intell Q Rep. (2013) 37(Suppl.):S1–95. [PubMed] [Google Scholar]
- 72.Li-Kim-Moy J. Influenza Disease Burden in Australia. Presented at: Influenza Specialist Group Annual Scientific Meeting, Melbourne: Available online at: https://immunisationcoalition.org.au/wp-content/uploads/2017/02/8-Jean-Li-Kim-Moy.pdf (accessed February 5, 2017). [Google Scholar]
- 73.Lopez L, Wood T, Prasad N, Huang S. Influenza Surveillance in New Zealand 2016. Institute of Environmental Science and Research Ltd (ESR) (2017). Available online at: https://surv.esr.cri.nz/PDF_surveillance/Virology/FluAnnRpt/InfluenzaAnn2016.pdf [Google Scholar]
- 74.Chea N, Yi SD, Rith S, Seng H, Ieng V, Penh C, et al. Two clustered cases of confirmed influenza A(H5N1) virus infection, Cambodia, 2011. Euro Surveill. (2014) 19:20839. 10.2807/1560-7917.ES2014.19.25.20839 [DOI] [PubMed] [Google Scholar]
- 75.Keynan Y, Malik S, Fowke KR. The role of polymorphisms in host immune genes in determining the severity of respiratory illness caused by pandemic H1N1 influenza. Public Health Genomics. (2013) 16:9–16. 10.1159/000345937 [DOI] [PubMed] [Google Scholar]
- 76.La D, Czarnecki C, El-Gabalawy H, Kumar A, Meyers AF, Bastien N, et al. Enrichment of variations in KIR3DL1/S1 and KIR2DL2/L3 among H1N1/09 ICU patients: an exploratory study. PLoS ONE. (2011) 6:e29200. 10.1371/journal.pone.0029200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keynan Y, Juno J, Meyers A, Ball TB, Kumar A, Rubinstein E, et al. Chemokine receptor 5Δ32 allele in patients with severe pandemic (H1N1) 2009. Emerg Infect Dis. (2010) 16:1621–2. 10.3201/eid1610.100108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zúñiga J, Buendía-Roldán I, Zhao Y, Jiménez L, Torres D, Romo J, et al. Genetic variants associated with severe pneumonia in A/H1N1 influenza infection. Eur Respir J. (2012) 39:604–10. 10.1183/09031936.00020611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J, To KK, Dong H, Cheng ZS, Lau CC, Poon VK, et al. A functional variation in CD55 increases the severity of 2009 pandemic H1N1 influenza A virus infection. J Infect Dis. (2012) 206:495–503. 10.1093/infdis/jis378 [DOI] [PubMed] [Google Scholar]
- 80.Steer AC, Carapetis JR. Acute rheumatic fever and rheumatic heart disease in indigenous populations. Pediatr Clin North Am. (2009) 56:1401–19. 10.1016/j.pcl.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 81.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, Regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. (2017) 377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 82.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. (2005) 5:685–94. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 83.Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res. (2013) 137:643–58. [PMC free article] [PubMed] [Google Scholar]
- 84.Carapetis JR. Rheumatic heart disease in Asia. Circulation. (2008) 118:2748–53. 10.1161/CIRCULATIONAHA.108.774307 [DOI] [PubMed] [Google Scholar]
- 85.Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol. (2013) 368:1–27. 10.1007/82_2012_280 [DOI] [PubMed] [Google Scholar]
- 86.Reeves BM, Kado J, Brook M. High prevalence of rheumatic heart disease in Fiji detected by echocardiography screening. J Paediatr Child Health. (2011) 47:473–8. 10.1111/j.1440-1754.2010.01997.x [DOI] [PubMed] [Google Scholar]
- 87.Parks T, Kado J, Miller AE, Ward B, Heenan R, Colquhoun SM, et al. Rheumatic heart disease-attributable mortality at ages 5–69 years in fiji: a five-year, national, population-based record-linkage cohort study. PLoS Negl Trop Dis. (2015) 9:e0004033. 10.1371/journal.pntd.0004033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weinberg J, Beaton A, Aliku T, Lwabi P, Sable C. Prevalence of rheumatic heart disease in African school-aged population: extrapolation from echocardiography screening using the 2012 World Heart Federation Guidelines. Int J Cardiol. (2016) 202:238–9. 10.1016/j.ijcard.2015.08.128 [DOI] [PubMed] [Google Scholar]
- 89.Abouzeid M, Katzenellenbogen J, Wyber R, Watkins D, Johnson TD, Carapetis J. Rheumatic heart disease across the Western Pacific: not just a Pacific Island problem. Heart Asia. (2017) 9:e010948. 10.1136/heartasia-2017-010948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abul-Fadl AMAM, Mourad MM, Ghamrawy A, Sarhan AE. Trends in deaths from rheumatic heart disease in the eastern mediterranean region: burden and challenges. J Cardiovasc Dev Dis. (2018) 5:E32. 10.3390/jcdd5020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. (2018) 12:e0006335. 10.1371/journal.pntd.0006335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.WHO Expert Consultation on Rheumatic Fever and Rheumatic Heart Disease Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation. Geneva: WHO Technical Report Series; (2001). p. 923. [Google Scholar]
- 93.Carapetis JR, Wolff DR, Currie BJ. Acute rheumatic fever and rheumatic heart disease in the top end of Australia's Northern Territory. Med J Aust. (1996) 164:146–9. [DOI] [PubMed] [Google Scholar]
- 94.Mirabel M, Tafflet M, Noël B, Parks T, Braunstein C, Rouchon B, et al. Prevalence of rheumatic heart disease in the pacific: from subclinical to symptomatic heart valve disease. J Am Coll Cardiol. (2016) 67:1500–2. 10.1016/j.jacc.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 95.Mirabel M, Bacquelin R, Tafflet M, Robillard C, Huon B, Corsenac P, et al. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. (2015) 8:e002324. 10.1161/CIRCIMAGING.114.002324 [DOI] [PubMed] [Google Scholar]
- 96.Mirabel M, Fauchier T, Bacquelin R, Tafflet M, Germain A, Robillard C, et al. Echocardiography screening to detect rheumatic heart disease: a cohort study of schoolchildren in French Pacific Islands. Int J Cardiol. (2015) 188:89–95. 10.1016/j.ijcard.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 97.Mirabel M, Tafflet M, Noël B, Parks T, Axler O, Robert J, et al. Newly diagnosed rheumatic heart disease among indigenous populations in the Pacific. Heart. (2015) 101:1901–6. 10.1136/heartjnl-2015-308237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baroux N, Rouchon B, Huon B, Germain A, Meunier JM, D'Ortenzio E. High prevalence of rheumatic heart disease in schoolchildren detected by echocardiography screening in New Caledonia. J Paediatr Child Health. (2013) 49:109–14. 10.1111/jpc.12087 [DOI] [PubMed] [Google Scholar]
- 99.Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis. (2018) 12:e0006577. 10.1371/journal.pntd.0006577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fink DL, Chaiter Y, Menahem S, Farkash R, Machluf Y. Valvular heart disease in a young israeli ethiopian immigrant population from the gondar region with implications for rheumatic heart disease. Front Public Health. (2018) 6:130. 10.3389/fpubh.2018.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Esposito S, Bianchini S, Fastiggi M, Fumagalli M, Andreozzi L, Rigante D. Geoepidemiological hints about Streptococcus pyogenes strains in relationship with acute rheumatic fever. Autoimmun Rev. (2015) 14:616–21. 10.1016/j.autrev.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 102.Dinis M, Plainvert C, Kovarik P, Longo M, Fouet A, Poyart C. The innate immune response elicited by Group A Streptococcus is highly variable among clinical isolates and correlates with the emm type. PLoS ONE. (2014) 9:e101464. 10.1371/journal.pone.0101464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guilherme L, Köhler KF, Postol E, Kalil J. Genes, autoimmunity and pathogenesis of rheumatic heart disease. Ann Pediatr Cardiol. (2011) 4:13–21. 10.4103/0974-2069.79617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azevedo PM, Merriman TR, Topless RK, Wilson NJ, Crengle S, Lennon DR. Association study involving polymorphisms in IL-6, IL-1RA, and CTLA4 genes and rheumatic heart disease in New Zealand population of Māori and Pacific ancestry. Cytokine. (2016) 85:201–6. 10.1016/j.cyto.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 105.Gupta U, Mir SS, Garg N, Agarwal SK, Pande S, Mittal B. Association study of inflammatory genes with rheumatic heart disease in North Indian population: a multi-analytical approach. Immunol Lett. (2016) 174:53–62. 10.1016/j.imlet.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 106.Bhatt M, Kumar S, Siddiqui MH, Garg N, Mittal B. Influence of cytokine gene polymorphism on the risk of rheumatic heart disease - A meta-analysis. Immunol Lett. (2018) 194:69–78. 10.1016/j.imlet.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 107.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. (2011) 3:67–84. 10.2147/CLEP.S12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol. (2007) 66:199–207. 10.1111/j.1365-3083.2007.01974.x [DOI] [PubMed] [Google Scholar]
- 109.Mocumbi AO. Rheumatic heart disease in Africa: is there a role for genetic studies? Cardiovasc J Afr. (2015) 26:S21–6. 10.5830/CVJA-2015-037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parks T, Mirabel MM, Kado J, Auckland K, Nowak J, Rautanen A, et al. Association between a common immunoglobulin heavy chain allele and rheumatic heart disease risk in Oceania. Nat Commun. (2017) 8:14946. 10.1038/ncomms14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. (2004) 430:242–9. 10.1038/nature02759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian C, Hromatka BS, Kiefer AK, Eriksson N, Noble SM, Tung JY, et al. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun. (2017) 8:599. 10.1038/s41467-017-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.World Health Organization Special Programme for Research and Training in Tropical Diseases. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control; Geneva: (2009). [Google Scholar]
- 114.Dupont-Rouzeyrol M, Aubry M, O'Connor O, Roche C, Gourinat AC, Guigon A, et al. Epidemiological and molecular features of dengue virus type-1 in New Caledonia, South Pacific, 2001–2013. Virol J. (2014) 11:61. 10.1186/1743-422X-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kiedrzynski T, Souarès Y, Stewart T. Dengue in the Pacific: an updated story. Pacific Health Dialog. (1998) 5:129–36. [Google Scholar]
- 116.Chungue E, Deparis X, Murgue B. Dengue in French Polynesia: major features, surveillance, molecular epidemiology and current situation. Pacific Health Dialog. (1998) 5:154–62. [Google Scholar]
- 117.Zulkipli MS, Dahlui M, Jamil N, Peramalah D, Wai HVC, Bulgiba A, et al. The association between obesity and dengue severity among pediatric patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2018) 12:e0006263. 10.1371/journal.pntd.0006263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. (2011) 377:557–67. 10.1016/S0140-6736(10)62037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parry J. Pacific islanders pay heavy price for abandoning traditional diet. Bull World Health Organ. (2010) 88:484–5. 10.2471/BLT.10.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sierra B, Triska P, Soares P, Garcia G, Perez AB, Aguirre E, et al. OSBPL10, RXRA and lipid metabolism confer African-ancestry protection against dengue haemorrhagic fever in admixed Cubans. PLoS Pathog. (2017) 13:e1006220. 10.1371/journal.ppat.1006220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oliveira M, Saraiva DP, Cavadas B, Fernandes V, Pedro N, Casademont I, et al. Population genetics-informed meta-analysis in seven genes associated with risk to dengue fever disease. Infect Genet Evol. (2018) 62:60–72. 10.1016/j.meegid.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 123.Oliveira M, Lert-Itthiporn W, Cavadas B, Fernandes V, Chuansumrit A, Anunciação O, et al. Joint ancestry and association test indicate two distinct pathogenic pathways involved in classical dengue fever and dengue shock syndrome. PLoS Negl Trop Dis. (2018) 12:e0006202. 10.1371/journal.pntd.0006202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xavier-Carvalho C, Cardoso CC, de Souza Kehdy F, Pacheco AG, Moraes MO. Host genetics and dengue fever. Infect Genet Evol. (2017) 56:99–110. 10.1016/j.meegid.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 125.Oliveira M, Ferreira J, Fernandes V, Sakuntabhai A, Pereira L. Host ancestry and dengue fever: from mapping of candidate genes to prediction of worldwide genetic risk. Future Virol. 13:647–55. 10.2217/fvl-2018-0073 [DOI] [Google Scholar]
- 126.Felzemburgh RD, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AX, et al. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the leptospira agent. PLoS Negl Trop Dis. (2014) 8:e2927. 10.1371/journal.pntd.0002927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. (2009) 7:736–47. 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lingappa J, Kuffner T, Tappero J, Whitworth W, Mize A, Kaiser R, et al. HLA-DQ6 and ingestion of contaminated water: possible gene-environment interaction in an outbreak of Leptospirosis. Genes Immun. (2004) 5:197–202. 10.1038/sj.gene.6364058 [DOI] [PubMed] [Google Scholar]
- 129.Fialho RN, Martins L, Pinheiro JP, Bettencourt BF, Couto AR, Santos MR, et al. Role of human leukocyte antigen, killer-cell immunoglobulin-like receptors, and cytokine gene polymorphisms in leptospirosis. Hum Immunol. (2009) 70:915–20. 10.1016/j.humimm.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 130.Esteves LM, Bulhoes SM, Branco CC, Mota FM, Paiva C, Cabral R, et al. Human Leptospirosis: seroreactivity and genetic susceptibility in the population of sao miguel Island (Azores, Portugal). PLoS ONE. (2014) 9:e108534. 10.1371/journal.pone.0108534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vinod Kumar K, Lall C, Raj RV, Vijayachari P. Coaggregation and biofilm formation of Leptospira with Staphylococcus aureus. Microbiol Immunol. (2019) 63:147–50. 10.1111/1348-0421.12679 [DOI] [PubMed] [Google Scholar]
- 132.Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, et al. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. (2015) 9:e0004122. 10.1371/journal.pntd.0004122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. (2015) 9:e0003898. 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bourke RM. History of agriculture in Papua New Guinea. In: Bourke RM, Harwood T. editors. Food and Agriculture in Papua New Guinea. Canberra, ACT: ANU E Press; The Australian National University; (2009). p. 10–26. 10.22459/FAPNG.08.2009 [DOI] [Google Scholar]
- 135.Feil DK. The Evolution of Highlands Papua New Guinea Societies. Cambridge: Cambridge University Press; (1987). [Google Scholar]
- 136.Bouke RM. Sweet potato (Ipomoea batatas) production and research in Papua New Guinea. Papua New Guinea J Agric Forestr Fish. (1985) 33:89–108. [Google Scholar]
- 137.Greenhill AR, Shipton WA, Blaney BJ, Brock IJ, Kupz A, Warner JM. Spontaneous fermentation of traditional sago starch in Papua New Guinea. Food Microbiol. (2009) 26:136–41. 10.1016/j.fm.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 138.Shipton WA, Baker A, Blaney BJ, Horwood PF, Warner JM, Pelowa D, Greenhill AR. Nitrogen fixation associated with sago (Metroxylon sagu) and some implications. Lett Appl Microbiol. (2011) 52:56–61. 10.1111/j.1472-765X.2010.02967.x [DOI] [PubMed] [Google Scholar]
- 139.Nunn PD, Kumar L, Eliot I, McLean RF. Classifying pacific islands. Geosci Lett. (2016) 3:7 10.1186/s40562-016-0041-8 [DOI] [Google Scholar]
- 140.Norgan NG, Ferroluz A, Durnin JVG. Energy and nutrient intake and energy expenditure of 204 New Guineas adults. Philos. Trans. Royal Soc. (1974) 268:309–48. 10.1098/rstb.1974.0033 [DOI] [PubMed] [Google Scholar]
- 141.Morita A, Natsuhara K, Tomitsuka E, Odani S, Baba J, Tadokoro K, et al. Development, validation, and use of a semi-quantitative food frequency questionnaire for assessing protein intake in Papua New Guinean Highlanders. Am J Hum Biol. (2015) 27:349–57. 10.1002/ajhb.22647 [DOI] [PubMed] [Google Scholar]
- 142.Bistrian BR. Recent advances in parenteral and enteral nutrition: a personal perspective. J Parenter Enteral Nutr. (1990) 14:329–34. 10.1177/0148607190014004329 [DOI] [PubMed] [Google Scholar]
- 143.Itoh S, Sugawa-Katayama Y, Koishi H, Izumi S. Serum concentration of protein, triglyceride, β-lipoproteins and cholesterol in Papua New Guinean highlanders. J Nutr Sci Vitaminol. (1982) 28:411–7. 10.3177/jnsv.28.411 [DOI] [PubMed] [Google Scholar]
- 144.Koishi H. Nutritional adaptation of Papua New guinea highlanders. Eur J Clin Nutr. (1990) 44:851–911. [PubMed] [Google Scholar]
- 145.Igai K, Itakura M, Nishijima S, Tsurumaru H, Suda W, Tsutaya T, et al. Nitrogen fixation and nifH diversity in human gut microbiota. Sci Rep. (2016) 6:31942. 10.1038/srep31942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dennett G, Connell J. Acculturation and health in the Highlands of Papua New Guinea: dissent on diversity, diets, and development. Curr Anthropol. (1988) 29:273–99. 10.1086/203631 [DOI] [PubMed] [Google Scholar]
- 147.Ohtsuka R, Ulijaszek SJ. Health change in the asia-pacific region. Cambridge: Cambridge University Press; (2007). [Google Scholar]
- 148.Hodge AM, Dowse GK, Zimmet PZ, Collins VR. Prevalence and secular trends in obesity in Pacific and Indian Ocean island populations. Obes Res. (1995) 3:77–87. 10.1002/j.1550-8528.1995.tb00450.x [DOI] [PubMed] [Google Scholar]
- 149.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Q. (2005) 83:731–57. 10.1111/j.1468-0009.2005.00398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. (2012) 97:E1677–85. 10.1210/jc.2012-1532 [DOI] [PubMed] [Google Scholar]
- 151.Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. (2017) 8:1745. 10.3389/fimmu.2017.01745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monteverde M, Noronha K, Palloni A, Novak B. Obesity and excess mortality among the elderly in the United States and Mexico. Demography. (2010) 47:79–96. 10.1353/dem.0.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. (2004) 32:998–1003. 10.1097/01.CCM.0000119422.93413.08 [DOI] [PubMed] [Google Scholar]
- 154.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. (2011) 378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 155.Lin S, Tukana I, Linhart C, Morrell S, Taylor R, Vatucawaqa P, et al. Diabetes and obesity trends in Fiji over 30 years. J Diabetes. (2016) 8:533–43. 10.1111/1753-0407.12326 [DOI] [PubMed] [Google Scholar]
- 156.Cockram CS. Diabetes mellitus: perspective from the Asia-Pacific region. Diabetes Res Clin Pract. (2000) 50:S3–7. 10.1016/S0168-8227(00)00202-3 [DOI] [PubMed] [Google Scholar]
- 157.Eschwege E, Basdevant A, Crine A, Moisan C, Charles MA. Type 2 diabetes mellitus in France in 2012: results from the ObEpi survey. Diabetes Metab. (2015) 41:55–61. 10.1016/j.diabet.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 158.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States. Available online at: https://www.cdc.gov/media/releases/2017/p0718-diabetes-report.html
- 159.Sundborn G, Metcalf P, Scragg R, Schaaf D, Dyall L, Gentles D, et al. Ethnic differences in the prevalence of new and known diabetes mellitus, impaired glucose tolerance, and impaired fasting glucose. Diabetes Heart and Health Survey (DHAH) 2002–2003, Auckland New Zealand. N Z Med J. (2007) 120:U2607. [PubMed] [Google Scholar]
- 160.Wang Z, Hoy WE, Si D. Incidence of type 2 diabetes in Aboriginal Australians: an 11-year prospective cohort study. BMC Public Health. (2010) 10:487. 10.1186/1471-2458-10-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barr ELM, Magliano DJ, Zimmet P, Polkinghorne KR, Atkins RC, Dunstan DW, et al. The Australian Diabetes Obesity and Lifestyle Study. Melbourne, VIC: The International Diabetes Institute; (2006). [Google Scholar]
- 162.Yu CH, Zinman B. Type 2 diabetes and impaired glucose tolerance in aboriginal populations: a global perspective. Diabetes Res Clin Pract. (2007) 78:159–70. 10.1016/j.diabres.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 163.Hegele RA, Zinman B, Hanley AJ, Harris SB, Barrett PH, Cao H. Genes, environment and Oji-Cree type 2 diabetes. Clin Biochem. (2003) 36:163–70. 10.1016/S0009-9120(03)00004-3 [DOI] [PubMed] [Google Scholar]
- 164.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA. (2010) 107:18933–8. 10.1073/pnas.1007028107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. (2015) 21:1228–34. 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. (2012) 486:222–7. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. (2011) 77:404–12. 10.1111/j.1574-6941.2011.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Greenhill AR, Tsuji H, Ogata K, Natsuhara K, Morita A, Soli K, et al. Characterization of the gut microbiota of Papua New Guineans using reverse transcription quantitative PCR. PLoS ONE. (2015) 10:e0117427. 10.1371/journal.pone.0117427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. (2009) 11:2574–84. 10.1111/j.1462-2920.2009.01982.x [DOI] [PubMed] [Google Scholar]
- 170.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. (2010) 107:14691–6. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren AM, Siba PM, Greenhill AR, et al. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep. (2015) 11:527–38. 10.1016/j.celrep.2015.03.049 [DOI] [PubMed] [Google Scholar]
- 173.Smits SA, Leach J, Sonnenburg ED, Gonzalez CG, Lichtman JS, Reid G, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. (2017) 357:802–6. 10.1126/science.aan4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brito IL, Yilmaz S, Huang K, Xu L, Jupiter SD, Jenkins AP, et al. Mobile genes in the human microbiome are structured from global to individual scales. Nature. (2016) 535:435–9. 10.1038/nature18927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. (2018) 16:e2006842. 10.1371/journal.pbio.2006842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martínez I, Maldonado-Gomez MX, Gomes-Neto JC, Kittana H, Ding H, Schmaltz R, et al. Experimental evaluation of the importance of colonization history in early-life gut microbiota assembly. Elife. (2018) 7:e36521. 10.7554/eLife.36521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, et al. US immigration westernizes the human gut microbiome. Cell. (2018) 175:962–72. 10.1016/j.cell.2018.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. (2019) 176:649–62. 10.1016/j.cell.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. (2012) 13:260–70. 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. (2007) 449:804–10. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1260–344. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. (1962) 14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 183.Schulz LO, Chaudhari LS. High-risk populations: the pimas of Arizona and Mexico. Curr Obes Rep. (2015) 4:92–8. 10.1007/s13679-014-0132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baier LJ, Muller YL, Remedi MS, Traurig M, Piaggi P, Wiessner G, et al. ABCC8 R1420H loss-of-function variant in a Southwest American Indian Community: association with increased birth weight and doubled risk of type 2 diabetes. Diabetes. (2015) 64:4322–32. 10.2337/db15-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Williams RC, Long JC, Hanson RL, Sievers ML, Knowler WC. Individual estimates of European genetic admixture associated with lower body-mass index, plasma glucose, and prevalence of type 2 diabetes in Pima Indians. Am J Hum Genet. (2000) 66:527–38. 10.1086/302773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. (2013) 341:1241214. 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. (2009) 457:480–4. 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. (2013) 5:178ra41. 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143:913–6. 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 190.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. (2013) 498:99–103. 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 191.Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. (2016) 280:339–49. 10.1111/joim.12508 [DOI] [PubMed] [Google Scholar]
- 192.Kho ZY, Lal SK. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. (2018) 9:1835. 10.3389/fmicb.2018.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. (2019) 51:600–5. 10.1038/s41588-019-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. (2009) 106:3698–703. 10.1073/pnas.0812874106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. (2011) 108:4680–7. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. (2011) 11:22. 10.1186/1471-230X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. (2004) 101:15718–23. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blottière HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. (2003) 62:101–6. 10.1079/PNS2002215 [DOI] [PubMed] [Google Scholar]
- 200.Chen T, Kim CY, Kaur A, Lamothe L, Shaikh M, Keshavarzian A, et al. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. (2017) 8:1166–73. 10.1039/C6FO01532H [DOI] [PubMed] [Google Scholar]
- 201.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. (2012) 143:1006–16. 10.1053/j.gastro.2012.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. (2014) 14:311. 10.1186/s12866-014-0311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arrieta MC, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol. (2018) 142:424–34. 10.1016/j.jaci.2017.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. (2014) 14:405–16. 10.1038/nri3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 19:865–73. 10.1016/j.chom.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. (2016) 7:185. 10.3389/fmicb.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strachan DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259–60. 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. (2004) 25:237–55. 10.1007/s00281-003-0148-9 [DOI] [PubMed] [Google Scholar]
- 210.Noverr MC, Huffnagle GB. The ‘microflora hypothesis' of allergic diseases. Clin Exp Allergy. (2005) 35:1511–20. 10.1111/j.1365-2222.2005.02379.x [DOI] [PubMed] [Google Scholar]
- 211.Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. (2013) 6:3. 10.1186/1939-4551-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. (2000) 130:403S−9S. 10.1093/jn/130.2.403S [DOI] [PubMed] [Google Scholar]
- 213.Steinkraus KH. Classification of fermented foods: worldwide review of household fermentation techniques. Food Control. (1997) 8:311–7. 10.1016/S0956-7135(97)00050-9 [DOI] [Google Scholar]
- 214.Weinstock JV. Do we need worms to promote immune health? Clin Rev Allergy Immunol. (2015) 49:227–31. 10.1007/s12016-014-8458-3 [DOI] [PubMed] [Google Scholar]
- 215.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. (2008) 118:1311–21. 10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zaiss MM, Harris NL. Interactions between the intestinal microbiome and helminth parasites. Parasite Immunol. (2016) 38:5–11. 10.1111/pim.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Martin I, Djuardi Y, Sartono E, Rosa BA, Supali T, Mitreva M, et al. Dynamic changes in human-gut microbiome in relation to a placebo-controlled anthelminthic trial in Indonesia. PLoS Negl Trop Dis. (2018) 12:e0006620. 10.1371/journal.pntd.0006620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bradbury RS, Harrington H, Kekeubata E, Esau D, Esau T, Kilivisi F, et al. High prevalence of ascariasis on two coral atolls in the Solomon Islands. Trans R Soc Trop Med Hyg. (2018) 112:193–9. 10.1093/trstmh/try041 [DOI] [PubMed] [Google Scholar]
- 219.Speare R, Latasi FF, Nelesone T, Harmen S, Melrose W, Durrheim D, et al. Prevalence of soil transmitted nematodes on Nukufetau, a remote Pacific island in Tuvalu. BMC Infect Dis. (2006) 6:110. 10.1186/1471-2334-6-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Burdam FH, Hakimi M, Thio F, Kenangalem E, Indrawanti R, Noviyanti R, et al. Asymptomatic vivax and falciparum parasitaemia with helminth co-infection: major risk factors for anaemia in early life. PLoS ONE. (2016) 11:e0160917. 10.1371/journal.pone.0160917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Phuanukoonnon S, Michael A, Kirarock WS, Pomat WS, van den Biggelaar AH. Intestinal parasitic infections and anaemia among pregnant women in the highlands of Papua New Guinea. P N G Med J. (2013) 56:119–25. [PubMed] [Google Scholar]
- 222.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. (2003) 19: 547–51. 10.1016/j.pt.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 223.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. Soil transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. (2006) 367:1521–32. 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- 224.Kline K, McCarthy JS, Pearson M, Loukas A, Hotez PJ. Neglected tropical diseases of oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. (2013) 7:e1755. 10.1371/journal.pntd.0001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim SH, Rinamalo M, Rainima-Qaniuci M, Talemaitoga N, Kama M, Rafai E, et al. Island-wide surveillance of gastrointestinal protozoan infection on fiji by expanding lymphatic filariasis transmission assessment surveys as an access platform. Am J Trop Med Hyg. (2018) 98:1179–85. 10.4269/ajtmh.17-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guittet V, Ménager C, Missotte I, Duparc B, Verhaegen F, Duhamel JF. Hepatic abscesses in childhood: retrospective study about 33 cases observed in New-Caledonia between 1985 and 2003. Arch Pediatr. (2004) 11:1046–53. 10.1016/j.arcped.2004.03.101 [DOI] [PubMed] [Google Scholar]
- 227.Navarro S, Pickering DA, Ferreira IB, Jones L, Ryan S, Troy S, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. (2016) 8:362ra143. 10.1126/scitranslmed.aaf8807 [DOI] [PubMed] [Google Scholar]
- 228.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. (2013) 14:1118–26. 10.1038/ni.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mabbott NA. The influence of parasite infections on host immunity to co-infection with other pathogens. Front Immunol. (2018) 9:2579. 10.3389/fimmu.2018.02579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Giacomin P, Zakrzewski M, Croese J, Su X, Sotillo J, McCann L, et al. Experimental hookworm infection and escalating gluten challenges are associated with increased microbial richness in celiac subjects. Sci Rep. (2015) 5:13797. 10.1038/srep13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chabé M, Lokmer A, Ségurel L. Gut protozoa: friends or foes of the human gut microbiota? Trends Parasitol. (2017) 33:925–34. 10.1016/j.pt.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 232.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. (2013) 14:685–90. 10.1038/ni.2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. (2017) 279:70–89. 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Malau E, Ford R, Valcanis M, Jennison AV, Mosse J, Bean D, et al. Antimicrobial sensitivity trends and virulence genes in Shigella spp. from the Oceania region. Infect Genet Evol. (2018) 64:52–6. 10.1016/j.meegid.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 235.Greenhill AR, Guwada C, Siba V, Michael A, Yoannes M, Wawarie Y, et al. Antibiotic resistant Shigella is a major cause of diarrhoea in the Highlands of Papua New Guinea. J Infect Dev Countr. (2014) 8:1391–7. 10.3855/jidc.4396 [DOI] [PubMed] [Google Scholar]
- 236.Benny E, Mesere K, Pavlin BI, Yakam L, Ford R, Yoannes M, et al. A large outbreak of shigellosis commencing in an internally displaced population, Papua New Guinea, 2013. Western Pac Surveill Response J. (2014) 5:18–21. 10.5365/wpsar.2014.5.2.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Horwood PF, Greenhill AR. Cholera in Oceania. In: Loukas A. editor. Neglected Tropical Diseases of Oceania. Basel: Springer International Publishing; (2016). p. 1–31. 10.1007/978-3-319-43148-2_1 [DOI] [Google Scholar]
- 238.Horwood PF, Soli KW, Maure T, Naito YI, Morita A, Natsuhara K, et al. A high burden of asymptomatic gastrointestinal infections in traditional communities in Papua New Guinea. Am J Trop Med Hyg. (2017) 97:1872–5. 10.4269/ajtmh.17-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McIver L, Kim R, Woodward A, Hales S, Spickett J, Katscherian D, et al. Health impacts of climate change in pacific island countries: a regional assessment of vulnerabilities and adaptation priorities. Environ Health Perspect. (2016) 124:1707–14. 10.1289/ehp.1509756 [DOI] [PMC free article] [PubMed] [Google Scholar]